Abstract
The mitochondrial phosphate carrier (PiC) is a mitochondrial solute carrier protein, which is encoded by SLC25A3 in humans. PiC delivers phosphate, a key substrate of oxidative phosphorylation, across the inner mitochondrial membrane. This transport activity is also relevant for allowing effective mitochondrial calcium handling. Furthermore, PiC has also been described to affect cell survival mechanisms via interactions with cyclophilin D and the viral mitochondrial-localized inhibitor of apoptosis (vMIA). The significance of PiC has been supported by the recent discovery of a fatal human condition associated with PiC mutations. Here, we present first the early studies that lead to the discovery and molecular characterization of the PiC, then discuss the very recently developed mouse models for PiC and pathological mutations in the human SLC25A3 gene.
Introduction
From measurements of mitochondrial swelling in sucrose or mannitol media, it was recognized very early on that the mitochondrial volume was not freely accessible to even small solutes [1, 2]. By varying the concentration of sucrose, it was further determined that the outer mitochondrial membrane (OMM) was quite permeant (<5 kDa) and that it was the inner mitochondrial membrane (IMM) that provided the barrier to solute movement [3]. Subsequently, and in parallel with studies to sort out oxidative phosphorylation, great efforts were made to understand the transport of solutes across the IMM. Earlier studies had already demonstrated that exogenous inorganic phosphate (Pi) was required to drive oxidative phosphorylation [4, 5]. Clearly, Pi was of importance as an anion whose transport mechanism across the IMM was in need of definition.
Functional characterization of Pi transport in mitochondria
It was again through the use of simple swelling experiments, as well as by measuring mitochondrial respiration in the presence of thiol-reactive mercurial compounds, that Pi transport across the IMM was initially characterized. For swelling experiments, mitochondria were suspended in iso-osmotic solutions containing an anion and a cation (plus EGTA and rotenone); swelling occurred only when both the anion and the cation permeated the mitochondria. Using this method, Chappell and co-workers determined, using NH4 as the cation, that mitochondria were permeable to Pi and postulated the existence of a carrier system for Pi uptake [2, 6]. At the same time, Fonyo and Bessman [7] sought to understand the basis for the inhibitory action of mercurial compounds on “the energy transfer sequence”; such an inhibition had been described earlier by Lehninger and Boyer [8–10]. This first study by Fonyo and Bessman, along with follow-up studies by Fonyo and colleagues and by Tyler, suggested that mercurial compounds suppressed respiration by inhibiting Pi uptake across the IMM [7, 11, 12]. Tyler further demonstrated that exogenous cysteine reverses the inhibitory effect of the mercurial compound, mersalyl, on respiration, and concluded that it was acting on a protein and that the inhibitory effect on respiration was not due to irreversible protein denaturation [12]. Then, using swelling experiments, with inactive respiration and ATP/ADP exchange, mersalyl was shown to prevent the swelling of mitochondria suspended in a solution of ammonium phosphate but not of other permeant ammonium salts [13]. It was subsequently established that H2PO4− is the transported species [14], and that it is exchanged for OH−, or taken up in symport with H+; either way, the uptake of Pi is electroneutral (Figure 1). The above studies measured Pi transport indirectly. The first direct measurements of Pi transport kinetics were undertaken by Coty and Pedersen using an “inhibitor-stop” assay which was performed at 0°C, and revealed Pi transport to be extremely rapid [15]. Indeed, the turnover number was estimated to be 50,000 per second at 25°C, which is extremely high among carriers [16].
Figure 1. Mitochondrial Pi transport and its coordination with ATP synthesis and Ca2+ homeostasis.
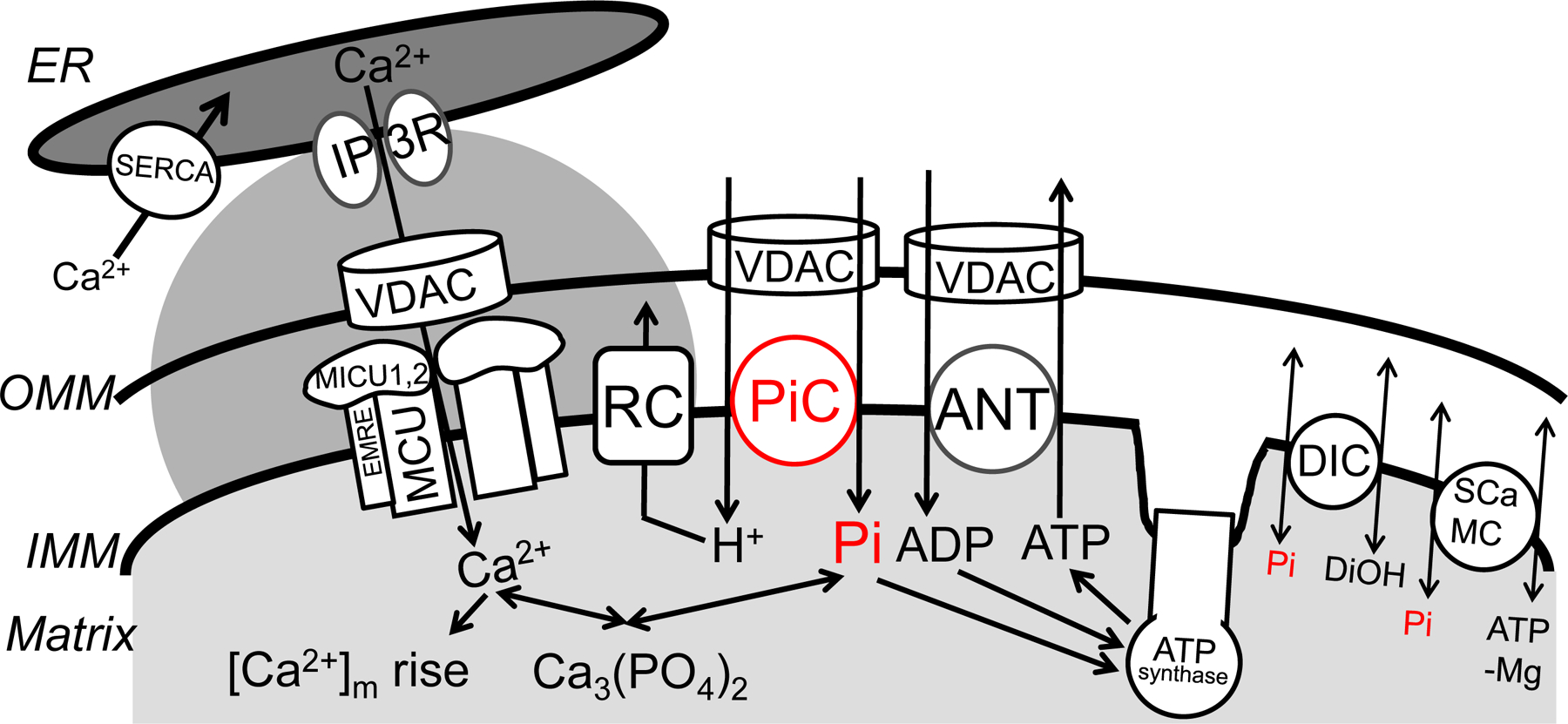
