Abstract
This study investigated the effects of SOD2 (MnSOD)-deficiency-induced excessive oxidative stress on ovarian steroidogenesis in vivo and isolated and cultured granulosa cells using WT and Sod2+/− mice. Basal and 48 h eCG-stimulated plasma progesterone levels were decreased ~50% in female Sod2+/− mice, whereas plasma progesterone levels were decreased ~70% in Sod2+/− mice after sequential stimulation with eCG followed by hCG. Sod2+/− deficiency caused about 50% reduction in SOD2 activity in granulosa cells. SOD2-deficiency also caused a marked reduction in progestins and estradiol in isolated granulosa cells. qRT-PCR measurements indicated that the mRNA expression levels of StAR protein and steroidogenic enzymes are decreased in the ovaries of Sod2+/− mice. Further studies showed a defect in the movement of mobilized cytosolic cholesterol to mitochondria. The ovarian membrane from Sod2+/− mice showed higher susceptibility to lipid peroxidation. These data indicates that SOD2-deficiency induced oxidative stress inhibits ovarian granulosa cell steroidogenesis primarily by interfering with cholesterol transport to mitochondria and attenuating the expression of Star protein gene and key steroidogenic enzyme genes.
Keywords: Antioxidant enzymes, Estradiol, Lipid peroxidation, Progestin, Reactive oxygen species (ROS), StAR protein
1. Introduction
Reactive oxygen species (ROS) are chemical radicals and non-radical molecules derived from molecular oxygen such as superoxide anion (O2●−), hydroxyl radical (OH●), peroxide (O2●2−), hydroxyl ion (OH−), peroxyl (ROO●) and alkoxyl radicals (RO●), singlet oxygen (1O2) and hydrogen peroxide (H2O2) (Kohen and Nyska, 2002; Krumova and Cosa, 2016; Pisoschi and Pop, 2015; Winterbourn, 2008). ROS originates from exogenous and endogenous sources (Bhattacharyya et al., 2014; Krumova and Cosa, 2016); among former are UV and ionizing radiation, pollutants and xenobiotics, the latter represented by numerous enzymatic reactions in various cell compartments, including the cytoplasm, cell membrane, endoplasmic reticulum and peroxisomes (Bhattacharyya et al., 2014; Auten and Davis, 2009; Forrester et al., 2018), mitochondria (Murphy, 2008; Turrens, 2003) and steroidogenic enzymes (Hanukoglu, 2006). At low-to-moderate levels of either intracellular or extracellular ROS (e.g., superoxide, H2O2) may play a role in cell signaling, including apoptosis, gene expression and the activation of cell signaling cascades (Dröge, 2002; Finkel, 2011; Ray et al., 2012; Schieber and Chandel, 2014; Wang et al., 2018). In contrast, increased production and/or inadequate removal of ROS (due to inefficient functioning by internal defense mechanisms such as antioxidants; tocopherols, ascorbic acid and glutathione) (Bhattacharyya et al., 2014; Birben et al., 2012; He et al., 2017) and/or enzymes/proteins involved in oxygen radical scavenging such as SODs [SOD1 and SOD2], catalase, glutathione peroxidases [GPXs], the glutathione redox system, the peroxiredoxin system, the thioredoxin redox system, the glutaredoxin system, and the mitochondrial nicotinamide nucleotide trans dehydrogenase (NNT)–pyridine nucleotide redox system (Bhattacharyya et al., 2014; Birben et al., 2012; Circu and Aw, 2010; Fukai and Ushio-Fukai, 2011; He et al., 2017; Pisoschi and Pop, 2015; Powis and Montfort, 2001; Rhee and Kil, 2017; Wang et al., 2018), especially superoxide, can initiate membrane lipid peroxidation (Hauck and Bernlohr, 2016), oxidize proteins to inactive states (Cecarini et al., 2007), and cause DNA strand breaks (Van Houten et al., 2018), all contributing to excessive oxidative stress (Bhattacharyya et al., 2014; Kohen and Nyska, 2002; Vitale et al., 2013) and potentially disruptive to normal cellular function. Excessive oxidative stress has been implicated in the pathology of many diseases including neurodegenerative disorders, cardiovascular diseases, diabetes, cancer, gastrointestinal mucosal diseases, aging including endocrine aging and male infertility (Barnham et al., 2004; Bhattacharyya et al., 2014; Bisht et al., 2017; Liguori et al., 2018; Vitale et al., 2013). Oxidative stress is also implicated in ovarian physiology (Sugino, 2005) and toxicity (Luderer, 2014), pregnancy and reproduction (Agarwal et al., 2006; Duhig et al., 2016), ovulation (Shkolnik et al., 2011), luteolysis (Foyouzi et al., 2005) and pathophysiology of many female reproductive complications (Duhig et al., 2016).
Mitochondrial superoxide dismutase (SOD2) is an essential antioxidant enzyme, which is exclusively localized in the matrix of mitochondria (Candas and Li, 2014; Fukai and Ushio-Fukai, 2011; Wang et al., 2018), and detoxifies superoxide into hydrogen peroxide (H2O2), the major by-product of mitochondrial oxidative phosphorylation (Murphy, 2008; Turrens, 2003) and steroidogenesis (Hanukoglu, 2006), which is quickly converted to water (H2O) by mitochondrial glutathione peroxidases 1 and 4 (GPX-1 and GPX-4) (Ribas et al., 2014) or peroxiredoxin III (PRXIII) (Rhee and Kil, 2016, 2017). It has been demonstrated that rodent steroidogenic tissues express high levels of SOD2 (Azhar et al., 1995; Cao et al., 2004; Matzuk et al., 1998). Elevated levels of rat ovarian SOD2 has also been observed during pseudo pregnancy and pregnancy (Shimamura et al., 1995; Sugino et al., 1993, 1998). In humans, increased expression of SOD2 is reported in granulosa and theca internal cells of steroid producing follicles, luteinized granulosa and theca cells of the functioning corpus luteum and steroid producing luteinized theca cells of degenerating corpus luteum (Suzuki et al., 1999; Tamate et al., 1995), whereas aging is associated with decreased expression of SODs and catalase in human ovarian granulosa cells (Tatone et al., 2006). In this study Sod2+/− mice were used to study the impact of SOD2 deficiency-induced oxidative stress, including mitochondrial oxidative stress, on steroid (progestin and estradiol) production in short-term cultured mouse ovarian granulosa cells. We selected granulosa cells because they are major steroid secreting ovarian cell type and these cells are exquisitely sensitive to cholesterol-rich exogenous lipoproteins and trophic hormones when maintained in a serum-free medium and secrete large amounts of progestin when incubated in the presence of lipoproteins and stimulatory hormones (Azhar et al., 1988; Reaven et al., 1999). In addition, granulosa cells are considered model cells to study conversion of androgens to estrogens, as well as progesterone synthesis (Havelock et al., 2004), Also, these cells, irrespective of their origin, are highly sensitive to oxidative insult and ROS-mediated inhibition of steroidogenesis (Ávila et al., 2016; Endo et al., 1993; Gatzuli et al., 1991; Masjedi et al., 2020; Miriyala et al., 2012; Musicki et al., 1994) and also express high levels of SOD2. We show that Sod2 gene ablation results in excessive oxidative stress (increased lipid peroxidation) and significant decreases in progestin production. Sod2+/− granulosa cells also show reduction in estradiol production and in mRNA levels of CYP11A1 (P450scc, gene Cyp11a1), CYP17 (P450c17, gene Cyp17a1), CYP19 (P450arom, gene Cyp19a1) and StAR (gene Star). These results provide the first in vivo evidence that ROS-induced excessive oxidative stress in response to SOD2 deficiency inhibits progestin and estradiol production in granulosa cells and direct evidence for the critical role of ROS/oxidative stress in the negative regulation of steroidogenesis.
2. Materials and Methods
2.1. Chemicals and reagents
Iodine-125 radionucleotide as sodium iodide carrier free (~17.4 mCi/μg) (~643.8 MBq/μg), 20α-hydroxyprogesterone [1,2–3H (N)] (40–60 Ci/mmol) (1.48–2.22 TBq/mmol), progesterone [1,2–3H] (40–60 Ci/mmol) (1.48–2.22 TBq/mmol) and cholesteryl [1a,2a(n)-3H]oleolyl ether (30–60 Ci/mmol) (1.11–2.22 TBq/mmol) were purchased from American Radiochemical, Inc., (St. Louis, MO). N6, 2′-dibutyryladenosine 3′,5′-cyclic monophosphate (Bt2cAMP), Sodium Cyanide (NaCN), nitroblue tetrazolium (NBT), diethylenetriaminepentaacetic acid (DETAPAC), bathocuproine disulfonic acid (BCS), xanthine, xanthine oxidase, bovine liver catalase, reduced glutathione (GSH), hydrogen peroxide (H2O2), NADPH, fatty acid free bovine serum albumin (BSA), progesterone, 3-isobutyl-1-methylxanthine (IBMX), aminoglutethimide (AMG), 20α-hydroxyprogesterone, 22(R)-hydroxycholesterol, 2-thiobarbituric acid, Tris-maleate salt, ascorbic acid, ADP, NADPH, insulin, transferrin, forskolin, human chorionic gonadotropin (hCG), equine chorionic gonadotropin (eCG) and mouse/rat estradiol ELISA kit were supplied by Sigma-Aldrich (St. Louis, MO). All other chemicals or reagents used were of analytical grade. For granulosa cell treatment, FSH was supplied by National Hormone & Peptide Program (NHPP) (National Hormone and Pituitary Program), NIDDK and Dr. Parlow. cAMP ELISA kit was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY). TRIzol® reagent and Power SYBR™ Green master mix from Applied Biosystems/Life Technologies and Invitrogen/Life Technologies (Grand Island, NY), respectively. Total cholesterol assay kit was obtained from Wako Diagnostics (Richmond, VA).
