Abstract
Introduction:
Cardiovascular disease is a leading cause of mortality in the U.S. Although the risk of cardiovascular disease can be mitigated substantially by following a healthy lifestyle, adhering to a healthy diet and other healthy behaviors are limited by reduced food security. This study aims to determine the association between food security and cardiovascular disease risk.
Methods:
Three samples from the 2007–2014 National Health and Nutrition Examination Survey were examined: (1) 7,340 non-fasting adults (aged 40–79 years); (2) 13,518 non-fasting adults (aged 20–64 years); and (3) 6,494 fasting adults (aged 20–64 years). Food security was assessed using the U.S. Household Food Security Survey Module, with households categorized as having full, marginal, low, or very low food security. Regressions were conducted in 2018 to test the associations between food security status and odds of ≥20% 10-year cardiovascular disease risk among middle-aged to older adults (OR, 95% CI) and cardiovascular disease risk factors among all adults (β, 95% CI).
Results:
Compared with adults with full food security, those with very low food security had higher odds of ≥20% 10-year cardiovascular disease risk (OR=2.36, 95% CI=1.25, 4.46), whereas those with marginal food security had higher systolic blood pressure (β=0.94 mmHg, 95% CI=0.09, 1.80). Compared with adults with full food security, adults with different levels of food security had higher BMIs (marginal: 0.76, 95% CI=0.26, 1.26; low: 0.97, 95% CI=0.34, 1.60; and very low: 1.03, 95% CI=0.44, 1.63) and higher odds of current smoking (marginal: OR=1.43, 95% CI=1.17, 1.75; low: OR=1.47, 95% CI=1.22, 1.77; and very low: OR=1.95, 95% CI=1.60, 2.37).
Conclusions:
Adults with food insecurity have elevated cardiovascular disease risk factors and excess predicted 10-year cardiovascular disease risk. Substantially improving food security may be an important public health intervention to reduce future cardiovascular disease in the U.S. population.
INTRODUCTION
Cardiovascular disease (CVD) is a leading cause of mortality in the U.S., with heart disease and stroke responsible for nearly 30% of all deaths each year.1 CVD poses a substantial economic burden on the U.S. healthcare system, resulting in direct and indirect costs of greater than $400 billion annually.2 More than 90 million Americans have at least one type of CVD, and it is projected that by 2030, 44% of the U.S. adult population will have CVD.3 Although the risk of CVD can be mitigated substantially by following a healthy lifestyle,4 the ability to adhere to a healthy diet and other healthy behaviors is severely limited by low SES and reduced food security.5
Food security is defined by the Food and Agriculture Organization as “physical and economic access to sufficient safe and nutritious foods to meet [one’s] dietary needs and food preferences for an active and healthy life.”6 Particularly in high-income countries, households lacking sufficient financial resources to purchase food may compensate by increasing reliance on inexpensive, energy-dense, and nutrient-poor foods.5,7 This poor diet quality, combined with the cyclical nature of reduced food security, may lead to metabolic dysregulation, fat accumulation, insulin resistance, and heightened CVD risk.8 Additionally, reduced food security may affect cardiovascular health through non-dietary pathways by activating a physiological stress response (e.g., elevated cortisol levels); triggering unhealthful coping behaviors (e.g., excessive drinking); or limiting the ability to properly manage chronic conditions (e.g., diabetes).8–11
Although studies have examined the association between food security status and individual CVD risk factors,11–16 few have studied combining these factors to estimate long-term CVD risk. Examining summary CVD risk may be more meaningful than individual CVD risk factors because it more broadly captures the multiple pathways through which food security is hypothesized to affect cardiovascular health.
The present study aims to examine the association between food security status and 10-year predicted CVD risk among a nationally representative sample of middle-aged and older adults. To inform these findings, the relationships between food security status, individual CVD risk factors, and anthropometric measures of adiposity are also examined.
METHODS
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional study released every 2 years and designed to be representative of the U.S. non-institutionalized, civilian population.17,18 During a home interview, data are collected on demographic; socioeconomic (including food security); and health-related topics. A separate examination collects standardized physical assessments (including weight, height, and blood pressure) and laboratory measurements. Additionally, a subsample of participants is randomly selected to undergo a fasting metabolic panel. A complete description of data collection procedures and analytic guidelines is available through NHANES.18
Study Population
Data from four survey cycles (2007–2008, 2009–2010, 2011–2012, and 2013–2014) were combined to ensure a sufficient analytic sample. The 2007–2008 cycle was chosen as the earliest cycle because at this point, physical activity (an important covariate controlled for in other analyses on food security and health outcomes11,19) was measured in a consistent and objective manner.
To predict 10-year CVD risk, the analytic sample was limited to 7,340 males and non-pregnant females (aged 40–79 years) with non-missing data on all covariates, who were free of self-reported CVD, and who did not report using cholesterol-lowering medication. Fasting biological samples were not required. The older age group of this analytic sample was selected to be consistent with the age range for which use of the Pooled Cohort Equations (PCEs) is recommended.20 To analyze individual CVD risk factors among adults, the analytic sample was limited to 13,518 males and non-pregnant females (aged 20–64 years) with non-missing data on all covariates. This age range was chosen to maximize comparisons with other studies.19 To analyze CVD risk factors requiring fasting blood samples (e.g., glucose), a subsample was created consisting of 6,494 adults who were randomly selected to undergo the fasting laboratory examination and reported fasting between 9 and 24 hours before measurement. Appendix Figure 1 (available online) shows the flow of participant inclusion into the analytic sample.
