Abstract
Purpose:
Despite improvements in frontline pediatric acute lymphoblastic leukemia (ALL) treatment, relapse remains a concern. Research in adult cancer patients suggests that patient-reported symptoms may predict survival, but the relationship between symptoms and relapse for pediatric ALL has received little attention.
Methods:
Pediatric patients with ALL (age 2–18 years) and/or their primary caregivers completed symptom surveys at: end of induction, start of delayed intensification (DI), start of maintenance cycle 1 (MC1), and start of maintenance cycle 2 (MC2). Symptom clusters for co-occurring fatigue, pain, sleep disruptions, and nausea were defined using latent profile analysis. Hazard ratios (HR) and 95% confidence intervals (CI) for the association between symptom clusters, individual symptoms and subsequent relapse were calculated using multivariable Cox proportional hazards models, adjusting for clinical and demographic factors.
Results:
Eligible patients (n=208) were followed an average of 2.6 years for the incidence of relapse (n = 22). Associations between relapse and symptoms were identified for fatigue at DI (HR = 1.83, 95%CI: 1.23–2.73) and MC1 (HR = 2.14, 95%CI: 1.62–2.84), pain at DI (HR = 1.80, 95%CI: 1.19–2.72), nausea at end induction (HR=1.19, 95%CI: 1.01–1.39), and sleep disturbances at end induction (HR=2.00, 95%CI: 1.11–3.62), DI (HR = 1.73, 95%CI: 1.01–2.96), and MC1 (HR = 2.19, 95%CI: 1.10–4.35). Symptom clusters comprised of individuals with a higher average symptom burden at DI were significantly (p < 0.05) associated with relapse.
Conclusion:
Patient-reported symptoms may provide prognostic information to aid in the identification of pediatric ALL patients at increased risk of relapse.
Keywords: Sleep, Fatigue, Pain, Nausea, Pediatric Acute Lymphoblastic Leukemia, Relapse
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most frequent malignancy diagnosed in children in the United States.1 Due to improvements in the treatment of pediatric ALL over the past several decades, five-year survival rates now exceed 90% in most developed countries.2 However, approximately 20% of cases experience relapse.3 Unlike survival rates for newly diagnosed pediatric ALL, little progress has been made in the treatment of relapsed ALL, with salvage therapy resulting in durable remission in approximately a third of cases.4 Due to the frequency of pediatric ALL and disappointing outcomes following disease recurrence, relapsed ALL remains the leading cause of death due to cancer in children.5 Although end of induction minimal residual disease (MRD) is the best established predictor of ALL relapse,6,7 nearly half of relapse cases occur among MRD negative individuals.6 Therefore, consideration of additional factors, which directly or indirectly contribute to ALL relapse, may be necessary to improve ALL risk prediction and stratification efforts.
Adverse symptoms of pediatric cancer and its treatment are a significant source of morbidity in children with cancer. In particular, pain, nausea, sleep disturbances, and fatigue are among the most pervasive symptoms reported during pediatric cancer therapy.8–11 In addition to negatively impacting quality of life and daily functioning,12,13 adverse symptom profiles may provide insight into the overall physical health of patients and their ability to tolerate cancer therapy. In fact, evidence from the adult oncology literature suggests that patient-reported symptoms may predict survival in individuals with cancer.14–16 To date, few studies have correlated patient-reported symptoms with treatment outcomes in pediatric oncology. Importantly, symptoms rarely remain static throughout childhood ALL treatment;17,18 therefore, there exists a need to evaluate the association between symptom profiles at critical phases of pediatric ALL therapy and subsequent relapse. Additionally, there is growing recognition that cancer-related symptoms rarely occur in isolation and characterizing the constellation of co-occurring symptoms that form “symptom clusters” may provide a more holistic estimation of overall symptom burden.19 Differences in clinical outcomes between symptom clusters remains largely undetermined, particularly among pediatric patients with ALL. Therefore, the objective of this study was to evaluate the prognostic potential of individual symptoms and symptom clusters systematically collected in a prospective cohort of pediatric patients during ALL therapy.
METHODS
Pediatric patients, aged 2–18 years at diagnosis, with newly-diagnosed ALL treated at Texas Children’s Cancer and Hematology Centers were enrolled between 2012 and 2017. Patients with pre-existing neurologic disorders or developmental disabilities were excluded from the study. Of the 236 eligible patients approached for the study, 208 (88%) agreed to participate. Most of the eligible patients who did not participate (21 of 28) cited lack of time or interest in the study as the primary reason for declining. The study protocol was reviewed and approved by an institutional review board at Baylor College of Medicine, and informed consent and/or assent, when indicated, was obtained from each research participant and legal guardian.
Leukemia Treatment
Eligible participants were treated on or according to recent Children’s Oncology Group (COG) ALL protocols: AALL0031, AALL0434, AALL0932, AALL1122, AALL1131, and AALL1231. Detailed information on each treatment protocol can be found on www.clinicaltrials.gov. Treatment typically consisted of one month of induction therapy with intrathecal methotrexate, corticosteroids, pegaspargase, and weekly vincristine and daunorubicin (for patients with high-and very-high risk disease). The next six to eight months of post-induction therapy consisted of several courses of vincristine, asparagine, doxorubicin, corticosteroids, mercaptopurine, cytarabine, intermediate- or high-dose intravenous methotrexate, and intrathecal methotrexate every 12 weeks. Finally, maintenance therapy typically lasted two to three years, depending on the sex of the child, and included a combination of daily oral mercaptopurine, weekly oral methotrexate, intravenous vincristine and oral corticosteroids, and intrathecal methotrexate every 12 weeks.
