Abstract
Purpose of Review
To review research on breast cancer mortality disparities, emphasizing research conducted in the Carolina Breast Cancer Study, with a focus on challenges and opportunities for integration of tumor biology and access characteristics across the cancer care continuum.
Recent Findings
Black women experience higher mortality following breast cancer diagnosis, despite lower incidence compared to white women. Biological factors, such as stage at diagnosis and breast cancer subtypes, play a role in these disparities. Simultaneously, social, behavioral, environmental, and access to care factors are important. However, integrated studies of biology and access are challenging and it is uncommon to have both data types available in the same study population. The central emphasis of Phase 3 of the Carolina Breast Cancer Study, initiated in 2008, was to collect rich data on biology (including germline and tumor genomics and pathology) and health care access in a diverse study population, with the long term goal of defining intervention opportunities to reduce disparities across the cancer care continuum.
Summary
Early and ongoing research from CBCS has identified important interactions between biology and access, leading to opportunities to build greater equity. However, sample size, population-specific relationships among variables, and complexities of treatment paths along the care continuum pose important research challenges. Interdisciplinary teams, including experts in novel data integration and causal inference, are needed to address gaps in our understanding of breast cancer disparities
Keywords: Breast cancer, racial disparities, access to health care, data integration, cancer care continuum
Introduction
Black women experience a 42% higher mortality rate compared to white women [1]. Since the 1980s, this mortality gap has increased, partly reflecting disproportionate increases in survival among white women [2]. The disparities are particularly pronounced among younger women, where black women age 45–54 years have a death rate from breast cancer double that of white women of the same age [3]. Younger and black women are more likely to have molecular subtypes of breast cancer that are more aggressive and that lack molecular targets for treatment [4–9]. However, our understanding of biologic factors that account for more aggressive breast cancers in black women remains incomplete, and moreover, is poorly integrated with understanding of how tumor biology interacts with other patient-level factors such as access to care and treatment adherence.
A recent review highlights how both tumor biology and quality of care increase the racial mortality gap [10]. One limitation of previous literature is that often depth of biological data is not found in the same study population as depth of data on treatment and access. For example, the Cancer Genome Atlas project (TCGA) has detailed tumor-level data on RNA, DNA, protein, methylation and other genomic features of breast cancer [11], but patient-level information on things like comorbidities, body mass index, and treatment are lacking. Conversely, SEER and SEER-Medicare or other studies linked to insurance or health care data [1, 12] may offer more detailed understanding of treatment patterns, but are lacking information on tumor subtype. Thus, much research continues to consider tumor biology and access separately, with little integration, and few studies are able to integrate granular and detailed information regarding both biology and access.
Based on early studies in the Carolina Breast Cancer Study (CBCS) Phase 1 and 2, a population-based case-control study that oversampled black and young women, it was demonstrated that tumor biology, specifically higher incidence of basal-like breast cancer in African American women [5], was an important plausible contributor to mortality disparities. However, as in many other studies of tumor biology, treatment data was lacking as was detailed follow up information. More than twenty-five years of research in the CBCS had generated a large and inter-connected body of research, but many of the more than 160 published studies were oriented toward understanding risk and tumor biology, rather than long term differences in survivorship. In 2008, the study began recruiting phase 3, and has now recruited 3000 women diagnosed with invasive breast cancer to develop a more complete understanding of breast cancer mortality disparities.
In this current report, we will review previous literature on racial mortality disparities in the CBCS, as well as available data for survivorship research. We will consider early attempts to integrate biology and access, and highlight needs and opportunities for future research. Throughout this review, we refer to biology, meaning the molecular, histological, and clinical characteristics of breast tumors and their microenvironments. We refer to access, meaning the patterns of health services availability, quality, utilization, and treatment adherence. The central hypothesis underlying our perspective and the driving motivation for the Carolina Breast Cancer Study is that disparities in outcomes are caused by complex interactions of biology and access. We will evaluate research across the cancer care continuum, from screening and diagnosis, to staging and treatment, to survivorship, to highlight research gaps and opportunities for data integration.
Designing Studies to Address Biology and Access to Care Interaction
Beginning in 1993, researchers at University of North Carolina had been studying breast cancer disparities, and the first phase of CBCS (CBCS1, 1993–1996) was designed as a case-control study to study risk factors for breast cancer. Early CBCS findings related to etiology and prevention such as genetic susceptibility, environmental exposures, gene-environment interactions, socio-behavioral and environmental risk factors, with a major emphasis on collecting tumor blocks and incorporating molecular epidemiologic methods into population-based research [13]. CBCS increased its sample size in phase 2 (1996–2001) and added new exposure information (Nonsteroidal anti-inflammatory drugs [NSAID] and anti-depressant use, and dietary practices). This phase also included cases with ductal carcinoma in situ, to begin developing a picture of the early stages of breast cancer etiology [14]. These studies helped elucidate reproductive and behavioral risk factors for breast cancer, including body size and physical activity, environmental exposures, alcohol, smoking and diet factors, and showed that many risk factors identified in white women were also relevant for black women [15–29]. In fact, many of the CBCS findings showed particularly strong effects in black women. For example, positive associations between cigarette smoking and increased Luminal breast cancer risk, but not Basal like, were significant only among black women [30].
These studies also emphasized etiologic heterogeneity according to tumor markers, showing that distinct molecular subtypes may have unique risk factors. Studies showed that 7 or more alcoholic beverages per week was associated with estrogen receptor negative and triple negative breast cancers [31] and highlighted risk associated with body size [32], NSAID use [33], mammographic density [34]. Studies also highlighted greater frequency of aggressive tumor features in young women‟s breast cancer [35]. Many of these findings were recapitulations of studies in other populations, but the contribution of CBCS1 and CBCS2 data was largely to extend these findings to a more diverse population. Ultimately, data and samples were contributed to large national projects in genetics [36–41] and consortium projects focused on understanding black women‟s risk such as the African American Breast Cancer Epidemiology and Risk (AMBER) consortium, a National Cancer Institute funded P01 project [42].
