Pharmacokinetic and pharmacodynamic (PK/PD) analysis required to advance drug compounds towards clinical testing is commonly carried out in animals, however, the results are often not predictive of human outcomes1. In vitro PK/PD modeling approaches exist2,3, but they fail to provide quantitative in vitro-to-in vivo translation (IVIVT) of human PK parameters4. Here we describe a predictive human IVIVT platform that uses physiologically-based PK (PBPK) in silico modeling with biomimetic scaling based on data generated from multiple, 2-channel, human Organ-on-a-Chip (Organ Chip) microfluidic culture devices that are fluidically linked to each other via their endothelium-lined vascular channels, and to an arterio-venous fluid mixing reservoir (AV Reservoir), by sequential robotic liquid transfers of a common blood substitute medium. Using a first-pass metabolism PBPK model with Gut, Liver and Kidney Chips for orally administered nicotine, and the same model with Bone Marrow, Liver, and Kidney Chips for intravenous cisplatin, predictions of human PK parameters for both drugs where obtained that were quantitatively similar to human values. Cisplatin PD (toxicity) also was modeled effectively in the Bone Marrow Chip. Thus, this microengineered IVIVT platform may enable predictions of drug absorption, distribution, metabolism, excretion, and toxicity (ADMET), in addition to predicting clinical PK/PD parameters which could help better design drug administration regimens in Phase I clinical trials in the future.
The high failure rate of drugs in clinical trials is in part due to fundamental interspecies differences between humans and animals used in preclinical testing, which often lead to incorrect predictions of critical human PK/PD parameters (e.g., clearance, safety margins, toxicity, efficacy)1. Isolated cells from relevant organs (e.g., hepatocytes from liver) and organ-specific cell cultures have been used for in vitro-in vivo correlation or extrapolation of certain drug compound and PK properties (e.g., hepatic clearance), but these approaches do not reliably or quantitatively predict human PK parameters or PD responses4. One potential way to confront this challenge is to use cultures of human organ-specific cells in microfluidic devices, transfer fluids between them, and analyze drug levels, cell-specific markers and metabolism in vitro5,6,7,8,9. Qualitative predictions of some drug toxicity responses have been made using PK/PD models with such multiphysiological systems (e.g., liver, lung, kidney, etc.)10,11,12,13, but they have not been able to quantitatively translate in vitro results to in vivo PK parameters (IVIVT). Moreover, the physiological relevance of these results is unclear as the drug-containing medium in these devices flows directly from one parenchymal tissue type to another (e.g., from hepatocytes to lung epithelium) without passing through the endothelial tissue barrier that is crucial for defining drug PK behavior in vivo7. Manual transfer of fluid between cultured intestine, liver, kidney, skeletal muscle, and blood-brain-barrier (BBB) microfluidic systems (only some of which contained endothelium-lined channels) combined with PK/PD modeling resulted in organ-specific processing of drug metabolism and barrier penetrance that were consistent with clinical data14; however, these results did not result in quantitative PBPK predictions that are needed by the clinical and pharmaceutical communities for meaningful compound assessment or design of optimal clinical drug administration regimens due to the lack of physiologically relevant organ-organ linking.
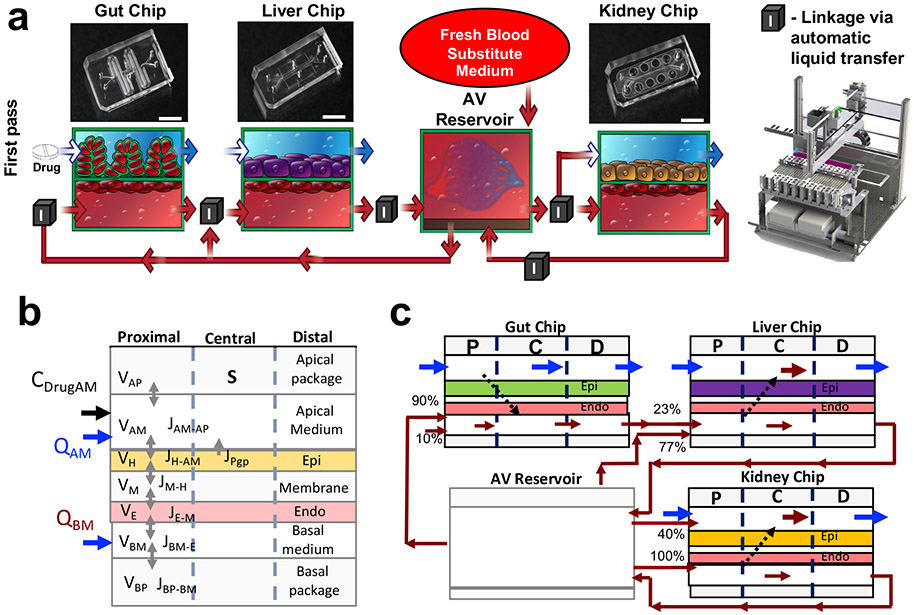
To address this challenge, we created a first pass model of human drug absorption, metabolism, and excretion by fluidically coupling 2-channel, microfluidic, human Organ Chip models of Gut, Liver, and Kidney15,16 ,17 through their vascular endothelium-lined channels, which are separated by a porous extracellular matrix-coated membrane from parallel channels lined by human organ-specific parenchymal cells, and integrating an AV Reservoir into the fluid path to enable drug mixing (Fig. 1a, Supplementary Information Table 1 & 2, Methods). The AV reservoir is a key design feature because it mimics the compartment representing the systemic circulation, and it can be used to measure blood and plasma concentrations of drug, which is critical for determining clinically relevant PK values. We modified the Gut Chip by lengthening its channels in a serpentine pattern (Fig. 1a) to increase the epithelial surface area by five times compared to the original design15, and thereby enhance its absorptive capacity. Importantly, the presence of endothelium-lined vascular channels in all Organ Chips enabled the entire multi-organ system to be perfused with a common ‘blood substitute’ in the form of an optimized low serum-containing endothelial cell medium16 (also see Methods). In this manner, fluid transfers between vascular channels of different Organ Chips both supports long term viability and allows us to mimic physiological systemic drug transport between organs specifically via the endothelium-lined vasculature. The parenchymal channel of each Organ Chip is perfused with a different medium that is optimized for the organ-specific epithelium, which provides another advantage over single channel microfluidic devices. The ability to directly interface parenchymal and vascular tissues separated by a porous membrane in these 2-channel Organ Chips is also crucial as drugs normally pass across the endothelium-parenchymal tissue interface of each organ in vivo, and the endothelium is a major contributor to drug toxicities as well as ADME and PK behaviors18.
Fig. 1. Development of a first pass multi-Organ Chip platform.
(a) Diagrammatic representations (bottom) of the Gut, Liver, and Kidney Chips containing apical parenchymal and basal vascular compartments separated by a porous matrix-coated membrane, as well as how they are fluidically linked to each other and to the AV Reservoir; photographs of the Organ Chips are shown at the top. Red arrows indicate medium flow path and direction; box with ‘I’ indicates sites where fluid was transferred by the automated liquid handling instrument between the AV Reservoir and input reservoirs of the channels of the different chips, as well as between the output and input reservoirs of different Chips. (b) Schematic of the multi-compartment reduced order (MCRO) in silico model of an individual Organ Chip. All organ chips have similar barrier configuration composed of horizontally stacked compartments with volume V shown schematically: Lower wall of the PDMS device (basal package), medium in the vascular channel (basal medium), endothelium (Endo), thin porous PDMS layer (membrane), epithelium (Epi), medical in the parenchymal channel (apical medium), and upper wall of the PDMS device (apical package). All organ devices are represented with similar mathematical equations based on drug mass balance in between the compartments, calculated for the drug flux J in between the compartments and Q, the volumetric medium flow to give the drug concentration C. Each organ device is further discretized into three axial zones (proximal, central, distal), creating a two-dimensional and less computationally demanding model to simulate a specific drug concentration over time. (c) Schematic of the first passage multi-Organ Chip linked system, where the organ-specific parenchymal epithelial cell layers of the Gut, Liver and Kidney Chips are represented by a drug-specific set of parameters for passive permeability, efflux, and metabolism determined experimentally in single Organ Chip studies and then calibrated for the linked Organ Chip platform. The direction of flow and the percentage of input flow distributions from other Organ Chips vs. the AV Reservoir are also indicated in the diagram.
The common blood substitute medium was transferred between the endothelium-lined channels of the different Organ Chips (i.e., via input and output mini-reservoirs on each chip), and between them and the AV Reservoir, using an automated Organ Chip fluid transfer instrument16. This instrument sequentially transfers multiple small volumes (down to 0.05 mL) of medium between up to 10 individual Organ Chips using a programmable robotic fluid handler (Fig. 1a, Supplementary Fig. 1, Supplementary Fig. 2) at given time points (from mins to hours or days) Supplementary Information Table 3. This provides a major advantage over continuous (serial) fluid coupling because there is much less dead space, the system is fully reconfigurable, and the same instrument can be used to automatically remove sample aliquots from the different fluid compartments for biochemical and mass spectrometry (MS) analysis. A detailed description of the chip handling, pipetting, perfusion and imaging capabilities of the instrument has been described16, and additional details of timelines, experimental requirements and workload are included in Organ Chip Linkage section of the Methods. Incorporation of the AV Reservoir into the system (Fig. 1a, Supplementary Fig. 1, Supplementary Fig. 2) also allows us to emulate drug dilution and systemic distribution due to blood flow through the entire human vasculature. This approach enabled us to normalize the total volume of the AV Reservoir to the total human blood volume, and distribute flows from the AV Reservoir to the vascular channel of each of the Organ Chips in a manner corresponding to the percentage of cardiac output that flows to each of these organs in vivo (Supplementary Table 4). The AV Reservoir also allowed us to overcome limitations of past studies that used a simple serial linkage between different microfluidic multiphysiological systems11,12,14, which resulted in cumulative drug loss with each organ linkage due to removal of experimental medium samples and replenishment with fresh medium; we observed the same limitation with serially linked 2-channel Organ Chips (Supplementary Fig. 3). This setup also overcomes other limitations of serial Organ Chip linking including maintaining even flow and pressure throughout the system as well as physiologically relevant drug distributions and organ-organ interactions16,12. The greatest advantage, however, is that drug concentrations within the vascular channels of all the chips can be measured with MS analysis by sampling only the AV Reservoir, and these quantitative values can then be compared directly to drug concentrations measured in blood samples from human clinical studies, thereby enabling IVIVT of PK parameters. As the automated platform is used to transfer the blood substitute medium from the AV reservoir in discrete time intervals every 12 hours (2 times a day) with each period of linking taking a maximum time of 40 min, this is to be taken into account in the experimental design, and it could pose a limitation for analysis of PK/PD of drugs with short half-lives. To circumvent this limitation, we used computational PK/PD modeling to transform the discrete linking time points into continuous flow (Supplementary Fig.1f,g, Supplementary info Table 3), as further described below.
To carry out quantitative IVIVT using this first pass multi-Organ Chip model, we developed a computational PBPK in silico model with biomimetic scaling based on a validated PK/PD framework18 using data generated by our experimental system. The workflow and rationale of our method are outlined in a three-step feed-back loop (Supplementary Fig.1f-h). Step one is to create initial in silico models using data obtained from experiments with individual Organ Chips where small molecules or drugs (e.g., inulin, nicotine) were perfused through each device over 24-72 hrs. For example, a small molecular drug was infused into the lumen of the Gut Chip with or without living cells to mimic oral administration, and its concentrations in the apical and basal channel inlets and outlets were quantified using MS; similar studies were carried with the Liver and Kidney Chips but the drug was perfused through the vascular channels. These data were then used in combination with the physicochemical parameters of the drug and published values for active metabolism and transport functions of the various human organ-specific epithelium, as well as results of quantifying passive drug absorption by the poly-dimethylsiloxane (PDMS) materials of the device and porous membrane (termed. ‘package loss’ and measured without cells)19, to develop computational models for each of the individual Organ Chips. These models were based on ordinary differential equation (ODE)-based, distributed (spatiotemporal), multi-compartment reduced-order (MCRO) models. The MCRO models were discretized in 3 axial zones (proximal, central, distal) along the flow axis, to allow for fast computation in linked Organ Chip studies (Fig. 1b, c, Supplementary Fig.1f, g, Supplementary Fig. 4), which was validated against computationally-demanding quasi-3D models. The MCRO model divides the Organ Chips in the following compartments: apical and basal medium channels, epithelial and endothelial cells, apical and basal PDMS package and the PDMS membrane (Fig. 1c, Supplementary Fig 4). A set of flux equations describe the drug properties in each compartment (Methods eq. 19-24). These chemical properties include passive permeability, efflux, and metabolism that were measured for nicotine in each Organ Chip with MS, as well as their published physicochemical parameters, including unbound fraction, pH, pKa, and logP/logD (Supplementary Fig. 4, Method). Compound losses were also incorporated into these model equations (Methods eq. 12-18) using tracer and MS data. Assuming volumetric plug flow of media through the channels, the solutions of the flux equations in between the compartments simulated specific drug concentrations over time, matching individual Organ Chip output drug concentrations from the drug infusion experiments.