H2PO4− (shown as Pi), H+, Ca2+, Mg2+, adenine nucleotides traverse the OMM via voltage-dependent anion selective channels (VDAC). The PiC mediates electroneutral co-transport of Pi and H+ across the IMM. Pi is used for both phosphorylation of ADP (entering the matrix through ANT) by the ATP synthase, and chelation of Ca2+ in the matrix to form osmotically neutral hydroxyapatite and whitlockite. H+-s entering through the PiC support respiration and are pumped out by the respiratory complexes (RC). Alternative, probably lesser capacity, Pi uptake mechanisms are provided by the DIC and ATP-Mg/Pi carriers (SCaMC). The scheme also shows a major source of mitochondrial Ca2+ uptake, the local Ca2+ transfer from the ER (endoplasmic reticulum), mediated by the IP3 receptor Ca2+ release channels (IP3R) in the ER and the uniporter, which is formed by proteins including MCU oligomers, MICUs and EMRE in the IMM. Rapid and intense matrix Ca2+ loads provided by this pathway are compensated by fast Pi uptake mediated by the PiC, and possibly by the DIC under some conditions. Please note that the relative sizes and shape of intermembrane space and cristae are altered to accommodate the symbols and their constituents.
Purification of the phosphate transport protein
Hofmann and Kadenbach, using rat liver mitochondria, isolated a candidate Pi transporter that was estimated to be ~31 kDa [17]. Their approach relied in part on the use of mersalyl, which at low concentration did not inhibit Pi transport but protected against irreversible SH reagents [18]. Further studies indicated that the 31 kDa band was in fact multiple bands. Therefore, Durand’s group and also Wohlrab refined the above technique by combining radioactive NEM, or mersalyl coupled to an agarose matrix, with hydroxylapatite which, during other purification attempts, was recognized as having a very low affinity for the Pi carrier. This was a more straightforward approach that apparently yielded a single protein band in one case [19] and two bands in another case [20]. In the latter study, the lower molecular weight band was easily confirmed as the adenine nucleotide translocase (ANT) [21], whereas the higher molecular weight band was identified, by reconstituting the protein into liposomes then measuring 32P transport, as the Pi carrier. Furthermore, because molecular weight markers were used, the molecular weight of the Pi carrier was revised from ~31 to ~34 kDa.
Kadenbach’s group observed that the reconstituted Pi transport activity measured by Wohlrab was very low (maximal rate just below 1 umol/min/mg protein; [20]). Subsequently, Pi carrier activity was found to depend on cardiolipin. When purified protein was reconstituted into liposomes, the addition of mitochondrial phospholipids almost doubled the phosphate transport rate [22, 23]. The effect was specific to cardiolipin because other phospholipids, including acidic ones, were ineffective [22]. Moreover, the activity of recombinant PiC could be increased by more than 5 times by adding ~7% purified cardiolipin to liposomes [24], decreasing the likelihood of an indirect effect of contaminants in mitochondrial phospholipid preparations. Palmieri used this specific requirement for cardiolipin to attempt to better isolate the Pi carrier [25]; indeed, higher resolution SDS gels had revealed multiple bands at ~33–34 kDa, despite the use of hydroxylapatite [26–28]. Inclusion of cardiolipin in the hydroxylapatite elution buffer largely resolved this issue, and also greatly enhanced protein recovery [25].
Cloning and molecular characterization of the PiC protein
PiC was first cloned from bovine heart [29] and subsequently, from rat liver [30] and human heart [31]. Further work demonstrated alternatively spliced forms in human and bovine tissues, giving rise to PiC-A and PiC-B isoforms, which show muscle-specific and broad tissue range expression, respectively [32, 33]. Based on the sequence and biochemical evidence, PiC has 6 transmembrane domains and its N and C termini are within the matrix, giving rise to a threefold symmetric organization [29]. It was recognized at the time of the sequencing that PiC is structurally similar to other IMM carrier proteins, the ANT and UCP1 (uncoupling protein 1) [29, 34]. This cohort of IMM proteins is known as mitochondrial carrier (MC) proteins [35, 36]. Many MCs of known function belong to the same protein family (encoded by slc25a3 genes), since their polypeptide chains consist of three tandemly related sequences of about 100 amino acids, and the repeats of the different carriers are homologous. They probably function as homodimers, each monomer being folded in the membrane into six transmembrane segments. Some MCs have isoforms encoded by different slc25a genes, whereas PiC has two tissue-specific variants arising from a single gene by mutually exclusive alternative splicing of slc25a3 exon 3. Human SLC25A3 is located at chromosome 12q23. PiC protein consists of 362 (isoform A) or 361 (isoform B) amino acids, and the 2 splice variants encoded by exon 3A and 3B are formed by 42 and 41 amino acids, respectively which show 70% sequence identity.
