Abstract
The RNA-dependent RNA polymerase (RdRp) and 3C-like protease (3CLpro) from SARS-CoV-2 play crucial roles in the viral life cycle and are considered the most promising targets for drug discovery against SARS-CoV-2. In this study, FDA-approved drugs were screened to identify the probable anti-RdRp and 3CLpro inhibitors by molecular docking approach. The number of ligands selected from the PubChem database of NCBI for screening was 1760. Ligands were energy minimized using Open Babel. The RdRp and 3CLpro protein sequences were retrieved from the NCBI database. For Homology Modeling predictions, we used the Swiss model server. Their structure was then energetically minimized using SPDB viewer software and visualized in the CHIMERA UCSF software. Molecular dockings were performed using AutoDock Vina, and candidate drugs were selected based on binding affinity (∆G). Hydrogen bonding and hydrophobic interactions between ligands and proteins were visualized using Ligplot and the Discovery Studio Visualizer v3.0 software. Our results showed 58 drugs against RdRp, which had binding energy of − 8.5 or less, and 69 drugs to inhibit the 3CLpro enzyme with a binding energy of − 8.1 or less. Six drugs based on binding energy and number of hydrogen bonds were chosen for the next step of molecular dynamics (MD) simulations to investigate drug-protein interactions (including Nilotinib, Imatinib and dihydroergotamine for 3clpro and Lapatinib, Dexasone and Relategravir for RdRp). Except for Lapatinib, other drugs-complexes were stable during MD simulation. Raltegravir, an anti-HIV drug, was observed to be the best compound against RdRp based on docking binding energy (− 9.5 kcal/mole) and MD results. According to the MD results and binding energy, dihydroergotamine is a suitable candidate for 3clpro inhibition (− 9.6 kcal/mol). These drugs were classified into several categories, including antiviral, antibacterial, anti-inflammatory, anti-allergic, cardiovascular, anticoagulant, BPH and impotence, antipsychotic, antimigraine, anticancer, and so on. The common prescription-indications for some of these medication categories appeared somewhat in line with manifestations of COVID-19. We hope that they can be beneficial for patients with certain specific symptoms of SARS-CoV-2 infection, but they can also probably inhibit viral enzymes. We recommend further experimental evaluations in vitro and in vivo on these FDA-approved drugs to assess their potential antiviral effect on SARS-CoV-2.
Keywords: SARS-CoV-2, COVID-19, Drug repurposing, FDA-approved drugs, RdRp, 3CLpro
Graphical Abstract
Docking drugs against the viral RdRp and 3CLpro enzymes. The candidate drugs were classified into several categories.
1. Introduction
The world is currently experiencing an emerging pandemic called COVID-19 (caused by SARS-CoV-2), to which no effective antiviral drugs or vaccines have been approved to date [1]. A recent hypothesis has proposed that COVID-19 may have three phases. Some of the drugs are probably more effective in each phase separately. These three phases are called the viral early infection phase, the pulmonary phase, and the hyper-inflammation phase [2]. In the early infection phase, antiviral drugs are probably the best option. In the second phase, due to the involvement of the immune system, the lungs become involved. Some symptoms, such as cough, shortness of breath, and hypoxia, are observed in this phase. Blood clots are also reported mostly in the second phase. In the hyper-inflammation phase, the cytokine storm is triggered by the activation of the immune system. The cytokine storm leads to more severe damage to the lungs, kidneys, heart, and other organs. In this phase, the anti-inflammatory category of drug candidates is probably better to be more investigated. Given that these phases overlap, no single drug is expected to be sufficient for all three phases, and a combination of drugs would probably be more efficient [2].
The rapid global spread of this virus has underscored the need to develop anti-Coronavirus therapies. Several approaches and strategies are typically used to detect a potential antiviral treatment against various infections, such as the new Coronavirus. One possible common approach is applying the existing broad-spectrum antiviral drugs using standard assays. Screening the previously approved chemical compounds by bioinformatics tools is another fast method in antiviral drug discovery. In this method, medications are evaluated for their potency to inhibit some essential elements of the new viruses [1], [3].
The 3CLpro is the prime enzyme responsible for proteolysis. It cleaves the viral polyprotein into distinct functional components [4]. The essential value of 3CLpro in the virus life cycle makes it a suitable target for developing effective antiviral drugs against different Coronaviruses [5], [6]. 3Clpro offers unconventional Cys catalytic residues with a unique diversification. Differently from other chymotrypsin-like enzymes and many SER (or Cys) hydrolases, including catalytic Cys-His Dyad instead of a canonical Ser (Cys)-His-Asp (Glu) triad8. The Cys145 and His41 catalytic residues in 3Clpro are entombed on the protein surface in an active site cavity. This cavity can contain four substrates in P1' to P4 positions and is flanked by both Domains I and II residues [7]. Another essential non-structural protein of the Coronavirus is the RNA-dependent RNA polymerase (RdRp, also known as nsp12) [8]. RdRp catalyzes the viral RNA synthesis and thus plays a pivotal role in the SARS-CoV-2 replication and transcription process, probably along with nsp7 and nsp8 as co-factors [9], [10]. Among coronaviruses, particularly in SARS-CoV-2, essential sites such as template entry and binding, polymerase activity reaction site followed by the exit through the tunnel (thumb) are highly conserved. Tyr618, Cys622, Asn691, Asn695, Met755, Ile756, Leu757, Leu758, Ser759, Asp760, Asp761, Ala762, Val763, Glu811, Phe812, Cys813 and Ser814 are the critical residues of interaction in the RDRP active site. The residue of active sites are adjoining aspartates, i.e. Asp761 and Asp762, participate in specific RdRp enzyme reactions [11].
Different anti-RNA polymerase drugs currently on the market have been previously approved for use against various viruses, including Ribavirin [12], Remdesivir [13], Galidesivir [14], and Tenofovir [15]. They are presently being examined against SARS-CoV-2 RNA-dependent RNA polymerase (RdRp). For the 3CLpro target, several studies and current clinical trials have proposed the Lopinavir [16], Ritonavir [17], Darunavir [18], Ganovo [19], ASC09F [20], and Cobicistat [21]. Ritonavir/Lopinavir (LPV) is one of the most commonly reported clinical trials for COVID-19. Even though some data indicate somewhat efficacy for LPV, its severe side effects are considerable [22], [23]. These confirm that RdRp and 3CLpro can be recommended as valuable targets for drug design against SARS-CoV-2, and inhibition of their activity seems a promising strategy to cure SARS-CoV-2 infection. In this study, we used a target-based virtual screening approach to identify novel inhibitors of SARS-CoV-2 RdRp and 3CLpro.
2. Methods
2.1. Retrieving drugs from databases and ligand minimizations
Drug repurposing using virtual screening (VS) techniques is one of the rapid and most promising strategies to candidate drugs against the Coronavirus [24].
In this study, 3D structures of 1760 FDA-approved drugs were retrieved from the NCBI PubChem database [25]. In fact, there were three-dimensional structures for approximately 2500 approved small molecule drugs (not proteins, etc); Therefore, We first removed some of the structures from our selection set including, the two-component structures, tiny compounds weighing less than 100 kDa, and the large-complex compounds with a high number of rotatable bonds. The remaining small molecules were filtered and selected for further docking analysis, including the 1760 small molecule drugs. The conjugate gradient geometry optimization was performed using Open Babel [26] and MMFF94 force fields for each drug geometry [27].
2.2. Molecular modeling and energy minimization of targets
RdRp and 3c-like proteinase (3CLpro) (from reference sequence of Accession number NC_045512) protein sequences were retrieved from the NCBI database. Then, homology modeling predictions were carried out using the Swiss model server (https://swissmodel.expasy.org/). The structures were energetically minimized using SPDB viewer software [28] and visualized by the CHIMERA UCSF v1.14 software [29]. The binding sites (active sites) in target proteins were identified by evaluating protein grooves in CHIMERA UCSF software 22 [30] and considering the previous studies [19], [31]. Since recently crystallography structures of the proteins were reported in PDB databank, we performed superimposing to check our homology modeling similarity with the crystallography results. Superimposing of the modeled structure with deposited crystallography structures available in PDB (Protein Databank) revealed the root mean square deviation (RMSD) value of < 2 angstroms (among 0.3–1.5 angstrom), which meant a perfect fit. Therefore, modeling has insignificant impacts on our overall results compared to using crystallography structures.
2.3. Preparation of protein structures for docking analysis
All nonpolar hydrogens were merged. Partial atomic charges were then assigned using the Gasteiger-Marsili approach for accurate ionization and tautomeric states of residues. Besides, charges were added to models, and Kollman United Atom charges and atomic salvation parameters were performed.
2.4. Molecular docking
Molecular docking was carried out to evaluate possible energy of interactions, hydrogen bonds, non-hydrogen bonds, and binding mode of FDA ligand datasets against RdRp and 3c-like proteinase binding sites. The docking studies were performed using AutoDock Vina v1.1.2 software in the PyRx v0.9.8 platform [32], [33]. In docking, targets were considered semi-rigid while ligands were flexible. To perform the suitable docking for each ligand, we set the search space box parameters on 32–37–39 Å (direction, x, y, and z), centered at (− 8, 15, and 67) Å, for 3c-like proteinase, and upon 35–39–42 Å, centered at (144, 133, 158) Å, for RdRp.
Final docked conformations were ranked based on binding energy (∆G) results, which meant the most favorable binding conformations had the lowest free energies. They were selected as suitable poses of binding and were then visually analyzed. Hydrogen bonds and the hydrophobic interactions between ligands and RdRp and 3c-like proteinase were analyzed (two-dimensionally) using LIGPLOT v.4.5.3 27 software [34]. Besides, the two-dimensional and three-dimensional structures of the selected ligands were analyzed using Discovery Studio Visualizer v3.0 software [35], [30].
2.5. Molecular dynamics simulations
An100 ns MD simulation for RdRp and 3clpro was used to confirm the docking results for identified candidate antiviral drugs. Molecular dynamics (MD) is a mathematical tool for analyzing the system dynamic structural behavior; in this process, atoms and molecules interact as a time-based function. The simulations of MD take the versatility of goals into account. The structural parameter RMSD and the number of intermolecular H-bonds have been used for determining the stability, dynamics and compactness of protein-drug complexes [36].
Six drugs were chosen for MD analysis based on binding energy and the number of hydrogen bonds in docking analysis. Six simulations were performed using the GROMACS 5.1.4 simulation suite for FDA-approved drugs containing Nilotinib, Imatinib, and dihydroergotamine for 3clpro and Lapatinib, Dexasone, and Relategravir for RdRp. The gromos54a7 force field was utilized for the complexes [37]. The ATB server was used for the preparation of the coordinates and topology of ligands [38]. The complexes were then solvated with TIP3P water molecules in a truncated octahedron periodic box with an 8 Å radius buffer zone of water molecules around the complexes using Gmx Editconf&Solvate softwares. Then counter ions have been added with the tool of Gromacs to neutralize the overall system charge. The surface charge of the structure was neutralized by adding several sodium ions. Reduction of energy on the structures was performed with 50,000 steps using the steepest descent method for eliminating van der Waals interactions and formation of hydrogen bonds between water molecules and the complex. In the next step, the system temperature was gradually increased from 0° to 310° K for 500 ps at constant volume, and then at constant pressure for 500 ps the system was equilibrated. Molecular dynamics simulations were performed at a temperature of 310 K and a duration of 100 nanoseconds. Non-bonded interactions with 10 Å intervals were calculated by the PME method. The SHAKE algorithm was used to limit the hydrogen atom bonds to increase computational speed. Finally, the simulation information was saved at 0.2 ps intervals for analysis.
3. Result
Molecular docking was performed on FDA-approved drugs to determine the potential drug candidates for inhibiting the SARS-CoV-2. The docking was based on the recognition of the binding pocket of Homology Modeled RdRp and 3CLpro enzymes. The SWISS online server modeled the viral proteins. The number of ligands selected from the PubChem database of NCBI for screening was 1760. All these drugs were docked against the two target enzymes of SARS-CoV-2 and ranked based on their binding affinity. The compounds with a binding affinity of − 8.5 or less were considered better compounds, possibly inhibiting the RdRp enzyme. The binding affinity of − 8.1 or less was considered the selection criterion against the 3CLpro protein. We used AutoDock Vina to dock the drugs to achieve more accurate medicines related to the two viral essential components. We first selected the top 100 medications for each viral target based on the order of their affinity energies. Depending on the rate of changes in the affinity energies among the drugs ordered, we selected 58 candidate drugs against the active site of the RdRp enzyme with an affinity of − 8.5 or less and 69 candidate drugs against the active site of the 3CLpro enzyme with affinity binding. − 8.1 or less. We observed that 20 drugs had binding affinity energy less than − 9 against the RdRp target. However, only seven drugs had binding affinity energy less than − 9 against the 3CLpro. They are likely to provide promising drugs against SARS-CoV-2. All the candidate drugs were then classified into several categories ( Table 1, Table 2). We sought further studies on COVID-19 drugs to validate our identified drugs. We found that some of these candidate drugs have already been introduced or validated by various other studies, including in-silico, preclinical, and clinical trials. These verifying studies are available in Table 4. We also compared the two identified drug lists using the online Venn diagram tool. Supplementary Fig. S1 depicts the Venn diagram comparing the two drug lists against RdRp and 3CLpro. We found that 32 drugs were shared between the two drug lists. They seem to be promising since they would probably inhibit both of the essential viral components. Supplementary Table S1 lists these 32 shared drugs (vs. RdRp and 3CLpro).
