Abstract
Background and Objectives:
Artificial intelligence (AI) as a field has recently gained a lot of importance and is expected to revolutionize the health-care scenario in the near future. There have been no studies done worldwide to review the status of research with respect to the use of AI in health care. Hence, we conceptualized this study to get an overview of the clinical studies being conducted in the field of AI, by analyzing those registered on the Food and Drug Administration trial registry website.
Methodology:
All the clinical studies conducted in the field of AI registered on the ClinicalTrials.gov website up to September 2019 were reviewed and analyzed. The variables such as geographical distribution, study design, status of study whether ongoing or completed, therapy area, type of intervention tested, type of funding, and year of initiation of study were recorded. The data were analyzed using descriptive statistics using SPSS for Windows, Version 16.0 (SPSS Inc. Chicago, IL, USA).
Results:
Out of all the studies registered, 156 were related to AI. Of these 156 studies, 84 were interventional and 72 were observational. The most common therapy area under study was oncology with 26.3% studies, followed by cardiology, ophthalmology, psychiatry, and neurology. Devices comprised the most common intervention being studied, accounting to 34% of studies, followed by diagnostics which included 28% of studies. In the first 8 months of 2019 itself, 65 studies had been registered.
Conclusion:
The study revealed an increasing trend in the studies being conducted using AI techniques, with majority being conducted in the area of oncology, with medical devices being the most common intervention being tested.
Keywords: Data mining, deep learning, machine learning, trial registry
INTRODUCTION
Artificial intelligence (AI) as a field has recently gained a lot of importance and is expected to revolutionize health-care scenario in the near future. It encompasses different analytical approaches such as machine learning (ML) (including deep learning), natural language processing, and computer or machine vision.[1] ML represents the most successful branch of AI and is concerned with the development of programs with the capacity to learn from data.[2] Broadly, AI in terms of health care can be divided into the virtual and physical aspects. The virtual part involves applications such as electronic health record systems to neural network-based guidance in treatment decisions, whereas the physical part deals with the use of performing surgeries assisted by robotic technology and intelligent prostheses for the differently abled.[3]
A remarkable application of AI is its increasing role at various stages of drug development, drug target identification and validation, new drug designing, drug repurposing, improving the research and development efficiency, and analyzing biomedicine information and refining the decision-making process to recruit patients for clinical trials.[4] It has also shown to aid diagnostic techniques and with some studies has tried to apply ML methods to neuroimaging data to assist with a stroke diagnosis.[5] A study revealed that the published literature mainly concentrates around a few disease types namely cancer, nervous system disease, and cardiovascular diseases.[5] Some success has also been observed with early proof-of-concept studies in medicine, ranging from medical imaging for detecting diabetic retinopathy and skin cancer, to the use of electronic health record data to predict important clinical parameters. Overall, AI has contributed at many different levels from sophisticated natural language-processing searches of the biomedical literature, to cancer subphenotyping using deep learning (DL), to predictions of gene targets of micro-RNAs, drug–target interactions, and drug-repositioning hypotheses.[6]
There have been no studies done worldwide to review the status of research in AI in health care. Hence, we conceptualized this study to get an overview of the clinical studies being conducted in the field of AI, by analyzing those registered on the Food and Drug Administration (FDA) trial registry website.
METHODOLOGY
All the clinical studies employing AI techniques registered on the ClinicalTrials.gov website since its inception up to September 2019 were reviewed and analyzed. The studies were searched in the “Other terms” section with the “Artificial Intelligence” keyword. The variables for capturing data were geographical distribution of studies; types of study designs used, whether observational or interventional; status of study, whether ongoing or completed; therapy area; type of intervention tested; type of funding; and year of initiation of study. The variables were analyzed using descriptive statistics using SPSS for Windows, Version 16.0 (SPSS Inc. Chicago, IL, USA).
RESULTS
Out of a total of 331,536 studies registered on ClinicalTrials.gov, 156 were related to AI. Of these 156 studies, 84 were interventional and 72 were observational. The most common therapy area under study was oncology with 41 (26.3%) studies, followed by cardiology, ophthalmology, psychiatry, and neurology. The therapy areas least studied were nephrology, pediatrics, and palliative care, with one study each. Figure 1 shows the detailed distribution of the use of AI in various therapy areas across studies.