SLC25A3 has a single transcriptional initiation site and lacks a TATA box but displays an activation domain (−223/−25) and an inhibition domain (−1017/−814). The most effective promoter activity in transfected HeLa cells corresponds to the region containing putative binding sites for Sp1 (−163/−142; where Sp1 stands for stimulating protein-1) and CREB (−138/−116) [37].
Role of the PiC in oxidative phosphorylation
Few studies aimed to establish the specific role of the PiC in oxidative phosphorylation. Rather, it was already understood that exogenous Pi was needed for oxidative phosphorylation, and respiration (both phosphorylating and uncoupled) was used as an outcome measure to study Pi transport across the IMM. However, Fonyo and Ligeti investigated the role of Pi in cation uptake using mersalyl and NEM, and concluded that PiC was an important H+ donor for respiration [38] (Figure 1). A small early onset burst remained in the presence of mersalyl, and this was found to be inhibited by butylmalonate, an inhibitor of the dicarboxylate carrier (DIC) which catalyzes the exchange of dicarboxylic acids (e.g., malate or succinate) for Pi [39]. It was concluded that both the PiC and the DIC have the possibility to support cation-stimulated respiration [40].
The functional importance of the PiC was first demonstrated in yeast (S. cerevisiae) that exhibited delayed growth or failed to grow on non-fermentable substrate when deleted for MIR1, the yeast homologue of PiC [41] or for MIR1 and PIC2 [42], respectively. PIC2 encodes a second mitochondrial Pi carrier in yeast and also in Arabidopsis thaliana, shares 40% homology to mir1, and appears to play a role under certain stress conditions [43]. That these yeast mutants fail to thrive specifically when grown in the presence of mitochondrial substrates suggests the relevance of the yeast homologs of the PiC for oxidative phosphorylation. It is only very recently that the role of the PiC in ATP production and oxidative phosphorylation has been confirmed in mammals. This was achieved using mouse models of PiC depletion [44, 45] and by studying a loss-of-function mutation in the human SLC25A3 gene [42] (see below).
An interesting line of recent evidence has implicated PiC as a mediator of inhibition of oxidative phosphorylation and cytopathic effect by a viral protein. Specifically, vMIA, an anti-apoptotic cytomegalovirus protein was shown to lower the cellular ATP level and inhibit the ADP-stimulated respiratory activity. No change was observed in the abundance or activity of the F1F0 ATP synthase or in ANT. The abundance of the PiC was also unaltered but the mitochondrial Pi uptake and Pi-stimulated ATP synthesis were suppressed [46]. Thus, PiC might serve as a target for the effect of viral infection on oxidative phosphorylation.
Role of PiC in calcium handling
The highly negative inner membrane potential (Δψm) presents a robust driving force for mitochondrial Ca2+ uptake. Ca2+ uptake occurs as uniport and is mediated by the mitochondrial calcium uniporter (mtCU) across the IMM (Figure 1). The mtCU is a highly Ca2+ selective ion channel formed by oligomers of pore forming MCU proteins and regulatory proteins (Figure 1) [47–49]. Mitochondrial Ca2+ uptake results in physiologically relevant [Ca2+] changes in both cytoplasm and mitochondrial matrix [50–52]. During cytoplasmic [Ca2+] ([Ca2+]c) elevations, strategically localized mitochondrial Ca2+ sequestration affects the spatial and temporal pattern of the [Ca2+]c signal, modulating a range of processes including muscle contraction and nerve function. Mitochondrial Ca2+ uptake also gives rise to a [Ca2+] increase in the mitochondrial matrix ([Ca2+]m), which controls oxidative metabolism through the Ca2+ sensitive mitochondrial matrix dehydrogenase enzymes and the pyruvate dehydrogenase phosphatase. However, when Ca2+ uptake exceeds a threshold that is dependent on the presence of sensitizers like ROS, it triggers opening of the permeability transition pore (PTP), which often initiates execution of the cell. These broadly relevant effects of mitochondrial Ca2+ uptake indicate that it has to be carefully controlled. Control of mitochondrial Ca2+ uptake can occur through a change in its driving force or the permeability of MCU. The latter is regulated for example by the Ca2+ sensing MICU1 protein that supports closure of the MCU at low and cooperative activation of the MCU at high [Ca2+]c [53–55]. Another regulatory factor that was recognized at the same time as mitochondrial Ca2+ uptake was discovered, is the availability of Pi [56].
Substantial net Ca2+ uptake by mitochondria requires the presence of anions, like Pi, acetate, β–hydroxybutyrate, glutamate, and bicarbonate but not nitrate, that can provide a H+ source for the H+ pumps of the respiratory chain. In the absence of an adequate anion, the Ca2+ uptake is suppressed, Δψm and O2 consumption are low and the matrix becomes highly alkalinic [57]. In addition, Pi uptake helps the formation of insoluble Pi salts, hydroxyapatite and whitlockite [56, 58] in the matrix and in turn, keeps the ionized [Ca2+]m at a relatively low level [59, 60] (Figure 1). This is important because the osmotically inactive precipitates help to control matrix expansion and because a progressive increase in the ionized [Ca2+]m would greatly stimulate mitochondrial Ca2+ efflux and in turn, would limit net mitochondrial Ca2+ uptake [61]. Thus, Pi can both stimulate the mtCU-mediated Ca2+ influx and suppress the exchanger-mediated Ca2+ efflux. None of the above listed anions can substitute for Pi in this regard.
Pi uptake can also be mediated by the DIC and the ATP-Mg/Pi carriers [39, 62] (Figure 1) but the primary pathway likely is provided by the PiC [40]; however, this evidence rests on potentially non-specific reagents such as NEM and mersalyl. Notably, PiC has also been proposed to undergo a Ca2+-induced cyclophilin D (CyPD)-mediated conformational change providing a mechanism central to the opening of the PTP [63]. However, a requirement for PiC in PTP opening remains debated [44, 64].