Table 1.
Drugs identified significantly interact with RdRp.
| Drug category | Drug Bank Drug ID | Drug Bank Drug Name | Drug mechanism | Binding affinity |
|---|---|---|---|---|
| Anti-inflammatory drug | ||||
| DB11611 | Lifitegrast | Lifitegrast inhibits an integrin | -8.7 | |
| DB08995 | Diosmin | A topical anesthetic and an anti-inflammatory agent | -8.7 | |
| DB04703 | Hesperidin | Tyrosin kinase activity | -8.9 | |
| Anti-allergy drug | ||||
| DB00549 | Zafirlukast | Leukotriene Receptor Antagonists | -8.5 | |
| DB01003 | Cromoglicic acid | Inhibiting the release of chemical mediators from sensitized mast cells | -8.9 | |
| Anti-bacterial drug | ||||
| DB12127 | Sultamicillin | Prevention and treatment of Ventilator-Associated Pneumonia and Chronic Obstructive Pulmonary Disease | -8.8 | |
| DB00430 | Cefpiramide | Inhibiting bacterial cell wall biosynthesis | -8.9 | |
| DB04918 | Zevtera; Ceftobiprole | Inhibiting bacterial cell wall biosynthesis is active against methicillin-resistant Staphylococcus aureus. | -8.7 | |
| DB01329 | Ceftobiprole | Inhibiting the bacterial cell wall synthesis | -8.7 | |
| DB01051 | Novobiocin | Novobiocin binds to DNA gyrase and blocks adenosine triphosphatase (ATPase) activity. | -8.5 | |
| DB09335 | Alatrofloxacin | Anti-bacterial effect by preventing bacterial DNA from unwinding and duplicating. | -9.0 | |
| DB09050 | Ceftolozane | Inhibiting bacterial cell wall biosynthesis | -8.9 | |
| DB01212 | Ceftriaxone | Inhibiting bacterial cell wall biosynthesis | -9.0 | |
| DB12434 | Steviolbioside | Inhibit mycobacterium | -8.5 | |
| Antidepressant drug | ||||
| DB13520 | Metergoline | Antipsychotics | -8.6 | |
| DB01267 | Paliperidone | Antipsychotics, 2nd Generation | -8.6 | |
| DB08815 | Lurasidone | Antipsychotics, 2nd Generation | -8.5 | |
| DB06684 | Vilazodone | Antidepressants, SSRI/5HT-1A Partial Agonist | -8.5 | |
| Antidiabetics drug | ||||
| DB08882 | Linagliptin | Dipeptyl Peptidase-IV Inhibitors | -9.2 | |
| DB00222 | Glimepiride | Sulfonylureas | -8.6 | |
| Antifungal drug | ||||
| DB01167 | Itraconazole | Inhibits cytochrome P-450-dependent enzymes resulting in impairment of ergosterol synthesis | -8.5 | |
| DB00826 | Natamycin | Inhibits fungal growth by binding to sterols | -9.4 | |
| Anti-migraine | ||||
| DB00696 | Ergotamine | Ergot Derivatives | -9.5 | |
| DB00320 | Dihydroergotamine | Ergot Derivatives | -9.4 | |
| Antiviral drug | ||||
| DB06817 | Raltegravir | HIV, Integrase Inhibitors | -9.5 | |
| BPH& impotency drug | ||||
| DB00590 | Doxazosin | Alpha-Blockers | -9.3 | |
| DB01126 | Dutasteride | 5-Alpha-Reductase Inhibitors | -8.8 | |
| DB00820 | Tadalafil | PAH, PDE-5 Inhibitors; Phosphodiesterase-5 Enzyme Inhibitors | -9.2 | |
| DB06237 | Avanafil | Phosphodiesterase-5 Enzyme Inhibitors | -9.0 | |
| Cardiovascular drug | ||||
| DB11577 | Indigotindisulfonic acid | Coloring Agents | -8.6 | |
| DB04861 | Nebivolol | Adrenergic beta-1 Receptor Agonizts | -8.7 | |
| DB08822 | Azilsartan medoxomil | ARBs (Angiotensin II receptor blocker) | -9.2 | |
| DB11691 | Naldemedine | Peripherally-Acting Mu-Opioid Receptor Antagonists (PAMORA) | -8.9 | |
| DB11995 | Avatrombopag | Thrombopoietic Agents (BCRP/ABCG2 Inhibitors) | -8.9 | |
| DB00872 | Conivaptan | Vasopressin-related | -8.8 | |
| DB06210 | Eltrombopag | Hematopoietic Growth Factors | -9.0 | |
| Anticoagulant | ||||
| DB09075 | Edoxaban | Factor Xa Inhibitors | -8.7 | |
| Anticancer drug | ||||
| DB11791 | Capmatinib | MET Tyrosine Kinase Inhibitors | -8.5 | |
| DB01259 | Lapatinib | HER2/ERBB2 and HER1/EGFR/ERBB1 tyrosine kinases inhibitor. | -9.4 | |
| DB05812 | Abiraterone | Antiandrogen | -8.7 | |
| DB00563 | Methotrexate | DMARDs, Immunomodulators; Immunosuppressants | -8.5 | |
| DB12001 | Abemaciclib | CDK Inhibitors; Immunosuppressives, | -8.5 | |
| DB00444 | Teniposide | Podophyllotoxin Derivatives | -8.8 | |
| DB00773 | Etoposide | Podophyllotoxin Derivatives | -8.6 | |
| DB00762 | Irinotecan | Topoisomerase Inhibitors | -9.4 | |
| DB11986 | Entrectinib | Tyrosine Kinase Inhibitor | -9.4 | |
| DB04868 | Nilotinib | Tyrosine Kinase Inhibitor | -9.2 | |
| DB06595 | Midostaurin | Tyrosine Kinase Inhibitor | -9.1 | |
| DB08901 | Ponatinib | Tyrosine Kinase Inhibitor | -8.6 | |
| DB01254 | Dasatinib | Tyrosine Kinase Inhibitor; Immunosuppressives | -9.1 | |
| DB00619 | Imatinib | Tyrosine Kinase Inhibitor; PDGFR-alpha Inhibitors; CYP3A4 Inhibitor | -8.9 | |
| DB09280 | Lumacaftor | CFTR Correctors; CFTR Potentiators | -9.1 | |
| DB11942 | Selinexor | Selective Inhibitors of Nuclear Export (SINE); tumor suppressor proteins (TSPs) | -9.2 | |
| Other drugs | ||||
| DB01452 | Diamorphine | Analgesics | -8.6 | |
| DB00137 | Lutein | Lutein helps protect from oxidative stress and high-energy light | -8.6 | |
| DB08827 | Lomitapide | Lipid-Lowering Agents, MTP Inhibitor | -8.7 | |
| DB00157 | NADH | Metabolic & Endocrine, Herbals; Neurology & Psychiatry, Herbals | -8.6 | |
| DB11176 | Zeaxanthin | Prevention of age-related macular degeneration | -8.5 | |
Table 2.
Drugs identified significantly interact with 3CLpro.
| Drug category | Drug Bank Drug ID | Drug Name | Drug mechanism | Binding affinity |
|---|---|---|---|---|
| Anti-inflammatory drug | ||||
| DB11611 | Lifitegrast | Lifitegrast inhibits an integrin | -8.6 | |
| DB04703 | Hesperidin | Tyrosin kinase activity | -8.4 | |
| DB01419 | Antrafenine | An analgesic and anti-inflammatory drug | -8.1 | |
| DB00554 | Piroxicam | NSAIDs | -8.2 | |
| DB00471 | Montelukast | Leukotriene Receptor Antagonists | -8.2 | |
| DB14632 | Prednisolone tebutate | Lipocortin I, p11/calpactin binding protein, secretory leukocyte protease inhibitor 1 (SLPI), and Mitogen-activated protein kinase phosphatase (MAPK phosphatase) | -8.3 | |
| Anti-allergic drug | ||||
| DB00637 | Astemizole | Second-generation H1-receptor antagonist. | -8.4 | |
| DB01003 | Cromoglicic acid | Inhibiting the release of chemical mediators from sensitized mast cells. | -8.6 | |
| DB00549 | Zafirlukast | Leukotriene Receptor Antagonists | -8.2 | |
| Anti-bacterial drug | ||||
| DB12329 | Eravacycline | Disrupts bacterial protein synthesis | -8.5 | |
| DB09335 | Alatrofloxacin | Anti-bacterial effect by preventing bacterial DNA from unwinding and duplicating. | -8.5 | |
| DB00845 | Clofazimine | Antitubercular Agents | -8.3 | |
| DB00685 | Trovafloxacin | Blocking the activity of DNA gyrase and topoisomerase IV. | -8.9 | |
| DB09050 | Ceftolozane | Inhibiting bacterial cell wall biosynthesis | -8.3 | |
| DB11943 | Delafloxacin | Inhibits the activity of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II) Label. | -8.4 | |
| Anticonvulsants | ||||
| DB08883 | Perampanel | AMPA Glutamate Antagonists | -8.6 | |
| Antidepressant drug | ||||
| DB04842 | Fluspirilene | Antagonist for D(2) dopamine receptor Voltage-dependent calcium channel gamma-1 subunit | -8.8 | |
| DB01100 | Pimozide | Antipsychotics, 1st Generation | -8.6 | |
| DB08815 | Lurasidone | Antipsychotics, 2nd Generation | -8.6 | |
| Antidiabetics drug | ||||
| DB01251 | Gliquidone | ATP-dependent K+ (KATP) channel blocker | -8.2 | |
| Antifungal drug | ||||
| DB00826 | Natamycin | Inhibits fungal growth by binding to sterols | -8.5 | |
| Antihistamines | ||||
| DB11614 | Rupatadine | Dual histamine H1 receptor and platelet-activating factor receptor antagonist | -8.4 | |
| Antihypertensive drug | ||||
| DB08822 | Azilsartan medoxomil | ARBs (Angiotensin II receptor blocker) | -8.2 | |
| Anti-migraine | ||||
| DB00696 | Ergotamine | Ergot Derivatives; vasoconstrictor and alpha adrenoreceptor antagonist. | -9.4 | |
| DB00320 | Dihydroergotamine | Ergot Derivatives | -9.6 | |
| Antiparkinson drug | ||||
| DB01200 | Bromocriptine | Dopamine Agonizts;Hyperprolactinemia; Metabolic & Endocrine, Other | -9.2 | |
| Antiplatelet and anticoagulant drug | ||||
| DB12364 | Betrixaban | Anticoagulants, Factor Xa Inhibitors | -8.8 | |
| DB09075 | Edoxaban | Factor Xa Inhibitors | -8.5 | |
| DB09030 | Vorapaxar | Antiplatelet Agents, Cardiovascular; Thrombin Inhibitors; Protease Activated Receptor-1 (PAR-1) Inhibitors | -8.6 | |
| DB08816 | Ticagrelor | An antagonist of P2Y12. This prevents ADP binding to the P2Y12 receptor | -8.2 | |
| Antiviral drug | ||||
| DB00224 | Indinavir | HIV protease inhibitor | -8.2 | |
| DB11799 | Bictegravir | HIV, Integrase Inhibitors | -8.6 | |
| DB08930 | Dolutegravir | HIV, Integrase Inhibitors | -8.3 | |
| DB06817 | Raltegravir | HIV, Integrase Inhibitors | -8.3 | |
| BPH& impotency drug | ||||
| DB01126 | Dutasteride | 5-Alpha-Reductase Inhibitors | -8.6 | |
| DB00820 | Tadalafil | PAH, PDE-5 Inhibitors; Phosphodiesterase-5 Enzyme Inhibitors | -9.3 | |
| Cardiovascular drug | ||||
| DB11577 | Indigotindisulfonic acid | Coloring Agents | -9.1 | |
| DB11691 | Naldemedine | Peripherally-Acting Mu-Opioid Receptor Antagonists (PAMORA) | -8.6 | |
| DB04861 | Nebivolol | Adrenergic beta-1 Receptor Agonizts | -8.1 | |
| DB01698 | Rutin | Capillary Stabilizing Agents | -8.6 | |
| DB06210 | Eltrombopag | Hematopoietic Growth Factors | -8.9 | |
| DB00872 | Conivaptan | Vasopressin-Related | -8.5 | |
| DB00966 | Telmisartan | Angiotensin II receptor blocker (ARBs) | -8.2 | |
| Anticancer drug | ||||
| DB01259 | Lapatinib | HER2/ERBB2 and HER1/EGFR/ERBB1 tyrosine kinases inhibitor. | -8.6 | |
| DB00762 | Irinotecan | Topoisomerase Inhibitors | -8.7 | |
| DB11986 | Entrectinib | Tyrosine Kinase Inhibitor | -8.8 | |
| DB09280 | Lumacaftor | CFTR Correctors; CFTR Potentiators | -8.9 | |
| DB00444 | Teniposide | Podophyllotoxin Derivatives | -8.7 | |
| DB00773 | Etoposide | Podophyllotoxin Derivatives | -8.3 | |
| DB11942 | Selinexor | Selective Inhibitors of Nuclear Export (SINE); tumor suppressor proteins (TSPs) | -8.8 | |
| DB13874 | Enasidenib | IDH2 Inhibitors | -8.4 | |
| DB11791 | Capmatinib | MET Tyrosine Kinase Inhibitors | -8.9 | |
| DB09079 | Nintedanib | Pulmonary, Tyrosine Kinase Inhibitors | -8.5 | |
| DB00619 | Imatinib | Tyrosine Kinase Inhibitor; PDGFR-alpha Inhibitors; CYP3A4 Inhibitor | -8.9 | |
| DB06595 | Midostaurin | Tyrosine Kinase Inhibitor | -9.9 | |
| DB09063 | Ceritinib | Anaplastic Lymphoma Kinase Inhibitor; CYP3A4 Inhibitor | -8.4 | |
| DB11718 | Encorafenib | BRAF Kinase Inhibitor; CYP3A4 Inhibitor, Moderate; CYP3A4 Inducers | -8.2 | |
| DB08911 | Trametinib | MEK Inhibitors | -8.6 | |
| DB11760 | Talazoparib | PARP Inhibitors | -8.2 | |
| DB09053 | Ibrutinib | Tyrosine Kinase Inhibitor | -8.4 | |
| DB12141 | Gilteritinib | Tyrosine Kinase Inhibitor | -8.3 | |
| DB08875 | Cabozantinib | Tyrosine Kinase Inhibitor | -8.3 | |
| DB04868 | Nilotinib | tyrosine kinase inhibitor | -9.4 | |
| DB09078 | Lenvatinib | VEGF Inhibitor | -8.1 | |
| Other drugs | ||||
| DB01395 | Drospirenone | Contraceptives, antimineralocorticoid and antiandrogenic activity; binding to the progesterone receptor | -8.5 | |
| DB00973 | Ezetimibe | Antilipemic agent; Inhibits sterol transporter at the brush border | -8.3 | |
| DB08827 | Lomitapide | Lipid-Lowering Agents, MTP Inhibitor | -8.1 | |
| DB00693 | Fluorescein | Diagnostics, Ophthalmics | -8.3 | |
| DB01138 | Sulfinpyrazone | Inhibition of the urate anion transporter (hURAT1) as well as the human organic anion transporter 4 (hOAT4) | -8.4 | |
Table 4.
