Abstract
OBJECTIVE:
The Veterans Health Administration (VHA) provides a continuum of care over the life course. Among US adults, bipolar disorder and schizophrenia are associated with increased risk for dementia. To inform service planning, this study assessed the incidence of dementia among Veteran VHA patients with bipolar disorder or schizophrenia, adjusting for comorbid medical conditions.
METHODS:
Using data from VHA Corporate Data Warehouse, we identified all Veterans who received VHA care in 2004 and 2005 without a dementia diagnosis and who were alive and 18–100 years old as of January 1, 2006. Individuals were categorized as having bipolar disorder, schizophrenia, or neither condition based on VHA diagnoses in 2004–2005. Among ongoing VHA users, incidence of dementia was assessed for up to 10 years, 2006–2015.
RESULTS:
The study cohort included 3,648,852 individuals. After controlling for baseline medical comorbidity and substance use, the incidence rate ratio for dementia for Veteran VHA patients with schizophrenia was 2.92 and it was 2.26 for those with bipolar disorder, as compared to Veteran VHA patients with neither condition.
CONCLUSIONS:
Among Veterans receiving ongoing VHA care, diagnoses of bipolar disorder and schizophrenia were each associated with increased risk for receiving a new diagnosis of dementia, controlling for medical comorbidity at baseline. Both conditions had elevated incidence ratios compared to patients with neither condition, and those with schizophrenia were at highest risk. VHA clinicians should evaluate for possible dementia when there are signs or symptoms of cognitive impairment. Findings may also be relevant to dementia risk in the general population.
Keywords: Dementia, bipolar disorder, schizophrenia, risk
In 2017, there were 19.8 million United States (US) Veterans, of whom approximately 9 million were enrolled in the Veterans Affairs health system, the Veterans Health Administration (VHA). Among VHA patients in 2010, approximately 1.8 percent had received diagnoses in the prior 24 months of schizophrenia and 2.5 percent of bipolar disorder (1). Veterans with bipolar disorder and schizophrenia have substantial ongoing treatment needs, and VHA provides a continuum of care over the life course.
Dementia is an illness with devastating impact on those affected and their families. In 2010 approximately 36 million people worldwide had dementia, a prevalence that is predicted to increase substantially in the coming decades (2). By 2040, 81 million people will have dementia (3). In 2013, 142,951 Veterans aged 65 and older who used VHA services had a diagnosis of dementia from VHA or Medicare providers. This represented a 7.4% prevalence rate for dementia in older veterans receiving care at VHA (4).
Schizophrenia and bipolar disorder are associated with an increased risk for dementia. Understanding that risk is paramount for treatment as persons with schizophrenia and bipolar illness age.
Prior studies indicate that individuals with schizophrenia are at increased risk for dementia. A recent meta-analysis of six studies with over 5 million participants calculated a relative increased risk of 2.2 times for the development of dementia in those with schizophrenia, compared to those without (5). In a Danish population-based cohort study, schizophrenia was also found to increase the risk of dementia two-fold (6). In that study, 7.4% of persons with schizophrenia developed dementia prior to age 80, compared to 5.8% of those without schizophrenia. The relative risk was nearly 4-fold in those under the age of 65. Estimates did not change significantly when adjusting for cardiovascular disease and diabetes.
There is also evidence that bipolar disorder is associated with an increased risk for dementia. In a meta-analysis that included 3,026 individuals and 6 studies (7), a history of bipolar disorder increased the risk of dementia with an overall calculated odds ratio of 2.36 (95% CI 1.36–4.0). A large cohort study in Australia showed an adjusted hazard ratio for dementia of 2.30 (95% CI 1.80–2.94) among those with bipolar disorder compared to the general population (8). A small population-based study demonstrated significant risk of subsequent dementia in bipolar patients over a 20-year period after adjusting for cardiovascular risk factors (9). An analysis of a large Taiwanese database of patients with dementia diagnosed over a decade matched 9,304 subjects by gender, age, and index date to those without dementia. In addition, medical conditions including cerebrovascular disease, diabetes, hypertension, head injury, COPD and substance use disorders were analyzed as covariates. Bipolar disorder was significantly associated with an increased risk of subsequent dementia with an adjusted odds ratio of 4.32 (95% CI 3.21–5.82) even after controlling for other co-morbidities (10). Preuss et al (11) compared 315,244 inpatient admissions for patients in the U.S. who were diagnosed with Alzheimer’s dementia and were at least 60 years of age. After controlling for age and gender, patients with Alzheimer’s disease (versus osteoarthritis) had a higher rate of bipolar disorder (OR 2.78). Kessing (12) analyzed all hospitalized patients in Denmark between 1977–1993, and found that those with a diagnosis of unipolar or bipolar illness were at a greater risk of being diagnosed with dementia at a subsequent readmission. Another longitudinal study of 345 bipolar patients over the age of 55 (13) found an increased risk of developing dementia when compared to age and sex matched controls, even after adjusting for medical co-morbidities (HR=5.58, 95% CI 4.26–7.32).
No previous study has examined the incidence of dementia among Veteran VHA patients with bipolar disorder or schizophrenia. To inform VHA operations, the purpose of this study was characterize the risk for the development of dementia among Veterans in VHA care, and to assess whether incidence differs among those with bipolar disorder and schizophrenia, as compared to patients with neither condition, adjusting for co-morbid medical diagnoses.