In summary, Pi transport-mediated control of mitochondrial Ca2+ handling has been well established and circumstantial evidence supports a major role for PiC in this paradigm. The high turnover rate of the PiC is an advantage to enable Pi to effectively buffer Ca2+ that enters at a high rate through an ion channel. The next section describes genetic strategies that have been developed to address more directly the role of PiC in PTP opening and calcium handling in murine models [44, 45].
Slc25a3 mouse phenotypes
Recently, the Baines and Molkentin groups have produced mice with both elevated and decreased cardiac expression of PiC. The two transgenic lines showed ~5 fold expression of PiC without a significant change in ANT and ATP synthase proteins, cardiac size and function [44]. Mitochondria isolated from the transgenic hearts showed unaltered respiration, ATP level, Ca2+ retention and activation of the PTP.
In the first set of complementing studies, shRNA was used to attain ~60% reduction in cardiac PiC protein abundance without altering ANT and ATP synthase [44]. The heart showed hypertrophy and slightly decreased fractional shortening. Mitochondrial respiration and Ca2+ retention were unaltered but the steady-state ATP level was decreased. In parallel studies, inducible and cardiac-specific deletion of PiC was established [45]. In this model, drug-induction resulted in >90% decrease in PiC protein, whereas other respiratory chain proteins were unaltered. Similar to the shRNA-mediated PiC silencing, cardiac hypertrophy was observed though cardiac shortening remained unaffected. Strikingly, time-dependent mitochondrial phosphate uptake was eliminated and the mitochondrial ATP level was decreased but Ca2+ retention was not impaired and was in fact increased.
The lack of a phenotype for the PiC transgenic mouse likely indicates that the abundance of PiC does not limit the transport and potential other functions of PiC in mouse heart, which is in accordance with the exceptionally high turnover high turnover rate of the PiC. The cardiac hypertrophy and energy deficit observed upon PiC depletion is in line with the phenotype of the ANT knockout mouse, in which another component of the ATP synthasome is missing [65]. Thus, a major reason for the phenotype can be insufficient Pi transport and oxidative phosphorylation. Although PiC has been shown to interact with CyPD, a regulator of the PTP, the lack of a change in PTP activation in the PiC deficient mitochondria is not unexpected because CyPD as a chaperone also interacts with several proteins unrelated to the PTP. Perhaps the most surprising finding is that PiC-deficient mitochondria are competent to take up Ca2+ and Ca2+ uptake happens without greater expansion of the matrix. Although the DIC and the ATP-Mg/Pi carriers could provide surrogate phosphate uptake pathways, no time-dependent mitochondrial Pi uptake was documented in this study [45]. Since Pi uptake was not measured during Ca2+ uptake, a possibility remains that Ca2+-stimulated Pi uptake also occurred in the mitochondria lacking PiC.
Pathogenic mutations in the human PIC gene
The first disease-associated PiC mutations were described in 2007. Two different mutations that affect only exon 3A and therefore the PiC-A isoform [42, 66, 67] were first observed in two different families of Turkish origin [42, 66]. More recently, one of these mutations (c.215G>A) was found in a third Turkish family (Mayr J.A. et al, unpublished). Remarkably the c.158–9A>G mutation, which results in a gain of a splice-acceptor, was also reported in a family of Guatemalan origin [67], suggesting that at least this mutation has a widespread prevalence. Furthermore, mutations in regions outside of exon 3 have also been described, which affect both PiC-A and B isoforms [67]. Every patient presented mainly with cardiomyopathy regardless of the involvement of only the A or A and B isoforms. Elevated lactate and skeletal myopathy were also displayed by every patient of PiC-A specific mutation. The prevalence of pathogenic PiC mutations may be significantly underestimated because the cardiac impairments can be fatal early after birth, or can mimic other forms of hypertrophic cardiomyopathy. Furthermore, symptoms may not be distinct enough to suggest a PiC dysfunction. One remarkable feature common to patients with SLC25A3 mutations is the lack of neurocognitive involvement, when compared to other mitochondrial disorders causing cardiomyopathy [66, 67].
The major difference in the symptoms associated with the PiC-A mutations compared to those associated with the mutations affecting both isoforms (see Table 1) might be explained by several factors, including tissue specific expression and distinct enzymatic activity of each isoform. The predominant involvement of cardiac and skeletal muscle in mitochondrial PiC deficiency can be explained by tissue specific isoforms of the enzyme, which are formed by tissue specific alternative splicing. It is also known that the transport affinity for Pi of PiC-A is 3-fold higher than that of PiC-B, whereas the maximal transport rate of PiC-B is 3-fold higher compared to PiC-A [24]. However, it is unknown how tissue function is affected by changes in the ratio of these two isoforms [67].
Table 1.
All reported cases of pathologic SLC25A3 variants.