Some of the candidate drugs have already been introduced or validated by various other studies, including in-silico, preclinical, and clinical trials.
| No | Drug category | Drug name/ID | Active against | The possible mechanism in COVID-19 treatment | Type of validation by other studies against SARS-CoV-2 |
Reference | ||
|---|---|---|---|---|---|---|---|---|
| Another in-silico | Preclinical studies (in vitro, in vivo) | Clinical trials, Case reports, Retrospectives | ||||||
| 1 | Antiviral | Raltegravir (DB06817) |
|
|
Yes | Kumar [41] | ||
| Mohamed [42] | ||||||||
| 2 | Antiviral | Indinavir (DB00224) |
|
|
Yes | Dong [10] | ||
| Chang [9] | ||||||||
| 3 | Antiviral | Dolutegravir (DB08930) |
|
|
Yes | Beck [39] | ||
| Khan [40] | ||||||||
| 4 | Anti bacterial | Eravacycline (DB12329) |
|
|
Yes | Wang [43] | ||
| 5 | Anti-inflammatory | Hesperidin (DB04703) |
|
|
Yes | [51] | ||
| [54] | ||||||||
| [19] | ||||||||
| [53] | ||||||||
| [48] | ||||||||
| [49] | ||||||||
| [50] | ||||||||
| [52] | ||||||||
| 6 | Anti-inflammatory | Diosmin (DB08995) |
|
|
Yes | Adem [48] | ||
| 7 | Anti-inflammatory | Rutin (DB01698) |
|
|
Yes | Adem [48] | ||
| Shanno [103] | ||||||||
| Wu [52] | ||||||||
| Das [50] | ||||||||
| 8 | Anti-allergic/asthmatic | Cromoglicic acid (DB01003) |
|
|
Yes | Han [60] | ||
| Shankar [61] | ||||||||
| 9 | Anti-allergic/asthmatic | Montelukast (DB00471) |
|
|
NCT | Fidan and Aydoğdu [57] | ||
| 04389411 | ||||||||
| Phase III | ||||||||
| 10 | Cardio vascular | Telmisartan (DB00966) |
|
|
NCT | Rothlin [104] Gurwitz [105] | ||
| 04360551 | ||||||||
| Phase II | ||||||||
| NCT 04355936 | ||||||||
| Phase II | ||||||||
| 11 | Cardio vascular | Avatrombopag (DB11995) |
|
|
Yes | Sajib [64] | ||
| 12 | Cardio vascular Cardio vascular | Azilsartan medoxomil (DB08822) |
|
|
Yes | Sato et al. [106] | ||
| Review | ||||||||
| Kai and Kai [65] | ||||||||
| Hypothesis | ||||||||
| [107] | ||||||||
| 13 | Cardio vascular | Conivaptan (DB00872) |
|
|
Yes | [52] | ||
| [108] | ||||||||
| [109] | ||||||||
| 14 | Cardio vascular | Eltrombopag (DB06210) |
|
|
Vero cells | Jeon et al. [68] | ||
| Arshad et al. [69] | ||||||||
| [67] | ||||||||
| [110] | ||||||||
| 15 | Cardio vascular | Nebivolol (DB04861) |
|
|
Yes | [111] | ||
| [87] | ||||||||
| 16 | Anti-coagulant | Ticagrelor (DB08816) | P2Y12 receptor antagonism Reduces levels of pro-inflammatory factors inhibits reactivation of platelets, decrease lung injury (by reducing thrombo-inflammatory) |
|
Letter | [72] | ||
| [73] | ||||||||
| Hypothesis | ||||||||
| [74] | ||||||||
| [75] | ||||||||
| 17 | Anti coagulant | Edoxaban (DB09075) |
|
|
Yes | Testa et al. [112] | ||
| Baker et al. [113] | ||||||||
| 18 | BPH | Dutasteride (DB01126) |
|
|
Yes | Kroumpouzos [84] | ||
| Chernyshev [79] | ||||||||
| Hosseini et al. [78] | ||||||||
| 19 | BPH | Doxazosin (DB00590) |
|
|
Yes | Gupta [80] | ||
| 20 | Impotency | Tadalafil (DB00820) |
|
|
Yes | Anwar [114] | ||
| Sharma [81] | ||||||||
| 21 | Anti psychotic | Fluspirilene (DB04842) |
|
|
Vero E6 cell line | Weston et al. [115] | ||
| Dyall et al. [116] | ||||||||
| 22 | Anti psychotic | Pimozide (DB01100) |
|
|
Yes | Yes | Vatansever et al. [117] | |
| Gul et al. [87] | ||||||||
| 23 | Anti psychotic | Lurasidone (DB08815) |
|
|
Yes | Gul et al. [87] | ||
| 24 | Antimigraine | Ergotamine(DB00696) |
|
|
Yes | [91] | ||
| [118] | ||||||||
| [90] | ||||||||
| 25 | Anti-diabetic | Linagliptin (DB08882) |
|
|
NCT 04341935 | [93] | ||
| [96] | ||||||||
| Phase IV | [97] | |||||||
| NCT 04371978 | [98] | |||||||
| [99] | ||||||||
| Phase III | ||||||||
| [94] | ||||||||
| [95] | ||||||||
| 26 | Analgesic | Diamorphine (DB01452) |
|
|
Hypothesis | Sawynok [102] | ||
| Marinelli [100] | ||||||||
| Hulin et al. [119] | ||||||||
| 27 | Contraceptive | Drospirenone (DB01395) |
|
|
Yes | Hosseini [78] | ||
All identified drug candidates were classified into several categories, including antiviral, anti-bacterial, anti-inflammatory, anti-allergic, cardiovascular, anticoagulant, BPH and impotence, antipsychotic, antimigraine, anticancer, and so on. Table 1, Table 2 represent these classifications for the identified drug candidates against RdRp and 3CLpro separately. The common prescription-indications for some of these medication categories appeared to be somewhat in line with manifestations of COVID-19. The docking binding interactions of the top ten active molecules (based on their binding energy) against the RdRp target are illustrated in Supplementary Fig. S2a and b.
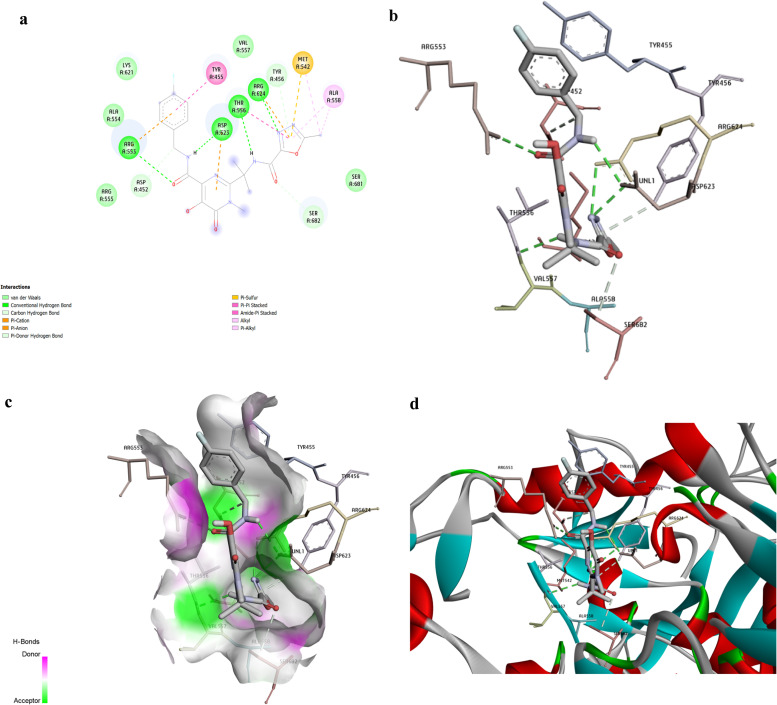
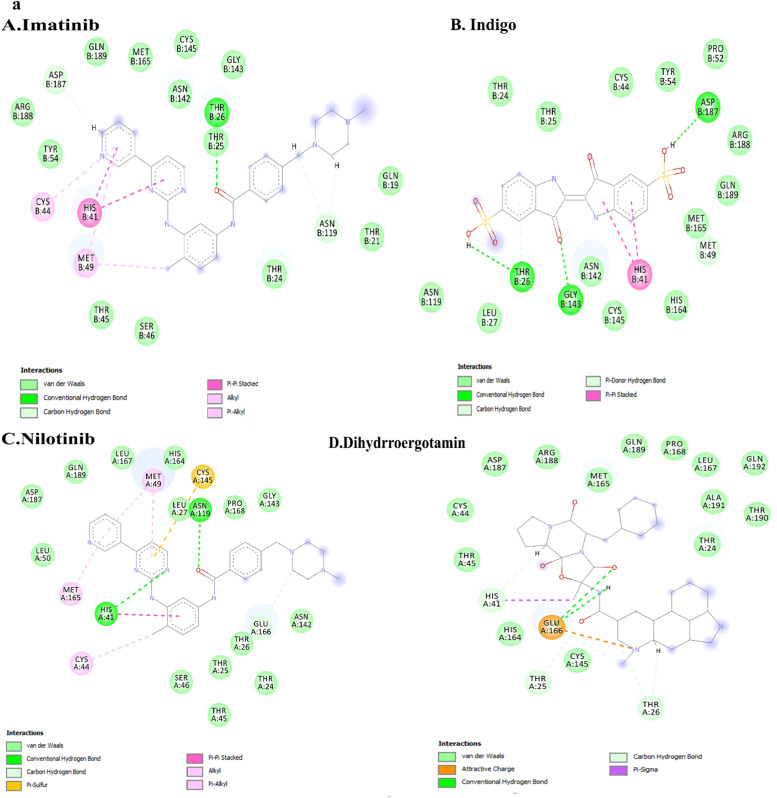
Raltegravir, an anti-HIV drug, was discovered to be the best compound against RdRp based on binding energy (− 9.5 kcal/mole). Doxazosin (− 9.3 kcal/mol) was also a BPH drug that appeared as the 9th drug on our list. Both Raltegravir and Doxazosin formed four types of bonds, including H-bonds, hydrophobic contacts, Pi contacts, and halogen interactions illustrated in Figures No. 1 and 2. As represented in Fig. 1a, Raltegravir formed four conventional H-bonds (ARG553, ASP623, THR556, and ARG624). Besides, Raltegravir made three cation and anion Pi interactions (with ARG553, ASP623, and a Pi-sulfur interaction with MET542). It also formed five van der Waals contacts (with LYS621, ALA554, VAL557, ARG555, and SER681). Besides, Raltegravir had two Pi-alkyl with MET542 and ALA558. It also made two Pi-Pi stacked with TYR455 and THR556. Fig. 1b–d provide the 3D indications of docking interactions between Raltegravir and RdRp active site residues.