More than a decade after the inception of CBCS 1 and 2, data were linked with the National Death Index to evaluate overall and breast cancer specific mortality. These analyses identified patterns of survivorship by exposure, and began to identify some racial differences. For example, analyses were conducted regarding the role of NSAIDs in survival [33], and the role of pregnancy and obesity in breast cancer mortality [43, 44]. A landmark paper for the study showed that breast cancer specific survival was worse for black women, even when stratifying on clinical markers such as estrogen receptor, progesterone receptor and HER2 [45]. This study was the impetus for a larger survivorship cohort study because of two limitations. The study had elucidated important mortality disparities, but it lacked information on treatment and there was no direct contact with patients to allow detailed resolution of survivorship experiences or intervention opportunities. In addition, treatment guidelines had changed over time, including the advent of HER2-targeted therapies and new guidelines for treating ER positive disease.
In response to the need for more detailed follow up and treatment data, CBCS3 was initiated as a case-only cohort study in 2008. Phase 3 continued to leverage the key interdisciplinary strength of the CBCS, and deepened engagement and collaboration with experts in molecular biology, epidemiology, genetics, and clinical expertise. Now, as CBCS3 begins its tenth year of patient contacts and detailed follow-up, the study is poised to integrate biology and access to identify the strongest contributors to differences between black and white women with breast cancer. The central emphasis of the study is deepening rich data on health care access and outcomes to better understand breast cancer mortality disparities across the cancer care continuum.
CBCS3 incorporates technical advances in intrinsic subtype analysis, including detailed RNA expression profiling, DNA sequencing, and immunoprofiling. Early results applied a robust gene expression platform called Nanostring that is optimized for RNA in formalin-fixed paraffin embedded specimens [46, 47], showing that multigene assays can better resolve biological differences between tumors (relative to single marker studies). Published CBCS3 data showed that while immunohistochemical (IHC) assays can identify Basal-like breast cancers from non-Basal-like with reasonable certainty (86% sensitivity), these IHC-based analyses cannot distinguish Luminal A from Luminal B breast cancers (46 of 60 tumors that were Luminal B by PAM50 in CBCS were inappropriately classified as Luminal A by IHC) [48]. IHC assays are also unable to differentiate molecular subtypes such the human epidermal growth factor receptor 2 (HER2)-enriched subtype from Luminal and other subtypes in clinically HER2-positive breast cancers. The study found that with improved PAM50 classification, significantly higher prevalence of poor prognosis tumors are observed in black women compared with white women. In particular there were significantly more Basal-like (35% vs 18%), particularly in younger women, and significantly fewer Luminal A tumors (31% vs 50%) [49].
CBCS3 is continuing to collect RNA-based data on a number of important pathways in breast cancer, and increasingly is integrating this data with other data types, including germline DNA [36] and ongoing efforts to integrate with DNA sequencing-derived mutational signatures. Expression data is also available to complement existing methylation findings [50, 51]. Histopathology data, including results from deep learning are now being integrated with gene expression data [52]. CBCS3 is also collecting recurrence and second primary tumors and to study etiologic heterogeneity by evaluating patterns of recurrence and concordance between first and subsequent tumors [53].
With this resource of tumor biologic data, important data integration steps have been made, but most complex data analyses have involved combining different types of biologic or genomic data. It is much more challenging to combine genomic data with detailed information on survivorship. In the sections that follow, we will detail how CBCS studies are beginning to address integration of biology and access, highlighting challenges and opportunities. To organize this analyses, we will consider the cancer care continuum in its entirety, beginning with screening and diagnosis, progressing to treatment and adherence, and finally considering recurrence and long term survivorship. Following these case studies, we will consider methodologic approaches and conceptual challenges for biology and access integration moving forward.
Screening and Diagnosis
Patients enter the cancer care continuum at screening and diagnosis, and this is the first opportunity for a more integrated conceptualization of breast cancer. Mammography is a screening tool intended to detect cancers at an earlier stage and thereby increase survivorship. However, access to mammography and mammography facilities are unequally distributed geographic and by racial/ethnic, poverty, work, insurance, education, transportation and cultural factors [54–56]. Access issues and neighborhood segregation create disparities, where black women are more likely to live in areas with higher proportions of inadequate and inappropriate treatment compared to white women [57]. Historically, affluent urban women have experienced greater benefit from screening compared to poorer women of color [58].
However, mammography effectiveness is also influenced by tumor biology. Mammography may have greater sensitivity to detect ER+ or luminal breast cancers [59]. Interval breast cancers are more likely to be triple negative [60–62]. Differences in screening adherence have been hypothesized to contribute to variation in subtype prevalence by race, with highly screened, older white women having the highest prevalence of the most readily detectable, Luminal A breast cancers. However, few studies have evaluated molecular subtype in association with mode of detection (symptomatic vs. screen-detected vs. interval detected) in the modern screening era (i.e. with digital mammography) [63, 64]. These previous papers did not incorporate multigene assays in their assessment of detection differences.
Through a data linkage between CBCS and the Carolina Mammography Registry, we observed that black women had similar rates of screening adherence to white women, but were more likely to develop interval breast cancers (cancers detected between regular screenings) [65]. Interval cancer was also associated with triple-negative clinical subtype and non-Luminal A molecular subtype (by more rapid progression and tumor features that are hard to detect by mammography), whereas screen-detected cancers tended to be more indolent, smaller and more frequently low genomic risk. These strong associations between interval cancers and poor-prognosis genomic features (non-Luminal A subtype and high risk of recurrence score) suggest that aggressive tumor biology is an important contributor to detection disparities by race. Moreover, these findings suggest that screening access or adherence differences interact with tumor subtype.