| Family 1 | Family 2 | Family 3 | Family 4 | ||||
|---|---|---|---|---|---|---|---|
| Patient 1 – 1 | Patient 1 – 2 | Patient 1 – 3 | Patient 2 – 1 | Patient 2 – 2 | Patient 3 – 1 | Patient 4 – 1 | |
| Reference | Mayr et al. 2011 | Mayr et al. 2011 | Mayr et al. 2011 | Mayr et al. 2007 | Mayr et al. 2007 | Bhoj et al. 2014 | Bhoj et al. 2014 |
| Ethnicity | Turkish | Turkish | Turkish | Turkish | Turkish | Guatemalan | Haitian/Dominican |
| Gender | Female | Male | Male | Female | Female | Male | Male |
| Current age | Died at 6 months | 9 years, stable | 17 years, stable | Died at 4 months | Died at 9 months | 12 months, stable | 10 months, stable |
| SLC25A3 variant, affected exon | Homozygous c.158–9A>G, exon 3A | Homozygous c.158–9A>G, exon 3A | Homozygous c.158–9A>G, exon 3A | Homozygous c.215G>A, exon 3A | Homozygous c.215G>A, exon 3A | Homozygous c.158–9A>G, exon 3A | Compound heterozygous c.599T>G (exon 4), c.886–898delins7 (exon 6) |
| Affected PiC isoform | A | A | A | A | A | A | A+B |
| Condition at birth, and onset of symptoms | Information not available | Prenatal hypertrophic cardiomyopathy. Gestation: born at term. After birth: artificial ventilation was required |
Gestation: born at term. Birth weight: 3000 g, Apgar score of 3 at 1 minute and 9 at 5 minutes. At 1.5 hrs: asphyxia resuscitation and artificial ventilation |
Gestation: 38 wks. Birth weight: 2600 g (10%), Apgar score of 9 at 1 minute and 10 at 5 minutes. At 12 hrs: cyanosis and muscular hypotonia |
Gestation: born at term. Birth weight: 2870 g (10%), Apgar score of 8 at 1 minute and 10 at 5 minutes, At 10 hrs: muscular hypotonia, metabolic and lactic acidosis |
Gestation: 41 wks. Birth weight: 3990 g (75%), Apgar score of 7 at 1 minute and 8 at 5 minutes. Within 2 hrs: muscular hypotonia, hypoxia followed rapidly by respiratory failure |
Prenatal hypertrophic cardiomyopathy. Gestation: born at term. Birth weight: 2849 g (50%), Apgar score of 8 at 1 minute and 9 at 5 minutes. At 4 wks: worsening heart failure and respiratory status |
| Clinical picture: | elevated lactate, neonatal hypertrophic cardiomyopathy, muscular hypotonia, limited information |
elevated lactate, neonatal hypertrophic cardiomyopathy, 9 yrs: skeletal myopathy, stable hypertrophic cardiomyopathy |
elevated lactate, neonatal hypertrophic cardiomyopathy 17 yrs: skeletal myopathy, stable hypertrophic cardiomyopathy |
elevated lactate, neonatal hypertrophic cardiomyopathy leading to death, skeletal myopathy, delayed development. |
elevated lactate, neonatal hypertrophic cardiomyopathy leading to death, skeletal myopathy, delayed development |
lactic acidosis, neonatal hypertrophic cardiomyopathy moderate skeletal myopathy, |
no elevated lactate (lactate/pyruvate ↑) Cardiac transplant at 7 months. no myopathy |
Another intriguing aspect of the human PiC mutations is that they appear to be at least partially functionally compensated to allow survival despite a severe clinical situation during infancy. Similar compensation has also been documented in Barth syndrome [68] and in TMEM70 deficiency [69], which, like PiC deficiency, are primary mitochondrial diseases caused by mutations in nuclear-encoded genes. These diseases also manifest as life threatening diseases during early infancy but show a stabilization or even improvement of the cardiac impairment if patients survive the first years of life. Thus PiC deficiency is not the only primary mitochondrial disease with a severe clinical presentation during infancy but with some surviving adults. The compensatory mechanisms have not been identified for PiC or for these other deficiencies. In a skeletal muscle biopsy from one of the surviving individuals harboring a pathological PiC-A variant, there was evidence for elevated mitochondrial biogenesis [66]. However, this alone would be unlikely to compensate for PiC-A deficiency. In this same individual, there was essentially no PiC protein evident on a western blot, and there was only a very low signal on immunohistochemistry. Since the antibody that was used detects both isoforms, no or only a small compensatory increase in PiC-B protein occurred [66]. It is more likely that, in this individual, functional compensation has been achieved by the alteration of one or more metabolic pathways. An upregulation of glycolytic ATP production is one possibility. But there are, at least theoretically, other less obvious candidates, such as increased PiC transport through the DIC. This possibility has not been formally tested. Even the entry of modest amounts of Pi into mitochondria could be beneficial. For example, a decreased level of Pi could continue to support the phosphorylation of ADP. This could prevent a large depolarization with consequent mitochondrial ATP consumption [58]; such a mechanism would spare cytosolic ATP pools. Interestingly, in yeast, destabilization of the ATP synthase was compensated by ATP production via substrate level phosphorylation of ADP within mitochondria [70], demonstrating that this type of metabolic reconfiguration can arise in an intact organism.
Future directions
The basic biophysical and molecular characteristics of mitochondrial Pi transport and the PiC were established many years ago. Yet, the questions, and even surprises, that arise from the more recently discovered human PiC mutations and the PiC mouse models bring fresh relevance to the study of the PiC. The clinical picture of individuals with PiC mutations raises new and fundamental questions about the relationship between the A and B isoforms, the basis for the healthy survival of some of affected individuals, and the lack of neurological symptoms despite severe cardiomyopathy due to mutations affecting both isoforms. Indeed, while the mutations affecting both isoforms are predicted to be pathogenic, a more complete understanding of these mutations is needed. Regarding Ca2+, its effective mitochondrial uptake in the mouse model, with almost complete depletion of both isoforms, is surprising [45]. Therefore, the precise role of the PiC in mitochondrial Ca2+ uptake requires further investigation. Since Pi likely is a primary factor in mitochondrial matrix Ca2+ buffering that shows remarkable adaptive changes during both acute and prolonged increases in mitochondrial Ca2+ uptake [53, 59], the mechanisms of the coordination of the transports of Pi and Ca2+ is of great interest. Although mitochondrial ATP production is an obvious major target of PiC deficiency, downstream effects of a limitation in mitochondrial ATP production may have their own consequences. In this regard, OPA1-mediated IMM fusion is sensitive to the level of oxidative phosphorylation (Mishra et al. 2014), and mitochondrial fusion can function to acutely preserve ATP production in response to stress [71–73]. Thus the PiC should be crucial to the effectiveness of this stress response. For all of the above questions, the PiC mouse models, as well as the natural models presented by the human PiC mutations will be valuable as both tools and sources of information. Finally, ongoing improvements in gene sequencing technology, coupled with a greater awareness of PiC mutations, may significantly expand the population of identified individuals, making a further understanding of these mutations, and of the PiC more generally, of great importance.