Symptom Battery
A detailed description of the study design and methods has been published.17 Briefly, symptom inventories were administered for participants at least three years of age at the time of the assessment. All evaluations were completed between January of 2013 and when the symptom study ended in August of 2018. Surveys were administered to each participant at four time points, approximately corresponding to the following treatment phases: end of induction (~1 month post-diagnosis), start of delayed intensification or day 113 of consolidation therapy for low-risk patients (~6 months post-diagnosis), start of the first cycle of maintenance therapy (~9 months post-diagnosis), and start of the second cycle of maintenance therapy (~12 months post-diagnosis). When appropriate, children ≥ 7 years of age self-reported symptoms, while guardian proxy reports were obtained for children < 7 years of age. To the extent possible, the individual completing the symptom inventory remained consistent across all time points evaluated. All surveys were administered in person during routine clinical appointments using electronic tablets or paper questionnaires to assess symptoms over the previous 2–4 week period. Assessments were structured around phases of therapy with participants completing questionnaires the day of the clinical appointment. In limited cases, participants were approached at the next scheduled office visit (within two weeks of designated time point) and allowed to complete the survey if not completed during the initial visit. Research coordinators were present to supervise completion of each questionnaire and offer assistance if needed. English and Spanish translations were available for all scales. Fatigue was measured using the Parent Fatigue Scale (ages 3–6 years), Child Fatigue Scale (ages 7–12 years), or Adolescent Fatigue Scale (ages 13–18 years).20–22 Cumulate scores were standardized to a T- score with mean of 50 and standard deviation (SD) of 10 (range: 20–80), with higher scores endorsing higher levels of fatigue. Sleep disturbances were recorded using the Child (ages 3–12) or Adolescent (ages 13–17) Sleep-Wake Scales.23 Responses were averaged across subscales to derive overall sleep quality scores (range: 1–6). In order to be consistent with the other symptom scales, scores were reverse coded such that higher scores provided evidence of poor sleep quality. Nausea was estimated using a visual analog scale (range 0–100), with higher scores indicating more severe nausea.24 Similarly, pain was measured using the Wong-Baker Faces Scale (range: 0–10),25 with higher scores reflecting higher levels of pain. Given the voluntary nature of the study, participants could elect to skip any questionnaire at any particular time point.
Clinical and Demographic Characteristics
Clinical information, including cancer diagnosis, age, post-induction treatment risk group, minimal residual disease (MRD) detected at day 29 of treatment, and height and weight at the time of diagnosis, was abstracted from participant medical records. Participants self-reported demographic information, including gender, race, and ethnicity. Diagnostic height (m) and weight (kg) were used to estimate body mass index (BMI) age- and sex-specific z-scores, based on the Centers for Disease Control and Prevention growth charts.26 Participants were prospectively followed for the incidence of relapse through February 2020. For relapse cases, date and site of relapse were abstracted from the patient’s medical records.
Statistical Analysis
Descriptive statistics, including means and standard deviations (SDs) for continuous covariates and counts and percent of the total for categorical variables, were estimated for the entire study sample. Differences in demographic and clinical factors between individuals with and without relapsed ALL were compared using a t-test or Fisher’s exact test. The associations between patient-reported symptoms and relapse were evaluated using unadjusted and adjusted Cox proportional hazards regression models, with participants contributing time from diagnosis until the date of relapse or the point at which they were censored (end of study follow-up or last contact). Deaths which occurred prior to relapse or progressive disease requiring bone marrow transplant were considered competing events in Cox models. Separate regression models were constructed for each symptom at each time point. Multivariable Cox proportional hazards models included age, gender, ethnicity, BMI z-score at diagnosis, ALL diagnosis (B-lineage or T-lineage ALL), and treatment risk group as covariates, which were selected a priori based on their suspected association with relapse and/or symptom severity. In order to capture the time-dependent nature of individual symptoms across treatment, we conducted latent class growth analysis to define clusters of individuals with similar severity reported across each time point. Similarly, to identify symptom clusters taking into consideration for the severity of all four symptoms evaluated simultaneously, we constructed four models (one for each time point) using latent profile analysis. Based on the distributions of the symptom variables, overall sleep quality and fatigue were modeled as continuous, normal variables while nausea and pain were fit with Poisson models. Consistent with previous work,17 a three-class solution was selected for each latent profile analysis model after consideration of Bayesian information criterion (BIC) for different solutions. Hazard ratios (HR) and corresponding 95% confidence intervals (CI) were estimated for the association between each latent class and relapse using Cox proportional hazards models, adjusting for the same covariates included in the individual symptom models. All analyses were conducted in Stata 15.1 (College Station, Texas, USA) with statistical significance defined at α < 0.05.
