Abstract
AIM
To assess the role of a severity score based on chest radiography (CXR) in predicting the risk of adverse outcomes in coronavirus disease 2019 (COVID-19).
MATERIALS AND METHODS
Of the patients who presented to L. Sacco Hospital (Milan, Italy) between 21 February and 31 March 2020, patients with a laboratory confirmation of COVID-19 who also underwent a CXR were included in the study. To quantify the extent of lung involvement, each CXR image was given a score (Milan score), ranging from 0 to 24, depending on the presence of reticular pattern and/or ground-glass opacities and/or extensive consolidations in each of the 12 areas in which the lungs were divided. The score was calculated by an expert radiologist, blinded to laboratory tests. The ability of the Milan score to predict hospital admission and mortality, after adjusting for some variables (age; gender; comorbidities; time between symptoms onset and admission), using univariate and multivariate statistical analysis was investigated retrospectively.
RESULTS
Among the 554 patients, 115 of which (21%) had a negative CXR, the in-hospital mortality was 16% (90/554). At univariate analysis, age, gender, and comorbidities were significant predictors of mortality and hospital admission. At multivariate analysis, adjusting for age and gender, the Milan score was an independent predictor of mortality and hospitalisation. In particular, patients with a Milan score ≥ 9 had a mortality risk five-times higher than those with a lower score. Other independent predictors of mortality were gender and age.
CONCLUSIONS
The CXR Milan score was an independent predictive factor of both in-hospital mortality and hospital admission.
Introduction
Since the first pneumonia cases related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 2019 in Wuhan, Hubei, China, the world has seen a rapidly increasing number of patients suffering from coronavirus disease 2019 (COVID-19), soon reaching pandemic status. Despite immense effort, the infection is still spreading at high rates worldwide, with many countries facing a second wave.
The reference standard for the diagnosis of SARS-CoV-2 infection is a positive result of real-time reverse transcriptase polymerase chain reaction (RT-PCR) on naso-oropharyngeal swab samples; however, chest imaging also plays a crucial role as an adjunct diagnostic tool, especially in settings where RT-PCR results are not readily available1, 2, 3, 4; moreover, it may help guide decisions about patient management.1 , 2 , 4 Computed tomography (CT) has proven to be more sensitive than chest radiography (CXR) in detecting imaging features of COVID-19,5 , 6 but it carries several disadvantages (e.g., high costs; high risk of cross-infection; low specificity; availability). For these reasons, many radiological professional organisations have advised against the adoption of CT as a screening tool,3 , 4 , 7 and have encouraged the use of portable CXR, which has become the primary imaging technique used for clinical management in different countries.
In our centre, one of the reference hospitals for COVID-19 in Italy, the diagnostic pathway includes a combination, the diagnostic pathway includes a combination of clinical, laboratory, and imaging tests in association with RT-PCR; in particular, CXR is performed routinely, whereas chest CT is reserved for selected cases. This strategy allowed us to collect a large consecutive series of CXRs performed in symptomatic patients with suspected COVID-19.
Evidence on the diagnostic performance of CXR in diagnosing COVID-19 pneumonia is still limited and few reports described the usefulness of CXR severity scores.8, 9, 10 Moreover, only two studies9 , 10 focused on the role of these scores in predicting patient clinical outcomes. In particular, to the authors' knowledge, there are no studies investigating the predictive value of imaging findings (and their scores) in a large cohort of >550 patients.
Therefore, the aim of the present was to evaluate the role of a novel Milan score, applied to the baseline CXR in a high COVID-19 prevalence setting, in predicting in-hospital mortality and hospital admission versus quarantine at home.
Materials and methods
This observational retrospective study was approved by the local ethics committee and written informed consent was waived.
Population
Adult symptomatic patients who consecutively presented to the Emergency Department (ED) of L. Sacco Hospital from 21 February to 31 March 2020 with clinical suspicion of COVID-19 pneumonia were considered eligible for inclusion. This cohort also included patients who had been transferred from the ED of other hospitals due to the lack of available beds. Only laboratory-confirmed COVID-19 patients who underwent CXR on the day of admission were included. Laboratory-confirmed patients were those with a positive RT-PCR result on a naso-oropharyngeal swab performed within 6 days of admission or with a positive serological test. Serological antibody testing was performed if patients with a high clinical and epidemiological suspicion of COVID-19 had multiple consecutive negative RT-PCR tests.