While screening mammography leads to early detection of some TNBCs and does benefit black women [66], it is important to develop improved understanding of where some of the failures may occur. Ongoing CBCS research is evaluating whether specific subtypes are more likely to present as masses (rather than as readily detectable calcifications). Notably, compared to white women, black women are less likely to present with calcifications and more likely to present with masses (mostly associated with aggressive subtypes) [67]. Better understanding tumor biology can also improve breast cancer screening methods. Other research has shown that mammographic features (e.g., calcification type and mass shape/margins) are associated with breast cancer subtypes [68].
Treatment/ Adherence
After diagnosis, there may be differences in types of treatment and also timeliness of treatment initiation or completion. Previous literature shows that timeliness of care improves survival, yet this relationship is a complex interaction of clinical factors, tumor biology, and SES factors [69]. Previous studies have shown that black women experience greater delays in care than white women at multiple points along the treatment pathway including diagnostic delay (time from detection to medical consultation/diagnosis) [70, 71] and treatment delay (time from diagnosis to the initiation of treatment) [70, 72–75]. Black women experience delay in radiation therapy, and these delays are partly explained by differences in geographic access to radiation facilities [76] [77]. In addition, black patients experience delays in initiating and completing chemotherapy [78–81] and experience longer delays in initiating appropriate endocrine therapy [82]. Although differential insurance coverage may explain some of these differences in time to treatment, black women are still more likely to experience delays [70, 74, 73, 76] and to fare worse[73, 83, 78, 79] after controlling for insurance type. These delays have been demonstrated to be particularly impactful on clinical outcomes of triple negative breast cancer [78, 83].
Similar to previous work, early papers in CBCS3 showed differences in access without incorporating biologic insights. For example, women of lower income and residents of rural areas were less likely to receive breast conserving surgery [84]. We also found that a larger proportion of black women (43%) had delays in first treatment (more than 30 days), compared to white women (38%) [75]. For younger women (under age 50), the racial disparity in treatment delay was even greater. Additionally, the disparity by race was present when stratified by treatment type (e.g. surgery, radiation, chemotherapy, etc.). It is likely that these treatment delays relate to a range of socioeconomic factors aside from race (e.g. marital status, insurance status, etc.). However, these analyses focused primarily on understanding differences in access, with less attention to biology.
A first attempt to integrate biology and access in understanding treatment decisions utilized genomic data to understand black-white differences [49]. Multigene precision medicine tools, such as Oncotype DX and Prosigna/PAM50, are important for optimizing treatment. The most frequently used genomic prognostic assay in ER+/HER2- patients is the Oncotype Dx. Through medical record abstraction, CBCS3 data showed that the uptake rates for Oncotype DX were similar in black and white node-negative patients of CBCS overall, but also found that among higher-risk women with node-positive disease, use of the test was higher in white women [85]. Application of Oncotype DX in higher risk women, is not guideline concordant. However, even guideline discordant utilization of genomic testing may influence treatment decisions. Our data showed that there were no racial differences in adjuvant chemotherapy initiation among women with similar Oncotype DX risk scores [85, 86]. This raises at least a couple of possibilities: (1) women who receive genomic testing differ systematically from those that do not, and (2) genomic tools could also function to create more equity in prescribing patterns by providing a clear decision aid.
Other ongoing work on treatment is addressing differences in endocrine adherence [87–89]. Oral adjuvant endocrine therapy (ET) dramatically reduces the risk of cancer-specific mortality and recurrence, but between 30% and 50% of women with HR+ disease never initiate ET, which can differ by race [90–92]. Using CBCS data, compared to white women, black women reported greater ET underuse and nonadherence. Major predictors of nonadherence by race included differential risk perception, lack of shared treatment decision making, and worse side effect burden [88].
A recent interesting finding that suggests potential for integration of tumor biology, chemotherapy, and endocrine adherence data. ER+ black women in SEER have higher rates of chemotherapy and lower rates of endocrine therapy initiation [93, 94]. It is unclear why apparently similar clinical indications are leading to different treatment patterns by race, but it would be helpful to understand whether chemotherapy treatment affects adherence rates. A recent study also showed that chemotherapy prevalence differed by race only among women who did not receive genomic testing [95]. These data suggest that treatment decisions vary by tumor biology in ways that are as yet not fully understood. Future research should evaluate not just chemotherapy initiation, but details on chemotherapy discontinuation, endocrine adherence, and genomic testing to gain a more complete picture of interactions among these variables.
Early Recurrence, Survival, and Quality of Life
For over 20 years we have known that African American (AA) women have a higher burden of breast cancer mortality. These differences are also attributable to black women being at greater risk of hormone receptor- and HER2 receptor-negative cancers [5, 96–98] more advanced stage [99] and higher grade at diagnosis [100], and other adverse tumor biology among black women [100, 101, 49]. Research conducted in the CBCS has demonstrated that black and young women have higher rates of the aggressive, treatment refractory basal-like subtype and lower rates of estrogen receptor positive Luminal A breast cancer than white women [5]. However, our research has also shown that the prevalence of the basal-like subtype does not fully explain mortality disparities [45]. Substantial disparities exist within subtype, such that even among a homogeneously defined group of Luminal A breast cancers, survival is substantially reduced for black women. Some of these differences may reflect inherent breast cancer biology, which may derive from ancestral genotype. Black women have significantly greater haplotype diversity, and GWAS studies from the CBCS have identified several new haplotypes/loci relevant in black and younger women [102]. In a recent transcript-wide association study published in CBCS, it was demonstrated that germline variation predicted gene expression in tumors, but that models derived in white women had poor predictive ability in black women, and vice versa [103]. However, race-stratified models were able to identify several alleles that had significant ability to predict survival. These findings underscore the need for larger studies and consortia where sufficient numbers of black and white patients can be included.