Figure 1.
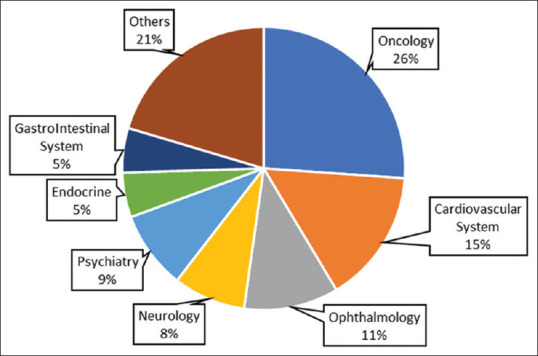
Distribution of AI studies across therapy areas
In oncology, the most common type of cancer studied was breast cancer (6 studies), and in cardiology, majority of the studies were on coronary artery diseases (9 studies).[2] Among all the studies analyzed, 66 were actively recruiting, whereas 32 were completed. One study was suspended (from the UK) and one had been terminated (from the USA). The status of the studies across different stages is illustrated in Figure 2.
Figure 2.
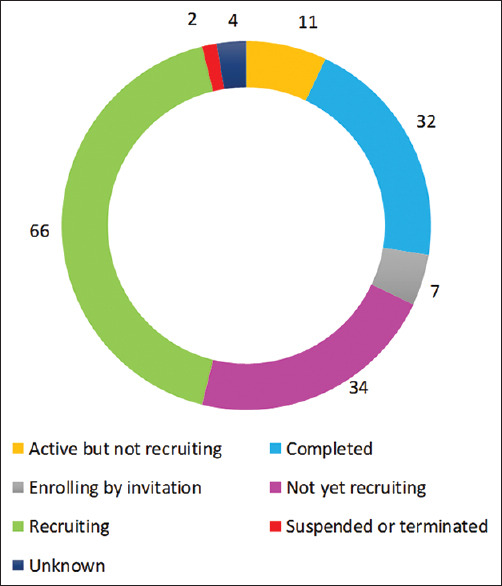
Study status
Fifty-three (34%) studies (maximum) were studying devices using AI. One such example is a study in the USA which is studying a device, which can identify intracranial bleeds on computed tomography and can notify the specialist, thus improving patient care by early detection and timely intervention by the specialist. Forty-four (28%) studies were studying the application of AI for diagnostic purposes. A study conducted in China is using an AI software for the diagnosis of glaucoma. Another example is a study conducted in Japan, which is a trial comparing the use of AI versus expert endoscopists for the diagnosis of gastric cancer in patients following upper gastrointestinal endoscopy. The type of interventions being studied by the remaining studies is represented in Figure 3. Examples of other studies using AI include studies for the identification of biomarkers in Parkinson's disease, studies using AI for measuring and improving medication adherence, and studies for the development of a predictive algorithm for the rehospitalization risk in heart failure.
Figure 3.
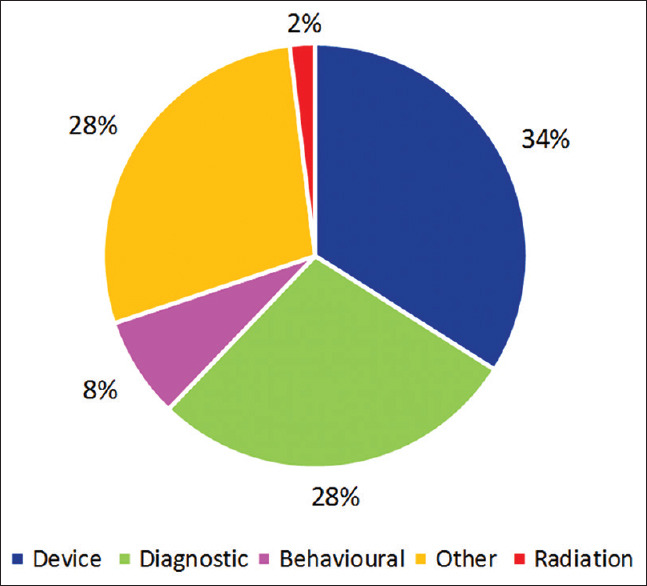
Type of intervention
Of the 156 studies, majority, i.e., 32.1%, were registered from the USA, closely followed by China with 30.8% of studies. The remaining countries from where studies are registered are summarized in Table 1.