Fig. 1.
The docking analysis of Raltegravir and RdRp enzyme interactions. Raltegravir, an anti-HIV drug, was discovered to be the best drug against RdRp, based on binding energy (− 9.5 kcal/mol). The Interactions between Raltegravir and RdRp were visualized using Discovery Studio software. a- The picture represents the results for the analysis of interactions between RdRp and Raltegravir. The colored circles are related to the RdRp residues interacting with Raltegravir. H bonds are represented in green color dashed lines. Conventional and Pi-donor H-bonds and hydrophobic interactions are depicted with various colors described in the picture guide of interaction colors below the shape. b- H bond interactions between Raltegravir and RdRp residues. c-Position of Raltegravir in the RdRp active site pocket; Electron donors and acceptors in the h-bonds. d- The picture indicates the3D interactions between Raltegravir and the RdRp essential amino acids. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
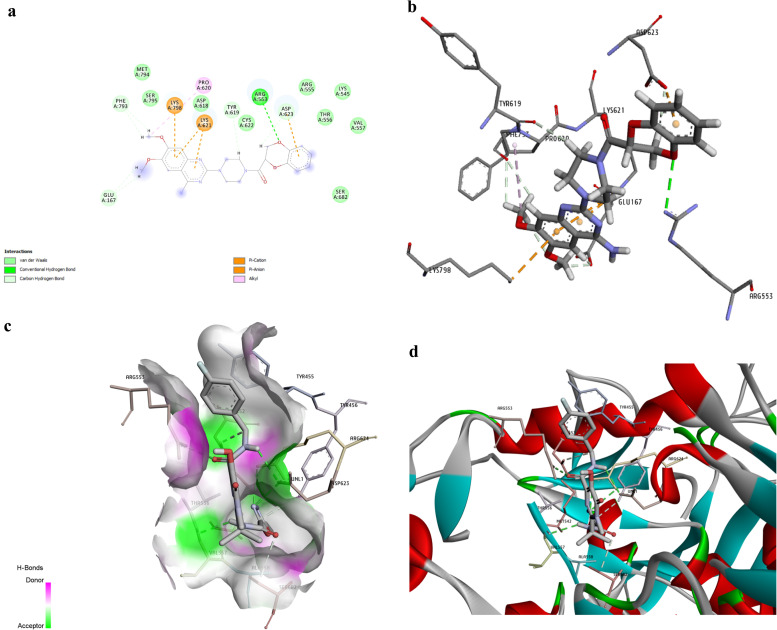
Fig. 2a indicates Doxazosin interactions. Doxazosin made three conventional H-bonds (with TYR455, ARG553 and ARG624). It also formed five van der Waals contacts (with LYS545, ALA554, THR556, VAL557ana LYS798). Besides, it made tree Pi-alkyl interactions with LYS551, ARG553, LYS621, and ARG624. Fig. 2b–d represent the 3D interactions between Doxazosin and RdRp active site residues.
Fig. 2.
The shape shows the interactions between Doxazosin and the viral RdRp enzyme established after docking analysis. Doxazosin (− 9.3 kcal/mol) is a BPH drug with the 9th rank in our drug list. a. The analysis of Binding interactions between Doxazosin and RdRp. The H bonds, conventional and Pi-donor H-bonds, and hydrophobic interactions are depicted with various colors described in the picture guide of interaction colors below the shape. b. 3D picture of H bond interactions between Doxazosin and RdRp residues; The H bonds are represented with green dashed lines. c. Position of Doxazosin in the pocket of the RdRp active site. d. A three-dimensional indication of interactions between Doxazosin and RdRp essential residues. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
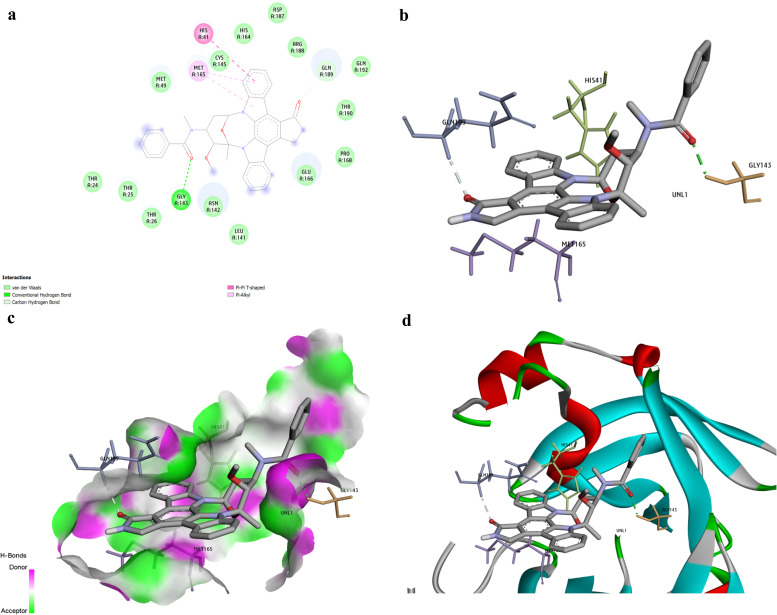
Docking interactions of the top ten active molecules (based on their binding energy) against the 3CLpro target are depicted in Supplementary Fig. S3a and b. The best compound was discovered to be Rydapt based on the binding energy (− 9.9 kcal/mol). Trovan (− 8.9 kcal/mol) was also the 9th drug on our list. The binding interactions of Rydapt are presented in Fig. 3a. The Hydroxyl group of Rydapt interacts by forming an H-bond with amino acid GLY143. Besides, it established 14 vans der Waals interactions with different residues, including MET49, ASP187, CYS146, THR24,25,26,190, ARG188, ASN142, GLU166, PRO168, LEU141, SYS145, and HIS164. A Pi-Pi T-shaped interaction was also visible between amino acid HIS41 and phenyl ring. Besides, a Pi-alkyl was observed between MET165 and the phenyl ring. The 3D pictures indicating the docking interaction of Rydapt and 3CLpro are shown in Fig. 3b–d.
Fig. 3.
The best compound identified against 3CLpro was Rydapt (− 9.9 kcal/mol) based on binding affinity. a. The picture represents the results for the analysis of interactions between Rydapt and the viral 3CLpro. The H bonds, conventional and Pi-donor H-bonds, and hydrophobic interactions are depicted using various colors described in the picture guide of interaction colors below the shape. b. The 3D picture shows the docking interactions between Rydapt and 3CLpro residues. c. Position of Rydapt in the pocket of 3CLpro active site. d. A three-dimensional indication of interactions between Rydapt and the 3CLpro active site amino acids. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
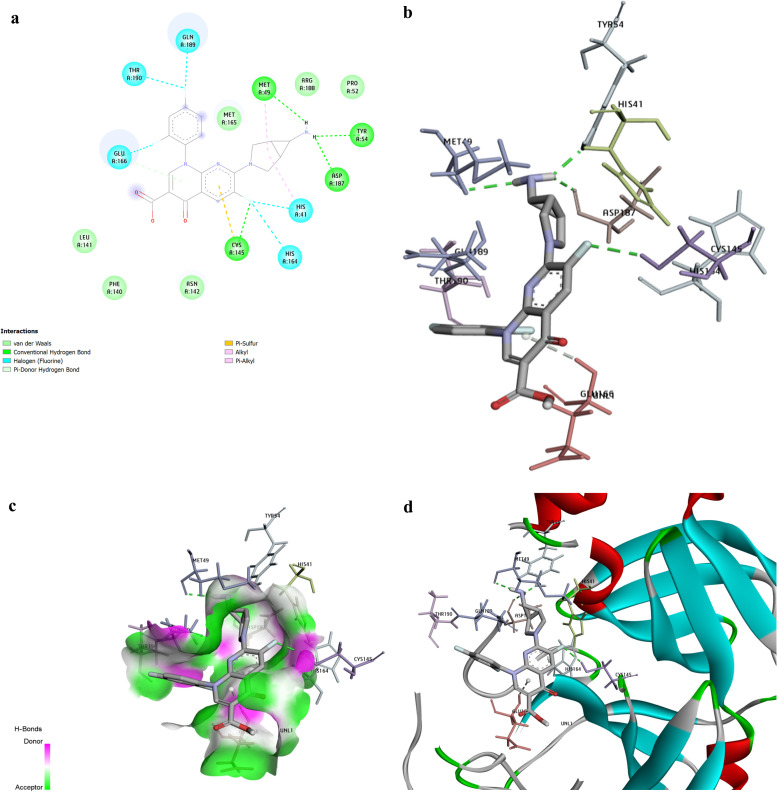
The drug called Trovan formed four H-bonds with amino acids MET49, ASP187, CYS145, and TRY54. It made a Pi-sulfur binding with CYS145 and made two Pi-alkyl interactions with MET49 and HIS41. It also formed five halogens (fluorine) bindings with GLN166, GLN189, THR190, HIS41, and HIS 164 (available in Fig. 4a). Fig. 4b–d show the 3D docking interactions between Trovan and the 3CLpro active site’ residues. Table 3 also represents the number of hydrogen bonding in the top seven drugs against RdRp and 3CLpro. The drugs are ranked based on their binding energy. Among them, Lapatinib is predicted to interact with RdRp forming six H bonds and Doxazosin-1, with seven H bonds. However, Indigo Carmine interacts with 3CLpro, forming possibly 12H bonds.
Fig. 4.
Trovafloxacin (Trovan) had a binding affinity of − 8.9 kcal/mol. The picture shows the interactions between Trovafloxacin (Trovan) and the viral 3CLpro. a. The picture represents the results for the analysis of interactions between Trovan and 3CLpro. H bonds, conventional and Pi-donor H-bonds, and hydrophobic interactions are depicted using various colors described in the picture guide of interaction colors below the shape. b. The picture shows the 3D docking interactions between Trovan and 3CLpro residues. H bonds are shown with green dashed lines. c. Position of Trovan in the pocket 3CLpro active site. d. The 3D picture shows the interactions between Trovan and 3CLpro active site’ critical residues. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Number of H bonds of top 10 drugs against RdRp and 3CLpro.