By creating a feedback design loop between experimental results generated in vitro with individual Organ Chips that were modeled computationally using our in silico platform, and then integrating the individual models into a fluidically coupled, first pass, computational model (Fig. 1c), we were able to develop, correlate, validate, and optimize methods for determining drug metabolism pharmacokinetic (DMPK) parameters for the individual Organ Chips, as well as for the linked multi-Organ Chip platform. Computationally, the AV Reservoir was integrated with the individual MCRO models for Gut, Kidney, and Liver Chips (Fig. 1c, Supplementary Fig.1f,g), and used to determine and implement optimal experimental linking parameters, such as dosing concentrations, timing of linking for drug infusion and wash out, and volumes that would be required for the analysis (Supplementary Table 3). The in vitro linking experiment involved fluidically linking Organ Chips twice a day using the automated Organ Chip instrument.
Under these conditions, all of the Organ Chips remained viable, as measured by low levels of lactate dehydrogenase (LDH) release, and maintained stable human organ-specific functions, including an intestinal barrier with a low apparent permeability (Papp) in the Gut Chip, albumin production in the Liver Chip, and proximal tubular reabsorption of albumin in the Kidney Chip for the 6 days of the study (Supplementary Fig. 5). Past studies that used the same Organ Chips individually (as opposed to fluidically linked) demonstrated physiologically relevant albumin reabsorption, alkaline phosphatase secretion, and glucose transport17 in the Kidney Chip; villus differentiation, mucus production, barrier function, increased CYP enzyme activity, and intestinal responses to radiation and microbiome in the Gut Chip15,20,21,22; and maintenance of high clinically relevant levels of albumin production and multiple CYP-enzyme activities, as well as human specific drug toxicities in the Liver Chip23.
We then used this first pass multi-Organ Chip model to study the DMPK of the small molecule (161 Da) drug, nicotine. Nicotine is used as a drug to promote smoking cessation, and it is being explored as a potential therapy for neurodegenerative disease and ulcerative colitis24, in addition to being the addictive component in tobacco products. Nicotine also has a low log P, binds minimally to serum proteins, and it is a well-studied tool compound. Moreover, clinical studies have shown that 20 to 45% of orally administered nicotine is bioavailable25 and that it is metabolized by cytochrome P450 CYP2A6 in the liver, which is expressed by hepatocytes in our Liver Chips (Supplementary Fig. 5); the functionality of these chips have been extensively characterized elsewhere23. As our Kidney Chip only recapitulates tubular functions16,17, we compensated for the lack of glomerular function by distributing 40% of the input medium into the parenchymal channel of the Kidney Chip from the AV Reservoir (Fig. 1a). This percentage was determined based on past clinical studies that revealed 40% of the nicotine is unbound in vivo25,26 and the known glomerular filtration rate for nicotine27.
We then explored whether we could carry out IVIVT of nicotine PK using our MCRO-based DMPK modeling approach in combination with this fluidically coupled, multi-Organ Chip, first pass platform by infusing a dose of nicotine (396±16 μM as detected by MS) into the lumen of the Gut Chip to mimic oral administration for 84 hrs followed by a 56 hrs wash-out period (Fig. 1a, c, Supplementary Fig. 1, Supplementary Information Table 3). This oral dose was determined using the linked first pass Organ Chip MCRO models to ensure that both nicotine and its metabolite, cotinine, could be detected by MS in the vascular channels of all the Organ Chips. Based on input from our model, combined with experimental constraints of medium loss due to sampling and evaporation, 90% of the input to the vascular channel of the Gut Chip was transferred from the AV Reservoir, and the remaining 10% was fresh medium (Fig. 1c); inulin-FITC tracer also was included in this medium to confirm the accuracy of the robotic fluidic transfers. A portion (350 μl) of the Gut Chip’s vascular outflow was then collected for sample MS analysis using the robotic sampler, and the remainder was transferred to the vascular channel of the Liver Chip, which represented 23% of the total fluid input into this channel, with the remaining 77% coming from the AV Reservoir (Fig. 1c). To include enterohepatic cycling that allows for recycling of small molecules in vivo, 350 μl of the Liver Chip’s vascular outflow was collected for sample analysis, and all of the remaining medium was transferred back into the AV Reservoir, which then served as a source for part of the blood substitute medium flowing into the vascular channel of the Gut Chip (Fig. 1c, Supplementary Table 3). The AV Reservoir also provided 100% of the input into the vascular channel of the Kidney Chip, as well as 40% of the input into its parenchymal channel (with 60% being fresh organ-specific medium) to account for the absence of glomerular filtration. The parenchymal channels of all other Organ Chips were perfused individually with 100% organ-specific medium (Fig. 1c). The AV Reservoir was replenished with fresh medium at every linking step to account for sampling and evaporative volume loss.
When we used MS to quantify nicotine in the AV Reservoir and the effluents of the vascular channels of the different Organ Chips, the predictions of the computational DMPK model matched well with the experimental results over the full 140 hrs time course (Fig. 2, Supplementary Fig.1f,g. When we compared the DMPK models and the Organ Chips (including the AV reservoir) using Bland-Altman analysis, the bias in the models was less than 10% (Supplementary Fig.7), resulting in good agreement between the model and experimental data. In addition, further analysis revealed that all Organ-Chips have good or very good scoring using Lin concordance coefficients. The Liver Chip exhibited the highest discrepancy between modeled and measured values (Pearson correlation r=0.68; Supplementary Fig. 7), which was likely due to variation in metabolic activity not considered by the model. Furthermore, there was a high in vitro-to-in vivo correlation for nicotine bioavailability as the maximum level we measured in the AV Reservoir (0.8 ±0.08μM) (Fig. 2) was quantitatively similar to levels previously reported in humans (peak plasma concentrations of 0.05 to 0.6 μM27,28. In addition, we detected production of cotinine (the hepatic breakdown product of nicotine) in the effluent from the apical hepatocyte channel of the Liver Chip, which independently confirmed that the Liver Chip expressed high levels of functional CYP2A6 activity that is responsible for this conversion (Supplementary Fig. 5). Importantly, cotinine was also present at levels that correlated very well with the DMPK model (Lin’s concordance coefficient 0.82) (Fig. 2, Supplementary Fig. 7). In these studies, CYP3A4 activity remained unchanged in the Gut Chip as did albumin production in the Liver Chip (Supplementary Fig. 6a), and we did not detect toxicity (LDH release) in any of the first pass human Organ Chips upon nicotine exposure (Supplementary Fig. 6b), which is consistent with clinical findings25. Nicotine use has been shown to increase intestinal barrier function in humans29, whereas this effect was not observed in nonsmokers30. We similarly observed that administration of nicotine to the epithelial lumen of the Gut Chip significantly increased intestinal barrier function (P < 0.03) when we used the low molecular weight hydrophilic tracer Cascade Blue to measure permeability (Supplementary Fig. 8); however, we could not detect any effect using the less sensitive higher molecular weight inulin (Supplementary Fig 6). We also did not detect a barrier change using TEER measurements when the same intestinal epithelial cells and endothelial cells were interfaced across a porous membrane in a similar manner but cultured in static Transwell inserts (Supplementary Fig. 8). While our finding is not consistent with a past study which reported that nicotine alters barrier function in Transwell cultures 32, a later study could not repeat the effect they observed33.
Fig. 2. Mass spectrometry data and DMPK model of first pass multi-Organ Chip platform.

Graphs showing nicotine levels measured over time by MS within the apical and basal channels of the linked Gut, Liver and Kidney Chips, as well as in the AV Reservoir, when nicotine was continuously infused through the lumen of the Gut chip at a dose of 396±16 μM for 84 hrs followed by a 56 hrs wash-out period (white bars) compared with predictions of the computational DMPK model (black bars). Values are also shown for the nicotine breakdown product, cotinine, in samples from the effluent of the epithelial channel of the Liver Chip (data are pooled from 3 separated experiments; error bars indicate standard deviation).
To be relevant for pharmaceutical development, regulatory assessment, and toxin testing, this fluidically coupled, multi-Organ Chip platform must be able to carry out IVIVT and computationally predict drug DMPK parameters that are measured in humans in vivo in a quantitative manner. The geometric average of the maximum concentration (Cmax) of nicotine measured in the blood of patients in a past study using nicotine gum administered for 30 min orally was 0.052 ± 0.009μM with half the time to reach that level (t1/2(RISE)) of 10.6 min (Table 1). While the human body has a closed continuous systemic circulation, these microengineered Organ Chips are discrete systems with extensive sampling requirements and experimental constraints, and so it is difficult to recapitulate rapid changes in drug levels. Experimentally, this linked system also requires that flow be stopped periodically at discrete time points to collect experimental samples, replenish medium, visually inspect the tissues, and transfer fluids between the different Organ Chips and the AV Reservoir.
Table 1.
Predictions of human PK parameters for oral nicotine obtained with two cohorts of multi-Organ Chip IVIVT platforms compared to clinical values and rodent clearance results.
| Nicotine PK Parameters (systemic compartment) |
Human (Clinical data)* | In Silico Model(Multi-Organ Chips) |
|---|---|---|
| Nicotine Gum (4.2 mg) | ||
| Cmax (μM) | 0.052 ±0.009 | 0.050 (δ=3,8 %, n.s.), 0.054 (δ=3,8 %, n.s.) |
| tmax (min) (Range: minimum – maximum) |
45 (19.8-90) |
40.0 (δ=11.1%) 38.9 (δ=13,6%) |
| t1/2, rise-phase (min) *** | 10.60 | 20.45 (δ=92,9 %) 19.9 (δ=87,7 %) |
| t1/2, fell-phase (min) *** | 203 | 160 (δ=21,1%) 263 (δ=29,7% |
| AUC(μM.hr)** | 0.08±0.02 | 0.062 (δ=22.5%, p=0.0007) 0.073 (δ=8,75%, n.s.) |
| Pouched snus(14.7 mg) | ||
| Cmax (μM) | 0.083±0.032 | 0.101 (δ=21.7%, n.s.), 0.088 (δ=6,0 %, n.s.) |
| tmax (min) (Range: minimum – maximum) |
60 (45-90) |
66.7 (δ=11.1%) 66.7(δ=11,1%) |
| t1/2, rise-phase (min) *** | 16.7 | 28,3 (δ=69.6 %) 31.7 (δ=89.9%) |
| t1/2, fell-phase (min) *** | 152.6 | 236.6 (δ=55.1%) 353.3 (δ=131.5% |
| AUC (μM.hr) ** | 0.13±0.05 | 0.13 (δ=1.5%, n.s. 0.12 (δ=9.2%, n.s.) |
| Loose snus(10.8mg) | ||
| Cmax (μM) | 0.064±0.022 | 0.081 (δ=26.5 %, p=0.003), 0.073 (δ=14.1 %, n.s.) |
| tmax (min) (Range: minimum – maximum) |
60 (45-90) |
66.7 (δ=11.1%) 66.7 (δ=11.1%) |
| t1/2, rise-phase (min) *** | 13.7 | 28.3 (δ=106.7,0 %) 30.0 (δ= 119.0%) |
| t1/2, fall-phase (min) *** | 155.6 | 238.3 (δ=53.2%) 353.3 (δ=127.1% |
| AUC (μM.hr) ** | 0.10±0.03 | 0.106 (δ=5.7%, n.s.) 0.098(δ=2.0%, n.s.) |
In vivo human clinical data from Digard et al.28
AUC was computed for t=0 to 2hrs (i.e., times when clinical measurements were available) for both in vivo and in silico studies
Estimated using curve fitting for in vivo (based on available clinical data); note that the estimated t1/2, fall-phase is beyond the measurement time window (therefore curve fit based estimates may not be reliable)
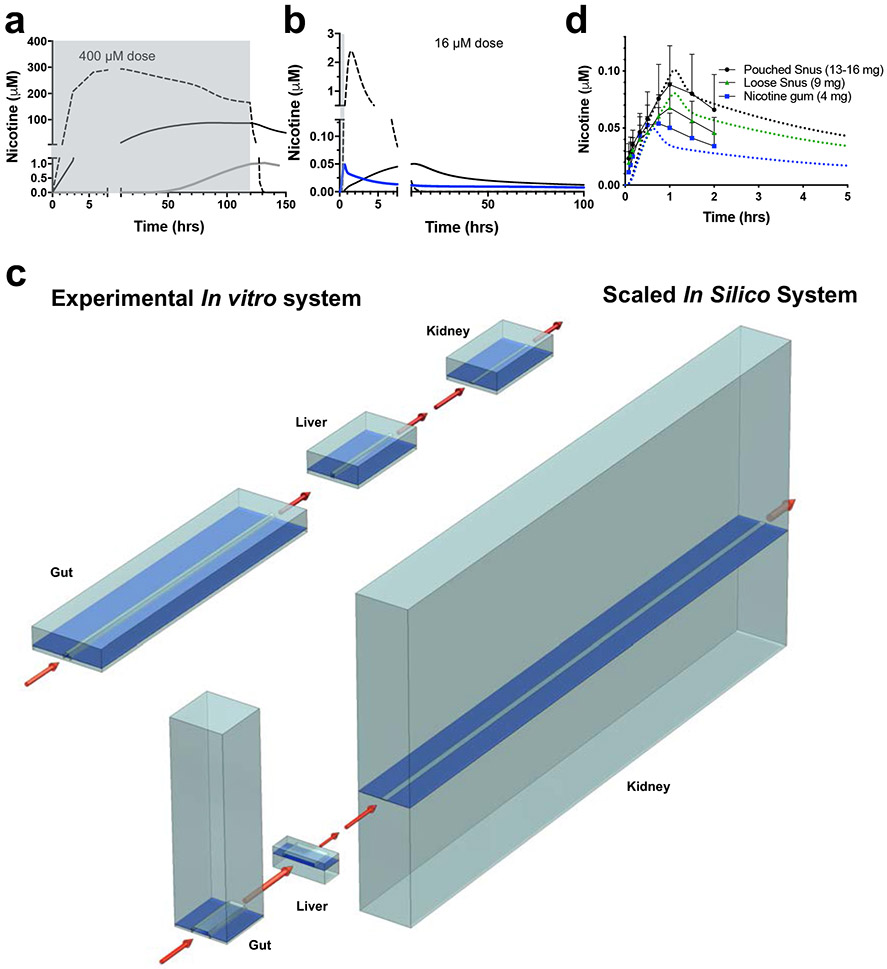
To overcome this limitation of discrete fluid transfers and predict clinically relevant blood nicotine concentrations, our second step in the work flow was to modify our validated DMPK model to computationally simulate continuous flow without fluid sampling based on measurements obtained from the experimental platform with discrete fluid transfers Supplementary Fig. 1,f,g. However, this continuous model did not produce a clinically relevant Cmax for nicotine when the high dose of 396 ± 16 μM as suggested by the MCRO model, even when the experimentally determined package loss of nicotine into the PDMS was integrated into the model (Fig. 3a). We then used the optimized DMPK model that simulates continuous linking in the Organ Chips and AV Reservoir based on experimental discrete sampling measurements, and input dose concentration and timing parameters from a previously published clinical study in patients who were given chewable nicotine gum28 (oral dose of 16 μM nicotine for 30 mins) . Impressively, the initial prediction of this continuous DMPK model based on our experimental data incorporating the optimized package loss into the PDMS materials estimated a Cmax of 0.055 μM, which is in the 95% clinical confidence interval, however, it predicted a t1/2(RISE) of 16 hrs that was far from the 10.6 min that are observed in vivo (Fig. 3b).