Measures
Household food security status over the past 12 months was measured using the U.S. Department of Agriculture’s 18-item Household Food Security Survey Module.21 Scores ranged from 0 to 10, with higher scores signifying reduced food security. Households were classified into categories based on validated cutpoints: full food security (score=0); marginal food security (score=1–2); low food security (score=3–5); and very low food security (score=6–10).
Blood pressure; total cholesterol; high-density lipoprotein cholesterol (HDL); and current smoking status were obtained from the full sample, whereas triglycerides, fasting glucose, and calculated low-density lipoprotein cholesterol were obtained from the fasting subsample only. Systolic and diastolic blood pressure were each calculated as the average of three readings. Participants who reported currently smoking were classified as current smokers (yes, no). BMI and waist circumference (centimeters) were also measured.
In 2013, the American College of Cardiology/American Heart Association introduced the PCE for estimation of 10-year risk for atherosclerotic CVD events using prediction data from several large cohorts with >12 years of follow-up each.20 Race- and sex-specific PCE were used to estimate 10-year risk for CVD events for each individual in the study population.20 The PCE includes the following variables as part of its estimation: age, total cholesterol, HDL, blood pressure, type 1 or 2 diabetes status, and smoking status. Participants self-reported if they were current smokers (yes, no), had ever been told they had diabetes by a doctor (yes, no), and were currently taking any blood pressure medications (yes, no). In line with previous studies and the cut off for immediate lipid-lowering drug therapy,22 participants with predicted CVD risk ≥20% were considered to be high risk.
In 2018, a revised version of the PCE was created to improve prediction accuracy and ameliorate criticisms that the original PCE overestimates CVD risk, particularly among non-Hispanic blacks.23 The results of both the original PCE (main paper) and the revised PCE (Appendix) are reported.
The following covariates were included: sex (male, female); age (continuous); race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other Hispanic, other race/ethnicity); marital status (single/no partner, married/partnered); educational attainment (less than high school, high school graduate or more); smoking status (never, former, current); physical activity (five questions; each yes, no); household Supplemental Nutrition Assistance Program (SNAP) participation in the past 12 months (participant, nonparticipant); and household income-to-poverty ratio (continuous).
Physical activity was assessed by asking participants if they typically (yes, no) engaged in physical activity in each of the following domains: vigorous work, moderate work, traveling to and from places by walking or bicycling, vigorous recreational activities, and moderate recreational activites.24 Participants who reported currently smoking were classified as current smokers, whereas those who had smoked <100 cigarettes during their life were classified as never smokers. Participants who reported smoking >100 cigarettes during their life but did not report currently smoking were classified as former smokers. Household income-to-poverty ratio was calculated by dividing household income by the poverty guidelines (specific to family size, year, and state).
Statistical Analysis
All analyses were weighted to account for the multistage, clustered probability sampling of NHANES. Chi-square and ANOVA were used to test for differences in characteristics across food security status categories. This comparison is intended to be descriptive in nature and does not imply causal relationships (e.g., SNAP participants appear to experience greater food insecurity, but this association has been shown to be diminished when one controls for nonrandom selection into the SNAP program25,26).
Logistic regression was used to assess the association between household food security status and the odds of having a 10-year CVD risk ≥20%. Three models were specified: Model 1 adjusted only for age; Model 2 adjusted for age, sex, and race/ethnicity; and Model 3 adjusted for all individual- and household-level covariates except smoking status. Sex-specific models were also fitted for each outcome.
Separate multiple linear regressions were conducted to analyze the association between food security status and individual CVD risk factors (systolic blood pressure, diastolic blood pressure, total cholesterol, HDL, low-density lipoprotein cholesterol, triglycerides, and fasting plasma glucose) and adiposity (BMI and waist circumference). Logistic regression was used to assess the association between food security status and the odds of being a current smoker. As previous studies have shown differing effects of food security on adiposity and CVD risk among males versus females,8,27 sex-specific models were also fit for each of the outcomes. Analyses were conducted in 2018 using SAS, version 9.4.
RESULTS
A total of 13,518 adults aged 20–64 years (weighted proportions=75.4% food security, 9.9% marginal food security, 8.2% low food security, and 6.5% very low food security) were included in the full analytic sample for adults (Table 1). Overall, adults in households with reduced food security were younger, less likely to be married, and more likely to be a racial/ethnic minority, smokers, and SNAP participants. On average, adults in households with reduced food security also had lower income-to-poverty ratios, educational attainment, and physical activity.
Table 1.