| Rank | complex | Drugbank ID | Binding affinity | Number of H bonds | Enzyme residue | Ligand atom | Distance |
|---|---|---|---|---|---|---|---|
| RdRp | |||||||
| 1 | Raltegravir (Dutrebis) | DB06817 | -9.5 | 3 | Arg624: NH2 | N5 | 2.9 |
| 2 | Ergotamine | DB00696 | -9.5 | 2 | Glue811: O | N5 | 3.14 |
| 2 | Arg553: NH1 | O2 | 3.14 | ||||
| 3 | Lapatinib | DB01259 | -9.4 | 6 | Arg624: NH1 | O4 | 2.95 |
| 4 | Irinotecan | DB00762 | -9.4 | 3 | Trp617: O | O4 | 2.89 |
| 5 | Entrectinib | DB11986 | -9.4 | 1 | Tyr456: OH | N5 | 2.67 |
| 6 | Dihydroergotamine | DB00320 | -9.4 | 1 | Arg618: ODN3 | O5 | 2.7 |
| 7 | Natamycin | DB00826 | -9.4 | 3 | Arg624: NH2 | O12 | 2.8 |
| 8 | Doxazosin | DB00590 | -9.3 | 7 | Arg624: NH2 | O5 | 2.93 |
| 9 | Linagliptin | DB08882 | -9.2 | 3 | Thr556: OG1 | N7 | 2.92 |
| 10 | Tadalafil | DB00820 | -9.2 | 3 | Ser682: OG | O4 | 3.1 |
| 3CLpro | |||||||
| 1 | Midostaurin (Rydapt) | DB06595 | -9.9 | 1 | Gly143: N | O3 | 2.78 |
| 3 | Dihydroergotamine | DB00320 | -9.6 | 2 | Gly143: N | O4 | 3.01 |
| 2 | Nilotinib | DB04868 | -9.4 | 1 | His163: NE2 | N4 | 2.98 |
| 4 | Ergotamine | DB00696 | -9.4 | 1 | Gly143: N | O3 | 3.01 |
| 5 | Tadalafil | DB00820 | -9.3 | 2 | Gly143: N | O3 | 3.14 |
| 6 | Bromocriptine | DB01200 | -9.2 | 3 | Gly143: N | O1 | 2.8 |
| 7 | Indigotindisulfonic acid (Indigo Carmine) | DB11577 | -9.1 | 12 | Ser46: OG | O6 | 2.65 |
| 8 | Eltrombopag | DB06210 | -8.9 | 1 | Thr24: O | O4 | 3.02 |
| 9 | Trovafloxacin (Trovan) | DB00685 | -8.9 | 2 | Tyr54: OH | N4 | 2.88 |
| 10 | Capmatinib | DB11791 | -8.9 | 3 | Thr26:N | O | 3.14 |
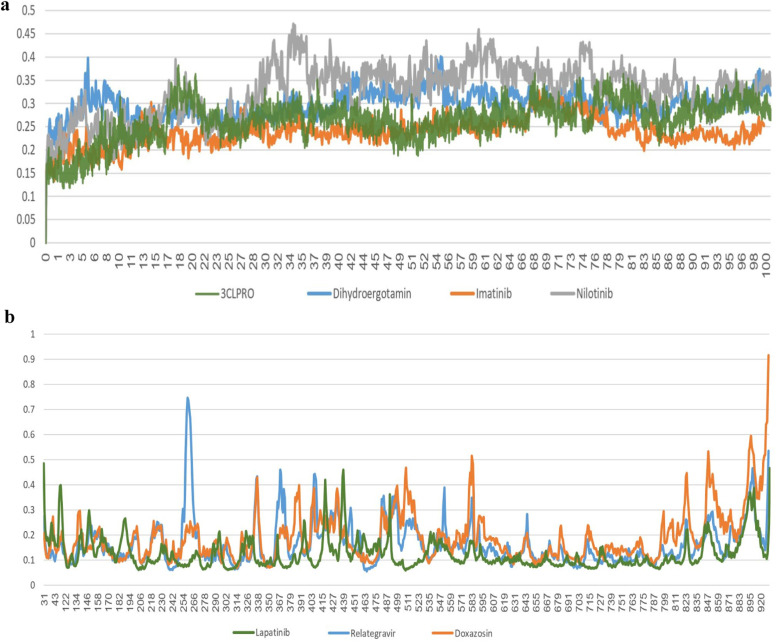
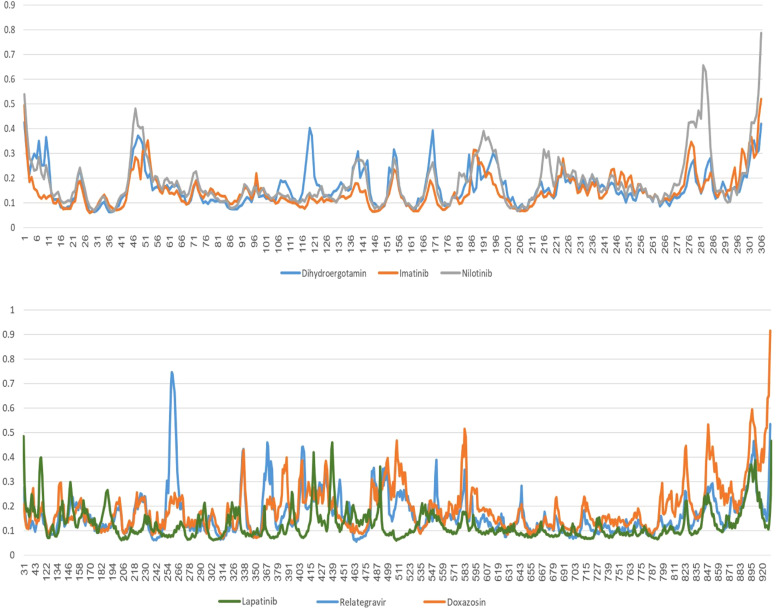
One of the best parameters for molecular dynamics simulation stability is the Root Mean Square Deviation (RMSD) factor. The root deviation of the mean RMSD squares between the structures created during the molecular dynamics simulation in the dimension of time is a common standard to confirm the protein structural stability alone and in the presence of the ligand. Therefore, the RMSD values for the alpha carbon atoms of the 3CLPRO and RdRp proteins complex with ligand during the simulation time (100 nm) relative to the primary structure were calculated and extracted. The results of this calculation for both simulations are shown in Fig. 5a,b.
Fig. 5.
a. RMSD graphs for ligands in complexes with 3CLpro during 100 ns of the molecular dynamics simulation period. b. RMSD graphs for ligands in complexes with RdRp during 100 ns of the molecular dynamics simulation period.
In Fig. 5a, the RMSD diagram of the nilotinib complex indicates that the slope increased slightly from the simulation beginning and after reaching 0.4 nm in 35 nm. The increasing process stopped and fluctuated around 0.4 from this time until the end of the simulation. The RMSD diagrams of the imatinib and dihydroergotamine complexes showed that the RMSD changes were stable and fluctuated around 0.3 nm from the simulation beginning to the end.
Hydrogen bonds have a crucial role in protein structure's overall stability and molecular recognition. In the 3clpro complex, the imatinib had two hydrogen bonds with HIS41 and ASN119, that HIS41 was one of the catalytic site residues in 3clpro. Moreover, dihydroergotamine had two hydrogen bonds with GLU166, but nilotinib did not have any hydrogen bond ( Fig. 6a).
Fig. 6.
a. The ligand molecule's orientation in the 3CLPRO complex. The ligand's orientation with its surrounding amino acids in the 3CLPRO complex is shown after 100 nm of simulation. b. The ligand molecule's orientation in the RdRp complex The ligand's orientation with its surrounding amino acids in the RdRP complex is shown after 100 nm of simulation.
Fig. 5b shows the RMSD diagram changes of the RdRp and ligand proteins during molecular dynamics simulations. The Doxazosin complex RMSD diagram was stable from the beginning of the simulation to 70 nm and reached about 0.4 nm. However, about 70 s to the end of the simulation, it had a slight increase to about 0.6 nm. The RMSD diagrams of the Relategravir complexes showed a slight increase to 0.4 after two ns and 60 ns; in sum, the RMSD changes were stable and fluctuated around 0.35.
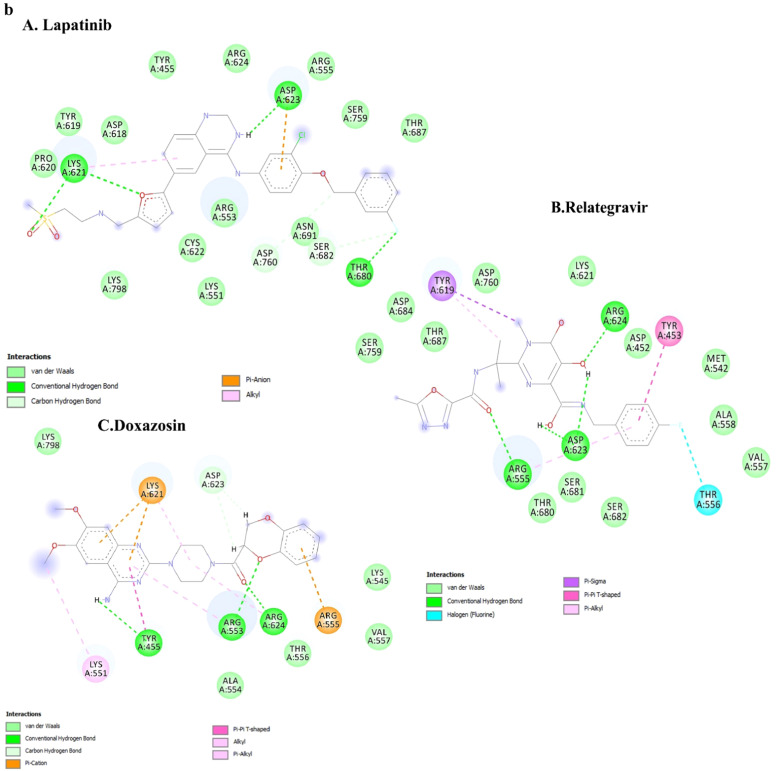
The graphs indicated that the ligand molecules in all complexes for 3Clpro and RdRp, at the junction of the beginning of the simulation, had proper orientation, and after about 35 and 70 nanoseconds, respectively, they reached stability. The ligands orientation and interaction in all complexes are shown in Fig. 6b. Raltegravir could make strong interactions with ARG555, ASP623 and ARG624 residues. Raltegravir had hydrogen bonds with these residues. Besides, Doxazosin had three hydrogen bonds with TYR455, ARG553 and ARG624. Despite the change in the residue direction in the MD lapatinib-RDRP complex, the drug established tree hydrogen bonds with LYS621, ASP623 and THR680 (Fig. 6b).
At the simulation beginning, the Lapatinib complex RMSD diagram (Fig. 5b) showed a sudden increase after 2000 ps to 0.2 nm. Then the trend increased so that at 25,000 ps, the RMSD value decreased abruptly and reached 0.18 nm at 60,000 ps, and then the RMSD value suddenly increased again and reached 0.4 nm and showed a slight fluctuation around this value until the end of the simulation. The ligand orientation in the complex showed a very significant change, which indicated that the ligand is unstable at the junction (Fig. 6b).
Then, protein flexibility was evaluated to examine the protein complex behavior in all simulations in more detail. The dynamic behavior of alpha carbon atoms in the structure contains sufficient information to investigate important motions in proteins and reflects the proteins structures general motions. Therefore, the root means square fluctuations (RMSF) of alpha carbon atoms were considered to investigate motion and structural flexibility. The last 20 nanoseconds of the simulation were used to prepare the RMSF diagram. The structural flexibility of each amino acid in two proteins is shown in Fig. 7. As shown in Fig. 7, in the 3Clpro protein, amino acids 15–25, 35–60 and 135–140 show more flexibility than other protein amino acids. In RdRp protein, amino acids 360–390 indicate more flexibility.
Fig. 7.
RMSF graphs in all six simulations.
The high number of receptor interactions with the ligand indicates the ligand stability at its position on the protein complex. Therefore, one of the crucial factors in the ligand stability at the protein binding site is the number of hydrogen interactions. A hydrogen interaction occurs between a hydrogen donor functional group and a hydrogen receptor group. At the beginning of a molecular dynamics simulation, the ligand changes position until it can interact with the protein the most. These interactions include van der Waals, electrostatic, and hydrogen interactions. Fig. 6a shows the changes in the number of hydrogen interactions between the protein and the ligand in all complexes. As shown in Fig. 6a, in the 3CLPRO complex, the number of hydrogen interactions was usually one and sometimes reached two interactions at the beginning of the simulation. The number of hydrogen interactions increased from about 40,000 picoseconds and reached four interactions between 45,000 and 50,000. Besides, four hydrogen interactions were formed between the protein molecule and the ligand at 51,000–52,000 picoseconds. Then, the number of hydrogen interactions decreased significantly, reaching one at the end of the simulation, and in some amounts, there was no hydrogen interaction between the protein and the ligand.
As shown in Fig. 6b, in the RdRp complex, at the beginning of the simulation, four hydrogen interactions between protein and ligand were formed, and with the continuation of simulation, the number of interactions reduced to 1 in 25,000 picoseconds. The number of hydrogen interactions fluctuateی between 1 and 2 from 30,000 to 80,000 ps. Also, in this process, in some cases, the number of interactions reached 3. Most of the two hydrogen interactions were established after 80,000 ps (to the end of simulation), in some cases, 1 and 3 interactions.
4. Discussion
Several laboratories worldwide are looking to develop drugs to decrease fever, cough, sore throat, difficulty breathing, or other manifestations of COVID-19. Almost every week, new research is published on COVID-19 and proposes a new drug among previously FDA-approved medicines for the possible Treatment of COVID-19. Herein, we targeted two essential viral enzymes (RdRp and 3CLpro) for candidating FDA-approved drugs, using in-silico analysis. The three-dimensional models of RdRp and 3CLpro proteins were constructed, based on their sequences in the NCBI protein databank, using the Swiss model, and then validated. Among drugs identified in this study as possible candidates, 32 medicines were shared between the two enzyme-related lists and are categorized in several drug classes introduced in Table 1, Table 2. Here we discuss some of our candidate drugs previously introduced or validated by other types of studies, including in-silico, preclinical, and clinical trials.
According to our results, some antiviral drugs were detected against 3CLpro including, Bictegravir, Dolutegravir, Raltegravir, and Indinavir; among them, Raltegravir was identified to have interaction with RdRp too. Indinavir was previously suggested as a repurposing candidate against nCoV-2019 [9], [10]. Dolutegravir is an Anti-HIV drug that has already been registered in clinical trials for COVID-19 treatment [39], [40]. Raltegravir was also reported as a possible drug against multi-targets, including 3CLpro targets in in-silico studies [41], [42]. However, Bictegravir, an anti-HIV drug, has not been studied in-silico or registered as any clinical trial.
We identified several antibacterials as potential candidates against RdRp and 3CLpro. These antibacterials included Eravacycline, Sultamicillin, Cefpiramide, Ceftobiprole, Cefoperazone, Novobiocin, Alatrofloxacin, Ceftolozane, and Ceftriaxone. Besides, Eravacycline is an antibiotic previously proposed by a virtual docking screening study [43]. The use of antibiotics is beneficial for patients with COVID-19 in two ways. Since bacterial diseases are the main challenges for patients admitted to the intensive care units (ICU), they can probably play dual roles as antiviral and antibacterial [44]. Due to the adverse side effects of both types of drugs on the immune system and the body, only one drug with two functions is likely to lead to fewer side effects.