CBCS3 is not fully mature for understanding long term survival outcomes, having only followed patients for a median of seven years. However, some early endpoints are showing important results. Physical activity after breast cancer diagnosis has been associated with improved survival, and in CBCS3, black women were less likely to meet national physical activity guidelines after diagnosis [104]. Using health-related quality of life (HRQOL) instruments, Whites reported physical and functional scores 2–2.5 points higher than blacks and these racial differences persisted more than two years after diagnosis [105]. Both modifiable patient-level factors, like smoking and obesity, and non-modifiable factors, including younger age, black race and comorbid conditions, were associated with poorer HRQOL [106].
Financial toxicity has been shown to adversely affect survival and overall quality of life. CBCS investigators found that treatment-related adverse financial impacts were reported by more than one-half of black women and more than one third of white women [107]. A majority of this adverse financial impact was due to lost income after breast cancer diagnosis. Black women with breast cancer experienced a significantly higher risk for all measured adverse financial impact (including decrease in income, financial barrier to care, transportation barrier to care, job loss and insurance loss). Higher cancer-related financial burden can affect treatment choice, treatment compliance, and cancer outcomes [107]. Thus, policies that help limit the effect of cancer-related financial strain are needed.
Long-term Survivorship
Long term survivorship is a large and growing area for further research. It is estimated that there are roughly 3.8 million women living as breast cancer survivors in the US [108]. Issues related to long term survivorship include side effects of treatment, adjuvant hormone therapy adherence, social support, body image, quality of life and acculturation. Figure 1 shows that CBCS is positioned to study several key domains, including the 5 major domains included in the American Society for Clinical Oncology (ASCO) survivorship guidelines [109]. The ASCO guidelines address issues in the transition from treatment to survivorship and include surveillance for breast cancer recurrence, screening for second primary cancers, management of long-term and late effects, health promotion, care coordination and practice implications. Continuing to ten years after diagnosis, CBCS has collected information that represents all of the ASCO domains.
Figure 1.
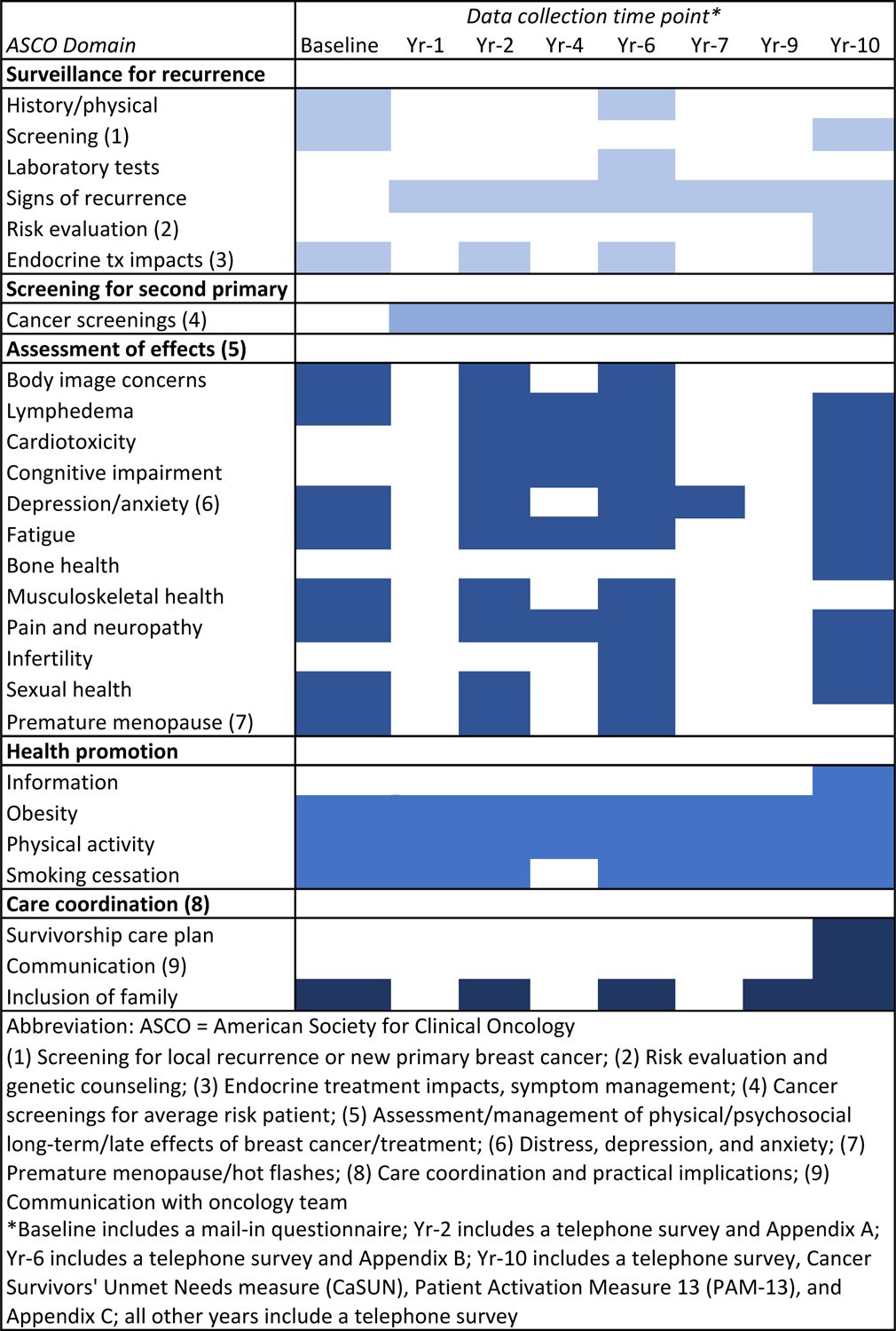
American Society of Clinical Oncology Survivorship Domains and Corresponding Data Collection Activities in CBCS3.
