Abstract
Introduction
Laboratory studies suggest an involvement of growth differentiation factor 15 (GDF-15) in metabolic dysregulation. However, the utility of GDF-15 for assessing risk of cardiometabolic outcomes has not been rigorously examined among older adults.
Methods
We conducted a cross-sectional analysis of older adults who attended visit 6 (2016–2017) of the Atherosclerosis Risk in Communities (ARIC) Study. We used multivariable logistic regression to quantify cross-sectional associations of GDF-15 (in quartiles) with prevalent diabetes, obesity, atherosclerotic cardiovascular disease (ASCVD), subclinical myocardial stress/injury (assessed by NT-proB-type Natriuretic Peptide [NT-proBNP] and high-sensitivity cardiac troponin T [hs-cTnT]), and heart failure (HF).
Results
Among 3792 ARIC study participants (mean age 80 years, 59% women, 23% Blacks and 77% Whites, mean GDF-15: 2094.9 pg/mL [SD: 1395.6]), higher GDF-15 concentrations (highest vs. lowest quartile) were positively associated with diabetes (adjusted odds ratio [aOR]:] : 2.48, 95% CI : 1.89, 3.26), ASCVD (aOR: 1.57, 95% CI: 1.16, 2.11), increased hscTnT (aOR: 2.27, 95%CI: 1.54, 3.34), increased NT-proBNP (aOR: 1.98, 95%CI: 1.46, 2.70), and HF (aOR: 3.22, 95%CI : 2.13, 4.85), in models adjusted for demographics and traditional cardiovascular risk factors.
Conclusions
In this sample of older US black and whites, increased GDF-15 was positively associated with diabetes, ASCVD, HF, and markers of subclinical myocardial stress or injury. These results illustrate the diverse aspects of the link between GDF-15 and diseases states, and its potential utility as robust biomarker of adverse cardiometabolic outcomes.
Keywords: GDF-15, diabetes, metabolism, cardiovascular disease
Introduction
Growth differentiation factor 15 (GDF-15) is a member of the transforming growth factor-β (TGF-β) cytokine superfamily (1). GDF-15 is primarily expressed in macrophages and epithelial cells (1), and its expression is positively associated with adiponectin production (2). The putative metabolic effects of GDF-15 have been described in animal studies (3), pointing to a role in energy balance and glucose homeostasis. However, the role of GDF-15 in human metabolism remains poorly understood. In small human and clinical studies, high circulating GDF-15 concentrations have been observed among individuals with diabetes, and positively associated with measures of hyperglycemia and insulin resistance (4, 5). Restoration of GDF-15 concentrations has also been described as a marker of response to obesity treatment using bariatric surgery (6).
Population-based studies have investigated the relation of GDF-15 with CVD, including heart failure and its prognosis (7). However, a limited number of community-based studies have examined the association of GDF-15 with diabetes (8, 9), or with obesity or metabolic syndrome (MetS). Moreover, extant studies of outcomes associated with GDF-15 have also had a limited scope seldom focusing on older adults and mainly including Caucasian participants (8, 9), and have yielded conflicting results with respect to diabetes (8–10). Given the emerging role of GDF-15 as a prognostic biomarker, it is important to clarify its relation with cardiometabolic outcomes among older adults.
Using data from the Atherosclerosis Risk in Communities (ARIC) Study, we comprehensively assessed the associations of GDF-15 with key cardiometabolic outcomes including diabetes, obesity, MetS, atherosclerotic cardiovascular disease (ASCVD), and heart failure (HF) with a diverse population of older White and Black adults.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study recruited 15 792 participants from 4 U.S. communities (11). The first study visit took place in 1987–1989. Since then, participants have returned for subsequent study visits and received annual initially and then semi-annual (since 2012) telephone calls. The sixth visit (visit 6) took place in 2016–2017. Of the 4003 eligible participants who attended visit 6, we excluded participants with missing GDF-15 measurements (n = 190), participants who were not Black or White (n = 10), or those who were Blacks from Minneapolis and Washington County (n = 11); leaving 3792 participants for this analysis.
All participants provided written informed consent and the study protocol was approved by the Institutional Review Board at each study site.
Laboratory Measures
Blood samples were collected, centrifuged, and stored at −70°C during ARIC visit 6 (2016–2017). GDF-15 was measured in stored samples in 2018 using an electrochemiluminescence immunoassay on a Cobas e 411 analyzer (Elecsys, Roche Diagnostics). The assay has a limit of detection (LOD) of 400 pg/mL, a measuring range of 400 to 20 000 pg/mL, and an inter-assay imprecision of 4.8%, 4.7%, and 5.1% at GDF-15 concentrations of 699 pg/mL, 1510 pg/mL, and 7264 pg/mL, respectively, in our cohort.
NT-proB-type natriuretic peptide (NT-proBNP) and high-sensitive cardiac troponin T (hs-cTnT) were also measured using an electrochemiluminescent immunoassay on an automated Cobas e411 analyzer (Roche Diagnostics). The NT-proBNP assay had an inter-assay imprecision of 4.8% and 4.3% at NT-proBNP concentrations of 152 pg/mL and 4824 pg/mL, respectively. The hs-cTnT assay had an inter-assay imprecision of 5.7% and 4.8% at hs-cTnT concentrations of 27 ng/L and 2230 ng/L, respectively.