RESULTS
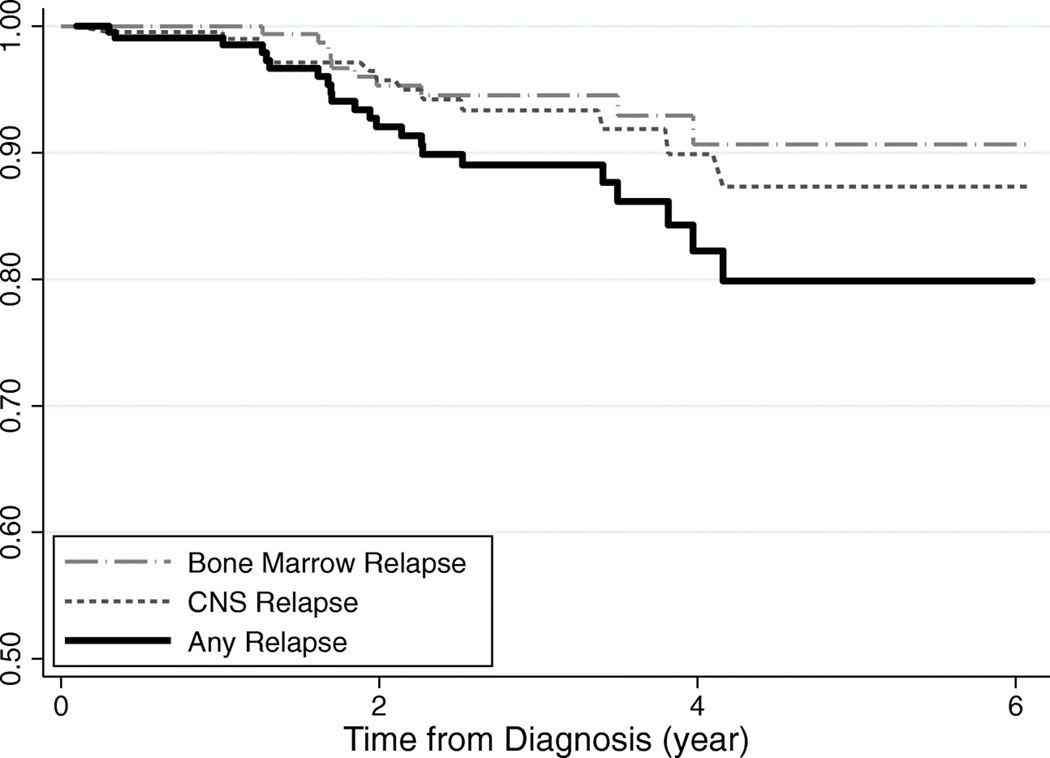
On average, eligible participants (n = 208) were 8.55 years of age at diagnosis, with the majority being male (55.8%), Hispanic (59.1%), cases diagnosed with Pre-B ALL (85.5%) and treated with high- or very high-risk post-induction therapy (63.0%). In univariate associations (Table 1), individuals who experienced relapse were more likely to have received high- or very high-risk post-induction therapy (90.9% vs 59.7%) and were older (10.54 years vs 8.31 years) with higher BMI z-scores (1.13 vs 0.26) at diagnosis. All the participants were followed for an average of 2.6 years (range: 0.1–6.1 years) for the incidence of relapse (Figure 1), which approached 20% by five-years post-diagnosis. During the observation period, 22 individuals experienced relapse, including eight cases of bone marrow relapse, 11 with central nervous system (CNS) relapse, two cases presenting with both bone marrow and CNS relapse, and one testicular relapse.
Table 1.
Clinical characteristics of childhood ALL patients treated at Texas Children’s Hospital, 2012–2017
| Overall (n=208) |
No Relapse (n=186) |
Relapse (n=22) |
|
|---|---|---|---|
| Mean age at diagnosis, year (SD) | 8.55 (4.32) | 8.31 (4.30) | 10.54 (4.00) |
| Mean BMI z-score at diagnosis, (SD) | 0.35 (1.31) | 0.26 (1.29) | 1.13 (1.23) |
| Gender, n (%) | |||
| Male | 116 (55.8) | 103 (55.4) | 13 (59.1) |
| Female | 92 (44.2) | 83 (44.6) | 9 (40.9) |
| Race/Ethnicity, n (%) | |||
| Non-Hispanic White | 50 (24.0) | 47 (25.3) | 3 (13.6) |
| Hispanic | 123 (59.1) | 105 (56.5) | 18 (81.8) |
| Non-Hispanic Black | 20 (9.6) | 19 (10.2) | 1 (4.6) |
| Non-Hispanic Other | 15 (7.2) | 0 (0.0) | 15 (8.1) |
| Diagnosis, n (%) | |||
| B-lineage | 180 (86.5) | 160 (86.0) | 20 (90.9) |
| T-lineage | 28 (13.5) | 26 (14.0) | 2 (9.1) |
| Treatment risk group, n (%) | |||
| Low/Standard | 77 (37.0) | 75 (40.3) | 2 (9.1) |
| High/Very High | 131 (63.0) | 111 (59.7) | 20 (90.9) |
| Induction/Postinduction protocol, n (%) | |||
| AALL0434/0434 | 8 (3.9) | 7 (3.8) | 1 (4.6) |
| AALL0932/0932 | 66 (31.7) | 65 (35.0) | 1 (4.6) |
| AALL0932/1131 | 21 (10.1) | 16 (8.6) | 5 (22.7) |
| AALL1131/1131 | 85 (40.9) | 72 (38.7) | 13 (59.1) |
| AALL1231/1231 | 20 (9.6) | 19 (10.2) | 1 (4.6) |
| Other | 8 (3.9) | 7 (3.8) | 1 (4.6) |
| Induction corticosteroid, n (%) | |||
| Prednisone | 67 (32.2) | 55 (29.6) | 12 (54.6) |
| Dexamethasone | 141 (67.8) | 131 (70.4) | 10 (45.5) |
| Induction intensity, n (%) | |||
| 3-drug induction< | 110 (52.9) | 103 (55.4) | 7 (31.8) |
| 4-drug induction | 98 (47.1) | 83 (44.6) | 15 (68.2) |
| Day 29 MRD, n (%) | |||
| Negative | 151 (72.6) | 137 (73.7) | 14 (63.6) |
| Positive | 57 (27.4) | 49 (26.3) | 8 (36.4) |
Acute lymphoblastic leukemia, ALL; standard deviation, SD; minimal residual disease, MRD
Figure 1.