Table 1.
Distribution of studies in artificial intelligence globally
| Country | Number of studies |
|---|---|
| USA | 50 |
| China | 48 |
| France | 11 |
| United Kingdom | 8 |
| Spain | 6 |
| Canada | 4 |
| Italy | 3 |
| Australia | 2 |
| Brazil | 2 |
| Hong Kong | 2 |
| Israel | 2 |
| Netherlands | 2 |
| Sweden | 2 |
| Switzerland | 2 |
| Taiwan | 2 |
| Austria | 1 |
| Belgium | 1 |
| Bolivia | 1 |
| India | 1 |
| Japan | 1 |
| Mexico | 1 |
| Portugal | 1 |
| Turkey | 1 |
| Zambia | 1 |
| Not provided | 1 |
A vast majority of the registered studies, i.e. 125 (80.1%) out of 156, were studying products not approved by the FDA. Only nine studies were studying FDA-approved products and for 22 (14.1%) studies, information about the approval status of the product under study was not available. The first study was registered in the year 2002. In the year 2018, there was an exponential increase in the studies applying AI in health care, with the number increasing from 13 studies in 2017 to 44 studies in 2018. In the first 8 months of 2019, 65 studies were registered, and three studies have already been registered for the years 2020 and 2021 on the website [Figure 4].
Figure 4.
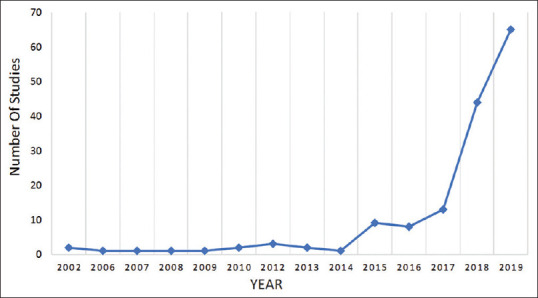
Trend of studies conducted in AI Till 2019
Five out of the 156 studies were funded by the national institutes of health (NIH) and 26 were pharma-sponsored studies. The remaining 125 had been funded by other agencies (including the USA and other international universities and organizations). Out of the 156 studies, 44 (28.2%) had a sample size of 100 or less. Sixty-eight (43.5%) studies had a sample size between 101 and 1000. Seventeen (10.8%) studies had sample size between 1001 and 2000, whereas 15 (9.6%) had a sample size between 2001 and 5000 and 10 (6.4%) studies had a sample size of more than 5000. For two studies (both from the USA), information about their sample size was not provided. The largest sample size was of 2,000,000 of a study in China from the year 2017 and the smallest sample size was of six, of a study on opioid addiction from the USA in the year 2016.
DISCUSSION
This study revealed an increase in the trend of the number of studies being conducted in AI over the years. Oncology was found to be the most common area of interest of this research, with breast cancer being the major focus. The USA was leading with the maximum number of studies in AI, followed by China.
Data mining has gained importance for the identification of novel diagnostic markers, which can help to precisely diagnose and classify the cancer as per its stages. The latest AI platform to be developed is “CURATE. AI” which has been used successfully for patients with metastatic castration-resistant prostate cancer and is being extrapolated to other forms of cancers.[7] A major advantage of deep learning is convolutional neural network (CNN) which can analyze pixel-level information from images and their ability to account for orientation of the pixels, which allows them to appreciate lines, curves, and eventually objects within images.[8] The CNN technology developed by training on 130,000 skin images was able to classify malignant lesions with higher sensitivity and specificity than a panel of 21 board-certified dermatologists.[8] A study stated that the applications of AI in precision oncology are largely due to its remarkable ability of classifying imaging data in different clinical domains and enumerated their role in the detection and classification of skin lesions, the identification and categorization of lung cancers, and the detection of metastases in women with breast cancer, all of which apply different versions of a DL technique known as CNN.[2] Similarly, at Google AI Healthcare, the researchers created a learning algorithm, LYmph Node Assistant (LYNA), that analyzed histology slides stained with tissue samples from lymph node biopsies. This could identify suspicious regions which could not be ascertained by the human eye and aid in the diagnosis of metastatic breast cancer tumors. LYNA not only showed a 99% accuracy rate of classifying a sample as cancerous or noncancerous, but also halved the average slide review time.[9] Our study also observed research developments with respect to the diagnosis of cancers, some of which included AI system for the detection and diagnosis of breast lesions using mammography, detection of lung cancer and colorectal cancer, and AI-based cytology analysis of oral cancers as well. These advances will serve in revolutionizing the prognosis, diagnosis, and treatment of cancer.