Characteristics of Sample Consisting of Adults (Aged 20–64 Years) in NHANES, 2007–2014
| Characteristic | Adults (aged 20–64 years, n=13,518) |
||||
|---|---|---|---|---|---|
| Food security (n=9,029) | Marginal food security (n=1,726) | Low food security (n=1,544) | Very low food security (n=1,219) | p-value | |
| Female | 4,428 (49.0) | 904 (52.0) | 814 (51.2) | 642 (52.7) | 0.06 |
| Race/ethnicity | <0.0001 | ||||
| Non-Hispanic white | 4,223 (73.1) | 528 (48.5) | 500 (47.3) | 481 (51.8) | |
| Non-Hispanic black | 1,661 (8.5) | 450 (18.5) | 377 (17.3) | 302 (18.6) | |
| Mexican American | 1,188 (6.6) | 410 (17.4) | 358 (18.7) | 222 (14.2) | |
| Other Hispanic | 816 (4.4) | 217 (9.5) | 207 (10.3) | 138 (9.2) | |
| Other | 1,141 (7.5) | 121 (6.1) | 102 (6.3) | 76 (6.3) | |
| High school graduate or more | 7,541 (89.1) | 1,209 (75.7) | 932 (64.9) | 788 (70.0) | <0.0001 |
| Married/partnered | 5,806 (67.6) | 979 (57.0) | 799 (52.5) | 577 (47.3) | <0.0001 |
| HH SNAP participation | 1,119 (8.1) | 584 (30.8) | 701 (41.7) | 621 (51.5) | <0.0001 |
| Smoking status | <0.0001 | ||||
| Never smoker | 5,408 (59.0) | 913 (50.9) | 762 (47.9) | 515 (38.7) | |
| Current smoker | 1,757 (18.5) | 513 (31.5) | 540 (36.5) | 524 (46.3) | |
| Former smoker | 1,864 (22.5) | 300 (17.6) | 242 (15.5) | 180 (15.0) | |
| Physical activity | |||||
| Vigorous work | 1,767 (20.8) | 423 (25.1) | 359 (26.3) | 358 (32.6) | <0.0001 |
| Moderate work | 3,395 (40.2) | 716 (43.5) | 602 (41.1) | 530 (48.0) | 0.0010 |
| Walk or bicycle | 2,366 (24.7) | 585 (33.3) | 488 (29.5) | 421 (33.6) | <0.0001 |
| Vigorous recreational | 2,575 (31.2) | 394 (25.3) | 283 (20.3) | 244 (20.1) | <0.0001 |
| Moderate recreational | 4,175 (51.2) | 637 (40.3) | 527 (35.0) | 400 (33.4) | <0.0001 |
| Age, M (SE) | 42.7 (0.26) | 38.8 (0.41) | 38.8 (0.50) | 39.3 (0.56) | <0.0001 |
| HH income to poverty ratio, M (SE) | 3.48 (0.05) | 1.88 (0.07) | 1.55 (0.06) | 1.30 (0.04) | <0.0001 |
Note: Categorical variables are reported as n (%), where n is number of NHANES sample and % is weighted percentage. Continuous variables are reported as M (SE). Physical activity refers to the proportion of individuals who self-reported engaging in specific type of physical activity in a given week (yes/no). Boldface indicates statistical significance (p<0.05).
FPL, federal poverty line; HH, household; NHANES, National Health and Nutrition Examination Survey; SNAP, Supplemental Nutrition Assistance Program.
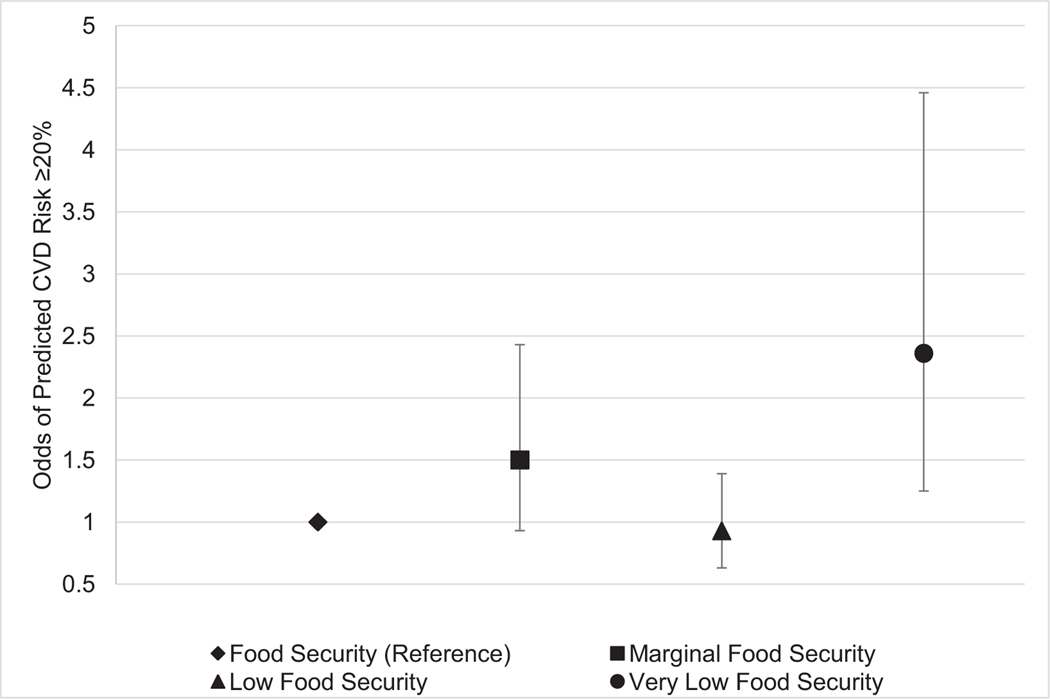
Table 2 reports ORs for the association between food security status and ≥20% predicted 10-year CVD risk for older adults. In the model adjusted only for age (Model 1) and the model adjusted for age, sex, and race/ethnicity (Model 2), adults in households with marginal and very low food security had significantly elevated odds of CVD risk ≥20% compared with those in households with full food security. After maximal adjustment (Model 3), only adults in households with very low food security had significantly greater odds of CVD risk ≥20% compared with adults in households with food security (OR=2.36, 95% CI=1.25, 4.46; Figure 1). In analyses of the recently revised PCE, similar, albeit even stronger, associations were found between severity of food insecurity and predicted 10-year risk of CVD (Appendix Table 1, available online).