Kaplan-Meier curve for time to relapse among pediatric patients with acute lymphoblastic leukemia, diagnosed 2012–2017
The distribution of symptoms reported across each study time point are presented in Supplemental Table 1. The observed associations between individual symptoms at each time point and subsequent relapse are presented in Table 2. Of the 208 individuals included in the study, at least one symptom assessment was completed by 192 (92.3%) at end of induction, 196 (94.2%) at start of delayed intensification, 173 (83.2%) at maintenance cycle 1, and 166 (79.8%) at maintenance cycle 2. Higher levels of fatigue were typically associated with an increased likelihood of relapse, with the effect estimates reaching statistical significance in adjusted models for fatigue assessed at the start of delayed intensification therapy (HR = 1.83, 95%CI: 1.23–2.73) and the first cycle of maintenance therapy (HR = 2.14, 95%CI: 1.62–2.84). Similarly, poorer sleep quality (i.e., higher sleep scores) was consistently associated with a statistically significant increased likelihood of relapse at each time point except maintenance cycle 2 (p-value = 0.055), independent of known clinical and demographic prognostic factors. For example, a one-unit increase in sleep disturbances measured at the end of induction was associated with a nearly two-fold increase in subsequent relapse (HR = 2.00, 95%CI: 1.11–3.62), after accounting for possible confounders. The adjusted associations between sleep and relapse revealed similar effect estimates for the other time points: delayed intensification (HR = 1.73, 95%CI: 1.01–2.96), maintenance cycle 1 (HR = 2.19, 95%CI: 1.10–4.35), maintenance cycle 2 (HR = 2.13, 95%CI: 0.98–4.60). Higher levels of pain reported at the start of delayed intensification were significantly associated with subsequent relapse in adjusted models (HR = 1.80, 95%CI: 1.19–2.72); however, the associations between relapse and pain reported at the other time points did not reach statistical significance. Finally, nausea was consistently associated with a slight increase in relapse in adjusted models across each time point, reaching statistical significance for nausea reported at end of induction (HR = 1.19, 95% CI: 1.01–1.39). Adjustment for corticosteroid exposure (prednisone vs. dexamethasone), induction therapy intensity (no daunorubicin vs daunorubicin), and day 29 MRD as a covariate in the regression models (results not shown) did not materially impact the effect estimates (i.e., <5% difference in effect estimates). In additional secondary analyses stratifying on MRD status (Supplemental Table 2), we did not find evidence of statistical differences in the relationship between symptoms and relapse by MRD status, suggesting that the observed associations between symptom distress and relapse occur independent of MRD at end of induction. Similarly, we compared the associations between symptoms and relapse stratifying on self-report and parent proxy-report (Supplemental Table 3), although sample size considerations limited our ability to draw meaningful inferences. With the possible exception of stronger associations observed for parent-reported symptoms at end of induction, associations for symptoms and relapse were similar between self- and parent-reported surveys.
Table 2.
Observed association between symptoms reported at four time points during therapy and relapse among cases of childhood ALL
| Median days from diagnosis (range) | Relapse Cases | Total Cases | Unadjusted Model HR (95% CI) | Adjusted Model1 HR (95% CI) | |
|---|---|---|---|---|---|
| End Induction | 47.5 (32.8 – 98.6) | ||||
| Fatigue | 20 | 177 | 1.19 (0.81–1.74) | 1.28 (0.83–1.98) | |
| Sleep | 20 | 180 | 1.64 (1.02–2.62) | 2.00 (1.11–3.62) | |
| Pain | 20 | 188 | 1.38 (1.09–1.74) | 1.31 (0.98–1.75) | |
| Nausea | 20 | 187 | 1.15 (1.01–1.32) | 1.19 (1.01–1.39) | |
| Delayed Intensification Day 1 | 186.2 (94.9 – 262.8) | ||||
| Fatigue | 20 | 182 | 1.39 (0.91–2.13) | 1.83 (1.23–2.73) | |
| Sleep | 19 | 177 | 1.93 (1.21–3.09) | 1.73 (1.01–2.96) | |
| Pain | 19 | 189 | 1.22 (0.87–1.73) | 1.80 (1.19–2.72) | |
| Nausea | 20 | 188 | 1.05 (0.88–1.24) | 1.13 (0.95–1.35) | |
| Maintenance Cycle 1 Day 1 | 288.4 (193.4 – 438.0) | ||||
| Fatigue | 16 | 166 | 1.65 (1.09–2.49) | 2.14 (1.62–2.84) | |
| Sleep | 16 | 165 | 1.39 (0.78–2.48) | 2.19 (1.10–4.35) | |
| Pain | 16 | 169 | 0.89 (0.53–1.49) | 0.90 (0.55–1.45) | |
| Nausea | 15 | 166 | 0.90 (0.72–1.14) | 1.08 (0.81–1.42) | |
| Maintenance Cycle 2 Day 1 | 390.5 (321.2 – 525.6) | ||||
| Fatigue | 14 | 160 | 1.02 (0.67–1.55) | 1.25 (0.80–1.96) | |
| Sleep | 11 | 153 | 1.82 (0.89–3.70) | 2.13 (0.98–4.60) | |
| Pain | 14 | 159 | 0.70 (0.32–1.55) | 0.83 (0.41–1.69) | |
| Nausea | 14 | 164 | 1.12 (0.87–1.44) | 1.19 (0.98–1.45) | |
Model adjusted for age, gender, ethnicity, BMI z-score at diagnosis, diagnosis, and treatment risk group
per standard deviation increase in fatigue, 1 unit increase in sleep, 2 unit increase in pain, 10 unit increase in nausea
Using latent class growth analysis to identify groups of individuals with similar symptom trajectories reported across all evaluation time points, we identified a three-cluster solution for each symptom (Table 3). In general, the classes captured consistently low symptom severity (Class 1), moderate symptom severity (Class 2), and persistently elevated symptom severity (Class 3). The incidence of ALL relapse was most frequent among individual belonging to classes with a higher overall symptom burden across treatment, with the associations reaching statistical significance (p-value for trend <0.05) for sleep disturbances and nausea. Adjustment for potential confounding factors did not substantially attenuate the observed associations between symptom classes and ALL relapse.
