Abstract
We present a case of acute myocarditis with left ventricular dysfunction and intracavitary thrombosis in a 55-year-old man with severe acute respiratory syndrome coronavirus 2 infection (coronavirus disease 2019) who was admitted with bilateral atypical pneumonia. The patient was treated with anticoagulation and optimal heart failure therapy and had an improvement of left ventricular function and thrombus resolution. (Level of Difficulty: Intermediate.)
Key Words: cardiovascular magnetic resonance (CMR), computed tomography, COVID-19, echocardiography, myocarditis, thrombus
Abbreviations and Acronyms: CMR, cardiac magnetic resonance; COVID-19, coronavirus disease-2019; Fio2, fraction of inspired oxygen; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro–B-type natriuretic peptide; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2
Central Illustration
History of Presentation
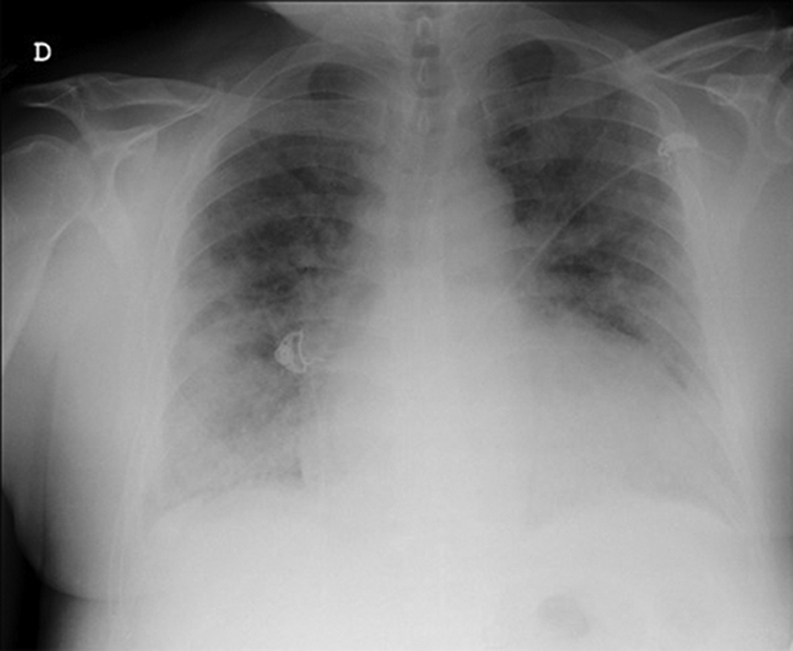
A 55-year-old man who tested positive for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) (the agent of coronavirus disease-2019 [COVID-19]) was admitted to the intensive care unit of Vall d'Hebron University Hospital, Barcelona, with a diagnosis of bilateral atypical pneumonia (Figure 1). Blood tests demonstrated leukopenia, elevated C-reactive protein of 12.35 mg/dl, and interleukin-6 levels of 1,946 pg/dl. High-sensitivity troponin I, D-dimer, and N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels were unremarkable. Blood pressure was 144/77 mm Hg, heart rate was 84 beats/min, body temperature was 36°C, and respiration rate was 24 breaths/min. The patient required high- flow oxygen through a nasal cannula (fraction of inspired oxygen [Fio2] 1) to maintain a peripheral oxygen saturation of 96%. However, 3 days after hospitalization, D-dimer levels increased significantly (up to 12,835 ng/ml), as did troponin I and NT-proBNP levels (4,162 ng/l and 7,460 pg/ml, respectively). He had no cardiovascular symptoms and was hemodynamically stable. A bedside echocardiogram showed a dilated left ventricle with moderate systolic dysfunction (left ventricular [LV] ejection fraction [LVEF] of 35% to 40%), global hypokinesia, and a huge multilobed, mobile, and hyperechogenic mass measuring 41 × 26 mm and attached to the apex. Right ventricular function was preserved (tricuspid annular plane systolic excursion of 22 mm and tissue Doppler systolic wave [Ś] of 12 cm/s), and there was no valve disease (Figures 2A and 2B, Videos 1 and 2). With these findings, anticoagulation with full-dose low-molecular-weight heparin and heart failure treatment (carvedilol and enalapril) were started.
Learning Objectives
-
•
To recognize cardiovascular complications among COVID-19 patients.
-
•
To learn the role of multimodality imaging in challenging cases.