Clinical data collection
Three investigators collected clinical data retrospectively (time between symptom onset and hospital admission; symptoms; comorbidities; length of hospital stay; in-hospital mortality) from the digital archives of the ED and Intensive Care Unit.
Image analysis
For all patients, only the first CXR acquired at admission was evaluated. All CXRs were acquired as digital radiograms following local protocols. In particular, CXRs were acquired in two projections (posteroanterior and laterolateral) when possible, and in one projection only (anteroposterior) in the case of seriously ill patients.
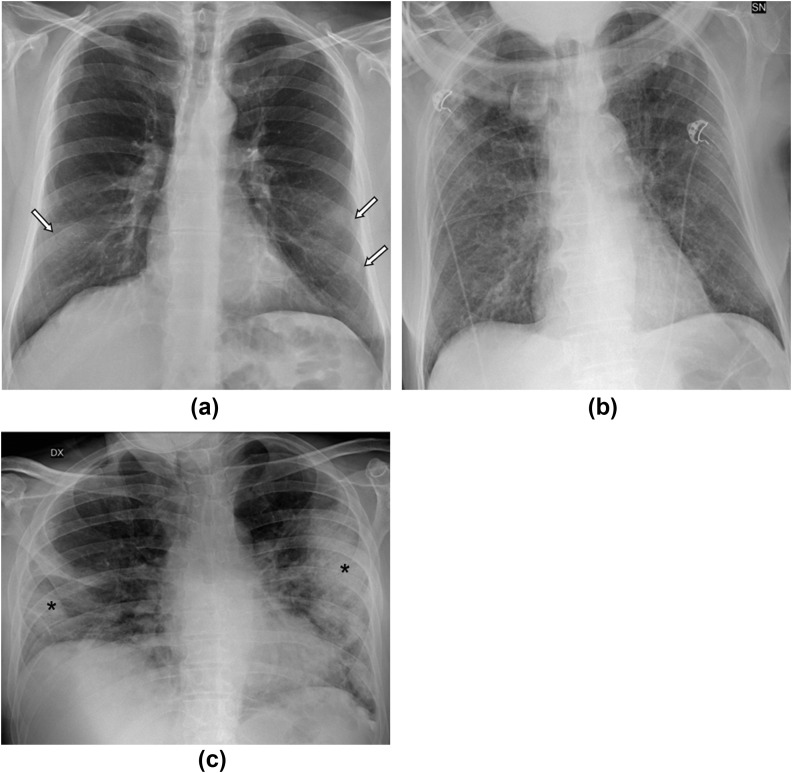
All CXRs were evaluated by a radiologist with >20 years of experience, blinded to the laboratory results and to the final outcomes, who classified CXR as positive or negative for COVID-19: according to the literature.11, 12, 13, 14, 15 The main features considered as suggestive for COVID-19 were interstitial reticular pattern, ground-glass opacities, and extensive consolidations, mostly involving the lower zones of both lungs with a preferred peripheral subpleural distribution (Fig 1 ).
Figure 1.
(a–c) Three typical CXR pattern of COVID-19 pneumonia. (a) Ground-glass opacities (white arrows) are seen in the lower zones of both lungs, with a typical peripheral distribution. (b) Alterations with a diffuse reticular pattern involve every zone of both lungs in a patient with continuous positive airway pressure (CPAP) face mask. (c) Extensive consolidations (asterisks) are seen in the lower zone of the right lung and in the middle and lower zones of the left lung, with a peripheral distribution.