Table 3.
Latent class growth analysis of four symptoms reported during childhood ALL therapy and association with ALL relapse
| Symptom Evaluation Time Point | |||||||
|---|---|---|---|---|---|---|---|
| N | Time 1 Median (IQR) | Time 2 Median (IQR) | Time 3 Median (IQR) | Time 4 Median (IQR) | Unadjusted HR (95% CI) | Adjusted1 HR (95% CI) | |
| Fatigue | |||||||
| Class 1 | 119 | 44.3 (38.4–48.9) | 42.9 (40.0–46.8) | 44.0 (40.3–47.7) | 44.5 (40.6–48.1) | Ref | Ref |
| Class 2 | 70 | 51.5 (45.7–57.2) | 55.1 (49.7–58.4) | 53.9 (49.9–59.1) | 56.0 (50.9–60.5) | 0.99 (0.39–2.51) | 1.69 (0.60–4.75) |
| Class 3 | 19 | 64.6 (58.4–68.8) | 67.7 (61.3–72.4) | 65.3 (62.8–71.4) | 64.0 (59.4–71.7) | 1.57 (0.46–5.38) | 2.78 (0.80–9.62) |
| Sleep | |||||||
| Class 1 | 79 | 1.9 (1.7–2.2) | 2.0 (1.7–2.2) | 2.0 (1.7–2.2) | 2.0 (1.6–2.2) | Ref | Ref |
| Class 2 | 111 | 2.9 (2.6–3.3) | 3.1 (2.6–3.3) | 2.9 (2.6–3.4) | 2.9 (2.5–3.3) | 3.58 (1.04–12.31) | 3.81 (1.03–14.06) |
| Class 3 | 24 | 3.7 (3.4–4.1) | 3.9 (3.7–4.4) | 4.0 (3.5–4.3) | 4.0 (3.7–4.5) | 3.62 (0.81–16.17) | 4.50 (0.75–26.82) |
| Pain | |||||||
| Class 1 | 43 | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | Ref | Ref |
| Class 2 | 92 | 2 (2–4) | 0 (0–2) | 0 (0–0) | 0 (0–0) | 6.24 (0.81–48.13) | 5.38 (0.65–44.26) |
| Class 3 | 79 | 2 (2–6) | 2 (0–4) | 2 (2–4) | 2 (2–4) | 4.81 (0.61–38.19) | 5.24 (0.62–44.18) |
| Nausea | |||||||
| Class 1 | 86 | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–2) | Ref | Ref |
| Class 2 | 68 | 20 (4–33) | 7 (1–20) | 10 (0–20) | 1 (0–10) | 2.86 (0.88–9.25) | 3.49 (1.04–11.72) |
| Class 3 | 60 | 10 (0–48) | 28 (9–50) | 30 (14–50) | 25 (10–40) | 3.64 (1.10–12.02) | 7.46 (2.27–24.54) |
Acute lymphoblastic leukemia, ALL; interquartile range, IQR; hazard ratio, HR; confidence interval, CI
Model adjusted for age, gender, ethnicity, BMI z-score at diagnosis, diagnosis, and treatment risk group
Time points: end induction (time 1), delayed intensification day 1 (time 2), maintenance cycle 1 day 1 (time 3), maintenance cycle 2 day 1 (time 4)
We used latent profile analysis to identify symptom clusters to account for correlation between symptoms at each time point (Supplemental Table 4). A description of the symptom clusters for each time point are presented in Table 4. In general, individuals in class 1 reported the least symptom distress, while those in class 3 reported the most. For example, individuals in class 3 generally reported higher levels of fatigue, poorer sleep quality, more pain, and higher levels of nausea than their counterparts in classes 1 or 2 across each time point. Depending on the phase of therapy, approximately half (range: 45.1% - 53.0%) of patients were categorized in class 1, a third (range: 29.7% - 38.2%) in class 2, and the remaining 16.3% - 24.1% in class 3. The clusters associated with a greater overall symptom burden were associated with a higher relative incidence of relapse (Table 5), with the associations reaching statistical significance for symptoms assessed during delayed intensification therapy (p < 0.05). Compared to individuals in class 1, individuals in class 3 experienced a three- to four-fold increase in the incidence of relapse for symptoms reported at delayed intensification (HR = 3.98, 95%CI: 1.38–11.46).
Table 4.