New evidence suggests that Cytokine storm Syndrome (CSS), a systemic inflammatory response, threatens a subset of patients with COVID-19 [45]. Acute Respiratory Distress Syndrome (ARDS) is also rooted in the pathogenesis of inflammatory mediators. It appears to be necessary to prevent increased inflammation for limiting the possible progression of ARDS [46]. Some of the drugs that have gained acceptable affinity scores in docking are classified as anti-inflammatory drugs in the DrugBank database [47]. Among these in-silico detected drugs, Hesperidin has been previously introduced in other in-silico studies to have antiviral potency by inhibiting SARS-CoV-2 main protease, PLpro (papain-like protease), and helicase (Nsp13) [19], [48], [49], [50], [51], [52]. It has also been previously computationally predicted to have a binding affinity to the ACE II receptor, so it might probably help treat COVID-19 in this way [53]. It has already treated cells against the influenza type-A virus in vitro by upregulating P38, JNK, and enhancing cell-autonomous immunity [54]. The two other inflammatory drugs, available on our result list, have also been suggested by other in-silico docking studies, including Diosmin [48] and Rutin [48], [50]. Rutin is predicted to inhibit the viral helicase (Nsp13) [52].
Asthma and airway allergies have similar pathogenetic mechanisms to some respiratory tract infections [55], and the main manifestations are related to respiration in both COVID-19 and allergy/asthma. Montelukast, an antiasthmatic drug identified in our result list, is registered for clinical trial against COVID-19 (NCT04389411). Antiasthmatic drugs stabilize mast cells to reduce the release of cytokines. They alleviate the inflammatory cell infiltration into the lungs [56]. Montelukast, a cysteinyl leukotriene receptor antagonist (cysLT), has anti-inflammatory effects. It reduces cytokine production. Montelukast may reduce the inflammatory response in severe cases of COVID-19. It might limit the progression of the disease [57].
A previous study has shown that an anti-allergy drug interfered with SARS-CoV replication. The SARS-CoV is a positive-strand RNA virus. The drug was called cyclosporin and was inhaled orally [58]. Some anti-allergy drugs have also appeared in our results table with appropriate docking scores (binding energies), such as Chromoglycic acid and Zafirlukast. They inhibit the release of chemical mediators from our sensitized mast cells and are used to prevent asthma [59]. Chromoglycic acid played a therapeutic role in Balb/c mice infected with influenza A (H5N1) compared to the PBS treated group. However, it did not affect the viral load [60]. It also has been predicted to have a binding affinity against the SARS-CoV-2 Nsp16 by another in-silico Study [61]. We recommend them to be further examined for their possible antiviral effect on the SARS-CoV-2 virus. Since the most severe symptoms of COVID-19 are respiratory distress, the use of certain anti-allergy medications may reduce the severity of respiratory manifestations of COVID-19 infection and may help breathe in patients with COVID-19.
Acute myocardial injury has been reported in some severe cases of COVID-19 [62]. Patients with chronic cardiovascular disease are among the most susceptible groups in severe COVID-19 and have the highest morbidity rate among COVID-19 severe cases [63]. Interestingly, ten cardiovascular drugs have appeared with proper docking scores (binding energies) in our results (Table 1, Table 2). Some previous studies have reported the effect of some of these cardiovascular drugs on various viral infections, including SARS-CoV, HCMV, and SARS-CoV-2. For example, Avatrombopag is predicted to bind to ACEII and ACEI (in-silico). Avatrombopag likely blocks SARS-CoV-2 interaction with host receptors [64]. Conivaptan, known as hyponatremia treatment, is also previously predicted to bind to 3CLpro in-silico, and it has also scored adequately on our result list [52].
An ARB is reported to prevent the aggravation of acute lung injury in mice infected with SARS-CoV, which is closely related to SARS-CoV-2 [65]. Eltrombopag is a Thrombopoietin Receptor Agonist and improves the low number of platelet counts in ITP and treats Thrombocytopenia. Interestingly, platelets have been shown to play a role in defense against respiratory viruses. Activated platelets engulf HIN1 virions and secrete antiviral molecules to destroy virions. The H1N1 virus is close to SARS-CoV-2. We can probably assume that it may show beneficial effects in SARS-CoV-2 Treatment. The Eltrombopag is also used in the Treatment of HCV and HIV-1. It is also an iron chelator and can prevent virus replication in human cytomegalovirus (HCMV). Interestingly an in-vitro study has confirmed its effect against SARS-CoV-2 in Vero cells [66], [67], [68], [69].
It has been reported that SARS-CoV-2 can also induce infection-associated Coagulopathies. Several recent studies have reported that patients infected by COVID-19 are at risk of disseminated intravascular coagulation (DIC) [70], [71]. Some anticoagulant drugs also have been theoretically shown in our in-silico analysis for their possible antiviral role against SARS-CoV-2, namely Ticagrelor, Edoxaban, Bevyxxa, and Zontivity. Ticagrelor (an antagonist of the P2Y12 receptor) is an anticoagulant drug. The usage of these drugs is recommended in one letter for COVID-19 [72]. Since COVID-19 pneumonia and myocardial infarction (MI) are concomitant, Ticagrelor seems to contribute to patient survival for various reasons. One reason is that the PLATO study has shown that sepsis and pulmonary infections were less common in individuals using Ticagrelor. It prevents DIC development by reducing pro-inflammatory factors and platelet reactivation [73]; besides, it reduces lung injury in pneumonia by reducing thromboinflammatory factors [74]. Surprisingly, recently it has also been reported as an antibacterial that acts against some antibiotic-resistant gram-positive bacteria [75].
Edoxaban, a direct oral anticoagulant (DOAC), has also appeared on our results. OACs are indicated for preventing thrombosis in susceptible patients and treating venous thromboembolism (VTE) [76]. The use of some antiviral drugs potentially enhances the OACs level in plasma. In one study, patients on OAC with COVID-19 started antiviral drugs, and their OAC plasma levels were measured and compared with those documented before treatment. Patients treated with both antiviral and OAC drugs showed an alarming increase in OAC plasma Levels. Physicians probably had better replace OACs with other anticoagulant medicines to prevent bleeding complications while using them concomitant with antivirals in COVID-19 [76]. It is crucial to adjust the serum levels of some anticoagulant drugs in the proper range, as both high and low levels might cause coagulation problems. The fact that taking some antiviral drugs can alter the serum stability of them makes it even more challenging to monitor the amount of them in patients’ blood. As a result, if we can propose a drug with both antiviral and anticoagulant effects for coagulation problems, it will probably be more comfortable to monitor the treatment [76], [77]. If Edoxaban shows sufficient antiviral effect in further in-vitro and in-vivo studies, it will likely be a suitable resort to overcome the dilemma.
Some drugs related to benign prostatic hyperplasia (BPH) or male erectile dysfunctional impotence have been identified to inhibit SARS-CoV-2 in our analysis, such as Dutasteride, Doxazosin, and Tadalafil. Dutasteride has been previously predicted to inhibit the main viral protease and E channel in-silico [78], [79]. Doxazosin is also predicted to inhibit the viral Mpro. The inhibiting effect of Doxazosin was validated by the MD trajectory clustering approach in-silico [80]. Tadalafil is predicted to have potential against nsp1 by the DeepDTA method and has also shown affinity as a 2’-O-methyltransferase inhibitor [81]. Besides, this category of drugs can be suitable choices against COVID-19 since they are androgen related. Androgen decrease has been associated with reduced ACE2 activity [82]. Besides, in type II pneumocytes, TMPRSS2 prims the viral spike surface, enabling the cell viral entry. Androgen receptor regulates the TMPRSS2 gene. The TMPRSS2 expression is also associated with an increase in androgen receptor (AR) [83]. It also blocks 5-AR isoform 3, which is expressed in the respiratory epithelium and fibroblasts. Based on these reasons, an androgen antagonist like Dutasteride could be a therapeutically beneficial drug for COVID-19. However, some cautions should be considered, and more preclinical studies seem to be required since inhibition of 5-AR impair the regeneration capacity of the respiratory epithelium [84].
Some antipsychotic drugs have also obtained suitable scores in our docking screening analysis, such as Fluspirilene, Lurasidone, and Pimozide. Fluspirilene, a neurotransmitter inhibitor, previously has shown activity against SARS-CoV and MERS-CoV in-vitro. In a recent study, it displayed activity against SARS-CoV-2 in-vitro in the Vero E6 cell line, too [85], [86].
Gul S et al. performed an in-silico screening against viral 3CLpro and RdRp. Lurasidone displayed binding affinity to RdRp, and Pimozide showed binding affinity to 3CLPro in their study [87]. Vatansever EC et al. examined Pimozide in-vitro. Interestingly Pimozide showed an IC50 value in inhibiting the viral MPro below 100 μM. Besides, Pimozide is likely to have a similar effect on hydroxychloroquine in increasing the endosome pH. Therefore, Pimozide probably slows the SARS-CoV-2 entry [88].
We observed drugs previously prescribed for migraine pains among the shared drugs, such as Ergotamine (a calcitonin gene-related peptide antagonist). Ergotamine was detected as a possible inhibitor for SARS-CoV-2 RNA-dependent RNA polymerase in our analysis. It has also been reported to have affinity binding for three viral proteins in COVID-19 by other in-silico studies [89]. It has been the drug of choice in some migraine sufferers who have long duration or infrequent headaches over 50 years. One study found that the over-release of neuropeptides, such as the calcitonin gene-related peptide (CGRP), may lead to an abnormal vascular response seen in acute lung injury. Therefore, the CGRP blockade may be helpful in some lung injuries [90], [91]. Headaches are also symptoms that emerge in a subset of patients with COVID-19, although they do not occur isolated [92].
In addition to previous drug categories, some drugs related to other drug categories appeared on our result list. One of these categories was anti-diabetic drugs, including Linagliptin (a DPP4 inhibitor). Considering that diabetes is a risk factor for critical manifestations of COVID-19 and increases the risk of severe symptoms in people with COVID-19, a clinical trial has been registered to assess its efficacy and safety in diabetic patients with COVID-19 (NTC04371978). Chronic hyperglycemia and inflammation can also lead to abnormalities in the immune system [93]. Based on our analysis results, we predict that taking Linagliptin can probably benefit these patients as an antiviral agent. Besides, recently a challenge was posed by a hypothesis against Metformin use in the COVID-19 crisis. A recent study in mice showed that Metformin and Chloroquine concomitant use (The first emergently approved drug for COVID-19) in mice has a high fatal toxicity rate [94]. The results of this study, though only in mice, caused concerns among diabetic metformin users during the Corona crisis. We suggest that Linagliptin can be studied alongside other drugs as a temporary alternative to diabetes in the Corona crisis. The potential antiviral effect of Linagliptin could also help overcome the viral infection. It also has previously shown attenuating effects on inflammation during wound healing in mice. This anti-inflammatory effect may increase the likelihood of its possible beneficial effects for COVID-19 pulmonary injuries [95]. Dipeptidyl peptidase 4 (DPP4 or the same CD26) is a serine exopeptidase expressed in many tissues, including kidney, liver, lung, intestine, and even immune cells. A previous study has hypothesized that DPP4 can probably contribute to the virus entry in SARS-CoV-2, using a dock model between the DPP4 and viral spike. The model had a significant interface. Since other CoVs use DPP4 as a receptor, they have assumed that DPP4 may contribute to the viral entry in SARS-CoV-2. However, they do not provide experimental data in this regard. There is also evidence that DPP4 inhibitors may modulate inflammation and have anti-fibrotic activity [96]. Therefore using Linagliptin, in patients even without type 2 diabetes can probably prevent the sustained cytokine storm indirectly [97]. However, some have presented nuanced debates that we must not rush to use DPP4 inhibitors since they may suppress the immune system or cause other life-threatening conditions [98], [99].
Diamorphine (heroin) is another drug that has properly scored against viral proteins in our analysis. Consuming high doses of some opioids and specifically, heroin affects the brain stem negatively and reduces the respiratory rate and has complications to the lungs and respiration system [100], [101]. However, it can still be used therapeutically in patients with a terminal disease (perhaps in severe pain to prevent neurotic shock). It can probably be investigated for patients in advanced and painful stages of cancers with moderate COVID-19 [102].
Drospirenone is another drug that has appeared on our list. Drospirenone is used to control acne and PMDD. It is a synthetic progestin contraceptive that contains estrogen and progesterone. Although the safety of its use is still controversial and it may increase venous thromboembolism, it is confirmed by another in-silico study to bind the three viral target proteins, including RdRp, Mpro, and PLpro [78].
This study has identified 69 small molecule drugs with higher binding affinity and interaction with the RdRp and 3clpro proteins active pocket residues. The top 10 small molecule drugs with docking binding energies lower than 9.2 kcal/mol for RdRp and lower than 8.9 kcal/mol are shown in Table 3. Moreover, the six drugs were selected for MD simulation, including Nilotinib, Imatinib, and dihydroergotamine for 3clpro and Lapatinib, Dexasone, Relategravir for RDRP.