2.2. Animals and hormonal treatment
Congenic wild-type and SOD2 (superoxide dismutase 2, mitochondrial; synonyms, manganese superoxide dismutase, manganese SOD, MnSOD) deficient heterozygous mice (Sod2+/+ [WT] and Sod2+/−, respectively) on the C57BL/6 J background were generated by crossing Sod2+/− mice with C57BL/6 J mice as described previously (Corniola et al., 2012; Van Remmen et al., 1999). Mice were maintained in the animal facility at the Veterans Affairs Palo Alto Health Care System with 12-h light and 12-h dark cycle and provided food and water ad libitum. All procedures were conducted in accordance with institution guidelines and approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Palo Alto Health Care System.
To study in vivo ovarian progestin secretory responses to exogenous gonadotropins, 2–3-month-old WT and Sod2+/− female mice were evaluated to avoid the complexity of ovarian functions associated with estrous cycles and endogenous surges of gonadotropins. Specifically, immature WT and Sod2+/− mice were injected (i.p.) with 5 IU eCG to stimulate pre-ovulatory follicle growth and ovaries were collected 48 h after the treatment for the isolation of granulosa cells. For other studies mice were treated with 5 IU eCG followed 48 h later with 5 IU hCG (human chorionic gonadotropin). Blood was collected from mice at time 0 and 4 h after hCG injection and serum samples were analyzed for progesterone by radioimmunoassay using specific antiserum described earlier (Azhar et al., 1988, 1990; Reaven et al., 1999). Ovaries were also collected 4 h post hCG and analyzed for mRNA levels of various genes by qRT-PCR. In some cases, eCG treated mice at 48 h were injected with ± amino glutethimide (AMG) (150 mg/kg BW; i.p.) and 2 h later with 5 IU hCG and euthanized 4 h later and subsequently used for the isolation of mitochondria and quantification of mitochondrial cholesterol.
2.3. Isolation and culture of granulosa cells
For the isolation and culture of ovarian granulosa cells, immature (2–3-month-old) WT or Sod2+/− mice were injected once with 5 IU of equine chorionic gonadotropin (eCG) for 48 h. The animals were killed by cervical dislocation, the ovaries were excised and placed in basal medium (DMEM:F12 with 20 mM HEPES, pH 7.4) supplemented with 1 mg/ml bovine serum albumin, plus penicillin (100 U/ml) and streptomycin (100 μg/ml). Granulosa cells were harvested from ovaries by puncturing follicles with a 25-gauge needle as described previously (Azhar et al., 1988; Reaven et al., 1999). Isolated granulosa cells were plated in basal medium (DMEM: F12 medium supplemented with growth factors (2 μg/ml insulin, 5 μg/ml transferrin, 100 ng/ml hydrocortisone), 100 U/ml penicillin and 100 μg/ml streptomycin and 1 mg/ml BSA in 6-well (1 × 106 cells) or 12-well (1 × 105 cells) culture dishes that were pre-coated with 1% fetal bovine serum and maintained at 37 °C in basal medium for 72 h.
2.4. Secretion of steroids
To assay for steroidogenesis (progestin [progesterone + 20α-hydroxyprogesterone] production), 72 h cultured granulosa cells from WT or Sod2+/− mice were treated with or without FSH (100 ng/ml) + testosterone (T, 10 ng/ml) (Dorrington et al., 1975), or Bt2cAMP (2.5 mM) (Reaven et al., 1999) for 24 h. Subsequently, triplicate culture dishes were supplemented with ± FSH (100 ng/ml), ± Bt2cAMP (2.5 mM), ± hHDL3 (500 μg protein/ml) or ± 22(R) hydroxycholesterol (20 μM) and incubated at 37 °C for an additional 24 h; samples of incubation medium were frozen and stored until assayed for progestin. Progesterone and its metabolite 20α-hydroxyprogesterone were quantified by radioimmunoassay using specific antiserum as described previously (Azhar et al., 1988; Reaven et al., 1999). Results are expressed as nanograms of progestin (the sum of progesterone and 20α-hydroxyprogesterone) produced per 1 × 105 cells and represent the Mean ± SE of duplicate determinations of three different dishes. To measure estradiol (E2) production by granulosa cells, 72 h cultured granulosa cells were incubated with FSH (100 ng/ml) + T (10 ng/ml) or Bt2cAMP (2.5 mM) for 48 h and following incubation, medium samples were collected and analyzed for estradiol production using an ELISA Kit (Sigma/Aldrich). Results are expressed as picograms of estradiol produced per 1 × 105 cells and represent the Mean ± SE of duplicate determinations of three different dishes.
2.5. cAMP production
Seventy-two-hour cultured granulosa cells from WT and Sod2+/− mice were treated with FSH (100 ng/ml) or forskolin (10 μM) for 48 h in basal medium supplemented with IBMX (0.1 mM). At the end of incubation, the cell plus medium were analyzed for cAMP content using an Enzo cAMP ELISA kit. Results are expressed as pmoles of cAMP produced per 1 × 105 cells and represent the Mean ± SE of duplicate determinations of three different dishes.
2.6. RNA isolation and real-time qRT-PCR analyses
Immature WT and Sod2+/− mice were injected intraperitoneally (i.p.) with 5 IU eCG followed 48 h later with 5 IU hCG. Ovaries were collected 4 h after hCG injection and subjected to RNA isolation and measurement of mRNA levels by quantitative real-time PCR. Total RNA was obtained using TRIzol® reagent (Invitrogen/Life Technologies) according to the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDrop Spectrophotometer. Two μg RNA were reverse transcribed to cDNA at 42 °C for 1 h using SuperScript II reverse transcriptase (Invitrogen), oligo (dt) and random hexamers. Quantitative RT-PCR was performed using specific primers (Supplemental Table S1) in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems® Life Technologies, Grand Island, NY) as described previously (Zaidi et al., 2014). The calculated relative levels of mRNAs were normalized to the levels of endogenous 36B4 (60 S acidic ribosomal protein P0) in the same sample. For each indicated gene, the relative transcript level of the WT control sample was set as 1. The relative transcript level of Sod2 ± sample was compared to the respective WT control, and the fold-changes shown in the graph. For each experiment qRT-PCR reactions were carried out in triplicate. Results are expressed as the Mean ± SE of four or six individual samples. Sequence of the primer pairs used for this study is listed in Supplemental Table S1.
2.7. Isolation of mitochondria and measurement of cholesterol content
Groups of four ovaries were homogenized in 1.0 ml homogenization medium (0.33 M sucrose, 1 mM EDTA and 10 mM Tris-HCl, pH 7.4) in a Potter-Elvehjem glass tube with the motor-driven Teflon pestle. The homogenate in each case was transferred to an eppendorf tube and tubes centrifuged at 400×g for 5 min at 4 °C to pellet unbroken tissue, cells and nuclei. The supernatant fraction was next centrifuged at 13,000×g for 10 min to sediment mitochondria. The sedimented mitochondrial pellets were washed twice in washing/resuspension medium (25 mM sucrose, 75 mM sorbitol, 100 mM KCl, 0.05 mM EDTA, 5 mM MgCl2, 10 mM Tris-HCl pH 7.4, and 10 mM H2PO4, pH 7.4) and re-suspended in a suitable volume of the same buffer. The mitochondrial preparations were quantified for their cholesterol content and assessed for their purity by following the enrichment of mitochondrial enzyme markers or markers for other contaminating subcellular organelles. The most common contaminants in a mitochondrial preparation are microsomes (endoplasmic reticulum), lysosomes and peroxisomes and their enrichment was monitored by measuring the activity of enzymes specific for these subcellular fractions. The activities of mitochondrial markers, inner mitochondrial membrane bound respiratory complex IV or cytochrome C oxidase (COX) and mitochondrial matrix enzyme citrate synthase (CS) were measured according to the procedures of Wharton and Tzagoloff (1967) and Srere (1969), respectively. The activity of microsomal marker glucose phosphatase was measured according to Aronson and Touster (1974). The colorimetric method of Johansson and Borg (1988) was used to measure catalase activity, a marker for peroxisomes. The activity of acid phosphatase, a lysosomal marker, was assayed using the procedure of Tulkens et al. (1974). The enrichment of COX activity in WT mitochondria and Sod2+/− mitochondria was 3.15 ± 0.56-fold and 3.25 ± 0.79-fold, respectively, whereas enrichment of CS was 3.08 ± 0.47-fold in WT mitochondria and 3.50 ± 0.64-fold in Sod2+/− mitochondria. No enrichment of acid phosphatase, catalase or glucose-6 phosphatase activity was observed in any of the mitochondrial preparations. The micro colorimetric method of Glick et al. (1964) was used to quantify mitochondrial cholesterol content.