METHODS
Study data were drawn from the VHA Corporate Data Warehouse (CDW), which includes information regarding inpatient and outpatient care, such as dates of service and clinical diagnoses. Using CDW data, we identified all Veterans who received VHA services in both 2004 and 2005, who were between 18 and 100 years old as of January 1, 2006, and who did not have a dementia diagnosis in 2004–2005. Veteran VHA users were identified based on Veteran status indicators from VA and the Department of Defense. This project was conducted in support of clinical operations and was approved by the Department of Veterans Affairs Office of Mental Health and Suicide Prevention
The cohort was followed for up to 10 years, 2006–2015, to assess onset or incidence of dementia. Prior to October 1, 2015, dementia was identified using the following ICD-9-CM codes: 046.11, 046.19, 046.3, 046.79, 290.0, 290.10, 290.11, 290.12, 290.13, 290.20, 290.21, 290.3, 290.40, 290.41, 290.42, 290.43, 291.2, 292.82, 294.10, 294.11, 294.20, 294.21, 294.8, 331.0, 331.11, 331.19, and 331.82. As of October 1, 2015, dementia was identified using the following singular ICD-10 codes: A8100, A8101, A8109, A812, A8189, A819, F0390, F0391, F1027, F1997, G231, G300, G301, G308, G309, G3101, G3109, G3183, or G903, and the following code combinations: B20, G10, G20, or G912 with F0280 or F0281 or a primary diagnosis of I6 with a secondary diagnosis of F0280 or F0281.
We assessed whether participants had a diagnosis of schizophrenia or bipolar disorder during the baseline period. This was categorized as bipolar disorder, schizophrenia disorder, or neither condition (“no serious mental illness”) based on the most frequent diagnosis in the baseline period, 2004–2005. Individuals with an equal number of encounters with diagnoses of schizophrenia and of bipolar disorder were categorized as schizophrenia. Bipolar disorder was identified using the following ICD-9-CM codes: 296.0, 296.1, 296.4, 296.5, 296.6, 296.7, and 296.8. Schizophrenia was identified using the following ICD-9-CM codes: 295.0, 295.1, 295.2, 295.3, 295.4, 295.6, 295.7, 295.8, and 295.9. Covariates included age as of January 1, 2006 (categorized as 18 to 49, 50 to 64, 65 to 79, or 80 to 100 years) and sex.
Analyses were specific to ongoing VHA users, assessed based on date of last VHA use. The outcome for this study was incident dementia, defined as the first ICD-9-CM or ICD-10 dementia diagnosis recorded during the follow-up period. In survival analyses, individuals were censored at death, age 100, the end of the study period, or if there was no VHA use in 2016 the date of last use through 2015, whichever came first.
Next, baseline medical comorbidities as well as substance use disorders were included in multivariable models of incidence rate ratios. These included cerebrovascular disease (ICD-9-CM codes 431, 434.01, 434.11, 434.91), diabetes (250), essential hypertension (401) dyslipidemia (272), traumatic brain injury (800, 801, 803, 804, 850–854.1), chronic obstructive pulmonary disease (416.8, 416.9, 490–498), alcohol use disorder (291, 303.0, 303.9, 305.0), substance use disorder (292, 304, 305.2–305.9), tobacco use (305.1, 989.84, 649.0), renal diseases (403, 404.02, 404.12, 404.92, 582, 583, 585, 586, 588, 593.9), ischemic heart disease (410–414), and congestive heart failure (428).
Data analysis:
Survival curves were created to assess dementia-free survival by diagnosis and age group using the actuarial method. Poisson regression was used to model the incidence rate of dementia by serious mental illness diagnosis, age, and sex. Baseline comorbidities were added to the multivariable model to calculate incidence rate ratios.