Serum glucose was measured using the hexokinase method. Glycosylated hemoglobin (HbA1C) was measured using a high-performance liquid chromatography (Tosoh G8 Analyzer) method certified by the National Glycohemoglobin Standardization Program and aligned to the Diabetes Control and Complications Trial assay. Serum total cholesterol, triglycerides, and high‐density lipoprotein (HDL) cholesterol concentrations were measured by using automated enzymatic assays. LDL cholesterol was calculated using the Friedewald equation.
Clinical Assessment and Outcomes
A physical examination and anthropometry were performed at sixth visit (2016–2017). Diabetes was defined as either nonfasting glucose ≥200 mg/dL(≥11.1 mmol/L), fasting serum glucose ≥126 mg/dL((≥6.99 mmol/L), HbA1C ≥6.5%, receiving drug treatment for increased glucose or self-reported physician diagnosis of diabetes. Systolic and diastolic blood pressure (BP) measurements were obtained 3 times and the mean of the second and third values were recorded. Hypertension was defined as systolic BP ≥130 mm Hg, diastolic BP ≥80 mm Hg, or use of antihypertension medications. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters, and obesity was defined as BMI ≥30 kg/m2. MetS was defined using criteria proposed by the National Cholesterol Education Program—Adult Treatment Panel III (12). The presence of MetS was defined by the presence of any 3 of the following 5 criteria: (1) high triglycerides (≥150 mg/dL[≥1.69 mmol/L) or use of lipid-lowering drugs, (2) increased systolic BP (≥130 mm Hg) or diastolic BP (≥85 mm Hg) or use of antihypertensive drugs, (3) increased fasting blood glucose [≥100 mg/dL(≥5.55 mmol/L)] or known diabetes, (4) low high-density lipoprotein (HDL)–cholesterol [< 40 mg/dL (<1,04 mmol/L) in men and < 50 mg/dL (<1.29 mmol/L) in women], and (5) waist circumference (WC) ≥ 40 inches (men) or 35 inches (women).
Using previously described cut-offs (13, 14), we defined increased hs-cTnT as a value ≥31 ng/L for male and hscTnT ≥17 ng/L for female, and increased NT-proBNP as a value ≥300 pg/mL. The cut-points for NTproBNP were provided by the manufacturer and are commonly used in clinical practice for the staging of heart failure. In the present study, we also used sex-specific hscTnT and NT-proBNP cut-points based on the distribution of our data (≥99th percentile) or 72 ng/dL for hs-cTnT and 416.2 pg/mL for NT-proBNP.
Estimated glomerular filtration rate (eGFR) was calculated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation (15). Information on medical history, medication use, alcohol use, and current smoking was obtained using standardized self-report questionnaires.
Statistical Analysis
In the statistical analyses, the values of GDF-15 that were below the LOD were set to 0.5 times LOD (200 pg/mL). We compared the baseline characteristics of participants across GDF-15 quartiles using the ANOVA procedure for continuous variables or the chi-square test for categorical variables. We used linear regression models to investigate the correlates of GDF-15 with adjustment for age, sex, and race-center. We conducted a similar analysis with GDF-15 modeled as a categorical independent variable (highest quartile vs. other 3 quartiles) using logistic regression.
We used logistic regression models to evaluate the associations of GDF-15 (quartiles) with the following cardiometabolic phenotypes: diabetes, obesity, MetS, ASCVD, and HF. For all the outcomes we initially adjusted for age, sex, and race-center (Model 1). The subsequent adjustments depended on the outcomes, as follows:
Model 2A when analyzing diabetes: Model 1+ current smoking, systolic BP, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL-cholesterol, triglycerides and BMI;
Model 2B when analyzing obesity: Model 1+ current smoking, systolic BP, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL-cholesterol, triglycerides and diabetes status;
Model 2C when analyzing ASCVD and HF: Model 1+ smoking, systolic BP, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL-cholesterol, triglycerides, BMI, and diabetes status.
Model 2D when analyzing increased hsTnT and NT-pro-BNP: Model 1+ smoking, systolic BP, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL-cholesterol, triglycerides, BMI, diabetes status, estimated glomerular filtration rate (eGFR), and prevalent HF.
Model 2E when analyzing MetS: Model 1+ current smoking;
For all the outcomes, we evaluated a third model (Model 3A) which additionally included high sensitivity C-reactive protein (CRP). In persons with diabetes, Model 3B additionally included metformin, as this medication can impact GDF-15 concentrations (16). In a sensitivity analysis, we additionally included adiponectin to evaluate if observed associations with obesity were independent of this variable (Model 4 A). For the diabetes, ASCVD, and HF outcomes, we additionally adjusted for increased hsTnT and NT-pro-BNP. For the diabetes, ASCVD and HF outcomes, we also assessed the additive predictive value of GDF-15 beyond traditional risk factors, by evaluating the changes in c-statistic (prediction statistic) associated with the addition of GDF-15 to traditional variables.