Fig. 3. In vitro-to-in vivo translation (IVIVT) of human pharmacokinetic (PK) parameters for nicotine using the multi-Organ Chip first pass platform.
(a) Graph showing the oral dose (~400 μM) of nicotine infused into the upper parenchymal channel of the Gut Chip (gray shaded area) and nicotine levels measured in the AV Reservoir over time in the real experimental system with discrete linkages every 12 hrs and package loss into the PDMS (gray solid line) versus computational DMPK model predictions of nicotine levels in which the same results were simulated as a continuous flow system with (black solid line) or without (dashed line) package loss. (b) Predictions of nicotine levels in the AV Reservoir using the same computational DMPK model as shown in a with a clinical nicotine dose of 16.15 μM and 30 min infusion (gray shaded area) compared with the same results were simulated as a continuous flow system with (black solid line) or without (dashed line) package loss. The blue line shows that the in silico multi-Organ Chip IVIVT platform made PK predictions for nicotine that much more closely match that rapid PK dynamics of human blood nicotine values using a continuous flow simulation after it was optimized for physiological differences in cell mass and blood flow between the different organs in vivo, drug loss into the chip material, and the geometry of endothelial channels to mimic drug transport. (c) Schematics comparing the actual relative Organ Chip channel volumes, flow rates, and geometries shown in cross-section in the linked multi-Organ system (top) and the scaled values for these properties used for the IVIVT simulations (bottom). (d) Graph showing that changes in nicotine blood concentrations over time predicted by the optimized, scaled, in silico multi-Organ Chip IVIVT platform for three different oral doses (different colored dashed lines) closely match previously published blood nicotine levels measured in human patients receiving orally administered nicotine in the form of nicotine gum (blue; same curve as the blue curve in b shown at different scale), pouched snus (black) or loose snus (green) at three different doses (4, 9, and 13-16 mg, respectively)28. Similar results were obtained in two separate studies, each with three independent fluidically linked multi-Organ Chip systems.
To further improve the accuracy of our ability to carry out IVIVT of these clinical DMPK parameters, we took a third step in our workflow and implemented a scaling approach in the MCRO model based on intrinsic PK parameters (e.g., intrinsic clearance)34 (Methods), which effectively changes the biomass and also accounts for PDMS adsorption in the various Organ Chips, with the goal of optimizing the model’s ability to predict the human nicotine Cmax and t1/2(RISE) previously reported in vivo28. This is possible because using this scaled IVIVT model, we are able to extend our in vitro DMPK studies into experimentally intractable regimes and computationally redesign the geometry of the Organ Chips, and create designs that are difficult or impossible to fabricate or implement experimentally. The optimal, scaled, first pass modeling platform included physiologically relevant changes including increased biomass in the in silico version of the Liver Chip, a larger surface area in the Kidney Chip, and decreased absorptive surface area in the Gut Chip (Fig. 3c, Supplementary Fig.1,h). Moreover, all vascular channel geometries were computationally changed to include dimensions with a smaller height and larger width (Supplementary Information Table 5), which effectively creates shorter diffusion distances in the vascular compartment. In the human body, there is also often significant drug loss into peripheral tissues such as fat, and so to incorporate this feature of drug behavior into the model, we systematically varied our package loss parameter from 0 to 100% as well (Supplementary Fig. 9, Supplementary Information Table 5); the highest correlation with clinical nicotine data corresponded to a package loss of 34% in our model (Supplementary Information Table 5). In addition, we integrated specific human in vivo anatomical parameters into the in silico continuous first pass model, including organ-specific fractional blood flows, blood flow rates, and dimensions of the major vessels (Supplementary Information Table 4), which were used to computationally scale the volumes and flow rates of the vascular channels, and the dimensions of tubing that connect fluid input and output reservoirs to each Organ Chip respectively.
Importantly, when this optimized, scaled, in silico IVIVT model was applied to our in vitro experimental data for oral administration of nicotine through the first pass, multi-Organ Chip platform and used it to simulate changes in nicotine levels in the AV Reservoir as a correlate for systemic blood levels. The IVIVT model quantitatively predicted a drug exposure profile and time course that closely mimicked that observed in vivo in patients after equivalent dosing with a nicotine gum (Fig. 3d, Supplementary Fig. 10). We have thereby demonstrated how we can use multi-Organ Chip platform experimental data generated over 140 hrs to create a IVIVT model that can predict drug PK over 30 mins in patients28 . This optimized model accurately predicted an effective nicotine concentration in the AV Reservoir (0.055 μM) is in the 95% confidence interval of the clinical Cmax (0.052± 0.009μM) previously measured in patients. The PK profile predictions from two data sets of experiments in which nicotine was tested in the multi-Organ Chip setups (Table 1, 3) also matched the clinical data, as predicted a tmax of 38.9 min that is in the clinical range of 18.9-90 min28. Even more impressively, when we compared predictions of the scaled in silico IVIVE model for two different nicotine-containing tobacco formulations (pouched snus and loose snus as two forms of oral administrated tobacco), dosing times, and doses, we again observed a close match (Table 1, 3) between the model’s predictions and the clinical drug dose time courses, even though our model was developed using a different drug dose and formulation (Fig. 3d, Supplementary Fig. 7 and 10, Supplementary Information Table 6). The predicted Cmax values, deviated at most by 26.5% from the clinical value for pouched snus, whereas for other formulations the discrepancy was not significant. Predicted times required to reach the maximum levels (tmax) were all within clinically measured range, and the AUC value deviated at most by 22% from the predicted value (Tables 1 & 3, Fig. 3d). The Bland-Altman analysis and Pearson correlations revealed that the bias of the model predictions was at most 16% of the Cmax values and Lin’s concordance coefficients were all rated good to very good (Supplementary Fig. 7). Furthermore, when we compared our IVIVT results with data using organ-specific PK parameters from rodents35, the human multi-Organ Chip system better predicted the clinical liver intrinsic clearance results than the rat (Tables 1 & 3). Importantly, while secondary intrinsic liver clearance cannot be observed in rodents, we can derive this kinetic parameter from AV Reservoir concentration curve. For renal clearance, however, the rodent model predictions were closer to the clinical data; this is likely because we lack a glomerulus in the Kidney Chip model and instead used simulations to computationally compensate for the urinary clearance.
Table 3.
Predictions of human PK parameters for oral nicotine (a) and intravenous cisplatin (b) obtained with the multi-Organ Chip IVIVT platform compared to clinical values and rodent clearance results.
| a | |||||
|---|---|---|---|---|---|
| Organ PK Parameters – Nicotine | |||||
| PK Parameter | Rodent (in vivo) |
Human (Clinical data) |
In Silico Model (Multi-Organ Chips) |
||
| Gut | Papp (10−6 m/s) | NA | 9.5 to 57.2 | 1.3 to 7.5 (δ=21-98%) **** | |
| Liver | Intrinsic Clearance, CLint (ml/min/kg.BW) | 300 | 12.5 |
10.0 (δ=20 %) 8.004 (δ=36,0 %) |
|
| Secondary Intrinsic Clearance, CLint, sec (ml/min/kq.BW) | NA | 0.74 | 0.15 (δ=80,0 %) | ||
| Kidney | Renal Clearance, CLR, (ml/min/kg.BW) | 5.5 | 0.5 to 1.29 | 0.03 to 0.13 (δ=75-98%) **** | |
| b | |||||
| Organ PK Parameters - Cisplatin | |||||
| PK Parameter | Rodent (in vivo) |
Human (Clinical data) |
In Silico Model (Multi-Organ Chips) |
||
| Liver | Intrinsic Clearance, CLint (ml/min/kg.BW) | 3.73 | 0.25 | 0.20 (δ=20,0 %) | |
| Kidney | Renal Clearance, CLR (ml/min/kg.BW) | 0.19 | 1.39 | 0.008 (δ=99,4 %) | |
Comments for Table 3a.