Figure 1.
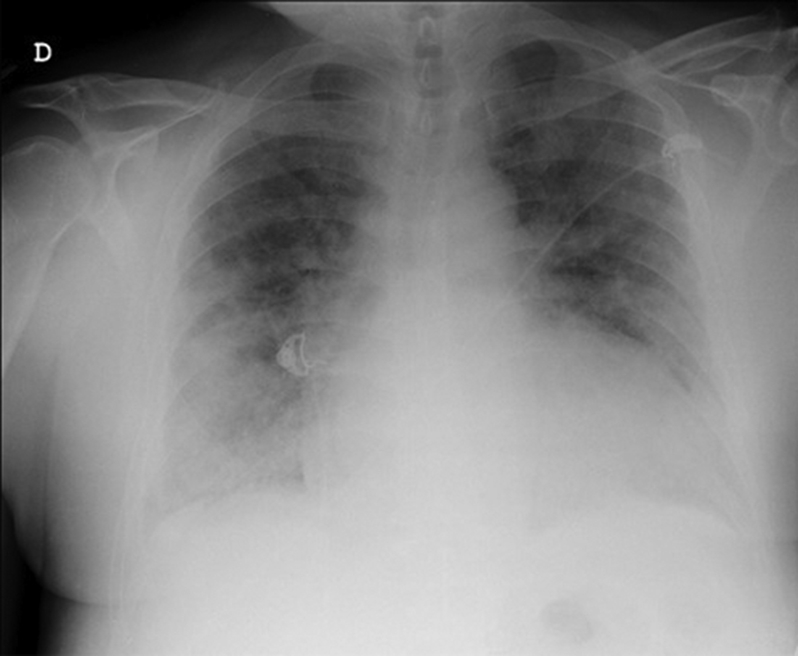
Chest Radiograph on Admission
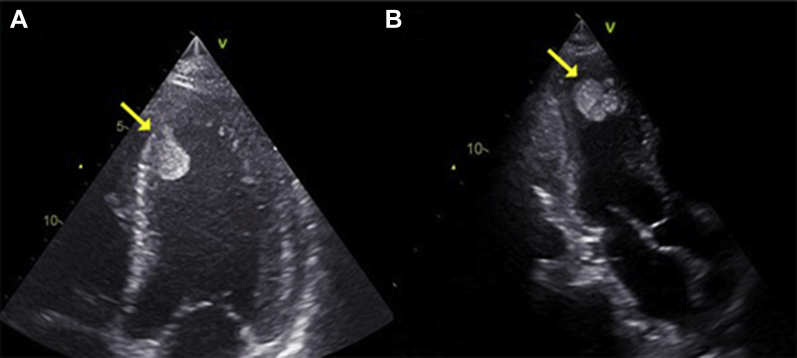
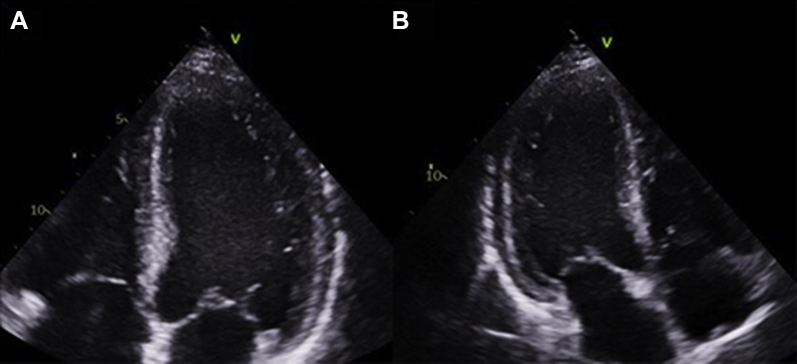
Figure 2.
Bedside 2-Dimensional Echocardiography
The images show a dilated left ventricle with a huge multilobed, mobile, and hyperechogenic mass attached to the apex (arrows). Left ventricle in (A) the apical 4 chamber view and (B) the apical 3-chamber view.
Past Medical History
His past medical history included obesity (body mass index, 31 kg/m2) and obstructive sleep apnea syndrome requiring continuous positive airway pressure. He had no previous cardiovascular disease.
Differential Diagnosis
Given the patient's clinical presentation with LV dysfunction, the differential diagnosis included ischemic cardiomyopathy, myocarditis, and decompensation of a previously unknown cardiomyopathy. Because of the presence of a huge intraventricular mass, thrombus versus intracardiac tumor was proposed.