2.8. Isolation and radiolabeling of human high-density lipoprotein 3 (hHDL3)
ApoE-free hHDL3 was isolated as previously described (Azhar et al., 1990). hHDL3 was used exclusively because it is not recognized by the LDL receptor/endocytic pathway. For uptake and internalization studies, hHDL3 preparations were conjugated with residualizing labels, i.e. [125I]-labeled dilactitol tyramine (DLT) and [3H] cholesteryl oleolyl ether ([3H] COE) ([125I] DLT-[3H]COE-hHDL3) (Azhar et al., 1990). Protein content of hHDL3 and [125I] DLT-[3H] COE-hHDL3 was determined by the procedure of Markwell et al. (1978). Cholesterol content of hHDL3 and [125I] DLT-[3H] COE-hHDL3 was determined using a total cholesterol assay kit from Wako diagnostics.
2.9. Uptake and internalization of hHDL3-derived cholesteryl esters
For these experiments, 72 h cultured granulosa cells from WT and Sod2+/− mice were treated with FSH (100 ng/ml) or Bt2cAMP (2.5 mM) for 24 h and then replaced with respective medium containing FSH (100 ng/ml) or Bt2cAMP (2.5 mM) and hHDL3 equipped with radio-labeled, non-releasable apolipoprotein and cholesteryl ester (CE) tags ([125I]DLT-[3H]COE-hHDL3) that accumulate within the cells even when degraded (Azhar et al., 1990). Incubations were carried out with [125I] DLT-[3H]COE-hHDL3 (100 μg protein/ml) + FSH (100 ng/ml) or Bt2cAMP (2.5 mM) for 24 h at 37 °C. At the end of incubation, the dishes were washed and then solubilized in 2 ml of 0.1 N NaOH. One‒ml aliquots were precipitated with an equal volume of 20% trichloroacetic acid (TCA) to determine insoluble 125I radioactivity or extracted with organic solvents to determine 3H-radioactivity (Azhar et al., 1990; Reaven et al., 1999). Endocytic uptake was calculated from the trichloroacetic acid‒soluble 125I label. The difference between total and TCA‒soluble radioactivity was taken as the cell surface associated radioactivity. Since both 125I and 3H labels are on the same particle, the surface bound radioactivity 125I is also equal to the surface bound 3H. Thus, total 3H minus surface bound 3H equals the total amount of 3H internalized. To calculate ‘selective’ uptake of CE, soluble 125I radioactivity is subtracted from soluble 3H radioactivity. Finally, to calculate the mass of CE internalized, these values are divided by the protein: cholesterol ratio of hHDL3 (i.e., 2.71).
2.10. Lipid peroxidation assay
Groups of forty-eight-hour eCG injected WT and Sod2+/− mice were euthanized, ovaries removed, cleaned, and rinsed with homogenization buffer (0.15 M KCl, 5 mM Tris-maleate, pH 7.4). The minced ovaries (four/sample) were homogenized in 10 vol of homogenization buffer using a Teflon Potter‒Elvehjem homogenizer, and subsequently centrifuged at 800×g for 10 min to sediment unbroken cells and nuclei. A total membrane fraction was pelleted by centrifugation of supernatant at 105, 000×g for 60 min, and resuspended in homogenization buffer, to contain approximately 2 mg of protein/ml. Protein concentration was determined by the procedure of Markwell et al. (1978).
Membrane lipid peroxidation was assessed by quantification of malondialdehyde (MDA) formed under basal condition and in response to enzymatic (NADPH-induced) or nonenzymatic pro-oxidants using the slight modification of the procedure described previously (Abidi et al., 2004). In brief, Ovarian membranes (200 μg of protein) were incubated at 37 °C for 40 min with 40 mM Tris-maleate buffer, pH 7.4 alone (basal), FeCl3 (50 μM) + ADP (4 mM) + NADPH (0.4 mM) or FeSO4 (10 μM) + ADP (1 mM) + ascorbate (0.5 mM) in a total volume of 0.5 ml. At the end of incubation, one milliliter of TBA/TCA/HCl/BHT reagent (15% w/v trichloroacetic acid [TCA]; 0.375% w/v thiobarbituric acid; 0.25 N hydrochloric acid; 0.045% w/v butylated hydroxytoluene) was added to individual incubation tubes and vortexed thoroughly. The tubes were heated for 15 min in a boiling water bath. After cooling, the tubes were centrifuged at 1000×g for 10 min and absorbance of the sample was determined at 535 nm against a blank that contained all the reagents minus the membrane. Lipid peroxidation is expressed as nmoles of MDA produced per milligram of membrane protein per 60 min ± SE. MDA values were calculated using a molar extinction coefficient of 1.56 × 105 M−1 cm−1 at 532 nm.
2.11. Measurement of superoxide dismutase 1 and 2, catalase and glutathione peroxidase 1 activities in granulosa cells from WT and Sod2+/− mice
For determination of SOD1, SOD2, catalase and GPX-1, granulosa cell pellets were homogenized in a suitable volume of 50 mM potassium phosphate buffer, pH 7.4–0.1 mM EDTA, sonicated in a bath-type sonicator on ice, and centrifuged at 600 g for 10 min; supernatant fractions were stored at −90 °C until assayed for antioxidant enzyme activities (Azhar et al., 1995) and protein content (Markwell et al., 1978).
SOD activity assay.
Total SOD activity was measured by the procedure of Spitz and Oberley (1989). In this assay, xanthine‒xanthine oxidase (XO) was used to generate O2•‒ and nitroblue tetrazolium (NBT) reduction was used an indicator of O2•‒ production. SOD competes with NBT for O2•‒; thus, the percentage inhibition of NBT was used as a measure of the amount of SOD present. The incubation mixture also contained catalase to remove H2O2 produced by SOD, and bathocuproine disulfonic acid (BCS) (an electron chain-associated free radical production inhibitor) and diethylenetriaminepentaacetic acid (DETAPAC) to inhibit iron-associated redox cycling and free radical production and bovine serum albumin (BSA) to keep the solution from forming precipitates following the addition of BCS. NaCN, 2 mM was added to inhibit SOD1 activity and measure SOD2 activity. SOD1 activity was calculated from total activity minus activity observed in the presence of 2 mM NaCN. One unit of SOD activity is defined as the amount of enzyme that inhibits the NBT reduction 50% of maximum inhibition. Specific activity is expressed as units per milligram of protein.
Catalase activity assay.
Catalase activity was measured by the method of Aebi (1984) with modifications (Azhar et al., 1995). In brief, granulosa cell extracts were treated with 0.01 volume of 95% of ethanol to decompose complex II. After 30-min incubation at 4 °C, 1% Triton X-100 was added, and subsequently extracts were then diluted with 100 mM phosphate buffer, pH 7.4 before assay. Specific activity is expressed as k units per milligram of protein.
Glutathione peroxidase activity assay.
Glutathione peroxidase activity was assayed according to Lawrence and Burk (1976) using H2O2, reduced glutathione and NADPH. One unit of enzyme activity is defined as the amount of enzyme that catalyzes oxidation 1 mmol of NADPH per minute. Specific activity is expressed as units per minute per milligram of protein.
2.12. Statistical analysis
Data are expressed as means ± SE. The numbers of independent experiments are indicated in the Figure and table legends. Statistical significance was performed using a two-tailed unpaired t-test for dual samples and Analysis of Variance (ANOVA) followed by the Bonferroni Post Test for groups using GraphPad Prism software, Prism 7 (GraphPad Software, Inc., San Diego, CA). P ≤ 0.05 was significant.
3. Results
3.1. Effect of SOD2 deficiency on the activity of granulosa cell SOD2 and other antioxidant enzymes
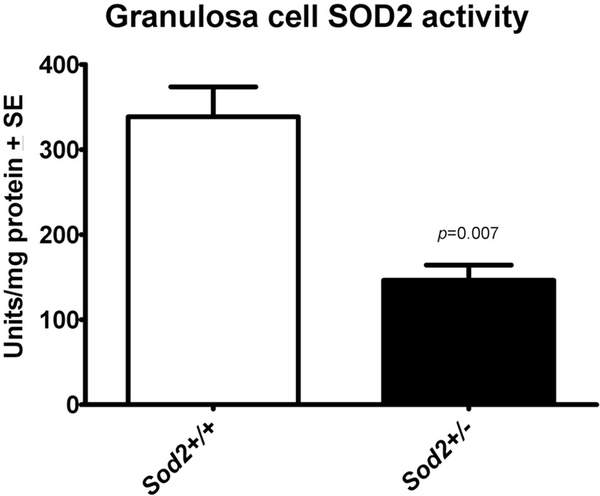
SOD2 activity was decreased close to 50% in granulosa cells isolated from Sod2+/− mice as compared to control (Sod2+/+) mice (Fig. 1). In contrast, SOD2-deficiency had no significant effect on the activity levels of SOD1, catalase or glutathione peroxidase 1 (Table 1).
Fig. 1. Comparison of SOD2 enzyme activity in granulosa cell extracts from WT and Sod2+/− mice.
SOD2 activity was measured as described under ‘Experimental Section’. Results presented are mean ± SEM of 6 independent experiments.
Table 1.