AUC was computed for t=0 to 2hrs (i.e., times when clinical measurements were available) for both in vivo and in silico studies
Comments for Table 3b
In vivo data from Rajkumar et al. 39 (human) and Gong et al.69 (rodent) – where total cisplatin clearance was used for liver clearance and clearance out of tissue for kidney
Minimum to maximum % relative error in the interval
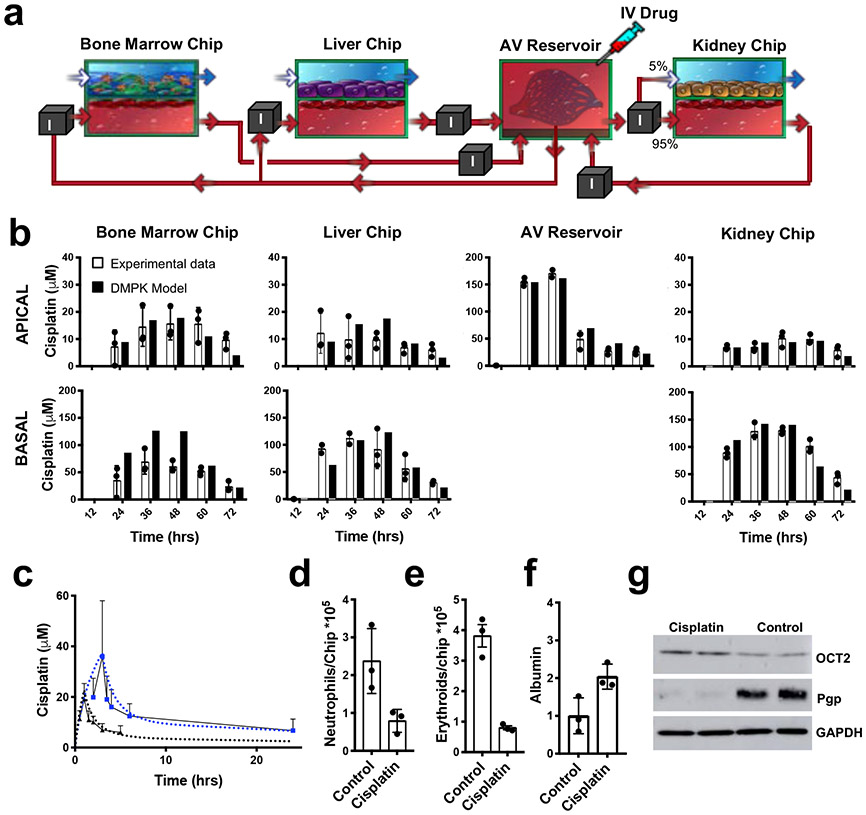
To explore whether this computational IVIVT approach using the multi-Organ Chip platform might have broader applicability, we applied the PBPK model developed for nicotine to predict human PK parameters for a second drug, the cancer chemotherapeutic cisplatin, using a different path of administration. Cisplatin is administered intravenously in patients and it inhibits cancer cell growth by interfering with DNA synthesis and repair36, which leads to a wide range of adverse effects, including myeloid toxicity in bone marrow and renal toxicity36. So by modifying the multi-Organ Chip platform by administering 160 μM cisplatin directly into the AV Reservoir to mimic intravenous injection and replacing the Gut Chip with a human Bone Marrow Chip (Fig. 4a, Supplementary Figs. 2e, 11a, Supplementary Information Table 3) that reconstitutes myeloid and erythroid cell production in vitro and recapitulates drug-induced bone marrow drug and radiation toxicities37 (Supplementary Fig. 11b), we were able to analyze drug PD as well as carry out PK modeling. To do this, we followed our workflow for IVIVT multi-Organ Chip models. First, a MCRO DMPK model of the Bone Marrow Chip was developed based on single-chip data (Supplementary Fig. 11c), and then it was integrated with the Liver Chip, Kidney Chip, and AV Reservoir in silico models developed for the nicotine study (Fig. 4a). Experimentally, the lack of glomerular function was compensated by transferring 5% and 95% of the AV Reservoir medium to the apical parenchymal and basal vascular channels of the Kidney chip, respectively (Fig. 4a), which is based on the ~5% unbound fraction of cisplatin in vivo38. We then monitored drug metabolism and clearance by the Liver and Kidney Chips, as well as toxicity in the Bone Marrow Chip when cisplatin was introduced into the AV Reservoir to mimic intravenous injection. Again, we obtained an good correlation between predictions of the DMPK model and experimental cisplatin levels measured in the effluents of each chip and the AV Reservoir (Fig. 4b, Tables 2 & 3, Supplementary Fig.12, 13)39. As in the case with nicotine, the Bland-Altman analysis revealed that errors in the DMPK models do not correlate with cisplatin concentration. In all cases but the Bone Marrow Chip basal data, the bias value did not exceed 11% of the maximum observed concentration in each chip (Supplementary Fig 12). The Lin concordance and Pearson correlation analyses also demonstrated that the model predicted cisplatin levels in the AV reservoir with high accuracy (ρ=0.98 and r=0.99, respectively), but that the liver and bone marrow apical channels were only moderately well captured by the DMPK model (ρ= 0.53 and 0.53, r=0.76 and 0.86 respectively) (Supplementary Fig 12).
Fig. 4. Prediction of cisplatin PK and pharmacodynamic (PD) parameters using the multi-Organ Chip IVIVT platform.
(a) Diagram showing the fluidic coupling path of the Bone Marrow, Liver and Kidney Chips linked to the AV Reservoir in the multi-Organ Chip platform used to study cisplatin PK. Red arrows indicate medium flow path and direction; box with ‘I’ indicates sites where fluid was transferred by the automated liquid handling instrument between the AV Reservoir and input reservoirs of the channels of the different chips, as well as between the output and input reservoirs of different Chips. Cisplatin (160 μM) was continuously infused into the AV reservoir for 24 hrs to simulate intravenous infusion followed by a 48 hrs wash-out period. (b) Cisplatin concentrations measured over time in medium samples collected from the effluent of each chip and the AV reservoir using MS (white bars) compared with predictions of the optimized scaled DMPK model (black bars) (error bars indicate standard deviation). (c) Graph showing that changes in cisplatin concentrations in blood over time predicted by the optimized, scaled, multi-Organ Chip IVIVT platform for either 1 (black) or 3 (blue) hour infusion periods (dotted lines) closely match previously published measurements of blood cisplatin levels measured in human patients who received cisplatin injected intravenously over these times39 (solid lines) (similar results were obtained in three replicate experiments). (d-g) Cisplatin infusion in the multi-Organ Chip platform resulted in suppression of total neutrophil (d) and erythroid (e) cell numbers in the Bone Marrow chip, as determined by FACS analysis (error bars indicate standard error of the mean), without significantly altering (f) albumin production in the Liver Chip, shown as albumin secretion normalized to control chips, thus, recapitulating cisplatin PD in vitro. (g) Western blot analysis also revealed that cisplatin increased OCT2 and decreased Pgp levels in Kidney Chips compared to controls (similar results were obtained in 2 different experiments; GAPDH is shown as a loading control).
Table 2.
Predictions of human PK parameters for intravenous cisplatin obtained with the multi-Organ Chip IVIVT platform compared to clinical values and rodent clearance results.
| Cisplatin PK Parameters (systemic compartment) |
Human (Clinical data) |
In Silico Model (Multi-Organ Chips) |
|---|---|---|
| Cisplatin (1 hour infusion) | ||
| Cmax (μM) | 18.02±4.93 | 22.72 (δ=26,1 %, p=0.0146) |
|
tmax (min) (Range: minimum – maximum) |
~1.0 | 1.0 |
| AUC (μM.hr) | 41.94±12.41 | 48.27 (δ=15.1 %, n.s.) |
| Cisplatin (3 hour infusion) | ||
| Cmax (μM) | 30.30±6.48 | 37.26 (δ=23.0 %, p=0.0079) |
|
tmax (min) (Range: minimum – maximum) |
~3.0 | 3.0 |
| AUC (μM.hr) | 253.33±100.32 | 252.6 (δ=0,4 %, n.s.) |
In vivo human clinical data Rajkumar et al. 39
AUC was computed for times when clinical measurements were available for both in vivo and in silico studies
In the cisplatin IVIVT analysis, we retained the same scaling parameters for Organ Chips, package loss and tubing that were used in the nicotine study except that for this setup, the Gut Chip was replaced by the Bone Marrow Chip (Fig. 4a), again using appropriate in vivo anatomic parameters (Supplementary Information Table 6) to scale Organ Chip dimensions and flows (Supplementary Information Table 7). The only change was that the medium volume in the AV Reservoir was decreased to account for the relative change attributed to the smaller volume of the major vessels in the bone marrow compared to the intestine in humans, as well as changes in geometry between the two chips and the mode of administration (Methods, Supplementary Information Tables 6,7)40,41. Importantly, our optimization showed that reducing the percentage of the AV reservoir volume from 93.83% (~7 mL) for nicotine to 30.23% (~ 2.5mL) for cisplatin resulted in better correlation (Supplementary Fig.12, 13) between the computational IVIVT predictions of the model using a cisplatin dose of 160 ± 11 μM administered through the AV Reservoir and the vascular channels of the linked Organ Chips. including the Bone Marrow Chip for 24 hrs. Results were compared to a previously published clinical study in which cisplatin was administered intravenously to patients at different doses and for different times (18 ± 5 or 30 ± 6 μM for 1 or 3 hrs, respectively) (Fig. 4c, Tables 2 & 3). Specifically, there was no significant difference between the AUC1hr (p=0.1412, One sample t-test) and the AUC3hr (p=0.9738, One sample t-test), whereas there was 26 and 23% discrepancy (p=0.015 and 0.008 respectively) between the clinical Cmax and the modeled data (Tables 2 & 3). However, Bland-Altman plots demonstrated that the bias was only ~13% of the maximum drug concentration and the Lin’s concordance and Pearson coefficients showed good to very good correlation (Supplementary Fig 13). Furthermore, when we compared the IVIVT predictions with rodent data, the linked human Organ Chip platform again provided better estimations of intrinsic liver clearance than renal clearance possibly due to the fact that we estimated glomerular function and the drug is highly bound to albumin42 (Tables 2 & 3). Importantly, these data show that same scaled in silico multi-Organ Chip model can be successfully applied to predict clinical PK profiles of two different drugs, each with a distinct dosing regimen, route of administration (oral and intravenous), and mechanism of toxicity.
It is important to note that we did not analyze the specific influence of fluid transfers on Organ Chip function because this is the focus of a separate study in which 8 Organ Chips (including Gut, Liver, Kidney, and Bone Marrow Chips) were linked for 3 weeks of culture using the same automated culture instrument16. However, the levels of albumin reabsorption in the Kidney chip, intestinal barrier function in the Gut Chip, and production of neutrophils and erythrocytes in the Bone Marrow Chip in this study are similar to or better than those measured in the 8-chip linking study. For example, albumin production in the Liver Chip measured here is similar to that in the stand-alone Liver chip, which is higher than levels detected when it was liked to 7 other Organ Chips, demonstrating that chip linkage procedures can influence Organ Metrics. More importantly, our finding that we can quantitatively predict human clinical PK for two different drugs using two different administration routes indicates that the Organ Chips functioned in a physiologically relevant way when fluidically linked in the present study.
Finally, we used the fluidically coupled multi-Organ Chip model to carry out human PD analysis in vitro. When cisplatin was flowed through the vascular channel of the linked Organ Chips at the same dose for 24 hrs, we detected myeloid toxicity as indicated by a 78% reduction in the total number of cells in the Bone Marrow Chip (P = 0.001) (Supplementary Fig. 14a). Additional fluorescence-activated cell sorting (FACS) analysis revealed 67% and 72% reductions in neutrophils (Fig. 4d; P < 0.04) and erythrocytes (Fig. 4e; P < 0.002), respectively, thus recapitulating the known side effects of cisplatin in patients (neutropenia and anemia, respectively) in vitro36. Cisplatin does not produce hepatotoxicity at clinically relevant doses in patients, and we similarly could not detect any change in albumin production (Fig. 4f) or CYP3A4 function (Supplementary Fig. 14b) in the Liver Chip. In contrast, cisplatin exposure resulted in a reduction in Pgp expression and upregulation of OCT2 in the Kidney Chip (Fig. 4g), which are membrane transporters that have been shown to be affected by cisplatin-mediated toxicity43. Interestingly, while the same patterns of upregulation of OCT2 and downregulation of Pgp have been observed in rat kidney slices exposed to cisplatin at doses similar to what we applied in the kidney chip, longer-term in vivo rat studies have shown the reverse pattern43,44. This discrepancy could be due to the fact that we used a lower dose and shorter time duration of cisplatin in our study. Alternatively, our human multi-Organ Chip model might more effectively recapitulate early cisplatin PD in the human kidney than rats.
Taken together, these findings demonstrate that this fluidically coupled, multi-Organ Chip-based, IVIVT platform can be used to predict quantitative human PK parameters in vitro which are highly similar to those obtained in human clinical studies. We carried out IVIVT using an in silico DMPK model built using experimental data obtained from multiple, 2-channel, human Organ Chips that were fluidically coupled by transferring a universal blood substitute between endothelium-lined vascular channels and incorporating an AV Reservoir to mimic drug distribution and dilution through the entire vasculature. This is in contrast to past studies that either only computationally simulated IVIVT using theoretical models of fluidically linked microfluidic culture systems without experimental validation12,14, or that applied PK/PD models using linked cell-containing microfluidic devices that lacked physiologically relevant endothelial barriers45. We have previously shown that the endothelium is vital to recapitulate many in vivo physiological functions, including Liver Chip metabolic function16. As a result of the more faithful physiological mimicry included here, and the use of more advanced MCRO-based DMPK models, we were able to model drug transport across the endothelial-parenchymal tissue barrier and, for the first time, develop an IVIVT approach that provides an excellent quantitative fit between predictions of human PK based on in vitro studies and data from clinical studies. It should be noted that some past models have been able to predict Cmax for drugs in humans, but they did not predict relevant drug residence times41 as we did here. In our studies with cisplatin, we also were able to recapitulate organ toxicities of this drug that are observed in humans, as well as the lack of hepatotoxicity. These data combined with the altered Gut Chip barrier function and kidney transporter changes upon nicotine exposure further demonstrate the biological relevance of this model. Together these data suggest that this IVIVT modeling platform may be useful for studying, and potentially predicting, drug PD in the future.