RESULTS
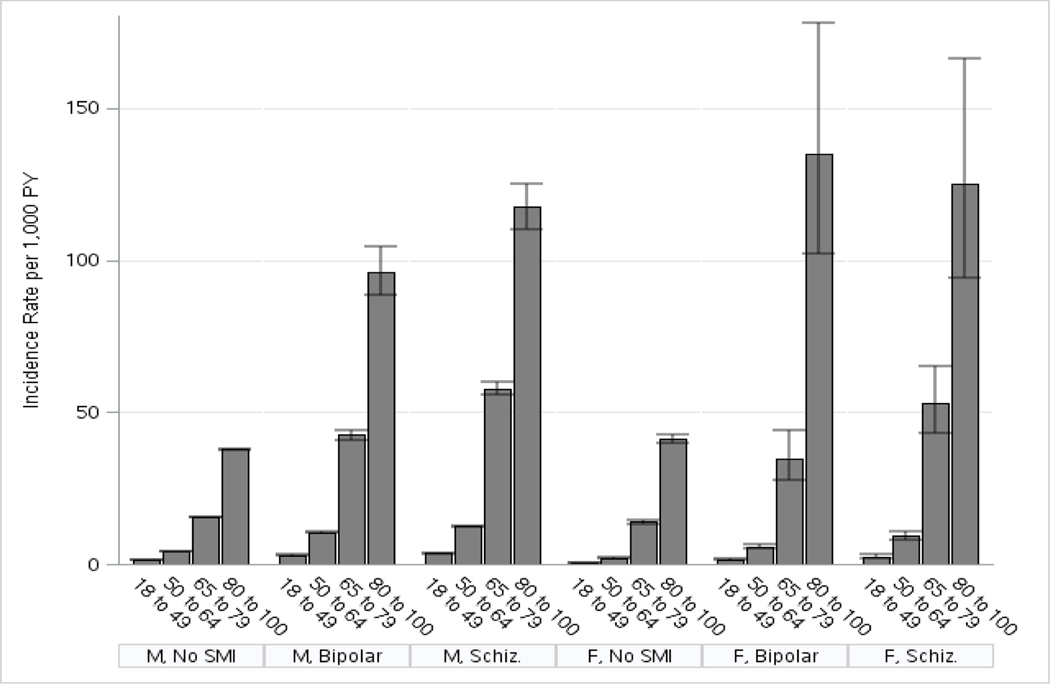
3,648,852 Veteran VHA patients were included in the study cohort: 3,407,752 men and 241,100 women. Men represented 93% and women accounted for 7%. The majority of individuals had no diagnosis of either bipolar disorder or schizophrenia (96% of men, 93% of women). The incidence of dementia per 1000 person-years was calculated by gender, age cohort, and diagnosis. For men, the mean incidence of dementia per thousand person-years in bipolar disorder was 11.5 and in schizophrenia 15.1, compared to the control group mean of 11.2. For women, the mean incidence of dementia in bipolar disorder per thousand person-years was 4.1 and in schizophrenia 8.4, compared to the control group mean of 4.0. The Results are displayed in Table 1 and figure 1. Among those diagnosed with dementia during the follow-up period, the mean (SD) age in years at first recorded dementia diagnosis was 78.8 (9.6) for individuals with no serious mental illness, 67.9 (11.0) for individuals with bipolar disorder, and 68.5 (10.9) for individuals with schizophrenia.
Table 1.
Incidence of dementia from 2006–2015 among Veterans who used VHA services in both 2004 and 2005, by diagnosis, gender, and age cohort (LCL- lower confidence limit, UCL- upper confidence limit, PY-person-years)
| Baseline Diagnoses | Gender/Age (years) | Total Population | Incident Cases | Person-Years at Risk | Incidence of Dementia per 1,000 PY | LCL | UCL |
|---|---|---|---|---|---|---|---|
| No serious mental illness | Men 18 to 49 | 390,595 | 4,527 | 3,420,969 | 1.3 | 1.3 | 1.4 |
| 50 to 64 | 1,139,206 | 40,254 | 9,709,530 | 4.2 | 4.1 | 4.2 | |
| 65 to 79 | 1,244,294 | 135,490 | 8,703,379 | 15.6 | 15.5 | 15.7 | |
| 80 to 100 | 483,070 | 89,838 | 2,372,556 | 37.9 | 37.6 | 38.1 | |
| Subtotal | 3,257,165 | 270,109 | 24,206,433 | 11.2 | 11.1 | 11.2 | |
| Women 18 to 49 | 111,233 | 568 | 951,809 | 0.6 | 0.6 | 0.7 | |
| 50 to 64 | 79,652 | 1,265 | 610,260 | 2.1 | 2.0 | 2.2 | |
| 65 to 79 | 17,867 | 1,760 | 126,085 | 14.0 | 13.3 | 14.6 | |
| 80 to 100 | 16,067 | 3,460 | 83,607 | 41.4 | 40.0 | 42.8 | |
| Subtotal | 224,819 | 7,053 | 1,771,761 | 4.0 | 3.9 | 4.1 | |
| Total | 3,481,984 | 277,162 | 25,978,194 | 10.7 | 10.6 | 10.7 | |
| Schizophrenia | Men 18 to 49 | 21,779 | 714 | 193,196 | 3.7 | 3.4 | 4.0 |
| 50 to 64 | 45,417 | 4,526 | 363,295 | 12.5 | 12.1 | 12.8 | |
| 65 to 79 | 9,880 | 3,173 | 54,963 | 57.7 | 55.8 | 59.8 | |
| 80 to 100 | 2,137 | 953 | 8,106 | 117.6 | 110.3 | 125.3 | |
| Subtotal | 79,213 | 9,366 | 619,560 | 15.1 | 14.8 | 15.4 | |
| Women 18 to 49 | 2,592 | 60 | 23,746 | 2.5 | 2.0 | 3.3 | |
| 50 to 64 | 2,052 | 158 | 17,007 | 9.3 | 8.0 | 10.9 | |
| 65 to 79 | 282 | 92 | 1,733 | 53.1 | 43.3 | 65.1 | |
| 80 to 100 | 96 | 48 | 383 | 125.2 | 94.4 | 166.2 | |
| Subtotal | 5,022 | 358 | 42,870 | 8.4 | 7.5 | 9.3 | |
| Total Schizophrenia | 84,235 | 9,724 | 662,431 | 14.7 | 14.4 | 15.0 | |
| Bipolar | Men 18 to 49 | 24,666 | 615 | 217,230 | 2.8 | 2.6 | 3.1 |
| 50 to 64 | 36,193 | 3,115 | 297,453 | 10.5 | 10.1 | 10.9 | |
| 65 to 79 | 9,039 | 2,339 | 55,108 | 42.4 | 40.8 | 44.2 | |
| 80 to 100 | 1,476 | 560 | 5,824 | 96.2 | 88.5 | 104.5 | |
| Subtotal | 71,374 | 6,629 | 575,614 | 11.5 | 11.2 | 11.8 | |
| Women 18 to 49 | 7,189 | 106 | 65,814 | 1.6 | 1.3 | 2.0 | |
| 50 to 64 | 3,656 | 183 | 31,327 | 5.8 | 5.1 | 6.8 | |
| 65 to 79 | 316 | 71 | 2,045 | 34.7 | 27.5 | 43.8 | |
| 80 to 100 | 98 | 50 | 370 | 135.0 | 102.3 | 178.1 | |
| Subtotal | 11,259 | 410 | 99,556 | 4.1 | 3.7 | 4.5 | |
| Total Bipolar | 82,633 | 7,039 | 675,170 | 10.4 | 10.2 | 10.7 | |
| Total | 3,648,852 | 293,925 | 27,315,795 | 10.8 | 10.7 | 10.8 |
Figure 1.