We also modeled GDF-15 using restricted cubic and linear splines to more flexibly evaluate its continuous associations with each cardiometabolic outcome.
We conducted sensitivity analyses examining the association of GDF-15 with diabetes, and with increased hsTnT or NT-pro-BNP, in the subset of individuals without a history of ASCVD or HF, as well as the associations of GDF-15 with prevalent ASCVD and HF among individuals without diabetes.
A P value <0.05 was used to denote a 2-sided statistical significance. All analyses were performed using Stata version 15.
Results
A total of 3792 individuals were included, with a mean age of 80 (SD: 5) years, 59% women, 23% Blacks, and 77% Whites. Age, alcohol use, current smoking, diastolic blood pressure, HbA1C, fasting glucose, BMI, waist circumference, triglycerides, as well as the proportion of individuals with diabetes, obesity, hypertension, MetS, and prevalent cardiovascular disease were higher across increasing GDF-15 quartiles (Table 1). HDL-cholesterol and eGFR were lower across GDF-15 quartiles.
Table 1.
Baseline characteristics of ARIC study participants at visit 6 (2016–2017), by quartiles of growth derived factor (GDF)-15.
| GDF-15 Quartiles |
|||||
|---|---|---|---|---|---|
| Characteristics | Q1 (470.1-1285 pg/mL) (n = 950) | Q2 (1286-1703 pg/mL) (n = 947) | Q3 (1704-2432 pg/mL) (n = 949) | Q4 (2433-17254 pg/mL)) (n = 946) | P-value |
| Age, mean (SD) | 77.5 (3.7) | 79.2 (4.4) | 80.5 (5.0) | 81.1 (5.0) | <0.001 |
| Female, n (%) | 658 (69.3%) | 596 (62.9%) | 513 (54.1%) | 465 (49.2%) | <0.001 |
| Race/Center, n (%) | <0.001 | ||||
| Whites, Forsyth Co. | 196 (20.6%) | 215 (22.7%) | 214 (22.6%) | 200 (21.1%) | |
| Whites, Minneapolis | 310 (32.6%) | 288 (30.4%) | 277 (29.2%) | 242 (25.6%) | |
| Whites, Washington Co. | 196 (20.6%) | 238 (25.1%) | 242 (25.5%) | 307 (32.5%) | |
| Blacks, Forsyth Co. | 22 (2.3%) | 19 (2.0%) | 8 (0.8%) | 14 (1.5%) | |
| Blacks, Jackson | 226 (23.8%) | 187 (19.7%) | 208 (21.9%) | 183 (19.3%) | |
| Drinking status, n (%) | <0.001 | ||||
| Current | 539 (57.7%) | 478 (51.9%) | 432 (45.6%) | 396 (43.0%) | |
| Former | 225 (24.1%) | 245 (26.6%) | 283 (30.5%) | 335 (36.4%) | |
| Never | 171 (18.3%) | 198 (21.5%) | 213 (23.0%) | 189 (20.5%) | |
| Current smoker, n (%) | 49 (5.2%) | 54 (5.9%) | 74 (8.0%) | 82 (8.9%) | 0.005 |
| Systolic blood pressure, mm Hg | 134.8 (18.5) | 135.3 (18.0) | 134.9 (18.7) | 135.6 (20.0) | 0.78 |
| Diastolic blood pressure, mm Hg | 69.6 (10.4) | 68.3 (10.2) | 67.1 (10.1) | 65.6 (10.9) | <0.001 |
| Hypertension, n (%) | 665 (71.7%) | 735 (78.9%) | 759 (81.4%) | 806 (87.4%) | <0.001 |
| Anti-hypertensive medication use, n (%) | 618 (65.5%) | 701 (74.6%) | 764 (80.9%) | 833 (88.5%) | <0.001 |
| HbA1C, % | 5.8 (0.6) | 5.9 (0.8) | 6.0 (0.9) | 6.3 (1.0) | <0.001 |
| Glucose, mg/dL | 100.6 (18.0) | 103.8 (27.4) | 107.0 (29.3) | 113.3 (30.8) | <0.001 |
| Triglycerides, mg/dL | 108.8 (54.4) | 110.2 (51.1) | 116.0 (54.7) | 123.5 (70.3) | <0.001 |
| Total cholesterol, mg/dL | 185.5 (38.2) | 177.2 (39.9) | 172.4 (39.3) | 161.8 (40.6) | <0.001 |
| HDL-cholesterol, mg/dL | 56.2 (13.7) | 53.6 (14.2) | 51.3 (13.9) | 47.8 (13.0) | <0.001 |
| eGFR-cr, mean (SD) | 76.3 (12.4) | 70.1 (13.7) | 65.2 (15.8) | 54.4 (19.2) | <0.001 |
| Prevalent CVD, n (%) | 118 (12.4%) | 170 (18.0%) | 236 (24.9%) | 341 (36.1%) | <0.001 |
| Prevalent heart failure, n (%) | 42 (4.4%) | 56 (5.9%) | 81 (8.5%) | 187 (19.8%) | |