Table 2.
ORs (95% CIs) for Association Between Food Security and 10-Year CVD Risk ≥20%
| Variable | Food security | Marginal food security | Low food security | Very low food security |
|---|---|---|---|---|
| Total, n | ||||
| High risk (≥20%) | 1,002 | 141 | 103 | 86 |
| Low risk (<20%) | 4,354 | 658 | 560 | 436 |
| Model 1 | ref | 1.54 (1.04, 2.27) | 1.34 (0.97, 1.87) | 2.55 (1.30, 4.98) |
| Model 2 | ref | 2.07 (1.36, 3.17) | 1.42 (0.95, 2.13) | 3.81 (1.94, 7.45) |
| Model 3 | ref | 1.50 (0.93, 2.43) | 0.93 (0.63, 1.39) | 2.36 (1.25, 4.46) |
| Males | ||||
| Model 1 | ref | 2.00 (1.23, 3.25) | 1.35 (0.87, 2.11) | 3.34 (1.39, 8.02) |
| Model 2 | ref | 2.08 (1.25, 3.45) | 1.42 (0.87, 2.32) | 3.74 (1.57, 8.90) |
| Model 3 | ref | 1.53 (0.86, 2.74) | 1.02 (0.63, 1.65) | 2.60 (1.14, 5.91) |
| Females | ||||
| Model 1 | ref | 2.25 (1.15, 4.39) | 1.70 (0.74, 3.91) | 5.30 (2.46, 11.41) |
| Model 2 | ref | 1.83 (0.89, 3.75) | 1.38 (0.61, 3.12) | 5.18 (2.28, 11.77) |
| Model 3 | ref | 1.39 (0.65, 2.95) | 0.83 (0.33, 2.07) | 2.67 (1.23, 5.80) |
Note: Model 1 is adjusted for age. Model 2 is adjusted for age; sex (except for sex-specific models); and race/ethnicity. Model 3 is adjusted for age; sex (except for sex-specific models); race/ethnicity; education; marital status; household income; household SNAP participation; and physical activity. Boldface indicates statistical significance (p<0.05).
CVD, cardiovascular disease.
Figure 1.
ORs (95% CIs) for association between food security and 10-year CVD risk ≥20%.
Notes: Error bars indicate the 95% CI. Figure represents Model 3 (fully adjusted).
CVD, cardiovascular disease.
Compared with adults in households with full food security, those in households with marginal food security had higher systolic blood pressure (β=0.94 mmHg, 95% CI=0.09, 1.80), whereas those in households with low and very low food security were not significantly different in systolic blood pressure than food secure adults. Adults in households with marginal, low, and very low food security had higher odds of being a current smoker than adults in households with full food security (marginal: OR=1.43, 95% CI=1.17, 1.75; low: OR=1.47, 95% CI=1.22, 1.77; and very low: OR=1.95, 95% CI=1.60, 2.37; Table 3). There were no significant differences for other CVD risk factors across food security status.
Table 3.
Beta Coefficients (95% CIs) for the Associations Between Food Security, CVD Risk Factors, and Adiposity
| Variable | Full food security | Marginal food security | Low food security | Very low food security |
|---|---|---|---|---|
| Total | ||||
| Full adult sample, n | 9,029 | 1,726 | 1,544 | 1,219 |
| Systolic blood pressure, mmHg | ref | 0.94 (0.09, 1.80) | 0.25 (−0.99, 1.48) | 0.89 (−0.67, 2.45) |
| Diastolic blood pressure, mmHg | ref | 0.21 (−0.48, 0.89) | 0.25 (−0.70, 1.20) | 0.88 (−0.26, 2.03) |
| Total cholesterol, mg/dL | ref | −0.59 (−2.73, 1.54) | 3.23 (−0.28, 6.74) | 4.04 (−0.51, 8.59) |
| HDL cholesterol, mg/dL | ref | −0.93 (−1.93, 0.08) | −0.08 (−1.20, 1.05) | −0.82 (−2.02, 0.39) |
| BMI | ref | 0.76 (0.26, 1.26) | 0.97 (0.34, 1.60) | 1.03 (0.44, 1.63) |
| Waist circumference, cm | ref | 1.74 (0.55, 2.93) | 2.20 (0.74, 3.66) | 2.22 (0.80, 3.66) |
| Smoking status (current smoker) | ref | 1.43 (1.17, 1.75) | 1.47 (1.22, 1.77) | 1.95 (1.60, 2.37) |
| Fasting adult subsample, n | 4,396 | 817 | 753 | 528 |
| Triglycerides, mg/dL | ref | 4.93 (−3.59, 13.46) | 6.06 (−2.36, 14.48) | 8.00 (−0.17, 16.16) |
| LDL cholesterol, mg/dL | ref | −1.06 (−4.32, 2.20) | 1.81 (−1.50, 5.13) | 5.14 (−0.55, 10.83) |
| Fasting plasma glucose, mg/dL | ref | 0.21 (−2.92, 3.33) | 2.44 (−0.51, 5.39) | 2.39 (−1.72, 6.50) |