Incidence of dementia from 2006–2015 among Veterans who used Veterans Health Administration services in both 2004 and 2005, by diagnosis, gender, and age cohort. (SMI-serious mental illness, Schiz.- schizophrenia, PY-person years, M-men, F-women)
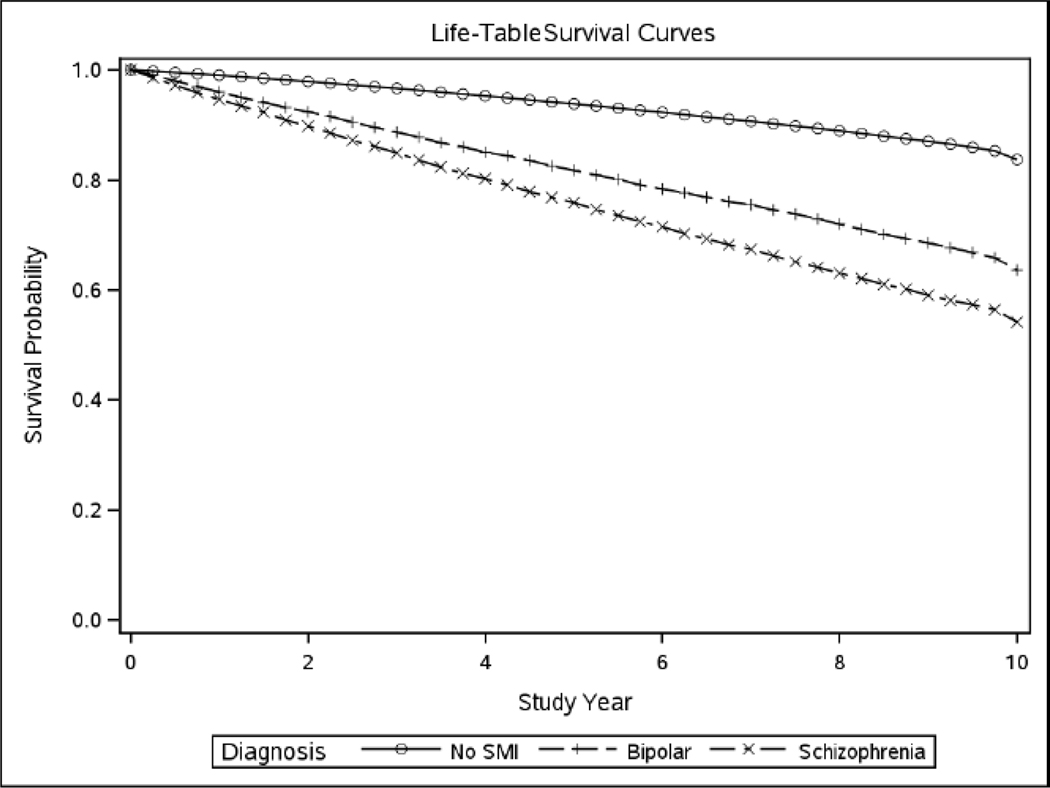
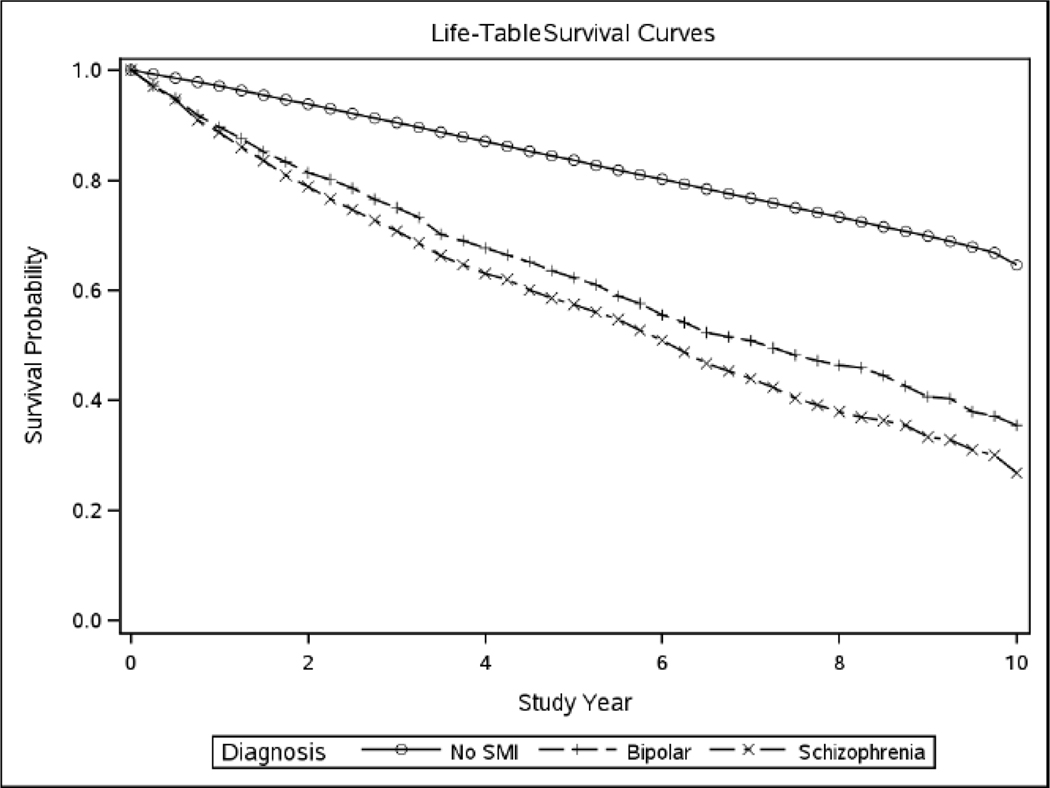
The incidence rate ratios (IRR) were calculated based on baseline diagnosis, gender, age and were adjusted for medical comorbidities and substance use disorders that can contribute to dementia risk. Results are displayed in Table 2. Even after accounting for medical comorbidity, the incidence ratios for dementia were higher for Veterans with a diagnosis of schizophrenia or bipolar disorder, compared to those with neither. The incidence ratios for dementia were higher for Veterans with schizophrenia compared to bipolar disorder. The highest incidence rate ratio was for Veterans with a baseline diagnosis of schizophrenia (2.92, 95% CI 2.86–2.98) compared to Veterans with no serious mental illness. The incidence rate was also elevated for bipolar disorder (2.26, 95% CI 2.20–2.31) compared to the unaffected baseline group. Finally, Figures 2 and 3 display dementia-free survival in the older age cohorts for men and women combined.
Table 2.
Baseline and adjusted incidence rate ratios for dementia, by baseline diagnosis, gender, age cohort, and medical comorbidity
| Bipolar Disorder vs. No Serious Mental Illness | Schizophrenia vs. No Serious Mental Illness | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Baseline IRR |