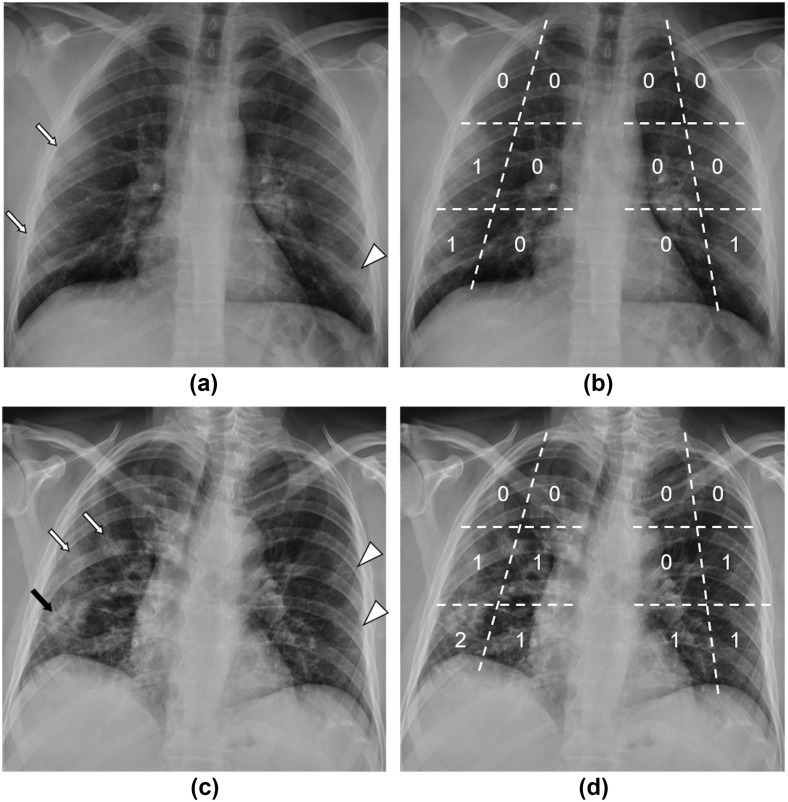
In order to evaluate the extension and distribution of disease, each lung was virtually divided into six areas (upper external, upper internal, middle external, middle internal, lower external, lower internal), for a total of 12 areas. Each area was given 0 points in case of no alterations, 1 point in case of interstitial reticular pattern or ground-glass opacities, and 2 points if extensive consolidations were observed; by summing the individual scores, a total score was obtained, ranging from 0 to 24 (Figure 2, Figure 3 ).
Figure 2.
Examples of CXR Milan score. (a) CXR of a 32-year-old male patient with no comorbidities who presented to the ED with fever, cough, ageusia, and normal pO2 values. Ground-glass opacities (white arrows) are visible in the middle and lower zones of the right lung, while alterations with a reticular pattern can be seen in the lower zone of the left lung (white arrowhead), both with a peripheral distribution. (b) The same CXR is shown with superimposed segmentation of each lung into six areas and relative scores, for a total Milan score = 3. This patient was sent home for quarantine after 6 days of hospital admission. (c) CXR of a 61-year-old male patient with diabetes mellitus and arterial hypertension who presented to the ED with fever, cough, and a pO2 of 70 mmHg. The CXR shows extensive consolidations (black arrow) in the peripheral half of the lower zone of the right lung and focal ground-glass opacities in the middle zone (white arrows); reticular pattern is present in the peripheral portions of the middle-lower zones of the left lung (white arrowheads). (d) Superimposed score grid with a total Milan score = 8. This patient was discharged after 29 days of hospitalisation.
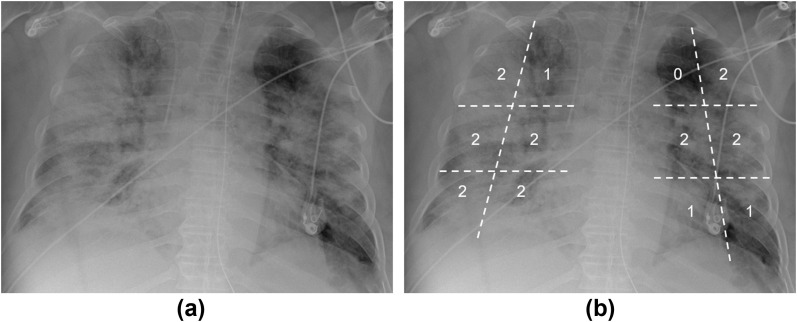
Figure 3.
CXR of a 68-year-old male patient who presented to the ED with fever, cough, and dyspnoea. (a) Bilateral extensive consolidations sparing the medial portion of the left lung apex; ground-glass opacities can be noted in the medial portion of the right lung apex and in the left lung base. (b) The same CXR is shown with a superimposed score grid, with a total Milan score = 19. This patient died after 13 days of hospitalisation.