| Males | ||||
| Full adult sample, n | 4,601 | 822 | 730 | 577 |
| Systolic blood pressure, mmHg | ref | 1.06 (−0.45, 2.57) | −0.61 (−2.28, 1.06) | 1.79 (−0.58, 4.17) |
| Diastolic blood pressure, mmHg | ref | −0.19 (−1.16, 0.77) | −0.42 (−1.71, 0.87) | 1.18 (−0.50, 2.86) |
| Total cholesterol, mg/dL | ref | 2.66 (−3.13, 8.44) | 6.93 (1.16, 12.70) | −0.03 (−8.16, 8.09) |
| HDL cholesterol, mg/dL | ref | −0.06 (−1.37, 1.25) | 0.41 (−1.05, 1.86) | −0.24 (−1.76, 1.29) |
| BMI | ref | 0.45 (−0.27, 1.17) | 0.25 (−0.47, 1.96) | 0.76 (−0.05, 1.56) |
| Waist circumference, cm | ref | 1.10 (−0.55, 2.75) | 0.71 (−1.14, 2.56) | 2.11 (0.07, 4.15) |
| Smoking status (current smoker) | ref | 1.38 (1.07, 1.78) | 1.41 (1.11, 1.78) | 1.88 (1.47, 2.39) |
| Fasting adult subsample, n | 2,152 | 369 | 358 | 235 |
| Triglycerides, mg/dL | ref | 5.77 (−9.53, 21.08) | 7.21 (−5.02, 19.44) | 6.48 (−7.02, 19.98) |
| LDL cholesterol, mg/dL | ref | −1.67 (−6.63, 3.29) | 3.01 (−2.48, 8.51) | 5.48 (−3.28, 14.25) |
| Fasting plasma glucose, mg/dL | ref | 2.13 (−2.87, 7.14) | 0.52 (−3.42, 4.46) | 2.43 (−1.75, 6.62) |
| Females | ||||
| Full adult sample, n | 4,428 | 904 | 814 | 642 |
| Systolic blood pressure, mmHg | ref | 0.86 (−0.21, 1.93) | 1.05 (−0.41, 2.51) | 0.00 (−1.62, 1.62) |
| Diastolic blood pressure, mmHg | ref | 0.60 (−0.33, 1.52) | 0.93 (−0.18, 2.03) | 0.68 (−0.74, 2.10) |
| Total cholesterol, mg/dL | ref | −0.59 (−2.73, 1.54) | 3.23 (−0.28, 6.74) | 4.04 (−0.51, 8.59) |
| HDL cholesterol, mg/dL | ref | −1.56 (−3.02, −0.09) | −0.48 (−1.84, 0.88) | −1.22 (−3.17, 0.72) |
| BMI | ref | 1.03 (0.41, 1.65) | 1.65 (0.81, 1.96) | 1.30 (0.45, 2.15) |
| Waist circumference, cm | ref | 2.18 (0.73, 3.63) | 3.61 (1.77, 5.46) | 2.33 (0.34, 4.33) |
| Smoking status (current smoker) | ref | 1.52 (1.18, 1.97) | 1.56 (1.22, 1.99) | 2.02 (1.49, 2.74) |
| Fasting adult subsample, n | 2,244 | 448 | 395 | 293 |
| Triglycerides, mg/dL | ref | 3.76 (−4.42, 11.95) | 5.89 (−3.07, 14.85) | 9.36 (0.97, 17.76) |
| LDL cholesterol, mg/dL | ref | −0.50 (−4.85, 3.85) | 0.74 (−3.69, 5.18) | 4.75 (−0.60, 10.11) |
| Fasting plasma glucose, mg/dL | ref | −1.47 (−4.23, 1.28) | 4.53 (0.37, 8.68) | 2.16 (−4.36, 8.69) |
Note: Reported as point estimate (CI). All models were adjusted for sex (except sex-specific models); race; age; education; marital status; household income; household SNAP participation; smoking status (except smoking model); and physical activity. Smoking status was estimated as the odds of being a current smoker. Boldface indicates statistical significance (p<0.05).
CVD, cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
In subgroup analyses, males in households with low food security had higher total cholesterol (β=6.93 mg/dL, 95% CI=1.16, 12.70) than males in households with full food security. Females in households with marginal food security had lower HDL (β= −1.56 mg/dL, 95% CI= −3.02, −0.09), females in households with very low food security had higher triglycerides (β=9.36 mg/dL, 95% CI=0.97, 17.76), and females in households with low food security had higher fasting plasma glucose (β = 4.53, 95% CI = 0.37, 8.68) compared with their fully food-secure counterparts.
Compared with adults in households with food security, adults in households with marginal, low, or very low food security had a higher BMI (marginal: β=0.76, 95% CI=0.26, 1.26; low: β=0.97, 95% CI=0.34, 1.60; very low: β=1.03, 95% CI=0.44, 1.63). A similar pattern was observed for waist circumference (marginal: β=1.74 cm, 95% CI=0.55, 2.93; low: β=2.20 cm, 95% CI=0.74, 3.66; and very low: β=2.22 cm, 95% CI=0.80, 3.66).