95% CI |
Adjusted † IRR |
95% CI |
Baseline IRR |
95% CI |
Adjusted † IRR |
95% CI |
| Male | 18 to 49 | 2.14 | 1.97–2.33 | 1.75 | 1.61–1.91 | 2.79 | 2.58–3.02 | 2.39 | 2.21–2.59 |
| 50 to 64 | 2.53 | 2.44–2.62 | 2.16 | 2.08–2.24 | 3.01 | 2.91–3.10 | 2.65 | 2.57–2.73 | |
| 65 to 79 | 2.73 | 2.62–2.84 | 2.48 | 2.38–2.59 | 3.71 | 3.58–3.84 | 3.36 | 3.24–3.48 | |
| 80 to 100 | 2.54 | 2.34–2.76 | 2.43 | 2.24–2.64 | 3.10 | 2.91–3.31 | 2.88 | 2.70–3.07 | |
| subtotal* | 2.61 | 2.54–2.67 | 2.29 | 2.23–2.35 | 3.27 | 3.20–3.34 | 2.92 | 2.86–2.98 | |
| Female | 18 to 49 | 2.70 | 2.19–3.32 | 2.32 | 1.88–2.85 | 4.23 | 3.24–5.52 | 3.63 | 2.78–4.74 |
| 50 to 64 | 2.82 | 2.41–3.29 | 2.46 | 2.11–2.87 | 4.48 | 3.80–5.29 | 3.95 | 3.34–4.65 | |
| 65 to 79 | 2.49 | 1.96–3.15 | 2.31 | 1.82–2.93 | 3.80 | 3.08–4.69 | 3.50 | 2.84–4.31 | |
| 80 to 100 | 3.26 | 2.47–4.31 | 3.21 | 2.43–4.24 | 3.03 | 2.28–4.02 | 2.96 | 2.23–3.94 | |
| subtotal* | 2.03 | 1.84–2.24 | 1.83 | 1.65–2.02 | 3.21 | 2.89–3.57 | 2.91 | 2.62–3.24 | |
| Total** | 2.57 | 2.50–2.63 | 2.26 | 2.20–2.31 | 3.27 | 3.20–3.33 | 2.92 | 2.86–2.98 | |
IRR- incidence risk ratio,
Adjusted for age,
Adjusted for age and sex,
Adjusted for baseline comorbidity: cerebrovascular disease, diabetes, essential hypertension, dyslipidemia, traumatic brain injury, chronic obstructive pulmonary disease, alcohol use disorder, substance use disorder, tobacco use, renal diseases, ischemic heart disease, and congestive heart failure.