Acknowledgements
This work was supported by a UMDF grant 14–145R to ELS and an NIH grant DK051526 to GH. The authors thank Drs. Jan Hoek and György Csordás (both at TJU, MitoCare) for helpful discussions.
References
- [1].Cleland KW, Permeability of isolated rat heart sarcosomes, Nature, 170 (1952) 497–499. [DOI] [PubMed] [Google Scholar]
- [2].Chappell JB, Crofts AB, Regulation of Metabolic Processes in Mitochondria (Tager JM, Papa S, Quagliariello E, and Slater EC, eds), (1966) 293–314.
- [3].Packer L, Wrigglesworth JM, Fortes PA, Pressman BC, Expansion of the inner membrane compartment and its relation to mitochondrial volume and ion transport, J Cell Biol, 39 (1968) 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chance B, Phosphorylation efficiency of the intact cell. III. Phosphorylation and dephosphorylation in yeast cells, The Journal of biological chemistry, 234 (1959) 3041–3043. [PubMed] [Google Scholar]
- [5].van den BS, Slater EC, The respiratory activity and respiratory control of sarcosomes isolated from the thoracic muscle of the housefly, Biochimica et biophysica acta, 40 (1960) 176–177. [DOI] [PubMed] [Google Scholar]
- [6].Chappell JB, Crofts AR, Gramicidin and Ion Transport in Isolated Liver Mitochondria, Biochem J, 95 (1965) 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fonyo A, Bessman SP, The action of oligomycin and of para-hydroxymercuribenzoate on mitochondrial respiration stimulated by ADP, arsenate and calcium, Biochemical and biophysical research communications, 24 (1966) 61–66. [DOI] [PubMed] [Google Scholar]
- [8].Lehninger AL, Phosphorylation coupled to oxidation of dihydrodiphosphopyridine nucleotide, The Journal of biological chemistry, 190 (1951) 345–359. [PubMed] [Google Scholar]
- [9].Cooper C, Lehninger AL, Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. V. The adenosine triphosphate-phosphate exchange reaction, The Journal of biological chemistry, 224 (1957) 561–578. [PubMed] [Google Scholar]
- [10].Boyer PD, Slater EC, Labelling rates and detection of intermediates in mitochondrial phosphorylations and other sequential reactions, Nature, 207 (1965) 409–412. [DOI] [PubMed] [Google Scholar]
- [11].Fonyo A, Phosphate carrier of rat-liver mitochondria: its role in phosphate outflow, Biochemical and biophysical research communications, 32 (1968) 624–628. [DOI] [PubMed] [Google Scholar]
- [12].Tyler DD, The inhibition of phosphate entry into rat liver mitochondria by organic mercurials and by formaldehyde, Biochem J, 107 (1968) 121–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tyler DD, Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria, Biochem J, 111 (1969) 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lofrumento NE, Hoek JB, Meyer AJ, Tager JM, Phosphate transport in rat-liver mitochondria, Biochimica et biophysica acta, 226 (1971) 297–308. [DOI] [PubMed] [Google Scholar]
- [15].Coty WA, Pedersen PL, Phosphate transport in rat liver mitochondria. Kinetics and energy requirements, The Journal of biological chemistry, 249 (1974) 2593–2598. [PubMed] [Google Scholar]
- [16].Ligeti E, Brandolin G, Dupont Y, Vignais PV, Kinetics of Pi-Pi exchange in rat liver mitochondria. Rapid filtration experiments in the millisecond time range, Biochemistry, 24 (1985) 4423–4428. [DOI] [PubMed] [Google Scholar]
- [17].Hofmann HD, Kadenbach B, Specific labeling of a phosphate-transporting protein from rat-liver mitochondria by [203Hg]mersalyl, Eur J Biochem, 102 (1979) 605–613. [DOI] [PubMed] [Google Scholar]
- [18].Fonyo A, Phosphate carrier of liver mitochondria: two equivalent SH-groups in the carrier unit, Biochemical and biophysical research communications, 57 (1974) 1069–1073. [DOI] [PubMed] [Google Scholar]
- [19].Touraille S, Briand Y, Durand R, Bonnafous JC, Mani JC, Purification of a phosphate carrier in pig heart mitochondria by affinity chromatography on mersalyl-ultrogel, FEBS letters, 128 (1981) 142–144. [DOI] [PubMed] [Google Scholar]
- [20].Wohlrab H, Purification of a reconstitutively active mitochondrial phosphate transport protein, The Journal of biological chemistry, 255 (1980) 8170–8173. [PubMed] [Google Scholar]
- [21].Klingenberg M, Riccio P, Aquila H, Isolation of the ADP, ATP carrier as the carboxyatractylate. protein complex from mitochondria, Biochimica et biophysica acta, 503 (1978) 193–210. [DOI] [PubMed] [Google Scholar]
- [22].Kadenbach B, Mende P, Kolbe HV, Stipani I, Palmieri F, The mitochondrial phosphate carrier has an essential requirement for cardiolipin, FEBS letters, 139 (1982) 109–112. [DOI] [PubMed] [Google Scholar]
- [23].Mende P, Kolbe HV, Kadenbach B, Stipani I, Palmieri F, Reconstitution of the isolated phosphate-transport system of pig-heart mitochondria, Eur J Biochem, 128 (1982) 91–95. [DOI] [PubMed] [Google Scholar]
- [24].Fiermonte G, Dolce V, Palmieri F, Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria, The Journal of biological chemistry, 273 (1998) 22782–22787. [DOI] [PubMed] [Google Scholar]
- [25].Bisaccia F, Palmieri F, Specific elution from hydroxylapatite of the mitochondrial phosphate carrier by cardiolipin, Biochimica et biophysica acta, 766 (1984) 386–394. [DOI] [PubMed] [Google Scholar]
- [26].Kolbe HV, Bottrich J, Genchi G, Palmieri F, Kadenbach B, Isolation and reconstitution of the phosphate-transport system from pig heart mitochondria, FEBS letters, 124 (1981) 265–269. [DOI] [PubMed] [Google Scholar]