This first pass multi-Organ Chip model does not include other important human organs that can influence drug PK, including drug absorbing fat tissues. Importantly, however, we are able to compensate for this limitation using in silico modeling that can create ‘virtual’ drug absorbing tissues, for example, by modulating the absorptive properties of the chip PDMS materials in our package loss estimations, and by changing the Organ Chip dimensions to best predict in vivo-like drug uptake. Drug absorption and package loss were optimized to the same level to faithfully recapitulate both nicotine and cisplatin PK in this study; however, for drugs with different properties and hepatic clearance rates, the PDMS absorption could need further optimization. The computational model also is used to compensate for the absence of glomerular function that normally contributes in a major way to urinary clearance, and to circumvent potential limitations due to absorption of drugs into the PDMS device materials. In this manner, this human-relevant, fluidically coupled, multi-Organ Chip PK/PD modeling platform allows users to carry out IVIVT to quantitatively predict human PK parameters in vitro (the full procedure from initial drug selection to IVIVE is outlined in Supplementary Fig. 15). Thus, the multi-Organ Chip PK/PD modeling platform may provide an experimentally tractable human platform for drug discovery, regulatory assessment, toxicological evaluation, and personalized medicine (using patient-specific cells) in addition to accelerating therapeutic development by enabling more effective design of drug regimens for Phase I clinical trials in the future.
METHODS
Organ Chip Fabrication
The Gut, Liver, and Bone Marrow Chips containing a central porous (7 μm diameter) membrane separating two linear parallel channels were fabricated from polydimethylsiloxane (PDMS) using a previously published soft lithography method46; channel dimensions and membrane features are described in Supplementary Information Table 1. The Kidney Chips were fabricated from PDMS upper and lower walls containing formed microchannels (0.1 mm height and width x 24 mm length) with a polyester terephthalate (PET) track-etched membrane (0.4 μm pores) sandwiched in between and bonded, as previously described47. The Gut Chip was fabricated containing a serpentine channel (Fig. 1a) to increase absorptive area; it was mechanically actuated to mimic peristalsis-like motions using a programmable vacuum regulator system built in-house that applies cyclic suction to hollow side chambers that run on either side of the central culture channels, thereby rhythmically deforming the porous membrane and attached cells, as previously described15. The Bone Marrow Chip used in this study had oval-shaped upper and lower chambers composed of PDMS (Supplementary Fig. 2 and 11), and the upper chamber had an open top that was closed with a resealable medical grade adhesive film (Adhesives Research) to enable loading of the matrix gel laden with cells.
Microfluidic Organ Chip Culture
Our methods for plating and culture of parenchymal and endothelial cells within the Gut, Liver, Kidney, and Bone Marrow Chips have been published15,16, 17,23, 37 , . We used the Gut Chip lined by Caco-2 intestinal epithelial cells to minimize cell source variability and ensure the robustness of this initial proof-of-principle IVIVT study as we have previously shown that when cultured in the presence of physiologically relevant luminal flow and peristalsis-like mechanical deformations, these chips display formation of intestinal villi lined by all four epithelial cell lineages of the small intestine and have enhanced barrier function, drug-metabolizing cytochrome P450 activity, and apical mucus accumulation compared with the same cells grown in conventional Transwell cultures15,22. However, this cell line could be replaced in the future with primary intestinal epithelial cells derived from organoids isolated from human patients as recently demonstrated21. A detailed description of the design, capabilities, and control systems of the automated multi-Organ Chip culture platform for transferring medium samples between chips, as well as for analysis, has been described as well16. Our methods for coating the chips and plating cells in the Gut, Liver, Kidney, and Bone Marrow Chips used in the present study are shown in Supplementary Information Table 2. In brief, to create a human Gut Chip, the PDMS chip was pre-coated with Matrigel (1%, Corning Inc., Corning, NY, USA) and Collagen type I (100 μg/mL Corning Inc., Corning, NY, USA) before Caco-2 BBe cells (acquired from the Harvard Digestive Disease center) were plated (1.5 x 105 cells/cm2) in the upper channel and human umbilical cord vascular endothelial cells (HUVECs; Lonza, Basel, CHE) were seeded (1.5 x 105 cells/cm2) on the opposite side of the porous membrane in the lower channel. The upper parenchymal channel was perfused (60 μL/hr) with DMEM-High Glucose (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated FBS (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA), and 1% P/S (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA). We used a common universal blood substitute’ endothelial medium to perfuse (60 μL/hr) the vascular channel of the Gut Chip, as well as all other Organ Chips and the AV Reservoir, which is composed of DMEM/F12 (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA) with EGM-2 supplements and growth factors (Lonza, Basel, CHE), 1% P/S (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA), and 0.5% FBS (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA). After 1 day of culture, cyclic suction was applied to side chambers to recreate peristalsis-like deformations on-chip (10% strain, 1.2Hz), which were maintained throughout the course of the experiment.
In the Liver Chip, human primary liver sinusoidal microvascular endothelial cells (Cell Systems Corp., Kirkland, WA, USA) were first plated (1 x 105/cm2) for 1 hr on the lower surface of the porous PDMS membrane in the lower channel of PDMS chips that were precoated with collagen I (300 μg/cm2; Corning Inc., Corning, NY, USA). The chips were then turned upside down and human primary hepatocytes (Thermo Fisher Scientific Inc., Waltham, MA, USA) were then seeded (2.5 x 105 cells/cm2) in the upper channel. After 3 hrs, the hepatocytes were overlaid with Matrigel (250 μg/mL; Corning Inc., Corning, NY, USA) in hepatocyte maintenance medium (Thermo Fisher Scientific Inc., Waltham, MA, USA). One day later, the Matrigel-containing medium was changed to fresh hepatocyte maintenance medium, which was then perfused (60 μL/hr) through the upper channel for the rest of the experiment, while the blood substitute medium was perfused at the same rate through the lower channel. The liver sinusoidal endothelial cells contribute significantly to the function of the chip, for examples, Liver Chips with liver sinusoidal endothelial cells exhibit is ~3 higher CYP3A4 metabolic activity than those without16.
In the Kidney Chip, primary human glomerular microvascular endothelial cells, GMVECs (Cell Systems Corp., Kirkland, WA, USA, Cat # ABRI128) were first plated (1 x 105 cells/cm2) for 1.5 hrs in the lower channel of chips containing PTE membranes that were precoated with collagen IV (500 ng/cm2 Sigma-Aldrich Corp, St. Louis, MO, USA) and laminin (500 ng/cm2 Sigma-Aldrich Corp, St. Louis, MO, USA). The chips were then inverted and primary human renal proximal tubule epithelial cells (ScienCell Research Laboratories, Carlsbad, CA, USA, cat # 4100 and donor lot 5110), were plated (1.2 x 105 cells/cm2) in the upper channel. After being allowed to attach for 2 hrs, they were perfused (60 μL/hr) with DMEM/F12 (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10 ng/mL EGF (Thermo Fisher Scientific Inc., Waltham, MA, USA), 440 μg/mL epinephrine (Sigma-Aldrich Corp, St. Louis, MO, USA), 36.25 μg/mL hydrocortisone (Sigma-Aldrich Corp, St. Louis, MO, USA), 67.3 μg/mL triiodothyronine (Sigma-Aldrich Corp, St. Louis, MO, USA), 5 μg/mL insulin (BioVision Inc., San Francisco, CA, USA), 5 μg/mL transferrin (Sigma-Aldrich Corp, St. Louis, MO, USA), 1% P/S (Gibco™ Thermo Fisher Scientific Inc., Waltham, MA, USA), and 0.5% fetal bovine serum (Lonza). Again, the universal endothelium medium was perfused at the same rate through the vascular channel.
In the Bone Marrow Chip, HUVECs were first plated (1 x 105 cells/cm2) on the lower surface of the porous membrane in the lower channel of PDMS chips that were precoated with fibronectin (100 μg/mL fibronectin; Gibco) and collagen I (50 μg/mL; Gibco). One hour later, 1 × 104 primary human CD34+ progenitor cells were added to StemSpan SFEM II basal medium (StemCell Technologies) supplemented with Collagen I (0.2 mg/mL; Sigma) and thrombin (0.5 U/mL; Sigma), and then 37 uL of this solution was combined with 14 μL of a solution containing fibrinogen (5mg/mL; Sigma) and aprotinin (0.025 mg/mL; Sigma). The total solution (50 μL) was introduced into the open apical channel of each chip and allowed to solidify at room temperature for 10 minutes; the upper channel was then sealed with clear medical grade adhesive film. The upper channel of the chips were perfused (at 67 μL/min with StemSpan SFEM II medium supplemented with 10% FBS, 1% Pen/Strep (12.5 μg/mL), aprotinin (25 ug/mL; Sigma), EPO (20 ng/mL; Peprotech), G-CSF (1 ng/mL; Peperotech), Flt3-L (100 ng/mL; Peprtoech), TPO (100 ng/mL; Peprotech), and SCF (50 ng/mL; Peprotech). The CD34+ cells and stromal cells were isolated from whole blood and apheresis samples from normal human donors undergoing peripheral blood mobilization at the Massachusetts General Hospital. The cells were purified using CD34+ microbeads (Miltenyi), frozen in RPMI (ThermoFisher) with 10% FBS and 10% DMSO (EMD Millipore), as perfused with universal blood substitute medium at the same rate as in the other chips. Transwells (24 well plate Corning 0.4 μm pore size PET membrane) were coated on both sides with coating solution as for the Gut chip. Caco-2 Bbe cells and HUVECS were seeded on either side of the membrane at 100 000 cells/cm2. Transwells were kept with apical and basal media as described for the Gut Chip changed every 2-3 days for four weeks and then assayed.
Organ Chip Linkage
The Organ Chips were linked using automated liquid handling instrument. A detailed description of platform is found in Novak et al.16. In summary, an automated liquid handling instrument which can hold and link up to 10 Organ Chip and AV reservoir was custom build so it will fit a standard incubator. Before linking the Organ Chips, each Organ was placed in “quarantine” and then perfused without linking for 24 – 48 hrs to ensure the Chips are in their optimal condition. Once all the Organ Chips were placed on the instrument, the linkage protocol was done as describe in Supplementary Information Table 3. Levels of flowinduced shear stress within all of the Organ Chips were in the same range as previously reported.17,22
A multi Organ-Chip study can take 2-3 weeks to carry out (Supplementary Information Table 8) if you have validated cells, seeded and matured the chips individually, and developed appropriate assay procedures, which is similar to the length of traditional preclinical evaluation studies in animals. Technically, Organ Chip handling, platform setup analysis, and IVIVT are different than classical preclinical models and thus, an additional training period (~2-4 week) is required as well. While use of a larger number of different Organs Chip demands several skilled technicians, the four Organ Chips used in this study are manageable with two trained staff members.
Analytical methods
To determine barrier function in the Gut Chip, the inert tracers Inulin-FITC (Sigma-Aldrich Corp, St. Louis, MO, USA) or Cascade Blue® hydrazide Trisodium Salt (Thermo Fisher Scientific Inc., Waltham, MA, USA) were included (100 μg/mL) in the parenchymal medium and flowed (60 μL/hr) through upper or basal channel of the chip. The tracers were chosen for low spectral overlap, which allows simultaneous fluorescent quantification and both tracers have been validated for measuring paracellular permeability changes16. The concentrations of the tracers were measured in the effluents of the upper (donor) and lower (receiver) channels. The apparent permeability formula has been derived from a barrier model between donor and receiver microchannels:
| (1) |
Assuming zero receiver inlet concertation (CRin=0) and zero initial receiver concentration (C0R=0) we can calculate the apparent permeability (Papp) as follows:
| (2) |
Using the measured concentration at the channel outlets (CD and CR) we can calculate the apparent permeability (Papp) from the final form:
| (3) |
where VR is the volume of the receiving (lower) channel effluent after time t, CR is the measured concentration of tracer in receiving channel effluent, t is time of effluent collection and A is the area of the barrier. Note that if there is no flow in the receiver channel (QR=0) Eq. 3 simplifies to a classical apparent permeability formula for a static barrier. TEER was measured in Transwells using an EndOhm chamber with an EVOM2 Meter (WPI, Sarasota, USA). The absolute value of TEER can vary depending on the Caco-2 clone used, medium composition, and co-culture conditions, as well as the measurement setup. When Papp was measured in Transwells, the tracers were added at the same concentrations into the apical compartment as described above, and the Transwells were incubated for 1 hr before samples were collected and analyzed in a fluorometer. All permeability measurements can vary ar depending on the device setup as well as the tracer molecular weight, with low molecular weight tracers being more sensitive to small changes in barrier function. The correlation between TEER and inulin permeability in Caco2 cells has been previously established in multiple publications48. TEER electrodes can be integrated into Organ Chips for real-time measurements49,50, but these TEER chips are not compatible with the fluidic handling system of the automated culture instrument used here; however, this approach could be modified for label-free monitoring of barrier function on this platform in the future.
Albumin concentrations in samples collected each day from the inlet and outlet channels of the apical and basal channels of the Liver Chip and Kidney Chip were quantified using a human albumin ELISA quantitation kit (Bethyl Laboratories Inc., Montgomery, TX, USA). Albumin production and reabsorption were calculated as a rate based on the difference between the inlet and outlet concentrations of apical and basal channels and normalized to total protein amount.