Figure 2.
Survival curves for dementia for combined sample of men and women age 65–79 years
(SMI-serious mental illness)
Figure 3.
Survival curves for dementia for combined sample of men and women age 80–100 years
(SMI-serious mental illness)
DISCUSSION
The Veterans Health Administration serves an older patient population, and it is important to understand risk factors for incident dementia among VHA patients. The present analysis confirms prior studies indicating that individuals with bipolar disorder or schizophrenia have increased risk of dementia onset, even when controlling for medical comorbidities. Both illnesses had a higher incidence ratio than patients with neither condition. Individuals with schizophrenia were at highest risk.
We have an incomplete understanding about why dementia develops at a higher rate in those with schizophrenia. Schizophrenia is associated with a wide range of cognitive deficits that can be severe and enduring. Seidman et al (14) demonstrated significant and clinically meaningful neurocognitive deficits in attention, working memory and declarative memory during the prodromal phase of schizophrenia in those that went on to develop psychosis. A study of stabilized patients with first episode psychosis found significant memory deficits in addition to motor and executive dysfunction compared to controls (15). In addition, severe neurocognitive deficits have been demonstrated to continue in the chronic phases of the illness, with 61–78% of patients scoring below the median on a wide range of neurocognitive measures in one study (16). It is therefore possible that patients with schizophrenia have cognitive and functional changes that are consistent with dementia over their lifespan, only to be formally diagnosed with dementia at a later age. Some studies suggest that in addition to being a neurodevelopmental condition, schizophrenia may also be a neurodegenerative disorder (9,17). Lyketsos (18) suggests that only a subpopulation of those with schizophrenia develop a neurodegenerative disorder, reflecting the heterogeneity of the disorder.
Increased medical comorbidity has been found in schizophrenia including medical conditions which are independent risk factors for dementia. These include diabetes, cerebrovascular disease, ischemic heart disease, congestive heart failure, and substance abuse (19–23). Yet the current study and other studies have found an increased risk of dementia even after controlling for medical comorbidity (24).
In a post-mortem study, neuropathologic findings in schizophrenia were different from Alzheimer’s disease, with high neurofibrillary tangle density (corresponding to dementia), but lower than expected neuritic plaque findings (25). The neuropathology of dementia may be different in important ways.
Similarly, the higher rates of dementia in bipolar disorder are as yet unexplained. Although baseline cognitive impairment has been demonstrated in about 30% of bipolar patients (26), longitudinal studies have failed to show evidence of neuroprogression (27–30). Allowing that baseline cognitive impairment does not inevitably lead to neuroprogression, there may be an increased overall risk for dementia. In addition, more refractory forms of bipolar disorder appear to be a risk factor, as a higher number of lifetime affective episodes have been associated with an increased risk for dementia (27,31).
Medical co-morbidities in bipolar disorder are serious and common, and include metabolic syndrome, hypertension, diabetes, cardiovascular disease, endocrine abnormalities (26, 32,33) as well as substance use disorders (34). These co-morbidities can contribute to the risk for dementia. And, psychiatric medications used to treat bipolar disorder can also contribute to medical comorbidities such as cardiovascular disorders, which in turn increase the risk for dementia. Moreover, the intensity of medical care may be lower for persons with bipolar disorder; at least one study noted a lower rate of prescribing for coronary heart disease and hypertension in these patients (35). Finally, negative health behaviors seen in bipolar disorder and schizophrenia such as poor diet, lack of exercise, and tobacco use can also increase the risk for dementia (7).
In bipolar disorder, there is compelling preclinical and clinical evidence that lithium has neuroprotective properties. Diniz (36) summarized the potential mechanisms of lithium including the inhibition of glycogen synthase kinase, leading to a reduction of amyloid Beta 42 production and tau protein phosphorylation. Lithium has also been shown to increase brain derived neurotropic factor (37,38), and increase the volumes of the hippocampus and amygdala (39). Moreover, higher white matter integrity has been demonstrated in older adults with bipolar disorder who remained on lithium (40). In a 10-year study, Kessing compared persons who purchased lithium at least once to a random sample from the general population (41) and found that continued lithium treatment was associated with a reduced incidence of dementia, approaching that of the general population. Lithium, therefore, appears to have substantial potential to lower risk for dementia in patients with bipolar disorder.