By conducting this research in a prospective cohort, we can assess evolution of patient concerns over time. For example, a common reason for choosing mastectomy over breast conservation therapy is the desire to avoid recurrence. Similarly, a major reason for choosing contralateral prophylactic mastectomy is for peace of mind about not having cancer recurrence in the future. Whether more extensive treatment such as mastectomy is actually associated with lower fear of recurrence is not known. Little is known about racial differences in fear of recurrence, although white women are more likely to choose more extensive surgery. An association between fear of recurrence and overutilization of services could have important implications for initial treatment, psychosocial management during survivorship, and policy. The 7-year and 10-year CBCS3 surveys include a 5-item instrument for assessing fear of recurrence, measuring the level of worry about diagnostic tests, other types of cancer, recurrence, death, and children‟s health [110]. The study is also following patients‟ unmet needs (using a measure called Cancer Survivors‟ Unmet Needs [CaSUN] [111]) and levels of health activation, using a patient activation measure [112]. These long-term survival questions lean toward questions about access and health services, but continuing to incorporate our knowledge about the original tumor biology and the risk of recurrence these women are likely to experience will provide key context for understanding these data.
Data Integration Approaches
Combining biological data, such as „omics data, with other classical epidemiologic data is not without challenges. Analytic approaches and machine learning tools are increasingly available to address some challenges, but there are several conceptual hurdles that remain. As outlined in Lopez de Maturana et al. [113], factors such as high correlation/collinearity between biological and access variables, the different nature of information, hierarchical dependence of data, heterogeneity of definitions, population substructure, disease heterogeneity, and dynamic nature of health processes are only a few of the possible hurdles. These problems are further compounded when working across multiple datasets.
One approach for coping with complex datasets that we have applied in the CBCS3 is latent class analysis (LCA). Our work with LCA illustrates some of the potential and challenges of data integration. LCA is a person-centered, dimensionality reduction analytical methods to more comprehensively characterize and understand this complex “social-contextual and biological mixture,” that can vary between people. Dimensionality reduction allow us to evaluate overall patterns that vary between people. A person-oriented approach can be contrasted with a variable-oriented approach like factor analysis, where the emphasis is on identifying relations between variables and it is assumed that these relations apply across all people.
In CBCS3, we used LCA to study barriers to care, tumor characteristics patterns, and individual level SES. We examined differences in more than 20 variables, and summarized these variables in only three sets of variables: SES, barriers to care, and tumor biological features [114]. We found that frequency distributions of all three classes varied by race and age, with lower SES, more barriers to care, and more aggressive aggregated tumor biological factors for younger and black women. The approach was data driven and can be contrasted with studies that use composite scores (e.g., area-level education or income and/or individual insurance) to capture SES [115–117]. While composite scores may be straightforward to calculate, they cannot be readily exported across datasets because SES manifests in different way in distinct populations [118]. Other studies have highlighted this challenge. For example, Palumbo et al. found lack of concordance when comparing the latent class variables with a continuous neighborhood SES index, concluding that SES characteristics were better represented by multiple latent classes than by a single index. Comparison of results from Palumbo et al. to our findings in CBCS3 show that the latent classes can be interpreted across studies. Despite formulating their latent classes differently, their work had findings similar to those from CBCS, showing that latent-class defined SES (i.e., higher proportions of neighborhoods with people single with dependents, below poverty line, low vehicle access, black race) was associated with more aggressive tumor characteristics (i.e., lower proportions of early stage, smaller size and lower grade) [119]. This work shows that data dimensionality reduction can be effective in defining some health determinants and can help elucidate relationships between factors.
However, a major limitation of data dimensionality reduction is that it often does not clearly elucidate underlying mechanisms or actionable public health strategies. It is very difficult to change SES or all the components that comprise barriers to care. Evaluation of single modifiable factors may seem more tractable for interventions. For example, if financial or transportation barriers exist, it may seem feasible to address these individually. However, previous research also suggests that seemingly simple solutions may not always have expected results [120–122]. Patients who report transportation and financial barriers may exhibit numerous other barriers to health care access, such that a simple intervention such as providing transportation services does not address the underlying barrier. Examining one barrier at a time may not adequately define the most effective interventions. Thus, balancing mechanistic understanding and appropriate modeling of complexity may sometimes be at odds. Or alternatively, they may be complementary approaches that should be used in concert.
Some recent novel approaches have developed data integration mechanisms that are pathway-focused or mechanistic by nature. These data integration approaches are particularly appealing because they hypothesize a mechanism that allows for targeted interventions. For example, Cheng and Levy considered disparities in treatment burden broadly, formulating a quantitative, multivariable measure of the workload that patients put into their care [123]. When patients are given more health care tasks than they can manage or afford, they are at risk for becoming overburdened, which can lead to decreased adherence. Workload varies by tumor aggressiveness, underscoring the inherent link between access and biology. Creative approaches that are inherently mechanistic, such as workload, are an important way to leverage epidemiologic data in concert with complex biological data.
Advancing Research in Biology and Access Disparities
In summary, this review retrospectively assessed research arising from the Carolina Breast Cancer Study, emphasizing the evolution of the field of breast cancer disparities and the current challenges faced by the field. There are many emerging research opportunities and resources, including analyses of rich electronic health care and insurance claims records, linkage of SEER data with detailed biological analyses, and efforts to improve representation of racial minorities in clinical research. As these efforts progress, our analysis of results from CBCS suggest that creating equity in health care access will require an interdisciplinary approach with creative approaches for dealing with data complexity and an emphasis on integrating biology and access.