Investigations
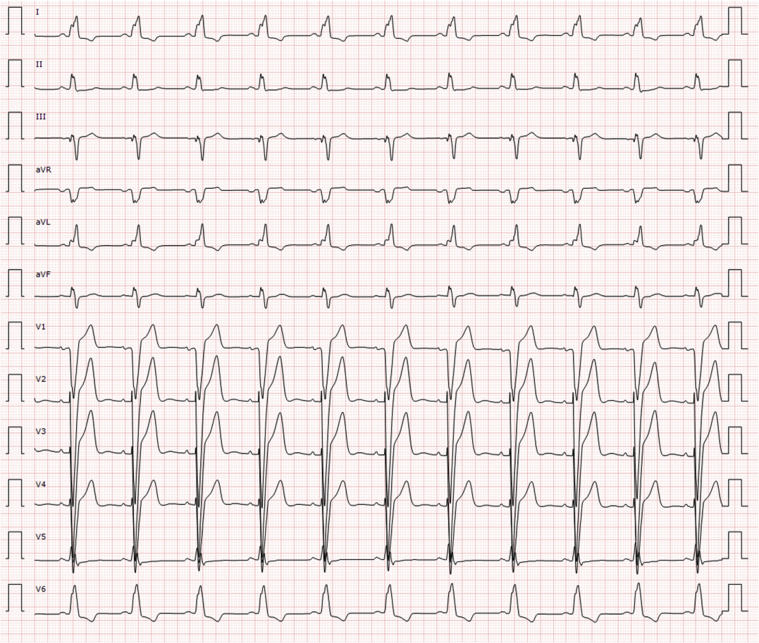
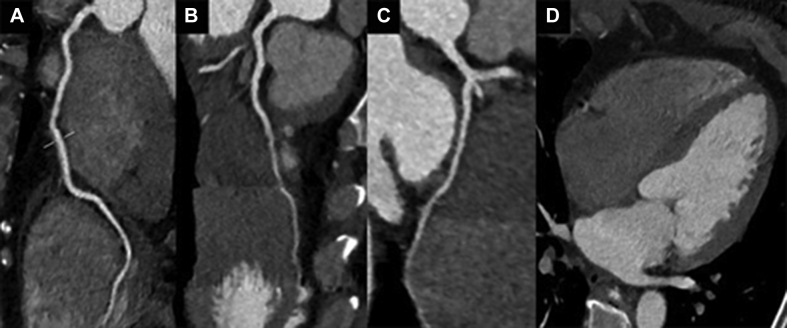
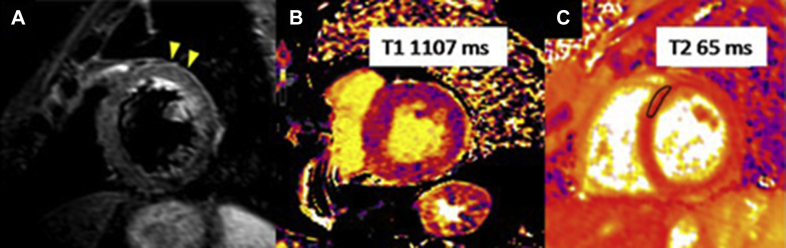
An electrocardiogram showed a previously unknown left bundle branch block (Figure 3). At 6 and 7 days after the echocardiogram and the start of anticoagulation, cardiac computed tomography and cardiac magnetic resonance (CMR) were performed. Computed tomography showed no coronary lesions and a dilated left ventricle with moderate systolic dysfunction (LVEF, 38%) (Figures 4A to 4D). No intracavitary mass was visualized. CMR revealed mildly increased diffuse wall thickness with an elevated myocardial signal intensity in the anterior middle and apical wall in balanced steady-state free precession cine sequences. In addition, short tau inversion recovery images showed mild hyperintensity of the anterior wall, with a T2 ratio of myocardium to skeletal muscle of 2.6. Native T1 mapping was elevated overall (native T1 of 1,107 ms [reference values 950 to 1,050 ms]), as was the extracellular volume ( 29%). T2 mapping sequences showed an elevated global T2 value of 62 ms (65 ms in the anterior and anteroseptal wall, 55 ms in the inferior wall). Late gadolinium enhancement sequences demonstrated no foci or areas of contrast uptake; nevertheless, there was a diffuse mild hyperintensity of the middle and apical anterior wall (slow contrast washout). Overall, these findings are suggestive of diffuse myocardial edema, predominantly in the anterior wall (Figures 5, 6A to 6C, and 7, Videos 3 and 4). First-pass perfusion and early and late gadolinium enhancement were performed, and no intraventricular mass was observed, a finding that suggested a thrombotic origin. Because 2 complementary studies had demonstrated the absence of a mass, a second echocardiogram during hospitalization was not performed to reduce the risk of contagion for the sonographer, according to international recommendations (1). The patient did not experience any symptoms or signs suggestive of peripheral embolism.