Primary care and mental health clinicians should be educated about the elevated risk for dementia among VHA patients with schizophrenia or bipolar disorder, as suggested by this study. They should be alert to memory or other cognitive complaints in this patient population and should initiate an evaluation for dementia when there are signs or symptoms of possible cognitive impairment. Possible prevention of dementia is also critical. Cardiovascular risk factors such as smoking, diabetes, hypertension, and hyperlipidemia should be clinically managed to help mitigate risk for dementia. Clinicians choosing medication treatment for schizophrenia or bipolar disorder should be aware of the increased risk for dementia and if possible select medications that will not increase cardiovascular risk. To the editor: sentence beginning w “Should further studies…..” has been eliminated
LIMITATIONS
This study has several limitations. We did not account for onset, course or duration of psychiatric illness. There were not baseline measures of neurocognition nor longitudinal measurements. The intensity of psychiatric and medical care was not measured and we did not evaluate the potential contribution of psychiatric medication exposure. Future studies should account for these potential factors in analysis as they may impact risk for dementia.
CONCLUSIONS
This study of 3.6 million U.S. Veterans in VHA care demonstrates an increased risk for dementia in schizophrenia and bipolar disorder even after controlling for medical co-morbidities. VHA clinicians should be educated on the potential elevated risk for dementia among VHA patients with schizophrenia or bipolar disorder and should be encouraged to evaluate for possible dementia when there are signs or symptoms of cognitive impairment in this patient population. Prevention of dementia in these populations is critical and includes management of co-morbid health conditions that can increase risk for dementia and careful choice of medications that do not generate increased cardiovascular risk. Lithium may be a promising agent in the prevention of dementia in bipolar disorder. Recommendations for providers working with persons with schizophrenia or bipolar disorder may be applicable to the general population.
A retrospective cohort of 3.6 million veterans with bipolar disorder, schizophrenia/schizoaffective disorder, or neither diagnosis was followed for a period of 10 years to assess the onset of dementia.
The incidence rate ratio for dementia was 2.92 for veterans with a baseline diagnosis of schizophrenia compared to no serious mental illness, even after controlling for medical co-morbidities.
The incidence rate ratio for dementia was 2.26 for veterans with bipolar disorder compared to no serious mental illness, even after controlling for medical co-morbidities.
This large study confirms that veterans with bipolar disorder or schizophrenia are at increased risk for dementia as they age even after accounting for co-morbid medical conditions.
Footnotes
Conflict of Interest: the authors report no conflict of interest
REFERENCES
- 1.McCarthy JF, Bossarte R, Katz IR, et al. Predictive Modeling and Concentration of the Risk of Suicide: Implications for Preventive Interventions in the US Department of Veterans Affairs. American Journal of Public Health 2015; 105(9):1935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and meta-analysis. Alzheimer’s and Dementia 2013; 9:63–75. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C., et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005; 366(9503): 2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei L, Cooley SG, Phibbs CS, Kinosian B, Allman RM, Porsteinsson AP, Intrator O. Attributable cost of dementia: Demonstrating pitfalls of ignoring multiple health care system utilization. Health Services Research 2018; 53(6): 5331–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai L, Huang J. Schizophrenia and risk of dementia: a meta-analysis study. Neuropsych. Disease and Treatment 2018; 14:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribe A, Laursen TM, Charles M, Katon W, Fenger-Gron M, Davydow D, Chwastiak L, Cerimele JM, Vetergaard M. Long term risk of dementia in persons with schizophrenia: A Danish population-based cohort study. JAMA Psych 2015; 72(11):1095–1101. [DOI] [PubMed] [Google Scholar]
- 7.Diniz BS Teixeira AL, Cao F, Gildengers A, Soares JC, Butters MA, Reynolds CF. History of bipolar disorder and risk of dementia: A systemic review and meta-analysis. J Geriatric Psych 2017; 25:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida OP, McCaul K, Hankey GJ, Yeap BB, Golledge J, Flicker L. Risk of dementia and death in community dwelling older men with bipolar disorder. Brit J Psych 2016; 209:121–126. [DOI] [PubMed] [Google Scholar]
- 9.Zilkens RR, Bruce DG, Duke J, Spilsbury K, Semmens JB. Severe psychiatric disorders in mid-life and risk of dementia in late life: A population based case control study. Curr Alz Res 2014; 11:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu KY, Chang CM, Liang HY, Wu Cs, Wu ECH, Chen CH, Chau YL, Tsai HJ. Increased risk of developing dementia in patients with bipolar disorder: a nested matched case-control study. Bipolar Disorder 2013; 15:787–794. [DOI] [PubMed] [Google Scholar]
- 11.Preuss UW, Watzke S, Choi JH. Diagnostic correlates of Alzheimer Dementia in a US nationwide inpatient sample. Amer. J Geri Psychiatry 2010; 18: 821–829. [DOI] [PubMed] [Google Scholar]
- 12.Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses. J Affective Disorders. 2003; 73: 261–169. [DOI] [PubMed] [Google Scholar]
- 13.Chen MH, Li CT, Tsai CF, Lin WC, Chang WH, Chen TJ, Pan TL Su TP, Bai YM. Risk of subsequent dementia among patients with bipolar disorder or major depression: A nationwide longitudinal study in Taiwan. JAMDA 2015; 16: 504–508. [DOI] [PubMed] [Google Scholar]