When stratified by sex, most of the associations between food security, BMI, and waist circumference among males were not significant. Findings for BMI and waist circumference among females remained strong.
DISCUSSION
This study provides evidence that adults in households with less than full food security have increased CVD risk factors and excess predicted 10-year risk of CVD, suggesting that improving food security may be an important public health intervention to reduce future CVD in the U.S. population. The results of this study demonstrate that adults in the least food secure households had the highest odds of excess predicted 10-year CVD risk, and adults with reduced food security had higher individual CVD risk factors, including systolic blood pressure; adiposity (females); triglycerides (females); and total cholesterol (males). Although differences in individual CVD risk factors between groups were small, these differences have a large impact at the population level. Furthermore, their combined effect on 10-year CVD risk was substantial. These findings are consistent with prior studies, which have found little or mixed associations between food security and individual risk factors, but stronger links with CVD outcomes.11–15
Researchers have posited several possible mechanisms by which reduced food security could contribute to increased CVD risk and adiposity. First, individuals in households with reduced food security may be constrained to purchasing inexpensive food, leading to diets high in refined grains, added sugars, and saturated fats.7 On a calorie for calorie basis, these diets cost less but are nutritionally poor and strongly associated with weight gain and CVD risk.28 Second, households with reduced food security often cycle in and out of food scarcity within a given month or year. This type of cyclic food restriction may be associated with a heightened desire for energy-dense, nutrient-poor foods, as well as metabolic dysregulation that heighten the risk of CVD.7,8 Furthermore, individuals who anticipate low food security may overconsume calories when available.29 Third, the presence of reduced food security may affect self-management of chronic diseases that, when poorly controlled, increase the risk of developing CVD. Studies have shown that among diabetic patients, reduced food security can affect self-management through cost-related medication underuse, impaired glucose self-monitoring, poor mental health, and gaps in food intake that cause poor glycemic control.30,31 Fourth, reduced food security may activate the stress response (e.g., increasing blood pressure) and trigger unhealthful coping behaviors (e.g., smoking). Given the substantial gradient in smoking status across food security status,32,33 smoking is likely a large driver of differences in CVD risk found in this study. Finally, a bidirectional relationship between food security and CVD risk is likely. Although reduced food security has the potential to reduce cardiovascular health through the above explanations, it is also plausible that worsening cardiovascular health may increase medical expenses and limit one’s ability to work, thereby reducing food security.
Consistent with prior studies,27,34,35 this study identified important sex differences in the associations between food security, individual CVD risk factors, and adiposity, with strong and consistent findings observed primarily among females. From a behavioral perspective, gender norms may place women at higher risk, as traditional discourse has emphasized the role of women (particularly in households with children) in feeding the family.36 Previous studies have demonstrated that the psychological burden of reduced food security may cause women to compromise their own nutritional intake to preserve the adequacy of their family’s diets (e.g., skipping meals).37,38 From a physiologic perspective, adiposity plays an important role in reproduction for women but is less important for men.39 If the relationship between reduced food security and obesity occurs through a metabolic response that preserves fat stores in times of food scarcity to ensure reproduction and survival, it is plausible that this response is stronger in women than in men, but more research in this area is needed.40
Lack of food security is increasingly being recognized as a key public health concern in the U.S.,41 with the most recent national estimates suggesting that 12% of American households experience reduced food security for at least some time during each year.42 This is particularly important given evidence demonstrating the high economic burden that reduced food security places on healthcare systems.43,44 The findings of this study support the need for the implementation of new strategies to improve food security, as well as the strengthening of existing strategies.45 For example, because research suggests that SNAP is effective in improving household food security,25,26 expansion of the program may result in further reductions in food insecurity, which could in turn lead to decreases in CVD burden.
This study helps to advance understanding of the role of food security as a modifiable determinant of health. Longitudinal cohort studies that track at-risk individuals over time will help to determine if there are critical developmental periods during which individuals are most susceptible to the CVD effects of reduced food security. Additionally, more research is needed examining the impact of interventions or policies aimed at reducing food insecurity on cardiovascular health.
This study has a number of strengths. First, these findings provide some of the first evidence for the relationship between food security and CVD risk by incorporating the PCE as a summary CVD risk measure. Furthermore, the utilization of both the original and revised versions of the PCE maximizes the validity of predictions. Second, a standardized measure of food security was used, promoting cross-study comparisons and pooling of results. Third, biological data were used to examine CVD risk, avoiding the potential biases present in self-reported measures.
Limitations
This study has several limitations. First, NHANES is a cross-sectional study, limiting the ability to make strong causal inferences, introducing the potential for reverse causation, and precluding the ability to examine how different factors (e.g., diet quality) mediate observed relationships between reduced food insecurity and CVD risk over time. In the analysis predicting 10-year CVD risk, this was mitigated by excluding participants with previous CVD history. Second, food security was measured at the household level, meaning there may be some risk of misclassification for the experience of individuals. Third, because NHANES assesses food insecurity status over the past 12 months, the measure does not shed light on the duration of food insecurity within that time period or on experiences of food insecurity prior to that point. Fourth, total and HDL cholesterol were obtained from a non-fasting sample. Although the vast majority of empirical evidence suggests that use of non-fasting lipids is similar or better in predicting CVD risk,46,47 there are no data in food insecure populations that non-fasting samples are a perfect proxy for usual fasting levels. Finally, NHANES may not represent people in the U.S. at the highest risk for reduced food security, such as undocumented immigrants, migrant workers, and the homeless.48
CONCLUSIONS
The results of this study demonstrate that adults in the least food secure households have heightened 10-year CVD risk and adults with reduced food security have higher individual CVD risk factors and measures of adiposity. Given the high prevalence of reduced food security and CVD in the U.S., this is a key public health concern. Substantially improving food security may be an important public health intervention to reduce future CVD.