Figure 3.
Electrocardiogram on Admission
Figure 4.
Coronary Computed Tomography Angiography
The images show no coronary stenosis in (A) the right coronary artery, (B) the left anterior descending artery, and (C) the circumflex artery. (D) No evidence of left ventricular thrombus is noted.
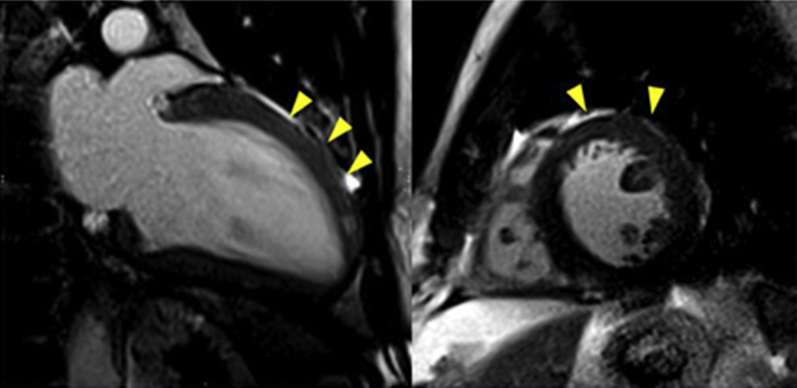
Figure 5.
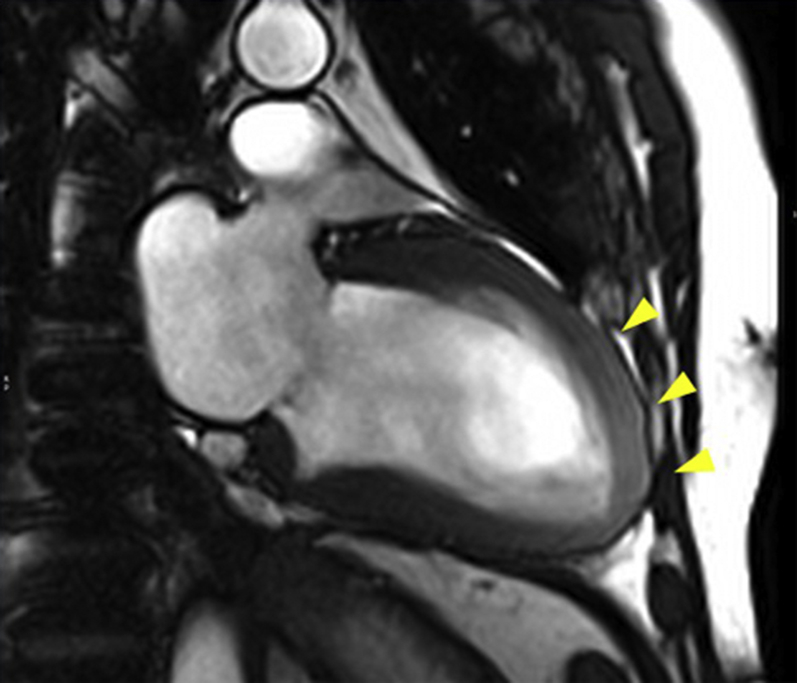
Balanced Steady-State Free Precession Cine Images in the 2-Chamber View
Mildly increased wall thickness and higher myocardial signal intensity in the anterior middle and apical wall (arrowheads).