For 3Clpro, approximately all 3Clpro complexes exhibited a similar fluctuation. The RMSF values indicated that Relategravir had more reasonable binding stability with RdRp. Moreover, an approximately similar RMSF value of residues was predicted for all RdRp complexes. Except for Lapatinib, the 5 of 6 drugs demonstrated significant interactions with critical residues indicating their binding stability in complexes with 3Clpro and RdRp. During MD simulation, drugs had maintained their original docking position thoroughly.
The results of this and other similar in-silico studies on FDA-approved drugs are promising for further in vitro and in vivo investigations of COVID-19 treatment. We recommend that some herbal extracts could also be similarly evaluated in-silico for their possible interaction with SARS-CoV-2 proteins in further investigations.
5. Conclusion
Several studies support that patients infected by SARS-CoV-2 are at risk of cytokine storm, inflammatory alterations, and disseminated intravascular coagulation. The lungs are the main target organ for the virus; patients develop acute lung damages, which can end to respiratory failure, although the defects in other organs, heart, nervous system, and skin are also reported. In this study, two crucial viral enzymes, RdRp and 3CLpro, were selected to dock against FDA-approved drugs. We identified and repurposed several medicines. We then categorized them based on their previous indications. These drugs were classified into several categories, including antiviral, antibacterial, anti-inflammatory, anti-allergic, cardiovascular, anticoagulant, BPH and impotence, antipsychotic, antimigraine, anticancer, and so on. Some of them were also previously reported as suitable repurposing candidates against SARS-CoV-2 by other in-silico or in-vitro studies. Some of them have also been recently registered in clinical trials to assess against COVID-19. However, many of them remain to be further experimentally examined against SARS-CoV-2 in vitro and in vivo. Those that successfully suppress SARS-CoV-2 in vitro and in vivo will probably be suitable candidates for further clinical investigations against SARS-CoV-2. Based on our in-silico analysis, Nilotinib, Imatinib and Dihydroergotamine, Dexasone, and Relategravir may be effective drugs to treat COVID-19 with need more confirming experimental studies. We hope that they limit the morbidity and mortality associated with the recent severe acute respiratory syndrome pandemic.
CRediT authorship contribution statement
Zahra Molavi: Writing - original draft. Sara Razi: Writing - original draft. Seyed Amir Mirmotalebisohi: Scientific investigation, Writing - reviewing & editing. Amirjafar Adibi: Investigation. Marzieh Sameni: Writing - original draft. Farshid Karami: Writing - original draft. Vahid Niazi: Writing - original draft. Zahra Niknam: Writing - original draft, Visualization, Software. Morteza Aliashrafi: Writing - reviewing & editing. Shabnam Jeibouei: Writing - original draft. Hakimeh Zali: Conceptualization, Supervision, Funding acquisition, Project administration. Mohammad Mehdi Ranjbar: Data curation, Visualization, Software, Methodology, Supervision. Mohsen Yazdani: Data curation, Visualization, Software, Methodology, Supervision.
Conflict of interest statement
The authors declare they have no conflict of interest.
Acknowledgments
The authors thank the Proteomics Research Center of Shahid Beheshti University of Medical Sciences for their support in conducting this work (NO. 22784).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2021.111544.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 2.Romagnoli S., Peris A., de Gaudio A.R., Geppetti P. SARS-CoV-2 and COVID-19: between pathophysiology complexity and therapeutic uncertainty. Physiol. Rev. 2020 doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim Y., Liu H., Kankanamalage A.C.G., Weerasekara S., Hua D.H., Groutas W.C., Chang K.-O., Pedersen N.C. Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog. 2016;12(3) doi: 10.1371/journal.ppat.1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.te Velthuis A.J. Common and unique features of viral RNA-dependent polymerases. Cell. Mol. Life Sci. 2014;71(22):4403–4420. doi: 10.1007/s00018-014-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand K., Yang H., Bartlam M., Rao Z., Hilgenfeld R. Springer; 2005. Coronavirus Main Proteinase: Target for Antiviral Drug Therapy, Coronaviruses with Special Emphasis on First Insights Concerning SARS; pp. 173–199. [Google Scholar]
- 6.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-Cov-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 7.Kneller D.W., Phillips G., O’Neill H.M., Jedrzejczak R., Stols L., Langan P., Joachimiak A., Coates L., Kovalevsky A. Structural plasticity of SARS-CoV-2 3CL M pro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020;11(1):1–6. doi: 10.1038/s41467-020-16954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368:779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Y.-C. Chang, Y.-A. Tung, K.-H. Lee, T.-F. Chen, Y.-C. Hsiao, H.-C. Chang, T.-T. Hsieh, C.-H. Su, S.-S. Wang, J.-Y. Yu, Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking, 2020.
- 10.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad J., Ikram S., Ahmad F., Rehman I.U., Mushtaq M. SARS-CoV-2 RNA dependent RNA polymerase (RdRp)–a drug repurposing study. Heliyon. 2020;6(7) doi: 10.1016/j.heliyon.2020.e04502. e04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.T.U.o.H. Kong, Lopinavir/ Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment, 2020.
- 13.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.B. Pharmaceuticals, A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19, 2020.
- 15.Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel (EPICOS), 2020.
- 16.Wang Z., Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye X., Luo Y., Xia S., Sun Q., Ding J., Zhou Y., Chen W., Wang X., Zhang W., Du W. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharmacol. Sci. 2020;24(6):3390–3396. doi: 10.26355/eurrev_202003_20706. [DOI] [PubMed] [Google Scholar]
- 18.De Meyer S., Bojkova D., Cinati J., Van Damme E., Buyck C., Van Loock M., Woodfall B., Ciesek S. Lack of antiviral activity of darunavir against SARS-CoV-2. medRxiv. 2020 doi: 10.1016/j.ijid.2020.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y.W., Yiu C.-P.B., Wong K.-Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL pro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research. 2020;9:129. doi: 10.12688/f1000research.22457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velavan T.P., Meyer C.G. The COVID‐19 epidemic. Trop. Med. Int. Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S. Nabirotchkin, A.E. Peluffo, J. Bouaziz, D. Cohen, Focusing on the unfolded protein response and autophagy related pathways to reposition common approved drugs against COVID-19, 2020.
- 22.Verdugo-Paiva F., Izcovich A., Ragusa M., Rada G., Group C.-L.-O.W. Lopinavir/ritonavir for the treatment of COVID-19: a living systematic review protocol. medRxiv. 2020 doi: 10.5867/medwave.2020.06.7966. [DOI] [PubMed] [Google Scholar]
- 23.Tobaiqy M., Qashqary M., Al-Dahery S., Mujallad A., Hershan A.A., Kamal M.A., Helmi N. Therapeutic management of patients with COVID-19: a systematic review. Infect. Prev. Pract. 2020;2 doi: 10.1016/j.infpip.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdian S., Ebrahim-Habibi A., Zarrabi M. Drug repurposing using computational methods to identify therapeutic options for COVID-19. J. Diabetes Metab. Disord. 2020;19:691–699. doi: 10.1007/s40200-020-00546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Cheng T., Wang Y., Bryant S.H. PubChem as a public resource for drug discovery. Drug Discov. Today. 2010;15(23–24):1052–1057. doi: 10.1016/j.drudis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Boyle N., Banck M., James C., Morley C., Vandermeersch T., Hutchison G. Open babel: an open chemical toolbox. J. Chemin. 2011;3(1):33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanal I.Y., Keith J.A., Hutchison G.R. A sobering assessment of small‐molecule force field methods for low energy conformer predictions. Int. J. Quantum Chem. 2018;118(5) [Google Scholar]
- 28.Kaplan W., Littlejohn T.G. Swiss-PDB viewer (deep view) Brief. Bioinform. 2001;2(2):195–197. doi: 10.1093/bib/2.2.195. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z., Lasker K., Schneidman-Duhovny D., Webb B., Huang C.C., Pettersen E.F., Goddard T.D., Meng E.C., Sali A., Ferrin T.E. UCSF Chimera, MODELLER, and IMP: an integrated modeling system. J. Struct. Biol. 2012;179(3):269–278. doi: 10.1016/j.jsb.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.T. Chowdhury, G. Roymahapatra, S.M. Mandal, In silico identification of a potent arsenic based approved drug darinaparsin against SARS-CoV-2: inhibitor of RNA dependent RNA polymerase (RdRp) and necessary proteases, 2020. [DOI] [PubMed]
- 32.Pagadala N.S., Syed K., Tuszynski J. Software for molecular docking: a review. Biophys. Rev. 2017;9(2):91–102. doi: 10.1007/s12551-016-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dallakyan S., Olson A.J. Chemical Biology. Springer; 2015. Small-molecule library screening by docking with PyRx; pp. 243–250. [DOI] [PubMed] [Google Scholar]
- 34.Patil R., Das S., Stanley A., Yadav L., Sudhakar A., Varma A.K. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inc A.S. Accelrys Software Inc; San Diego: 2012. Discovery Studio Modeling Environment, Release 3.5, Accelrys Discovery Studio. [Google Scholar]
- 36.Mosquera-Yuqui F., Lopez-Guerra N., Moncayo-Palacio E.A. Targeting the 3CLpro and RdRp of SARS-CoV-2 with phytochemicals from medicinal plants of the Andean Region: molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1835716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Best R.B., Zhu X., Shim J., Lopes P.E., Mittal J., Feig M., MacKerell A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 2012;8(9):3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malde A.K., Zuo L., Breeze M., Stroet M., Poger D., Nair P.C., Oostenbrink C., Mark A.E. An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theory Comput. 2011;7(12):4026–4037. doi: 10.1021/ct200196m. [DOI] [PubMed] [Google Scholar]
- 39.Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan R.J., Jha R.K., Amera G., Jain M., Singh E., Pathak A., Singh R.P., Muthukumaran J., Singh A.K. Targeting SARS-CoV-2: a systematic drug repurposing approach to identify promising inhibitors against 3C-like proteinase and 2′-O-ribose methyltransferase. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1753577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Y. Kumar, H. Singh, In silico identification and docking-based drug repurposing against the main protease of SARS-CoV-2, causative agent of COVID-19, 2020.
- 42.K. Mohamed, N. Yazdanpanah, A. Saghazadeh, N. Rezaei, Computational drug discovery and repurposing for the treatment of COVID-19: a systematic review, Available at SSRN 3583748, 2020. [DOI] [PMC free article] [PubMed]
- 43.Wang J. Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020;60:3277–3286. doi: 10.1021/acs.jcim.0c00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.F. da Silveira Silva, B. Brixner, C.F. de Oliveira, J.D.P. Renner, What are the risk factors and agents responsible for bacterial infections in ICUs?
- 45.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatia M., Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. J. Pathol. Soc. G. B. Irel. 2004;202(2):145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 47.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Sajed T., Johnson D., Li C., Sayeeda Z. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.S. Adem, V. Eyupoglu, I. Sarfraz, A. Rasul, M. Ali, Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in silico strategy unveils a hope against CORONA, 2020. [DOI] [PMC free article] [PubMed]
- 49.Mittal L., Kumari A., Srivastava M., Singh M., Asthana S. Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J. Biomol. Struct. Dyn. 2020:1–19. doi: 10.1080/07391102.2020.1768151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das S., Sarmah S., Lyndem S., Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020:1–18. doi: 10.1080/07391102.2020.1763201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R.Y. Utomo, E. Meiyanto, Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection, 2020.
- 52.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.L. Cheng, W. Zheng, M. Li, J. Huang, S. Bao, Q. Xu, Z. Ma, Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2, 2020. [DOI] [PMC free article] [PubMed]
- 54.Dong W., Wei X., Zhang F., Hao J., Huang F., Zhang C., Liang W. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci. Rep. 2014;4:7237. doi: 10.1038/srep07237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Åberg N. The use of antiallergic and antiasthmatic drugs in viral infections of the upper respiratory tract. Clin. Immunother. 1996;6(3):171–179. doi: 10.1007/BF03259516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagai H., Teramachi H., Tuchiya T. Recent advances in the development of anti-allergic drugs. Allergol. Int. 2006;55(1):35–42. doi: 10.2332/allergolint.55.35. [DOI] [PubMed] [Google Scholar]
- 57.Fidan C., Aydoğdu A. As a potential treatment of COVID-19: Montelukast. Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., Shimojima M., Fukushi S. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. 2020 doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dekhuijzen P.N., Koopmans P.P. Pharmacokinetic profile of zafirlukast. Clin. Pharmacokinet. 2002;41(2):105–114. doi: 10.2165/00003088-200241020-00003. [DOI] [PubMed] [Google Scholar]
- 60.Han D., Wei T., Zhang S., Wang M., Tian H., Cheng J., Xiao J., Hu Y., Chen M. The therapeutic effects of sodium cromoglycate against influenza A virus H5N1 in mice. Influenza Other Respir. Virus. 2016;10(1):57–66. doi: 10.1111/irv.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.U. Shankar, N. Jain, P. Majee, S.K. Mishra, B. Rathi, A. Kumar, Potential drugs targeting Nsp16 protein may corroborates a promising approach to combat SARS-CoV-2 virus, 2020.