| Waist circumference, inches | 38.3 (5.2) | 39.3 (5.4) | 40.1 (5.5) | 40.5 (5.4) | <0.001 |
| Body mass index, kg/m2 | 27.7 (5.3) | 28.4 (5.4) | 28.5 (5.5) | 28.4 (5.4) | 0.010 |
| Obese, n (%) | 279 (29.6%) | 321 (34.3%) | 297 (31.8%) | 306 (33.3%) | 0.16 |
| Diabetes, n (%) | 195 (20.9%) | 251 (27.3%) | 312 (34.0%) | 525 (58.1%) | <0.001 |
| Metformin Use n (%) | 22 (2.3%) | 64 (6.8%) | 114 (12.0%) | 254 (26.8%) | <0.001 |
| Metabolic syndrome components, n (%) | 501 (52.7%) | 566 (59.8%) | 622 (65.5%) | 710 (75.1%) | <0.001 |
| Increased triglycerides | 530 (55.8%) | 557 (58.8%) | 612 (64.5%) | 694 (73.4%) | <0.001 |
| Increased blood pressure | 797 (83.9%) | 847 (89.4%) | 867 (91.4%) | 892 (94.3%) | <0.001 |
| Increased glucose | 371 (39.1%) | 418 (44.2%) | 463 (48.9%) | 572 (60.6%) | <0.001 |
| Low HDL-cholesterol | 219 (23.1%) | 269 (28.5%) | 330 (34.8%) | 405 (42.9%) | <0.001 |
| Large waist circumference | 577 (62.2%) | 621 (68.2%) | 610 (67.7%) | 612 (70.1%) | 0.003 |
| Metabolic syndrome, n (%) | 340 (46.1%) | 349 (52.2%) | 353 (58.4%) | 217 (57.4%) | <0.001 |
Values are mean±SD for continuous variables, and n (%) for categorical variables. BMI, body mass index; CVD, cardiovascular diseases; HDL, high‐density lipoprotein; Metabolic syndrome was estimated among those without diabetes (n = 2390).
To convert glucose, triglycerides, total cholesterol and HDL cholesterol from mg/dL to mmol/L multiply by 0.0555, 0.0113, 0.0259, and 0.0259, respectively.
Clinical Correlates of GDF-15
In age‐, sex-, and race‐center—adjusted analyses, GDF-15 was correlated with various traits (Table 2). The main correlates of GDF-15 included current smoking (β coefficient: 0.13, 95% confidence interval (CI): 0.07, 0.19), diabetes status (β: 0.31, 95%CI: 0.28, 0.35), BMI (β per 1 SD change: 0.036, 95%CI: 0.021, 0.052), total cholesterol (β per 1 SD change: -0.089, 95%CI: -0.105, -0.073), HDL-cholesterol (β per 1 SD change: -0.097, 95%CI: –0.113, -0.081)) and triglycerides (β per 1SD change: 0.057, 95%CI: 0.042, 0.072). Similar correlates were identified in models including GDF-15 as a categorical outcome, comparing the highest quartile to the other 3 quartiles (Table 2); for example the odds ratio for the diabetes status and GDF-15 association was 4.15 (95%CI: 3.51, 4.91).
Table 2.
Correlates of GDF-15 among ARIC study participants at visit 6 (2016–2017).
| GDF-15 (log-transformed) | GDF-15—quartile 4 vs. quartiles 1 to 3 | |
|---|---|---|
| Predictors | Beta Coefficient (95% CI) i | Odds Ratio (95% CI) i |
| Current smoking | 0.13 (0.07, 0.19) | 1.64 (1.23, 2.18) |
| Systolic blood pressure (mm Hg)ii | 0.002 (-0.013, 0.018) | 1.02 (0.94, 1.10) |
| Use of antihypertensive medications | 0.21 (0.18, 0.25) | 2.63 (2.10, 3.29) |
| Use of cholesterol lowering medication | 0.13 (0.10, 0.16) | 1.72 (1.47, 2.02) |
| Total cholesterol (mg/dL)ii | −0.089 (-0.105, -0.073) | 0.67 (0.62, 0.74) |
| High‐density lipoprotein—cholesterol (mg/dL)ii | −0.097 (-0.113, -0.081) | 0.65 (0.59, 0.71) |
| Triglycerides (mg/dL)ii | 0.057 (0.042, 0.072) | 1.23 (1.15, 1.33) |
| Body mass index (kg/m2)ii | 0.036 (0.021, 0.052) | 1.11 (1.02, 1.20) |
| Diabetes status | 0.31 (0.28, 0.35) | 4.15 (3.51, 4.91) |
Estimates are adjusted for age, sex, and race/center.
Per 1 SD change (1 SD: Systolic blood pressure = 18.80 mm Hg; Total cholesterol = 40.41 mg/dL; High-density lipoprotein- cholesterol =14.02 mg/dL; Triglycerides= 58.37 mg/dL; Body mass index = 5.41 kg/m2).