The activity of cytochrome P450 CYP3A4 within the Organ Chips was determined using the P450 Glo kit (Promega, Madison, WI, USA). Briefly, luciferin-IPA was diluted (1:1000) in the inlet medium of the parenchymal channel and flowed through the Organ Chip at a rate of 60 μL/hr. After at least 2 hrs, chips were purged to collect remaining metabolite. From these data, metabolite production rate was determined and standardized to total protein. Total protein was determined in lysates from the parenchymal cells after removal of the endothelium from the basal channel by trypsinization. The remaining cells were dissolved in RIPA Buffer supplemented with 1x Halt™ Protease and Phosphatase Inhibitor Single-Use Cocktail (Thermo Fisher Scientific Inc., Waltham, MA, USA). Total protein was determined using Pierce BCA assay kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Western blot analysis was carried out using 10% Tris-Glycine (Novex WedgeWell 10-well) gels run at 150V with protein ladder SeeBlue Plus2 pre-stained protein ladder (Invitrogen). Transfer to nitrocellulose membranes was carried out using an iBlot 2 machine (Thermo Fisher). Primary CYP2A6 antibody (Abcam), Pgp Rabbit andti-P-Glycoprotein-1 141KDa(Abcam, ab170904) 1:1000 in 5% BSA in TBST, OCT2 (Rabbit anti-SLC22A2 (OCT2) 63KDa (Abcam, ab198800) 1:1000 in 5% BSA in TBST and Rabbit anti-GAPDH (Cell Signaling, 5174S) 1:10000 in 5% BSA in TBST) were used, antibodies were visualized using donkey anti-mouse secondary antibodies conjugated to HRP (Jackson Immunoresearch Laboratories).
To assess bone marrow cell populations, cells from the apical channel were removed by digesting the fibrin gel in buffer containing DMEM supplemented with Nattokinase (2.5 mg/mL; Japan Bio Science Laboratory), 25mM HEPES (ThermoFisher Scientific Inc), Collagenase type I (1 mg/mL Gibco), and 10% FBS for 1 hr at 37°C. Cells were then harvested, mixed with 5,000 counting beads (Spherotech), and pelleted at 400g for 4 mins at 4°C. To identify different hematopoietic populations, Cells from the apical channel were harvested for flow cytometry analysis by digesting the fibrin gel in buffer containing DMEM supplemented with Nattokinase (2.5mg/mL; Japan Bio Science Laboratory), 25mM HEPES (ThermoFisher Scientific Inc.), Collagenase type I (1mg/mL; Gibco), and 10% FBS for 1 hr at 37°C. Cells were then mixed with 5 x 10Λ3 counting beads (Spherotech, ACRFP-100-3) to enable quantification of cell numbers. The mixture was pelleted at 400g for 4 mins at 4°C. To identify different hematopoietic populations, surface staining was performed for 30 mins at 4°C in flow cytometry buffer composed of PBS, 1% FBS, 25mM HEPES (ThermoFisher), 1mM EDTA (Thermo Fisher), and 0.05% sodium azide (VWR) using the following antibodies: anti-CD235a-Pacific Blue (HI264 clone, BioLegend, 349108, dilution 1:80), anti-CD15-Brilliant Violet 510 (W6D3 clone, BioLegend, 323028, dilution 1:50) and anti-CD71-PerCP/Cy5.5 (CY1G4 clone, BioLegend, 334114, dilution 1:50) for erythrocytes and anti-CD16-PE/Dazzle 594 (3G8 clone, BioLegend, 302054, dilution 1:80) and anti-CD13-APC (WM15 clone, BioLegend, 301706, dilution 1:80) for neutrophils, as well as anti-CD34-PE/Cy7 (581 clone, BioLegend, 343516, dilution 1:50) for additional characterization. Additionally, the staining panel included 20 nM Syto16 (Thermo Fisher Scientific, S7578), Zombie NIR dye (BioLegend, 423106, dilution: 1:500), Fc Block (BioLegend, 422302, dilution 1:20), Monocyte block (BioLegend, 426103, dilution 1:20), and Brilliant stain buffer (BD, 566385, dilution 1:20). Data acquisition was performed using a BD LSRFortessa, and samples were analyzed using FlowJo software.
Mass spectrometry (MS) was carried by PureHoney Technologies (Billerica, MA, USA). Their RapidFire-MS/MS system consisted of an Agilent RapidFire 300 interfaced to a Sciex API4000 triple quadrupole mass spectrometer using Analyst 1.6 data acquisition software. All quantitative analysis was performed using Agilent RapidFire Integrator software. Nicotine and cotinine, together with stable isotope internal standards, were simultaneously quantified in positive ion turbospray mode. Medium samples were diluted such that the maximum concentration would be 10 μM or less, which was determined to be upper limit of the linear range of the assay. All standard curve injections were performed in triplicate; the limit of detection of the assay ranged from 10 to 40 nM. All reagents were purchased from Sigma-Aldrich, St. Louis, MO with the exception of the stable isotope internal standards which were purchased from Cerilliant, Round Rock, TX. All reagents were HPLC grade except for methanol used in Buffer B which was UHPLC grade.
Inductively coupled plasma mass spectrometer (ICP-MS) analysis was carried out by PureHoney Technologies (Billerica, MA, USA). The system was composed of an Agilent 7500CX ICP-MS equipped with an ASX-500 autosampler.
Materials:
Platinum standard solutions (1,000 μg/mL) was purchased from Agilent Technologies. Trace metal grade nitric acid was purchased from Fisher Scientific Inc. (Waltham, MA, USA) while all water used was HPLC grade.
Method:
A mass of 195 was monitored for platinum quantitation. A total of 3 independent measurements of 5.5 seconds each were performed on each sample and the average instrument response was reported. 40 μL of test sample consisting of cell culture media was diluted to 4 mL in 2% nitric acid in a 13 x 100 mm polypropylene vial, heated to 50°C for 30 mins and allowed to cool to room temperature. An 11-point, 2x serial dilution of platinum starting at 10 micromolar was prepared as a standard curve and a 12th concentration with 0 ppm Pt was included as a blank. 40 μL of blank cell culture media was spiked into each of the 12 standard curve samples accurately model the test samples
Data Analysis:
Two separate standard curves, each consisting of 6 calibration levels were created for quantitative platinum analysis: a low Pt concentration curve from 0 to 10 nanomolar using the pulse detector and a high Pt concentration curve for samples between 10 and 1000 nanomolar using the analog detector of the 7500 ICP-MS were generated to analyze the samples. The appropriate standard curve based on the signal level of the test samples (ie: pulse or analog) was used to calculate the platinum concentration of the test samples. The method had a lower limit of detection below 0.5 nanomolar and was linear to 1000 nanomolar, the lowest and highest concentration standards tested. Linear curve fitting was used for both the pulse and analog detector standard curves.
Statistical Analysis
All experiments contain a statement of number of replicates in the text. Data are presented as mean ± standard deviation or standard error of the mean, as indicated. Significance values are based on two-tailed t-tests; significance is determined as P < 0.05. Bland-Altman analysis was carried out of nicotine, cotinine and cisplatin and displayed as the average of the data quantified with mass spectroscopy and the DMPK model vs the difference between the measured and modelled data. Additionally, the IVIVT models were analyzed versus the clinical data (geometric mean and standard deviation) in a similar way. The dotted lines show the bias, which is the average of the differences, and the 95% limits of agreement. Correlation analysis was carried out with Pearson correlation between measured, mass spectroscopy or clinical data, versus the modelled data. Two-tailed tests were carried out. Lin’s concordance coefficient calculations were also carried out on these data sets. Prism 8.0 (Graphpad Software Inc, Sand Diego USA) and R environment were used for the Bland-Altman, Pearson correlation and Lin’s concordance analysis.
Computational Modeling Methods
The overview of the in silico workflow is presented in Supplementary Fig. 15.
Development of models of individual Organ Chips.
DMPK models of each Organ Chip were developed using ordinary differential equation (ODE)-based distributed (spatio-temporal) multi-compartment reduced-order (MCRO) fast running models. These models were applied to simulate in vitro ADME and DMPK/PD, in vivo human DMPK/PD, and model-based IVIVT. All models were developed using CFDRC’s Computational Biology (CoBi) tools51 (available in: http://medicalavatars.cfdrc.com/index.php/cobi-tools/).
Construction of Blank MCRO Organ Chip Model.
The geometry in each MCRO Organ Chip model (Fig. 1b,c and Supplementary Fig. 4 and 8) is represented with a coarse spatial computational mesh represented by control volumes in stream-wise direction and one control volume in cross-stream direction: PDMS, top channel, epithelial layer, porous membrane, endothelial layer, bottom channel, PDMS, respectively. The MCRO model is derived for general spatio-temporal transport equations accounting for accumulation, convection, diffusion and reaction:
| (4) |
Where C is the compound concentration, v is the fluid velocity, D is the diffusion coefficient and S is the generalized source term. The MCRO model is derived from above equation by integrating spatial terms for convection and diffusion into individual fluxes across control volume boundaries and treating them as source terms in the ordinary differential equations (ODEs). For a simplified barrier configuration (Fig. 1b, Supplementary Fig. 4), the above transport equation, assuming negligible stream-wise diffusion, can be integrated into three ODEs with the spatial convection and transmembrane diffusive transport terms expressed as individual fluxes:
| (5) |
| (6) |
| (7) |
where CB, CA, CM are compound concentrations in individual compartments (basal channel medium, membrane and apical channel medium), V’s are compartment volumes, QA and QB are the volumetric flow rates in basal and apical microchannels respectively, and CA,in and CB,in are concentrations at microchannel inlets. The transmembrane fluxes are calculated as follows:
| (8) |
| (9) |
Where S is the surface area of the barrier and PB-M and PM-A are the effective permeability coefficients between compartments. Note that depending on the compound partition coefficient, k, there may be a concentration discontinuity at the medium-membrane interfaces (illustrated with dashed lines in Supplementary Fig. 4). A similar approach was used to calculate fluxes between the channel medium and the PDMS.
Discretization of MCRO Organ Chip Models and the linked configuration.
Each Organ Chip lined by an epithelium interfaced with an endothelium was further discretized into thee axial zones (proximal, central, distal) making the problem two-dimensional (2D), which allowed for faster computing compared to whole 3D models. Additionally, this enabled us to calculate both passive and active transport parameters for each Organ Chip. The MCRO model is thus segmented into three axial zones, i=1,2,3, in the stream-wise direction (proximal, central, distal) involving convective and diffusive fluxes. For example, for the basal medium (B), three equations are solved:
| (10) |
where ΔJdiff.i is the diffusive flux difference across the downstream and upstream control volume faces in the stream-wise direction (along the microchannel axis):
| (11) |
where A is the channel cross-section area and Δx is the channel sub-compartment length. The following sections briefly summarize the transport equation for each organ device.
All organ device configurations (i.e., Gut, Liver, Kidney and Bone Marrow Chips) have similar barrier configuration involving several layers: basal PDMS package (BP), basal channel medium (BM), endothelium (E), thin porous PDMS membrane (M), epithelium (H), apical channel medium (AM), and apical PDMS package (AP) (Fig. 1c, Supplementary Fig. 4). As a result, all Organ Chip devices are represented with similar mathematical equations describing species mass balance in all layers. Specific Organ Chips were modeled using the parameters listed in Supplementary Information Table 8. In the following we describe details of the MCRO model shared by all organ chips used in this study.
MCRO Organ Chip model of passive and active transport plus metabolism for calculating intrinsic PK parameters.
The organ barrier model for each axial zone (i=1,2,3) in both the individual chip and linked system (Fig. 1b, c) involves compound conservation equations in: basal package (BP), basal medium (BM), endothelial barrier (E), membrane (M), epithelial barrier (H) apical medium (AM) and apical package (AP):
| (12) |
| (13) |
| (14) |
| (15) |
| (16) |
| (17) |
| (18) |
where QBM and QAM are the media volumetric flow rates in the basal and apical channels respectively and index, i=1,2,3, represents three stream-wise sections of the organ device (proximal, central and distal). The epithelial cell layer (H) metabolic clearance term in Eq. 16 with ClH,int represents the intrinsic clearance. The last terms in Eq. 16 and 17 represent the active transport between the epithelial cells and the apical media where kact is the efflux rate constant for each different Organ Chip.
The cross-stream passive diffusion fluxes between individual barrier layers are:
| (19) |
| (20) |
| (21) |
| (22) |
| (23) |
| (24) |
The stream-wise diffusive flux terms, ΔJdiff, in BM and AM media, membrane (M) and PDMS package layers (BP, AP) are calculated using Eq. 11. In the above equations, kp is the partition coefficient for a specific compound, fu is the unbound fraction of the compound and, S is the surface area normal to the cross-stream direction and P’s are the permeabilities for each barrier interface estimated using Eq. 8 and 9 and then calibrated computationally for each organ device according to the procedure described below.