Statistical analysis
Categorical variables were reported as counts (percentages), whereas continuous variables were reported as mean (standard deviation) or median (interquartile range, IQR), as appropriate. The CXR Milan score was categorised according to tertiles. In order to assess the predictive accuracy of CXR Milan score on the risk of the two outcomes (hospitalisation and death), a logistic regression approach was adopted. The following variables were considered for adjustment in statistical models: age (categorised in quartiles); gender; comorbidities (categorised as 0, 1, 2 or more); radiological pattern (negative; ground-glass opacities; interstitial reticular pattern; extensive consolidations); time from symptoms onset before ED admission (categorised as <7 or ≥7 days). First, univariate logistic models were fitted to assess the association between each of the potential predictors and the outcome. Then, a multivariate regression analysis was performed by including only the predictors that showed a significant effect at univariate analysis. A stepwise strategy was then adopted to identify the best multivariate model. Results of the logistic regression analysis were reported as odds ratios (OR) with their 95% confidence intervals (CI). The predictive ability of the multivariate model was assessed calculating the c-statistic. Two-sided p-values <0.05 were considered statistically significant. Statistical analyses were performed using SAS statistical software (release 9.4).
Results
Population
The patient flowchart is shown in Fig 4 . Of the 554 included patients, 445 presented directly to the ED; 109 patients were transferred to the ED from other EDs. Of the 554 included patients, 547 had a COVID-19 diagnosis confirmed by RT-PCR, while seven (1%) were confirmed by serological testing. The demographic, radiological findings, and clinical data are summarised in Table 1 .
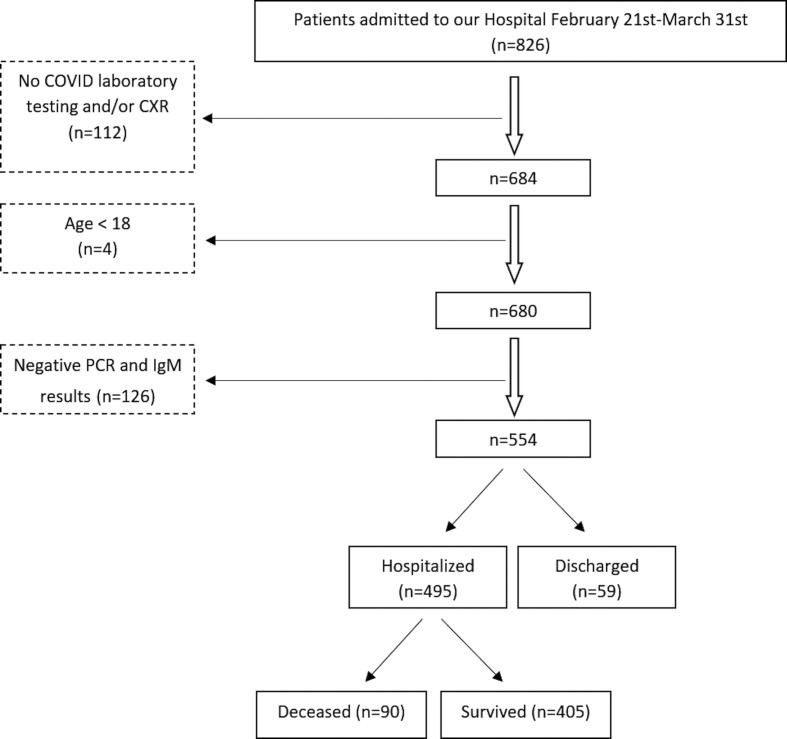
Figure 4.
Study flowchart. Out of the 826 eligible patients, 112 were excluded due to the lack of COVID-19 laboratory testing and/or CXR, four because <18 years, and 126 due to negative PCR and serologic testing.
Table 1.
Patient demographic, radiologic patterns and clinical data for 554 patients who tested positive for COVID-19.
| Patient characteristics | |
|---|---|
| Age, years, median (IQR) | 60 (47–72) |
| Age (years) | |
| ≤47 | 139 (25.1%) |
| 47–60 | 147 (26.5%) |
| 60–72 | 145 (26.2%) |
| >72 | 123 (22.2%) |
| Gender | |
| Female | 203 (36.6%) |
| Male | 351 (63.4%) |
| Comorbidities | |
| 0 | 285 (51.4%) |
| 1 | 142 (25.6%) |
| 2 | 89 (16.1%) |
| 3 | 27 (4.9%) |
| 4 | 11 (2) |
| Chest radiography prevalent pattern | |
| Negative | 115 (20.8%) |
| Ground-glass opacities | 105 (18.9%) |
| Reticular pattern | 186 (33.6%) |
| Extensive consolidation | 148 (26.7%) |
| Time from symptoms onset before hospital admission | |
| 0–7 days | 282 (64.4%) |
| >7 days | 156 (35.6%) |
Among the 495 (89%) hospitalised patients, 470 (95%) stayed for <15 days, while the remaining 5% stayed for >15 days. The in-hospital mortality was 16% (90/554); in particular, death occurred within 30 days from hospital admission in about 95% of cases. The median time between symptom onset and presentation to the ED was 7 days; data on symptom onset was missing for 116 patients (21%). Two hundred and sixty-nine patients (269/554; 49%) had comorbidities. The most common comorbidities were hypertension, cardiovascular diseases, diabetes mellitus, and respiratory system diseases.