Acknowledgements:
The authors wish to acknowledge Beth Newman and Bob Millikan, the initial PIs for CBCS phases 1 and 2/3, respectively, who made seminal contributions to the design of the study. We are also grateful to the Carolina Breast Cancer Study participants and staff. Research reported in this publication was supported by a Specialized Program of Research Excellence (SPORE) in breast cancer (P50 CA058223), an award from the Susan G. Komen Foundation (OGUNC1202), the North Carolina University Cancer Research Fund, and a Cancer Center Support Grant (P30 CA016086).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Marc A. Emerson, Katherine E. Reeder-Hayes, Heather J. Tipaldos, Mary E. Bell, Marina R. Sweeney, Lisa A. Carey, H. Shelton Earp, Andrew F. Olshan and Melissa A. Troester declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
REFERENCES
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: a cancer journal for clinicians. 2016;66(1):31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Siegel RL, Sauer AG, Miller KD, Fedewa SA, Alcaraz KI et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA: a cancer journal for clinicians. 2016;66(4):290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 3.National Center for Health Statistics, National Vital Statisitcs System. Table 26. Death rates for malignant neoplasm of breast among females, by race, Hispanic origin and age: United States 2017. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 4.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast cancer research : BCR. 2007;9(1):R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 6.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109(1):123–39. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 8.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 9.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–70. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 10.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA: a cancer journal for clinicians. 2015;65(3):221–38. doi: 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 11.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5). doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman B, Moorman PG, Millikan R, Qaqish BF, Geradts J, Aldrich TE et al. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Cancer Res Treat. 1995;35(1):51–60. doi: 10.1007/bf00694745. [DOI] [PubMed] [Google Scholar]
- 14.Phillips LS, Millikan RC, Schroeder JC, Barnholtz-Sloan JS, Levine BJ. Reproductive and hormonal risk factors for ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1507–14. doi: 10.1158/1055-9965.epi-08-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn BK, Agurs-Collins T, Browne D, Lubet R, Johnson KA. Health disparities in breast cancer: biology meets socioeconomic status. Breast Cancer Res Treat. 2010;121(2):281–92. doi: 10.1007/s10549-010-0827-x. [DOI] [PubMed] [Google Scholar]
- 16.Andaya AA, Enewold L, Horner MJ, Jatoi I, Shriver CD, Zhu K. Socioeconomic disparities and breast cancer hormone receptor status. Cancer Causes Control. 2012;23(6):951–8. doi: 10.1007/s10552-012-9966-1. [DOI] [PubMed] [Google Scholar]
- 17.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20(4):417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson BM, MacLennan MB, Hillyer LM, Ma DW. Lifelong exposure to n-3 PUFA affects pubertal mammary gland development. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39(6):699–706. doi: 10.1139/apnm-2013-0365. [DOI] [PubMed] [Google Scholar]
- 19.Krieger N, Kiang MV, Kosheleva A, Waterman PD, Chen JT, Beckfield J. Age at menarche: 50-year socioeconomic trends among US-born black and white women. Am J Public Health. 2015;105(2):388–97. doi: 10.2105/ajph.2014.301936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–27. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Progress in increasing breastfeeding and reducing racial/ethnic differences - United States, 2000–2008 births. MMWR Morbidity and mortality weekly report. 2013;62(5):77–80. [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital and health statistics Series 23, Data from the National Survey of Family Growth. 2005(25):1–160. [PubMed] [Google Scholar]
- 23.McDowell MM, Wang CY, Kennedy-Stephenson J. Breastfeeding in the United States: findings from the national health and nutrition examination surveys, 1999–2006. NCHS data brief. 2008(5):1–8. [PubMed] [Google Scholar]
- 24.Racial and ethnic differences in breastfeeding initiation and duration, by state - National Immunization Survey, United States, 2004–2008. MMWR Morbidity and mortality weekly report. 2010;59(11):327–34. [PubMed] [Google Scholar]
- 25.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64(1):1–65. [PubMed] [Google Scholar]
- 26.Daniels K, Mosher WD. Contraceptive methods women have ever used: United States, 1982–2010. National health statistics reports. 2013(62):1–15. [PubMed] [Google Scholar]
- 27.Bethea TN, Rosenberg L, Hong CC, Troester MA, Lunetta KL, Bandera EV et al. A case– control analysis of oral contraceptive use and breast cancer subtypes in the African American Breast Cancer Epidemiology and Risk Consortium. Breast cancer research : BCR. 2015;17(1). doi: 10.1186/s13058-015-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315(21):2284–91. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention. US Dept of Health and Human Services. 2017. [Google Scholar]