Calibration of MCRO organ model for compound partition into PDMS.
Simulations of transport of highly lipophilic drugs in Organ Chips made with the PDMS have to account for drug distribution into the package materials19. The MCRO model of each Organ Chip involves drug conservation equations in the PDMS package layers adjacent to basal and apical channels (Eq. 12 and 18). To calculate the medium-PDMS permeability and partition coefficients in flux terms, Eq. 19 and 24, we use experimental data that is generated using a blank chip (with no cell layers) perfused with drug in both channels for a specific time period Experimental drug concentration-time data are collected from the apical and basolateral outlets during the loading. We use the blank Organ Chip MCRO model (Eq. 12,13,15,17,18) with the medium-PDMS partition coefficients and permeabilities as calibration parameters. Damped least-squares optimization solver in CoBi and Dakota optimization tools (https://dakota.sandia.gov/) were used to calibrate the parameters. The Dakota toolkit52, developed by Sandia National Laboratories (available in open source) provides a flexible interface between the analysis solver code (CoBi) and iterative optimization analysis methods. The Dakota framework contains algorithms for optimization with gradient and nongradient-based methods; uncertainty quantification with sampling, reliability, stochastic expansion and epistemic methods, parameter estimation with nonlinear least squares methods; and sensitivity/variance analysis with design of experiments and parameter study methods52. The described calibrated model has been validated for various hydrophilic and lipophilic tests compounds including FITC-inulin, Dextran Texas Red, and Lucifer Yellow (as described in Methods and ref 16). The calibrated Organ Chip MCRO model for a specific compound enables predictive calculation of compound concentrations in the epithelial and endothelial cell compartments, as well as in the barrier, and the associated absorption, metabolism, clearance, toxicity and other PK/PD responses. Moreover, this in silico calibration method allows us to use cost effective device fabrication processes and calculate Organ Chip PK performance as it would behave in idealized (non-absorbing) designs and materials.
Dynamic Organ Chip assumptions in the model.
The Gut Chip is subject to oscillatory membrane/channel stretching15, which is essential to achieve the in vivo observed physiological functions of the endothelial-epithelial cell barriers in this Organ Chip18. Because of small strain and very short time constants of the membrane stretching (~ 6% strain with 0.15 Hz frequency) compared to the long duration (hours to days) of the PK studies, the direct effects of mechanical strain application on organ geometry can be neglected in the present MCRO organ model. Therefore, the stretch effects are indirectly accounted for in the calibrated permeability parameters (Eqs. 19-24).
Drug metabolism and modeling metabolite flux.
The epithelial layer (H) equation (Eq. 16) includes the compound metabolism term calculated using the organ specific intrinsic clearance, CLH,int. For example, the equation for the hepatocyte cell layer involves the hepatic intrinsic clearance. For practical cases, conservation equations have to be solved for both the primary compound and for its metabolites. Both sets of equations for both the primary compound and for its metabolites are coupled via the drug metabolism reaction term in the epithelial cell layers (e.g. in liver hepatocytes) where a sink term of the primary compound (e.g. a prodrug or nicotine) becomes a source term in the equation for the metabolite conservation equation.
MCRO Organ Chip Model Parameters
Each Organ Chip has its own set of parameters including channel dimensions, flow rates in the apical and basal channels and metabolic and transport properties in the organ-specific epithelial cells (intestinal cells in the Gut Chip, hepatocytes in the Liver Chip, and proximal tubular epithelial cells in the Kidney Chip). The geometric dimensions which were used to calculate areas (A and S) and volumes for each compartment in Eqs. 12 - 24 for each Organ Chip are provided in Supplementary Information Table 1. The cell types used of each Organ Chip are described in Supplementary Information Table 2.
The fluidically linked multi Organ Chip model.
Computational simulation of fluidically linked Organ Chips with intermittent medium exchange between the chips involves the above MCRO barrier model equations (Eqs. 12-24) and additional compound conservation equations for four compartments: inlet/outlet tubing and inlet/outlet micro-reservoirs associated with each chip. Because the organ micro-reservoirs are periodically emptied and replenished with medium, additional fluid volume conservation equations have to be solved for each micro-reservoir. The conservation equations for the Basal (Endo) and Apical (Epi) side inlet and outlet micro-reservoirs (IR, OR) are (only the basal side is described as both sides have the same equation forms):
| (25) |
| (26) |
| (27) |
| (28) |
where and are the intermittent medium injections into the inlet micro-reservoir from the AV reservoir and sampling from the outlet micro-reservoir, respectively. CBIR,inj is the injected concentration into the inlet basal micro-reservoir (AV Reservoir concentration for a linked system) and CBOt is the basal outlet tubing concentration. The inlet/outlet tubing (IT, OT) equations are:
| (29) |
| (30) |
Where CBI,3 is the concentration at basal medium outlet section (i=3 in Eq. 13). The conservation equations for the apical side have similar form. Because the time scale of the intermittent medium exchange in micro-reservoirs (few minutes) is much smaller than the organ operation time sale (several hours), the above equations can be expressed as algebraic equations for mass balance in micro-reservoirs before and after the sampling event. Two linked organ systems have been simulated using the above MCRO Organ Chip models:
Gut, Liver, and Kidney Chips to study gut (oral) nicotine absorption, hepatic metabolism and systemic liver and renal clearance (Fig. 1, 2), and
Liver, Kidney and Bone Marrow Chips to investigate intravenous cisplatin administration, systemic PK and PD effects on bone marrow hematopoietic stem cells, (Fig. 4)
The major difference is the route of drug administration: “oral” via gut for the nicotine studies and “intravenous” into the AV Reservoir for cisplatin.
For the nicotine system simulations, the flow path of the basal media between individual Organ Chips and the AV Reservoir, which collects medium from basal micro-reservoir outlets from all Organ Chips is shown in Fig. 1a,c. The Liver Chip inlet micro-reservoir is supplied from the Gut Chip basal outlet micro reservoir (representing a portal vein) and from the AV Reservoir (representing the hepatic artery). The medium transferred to the Kidney Chip is divided into two paths: the glomerular filtrate flow and the glomerular efferent arteriole flow supplying the kidney tubular channel. A virtual glomerulus model is applied in both the experimental and computational platforms by extracting a medium volume filtered by the virtual glomerulus into the filtrate path (apical epithelial channel of the Kidney Chip), and the rest is supplied to the medium inlet mini-reservoir of the basal vascular channel. The filtrate flow rate is calculated based on renal physiological data and compound physicochemical properties. A similar medium circulation pattern was used for modeling the cisplatin system, except the Gut Chip is replaced by the Bone Marrow chip. In this case, due to lack of the Gut chip, the inlet mini-reservoir of the basal vascular channel of the Liver Chip is fully supplied with blood substitute medium directly from the AV Reservoir.
Simulating the arterio-venous compartment.
The physiological arterio-venous pool is represented by the AV Reservoir with a dynamic medium volume, VAV(t) periodically resupplied from the basal (venous) outlet micro-reservoirs of the Liver and Kidney Chips, drained to supply inlet micro-reservoirs of the basal vascular channels of all Organ Chip devices, replenished with fresh medium, and sampled for analytics. The total and compound mass balance equations describe the AV Reservoir dynamics:
| (31) |
| (32) |
where the indices g, l, k and fM stand for gut, liver, kidney and fresh medium, respectively. and are the volumetric rates of sampling and fresh medium supply to the AV. As for the micro-reservoirs, the above equations can be expressed as algebraic mass balance equations in the AV reservoir before and after the sampling event. In the nicotine studies, pure medium is injected, CfM=0, (experimentally delivered to the gut), while for the cisplatin study the drug is supplied at a specified concentration, CfM>0 to mimic IV infusion experimentally.
Solving MCRO simulations.
The above system of ODE equations for each organ is programmed in the form of text (*.Sim) input files and solved using CoBi tools, using a standard PC workstation. These tools can be accessed at https://www.cfdrc.com/CoBi-SUITE-10-47.html with provided instructions for running simulation solvers.
Implementation of in vitro-to-in vivo translation (IVIVT) of human PK parameters.
Even the most advanced in vitro “human-body-on-chip” (HuBoC) systems are an enormous simplification of the human body. Thus, there will be always a need for mathematical model-based extrapolation of in vitro results to humans in vivo. In vitro-to-in vivo correlation (IVIVC) described previously involves “a predictive mathematical model describing the relationship between an in vitro property of a dosage form and an in vivo response2. This dose-response model establishes a statistical correlation between in vitro dissolution or permeation rate and in vivo PK properties such as Cmax and AUC53,54, but it does not quantitatively predict these parameters. In contrast, in vitro-to-in vivo extrapolation (IVIVE) applies mechanistic, but relatively simple models to extrapolate the in vitro drug metabolism in cell cultures or transport across barriers to in vivo organ clearance or absorption55,56. In vitro organ data along with drug physicochemical properties are then used in PBPK models57,58. Physiologically interconnected multi-Organ Chip systems, such as the one described here, can generate huge amounts of data that require new computational tools for both interpretation of in vitro data and for translation to humans in vivo (IVIVT)3,59. In this study, we used multiphysics CoBi tools for first principles-based modeling of multiple fluidically linked Organ Chips and to develop novel quantitative IVIVT approach.
Conventional IVIVE methods rely on static cell culture or barrier experiments to estimate single lumped PK parameters in vivo using in vitro data. Separate in vitro experiments are used to estimate drug dissolution, intestinal permeability (absorption)60, hepatic intrinsic clearance25,61 or renal clearance rates25. For example, the in vivo hepatic clearance, CLh and the hepatic availability (Fh= 1–CLh /Qh) can be calculated using in vitro primary human hepatocytes culture data and the well-stirred liver model as previously shown62,63:
| (33) |
where Qh is the liver blood flow (20 ml/min/kg), fup is the unbound fraction in plasma and the in vivo clearance is estimated using a physiology based scaling factor, SF that converts the units of CLint,in vitro [μL/min/106 cells] to CLint,in vivo [mL/min/kg]:
| (34) |
where HPGL is the hepatocellularity (i.e. number of hepatocytes per gram of liver = 99-120*106). Liver Wt is the liver weight (1500–1800 g liver/70 kg) and REF is the relative expression factor (a ratio of in vivo/in vitro expression of metabolic enzymes or transporters, often REF ~1)62,64. Such scaled intrinsic clearance is directly used in a PBPK model as a sink term in a liver model – typically a single equation well stirred blood/tissue compartment.
Complementary experimental and computational models of in vitro physiologically connected Organ Chips provide a unique opportunity to establish next generation multiscale PBPK models coupling the whole body with spatially and morphologically resolved barrier models65,66,67. It has been proposed that computational models of morphologically resolved barriers in organs (gut, liver, lung, brain, kidney, skin, bone), validated in vitro in Organ Chip and multiple linked HuBoC platforms could be directly applied in barrier resolved multiscale PBPK models34. In the present study, we integrated computational models of Organ Chips with functionalized barriers, calibrated and validated on experimental data are used to construct corresponding spatially resolved in vivo barrier models within a multiscale PBPK framework, such as that incorporated in CoBi tools67, to develop a quantitative IVIVT protocol.
Comparison with other Organ Chip models.
The key design principle for Organ Chips is to recapitulate in vitro anatomical, physiological and PK parameters exhibited by living tissue barriers within living organs in vivo. Past models of fluidically perfused Organ Chip designs represent the tissue barrier in the form of vertically stacked barriers18, horizontally stacked barriers, or Transwells with fluidic connection to perfusion chambers57. As discussed above, each approach has some advantages and limitations, but from the computational model point of view, the parallel channels and Transwell designs have significant disadvantages. Note that in past formulations, the liver model is subdivided into two compartments, basal and apical, and the hepatocytes are lumped with the apical compartment57. In the open surface Transwell device, the apical volume is treated as time dependent. In other models previously described41,68 where the liver model is represented as a single well-stirred reactor with medium flowing across the barrier and the clearance term applied to the entire liver volume instead of hepatocyte cell layer. The model described here contained an additional hepatocyte compartment.
One of the goals of this study was to explore how to adapt the in silico model of the in vitro linked multi-Organ Chip to achieve compound DMPK profiles observed in humans in vivo. To evaluate the feasibility of a potential IVIVT protocol, we decided to retain the in vitro MCRO model of the barrier between the basal and apical medium, but we explored adaptation and scaling of other components of the in silico model as well, including:
Connect all organs microfluidically with continuous, rather than intermittent, medium exchange, to emulate physiological conditions
Directly connect individual Organ Chips to an AV reservoir (i.e. basal “venous” outlets supplying the AV reservoir), which then feeds basal “arterial” inlets
Adjust the geometry of microfluidic channels to replicate more physiological conditions by increasing the surface area, decreasing channel height, and scaling the organ vascular volume to achieve human values (as in typical PBPK models) Fig. 3c
Calibrate the flow rate ratios through the Organ Chips to achieve physiological flow distribution exhibited by those organs in vivo (as in human PBPK models based on Supplementary Table 4)
Calibrate the AV reservoir volume to account for missing organs and tissues (e.g. fat) and for drug specific volume of distribution
With these novel modifications of the modeling approach, we were able to computationally reproduce the in vivo PK profiles of human clinical data for both nicotine and cisplatin at different doses and different routes of administration.