Hospital admission versus home quarantine
Tertiles of CXR Milan score were 2 and 9. Accordingly, for multivariate and univariate analyses, the score was categorised into the following ranges: 0–2, 3–8 and ≥9. At univariate analysis, age (p<0.0001), gender (p=0.018), number of comorbidities (p<0.001), chest radiographic pattern (p<0.0001), and the CXR Milan score (p<0.0001) were significant predictors of the choice between hospital admission and quarantine at home (Table 2 ).
Table 2.
Univariate and multivariate analysis show how clinical variables and radiological variables (pattern and Milan score) correlate with the risk of hospital admission.
| Variable | Univariate |
p-Value | Multivariate |
p-Value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (years) | ||||
| <47 | 1a | <0.0001 | 1a | 0.0051 |
| 47–60 | 2.73 (1.38–5.38) | 2.14 (1.06–4.32) | ||
| 60–72 | 3.5 (1.68–7.28) | 2.25 (1.04–4.88) | ||
| >72 | 11.48 (3.41–38.62) | 7.04 (2.03–24.37) | ||
| Gender | ||||
| F | 1a | 0.0180 | - | ns |
| M | 1.93 (1.12–3.31) | |||
| Comorbidities | ||||
| 0 | 1a | 0.0014 | - | ns |
| 1 | 2.7 (1.28–5.7) | |||
| >1 | 3.68 (1.53–8.88) | |||
| Time from symptom onset before admission (days) | ||||
| 0–7 | 1a | 0.0617 | ns | |
| >7 | 1.87 (0.97–3.61) | |||
| Chest radiography pattern | ||||
| Negative | 1a | <0.0001 | - | ns |
| Ground-glass opacity | 2.74 (1.32–5.68) | |||
| Reticular pattern | 4.34 (2.19–8.61) | |||
| Ext consolidation | 17.05 (5.05–57.52) | |||
| CXR Milan score | ||||
| 0–2 | 1a | <0.0001 | 1a | <0.0001 |
| 3–8 | 4.22 (2.1–8.48) | 3.92 (1.93–7.95) | ||
| ≥9 | 10.3 (3.98–26.68) | 6.85 (2.56–18.35) | ||
c-Statistic for the multivariate model: 0.78.
Reference category.
From the multivariate analysis, only age (p=0.009) and the CXR score (p<0.0001) were significant predictors of hospital admission. In particular, considering a score ≤2 as the reference category, patients with a score between 3 and 8 (OR=3.92, 95% CI 1.93 to 7.95) and patients with a score ≥9 (OR=6.85, 95% CI 2.56 to 18.35) were at higher risk of hospital admission. Similarly, considering age <47 years as a reference, patients >72 years had a higher risk of hospital admission (OR=7.04, 95% CI 2.03 to 24.37; Table 2).
In-hospital mortality
The relationship between in-hospital mortality and the mentioned predictors (age, gender, comorbidities, time between symptoms onset and admission, CXR pattern, CXR score) is summarised in Table 3 .
Table 3.
Univariate and multivariate analysis show how clinical variables and radiological variables (pattern and Milan score) correlate with the risk of in hospital mortality.