- 30.Butler EN, Tse CK, Bell ME, Conway K, Olshan AF, Troester MA. Active smoking and risk of Luminal and Basal-like breast cancer subtypes in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27(6):775–86. doi: 10.1007/s10552-016-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams LA, Olshan AF, Tse CK, Bell ME, Troester MA. Alcohol intake and invasive breast cancer risk by molecular subtype and race in the Carolina Breast Cancer Study. Cancer Causes Control. 2016;27(2):259–69. doi: 10.1007/s10552-015-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson WR, Tse CK, Olshan AF, Troester MA. Body size across the life course and risk of premenopausal and postmenopausal breast cancer in Black women, the Carolina Breast Cancer Study, 1993–2001. Cancer causes & control : CCC. 2014;25(9):1101–17. doi: 10.1007/s10552-014-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allott EH, Tse CK, Olshan AF, Carey LA, Moorman PG, Troester MA. Non-steroidal anti-inflammatory drug use, hormone receptor status, and breast cancer-specific mortality in the Carolina Breast Cancer Study. Breast cancer research and treatment. 2014;147(2):415–21. doi: 10.1007/s10549-014-3099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Razzaghi H, Troester MA, Gierach GL, Olshan AF, Yankaskas BC, Millikan RC. Association between mammographic density and basal-like and luminal A breast cancer subtypes. Breast cancer research : BCR. 2013;15(5):R76. doi: 10.1186/bcr3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chollet-Hinton L, Anders CK, Tse CK, Bell MB, Yang YC, Carey LA et al. Breast cancer biologic and etiologic heterogeneity by young age and menopausal status in the Carolina Breast Cancer Study: a case-control study. Breast cancer research : BCR. 2016;18(1):79. doi: 10.1186/s13058-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bensen JT, Tse CK, Nyante SJ, Barnholtz-Sloan JS, Cole SR, Millikan RC. Association of germline microRNA SNPs in pre-miRNA flanking region and breast cancer risk and survival: the Carolina Breast Cancer Study. Cancer causes & control : CCC. 2013;24(6):1099–109. doi: 10.1007/s10552-013-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F, Chen GK, Stram DO, Millikan RC, Ambrosone CB, John EM et al. A genome-wide association study of breast cancer in women of African ancestry. Human genetics. 2013;132(1):39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song C, Chen GK, Millikan RC, Ambrosone CB, John EM, Bernstein L et al. A genome-wide scan for breast cancer risk haplotypes among African American women. PloS one. 2013;8(2):e57298. doi: 10.1371/journal.pone.0057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien KM, Cole SR, Engel LS, Bensen JT, Poole C, Herring AH et al. Breast cancer subtypes and previously established genetic risk factors: a bayesian approach. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23(1):84–97. doi: 10.1158/1055-9965.EPI-13-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Family L, Bensen JT, Troester MA, Wu MC, Anders CK, Olshan AF. Single-nucleotide polymorphisms in DNA bypass polymerase genes and association with breast cancer and breast cancer subtypes among African Americans and Whites. Breast cancer research and treatment. 2015;149(1):181–90. doi: 10.1007/s10549-014-3203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haiman CA, Chen GK, Vachon CM, Canzian F, Dunning A, Millikan RC et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nature genetics. 2011;43(12):1210–4. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer JR, Ambrosone CB, Olshan AF. A collaborative study of the etiology of breast cancer subtypes in African American women: the AMBER consortium. Cancer causes & control : CCC. 2014;25(3):309–19. doi: 10.1007/s10552-013-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X, Nichols HB, Robinson W, Sherman ME, Olshan AF, Troester MA. Post-diagnosis adiposity and survival among breast cancer patients: influence of breast cancer subtype. Cancer Causes Control. 2015;26(12):1803–11. doi: 10.1007/s10552-015-0673-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, Nichols HB, Tse CK, Bell MB, Robinson WR, Sherman ME et al. Association of Parity and Time since Last Birth with Breast Cancer Prognosis by Intrinsic Subtype. Cancer Epidemiol Biomarkers Prev. 2016;25(1):60–7. doi: 10.1158/1055-9965.epi-15-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(24):6100–10. doi: 10.1158/1078-0432.ccr-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nature biotechnology. 2008;26(3):317–25. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 47.Malkov VA, Serikawa KA, Balantac N, Watters J, Geiss G, Mashadi-Hossein A et al. Multiplexed measurements of gene signatures in different analytes using the Nanostring nCounter Assay System. BMC research notes. 2009;2:80. doi: 10.1186/1756-0500-2-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allott EH, Cohen SM, Geradts J, Sun X, Khoury T, Bshara W et al. Performance of Three-Biomarker Immunohistochemistry for Intrinsic Breast Cancer Subtyping in the AMBER Consortium. Cancer Epidemiol Biomarkers Prev. 2016;25(3):470–8. doi: 10.1158/1055-9965.epi-15-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. ••.Troester MA, Sun X, Allott EH, Geradts J, Cohen SM, Tse CK et al. Racial Differences in PAM50 Subtypes in the Carolina Breast Cancer Study. J Natl Cancer Inst. 2018;110(2). doi: 10.1093/jnci/djx135.Using RNA expression data, this analysis classified breast cancer PAM50 subtype in a population-based sample. Multi-gene assays showed racial disparities in frequency of Basal-like breast cancer and implicate differences in tumor biology as an important contributor to mortality disparities among both younger and older black patients with HR-positive/HER2-negative disease.