Associations of GDF-15 with Cardiometabolic Outcomes
In analyses examining categorical associations of GDF-15 with outcomes, compared to the lowest quartile, the highest quartile of GDF-15 was significantly associated with diabetes (adjusted odds ratio [aOR]: 2.48, 95% CI1.89, 3.26), ASCVD (aOR: 1.57, 95%CI: 1.16, 2.11), increased hscTnT (aOR: 2.27, 95%CI: 1.54, 3.34), increased NT-proBNP (aOR: 1.98, 95%CI: 1.46, 2.70), and HF (aOR: 3.22, 95%CI: 2.13, 4.85) in multivariable-adjusted models (Table 3). For the diabetes, ASCVD, and HF outcomes, additionally adjusting for hscTnT and NT-proBNP did not materially affect our results (Model 4A, Table 3). For the obesity outcome, additionally accounting for adiponectin did not significantly change our results (Model 4B, Table 3). Regarding the increased NT-proBNP and hscTnT outcomes, the use of alternative definitions based on the cohort and sex-specific distributions of these markers showed similar results (Supplemental Table 1).
Table 3.
Association of growth derived factor (GDF)-15 with cardiometabolic outcomes among ARIC study participants at visit 6 (2016–2017).
| Odds Ratio (95% Confidence Interval) |
|||||||
|---|---|---|---|---|---|---|---|
| Models | GDF-15 quartiles | Diabetes | Obesity | Prevalent ASCVD | Prevalent HF | High TnT (hscTnT ≥ 31 ng/L for male and hscTnT ≥ 17 ng/L for female) | High NT-proBNP (BNP ≥ 300 pg/mL) |
| Model 1 | Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.60 (1.28, 1.99) | 1.44 (1.18, 1.76) | 1.31 (0.99, 1.72) | 1.31 (0.87, 1.98) | 1.74 (1.24, 2.46) | 1.45 (1.14, 1.86) | |
| Q3 | 2.33 (1.87, 2.90) | 1.41 (1.15, 1.74) | 1.80 (1.38, 2.35) | 1.84 (1.24, 2.73) | 2.72 (1.95, 3.78) | 1.98 (1.55, 2.52) | |
| Q4 | 6.77 (5.41, 8.48) | 1.57 (1.28, 1.94) | 2.46 (1.90, 3.20) | 4.96 (3.45, 7.14) | 8.06 (5.88, 11.05) | 4.78 (3.77, 6.05) | |
| Model 2 | Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.41 (1.11, 1.79) | 1.28 (1.03, 1.60) | 1.21 (0.90, 1.63) | 1.08 (0.69, 1.69) | 1.13 (0.78, 1.63) | 1.12 (0.85, 1.48) | |
| Q3 | 1.78 (1.40, 2.26) | 1.03 (0.82, 1.29) | 1.48 (1.11, 1.98) | 1.47 (0.96, 2.24) | 1.36 (0.95, 1.97) | 1.21 (0.91, 1.60) | |
| Q4 | 4.86 (3.80, 6.21) | 0.89 (0.70, 1.14) | 1.58 (1.17, 2.13) | 3.20 (2.13, 4.82) | 2.28 (1.55, 3.35) | 2.03 (1.49, 2.77) | |
| Model 3 | Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.12 (0.87, 1.45) | 1.27* (1.02, 1.58) | 1.21 (0.89, 1.63) | 1.08 (0.69, 1.69) | 1.13 (0.78, 1.63) | 1.11 (0.85, 1.47) | |
| Q3 | 1.17 (0.90, 1.53) | 1.00 (0.80, 1.25) | 1.47 (1.10, 1.97) | 1.47 (0.96, 2.25) | 1.36 (0.95, 1.96) | 1.19 (0.90, 1.58) | |
| Q4 | 2.48 (1.89, 3.26) | 0.84 (0.65, 1.07) | 1.57 (1.16, 2.11) | 3.22 (2.13, 4.85) | 2.27 (1.54, 3.34) | 1.98 (1.46, 2.70) | |
| Model 4 | Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||
| Q2 | 1.10 (0.85, 1.43) | 1.26 (1.01, 1.58) | 1.19 (0.88, 1.60) | 1.05 (0.67, 1.64) | |||
| Q3 | 1.14 (0.88, 1.49) | 0.97 (0.77, 1.23) | 1.42 (1.06, 1.90) | 1.36 (0.89, 2.10) | |||
| Q4 | 2.23 (1.68, 2.96) | 0.88 (0.68, 1.13) | 1.27 (0.93, 1.74) | 2.19 (1.43, 3.37) | |||
ASCVD: atherosclerotic cardiovascular disease, HF: heart failure, hs-cTnT: High-sensitive cardiac troponin T, NT-proBNP: NT-proB-type Natriuretic Peptide.
Model 1 adjusts for age, sex, and race-center.
Model 2a (diabetes outcome): Model 1 + current smoking, systolic blood pressure, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL cholesterol, triglycerides and body mass index; Model 2 b (obesity outcome): Model 1 + current smoking, systolic blood pressure, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL cholesterol, triglycerides, and diabetes status; Model 2c (ASCVD or HF): outcomes includes for Model 1 + current smoking, systolic blood pressure, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL cholesterol, triglycerides, BMI, and diabetes status; Model 2d: (increased hsTnT or BNP): Model 1 + current smoking, systolic blood pressure, use of antihypertensive medications, use of cholesterol lowering medications, total cholesterol, HDL cholesterol, triglycerides, body mass index, diabetes status, eGFR and prevalent CVD/HF.