Latent profile analysis marginal means and 95% confidence intervals of symptoms reported by phase of childhood ALL therapy
| N (%) | Fatigue | Sleep | Pain | Nausea | |
|---|---|---|---|---|---|
| End Induction | |||||
| Class 1 | 95 (49.5) | 46.02 (44.13–47.91) | 2.56 (2.40–2.72) | 1.84 (1.56–2.11) | 0.20 (0.09–0.31) |
| Class 2 | 62 (32.3) | 48.88 (46.56–51.21) | 2.65 (2.46–2.84) | 2.47 (2.06–2.87) | 14.38 (13.15–15.62) |
| Class 3 | 35 (18.2) | 54.36 (51.19–57.52) | 3.14 (2.88–3.40) | 4.05 (3.36–4.75) | 55.14 (52.01–58.27) |
| Delayed Intensification Day 1 | |||||
| Class 1 | 90 (46.2) | 46.25 (44.40–48.09) | 2.50 (2.34–2.66) | 0.71 (0.53–0.88) | 0.09 (0.01–0.17) |
| Class 2 | 58 (29.7) | 48.16 (45.8O-5O.52) | 2.95 (2.75–3.16) | 1.65 (1.31–1.98) | 9.19 (8.28–10.10) |
| Class 3 | 47 (24.1) | 56.66 (53.98–59.34) | 3.01 (2.73–3.29) | 2.50 (2.03–2.97) | 43.83 (41.74–45.92) |
| Maintenance Cycle 1 Day 1 | |||||
| Class 1 | 78 (45.1) | 45.20 (43.24–47.15) | 2.50 (2.33–2.68) | 0.72 (0.52–0.91) | 0.13 (0.04–0.23) |
| Class 2 | 66 (38.2) | 51.92 (49.65–54.19) | 2.87 (2.68–3.07) | 1.46 (1.14–1.77) | 12.83 (11.70–13.97) |
| Class 3 | 29 (16.8) | 57.06 (53.62–60.50) | 3.02 (2.69–3.35) | 1.97 (1.42–2.52) | 50.85 (47.19–54.51) |
| Maintenance Cycle 2 Day 1 | |||||
| Class 1 | 88 (53.0) | 47.08 (45.13–49.04) | 2.56 (2.37–2.74) | 0.64 (0.47–0.81) | 0.25 (0.12–0.39) |
| Class 2 | 51 (30.7) | 53.62 (51.01–56.24) | 2.85 (2.62–3.08) | 1.42 (1.05–1.78) | 11.84 (10.59–13.09) |
| Class 3 | 27 (16.3) | 56.30 (52.80–59.80) | 2.81 (2.44–3.18) | 2.44 (1.82–3.06) | 43.87 (40.83–46.92) |
Table 5.
Observed association between latent profile classes at four time points during therapy and relapse among cases of childhood ALL
| Relapse Cases | Total Cases | Adjusted HR (95% CI)1 | P for Trend | |
|---|---|---|---|---|
| End Induction | ||||
| Class 1 | 6 | 95 | Ref. | 0.07 |
| Class 2 | 7 | 62 | 1.66 (0.60–4.56) | |
| Class 3 | 7 | 35 | 3.31 (0.95–11.58) | |
| Delayed Intensification Day 1 | ||||
| Class 1 | 6 | 90 | Ref. | 0.01 |
| Class 2 | 8 | 58 | 2.26 (0.76–6.69) | |
| Class 3 | 6 | 47 | 3.98 (1.38–11.46) | |
| Maintenance Cycle 1 Day 1 | ||||
| Class 1 | 7 | 78 | Ref. | 0.32 |
| Class 2 | 7 | 66 | 1.41 (0.47–4.23) | |
| Class 3 | 2 | 29 | 2.39 (0.39–14.53) | |
| Maintenance Cycle 2 Day 1 | ||||
| Class 1 | 6 | 88 | Ref. | 0.11 |
| Class 2 | 6 | 51 | 2.68 (0.82–8.79) | |
| Class 3 | 3 | 27 | 2.29 (0.59–8.92) | |
Model adjusted for age, gender, ethnicity, BMI z-score at diagnosis, diagnosis, and treatment risk group
DISCUSSION
Although much progress has been made in the frontline treatment of pediatric ALL, relapsed ALL remains the leading cause of cancer death in children.5 End of induction MRD is the most reliable established predictor of ALL relapse.6,7 However, approximately half of relapse cases occur in individuals who were MRD negative at end of induction,6 highlighting the need to consider other potential predictors of relapse. This study systematically evaluated the association between symptoms commonly reported during childhood cancer therapy and ALL relapse. In particular, this study reports significant associations between the individual symptoms of fatigue, sleep disturbance, and pain and later relapse. Notably, these associations occurred independent of known clinical and demographic risk factors for relapse, including MRD. For some of these symptoms (i.e., fatigue, pain, nausea) significant associations with relapse were only observed during specific phases of therapy, while the associations between impaired sleep and relapse was consistent across most phases assessed. The higher incidence of relapse appears to be most pronounced among individuals with persistently elevated symptoms across therapy. Additionally, this study identified a higher incidence of relapse in symptom clusters characterized by a greater overall symptom burden, particularly for symptom profiles evaluated prior to maintenance therapy. Considered collectively, these findings underscore the potential prognostic significance of adverse symptoms experienced during childhood ALL therapy.
To our knowledge, this is the first study to prospectively evaluate the association between patient-reported symptoms during chemotherapy and relapse in pediatric patients with ALL. Our major finding that symptoms experienced during treatment are associated with adverse clinical outcomes is largely consistent with more than a decade of research in adults with cancer. For example, higher levels of fatigue have been linked to increased mortality in numerous adult oncology populations, including patients with breast,27 lung,28 esophageal,29 and colorectal cancers.30 We also observed a trend towards a higher incidence of relapse in patients who reported more nausea, consistent with some adult studies.31 Pain has been extensively studied in adult populations but not consistently associated with overall survival or clinical outcome independent of other prognostic factors.32 In the current study, relapse was only significantly associated with pain reported at the start of delayed intensification therapy, underscoring the value of prospective symptom assessment to better understand the potentially dynamic relationship between symptoms and treatment outcomes.