- [27].Kolbe HV, Mende P, Kadenbach B, The protein component(s) of the isolated phosphate-transport system of mitochondria, Eur J Biochem, 128 (1982) 97–105. [DOI] [PubMed] [Google Scholar]
- [28].de Pinto V, Tommasino M, Palmieri F, Kadenbach B, Purification of the active mitochondrial phosphate carrier by affinity chromatography with an organomercurial agarose column, FEBS letters, 148 (1982) 103–106. [DOI] [PubMed] [Google Scholar]
- [29].Runswick MJ, Powell SJ, Nyren P, Walker JE, Sequence of the bovine mitochondrial phosphate carrier protein: structural relationship to ADP/ATP translocase and the brown fat mitochondria uncoupling protein, The EMBO journal, 6 (1987) 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferreira GC, Pratt RD, Pedersen PL, Energy-linked anion transport. Cloning, sequencing, and characterization of a full length cDNA encoding the rat liver mitochondrial proton/phosphate symporter, The Journal of biological chemistry, 264 (1989) 15628–15633. [PubMed] [Google Scholar]
- [31].Dolce V, Fiermonte G, Messina A, Palmieri F, Nucleotide sequence of a human heart cDNA encoding the mitochondrial phosphate carrier, DNA sequence : the journal of DNA sequencing and mapping, 2 (1991) 133–135. [DOI] [PubMed] [Google Scholar]
- [32].Dolce V, Iacobazzi V, Palmieri F, Walker JE, The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms, The Journal of biological chemistry, 269 (1994) 10451–10460. [PubMed] [Google Scholar]
- [33].Dolce V, Fiermonte G, Palmieri F, Tissue-specific expression of the two isoforms of the mitochondrial phosphate carrier in bovine tissues, FEBS letters, 399 (1996) 95–98. [DOI] [PubMed] [Google Scholar]
- [34].Palmieri F, Bisaccia F, Capobianco L, Dolce V, Fiermonte G, Iacobazzi V, Zara V, Transmembrane topology, genes, and biogenesis of the mitochondrial phosphate and oxoglutarate carriers, Journal of bioenergetics and biomembranes, 25 (1993) 493–501. [DOI] [PubMed] [Google Scholar]
- [35].Palmieri F, Pierri CL, Structure and function of mitochondrial carriers - role of the transmembrane helix P and G residues in the gating and transport mechanism, FEBS letters, 584 (2010) 1931–1939. [DOI] [PubMed] [Google Scholar]
- [36].Palmieri F, The mitochondrial transporter family SLC25: identification, properties and physiopathology, Molecular aspects of medicine, 34 (2013) 465–484. [DOI] [PubMed] [Google Scholar]
- [37].Iacobazzi V, Infantino V, Costanzo P, Izzo P, Palmieri F, Functional analysis of the promoter of the mitochondrial phosphate carrier human gene: identification of activator and repressor elements and their transcription factors, The Biochemical journal, 391 (2005) 613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fonyo A, Ligeti E, The role of intramitochondrial Pi in stimulation of respiration by calcium and strontium, FEBS Lett, 93 (1978) 289–292. [DOI] [PubMed] [Google Scholar]
- [39].Palmieri F, Prezioso G, Quagliariello E, Klingenberg M, Kinetic study of the dicarboxylate carrier in rat liver mitochondria, European journal of biochemistry / FEBS, 22 (1971) 66–74. [DOI] [PubMed] [Google Scholar]
- [40].Ligeti E, Ikrenyi K, Fonyo A, The inhibitor-sensitivity and pathways of Pi uptake during calcium and strontium accumulation in liver mitochondria, FEBS letters, 107 (1979) 205–208. [DOI] [PubMed] [Google Scholar]
- [41].Zara V, Dietmeier K, Palmisano A, Vozza A, Rassow J, Palmieri F, Pfanner N, Yeast mitochondria lacking the phosphate carrier/p32 are blocked in phosphate transport but can import preproteins after regeneration of a membrane potential, Molecular and cellular biology, 16 (1996) 6524–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mayr JA, Merkel O, Kohlwein SD, Gebhardt BR, Bohles H, Fotschl U, Koch J, Jaksch M, Lochmuller H, Horvath R, Freisinger P, Sperl W, Mitochondrial phosphate-carrier deficiency: a novel disorder of oxidative phosphorylation, Am J Hum Genet, 80 (2007) 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hamel P, Saint-Georges Y, de Pinto B, Lachacinski N, Altamura N, Dujardin G, Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana, Molecular microbiology, 51 (2004) 307–317. [DOI] [PubMed] [Google Scholar]
- [44].Gutierrez-Aguilar M, Douglas DL, Gibson AK, Domeier TL, Molkentin JD, Baines CP, Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition, Journal of molecular and cellular cardiology, 72 (2014) 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kwong JQ, Davis J, Baines CP, Sargent MA, Karch J, Wang X, Huang T, Molkentin JD, Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy, Cell death and differentiation, 21 (2014) 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Poncet D, Pauleau AL, Szabadkai G, Vozza A, Scholz SR, Le Bras M, Briere JJ, Jalil A, Le Moigne R, Brenner C, Hahn G, Wittig I, Schagger H, Lemaire C, Bianchi K, Souquere S, Pierron G, Rustin P, Goldmacher VS, Rizzuto R, Palmieri F, Kroemer G, Cytopathic effects of the cytomegalovirus- encoded apoptosis inhibitory protein vMIA, J Cell Biol, 174 (2006) 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK, Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter, Nature, (2011). [DOI] [PMC free article] [PubMed]
- [48].De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R, A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter, Nature, (2011). [DOI] [PMC free article] [PubMed]
- [49].Kirichok Y, Krapivinsky G, Clapham DE, The mitochondrial calcium uniporter is a highly selective ion channel, Nature, 427 (2004) 360–364. [DOI] [PubMed] [Google Scholar]