Model 3 adjusts for Model 2 + C-reactive protein—Model 3 for the diabetes outcome adjusts for Model2 + C reactive protein+ metformin use.
Model 4a (diabetes, ASCVD and HF): Model 3 + hs-cTnT and NT-proBNP.
Model 4 b (obesity): Model 3 + adiponectin (Visit 5).
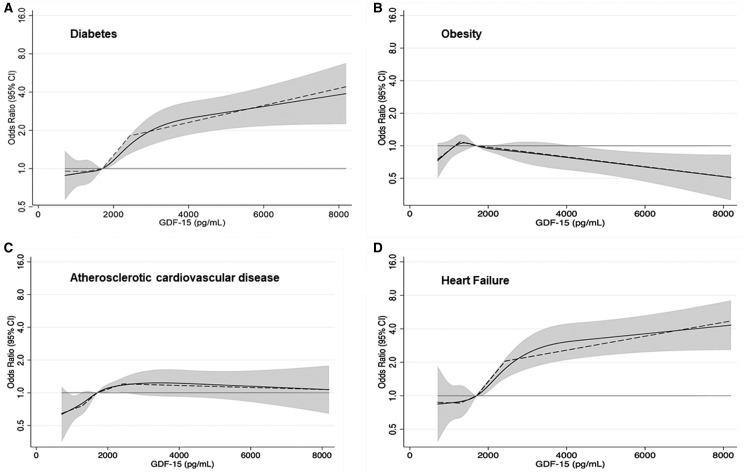
GDF-15 was not significantly associated with obesity (aOR for highest vs. lowest quartile: 0.84, 95%CI: 0.65, 1.07). We observed roughly linear associations of GDF-15 with diabetes, obesity, ASCVD, and HF (Fig. 1). GDF-15 appeared to have a J-shaped association with obesity. The exploration of MetS revealed a positive association with GDF-15 (aOR: 3.09, 95% CI; 2.49, 3.82) (Supplemental Table 2).
Fig. 1.
Restricted cubic (solid) and piece-wise linear (dashed) splines of GDF-15 associations with various outcomes—Panel (A) diabetes, panel (B) obesity, panel (C) atherosclerotic cardiovascular disease, and panel (D) heart failure.
In the subpopulation of ARIC participants without a history of ASVCD or HF, we found a significant association of GDF-15 with diabetes (aOR for diabetes for highest vs. lowest quartile: 4.81, 95% CI 3.61, 6.40) (Supplemental Table 3). In the participants without diabetes, GDF-15 was significantly associated with HF (aOR 3.99, 95%CI: 2.31, 6.9) but not significantly associated with ASCVD (aOR: 1.41, 95%: 0.95, 2.11) (Supplemental Table 3).
Among individuals without prevalent CVD or HF (Supplemental Table 4), GDF-15 remained significantly associated with increased hscTnT (aOR: 2.22, 95%CI: 1.42, 3.48), and increased NT-proBNP (aOR: 1.90, 95%CI: 1.32, 2.75).
The addition of GDF-15 to a model including traditional risk factors (Model 3A or 3B, Table 3) showed that GDF-15 significantly improved risk prediction for diabetes (c-statistic for model without GDF-15: 0.829 vs. c-statistic for model with GDF-15: 0.836, P value for difference: 0.007), and for HF (0.714 vs. 0.742, P value for difference: 0.001), but not for ASCVD (0.753 vs. 0.756, P value for difference: 0.071).
Discussion
In a cross-sectional investigation of a large community-based sample of Black and White older adults, we examined the association of GDF-15 with major cardiometabolic outcomes. Circulating GDF-15 was positively associated with diabetes, ASCVD, subclinical myocardial injury and stress, and HF. However, the association of GDF-15 with obesity was more complex, with evidence for a J-shape. The divergent directions of the associations of GDF-15 with diabetes and obesity suggest that the former is most probably the main driver of the association with the MetS outcome. The observations made suggest that GDF-15 is potentially a robust biomarker for various disease states. Our findings reflect the complexity of GDF-15 metabolism which involves various tissues (e.g., myocardium, vessels, and adipose tissue) and multiple pathways such as those involved in glucose regulation. Indeed, GDF-15 has been implicated in various processes including inflammation, apoptosis, and vascular injury (3, 17).
The correlates of GDF-15 identified in our study were similar to those described in prior reports, including the PIVUS study which included older adults (18), the Rancho Bernardo study (19), and younger individuals in the Framingham Heart Study (20). Our study complements and extends the later findings. Our findings of GDF-15 associations with most of the examined cardiometabolic outcomes are consistent with results from previous studies. Higher GDF-15 concentrations have been described among individuals with diabetes or impaired glucose tolerance as compared to individuals without glycemic impairment (4, 5, 8, 20), and have also been prospectively associated with insulin resistance and diabetes (9, 21). Our ASCVD results complement the evidence from prior investigations, which showed a positive relation of GDF-15 with overt ASCVD (7, 18, 22–24) and subclinical atherosclerosis (25, 26). Similarly a number of studies have also shown high concentrations of GDF-15 among individuals with prevalent HF (27–30). While prior studies have investigated the HF prognostic utility of GDF-15 above and beyond that of hsTcnT and/or NT-proBNP (27, 31), these have seldom examined the direct link between GDF-15 and subclinical measures of myocardial injury and stress (27). The observed relations of GDF-15 with diabetes and other metabolic traits as captured in the MetS entity may partially explain the associations between GDF-15 and CVD, including atherosclerotic conditions and HF (7, 26, 32). The observation of an attenuation of the GDF-15 and ASCVD association after the exclusion of individuals with diabetes in our study, suggests that dysglycemia may play a role in the GDF-15 and atherosclerosis pathway.