SOD1, catalase and glutathione peroxidase activities in granulosa cells from Sod2+/+ and Sod2+/− mice.
| Antioxidant Enzymes | Sod2+/+ mice | Sod2+/− mice |
|---|---|---|
| SOD1 | 577.8 ± 72.05 | 565.5 ± 86.04 |
| Catalase | 256.0 ± 43.58 | 337.6 ± 87.40 |
| Glutathione peroxidase 1 | 673.8 ± 137.60 | 573.2 ± 112.40 |
Results are expressed as Mean ± SEM (n = 6). Activities are expressed as units/mg protein.
3.2. Impact of SOD2 deficiency on circulating progesterone levels in female mice
To evaluate the effect of SOD2 deficiency on serum progesterone levels, young (2–3 mo of age) female animals were treated with eCG (5 IU/mouse) for 48 h, followed by administration of hCG (5 IU/mouse) for 4 h, blood was collected at 0 time and 4 h post hCG and serum samples analyzed for progesterone levels by RIA. As shown in Table 2, serum progesterone levels were decreased ~50% in Sod2+/− mice both under basal conditions and in response to eCG treatment for 48 h. Serum progesterone levels, however, were further reduced by ~70% in SOD2-deficient mice stimulated with eCG followed hCG.
Table 2.
Effects of eCG and hCG treatment of female WT and Sod2 ± mice on plasma progesterone levels.
| Treatment | WT |
Sod2+/− |
|---|---|---|
| Progesterone (ng/ml) | Mean ± SE | |
| Control (basal) | 4.75 ± 0.621 | 2.217 ± 0.388* |
| eCG (5 IU, sc, 48 h) | 8.32 ± 0.815 | 4.000 ± 0.700¶ |
| eCG (5 IU, sc, 48 h) + hCG (5 IU, 4 h) | 24.82 ± 5.725 | 7.783 ± 1.377§ |
Two to three-month-old WT and Sod2+/− female mice were injected with 5 IU eCG followed 48 h later with 5 IU hCG. Blood was collected from mice at time zero and 4 h after hCG injection and serum progesterone levels were analyzed using RIA. Results are shown as mean ± SE; n = 4 each.
p = 0.0039
p = 0.0061
p = 0.0024.
3.3. SOD2 deficiency-induced alteration in progestin and estradiol production by isolated and cultured granulosa cells
Initially, experiments were carried out to determine the hormone-stimulated and lipoprotein (HDL3)-supported progestin (progesterone + 20α-hydroxyprogesterone) production by cultured granulosa cells from WT, and Sod2+/− mice. Fig. 2 shows progesterone, 20α-hydroxyprogesterone and total progestin production by cultured granulosa cells from WT and Sod2+/− mice under basal conditions and in response to various combinations of FSH (100 ng/ml), Bt2cAMP (2.5 mM) and hHDL3 (500 μg protein/ml). The mean basal progesterone, 20α-hydroxyprogesterone and total progestin production was decreased significantly in Sod2+/− granulosa cells as compared to WT cells. Likewise, FSH, FSH + hHDL3, Bt2AMP and Bt2cAMP + hHDL3 stimulated individual and total progestin production was also reduced significantly albeit to a varying degree in granulosa cells from SOD2 deficient mice relative to WT control.
Fig. 2. Progestin production by primary granulosa cells from WT and Sod2+/− mice.
Granulosa cells from WT and Sod2+/− mice were isolated and cultured as described in Materials and Methods. Granulosa cells were plated at a density of 1 × 105 cells/well and incubated with medium alone, FSH (100 ng/ml), Bt2cAMP (2.5 mM), hHDL3 (500 μg protein/ml), FSH + hHDL3 or Bt2cAMP + hHDL3 for 48 h. Media were collected for analysis of progesterone and 20α-hydroxyprogesterone by specific RIAs. Results are Mean ± SE of four independent experiments. [A] Progesterone; [B] 20α-Hydroxyprogesterone; [C] Total Progestin (Progesterone + 20α-Hydroxyprogesterone). *p < 0.05; **p < 0.01; ***p < 0.001.
To determine whether the ovarian steroidogenic pathway is compromised by SOD2 deficiency-induced oxidative stress, we also measured 22(R) hydroxycholesterol (5-cholestene-3β, 22(R)-diol) supported progestin production. 22(R) hydroxycholesterol is an endogenous metabolic intermediate in the biosynthesis of the steroid hormone from cholesterol and when supplied exogenously, it is freely transported to mitochondria where it is converted to pregnenolone (and subsequently other steroids) with high efficiency by mitochondrial side chain cleavage (P450scc) enzyme activity. The extent of conversion of 22(R) hydroxycholesterol into steroids is indicative of the functional efficiency of the steroidogenic pathway. As shown in Fig. 3, cultured granulosa cells from wild-type mice secreted high levels of progesterone and 20α-hydroxyprogesterone when supplied with 22(R) hydroxycholesterol. However, 22(R) hydroxycholesterol-supported responses were significantly decreased in granulosa cells from Sod2+/− mice. These results suggest that SOD2 deficiency not only leads to inhibition of steroidogenesis, but also negatively impacts the efficiency of the steroidogenic pathway.
Fig. 3. 22(R) Hydroxycholesterol supported progestin production by primary granulosa cells from WT and Sod2+/− mice.
Granulosa cells from WT and Sod2+/− mice were isolated and cultured as described in Materials and Methods. Granulosa cells were plated at a density of 1 × 105 cells/well and incubated with ± 22(R) hydroxycholesterol (20 μM) for 48 h. Media were collected for analysis of progesterone and 20α-hydroxyprogesterone by specific RIAs. Results are Mean ± SE of four independent experiments. *p < 0.05; **p < 0.01.
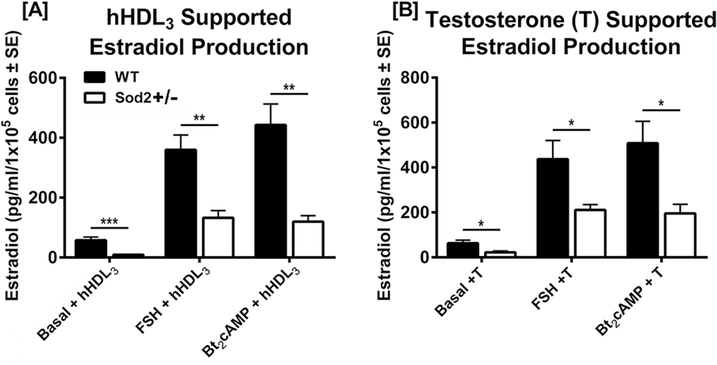
Since granulosa cells actively synthesize and secrete estrogens (Dorrington et al., 1975), we also assessed the effect of SOD2 deficiency on 17β-estradiol (E2) production. WT and Sod2+/− granulosa cells that had been cultured for 72 h in basal medium were washed and re-cultured for an additional 48 h in medium containing ± aromatase (CYP19) substrate (50 nM testosterone), ± hHDL3 (500 μg protein/ml), ± FSH (100 ng/ml) or ± Bt2cAMP (2.5 mM), and E2 levels in the medium samples were measured using an ELISA kit. Basal HDL3-supported E2 production was decreased > 80% in Sod2+/− granulosa cells as compared with WT granulosa cells (Fig. 4A). E2 production by Sod2+/− granulosa cells was also reduced by 60–70% relative to WT cells in response to FSH + hHDL3 or Bt2cAMP + hHDL3. Likewise, testosterone-supported E2 production was significantly reduced in Sod2+/− granulosa cells both under basal conditions and in response to FSH + testosterone or Bt2cAMP + testosterone (Fig. 4B).
Fig. 4. Estradiol production by primary rat granulosa cells from WT and Sod2+/− mice.
Granulosa cells from WT and Sod2+/− mice were isolated and cultured as described in Materials and Methods. Granulosa cells were plated at a density of 1 × 105 cells/well and incubated with hHDL3 (500 μg protein/ml), FSH (100 ng/ml) + hHDL3, or Bt2cAMP (2.5 mM) + hHDL3 for 48 h. Media were collected for analysis of estradiol by using an ELISA kit (Sigma-Aldrich). Results are Mean ± SE of four independent experiments. *p < 0.05; **p < 0.01.
3.4. SOD2-deficiency induced alterations in gene expression of steroidogenic transcription factors, receptors of endocytic and selective cholesterol transport pathways, cholesterogenic enzymes and steroidogenic enzymes and proteins
To examine the possibility that SOD2-deficiency inhibits steroidogenesis by impacting one or more pathways involved in cholesterol acquisition, its intracellular transport and utilization, steroidogenic transcription factors or transcriptional regulation of genes involved in steroid biosynthesis, we used quantitative real-time RT-PCR to measure mRNA levels of Nr0b1, Nr5a1, Nr4a2, Srebf2, Fshr, Lhcgr, Ldlr, Scab1, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, Cyp19a1, Star, Hmgcs1 and Fdft1in WT and Sod2+/− granulosa cells. The mRNA expression of CYP11A1, CYP17, and CYP19 decreased significantly in response to MnSOD deficiency (Fig. 5). The expression of Hsd3b1 and Hsd17b1 steroidogenic enzymes was unchanged, while expression of Star gene decreased significantly in granulosa cells of Sod2+/− mice. In contrast, the expression of genes for steroidogenic transcription factors, DAX-1 (gene Nr0b1), SF-1 (gene Nr5a1), NURR1 (gene Nr4a2), SREBP-2 (gene Srebf2), trophic hormone receptors, FSH-R (gene Fshr), and LH/CG-R (gene Lhcgr), proteins involved in endocytic (LDL Receptor, gene Ldlr) and selective (SRB1, gene Scarb1) CE uptake, and enzymes involved in cholesterol biosynthesis, HMG-CoA synthase (gene Hmgcs1) and squalene synthase (gene Fdft1) was similar in granulosa cells from WT and Sod2+/− mouse granulosa cells.