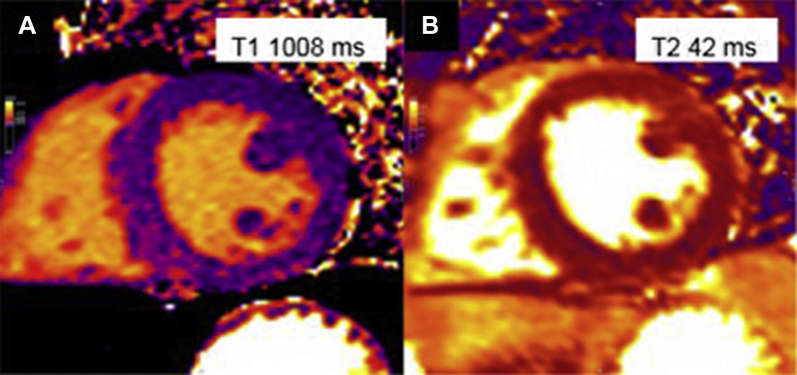
Figure 6.
Short Tau Inversion Recovery and Mapping Images
(A) Short tau inversion recovery images in the short axis with mild hyperintensity of the anterior wall (arrowheads). (B) T1 mapping study shows an overall elevated native T1 value of 1,107 ms and an extracellular volume of 29%. (C) T2 mapping shows an elevated global T2 value of 62 ms (65 ms in the anterior and anteroseptal wall, 55 ms in the inferior wall).
Figure 7.
Late Gadolinium Enhancement Images
Late gadolinium enhancement images in the 2-chamber (left) and mid–short-axis views (right) with no evidence of focal necrosis or fibrosis. Nevertheless, there is diffuse mild hyperintensity of the middle and apical anterior wall (arrows).
Management
The patient required only high-flow oxygen (Fio2 1) with no requirements of ventilation or hemodynamic support. He was treated with ceftriaxone, azithromycin, hydroxychloroquine, lopinavir and ritonavir in combination, and tocilizumab, according to the local protocol at the time. Anticoagulation with full-dose low-molecular-weight heparin was also prescribed, as well as complete heart failure therapy.
Discussion
Severe acute respiratory syndrome associated with SARS-CoV-2 infection appears to affect the myocardium by different mechanisms (2): infection-related myocarditis, hypoxemia, and/or ischemia, and it is considered an important prognostic factor (3). Moreover, COVID-19 may be associated with a hypercoagulable state. There is evidence of a high prevalence of clinically relevant thrombosis, essentially pulmonary embolisms (16.7%), in patients with hypoxemic acute respiratory failure (4). Furthermore, venous and arterial thromboembolisms are emerging as among the most severe sequelae of the disease associated with poorer outcomes (5). In addition, acute coronary syndromes are noted to occur after severe acute respiratory syndrome (6).
We report a case of myocarditis associated with COVID-19 with LV dysfunction and a large mobile LV thrombus in a young patient with no cardiovascular history. This thrombus could have resulted in severe complications without prompt diagnosis and treatment initiation. Because endomyocardial biopsy is not recommended in patients with COVID-19 and suspected myocarditis (1), and given that the patient had a good evolution, a biopsy was not performed. Moreover, recent biopsy data revealed that T1 and T2 increase in this population is related to myocarditis (lymphocytic infiltrates) (7).
Although the presence of elevated troponin I is frequently seen in patients with myocarditis and is usually associated with myocyte necrosis, our patient had elevated troponin but no late gadolinium enhancement. We believe that these findings can be explained by the marked myocardial inflammation and associated submillimetric and nonhomogeneous areas of necrosis (8). The lesser severity of myocardial injury also explains the overall improvement of myocardial systolic function during follow-up, whereas the expansion of the extracellular space is supported by the finding of increased native T1 and T2 mapping, as well as increased extracellular volume.
Echocardiography is helpful in assessing cardiac hemodynamics in the presence of chest pain, electrocardiographic changes, or biomarker elevation suggestive of myocardial injury, myopericarditis, or fulminant myocarditis (9), and it should be planned in patients with new onset of malignant ventricular arrhythmias not associated with a prolonged QT interval (10). As demonstrated in our case, echocardiography permitted accurate diagnosis and management.
We highlight the importance of echocardiography in patients with SARS-CoV-2 infection and elevated heart injury biomarkers, to establish a correct diagnosis, guide management, and reduce complications. To the best of our knowledge, no similar case has been published.
Follow-Up
The patient was discharged in good clinical condition. He is currently receiving the following medications: carvedilol, 6.25 mg b.i.d.; enalapril, 5 mg o.d.; and acenocoumarol. Two months after discharge, he is asymptomatic, and an echocardiogram revealed improvements in contractility and ventricular function (LVEF, 50%), as well as the absence of intracavitary thrombus (Figures 8A and 8B, Videos 5 and 6). Moreover, control CMR showed a normalization of T1 and T2 values (Figures 9A and 9B, Videos 7 and 8).