The exact mechanisms by which GDF-15 may modulate the risk of cardiovascular disease and affects metabolic regulation are still not clearly understood. GDF-15 appears to be a stress-induced cytokine reflecting damages in a variety of tissues, including the heart and the vessels (17). The effects on GDF-15 on the vascular system include pro-atherogenic effects possibly through LDL oxidization (33). GDF-15 has been described as a marker of myocardial fibrosis (34), though it may also limit excessive myocardial hypertrophy (35). Regarding its metabolic effects, GDF-15 may act as an adipokine (2), thus its link to an activation of the transcription factor p53, which contributes to inflammation and insulin resistance (36). Increased GDF-15 concentrations may also reflect mitochondrial dysfunction (3), which contributes to the adverse vascular and myocardial effects and impairment of glucose tolerance. Our findings on obesity are congruent with data from experimental models showing that GDF-15 has anorexic or weight loss effects (37, 38), through a counter-regulatory energy feedback loop including the hypothalamus (where GFD-15 receptors are located (39)), which upon stimulation by GDF-15 attempts to limit excess energy intake in the setting of obesity (40).
Strengths of our study include the community-based design, the large sample of Blacks and White older individuals, the examination of a wide spectrum of cardiometabolic outcomes, and the rigorous measurement of potential confounding factors. Nonetheless, there are limitations that should be considered in the interpretation of our results. First, the observational cross-sectional nature of our study limits causal inferences, especially as GDF-15 may both contribute to and be a marker of cardiometabolic risk. Second, while we were able to account for many measured clinical factors, our effect estimates may be subject to residual confounding. Finally, we included many comparisons in this study which raises the concern of a false positive result.
Conclusion
In conclusion, GDF-15 concentrations were associated with diabetes, MetS, prevalent ASCVD, and HF in a large community‐based sample of older individuals. Our findings illustrate the complexity of the link between GDF-15 and diseases states. Specifically, this points to a potential adverse impact of increased concentrations GF-15 on glucose metabolism, vascular biology, and myocardial function, whereas of the association of GDF-15 with obesity was not robust. The findings also support a potential role for GDF-15 as a clinical biomarker of cardiometabolic risk in the community. Prospective investigations and interventional studies are needed to further characterize how the GDF-15 pathway modulates the occurrence of cardiometabolic outcomes.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations
- ARIC
Atherosclerosis Risk in Communities Study
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CRP
C-reactive protein
- eGFR
estimated glomerular filtration rate
- GDF-15
growth differentiation factor 15
- HDL
high density lipoprotein
- HF
heart failure
- hs-cTnT
high-sensitivity cardiac troponin T
- LDL
low density lipoprotein
- LOD
limit of detection
- MetS
metabolic syndrome
- NT-proBNP
NT-proB-type natriuretic peptide
- WC
waist circumference
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
J.B. Echouffo-Tcheugui, study design and conception. N. Daya, statistical analysis; D. Wang, statistical analysis; E. Selvin, financial support, statistical analysis, administrative support.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest.
Employment or Leadership
None declared.
Consultant or Advisory Role
R.C. Hoogeveen has received consulting fees from Denka Seiken outside the scope of the current research study.
Stock Ownership
None declared.
Honoraria
None declared.
Research Funding
The Atherosclerosis Risk in Communities Study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). J.B. Echouffo-Tcheugui was supported by NIH/NHLBI grant K23 HL153774. E. Selvin was supported by NIH/NHLBI grant K24 HL152440, and NIH/NIDDK grant R01DK089174. E. Selvin and C.M. Ballantyne were supported by R01-HL134320. Roche supplied reagents for the measurement of GDF-15, NT-proBNP and hs TnT. C.E. Ndumele is supported by NIH grant R01HL146907. R.C. Hoogeveen has received grant support from Denka Seiken outside the scope of the current research study.
Expert Testimony
None declared.