Fig. 5. Gene expression in ovary of WT and Sod2+/− mice.
Groups of WT and Sod2+/− mice were primed with 5 IU of hCG for 48 h followed by treatment with hCG for 4 h. At the end of hCG treatment, ovaries were collected, RNA extracted and used for analysis of gene expression levels using qRT-PCR. Results are Mean ± SE of four independent experiments. *p < 0.05; **p < 0.01.
3.5. Impaired translocation of cytosolic cholesterol to mitochondria for cholesterol side-chain cleavage (P450scc) in ovaries of Sod2+/− mice
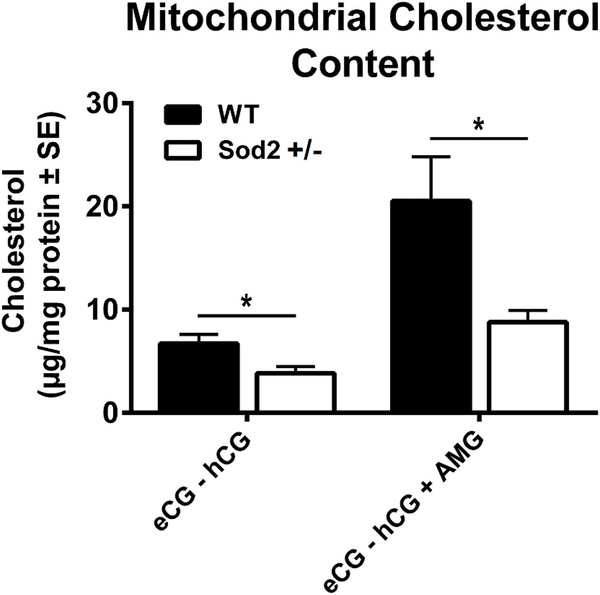
Because StAR mediates rate-limiting transfer of cholesterol from OMM to IMM P450scc sites for mitochondrial steroidogenesis and its expression is downregulated in eCG-hCG primed Sod2+/− mice, this raised the possibility that an inadequate amount of cholesterol is transported to mitochondria for CYP11A1 (side-chain cleavage [P450scc] enzyme) catalyzed conversion of cholesterol to the steroid precursor, pregnenolone. To address this issue, we measured total cholesterol content in ovarian mitochondrial preparations from WT and Sod2+/− mice pre-treated with eCG-hCG or eCG-hCG + AMG. As shown in Fig. 6, ovarian mitochondrial cholesterol content was decreased significantly in eCG primed and hCG stimulated (eCG-hCG) Sod2+/− mice as compared to similarly treated WT mice. Such decreases in ovarian mitochondrial cholesterol content in Sod2+/− mice were further exaggerated when steroidogenesis was blocked by treatment of mice with aminoglutethimide (AMG). In contrast, total cholesterol levels were comparable in ovaries of WT and Sod2+/− mice (data not shown).
Fig. 6. Transport of mobilized cytosolic cholesterol to mitochondria in ovaries of WT and Sod2+/− mice.
Groups of WT and Sod2+/− mice were primed with eCG for 48 h and subsequently treated with aminoglutethimide for 6 h and hCG for 4 h before the animals were euthanized. Ovaries were collected, mitochondria isolated by differential centrifugation and cholesterol content quantified. Results are Mean ± SE of four independent experiments. *p < 0.05.
3.6. HDL-CE-selective uptake in WT and Sod2+/− mouse granulosa cells
Since SOD2-deficiency leads to impaired ovarian HDL3-supported steroidogenesis and decreased cholesterol availability for pregnenolone production in mitochondria, we next determined whether altered selective delivery of HDL-CE in Sod2+/− mouse granulosa cells could account for such changes. [SR-BI-mediated selective uptake of HDL-CE provides the bulk of the cholesterol needed for steroidogenesis in the steroidogenic cells of the rodent adrenal gland and gonads]. For these studies, groups of dishes with growing WT and Sod2+/− granulosa cells were treated with either FSH (100 ng/ml) or Bt2cAMP (2.5 mM) for 24 h and subsequently, incubated with [125I]DLT-[3H]COE-hHDL3 in the presence of a respective hormone for an additional 24 h. The dishes were then processed for the quantification of HDL-CE delivered to cells via the SRB1-mediated selective pathway. As shown in Table 2, both WT and Sod2+/− granulosa cells internalized a comparable amount of selectively internalized HDL-CE in response to either FSH or Bt2cAMP stimulation. These results led us to conclude that SOD2 deficiency has no discernible effect on SRB1-mediated selective HDL-CE uptake in mouse granulosa cells.
3.7. SOD2 deficiency and basal (endogenous) and pro-oxidant induced ovarian membrane lipid peroxidation
TBARS (thiobarbituric acid-reactive substances) measurements were made to assess the extent of lipid peroxidation (oxidative/peroxidative damage), a most commonly employed index of oxidative stress, in ovarian membrane preparations from WT and Sod2+/− mice. These measurements were made both under basal conditions and in response to an enzymatic (Fe3+/ADP/NADPH) or non-enzymatic (Fe2+/ascorbate) lipid peroxidation initiator (Abidi et al., 2004). While the TBAR technique has some limitations (Devasagayam et al., 2003), it was employed to simply quantify oxidative damage that may be occurring in SOD2 deficient ovaries. The results presented in Table 4 indicate that ovarian membranes from WT animals contained only minimal levels of endogenous TBARS, but these levels increased roughly 2.5-fold in membrane preparations from Sod2+/− mice. Exposure of control membranes to either enzymatic or non-enzymatic prooxidants enhanced lipid peroxidation by approximately 5-fold and this peroxidation was roughly doubled in membranes from Sod2+/− mice (Table 3).
Table 4.
Basal, enzymatic and non-enzymatic lipid peroxidation in ovarian membranes from eCG-hCG primed WT and Sod2+/− mice.
| Lipid Peroxidation | WT |
Sod2+/− |
|---|---|---|
| TBARS (nmol MDA produced/mg protein/h) Mean ± SE | ||
| Basal | 2.787 ± 0.5326 | 7.049 ± 1.277** |
| Enzymatic (Fe3+/ADP/NADPH) | 14.86 ± 2.854 | 25.33 ± 2.531* |
| Non-enzymatic (Fe2+/Ascorbate) | 16.23 ± 3.246 | 32.23 ± 4.771§ |
Membranes (200 μg of protein) from WT or Sod2+/− mouse ovaries were incubated at 37 °C for 40 min with 40 mM Tris-maleate buffer, pH 7.4 alone (basal), FeCl3 (50 μM) + ADP (4 mM) + NADPH (0.4 mM) or FeSO4 (10 μM) + ADP (1 mM) + ascorbate (0.5 mM) in a total volume of 0. 5 ml. At the end of incubation, MDA levels were determined by the using the thiobarbituric acid assay (TBA) of Beuege & Aust (1978). Results are Mean ± SE of 4 separate experiments (n = 4).
p = 0.0334
p = 0.0216
p = 0.0322.
Table 3.
Selective uptake of hHDL3-derived CE by granulosa cells from WT and Sod2 ± mice in response to FSH or Bt2cAMP stimulation.
| Treatment | WT |
Sod2+/− |
|---|---|---|
| Selective HDL-CE uptake (ng/105 cells/24 h) Mean ± SE | ||
| FSH (100 ng/ml) | 6791 ± 1296 | 6801 ± 1523 |
| Bt2cAMP (2.5 mM) | 8423 ± 1300 | 7905 ± 1070 |
Seventy two hours cultured granulosa cells from WT and Sod2+/- mice were treated with FSH (100 ng/ml) or Bt2cAMP (2.5 mM) for 24 h and then replaced with respective medium containing fresh FSH (100 ng/ml) or Bt2cAMP (2.5 mM) and [125I]DLT-[3H]COE-hHDL3 (100 μg protein/ml) for an additional 24 h. At the end of incubation, the dishes were processed for the determination 125I and 3H radioactivity and calculation of amount of HDL-CE selectively taken up by cells. Results are mean ± SE of four separate determinations.