Almost one-third of the studies registered were being conducted on AI-based medical devices. The studies ranged from stethoscopes working on AI to devices to diagnose glaucoma. The FDA released its Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a medical device (SaMD) – discussion paper and request for feedback” in April 2019. This described the potential approach to premarket review for AI and ML-driven software modifications. It will enable the FDA and manufacturers to evaluate and monitor a software product from its premarket development phase to postmarket performance. This new set of recommendations was much needed as AI-based devices differ significantly from routine medical devices and need a shift from the existing regulations.[10]
There has been a phenomenal peak in the number of studies using AI starting from 2017, with more than one-third of the total number of studies being registered in the year 2019 itself. AI is already being utilized in medicine, right from scheduling of appointments and follow-ups, online check-ins in medical centers, digitization of medical records, immunization dates for children, and pregnant females to drug dosage algorithms.[3] Thus, the growth in this sector of research will pave a new way for medicine in the near future.
Our study was limited to one registry and hence cannot give a comprehensive idea about all global clinical researches in AI. However, it helps to outline the trend toward which research in AI is moving.
CONCLUSION
The study revealed an increasing trend in the studies being conducted using AI, with majority being conducted in the area of oncology, with medical devices being the most common intervention being tested.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lamberti MJ, Wilkinson M, Donzanti BA, Wohlhieter GE, Parikh S, Wilkins RG, et al. A study on the application and use of artificial intelligence to support drug development. Clin Ther. 2019;41:1414–26. doi: 10.1016/j.clinthera.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Azuaje F. Artificial intelligence for precision oncology: Beyond patient stratification. NPJ Precis Oncol. 2019;3:6. doi: 10.1038/s41698-019-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amisha, Malik P, Pathania M, Rathaur VK. Overview of artificial intelligence in medicine. J Family Med Prim Care. 2019;8:2328–31. doi: 10.4103/jfmpc.jfmpc_440_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak KK, Pichika MR. Artificial intelligence in drug development: Present status and future prospects. Drug Discov Today. 2019;24:773–80. doi: 10.1016/j.drudis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: Past, present and future. Stroke Vasc Neurol. 2017;2:230–43. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrer S, Shah P, Antony B, Hu J. Artificial intelligence for clinical trial design. Trends Pharmacol Sci. 2019;40:577–91. doi: 10.1016/j.tips.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Londhe VY, Bhasin B. Artificial intelligence and its potential in oncology. Drug Discov Today. 2019;24:228–32. doi: 10.1016/j.drudis.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Kann BH, Thompson R, Thomas CR, Jr, Dicker A, Aneja S. Artificial intelligence in oncology: Current applications and future directions. Oncology (Williston Park) 2019;33:46–53. [PubMed] [Google Scholar]
- 9.Artificial Intelligence in Medicine: Applications, Implications, and Limitations - Science in the News. 2019. [[Last accessed on 2019 Oct 12]]. Science in the News. Available from: http://sitn.hms.harvard.edu/flash/2019/artificial-intelligencein- medicine-applications-implications-and-limitations/
- 10.Artificial Intelligence and Machine Learning in Software. U.S. Food and Drug Administration. 2019. [[Last accessed on 2019 Oct 12]]. Available from: https://www.fda. gov/medical-devices/software-medical-device-samd/artificialintelligence- and-machine-learning-software-medical-device.