Patents
None declared.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Disclaimer: The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Unsicker K, Spittau B, Krieglstein K.. The multiple facets of the TGF-β family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev 2013;24:373–84. [DOI] [PubMed] [Google Scholar]
- 2. Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, et al. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 2009;150:1688–96. [DOI] [PubMed] [Google Scholar]
- 3. Lockhart SM, Saudek V, O’Rahilly S.. GDF15: A hormone conveying somatic distress to the brain. Endocr Rev 2020;41:610–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dostálová I, Roubíček T, Bártlová M, Mráz M, Lacinová Z, Haluzíková D, et al. Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: The influence of very low calorie diet. Eur J Endocrinol 2009;161:397–404. [DOI] [PubMed] [Google Scholar]
- 5. Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem 2011;57:309–16. [DOI] [PubMed] [Google Scholar]
- 6. Kleinert M, Bojsen-Møller KN, Jørgensen NB, Svane MS, Martinussen XC, Kiens B, et al. Effect of bariatric surgery on plasma GDF15 in humans. Am J Physiol - Endocrinol Metab 2019;316:E615–E621. [DOI] [PubMed] [Google Scholar]
- 7. Wollert KC, Kempf T, Wallentin L.. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin. Chem 2017;63:140–51. [DOI] [PubMed] [Google Scholar]
- 8. Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, et al. Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: The Whitehall II study. Eur J Endocrinol 2010;162:913–7. [DOI] [PubMed] [Google Scholar]
- 9. Bao X, Borné Y, Muhammad IF, Nilsson J, Lind L, Melander O, et al. Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmö Diet and Cancer–Cardiovascular Cohort. Diabetologia 2019;62:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Au Yeung SL, Luo S, Schooling CM.. The impact of GDF-15, a biomarker for metformin, on the risk of coronary artery disease, breast and colorectal cancer, and type 2 diabetes and metabolic traits: a Mendelian randomisation study. Diabetologia 2019;62:1638–46. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 13. Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, Hashim IA, et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014;63:1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Januzzi JL, Van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The international collaborative of NT-proBNP study. Eur Heart J 2006;27:330–7. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration), et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coll AP, Chen M, Taskar P, Rimmington D, Patel S, Tadross JA, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020;578:444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emmerson PJ, Duffin KL, Chintharlapalli S, Wu X.. GDF15 and Growth Control. Front Physiol 2018;9:1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur Heart J 2009;30:2346–53. [DOI] [PubMed] [Google Scholar]
- 19. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E.. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The rancho bernardo study. Circulation 2011;123:2101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho JE, Mahajan A, Chen MH, Larson MG, McCabe EL, Ghorbani A, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem 2012;58:1582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: Results from the XENDOS trial. Eur J Endocrinol 2012;167:671–8. [DOI] [PubMed] [Google Scholar]
- 22. Schopfer DW, Ku IA, Regan M, Whooley MA.. Growth differentiation factor 15 and cardiovascular events in patients with stable ischemic heart disease (The Heart and Soul Study). Am Heart J 2014;167:186–92. [DOI] [PubMed] [Google Scholar]
- 23. Brown DA, Breit SN, Buring J, Fairlie WD, Bauskin AR, Liu T, et al. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: A nested case-control study. Lancet 2002;359:2159–63. [DOI] [PubMed] [Google Scholar]
- 24. Kempf T, Sinning JM, Quint A, Bickel C, Sinning C, Wild PS, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: Results from the atherogene study. Circ Cardiovasc Genet 2009;2:286–92. [DOI] [PubMed] [Google Scholar]
- 25. Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the dallas heart study. Clin Chem 2012;58:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gopal DM, Larson MG, Januzzi JL, Cheng S, Ghorbani A, Wollert KC, et al. Biomarkers of cardiovascular stress and subclinical atherosclerosis in the community. Clin Chem 2014;60:1402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1054–60. [DOI] [PubMed] [Google Scholar]
- 28. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, et al. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the valsartan heart failure trial. Circulation 2010;122:1387–95. [DOI] [PubMed] [Google Scholar]
- 29. Stahrenberg R, Edelmann F, Mende M, Kockskämper A, Düngen HD, Lüers C, et al. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail 2010;12:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santhanakrishnan R, Chong JPC, Ng TP, Ling LH, Sim D, Toh G. Leong K, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2012;14:1338–47. [DOI] [PubMed] [Google Scholar]
- 31. Sharma A, Stevens SR, Lucas J, Fiuzat M, Adams KF, Whellan DJ, et al. Utility of growth differentiation factor-15, a marker of oxidative stress and inflammation, in chronic heart failure: insights from the HF-ACTION Study . JACC Heart Fail 2017;5:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ho JE, Lyass A, Courchesne P, Chen G, Liu C, Yin X, et al. Protein biomarkers of cardiovascular disease and mortality in the community. J Am Heart Assoc 2018;7:e008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res 2004;318:325–33. [DOI] [PubMed] [Google Scholar]
- 34. Lok SI, Winkens B, Goldschmeding R, Van Geffen AJP, Nous FMA, Van Kuik J, et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail 2012;14:1249–56. [DOI] [PubMed] [Google Scholar]
- 35. Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 2006;98:342–50. [DOI] [PubMed] [Google Scholar]
- 36. Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 2009;15:1082–7. [DOI] [PubMed] [Google Scholar]
- 37. Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, et al. NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes 2014;38:1555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 2017;23:1150–7. [DOI] [PubMed] [Google Scholar]
- 39. Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 2017;23:1215–9. [DOI] [PubMed] [Google Scholar]
- 40. Baek SJ, Eling T.. Growth differentiation factor 15 (GDF15): A survival protein with therapeutic potential in metabolic diseases. Pharmacol Ther 2019;198:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.