4. Discussion
SOD2 is a critical antioxidant enzyme, which is located in the matrix of mitochondria (Candas and Li, 2014; Fukai and Ushio-Fukai, 2011; Wang et al., 2018), protecting macromolecules, proteins, lipids and DNA against oxidative damage. It catalyzes the dismutation of superoxide anion to hydrogen peroxide, which is quickly converted to water by the mitochondrial GPX-1 and GPX-4 (Ribas et al., 2014) or PRXIII (Rhee and Kil, 2016). Interestingly PRXIII, a typical 2-Cys peroxiredoxin located exclusively in the mitochondrial matrix, is the principal peroxidase, especially in steroidogenic cells, responsible for metabolizing mitochondrial H2O2 (Kil et al., 2012; Rhee and Kil, 2016). Mitochondrial electron transport chains I and III are the major sites of superoxide production as a result of electron leakage during oxidative phosphorylation (Murphy, 2008; Turrens, 2003) and in steroidogenic cells as a by-product of enzymatic reactions of mitochondrial P450 systems (Hanukoglu, 2006). While Sod2−/− mice are neonatal lethal (Huang et al., 2001), the heterozygous mice with a partial SOD2 deficiency (Sod2 ± mice) exhibit impaired mitochondrial enzyme activity and respiration (Williams et al., 1998), increased mitochondrial oxidative stress, age-related decline in mitochondrial function and apoptosis, oxidative damage to mitochondria, and increased seizure susceptibility (Kokoszka et al., 2001; Strassburger et al., 2005; Liang and Patel, 2004). In contrast, overexpression of SOD2 in transgenic mice has been shown to provide protection against oxidative stress in various tissues (Kawak et al., 2015).
Several studies have shown that ROS-induced excessive oxidative stress exerts a negative effect on steroidogenesis, for example, exposure of primary rat luteal cells, human granulosa luteal cells and other model steroidogenic cell lines to ROS leads to a robust inhibition of steroid hormone production. Studies carried out by Behrman and colleagues demonstrated that exposure of isolated and cultured primary rat granulosa cells to hydrogen peroxide caused inhibition of steroidogenesis in response to LH, cholera toxin, forskolin and 8-bromo-cAMP (Margolin et al., 1990). Follow-up studies demonstrated that treatment of rat luteal cells with superoxide and hydrogen peroxide (generated by hypoxanthine and xanthine oxidase) (Gatzuli et al., 1991), lipid hydroperoxide (cumene hydroperoxide, hydroperoxyeicosatetraenoic acid) (Kodaman et al., 1994) or hydrogen peroxide (Musicki et al., 1994) resulted in a significant inhibition of LH-stimulated progesterone production. Such anti-gonadotropic and anti-steroidogenic actions of superoxide anion, lipid hydroperoxide and hydrogen peroxide, however, were independent of their effects on LH receptor activity or LH-stimulated cAMP production. It was further demonstrated that hydrogen peroxide inhibits rat luteal cell steroidogenesis by blocking intracellular transport of cholesterol to mitochondria or translocation of cholesterol across the outer mitochondrial membrane (Behrman and Aten, 1991). Studies by Sawada and Carlson (1996), however, had provided evidence that in situ generated superoxide anion (xanthine/xanthine oxidase) exerts a biphasic effect on progesterone production in isolated rat luteal cells: small increases in superoxide levels were associated with stimulation of progesterone production, whereas high levels were associated with a significant reduction in the extent of progesterone synthesis and secretion. Additional studies demonstrated that hydrogen peroxide also exerts anti-gonadotropic and anti-steroidogenic actions in human granulosa luteal cells (Endo et al., 1993). However, in these cells, it was shown that hydrogen peroxide not only inhibited progesterone and estrogen synthesis, but also interfered with gonadotropin-stimulated cAMP production. In addition, hydrogen peroxide also inhibited key steroidogenic enzymes including CYP11A1, HSD3B1, CYP19A1 and HSD17B1. Likewise, treatment of either LH- or cAMP analog Bt2cAMP-stimulated MA-10 mouse Leydig tumor cells with hydrogen peroxide resulted in a dose dependent inhibition of progesterone production. Hydrogen peroxide inhibited steroidogenesis primarily by inhibiting Hsd3b1 activity but may also have inhibitory effects on protein synthesis and cholesterol transport to mitochondria for side chain cleavage (Stocco et al., 1993). In contrast, Diemer et al. (2003) demonstrated that both superoxide anion (xanthine/xanthine oxidase generated) and hydrogen peroxide inhibit progesterone production in MA-10 Leydig cells by selectively inhibiting steroidogenic acute regulatory protein (StAR) expression and its mitochondrial import and processing. We previously demonstrated that treatment of mouse Y1-BS1 adrenocortical tumor cells with in situ generated superoxide anion, hydrogen peroxide or lipid peroxidation product 4-hydroxy-2-nonenal (HNE) significantly inhibited steroid production and that such inhibitory effect was mediated by oxidant-stimulated p38 MAPK (Abidi et al., 2008). Furthermore, pretreatment of Y1-BS1 cells with MnTMPyP, a cell permeable superoxide-dismutase/catalase mimetic ROS scavenger, completely prevented the superoxide anion and hydrogen peroxide induced inhibition of steroid production. Likewise, the antioxidant N-acetylcysteine completely blocked the HNE-induced loss of steroidogenic response. These later studies further confirmed the inhibitory effects of ROS-induced excessive oxidative stress on steroid synthesis.
While the above studies establish a direct link between ROS-induced excessive oxidative stress and inhibition of steroidogenesis using primary ovarian steroidogenic cells or model steroidogenic cell lines and specific ROS species under in vitro conditions, to our knowledge the finding that excessive oxidative stress leads to inhibition of steroidogenesis under a pathophysiological setting in vivo has not previously been reported. The present study demonstrates that oxidative stress induced by genetic deletion of mitochondrial Sod2 inhibits steroid secretion both in intact mice in vivo (progesterone) and in isolated mouse granulosa cells in vitro (progesterone, 20α-hydroxyprogesterone and estradiol). More specifically, we observed that SOD2 deficiency inhibits progesterone secretion in vivo under basal conditions, in eCG primed, as well as eCG primed and hCG-stimulated mice. Likewise, isolated MnSOD-deficient granulosa cells secreted significantly less progestin both under basal conditions and in response to hHDL3 and/or Bt2cAMP stimulation. Similarly, granulosa cells isolated from Sod2+/− mice exhibited attenuation of estradiol production under basal conditions and in response to hormonal and/or substrate stimulation. Studies into the possible underlying mechanisms examined FSH or forskolin stimulation of cAMP production and selective uptake of HDL3-derived cholesteryl esters by the cultured granulosa cells and in vivo transport of hormone-stimulated cholesterol in mitochondria of ovaries. We show there are no defects in hormone-stimulated cAMP production. Thus, the attenuation of steroidogenesis in SOD2-deficient granulosa cells does not appear due to impaired cAMP-cAMP-dependent protein kinase (PKA) signaling cascade. When delivery of HDL3-cholesteryl esters to the granulosa cells was assayed using doubly labeled lipoprotein particles, selective delivery of CE was similar in WT and Sod2+/− granulosa cells. Taken together, the observations that uptake of lipoprotein derived cholesterol in granulosa cells of WT and Sod2+/− mice and that total ovarian cholesterol is not different suggest that the defect in steroidogenesis is at the level of mitochondria, with either a defect in cholesterol transport to the mitochondria, or a defect in mitochondrial processing of cholesterol. Quantitative real-time measurement of genes involved in cholesterol uptake, synthesis and processing in mitochondria showed no significant difference between WT and Sod2+/− mice except for Cyp11a1 and Star genes. The demonstration that mitochondrial cholesterol content was markedly lower in ovaries of Sod2+/− mice with or without inhibition of CYP11A1 by treatment of mice with aminoglutethimide suggests that the defect resides in the transport of cholesterol from its site of mobilization in cytoplasm to mitochondria. It should be noted that mRNA expression of StAR was also decreased and raised the possibility that transfer of cholesterol from the outer mitochondrial membrane (OMM) to the inner mitochondrial membrane (IMM) for side-chain cleavage activity and pregnenolone production may also be impaired.
Our results also demonstrated that steroid production was not only reduced in Sod2+/− granulosa cells treated with FSH or Bt2cAMP but also when cells were treated with freely diffusible 22(R) hydroxycholesterol as a cholesterol substrate for progestin production or treated with testosterone for estradiol production. These results led us to conclude that inhibition of both progestin and estradiol production in response to SOD2-deficiency may also be due to inhibition of one or more steroidogenic enzymes. This conclusion is in keeping with our quantitative RT-PCR data showing a significant reduction in mRNA levels of CYP11A1, CYP17 and CYP19. The latter results are also in agreement with the previous in vitro studies showing that oxidant treatment of steroidogenic cells leads to inhibition of one or more steroidogenic enzymes (Endo et al., 1993; Stocco et al., 1993).
In summary, the present result show for the first time that deficiency of mitochondrial SOD is accompanied by excessive oxidative stress and inhibition of progestin and estradiol production in ovarian granulosa cells. The inhibitory actions of SOD2 deficiency-induced oxidative stress on granulosa cell steroidogenesis is characterized by downregulation of StAR expression, suppression of tropic hormone stimulated cholesterol transport to mitochondria for side chain cleavage (P450scc), and inhibition of CYP P450 enzymes involved in progesterone/20α-hydroxyprogesterone and estradiol production. The inhibition of progestin synthesis by SOD2-deficiency occurred under both basal and hormone-stimulated ± hHDL3-supported conditions. Likewise, reduced Sod2 expression resulted in significant inhibition of estradiol production under basal conditions and in response to hHDL3 without or with FSH/Bt2cAMP treatments. SOD2 deficiency, however, had no significant effect on FSH or forskolin-stimulated cAMP production and on selective HDL-CE uptake.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health grant P30DK116074 (FBK), and by Merit Review Awards #I01BX001923 (SA), #I01BX000398 (FBK), and 1BX004487-01A1(TTH), and Senior Research Career Scientist Award # IK6B004200 (SA) from the United States Department of Veterans Affairs, Biomedical Laboratory Research Development Program and Department of Defense the Gulf War Illness Research Program #W81XWH-17-2-0025(TTH).