| Variable | Univariate |
p-Value | Multivariate |
p-Value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Age (years) | ||||
| <47 | 1a | <0.0001 | 1a | <0.0001 |
| 47–60 | 1.94 (0.57–6.60) | 1.23 (0.35–4.32) | ||
| 60–72 | 8.80 (3.01–25.7) | 4.62 (1.51–14.08) | ||
| >72 | 21.6 (7.49–62.2) | 12.55 (4.19–37.6) | ||
| Gender | ||||
| F | 1a | 0.0005 | 1a | 0.0042 |
| M | 2.65 (1.53–4.59) | 2.46 (1.33–4.57) | ||
| Comorbidities | ||||
| 0 | 1a | 0.0173 | - | Ns |
| 1 | 1.81 (1.05–3.13) | |||
| >1 | 2.09 (1.20–3.63) | |||
| Time from symptom onset before admission (days) | ||||
| 0–7 | 1a | 0.6244 | - | - |
| >7 | 1.17 (0.63–2.15) | |||
| Chest radiography pattern | ||||
| Negative | 1a | <0.0001 | - | Ns |
| Ground-glass opacity | 0.71 (0.25–2.08) | |||
| Reticular pattern | 1.58 (0.70–3.56) | |||
| Extensive consolidation | 6.57 (3.08–14.04) | |||
| CXR Milan score | ||||
| 0–2 | 1a | <0.0001 | 1a | <0.0001 |
| 3–8 | 1.12 (0.5–2.57) | 0.91 (0.38–2.17) | ||
| ≥9 | 8.34 (4.33–16.1) | 4.69 (2.32–9.5) | ||
c-Statistic for the multivariate model: 0.852.
Reference category.
At univariate analysis, age (p<0.0001), gender (p<0.001), number of comorbidities (p=0.0026), CXR pattern (p<0.0001), and Milan score were significant predictors of mortality. In the multivariate logistic regression model, only CXR Milan score, age and gender were independent predictive factors of mortality (p<0.0001, p<0.0001, p=0.004, respectively). In particular, patients >72 years compared to patients <47 years, were at higher risk of death (OR=12.54, 95% CI 4.19 to 37.59). As for the Milan score, with score ≤2 as the reference category, patients with a Milan score ≥9 were at higher risk of death (OR=4.69, 95% CI 2.31 to 9.49).
Discussion
In our Hospital, a tertiary reference centre for infectious diseases in Northern Italy, a combination of clinical, a combination of clinical, laboratory, and imaging tests are used to triage patients with suspected COVID-19. Along with RT-PCR, whose results may be not readily available, especially during incidence peaks, all suspected patients undergo CXR, while chest CT is reserved for selected cases.
From the analysis of a large cohort of symptomatic patients with laboratory-confirmed COVID-19, it was found that 80% had CXR abnormalities, a result consistent with other studies. Wong et al. 12 described CXR abnormalities in 69% of cases in a small cohort of 64 patients, while Vancheri et al. 16 reported the presence of pathological findings in as much as 75% of cases in a larger cohort of 240 patients. On the contrary, Toussie et al.,10 evaluating 338 young and middle-aged patients with COVID-19 pneumonia, found abnormalities at baseline CXR in 50% of cases, and Hui et al. 17 reported pathological findings in only 18% of patients. In the latter two studies, the lower percentage of positive CXRs might be explained by the earlier presentation of patients to the ED, with a median of 4 days between symptom onset and hospital admission, in contrast to 7 days in the present study: indeed, some authors described a correlation between disease severity and time from symptom onset.14 , 15 , 18
In a setting of high disease prevalence, the present results support the choice of using CXR instead of chest CT; in fact, the positivity of CXR, in combination with a clinical suspicion, does not justify the need for a chest CT.
As a main result of this study, the Milan score, a novel easy to assess CXR severity score, was found to be an independent predictor of COVID19-related in-hospital mortality. In particular, patients with a Milan score ≥9 had a mortality rate five-times higher than those with a score ≤ 2.
The Milan score, which considers six zones per lung, is a semiquantitative score that combines qualitative and quantitative features, as it is based on the extension of lung involvement and on the pattern of parenchymal abnormalities. In particular, the division between internal and peripheral lung areas reflects the typical distribution of lung abnormalities in COVID-19 pneumonia. Thanks to these features, the score has the potential to be more precise and informative than those focused on abnormalities extension alone.11
The role of the score in predicting mortality bears similarities to two recent reports, involving smaller cohorts (approx. 300 patients)9 , 10: in particular, Borghesi et al. 9 found their CXR score to be predictive of in-hospital mortality, in association with patient age and immunosuppression. Their score, matching qualitative assessment of lung abnormalities with their extent, is similar to the Milan score, but it splits each lung in three zones instead of six and assigns a score to lung abnormalities in a different way. Toussie et al.,10 evaluating 334 young and middle-age adult patients, used a CXR score based on the presence of lung opacities in the three zones in which each lung was divided, finding it to be a good predictor of hospital admission and intubation. Some authors investigated the role of artificial intelligence (AI) applied to chest imaging in predicting clinical outcomes; in particular, Mushtaq et al. 19 recently described a predictive value for an AI-based score applied to the initial CXR and focused on density and extent of opacities.