Supplementary Material
ACKNOWLEDGMENTS
This content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. Dr. Moran is supported by a T32 training grant (DK 007703) and Dr. McClain is supported by a NIH Ruth L. Kirschstein Institutional Training Grant Postdoctoral Fellowship (T32 DK 7703-23) from NIH under the PI, Dr. Frank Hu.
KAV developed the research question, conducted the statistical analysis, interpreted the data, and drafted the manuscript. EBR developed the research question, interpreted the data, provided manuscript revisions, and approved the final version of the manuscript. AJM, APF, ACM, and ANT provided manuscript revisions and approved the final version of the manuscript.
No financial disclosures were reported by the authors of this paper.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2018.11.016.
REFERENCES
- 1.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1–119. [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HHS. 2015–2020 Dietary Guidelines for Americans. Washington, DC: U.S. Department of Agriculture, 2015. [Google Scholar]
- 5.Hanson KL, Connor LM. Food insecurity and dietary quality in U.S. adults and children: a systematic review. Am J Clin Nutr. 2014;100 (2):684–692. 10.3945/ajcn.114.084525. [DOI] [PubMed] [Google Scholar]
- 6.FAO. Introduction to the Basic Concepts of Food Security. Rome, Italy: FAO, 2008. [Google Scholar]
- 7.Seligman HK, Schillinger D. Hunger and socioeconomic disparities in chronic disease. N Engl J Med. 2010;363(1):6–9. 10.1056/NEJMp1000072. [DOI] [PubMed] [Google Scholar]
- 8.Laraia BA. Food insecurity and chronic disease. Adv Nutr. 2013;4 (2):203–212. 10.3945/an.112.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClain AC, Xiao RS, Gao X, Tucker KL, Falcon LM, Mattei J. Food insecurity and odds of high allostatic load in Puerto Rican adults: the role of participation in the Supplemental Nutrition Assistance Program during 5 years of follow-up. Psychosom Med. 2018;80(8):733–741. 10.1097/PSY.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golin CE, Haley DF, Wang J, et al. Post-traumatic stress disorder symptoms and mental health over time among low-income women at increased risk of HIV in the U.S. J Health Care Poor Underserved. 2016;27(2):891–910. 10.1353/hpu.2016.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seligman HK, Bindman AB, Vittinghoff E, Kanaya AM, Kushel MB. Food insecurity is associated with diabetes mellitus: results from the National Health Examination and Nutrition Examination Survey (NHANES) 1999–2002. J Gen Intern Med. 2007;22(7):1018–1023. 10.1007/s11606-007-0192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz SA, Berkowitz TS, Meigs JB, Wexler DJ. Trends in food insecurity for adults with cardiometabolic disease in the United States: 2005–2012. PLoS One. 2017;12(6):e0179172. 10.1371/journal.pone.0179172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ford ES. Food security and cardiovascular disease risk among adults in the United States: findings from the National Health and Nutrition Examination Survey, 2003–2008. Prev Chronic Dis. 2013;10:E202. 10.5888/pcd10.130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holben DH, Taylor CA. Food insecurity and its association with central obesity and other markers of metabolic syndrome among persons aged 12 to 18 years in the United States. J Am Osteopath Assoc. 2015;115(9):536–543. 10.7556/jaoa.2015.111. [DOI] [PubMed] [Google Scholar]
- 15.Parker ED, Widome R, Nettleton JA, Pereira MA. Food security and metabolic syndrome in U.S. adults and adolescents: findings from the National Health and Nutrition Examination Survey, 1999–2006. Ann Epidemiol. 2010;20(5):364–370. 10.1016/j.annepidem.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory CA, Coleman-Jensen A. Food insecurity, chronic disease, and health among working-age adults. U.S. Department of Agriculture. Economic Research Service. 2017. [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). About the National Health and Nutrition Examination Survey. www.cdc.gov/nchs/nhanes/about_nhanes.htm. Published 2017. Accessed November 30, 2018.
- 18.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics. National Health and Nutrition Examination Survey. www.cdc.gov/nchs/nhanes/index.htm. Published 2017. Accessed September 30, 2017.