Abbreviations:
- ADP
adenosine diphosphate
- AMG
aminoglutethimide
- Bt2cAMP
N6, 2′-dibutyryladenosine 3′5′-cyclic monophosphate
- COX
Cytochrome c oxidase
- CS
citrate synthase
- CYP11A1
Cytochrome P450 Family 11 Subfamily a, Member 1
- CYP17A1
Cytochrome P450 Family 17 Subfamily a, Member 1
- Cyp11a1 gene
protein coding gene of CYP11A1
- Cyp17a1 gene
protein coding gene of CYP17A1
- [125I]DLT
[125I]-labeled dilactitol tyramine
- E2
estradiol
- eCG
equine chorionic gonadotropin
- FeCl3
iron(III) chloride or ferric chloride
- FeSO4
iron(II) sulfate or ferrous sulfate
- FSH
follicle-stimulating hormone
- [3H]COE
[3H] cholesteryl oleolyl ether
- GPX-1
glutathione peroxidase 1
- hCG
human chorionic gonadotropin
- IBMX
3-isobutyl-1-methylxanthine
- IMM
inner mitochondrial membrane
- MDA
malondialdehyde
- OMM
outer mitochondrial membrane
- P450scc ((CYP11A1)
cholesterol side chain cleavage enzyme, mitochondrial
- Progestin
progesterone + 20α-hydroxy-progesterone
- SOD1
superoxide dismutase 1, also called copper, zinc superoxide dismutase (Cu,Zn-SOD), Sod2+/−, heterozygous SOD2 knockout mice
- SOD2
superoxide dismutase 2, also called manganese superoxide dismutase (Mn-SOD)
- ROS
reactive oxygen species
- StAR
steroidogenic acute regulatory protein
- Star gene
protein coding gene of StAR
- T
testosterone
- TBA
thiobarbituric acid
- TCA
trichloroacetic acid
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mce.2020.110888.
References
- Abidi P, Leers-Sucheta S, Azhar S, 2004. Suppression of steroidogenesis and activator protein-1 transcription factor activity in rat adrenals by vitamin E deficiency‒induced chronic oxidative stress. J. Nutr. Biochem 15, 210–219. 10.1016/j.jnutbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Abidi P, Zhang H, Zaidi SM, Shen W-J, Leers-Sucheta S, Cortez Y, Han J, Azhar S, 2008. Oxidative stress-induced inhibition of adrenal steroidogenesis requires participation of p38 mitogen-activated protein kinase signaling pathway. J. Endocrinol 198, 193–207. 10.1677/JOE-07-0570. [DOI] [PubMed] [Google Scholar]
- Aebi H, 1984. Catalase in vitro. Methods Enzymol. 105, 121–126. 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sikka S, 2006. The role of free radicals and antioxidants in reproduction. Curr. Opin. Obstet. Gynecol 18, 325–332. 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- Aronson NA Jr., Touster O, 1974. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 31, 90–102. 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Auten RL, Davis JM, 2009. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res 66, 121–127. 10.1203/PDR.0b013e3181a9eath. [DOI] [PubMed] [Google Scholar]
- Ávila J, González-Fernández, Rotoli D, Hernández J, Palumbo A, 2016. Oxidative stress in granulosa-lutein cells from in vitro fertilization patient. Reprod. Sci 23, 1656–1661. 10.1177/1933719116674000. [DOI] [PubMed] [Google Scholar]
- Azhar S, Cao L, Reaven E, 1995. Alteration of the adrenal antioxidant system during aging in rats. J. Clin. Invest 96, 1414–1424. 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar S, Tsai L, Maffe W, Reaven E, 1988. Cultivation of rat granulosa in a serum-free chemically defined medium—a useful model to study lipoprotein metabolism. Biochim. Biophys. Acta 963, 139–150. 10.1016/ooo5-2760(88)90275-5. [DOI] [PubMed] [Google Scholar]
- Azhar S, Tsai L, Reaven E, 1990. Uptake and utilization of lipoprotein cholesteryl esters by rat granulosa cells. Biochim. Biophys. Acta 1047, 148–160. 10.1016/0005-2760(90)90041-U. [DOI] [PubMed] [Google Scholar]
- Barnham K, Masters C, Bush A, 2004. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov 3, 205–214. 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Aten RF, 1991. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology 128, 2958–2966. 10.1210/endo-128-6-2958. [DOI] [PubMed] [Google Scholar]
- Bisht S, Faiq M, Tolahunase M, Dada R, 2017. Oxidative stress and male infertility. Nat. Rev. Urol 14, 470–485. 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE, 2014. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev 94, 329–354. 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O, 2012. Oxidative stress and antioxidant defense. World Allergy Organ J. 5, 9–19. 10.1097/WOX0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD, 1978. Microsomal lipid peroxidation. Methods Enzymol 52, 302–310. 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S, 2004. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J. Steroid Biochem. Mol. Biol 88, 61–67. 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Candas D, Li JJ, 2014. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxidants Redox Signal. 20, 1599–1617. 10.1089/ars.2013.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN, 2007. Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim. Biophys. Acta 1773, 93–104. 10.1016/j.bbamer.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Circu ML, Aw TY, 2010. Reactive oxygen species, cellular redox system and apoptosis. Free Radic. Biol. Med 48, 749–762. 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corniola R, Zou Y, Leu D, Fike JR, Huang T-T, 2012. Paradoxical relationship between Mn superoxide dismutase deficiency and radiation-induced cognitive defects. PloS One 7, e49367. 10.1371/journal.pone.0049367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Allen JA, Hales KH, Hales DB, 2003. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 144, 2882–2891. 10.1210/en.2002-0090. [DOI] [PubMed] [Google Scholar]
- Dorrington JH, Moon YS, Armstrong DT, 1975. Estraduiol-17β biosynthesis in cultured granulosa cells from hypophysectomized immature rats; stimulation by follicle stimulating hormone. Endocrinology 97, 1328–1331. [DOI] [PubMed] [Google Scholar]
- Devasagayam TP, Boloor KK, Ramasarma T, 2003. Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J. Biochem. Biophys 40, 300–308. [PubMed] [Google Scholar]
- Dröge W, 2002. Free radicals in the physiological control of cell function. Physiol. Rev 82, 47–95. 10.1152/physerv.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duhig K, Chappell LC, Shennan AH, 2016. Oxidative stress in pregnancy and reproduction. Obstet. Med 9, 113–116. 10.1177/1753495X16648495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Aten RF, Leykin L, Behrman HR, 1993. Hydrogen peroxide evokes anti-steroidogenic and antigonadotropic actions in human granulosa luteal cells. J. Clin. Endocrinol. Metab 76, 337–342. 10.1210/jcem.76.2.7679398. [DOI] [PubMed] [Google Scholar]
- Finkel T, 2011. Signal transduction by reactive oxygen species. J. Cell Biol 194, 7–15. www.jcb.org/cgi/doi/10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK, 2018. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res 122, 877–902. 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyouzi N, Cai Z, Sugimoto Y, Stocco C, 2005. Changes in the expression of steroidogenic and antioxidant genes in the mouse corpus luteum during luteolysis. Biol. Reprod 72, 1134–1141. 10.1095/biolreprod.104.037598. [DOI] [PubMed] [Google Scholar]
- Fukai T, Ushio-Fukai M, 2011. Superoxide dismutases: role in redox signaling, vascular function and diseases. Antioxidants Redox Signal. 15, 1583–1606. 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzuli E, Aten R, Behrman HR, 1991. Inhibition of gonadotropin action and progesterone synthesis by xanthine oxidase in rat luteal cells. Endocrinology 128, 2253–2258. 10.1210/endo-128-5-2253. [DOI] [PubMed] [Google Scholar]
- Glick D, Fell BR, Sjølin K-E, 1964. Spectrophotometric determination of nanogram amounts of total cholesterol in microgram quantities of tissue or microliter volumes of serum. Anal. Chem 36, 1119–1121. 10.1021/ac60212a050. [DOI] [Google Scholar]
- Hanukoglu I, 2006. Antioxidative protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab. Rev 38, 171–196. 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Hauck AK, Bernlohr DA, 2016. Oxidative stress and lipotoxicity. J. Lipid Res 57, 1976–1986. 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock JC, Rainey WE, Carr BR, 2004. Ovarian granulosa cell lines. Mol. Cell. Endocrinol 228, 67–78. https://10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- He L, He T, Farrar S, Ji L, Liu T, Ma X, 2017. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem 44, 532–553. 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- Huang TT, Carlson EJ, Kozy HM, Mantha S, Goodman SI, Ursell PC, Epstein CJ, 2001. Genetic modification of prenatal lethality and dilated cardiomyopathy in Mn superoxide dismutase mutant mice. Free Radic. Biol. Med 31, 1101–1110. 10.1016/s0891-5849(01)00694-3. [DOI] [PubMed] [Google Scholar]