We observed the most consistent associations between relapse and sleep, with poorer sleep quality predicting an increased incidence of relapse at each phase of therapy evaluated. There is growing awareness that sleep disturbances pose a considerable problem for children with cancer.33 For some patients, it is possible that sleep disturbances or poor sleep hygiene predate the pediatric leukemia diagnosis, information which was not available in the current study. However, sleep problems are commonly reported by parents for children with ALL after induction therapy,34 likely reflecting circadian rhythm disruptions due to hospitalization or corticosteroid exposure.35,36 Both objective and subjective measures of sleep have been implicated in poor treatment outcomes in adults with cancer.37–39 In the general population, research has identified a complex, non-linear correlation between sleep duration and all-cause mortality,40 but generally supports a robust association between sleep quality and health outcomes.41 Additional research is warranted to better characterize the relationship between sleep and ALL relapse to identify patient subgroups most likely to benefit from intervention.
Strengths of the current study include a prospective design with systematically evaluated symptoms in a contemporary, ethnically diverse cohort. Notably, research has traditionally focused on a single symptom and the study of symptom clusters on survival and treatment outcomes has received relatively limited attention.42 Because symptoms rarely occur in isolation, we compared relapse between symptom clusters statistically defined by the co-occurrence of pain, sleep, fatigue, and nausea during childhood ALL therapy. This study provides novel insight into the potential detrimental impact of adverse symptom clusters and ALL relapse, particular for symptom clusters identified prior to maintenance therapy. Still, these results should be considered in light of several limitations. First, although the sample is relatively large for a study of a single pediatric malignancy, the current study had limited statistical power to fully evaluate associations with relapse. The study sample was also comprised of large proportion of Hispanic patients (59%) and patients treated on high or very high risk protocols (63%), populations with an elevated risk of relapse.43 Thus, the study findings may not be generalizable to all pediatric populations treated for ALL. Additionally, although visual analog scales are commonly used in symptom reporting,24 the validity of the nausea visual analog scale has not been established in pediatric oncology populations. As a result, other scale may be more appropriate for evaluating nausea in this population. Finally, this study only provides correlations between symptoms and relapse, and does not necessarily support a causal link between the two. It is unclear if patient-reported symptoms impact treatment outcomes directly or indirectly. Symptom severity may be a surrogate for disease aggressiveness, compromised physical health of the patients, treatment-related complications, or other factors likely to unfavorably affect outcomes. Because the impact of these factors was not characterized in the current study, further research is needed to elucidate the mechanisms linking patient-reported symptoms to adverse treatment outcomes.
The results of this study add to the growing body of evidence from the adult oncology literature suggesting patient-reported symptoms contribute significant prognostic information beyond traditional clinical risk factors. In our prospective evaluation of symptoms reported at four time points during the first year of pediatric ALL chemotherapy, we observed associations between relapse and fatigue, sleep disturbances, nausea, or pain at one or more time points. In particular, sleep disruptions were consistently associated with subsequent relapse, independent of established clinical risk factors. The mechanisms responsible for the observed associations between patient-reported symptoms and relapse as well as the potential for patient-reported symptoms to improve risk stratification efforts in pediatric patients with ALL remain speculative. However, given the pervasiveness of adverse symptoms during pediatric ALL therapy and their inverse relationship with quality of life,12,13 efforts to better manage symptoms deserve increased attention. Based on the results of this study, future interventions intended to ameliorate the burden of symptoms in pediatric patients with ALL should consider the possible impact on relapse and other clinical endpoints.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health National Cancer Institute (R01CA1693398, K07CA218362) and the St. Baldrick’s Foundation Consortium Research Grant (522277) Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium.
Funding: This work was supported in part by the National Institutes of Health National Cancer Institute (R01CA1693398, K07CA218362) and the St. Baldrick’s Foundation Consortium Research Grant (522277) Reducing Ethnic Disparities in Acute Leukemia (REDIAL) Consortium.
Footnotes
DECLARATIONS
Data Availability: Data are available from the EpiCenter at Baylor College of Medicine (contact via epicenter@bcm.edu) for researchers who meet the criteria for access to confidential data.
Ethics Approval: All research procedures involving human participants were in accordance with the ethical standards of the 1964 Helsinki Declaration and were approved by the Institutional Review Board of Baylor College of Medicine.