Supplementary Material
ACKNOWLEDGMENTS
This research was sponsored by the Wyss Institute for Biologically Inspired Engineering at Harvard University, the Defense Advanced Research Projects Agency under Cooperative Agreement Number W911NF-12-2-0036, and US Food and Drug Administration grant HHSF223201310079C. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Defense Advanced Research Projects Agency, or the U.S. Government. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. CNS is part of Harvard University, the Harvard Materials Research Science and Engineering Center (DMR-1420570). We thank M. Rosnach for his artwork, B. Fountaine and S. Kroll for their help with photography, N. Dimitrakakis for statistical analysis and C. Vidoudez for M.S analysis.
Footnotes
COMPETING INTERESTS
D.E.I. is a founder and holds equity in Emulate, Inc., and chairs its scientific advisory board. K.K.P. is a consultant to Emulate, Inc. S.S.F.J, J.F-A., G.A.H., C.H., K-J.J., V.K., L.L., D.L., J.N., J.S, G.T.II, and N.W. are employees of and hold equity in Emulate, Inc. A.B., Y.C., M.C., S.D., J.F-A., T.F., E.A.F., J.A.G., G.A.H., T.H-I. O.H., A.H., C.H., D.E.I., M.I., K-J.J., V.K., L.L., D.L., O.L., B.M.M., Y.M., J.N., R.N., T-E.P., K.K.P., J.S, A.S-P., G.T.II, N.W., and D.E.I. are inventors on intellectual property licensed to Emulate, Inc.
DATA AVAILABILITY
Code availability:
The CoBi code used to simulate individual organs and their network and individual organ models are freely available at http://medicalavatars.cfdrc.com/index.php/cobi-tools/ under the folder entitled: Microphysiological Organs and Systems Models.
Datasets:
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Shanks N, Greek R & Greek J Are animal models predictive for humans? Philos. Ethics Humanit. Med. PEHM 4, 2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malinowski H et al. Draft guidance for industry extended-release solid oral dosage forms. Development, evaluation and application of in vitro-in vivo correlations. Adv. Exp. Med. Biol 423, 269–288 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Stokes CL, Cirit M & Lauffenburger DA Physiome-on-a-Chip: The Challenge of “Scaling” in Design, Operation, and Translation of Microphysiological Systems. CPT Pharmacomet. Syst. Pharmacol 4, 559–562 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danhof M, de Lange ECM, Della Pasqua OE, Ploeger BA & Voskuyl RA Mechanism-based pharmacokinetic-pharmacodynamic (PK-PD) modeling in translational drug research. Trends Pharmacol. Sci 29, 186–191 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Abaci HE & Shuler ML Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr. Biol 7, 383–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esch MB, Ueno H, Applegate DR & Shuler ML Modular, pumpless body-on-a-chip platform for the co-culture of GI tract epithelium and 3D primary liver tissue. Lab. Chip 16, 2719–2729 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Coppeta JR et al. A portable and reconfigurable multi-organ platform for drug development with onboard microfluidic flow control. Lab. Chip 17, 134–144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao S et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun 8, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner I et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab. Chip 13, 3538–3547 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Bovard D et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab. Chip 18, 3814–3829 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Oleaga C et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep 6, 20030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maschmeyer I et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab. Chip 15, 2688–2699 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Edington CD et al. Interconnected Microphysiological Systems for Quantitative Biology and Pharmacology Studies. Sci. Rep 8, 4530 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernetti L et al. Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci. Rep 7, 42296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HJ, Li H, Collins JJ & Ingber DE Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl. Acad. Sci. U. S. A 113, E7–15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novak R et al. A robotic platform for fluidically-linked human body-on-chips experimentation. Nature Biomed. Engin - in press [Google Scholar]
- 17.Jang K-J et al. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol. Quant. Biosci. Nano Macro 5, 1119–1129 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Prantil-Baun R et al. Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu. Rev. Pharmacol. Toxicol 58, 37–64 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Auner AW, Tasneem KM, Markov DA, McCawley LJ & Hutson MS Chemical-PDMS binding kinetics and implications for bioavailability in microfluidic devices. Lab. Chip (2019) doi: 10.1039/C8LC00796A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalili-Firoozinezhad S et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng 3, 520–531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasendra M et al. Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids. Sci. Rep 8, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Huh D, Hamilton G & Ingber DE Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab. Chip 12, 2165–2174 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Jang K-J et al. Liver-Chip: Reproducing Human and Cross-Species Toxicities. Science Translation Medicine - in press. [DOI] [PubMed] [Google Scholar]
- 24.Pullan RD et al. Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med 330, 811–815 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL, Hukkanen J & Jacob P Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol 29–60 (2009) doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dancik Y, Anissimov YG, Jepps OG & Roberts MS Convective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical application. Br. J. Clin. Pharmacol 73, 564–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varma MVS et al. Physicochemical determinants of human renal clearance. J. Med. Chem 52, 4844–4852 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Digard H, Proctor C, Kulasekaran A, Malmqvist U & Richter A Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob 15, 255–261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prytz H, Benoni C & Tagesson C Does Smoking Tighten the Gut? Scand. J. Gastroenterol 24, 1084–1088 (1989). [DOI] [PubMed] [Google Scholar]
- 30.Suenaert P et al. In vivo Influence of Nicotine on Human Basal and NSAID-induced Gut Barrier Function. Scand. J. Gastroenterol 38, 399–408 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Prytz H, Benoni C & Tagesson C Does smoking tighten the gut? Scand. J. Gastroenterol 24, 1084–1088 (1989). [DOI] [PubMed] [Google Scholar]
- 32.McGilligan VE, Wallace JMW, Heavey PM, Ridley DL & Rowland IR The effect of nicotine in vitro on the integrity of tight junctions in Caco-2 cell monolayers. Food Chem. Toxicol 45, 1593–1598 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Gaztelumendi A, Alvehus M, Andersson T & Jacobsson SOP Comparison of the effects of nicotine upon the transcellular electrical resistance and sucrose permeability of human ECV304/rat C6 co-cultures and human CaCo2 cells. Toxicol. Lett 207, 1–6 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Somayaji MR, Das D & Przekwas A Computational approaches for modeling and analysis of human-on-chip systems for drug testing and characterization. Drug Discov. Today 21, 1859–1862 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki H et al. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int. J. Environ. Res. Public. Health 7, 3406–3421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartmann JT & Lipp H-P Toxicity of platinum compounds. Expert Opin. Pharmacother 4, 889–901 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Human bone marrow disorders recapitulated in vitro using organ chip technology ∣ bioRxiv. https://www.biorxiv.org/content/10.1101/458935v1.abstract. [Google Scholar]
- 38.Sparreboom A, Nooter K, Loos WJ & Verweij J The (ir)relevance of plasma protein binding of anticancer drugs. Neth. J. Med 59, 196–207 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Rajkumar P et al. Cisplatin Concentrations in Long and Short Duration Infusion: Implications for the Optimal Time of Radiation Delivery. J. Clin. Diagn. Res. JCDR 10, XC01–XC04 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wikswo JP et al. Scaling and systems biology for integrating multiple organs-on-a-chip. Lab. Chip 13, 3496–3511 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maass C, Stokes CL, Griffith LG & Cirit M Multi-functional scaling methodology for translational pharmacokinetic and pharmacodynamic applications using integrated microphysiological systems (MPS). Integr. Biol. Quant. Biosci. Nano Macro 9, 290–302 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neault JF & Tajmir-Riahi HA Interaction of cisplatin with human serum albumin. Drug binding mode and protein secondary structure. Biochim. Biophys. Acta 1384, 153–159 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Vickers AEM et al. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol. Pathol 32, 577–590 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Huang Q et al. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol. Sci. Off. J. Soc. Toxicol 63, 196–207 (2001). [DOI] [PubMed] [Google Scholar]
- 45.Maass C et al. Establishing quasi-steady state operations of microphysiological systems (MPS) using tissue-specific metabolic dependencies. Sci. Rep 8, 8015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huh D et al. Microfabrication of human organs-on-chips. Nat. Protoc 8, 2135–2157 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Park T-E et al. Hypoxia-enhanced Blood-Brain Barrier Chip recapitulates human barrier function, drug penetration, and antibody shuttling properties. bioRxiv 482463 (2018) doi: 10.1101/482463. [DOI] [Google Scholar]
- 48.Molecular Aspects of Alcohol and Nutrition - 1st Edition. https://www.elsevier.com/books/molecular-aspects-of-alcohol-and-nutrition/patel/978-0-12-800773-0.
- 49.Henry OYF et al. Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function. Lab. Chip 17, 2264–2271 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maoz BM et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab. Chip 17, 2294–2302 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Przekwas A, Friend T, Teixeira R, Chen Z & Wilkerson P Spatial Modeling Tools for Cell Biology. Afrl-If-Rs-Tr-2006-318 vol. Final Repo (2006). [Google Scholar]
- 52.Adams BM et al. Dakota, a multilevel parallel object-oriented framework for design optimization, parameter estimation, uncertainty quantification, and sensitivity analysis : https://www.osti.gov/biblio/1177077 (2014) doi: 10.2172/1177077. [DOI] [Google Scholar]
- 53.Amidon GL, Lennernäs H, Shah VP & Crison JR A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res 12, 413–420 (1995). [DOI] [PubMed] [Google Scholar]
- 54.O’Hara T et al. In vivo-in vitro correlation (IVIVC) modeling incorporating a convolution step. J. Pharmacokinet. Pharmacodyn 28, 277–298 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Howell BA et al. In vitro to in vivo extrapolation and species response comparisons for drug-induced liver injury (DILI) using DILIsym™: a mechanistic, mathematical model of DILI. J. Pharmacokinet. Pharmacodyn 39, 527–541 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Poulin P & Haddad S Toward a new paradigm for the efficient in vitro-in vivo extrapolation of metabolic clearance in humans from hepatocyte data. J. Pharm. Sci 102, 3239–3251 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Jin JY, Mukadam S, Malhi V & Kenny JR Application of IVIVE and PBPK modeling in prospective prediction of clinical pharmacokinetics: strategy and approach during the drug discovery phase with four case studies. Biopharm. Drug Dispos 33, 85–98 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Rostami-Hodjegan A Physiologically based pharmacokinetics joined with in vitro-in vivo extrapolation of ADME: a marriage under the arch of systems pharmacology. Clin. Pharmacol. Ther 92, 50–61 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Cirit M & Stokes CL Maximizing the impact of microphysiological systems with in vitro-in vivo translation. Lab. Chip 18, 1831–1837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu C, Jiang L, Chen T-M & Hwang K-K A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem 37, 399–407 (2002). [DOI] [PubMed] [Google Scholar]
- 61.Hukkanen J, Jacob P & Benowitz NL Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 57, 79–115 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Riley RJ, McGinnity DF & Austin RP A unified model for predicting human hepatic, metabolic clearance from in vitro intrinsic clearance data in hepatocytes and microsomes. Drug Metab. Dispos. Biol. Fate Chem 33, 1304–1311 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Chiba M, Ishii Y & Sugiyama Y Prediction of hepatic clearance in human from in vitro data for successful drug development. AAPS J. 11, 262–276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jamei M et al. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drug-drug interaction between rosuvastatin and cyclosporine. Clin. Pharmacokinet 53, 73–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sluka JP et al. A Liver-Centric Multiscale Modeling Framework for Xenobiotics. PloS One 11, e0162428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clancy CE et al. Multiscale Modeling in the Clinic: Drug Design and Development. Ann. Biomed. Eng 44, 2591–2610 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kannan RR, Singh N & Przekwas A A compartment-quasi-3D multiscale approach for drug absorption, transport, and retention in the human lungs. Int. J. Numer. Methods Biomed. Eng 34, e2955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsamandouras N et al. Integrated Gut and Liver Microphysiological Systems for Quantitative In Vitro Pharmacokinetic Studies. AAPS J. 19, 1499–1512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong C et al. Hepatotoxicity and pharmacokinetics of cisplatin in combination therapy with a traditional Chinese medicine compound of Zengmian Yiliu granules in ICR mice and SKOV-3-bearing nude mice. BMC Complement. Altern. Med 15, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code availability:
The CoBi code used to simulate individual organs and their network and individual organ models are freely available at http://medicalavatars.cfdrc.com/index.php/cobi-tools/ under the folder entitled: Microphysiological Organs and Systems Models.
Datasets:
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.