- 62.Bonow R.O., Fonarow G.C., O’Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 63.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.A. Sajib, Repurposing of approved drugs with potential to block SARS-CoV-2 surface glycoprotein interaction with host receptor, 2020.
- 65.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020;43:648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogel J.U., Schmidt S., Schmidt D., Rothweiler F., Koch B., Baer P., Rabenau H., Michel D., Stamminger T., Michaelis M., Cinatl J. The thrombopoietin receptor agonist eltrombopag inhibits human cytomegalovirus replication via iron chelation. Cells. 2019;9(1):31. doi: 10.3390/cells9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu J., Ma J., Zhang S., Han J., Liu S. Promoting platelets is a therapeutic option to combat severe viral infection of the lung. Blood Adv. 2020;4(8):1640–1642. doi: 10.1182/bloodadvances.2020001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00819-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arshad U., Pertinez H., Box H., Tatham L., Rajoli R.K., Curley P., Neary M., Sharp J., Liptrott N.J., Valentijn A. Prioritisation of potential anti-SARS-CoV-2 drug repurposing opportunities based on ability to achieve adequate plasma and target site concentrations derived from their established human pharmacokinetics!Abstract. medRxiv. 2020 doi: 10.1002/cpt.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan W.-j, Ni Z.-y, Hu Y., Liang W.-h, Ou C.-q, He J.-x, Liu L., Shan H., Lei C.-l, Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lillicrap D. Disseminated intravascular coagulation in patients with 2019–nCoV pneumonia. J. Thromb. Haemost. 2020;18(4):786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akşit E., Kırılmaz B., Gazi E., Aydın F. Ticagrelor can be an important agent in the treatment of severe COVID-19 patients with myocardial infarction. Balk. Med. J. 2020;37(4) doi: 10.4274/balkanmedj.galenos.2020.2020.4.100. 233-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salvati M., D’Elia A., Brogna C., Frati A., Antonelli M., Giangaspero F., Raco A., Santoro A., Delfini R. Cerebral astroblastoma: analysis of six cases and critical review of treatment options. J. Neuro-Oncol. 2009;93(3):369–378. doi: 10.1007/s11060-008-9789-9. [DOI] [PubMed] [Google Scholar]
- 74.Janz C., Buhl R. Astroblastoma: report of two cases with unexpected clinical behavior and review of the literature. Clin. Neurol. Neurosurg. 2014;125:114–124. doi: 10.1016/j.clineuro.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Merfeld E.C., Dahiya S., Perkins S.M. Patterns of care and treatment outcomes of patients with astroblastoma: a National Cancer Database analysis. CNS Oncol. 2018;7(02):CNS13. doi: 10.2217/cns-2017-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Testa S., Prandoni P., Paoletti O., Morandini R., Tala M., Dellanoce C., Giorgi‐Pierfranceschi M., Betti M., Danzi G.B., Pan A. Direct oral anticoagulant plasma levels’ striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J. Thromb. Haemost. 2020;18:1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosengren A.M., Karlsson Br.C., Nicholls I.A. Monitoring the distribution of warfarin in blood plasma. ACS Med. Chem. Lett. 2012;3(8):650–652. doi: 10.1021/ml300112e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.M. Hosseini, W. Chen, C. Wang, Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs, 2020. [DOI] [PMC free article] [PubMed]
- 79.A. Chernyshev, Pharmaceutical targeting the envelope protein of SARS-CoV-2: the screening for inhibitors in approved drugs, 2020.
- 80.A. Gupta, Profiling molecular simulations of SARS-CoV-2 main protease (Mpro) binding to repurposed drugs using neural network force fields, 2020. [DOI] [PMC free article] [PubMed]
- 81.K. Sharma, S. Morla, A. Goyal, S. Kumar, Computational guided drug repurposing for targeting 2'-O-ribose methyltransferase of SARS-CoV-2, 2020. [DOI] [PMC free article] [PubMed]
- 82.Qiu Y., Zhao Y.-B., Wang Q., Li J.-Y., Zhou Z.-J., Liao C.-H., Ge X.-Y. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020;22:221–225. doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikkonen L., Pihlajamaa P., Sahu B., Zhang F.-P., Jänne O.A. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Kroumpouzos G. Effects of 5–alpha reductase inhibitors on lung function: a reason for discontinuation during COVID‐19 pandemic? Dermatol. Ther. 2020;33 doi: 10.1111/dth.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weston S., Haupt R., Logue J., Matthews K., Frieman M. Broad anti-coronaviral activity of FDA approved drugs against SARS-CoV-2in vitroand SARS-CoVin vivo!Abstract. BioRxiv. 2020 doi: 10.1128/JVI.01218-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gul S., Ozcan O., Okyar A., Barıs I., Kavakli I.H. In silico identification of widely used and well tolerated drugs that may inhibit SARSCov-2 3C-like protease and viral RNA-dependent RNA polymerase activities, and may have potential to be directly used in clinical trials. Chemrxiv. 2020 [Google Scholar]
- 88.Vatansever E.C., Yang K., Kratch K.C., Drelich A., Cho C.-C., Mellot D.M., Xu S., Tseng C.-T.K., Liu W.R. Targeting the SARS-CoV-2 main protease to repurpose drugs for COVID-19. bioRxiv. 2020 [Google Scholar]
- 89.Mevada V., Dudhagara P., Gandhi H., Vaghamshi N., Beladiya U., Patel R. Drug repurposing of approved drugs Elbasvir, Ledipasvir, Paritaprevir, Velpatasvir, Antrafenine and Ergotamine for combating COVID19. ChemRxiv. 2020 [Google Scholar]
- 90.Lange M., Enkhbaatar P., Traber D.L., Cox R.A., Jacob S., Mathew B.P., Hamahata A., Traber L.D., Herndon D.N., Hawkins H.K. Role of calcitonin gene-related peptide (CGRP) in ovine burn and smoke inhalation injury. J. Appl. Physiol. 2009;107(1):176–184. doi: 10.1152/japplphysiol.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tfelt-Hansen P., Saxena P., Dahlöf C., Pascual J., Lainez M., Henry P., Diener H.-C., Schoenen J., Ferrari M., Goadsby P. Ergotamine in the acute treatment of migraine: a review and European consensus. Brain. 2000;123(1):9–18. doi: 10.1093/brain/123.1.9. [DOI] [PubMed] [Google Scholar]
- 92.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J. Neurol. Sci. 2020;413 doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajeshkumar N., Yabuuchi S., Pai S.G., Maitra A., Hidalgo M., Dang C.V. Fatal toxicity of chloroquine or hydroxychloroquine with metformin in mice. Biorxiv. 2020 [Google Scholar]
- 95.Schürmann C., Linke A., Engelmann-Pilger K., Steinmetz C., Mark M., Pfeilschifter J., Klein T., Frank S. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharmacol. Exp. Ther. 2012;342(1):71–80. doi: 10.1124/jpet.111.191098. [DOI] [PubMed] [Google Scholar]
- 96.Strollo R., Pozzilli P. DPP4 inhibition: preventing SARS‐CoV‐2 infection and/or progression of COVID‐19? Diabetes Metab. Res. Rev. 2020;36 doi: 10.1002/dmrr.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solerte S.B., Di Sabatino A., Galli M., Fiorina P. Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19. Acta Diabetol. 2020;57:779–783. doi: 10.1007/s00592-020-01539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalan R. Is DPP4 inhibition a comrade or adversary in COVID-19 infection. Diabetes Res. Clin. Pract. 2020;164 doi: 10.1016/j.diabres.2020.108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Males V.K. Letter to the Editor in response to the article “COVID-19 and diabetes: can DPP4 inhibition play a role?”. Diabetes Res. Clin. Pract. 2020 doi: 10.1016/j.diabres.2020.108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marinelli E. The impact of heroin illicit market in the framework of COVID 19 pandemic. Eur. Rev. Med. Pharmacol. Sci. 2020;24(10):5197–5198. doi: 10.26355/eurrev_202005_21299. [DOI] [PubMed] [Google Scholar]
- 101.Jolley C.J., Bell J., Rafferty G.F., Moxham J., Strang J. Understanding heroin overdose: a study of the acute respiratory depressant effects of injected pharmaceutical heroin. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sawynok J. The therapeutic use of heroin: a review of the pharmacological literature. Can. J. Physiol. Pharmacol. 1986;64(1):1–6. doi: 10.1139/y86-001. [DOI] [PubMed] [Google Scholar]
- 103.Shanno R.L. Rutin: a new drug for the treatment of increased capillary fragility. Am. J. Med. Sci. 1946;211:539–543. [PubMed] [Google Scholar]
- 104.Rothlin R.P., Vetulli H.M., Duarte M., Pelorosso F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID‐19. Drug Dev. Res. 2020;81:768–770. doi: 10.1002/ddr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato K., Hosonuma K., Yamazaki Y., Kobayashi T., Takakusagi S., Horiguchi N., Kakizaki S., Kusano M., Ohnishi H., Okamoto H. Combination therapy with ombitasvir/paritaprevir/ritonavir for dialysis patients infected with hepatitis C virus: a prospective multi-institutional study. Tohoku J. Exp. Med. 2017;241(1):45–53. doi: 10.1620/tjem.241.45. [DOI] [PubMed] [Google Scholar]
- 107.Feng S., Luan X., Wang Y., Wang H., Zhang Z., Wang Y., Tian Z., Liu M., Xiao Y., Zhao Y. Eltrombopag is a potential target for drug intervention in SARS-CoV-2 spike protein. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Odhar H.A., Ahjel S.W., Albeer A.A.M.A., Hashim A.F., Rayshan A.M., Humadi S.S. Molecular docking and dynamics simulation of FDA approved drugs with the main protease from 2019 novel coronavirus. Bioinformation. 2020;16(3):236–244. doi: 10.6026/97320630016236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gul S., Ozcan O., Asar S., Okyar A., Barıs I., Kavakli I. In silico identification of widely used and well tolerated drugs that may inhibit SARSCov-2 3C-like protease and viral RNA-dependent RNA polymerase activities, and may have potential to be directly used in clinical trials. ChemRxiv. 2020 [Google Scholar]
- 110.Vogel J.-U., Schmidt S., Schmidt D., Rothweiler F., Koch B., Baer P., Rabenau H., Michel D., Stamminger T., Michaelis M. The thrombopoietin receptor agonist eltrombopag inhibits human cytomegalovirus replication via iron chelation. Cells. 2020;9(1):31. doi: 10.3390/cells9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Silkauskaite V., Kupčinskas J., Pranculis A., Jonaitis L., Petrenkiene V., Kupčinskas L. Acute and 14-day hepatic venous pressure gradient response to carvedilol and nebivolol in patients with liver cirrhosis. Medicina. 2013;49(11):73. [PubMed] [Google Scholar]
- 112.Testa S., Prandoni P., Paoletti O., Morandini R., Tala M., Dellanoce C., Giorgi‐Pierfranceschi M., Betti M., Battista Danzi G., Pan A. Direct oral anticoagulant plasma levels’ striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J. Thromb. Haemost. 2020;18(6):1320–1323. doi: 10.1111/jth.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baker J.V., Wolfson J., Peterson T., Mooberry M., Gissel M., Mystakelis H., Henderson M.W., Garcia-Myers K., Rhame F.S., Schacker T.W. Oxford University Press US; 2020. Factor Xa Inhibition Reduces Coagulation Activity but Not Inflammation Among People with HIV: A Randomized Clinical Trial, Open Forum Infectious Diseases; p. ofaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anwar M.U., Adnan F., Abro A., Khan M.R., Rehman A.U., Osama M., Javed S., Baig A., Shabbir M.R., Assir M.Z. Combined deep learning and molecular docking simulations approach identifies potentially effective FDA approved drugs for repurposing against SARS-CoV-2. ChemRxiv. 2020 doi: 10.1016/j.compbiomed.2021.105049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.S. Weston, R. Haupt, J. Logue, K. Matthews, M.J.B. Frieman, FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro, 2020. [DOI] [PMC free article] [PubMed]
- 116.J. Dyall, C.M. Coleman, B.J. Hart, T. Venkataraman, M.R. Holbrook, J. Kindrachuk, R.F. Johnson, G.G. Olinger, P.B. Jahrling, M.J. Laidlaw, Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection, 58 (8) (2014) 4885–4893. [DOI] [PMC free article] [PubMed]
- 117.E.C. Vatansever, K. Yang, K.C. Kratch, A. Drelich, C.-C. Cho, D.M. Mellot, S. Xu, C.-T.K. Tseng, W.R.J.b. Liu, Targeting the SARS-CoV-2 main protease to repurpose drugs for COVID-19, 2020.
- 118.V. Mevada, P. Dudhagara, H. Gandhi, N. Vaghamshi, U. Beladiya, R. Patel, Drug repurposing of approved drugs Elbasvir, Ledipasvir, Paritaprevir, Velpatasvir, Antrafenine and Ergotamine for combating COVID19, 2020.
- 119.Hulin J., Brodie A., Stevens J., Mitchell C. Prevalence of respiratory conditions among people who use illicit opioids: a systematic review. Addiction. 2020;115(5):832–849. doi: 10.1111/add.14870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material