- Johansson LH, Borg LA, 1988. A spectrophotometric method for the determination of catalase activity in small tissue samples. Anal. Biochem 174, 331–336. 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- Kawak H-B, Lee Y, Kim JH, Van Remmen H, Richardson AG, Lawler JM, 2015. MnSOD overexpression reduces fibrosis and proapoptotic signal in the aging mouse heart. J. Gerontol. A Biol. Sci. Med. Sci 70, 533–544. 10.1093/gerona/glu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil IS, Lee SK, Ryu KW, Woo HA, Hu M-C, Bae SH, Rhee SG, 2012. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Mol. Cell 46, 584–594. 10.1016/j.molcel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Kodaman PH, Aten RF, Behrman HR, 1994. Lipid hydroperoxides evoke anti-gonadotropic and antisteroidogenic activity in rat luteal cells. Endocrinology 135, 2723–2730. 10.1210/endo.135.6.7988463. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A, 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30, 620–650. 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kokoszka JE, Coskin P, Espossito LA, Wallace DE, 2001. Increased mitochondrial oxidative stress in the Sod2 (+/−) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc. Natl. Acad. Sci. USA 98, 2278–2283. 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumova K, Cosa G, 2016. Overview of Reactive Oxygen Species. Chapter 1. Overview of Reactive Oxygen Species in Singlet Oxygen: Application in Biosciences and Neurosciences, vol. 1. pp. 1–21. 10.1039/9781782622208-00001. eISBN: 978=1–78262-220–8. From Book Series: Comprehensive series in Photochemical and Photobiological Sciences. [DOI] [Google Scholar]
- Lawrence RA, Burk RF, 1976. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 71, 952–958. 10.1016/0006-291x(76)90746-6. [DOI] [PubMed] [Google Scholar]
- Liang L-P, Patel M, 2004. Mitochondrial oxidative stress and increased seizure susceptibility in Sodd2−/+ mice. Free Radical Biol. Med. 36, 542–554. 10.1016/j.freeradbiomed.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Liguori I, Russo G, Curcio F, Bulli G, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P, 2018. Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 913–927. 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer U, 2014. Ovarian toxicity from reactive oxygen species. Vitam. Horm 94, 99–127. 10.1016/B978-0-12-800095-3.00004-3. [DOI] [PubMed] [Google Scholar]
- Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, Kazeroni M, 2020. Effect of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J. Steroid Biochem. Mol. Biol 197, 105521. 10.1016/j.jsbmb.2019.105521. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz LM, 1998. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139, 4008–4011. 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Margolin Y, Aten RF, Behrman HR, 1990. Antigonadotropic and antisteroidogenic actions of peroxide in rat granulosa cells. Endocrinology 127, 245–250. 10.1210/endo-127-1-245. [DOI] [PubMed] [Google Scholar]
- Markwell MAK, Hass SM, Bieber LL, Tolbert NE, 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem 87, 206–210. 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic-Haberle I, 2012. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 1822, 794–814. 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, 2008. How mitochondria produce reactive oxygen species. Biochem. J 417, 1–13. 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musicki B, Aten RF, Behrman HR, 1994. Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology 13434, 588–595. 10.1210/endo.134.2.7507829. [DOI] [PubMed] [Google Scholar]
- Pisoschi AM, Pop A, 2015. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem 97, 55–74. 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Powis G, Montfort W, 2001. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct 30, 421–455. 10.1158/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- Ray PD, Huang B-W, Tsuji Y, 2012. Oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal 24, 981–990. 10.1016/j.cellsig.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E, Lua Y, Nomoto A, Temel R, Williams DL, van der Westhuyzen DR, Azhar S, 1999. The selective pathway and a high-density lipoprotein receptor (SR-BI) in ovarian granulosa cells of the mouse. Biochim. Biophys. Acta 1436, 565–576. 10.1016/s0005-2760(98)00169-6. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kil IS, 2016. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic. Biol. Med 99, 120–127. 10.1016/j.freeradiomed.2016.07.029. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kil IS, 2017. Multiple functions and regulation of mammalian peroxiredoxins. Annu. Rev. Biochem 86, 749–775. 10.1146/annurev-biochem-060815-014431. [DOI] [PubMed] [Google Scholar]
- Ribas V, García-Ruiz G, Fernández-Checa JC, 2014. Glutathione and mitochondria. Front. Pharmacol 5, 151. 10.3389/fpharm.201400151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Carlson JC, 1996. Intracellular regulation of progesterone secretion by the superoxide radical in rat corpus luteum. Endocrinology 137, 1580–1584. 10.1210/endo.137.5.8612488. [DOI] [PubMed] [Google Scholar]
- Schieber M, Chandel S, 2014. ROS function in redox signaling and oxidative stress. Curr. Biol 24, R453–R462. 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K, Sugino N, Yoshida Y, Nakamura K, Ogino K, Kato H, 1995. Changes in lipid peroxide and antioxidant enzyme activities in corpora lutea during pseudopregnancy in rats. J. Reprod. Fertil 105, 2530257. 10.1530/jrf.0.1050253. [DOI] [PubMed] [Google Scholar]
- Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, Dekel N, 2011. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. U.S.A 108, 1462–1467. www.pnas.org/cgi/doi/10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW, 1989. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem 179, 8–18. 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Srere PA, 1969. Citrate synthase: [EC 4.1.3.7. Citrate oxaloacetate-lyase (CoA-acelyting)]. Methods Enzymol. 13, 3–11. 10.1016/0076-6879(69)12005-0. [DOI] [Google Scholar]
- Stocco DM, Wells J, Clark BJ, 1993. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig Tumor cells. Endocrinology 133, 2827–2832. 10.1210/endo.133.6.8243310. [DOI] [PubMed] [Google Scholar]
- Strassburger M, Bloch W, Sulyok S, Schüller J, Keist AF, Schmidt A, Wenk J, Peters T, Wlaschek M, Lenart J, Krieg T, Hafner M, Kümin A, Werner S, Müller W, Scharffetter-Kochanek K, 2005. Heterozygous deficiency of mammalian superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium in vivo. Free Radic. Biol 38, 1458–1470. 10.1016/j.freeradbiomed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Sugino H, 2005. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol 4, 41–44. 10.1111/j.1447-0578.2005.00085x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino H, Nakamura Y, Takeda O, Ishimatsu M, Kato H, 1993. Changes in activities of superoxide dismutase and lipid peroxide incorpus luteum during pregnancy in rats. J. Reprod. Fertil 97, 347–351. 10.1530/jrf.0970347. [DOI] [PubMed] [Google Scholar]
- Sugino N, Telleria CM, Gibori G, 1998. Differential regulation of copper-zinc superoxide dismutase in the rat corpus luteum: induction of manganese superoxide dismutase messenger ribonucleic acid by inflammatory cytokines. Biol. Reprod 59, 208–215. 10.1095/biolreprod59.1.208. [DOI] [PubMed] [Google Scholar]
- Suzuki T, sugino N, fukaya T, Sugiyama U, Uda T, Takaya R, Yajima A, Sasano H, 1999. Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization. Fertil. Steril 72, 720–726. 10.1016/s0015-0282(99)00332-5. [DOI] [PubMed] [Google Scholar]
- Tamate K, Sengoku K, Ishikawa M, 1995. The role of superoxide dismutase in the human ovary and fallopian tube. J. Obstet. Gynecol 21, 401–409. 10.1111/j.1447-0756.1995.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Turrens JF, 2003. Mitochondrial formation of reactive oxygen species. J. Physiol 552 (2), 335–344. 10.1113/physiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, Amicarelli F, 2006. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in granulosa cells. Mol. Reprod 12, 655–660. 10.1093/mderhr/gal080. [DOI] [PubMed] [Google Scholar]
- Tulkens P, Beaufy H, Trouet A, 1974. Analytical fractionation of homogenates from cultured rat embryo fibroblasts. J. Cell Biol 63, 383–401. 10.1083/jcb.63.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B, Santa-Gonzalez GA, Camargo M, 2018. DNA repair after oxidative stress: current challenges. Curr. Opin. Toxicol 7, 9–16. 10.1016/j.cotox.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H, Salvador C, Yang H, Huang T-T, Epstein CJ, Richardson A, 1999. Characterization of the antioxidant status of the heterozygous manganese superoxide dismutase knoc+/−ut mouse. Arch. Biochem. Biophys 363, 91–97. 10.1006/abbi.1998.1060. [DOI] [PubMed] [Google Scholar]
- Vitale G, Salvioli S, Franceschi G, 2013. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol 9, 228–240. 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- Wang Y, Branicky R, Noë A, Hekimi S, 2018. Superoxide dismutases: dual roles in controlling ROS damage and regulating signaling. J. Cell Biol 217, 1915–1928. 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton DC, Tzagoloff A, 1967. Cytochrome oxidase from beef heart mitochondria. Methods Enzymol. 10, 245–250. 10.1016/0076-6879(67)0048-7. [DOI] [Google Scholar]
- Williams MD, Van Remmen H, Conrad CC, Huang TT, Epstein CJ, Richardson A, 1998. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem 273, 28510–28515. 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC, 2008. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol 4, 278–286. 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Shen W-J, Bittner S, Bittner A, McLean NP, Han J, Davis RJ, Kraemer FB, Azhar S, 2014. p38 MAPK regulates steroidogenesis through transcriptional repression of StAR gene. J. Mol. Endocrinol 53, 1–16. 10.1530/JME-13-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.