Consent: Informed consent and/or assent, when indicated, was obtained from each research participant and legal guardian
Conflict of Interest: The authors declare no potential conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112(2):416–432. [DOI] [PubMed] [Google Scholar]
- 2.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. The New England journal of medicine. 2015;373(16):1541–1552. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Moretta F, Rutella S. Management of relapsed acute lymphoblastic leukemia in childhood with conventional and innovative approaches. Curr Opin Oncol. 2013;25(6):707–715. [DOI] [PubMed] [Google Scholar]
- 4.Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–136. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe J, Orellana L, Ullrich C, et al. Symptoms and Distress in Children With Advanced Cancer: Prospective Patient-Reported Outcomes From the PediQUEST Study. J Clin Oncol. 2015;33(17):1928–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hockenberry MJ, Taylor OA, Pasvogel A, et al. The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncology nursing forum. 2014;41(4):E238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kestler SA, LoBiondo-Wood G. Review of symptom experiences in children and adolescents with cancer. Cancer nursing. 2012;35(2):E31–49. [DOI] [PubMed] [Google Scholar]
- 11.Hooke MC, Linder LA. Symptoms in Children Receiving Treatment for Cancer-Part I: Fatigue, Sleep Disturbance, and Nausea/Vomiting. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2019;36(4):244–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodgers CC, Hooke MC, Taylor OA, et al. Childhood Cancer Symptom Cluster: Leukemia and Health-Related Quality of Life. Oncology nursing forum. 2019;46(2):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunes MDR, Nascimento LC, Fernandes AM, et al. Pain, sleep patterns and health-related quality of life in paediatric patients with cancer. Eur J Cancer Care (Engl). 2019;28(4):e13029. [DOI] [PubMed] [Google Scholar]
- 14.Quinten C, Maringwa J, Gotay CC, et al. Patient self-reports of symptoms and clinician ratings as predictors of overall cancer survival. J Natl Cancer Inst. 2011;103(24):1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 16.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. [DOI] [PubMed] [Google Scholar]
- 17.Hockenberry MJ, Hooke MC, Rodgers C, et al. Symptom Trajectories in Children Receiving Treatment for Leukemia: A Latent Class Growth Analysis With Multitrajectory Modeling. Journal of pain and symptom management. 2017;54(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng DJ, Lu X, Schore RJ, et al. Longitudinal analysis of quality-of-life outcomes in children during treatment for acute lymphoblastic leukemia: A report from the Children’s Oncology Group AALL0932 trial. Cancer. 2018;124(3):571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers CC, Hooke MC, Hockenberry MJ. Symptom clusters in children. Curr Opin Support Palliat Care. 2013;7(1):67–72. [DOI] [PubMed] [Google Scholar]
- 20.Hinds PS, Yang J, Gattuso JS, et al. Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. Journal of pain and symptom management. 2010;39(3):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrell BN, Yang J, Hooke MC, et al. Psychometric and clinical assessment of the 13-item reduced version of the fatigue scale-adolescent instrument. Journal of pediatric oncology nursing : official journal of the Association of Pediatric Oncology Nurses. 2011;28(5):287–294. [DOI] [PubMed] [Google Scholar]
- 22.Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: the child, parent and staff perspectives. Journal of pain and symptom management. 2003;25(4):319–328. [DOI] [PubMed] [Google Scholar]
- 23.LeBourgeois MK, Giannotti F, Cortesi F, Wolfson AR, Harsh J. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(1 Suppl):257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976;31(9):1191–1198. [DOI] [PubMed] [Google Scholar]
- 25.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9–17. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. 2000 CDC Growth Charts for the United States: Methods and Development. http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf. Published 2010. Accessed 17 July, 2015.
- 27.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105(2):209–219. [DOI] [PubMed] [Google Scholar]
- 28.Martins SJ, Ho N, Cavamura SO, Harada CM, Yamamoto CA, Takagaki TY. Lung cancer symptoms and pulse oximetry in the prognostic assessment of patients with lung cancer. BMC Cancer. 2005;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stauder MC, Romero Y, Kabat B, et al. Overall survival and self-reported fatigue in patients with esophageal cancer. Support Care Cancer. 2013;21(2):511–519. [DOI] [PubMed] [Google Scholar]
- 30.Adam S, van de Poll-Franse LV, Mols F, et al. The association of cancer-related fatigue with all-cause mortality of colorectal and endometrial cancer survivors: Results from the population-based PROFILES registry. Cancer Med. 2019;8(6):3227–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woopen H, Richter R, Chekerov R, et al. Prognostic role of chemotherapy-induced nausea and vomiting in recurrent ovarian cancer patients: results of an individual participant data meta-analysis in 1213. Support Care Cancer. 2020;28(1):73–78. [DOI] [PubMed] [Google Scholar]
- 32.Zylla D, Steele G, Gupta P. A systematic review of the impact of pain on overall survival in patients with cancer. Support Care Cancer. 2017;25(5):1687–1698. [DOI] [PubMed] [Google Scholar]
- 33.Daniel LC, van Litsenburg RRL, Rogers VE, et al. A call to action for expanded sleep research in pediatric oncology: A position paper on behalf of the International Psycho-Oncology Society Pediatrics Special Interest Group. Psychooncology. 2020;29(3):465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steur LMH, Grootenhuis MA, Van Someren EJW, et al. High prevalence of parent-reported sleep problems in pediatric patients with acute lymphoblastic leukemia after induction therapy. Pediatric blood & cancer. 2020;67(4):e28165. [DOI] [PubMed] [Google Scholar]
- 35.Rosen G, Harris AK, Liu M, Dreyfus J, Krueger J, Messinger YH. The effects of dexamethasone on sleep in young children with acute lymphoblastic leukemia. Sleep medicine. 2015;16(4):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers VE, Zhu S, Mandrell BN, Ancoli-Israel S, Liu L, Hinds PS. Relationship between circadian activity rhythms and fatigue in hospitalized children with CNS cancers receiving high-dose chemotherapy. Support Care Cancer. 2020;28(3):1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottfried T, Kamer I, Salant I, et al. Self-reported sleep quality as prognostic for survival in lung cancer patients. Cancer Manag Res. 2020;12:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins KP, Geller DA, Antoni M, et al. Sleep duration is associated with survival in advanced cancer patients. Sleep medicine. 2017;32:208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheville AL, Novotny PJ, Sloan JA, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. Journal of pain and symptom management. 2011;42(2):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–1964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.