- 19.Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income NHANES participants. J Nutr. 2010;140(2):304–310. 10.3945/jn.109.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(25) (suppl 2):S49–S73. 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 21.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to Measuring Household Food Security, Revised 2000. Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service, Office of Analysis, Nutrition, and Evaluation, 2000. [Google Scholar]
- 22.Lloyd-Jones DM. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121(15):1768–1777. 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 23.Yadlowsky S, Hayward RA, Sussman JB, McClelland RL, Min Y-I, Basu S. Clinical implications of revised pooled cohort equations for estimating atherosclerotic cardiovascular disease risk. Ann Intern Med. 2018;169(1):20–29. 10.7326/M17-3011. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Global physical activity questionnaire (GPAQ) analysis guide. Geneva: WHO, 2012. [Google Scholar]
- 25.Gundersen C, Kreider B, Pepper JV. Partial identification methods for evaluating food assistance programs: a case study of the causal impact of SNAP on food insecurity. Am J Agric Econ. 2017;99(4):875–893. 10.1093/ajae/aax026. [DOI] [Google Scholar]
- 26.Swann CA. Household history, SNAP participation, and food insecurity. Food Policy. 2017;73:1–9. 10.1016/j.foodpol.2017.08.006. [DOI] [Google Scholar]
- 27.Larson NI, Story MT. Food insecurity and weight status among U.S. children and families: a review of the literature. Am J Prev Med. 2011;40(2):166–173. 10.1016/j.amepre.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 28.Drewnowski A, Specter S. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79(1):6–16. 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- 29.Dinour LM, Bergen D, Yeh M-C. The food insecurity–obesity paradox: a review of the literature and the role food stamps may play. J Acad Nutr Diet. 2007;107(11):1952–1961. 10.1016/j.jada.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Gundersen C, Ziliak JP. Food insecurity and health outcomes. Health Aff (Millwood). 2015;34(11):1830–1839. 10.1377/hlthaff.2015.0645. [DOI] [PubMed] [Google Scholar]
- 31.Essien UR, Shahid NN, Berkowitz SA. Food insecurity and diabetes in developed societies. Curr Diab Rep. 2016;16(9):79. 10.1007/s11892-016-0774-y. [DOI] [PubMed] [Google Scholar]
- 32.Cutler-Triggs C, Fryer GE, Miyoshi TJ, Weitzman M. Increased rates and severity of child and adult food insecurity in households with adult smokers. Arch Pediatr Adolesc Med. 2008;162(11):1056–1062. 10.1001/archpediatrics.2008.2. [DOI] [PubMed] [Google Scholar]
- 33.Armour BS, Pitts MM, Lee C-w. Cigarette smoking and food insecurity among low-income families in the United States, 2001. Am J Health Promot. 2008;22(6):386–390. 10.4278/ajhp.22.6.386. [DOI] [PubMed] [Google Scholar]
- 34.Franklin B, Jones A, Love D, Puckett S, Macklin J, White-Means S. Exploring mediators of food insecurity and obesity: a review of recent literature. J Community Health. 2012;37(1):253–264. 10.1007/s10900-011-9420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung NM, de Bairros FS, Pattussi MP, Pauli S, Neutzling MB. Gender differences in the prevalence of household food insecurity: a systematic review and meta-analysis. Public Health Nutr. 2017;20(5):902–916. 10.1017/S1368980016002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin MA, Lippert AM. Feeding her children, but risking her health: the intersection of gender, household food insecurity and obesity. Soc Sci Med. 2012;74(11):1754–1764. 10.1016/j.socscimed.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntyre L, Glanville NT, Raine KD, Dayle JB, Anderson B, Battaglia N. Do low-income lone mothers compromise their nutrition to feed their children? CMAJ. 2003;168(6):686–691. [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens CA. Exploring food insecurity among young mothers (15–24 years). J Spec Pediatr Nurs. 2010;15(2):163–171. 10.1111/j.1744-6155.2010.00235.x. [DOI] [PubMed] [Google Scholar]
- 39.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, andthe health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99(5):931–940. 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 40.Dhurandhar EJ. The food-insecurity obesity paradox: a resource scarcity hypothesis. Physiol Behav. 2016;162:88–92. 10.1016/j.physbeh.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holben DH, Marshall MB. Position of the Academy of Nutrition and Dietetics: food insecurity in the United States. J Acad Nutr Diet. 2017;117(12):1991–2002. 10.1016/j.jand.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Coleman-Jensen A, Rabbitt MP, Gregory CA, Singh A. Household food security in the United States in 2017. U.S. Department of Agriculture, Economic Research Service. www.ers.usda.gov/webdocs/publications/90023/err-256.pdf?v=0. Published 2018. Accessed November 30, 2018. [Google Scholar]
- 43.Berkowitz SA, Basu S, Meigs JB, Seligman HK. Food insecurity and health care expenditures in the United States, 2011–2013. Health Serv Res. 2018;53(3):1600–1620. 10.1111/1475-6773.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarasuk V, Cheng J, de Oliveira C, Dachner N, Gundersen C, Kurdyak P. Association between household food insecurity and annual health care costs. CMAJ. 2015;187(14):E429–E436. 10.1503/cmaj.150234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Department of Agriculture. Supplemental Nutrition Assistance Program (SNAP). www.fns.usda.gov/snap/supplemental-nutritionassistance-program-snap. Published 2018. Accessed November 30, 2018.
- 46.Mora S.Nonfasting for routine lipid testing: from evidence to action. JAMA Intern Med. 2016;176(7):1005–1006. 10.1001/jamainternmed.2016.1979. [DOI] [PubMed] [Google Scholar]
- 47.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with non-fasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118(10):993–1001. 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention (CDC). About the National Health and Nutrition Examination Survey. www.cdc.gov/nchs/nhanes/about_nhanes.htm. Published 2017. Accessed November 30, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.