Figure 8.
Control Echocardiogram 2 Months After Discharge Showing Left Ventricular Function Improvement and the Absence of Intraventricular Thrombus
Left ventricle in (A) the apical 4-chamber view and (B) the apical 3-chamber view.
Figure 9.
Control Cardiac Magnetic Resonance
The images show normalization of (A) T1 and (B) T2 values.
Conclusions
This is a rare case of myocarditis with acute LV thrombosis and dysfunction in a young patient with SARS-CoV-2 pneumonia. The patient had a favorable clinical evolution under anticoagulation and heart failure treatment. We highlight the importance of echocardiography in patients with SARS-CoV-2 infection and biomarker elevation suggestive of myocardial injury, to guide management and reduce complications and mortality.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this article.
Contributor Information
Ignacio Ferreira González, Email: nachoferreira@secardiologia.es, iferreir@vhebron.net.
José F. Rodríguez-Palomares, Email: jfrodriguezpalomares@gmail.com, jfrodrig@vhebron.net.
Appendix
Bedside 2-Dimensional Echocardiogram With a Huge Mass Attached to the Left Ventricle Apex in the Apical 4-Chamber View
Bedside 2-Dimensional Echocardiogram With a Huge Mass Attached to the Left Ventricle Apex in the Apical 3-Chamber View
CMR bSSFP Cine Images in the 2-Chamber View Showing Moderate Systolic Dysfunction Secondary to Global Hypokinesia and Increased Myocardial Signal Intensity in the Anterior Middle and Apical Wall
CMR bSSFP Cine Images in the Short-Axis View Showing Moderate Systolic Dysfunction
Control Echocardiogram, Apical 4-Chamber View, 2 months After Discharge With Improvements in Contractility and Ventricular Function and With No Evidence of Intracavitary Thrombus
Control Echocardiogram, Apical 2-Chamber View, 2 Months After Discharge With Improvements in Contractility and Ventricular Function and With No Evidence of Intracavitary Thrombus
Follow-Up CMR bSSFP Cine Images in the 2-Chamber View Showing Improvement in LVEF
Follow-Up CMR bSSFP Cine Images in the Short-Axis View Showing Improvement in LVEF
References
- 1.The European Society for Cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance Last updated June 2020. Available at:
- 2.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madjid M., Safavi-Naeini P., Solomon S. Potential effects of coronaviruses on the cardiovascular system. A review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 4.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abou-Ismail M., Diamond A., Kapoor S. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rey J., Jiménez Valero S., Poveda Pinedo D. COVID-19 and simultaneous thrombosis of two coronary arteries. Rev Esp Cardiol. 2020;73:665–687. doi: 10.1016/j.rec.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira V.M., Schulz-Menger J., Holmvang G. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 9.Zeng J.H., Liu Y.X., Yuan J. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capotosto L., Nguyen B., Ciardi M. Heart, COVID-19, and echocardiography. Echocardiography. 2020;37:1454–1464. doi: 10.1111/echo.14834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bedside 2-Dimensional Echocardiogram With a Huge Mass Attached to the Left Ventricle Apex in the Apical 4-Chamber View
Bedside 2-Dimensional Echocardiogram With a Huge Mass Attached to the Left Ventricle Apex in the Apical 3-Chamber View
CMR bSSFP Cine Images in the 2-Chamber View Showing Moderate Systolic Dysfunction Secondary to Global Hypokinesia and Increased Myocardial Signal Intensity in the Anterior Middle and Apical Wall
CMR bSSFP Cine Images in the Short-Axis View Showing Moderate Systolic Dysfunction
Control Echocardiogram, Apical 4-Chamber View, 2 months After Discharge With Improvements in Contractility and Ventricular Function and With No Evidence of Intracavitary Thrombus
Control Echocardiogram, Apical 2-Chamber View, 2 Months After Discharge With Improvements in Contractility and Ventricular Function and With No Evidence of Intracavitary Thrombus
Follow-Up CMR bSSFP Cine Images in the 2-Chamber View Showing Improvement in LVEF
Follow-Up CMR bSSFP Cine Images in the Short-Axis View Showing Improvement in LVEF