The Milan score also proved to be an independent predictor of the final decision to admit the patient to the hospital; the only other variable that emerged as an independent predictor of hospital admission at multivariate analysis was patient age. This result supports the role of CXR in managing COVID-19 patients, especially decision-making regarding whether to hospitalise or discharge the patient with the instruction to self-quarantine at home. This has clinical and logistic implications, most notably when a surge in cases puts a strain on hospitals, overcrowding wards and Eds, and reducing bed availability, therefore making it even more important to be able to quickly and reliably distinguish those who need hospital care from those who can be treated conservatively at home. In this context, the presence of a prevalent extensive consolidations pattern and/or the evidence of an extended lung disease at CXR, which correspond to a higher Milan score, can support the clinician in making this decision, which is particularly challenging in times of emergency.
Finally, the use of CXR in the diagnostic pathway has advantages in terms of low cost, availability, and low risk of spreading the infection to the radiology staff.
The present study has some limitations. Firstly, CXR positivity and severity might have influenced the clinical decision regarding ED admission or discharge, but it is only a part of the decision process, which is mainly guided by symptoms and respiratory function. Secondly, this study does not include an evaluation of the reproducibility of this novel score; this will be investigated in a separate study. Finally, considering the strict relationship between symptom duration and CXR findings, the absence of data on symptom onset for 21% of patients is acknowledged as a limitation.
In conclusion, the CXR Milan score was found to be an independent predictive factor of both in-hospital mortality and hospital admission versus quarantine at home. This result, also considering the diagnostic value of CXR, reinforces the pivotal role of chest radiography in the management of COVID-19 patients at triage.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Akl E.A., Blazic I., Yaacoub S. Use of chest imaging in the diagnosis and management of COVID-19: a WHO rapid advice guide. Radiology. 2021;298:E63–E69. doi: 10.1148/radiol.2020203173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair A., Rodrigues J.C.L., Hare S. A British Society of Thoracic Imaging statement: considerations in designing local imaging diagnostic algorithms for the COVID-19 pandemic. Clin Radiol. 2020;75:329–334. doi: 10.1016/j.crad.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ACR Recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection Available at:
- 4.Rubin G.D., Ryerson C.J., Haramati L.B. The role of chest imaging in patient management during the covid-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158:106–116. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson S., Kay F.U., Abbara S. Radiological society of north America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues J.C.L., Hare S.S., Edey A. An update on COVID-19 for the radiologist — a British society of Thoracic Imaging statement. Clin Radiol. 2020;75:323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagnera S., Bisanti F., Tibaldi C. Performance of radiologists in the evaluation of the chest radiography with the use of a “new software score” in coronavirus disease 2019 pneumonia suspected patients. J Clin Imaging Sci. 2020;10:40. doi: 10.25259/JCIS_76_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghesi A., Zigliani A., Golemi S. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toussie D., Voutsinas N., Finkelstein M. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology. 2020;297:E197–E206. doi: 10.1148/radiol.2020201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan F., Ye T., Sun P. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong H.Y.F., Lam H.Y.S., Fong A.H.T. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296:E72–E78. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cozzi D., Albanesi M., Cavigli E. Chest X-ray in new coronavirus disease 2019 (COVID-19) infection: findings and correlation with clinical outcome. Radiol Med. 2020;125:730–737. doi: 10.1007/s11547-020-01232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ippolito D., Pecorelli A., Maino C. Diagnostic impact of bedside chest X-ray features of 2019 novel coronavirus in the routine admission at the emergency department: case series from Lombardy region. Eur J Radiol. 2020;129:109092. doi: 10.1016/j.ejrad.2020.109092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobi A., Chung M., Bernheim A. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vancheri S.G., Savietto G., Ballati F. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;30:6161–6169. doi: 10.1007/s00330-020-06967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui T.C.H., Khoo H.W., Young B.E. Clinical utility of chest radiography for severe COVID-19. Quant Imaging Med Surg. 2020;10:1540–1550. doi: 10.21037/QIMS-20-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernheim A., Mei X., Huang M. Chest CT findings in coronavirus disease 2019 (COVID-19): relationship to duration of infection. Radiology. 2020;295:685–691. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mushtaq J., Pennella R., Lavalle S. Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in COVID-19 patients: analysis of 697 Italian patients. Eur Radiol. 2021;31:1770–1779. doi: 10.1007/s00330-020-07269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]