- [50].Rizzuto R, Bernardi P, Pozzan T, Mitochondria as all-round players of the calcium game, J Physiol, 529 Pt 1 (2000) 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hajnoczky G, Csordas G, Madesh M, Pacher P, The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria, J Physiol, 529 Pt 1 (2000) 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Duchen MR, Mitochondria and calcium: from cell signalling to cell death, J Physiol, 529 Pt 1 (2000) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G, MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter, Cell metabolism, 17 (2013) 976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK, MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake, Nature, 467 (2010) 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M, MICU1 Is an Essential Gatekeeper for MCU-Mediated Mitochondrial Ca(2+) Uptake that Regulates Cell Survival, Cell, 151 (2012) 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Carafoli E, The fateful encounter of mitochondria with calcium: how did it happen?, Biochimica et biophysica acta, 1797 (2010) 595–606. [DOI] [PubMed] [Google Scholar]
- [57].Ligeti L, Barlow C, Chance B, Kovach AG, O’Connor M, 31P NMR spectroscopy of brain and heart, Advances in experimental medicine and biology, 159 (1983) 281–292. [DOI] [PubMed] [Google Scholar]
- [58].Chinopoulos C, Adam-Vizi V, Mitochondrial Ca2+ sequestration and precipitation revisited, The FEBS journal, 277 (2010) 3637–3651. [DOI] [PubMed] [Google Scholar]
- [59].Chalmers S, Nicholls DG, The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria, J Biol Chem, 278 (2003) 19062–19070. [DOI] [PubMed] [Google Scholar]
- [60].Wei AC, Liu T, Winslow RL, O’Rourke B, Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering, J Gen Physiol, 139 (2012) 465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zoccarato F, Nicholls D, The role of phosphate in the regulation of the independent calcium-efflux pathway of liver mitochondria, Eur J Biochem, 127 (1982) 333–338. [DOI] [PubMed] [Google Scholar]
- [62].Fiermonte G, De Leonardis F, Todisco S, Palmieri L, Lasorsa FM, Palmieri F, Identification of the mitochondrial ATP-Mg/Pi transporter. Bacterial expression, reconstitution, functional characterization, and tissue distribution, The Journal of biological chemistry, 279 (2004) 30722–30730. [DOI] [PubMed] [Google Scholar]
- [63].Leung AW, Varanyuwatana P, Halestrap AP, The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition, The Journal of biological chemistry, 283 (2008) 26312–26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Basso E, Petronilli V, Forte MA, Bernardi P, Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation, The Journal of biological chemistry, 283 (2008) 26307–26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Narula N, Zaragoza MV, Sengupta PP, Li P, Haider N, Verjans J, Waymire K, Vannan M, Wallace DC, Adenine nucleotide translocase 1 deficiency results in dilated cardiomyopathy with defects in myocardial mechanics, histopathological alterations, and activation of apoptosis, JACC. Cardiovascular imaging, 4 (2011) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mayr JA, Zimmermann FA, Horvath R, Schneider HC, Schoser B, Holinski-Feder E, Czermin B, Freisinger P, Sperl W, Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children, Neuromuscul Disord, 21 (2011) 803–808. [DOI] [PubMed] [Google Scholar]
- [67].Bhoj EJ, Li M, Ahrens-Nicklas R, Pyle LC, Wang J, Zhang VW, Clarke C, Wong LJ, Sondheimer N, Ficicioglu C, Yudkoff M, Pathologic Variants of the Mitochondrial Phosphate Carrier SLC25A3: Two New Patients and Expansion of the Cardiomyopathy/Skeletal Myopathy Phenotype With and Without Lactic Acidosis, JIMD Reports, (2014). [DOI] [PMC free article] [PubMed]
- [68].Christodoulou J, McInnes RR, Jay V, Wilson G, Becker LE, Lehotay DC, Platt BA, Bridge PJ, Robinson BH, Clarke JT, Barth syndrome: clinical observations and genetic linkage studies, American journal of medical genetics, 50 (1994) 255–264. [DOI] [PubMed] [Google Scholar]
- [69].Honzik T, Tesarova M, Mayr JA, Hansikova H, Jesina P, Bodamer O, Koch J, Magner M, Freisinger P, Huemer M, Kostkova O, van Coster R, Kmoch S, Houstek J, Sperl W, Zeman J, Mitochondrial encephalocardio-myopathy with early neonatal onset due to TMEM70 mutation, Archives of disease in childhood, 95 (2010) 296–301. [DOI] [PubMed] [Google Scholar]
- [70].Schwimmer C, Lefebvre-Legendre L, Rak M, Devin A, Slonimski PP, di Rago JP, Rigoulet M, Increasing mitochondrial substrate-level phosphorylation can rescue respiratory growth of an ATP synthase-deficient yeast, The Journal of biological chemistry, 280 (2005) 30751–30759. [DOI] [PubMed] [Google Scholar]
- [71].Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC, SLP-2 is required for stress-induced mitochondrial hyperfusion, The EMBO journal, 28 (2009) 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gomes LC, Di Benedetto G, Scorrano L, During autophagy mitochondria elongate, are spared from degradation and sustain cell viability, Nature cell biology, 13 (2011) 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J, Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation, Proceedings of the National Academy of Sciences of the United States of America, 108 (2011) 10190–10195. [DOI] [PMC free article] [PubMed] [Google Scholar]