- 14.Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW. Neurocognition and transition to psychosis: Baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA PSychiatiatry 73(12): 1239–1248, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Wilson DF, Alvier JMJ, Woerner MG, Geisler S, Kane JM, and Lieberman JA. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. Am J Psychiatry 157:549–559, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs RW, Zakzanis KK. Neurocognitive deficits in schizophrenia: a quantitative review of the evidence. Neuropsychology 12(3):426–445, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Kulhara P. What is schizophrenia: a neurodevelopmental or neurodegenerative disorder or a combination of both? A Critical analysis. Indian J Psych 2010; 52(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyketsos CG, Peters ME. Dementia in patients with schizophrenia: Evidence for heterogeneity. JAMA Psych 2015; 72(11): 1075–6. [DOI] [PubMed] [Google Scholar]
- 19.Fan Z, Wu Y, Shen J, Ji T, Zhan R. Schizophrenia and risk of Cardiovascular diseases: A meta-analysis of thirteen cohort studies. J Psychiatric Res. 2013; 47(11): 1549–1556. [DOI] [PubMed] [Google Scholar]
- 20.Breese LC, Majundar SR, Patten SB, Johnson JA. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: A population based study. Schizophrenia Res. 2010: 117(1): 75–82. [DOI] [PubMed] [Google Scholar]
- 21.Dixon L, Weiden P, Delahanty J, et al. Prevalence and corrleates of diabetes in national schizophrenia samples. Schizophrenia Bull 2000; 26(4); 903–912. [DOI] [PubMed] [Google Scholar]
- 22.Rusanen M, Kivipelto M, Levalahti E, et al. Heart diseases and long term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimer’s Disease. 2014; 42(1) 183–191. [DOI] [PubMed] [Google Scholar]
- 23.Watkins KE, Pincus HA, Smith B, Paddock SM, Mannle TE, Woodroffe A, Solomon J, Sorbero ME, Farmer CM, Hepner KA, Adamson DM, Forrest L, Call C. (2011). Veterans Health Administration Mental Health program evaluation: Capstone report. http://www.rand.org/pubs/technical_reports/TR956.html [Google Scholar]
- 24.Lin CE, Chung CH, Chen LF, Chi MJ. Increased risk of dementia in patients with Schizophrenia: A population-based cohort study in Taiwan. Eur. Psychiatry 2018; 53:7–16. [DOI] [PubMed] [Google Scholar]
- 25.Rapp MA, Schnaider-Beeri M Purohit DP, et al. Cortical neuritic plaques and hippocampal neurofibrillary tangles are related to dementia severity in elderly schizophrenia patients. Schiz. Res 2010; 116(1):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatham LN, Kennedy SH, Parikh S, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety disorders and International Society for Bipolar Disorders 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders 2018; 20:97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Morla EM, Lopez-Villarreal A, Jimenez-Lopez E, Aparicio AI, Martinez-Vizcaino V, Roberto RJ, Vieta E, Santos JL. Impact of number of episodes on neurocognitive trajectory in bipolar disorder patients: a 5-year follow-up study. Psychological Medicine 2018; 1–9. [DOI] [PubMed] [Google Scholar]
- 28.Gildengers AG, Chisholm D, Butters MA et al. Two year course of cognitive function and instrumental activities of daily living in older adults with bipolar disorder: evidence for neuroprogression? Psychological Medicine 2013; 43–801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bora E, Ozerdem A. Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol Medicine 2017; 47:2753–2766. [DOI] [PubMed] [Google Scholar]
- 30.Santos JL, Aparicio A, Bagney A, Sanchez-Morla EM, Sanchez-Moria EM, Rodriguez-Jimenez R, Mateo J, Jimenez-Arriero MA. A five year follow up study of neurocognitive functioning in bipolar disorder. Bipolar Disorder 2014; 16:722–731. [DOI] [PubMed] [Google Scholar]
- 31.Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J Neurol Neurosurg Psych 2004; 75: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012; 25:20–5. [DOI] [PubMed] [Google Scholar]
- 33.Rise IV, Haro JM, Gjervan B. Clinical features, comorbidity, and cognitive impairment in elderly bipolar patients. Neuropsych Disease and Treatment 2016; 12:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt GE, Malhi GS, Cleary M et al. Comorbidity of bipolar and substance use disorders in national surveys of general populations, 1990–2015: systematic review and meta-analysis. J Affect Disorders 2016; 206:321–330. [DOI] [PubMed] [Google Scholar]
- 35.Smith DJ, Martin D, McLean G, Langan J, Guthrie B, Mercer SW. Multi-morbidity in bipolar disorder and under-treatment of cardiovascular disease: a cross sectional study. BMC Med 2013, 11:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diniz BS, Machado-Viera R, Forlenza OV. Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders. Neuropsych Dis and Treatment 2013; 9:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukomoto T, Morinobu S, Okamato Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharm 2001; 158:100–106. [DOI] [PubMed] [Google Scholar]
- 38.Forlenza OV, De-Paula VJR, Diniz BSO. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem Neurosci 2014; 5:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 2008; 19(2):221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gildengers AG, Butters MA, Alzenstein HJ, Marron MM, Emanuel J, Anderson SJ, Weissfeld LA, Becker JT, Lopez OL, Mulsant BH, Reynolds CF. Longer lithium exposure is associated with better white matter integrity in older adults with bipolar disorder. Bipolar Disorder. 2015; 17(3): 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch Gen Psych 2008; 65(11):1331–1335. [DOI] [PubMed] [Google Scholar]