- 50.Conway K, Edmiston SN, May R, Kuan PF, Chu H, Bryant C et al. DNA methylation profiling in the Carolina Breast Cancer Study defines cancer subclasses differing in clinicopathologic characteristics and survival. Breast cancer research : BCR. 2014;16(5):450. doi: 10.1186/s13058-014-0450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hair BY, Troester MA, Edmiston SN, Parrish EA, Robinson WR, Wu MC et al. Body mass index is associated with gene methylation in estrogen receptor-positive breast tumors. Cancer Epidemiol Biomarkers Prev. 2015;24(3):580–6. doi: 10.1158/1055-9965.epi-14-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couture HD, Williams LA, Geradts J, Nyante SJ, Butler EN, Marron JS et al. Image analysis with deep learning to predict breast cancer grade, ER status, histologic subtype, and intrinsic subtype. NPJ breast cancer. 2018;4:30. doi: 10.1038/s41523-018-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benefield HC, Zabor EC, Shan Y, Allott EH, Begg CB, Troester MA. Evidence for Etiologic Subtypes of Breast Cancer in the Carolina Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2019;28(11):1784–91. doi: 10.1158/1055-9965.epi-19-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA: a cancer journal for clinicians. 2019;69(3):211–33. doi: 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 55.White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV et al. Cancer Screening Test Use - United States, 2015. MMWR Morbidity and mortality weekly report. 2017;66(8):201–6. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jewett PI, Gangnon RE, Elkin E, Hampton JM, Jacobs EA, Malecki K et al. Geographic access to mammography facilities and frequency of mammography screening. Annals of epidemiology. 2018;28(2):65–71.e2. doi: 10.1016/j.annepidem.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. 2008;113(8):2166–72. doi: 10.1002/cncr.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porter P “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med. 2008;358(3):213–6. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 59.Sihto H, Lundin J, Lehtimaki T, Sarlomo-Rikala M, Butzow R, Holli K et al. Molecular subtypes of breast cancers detected in mammography screening and outside of screening. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(13):4103–10. doi: 10.1158/1078-0432.ccr-07-5003. [DOI] [PubMed] [Google Scholar]
- 60.Kirsh VA, Chiarelli AM, Edwards SA, O’Malley FP, Shumak RS, Yaffe MJ et al. Tumor characteristics associated with mammographic detection of breast cancer in the Ontario breast screening program. J Natl Cancer Inst. 2011;103(12):942–50. doi: 10.1093/jnci/djr138. [DOI] [PubMed] [Google Scholar]
- 61.Caldarella A, Puliti D, Crocetti E, Bianchi S, Vezzosi V, Apicella P et al. Biological characteristics of interval cancers: a role for biomarkers in the breast cancer screening. Journal of cancer research and clinical oncology. 2013;139(2):181–5. doi: 10.1007/s00432-012-1304-1. [DOI] [PubMed] [Google Scholar]
- 62.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ breast cancer. 2017;3:12. doi: 10.1038/s41523-017-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson K, Zackrisson S, Rosso A, Sartor H, Saal LH, Andersson I et al. Tumor Characteristics and Molecular Subtypes in Breast Cancer Screening with Digital Breast Tomosynthesis: The Malmo Breast Tomosynthesis Screening Trial. Radiology. 2019;293(2):273–81. doi: 10.1148/radiol.2019190132. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Ivansson E, Klevebring D, Tobin NP, Lindstrom LS, Holm J et al. Molecular Differences between Screen-Detected and Interval Breast Cancers Are Largely Explained by PAM50 Subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(10):2584–92. doi: 10.1158/1078-0432.ccr-16-0967. [DOI] [PubMed] [Google Scholar]
- 65. •.Puvanesarajah S, Nyante SJ, Kuzmiak CM, Chen M, Tse CK, Sun X et al. PAM50 and Risk of Recurrence Scores for Interval Breast Cancers. Cancer prevention research (Philadelphia, Pa). 2018;11(6):327–36. doi: 10.1158/1940-6207.capr-17-0368.This study examined the association of mode of detection with cancer characteristics (clinical, IHC, and genomic), and stratified analyses on mammographic density and race. Interval cancers were less likely, whereas screen-detected cancers were more likely, to have non-Luminal A subtype.
- 66.Chen Y, Susick L, Davis M, Bensenhaver J, Nathanson SD, Burns J et al. Evaluation of Triple-Negative Breast Cancer Early Detection via Mammography Screening and Outcomes in African American and White American Patients. JAMA surgery. 2020. doi: 10.1001/jamasurg.2019.6032. [DOI] [PMC free article] [PubMed]
- 67.Killelea BK, Chagpar AB, Bishop J, Horowitz NR, Christy C, Tsangaris T et al. Is there a correlation between breast cancer molecular subtype using receptors as surrogates and mammographic appearance? Annals of surgical oncology. 2013;20(10):3247–53. doi: 10.1245/s10434-013-3155-7. [DOI] [PubMed] [Google Scholar]
- 68.Boisserie-Lacroix M, Bullier B, Hurtevent-Labrot G, Ferron S, Lippa N, Mac Grogan G. Correlation between imaging and prognostic factors: molecular classification of breast cancers. Diagnostic and interventional imaging. 2014;95(2):227–33. doi: 10.1016/j.diii.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–26. [DOI] [PubMed] [Google Scholar]
- 70.George P, Chandwani S, Gabel M, Ambrosone CB, Rhoads G, Bandera EV et al. Diagnosis and surgical delays in African American and white women with early-stage breast cancer. J Womens Health (Larchmt). 2015;24(3):209–17. doi: 10.1089/jwh.2014.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166(20):2244–52. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 72.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA surgery. 2013;148(6):516–23. doi: 10.1001/jamasurg.2013.1680. [DOI] [PubMed] [Google Scholar]
- 73.Polverini AC, Nelson RA, Marcinkowski E, Jones VC, Lai L, Mortimer JE et al. Time to Treatment: Measuring Quality Breast Cancer Care. Annals of surgical oncology. 2016;23(10):3392–402. doi: 10.1245/s10434-016-5486-7. [DOI] [PubMed] [Google Scholar]
- 74.Halpern MT, Schrag D. Effects of state-level medicaid policies and patient characteristics on time to breast cancer surgery among medicaid beneficiaries. Breast Cancer Res Treat. 2016;158(3):573–81. doi: 10.1007/s10549-016-3879-8. [DOI] [PubMed] [Google Scholar]
- 75.McGee SA, Durham DD, Tse CK, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1227–38. doi: 10.1158/1055-9965.epi-12-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(1):333–45. doi: 10.1007/s10549-012-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold HT, Thwin SS, Buist DS, Field TS, Wei F, Yood MU et al. Delayed radiotherapy for breast cancer patients in integrated delivery systems. The American journal of managed care. 2009;15(11):785–9. [PMC free article] [PubMed] [Google Scholar]
- 78.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA oncology. 2016;2(3):322–9. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nurgalieva ZZ, Franzini L, Morgan RO, Vernon SW, Liu CC, Du XL. Impact of timing of adjuvant chemotherapy initiation and completion after surgery on racial disparities in survival among women with breast cancer. Medical oncology (Northwood, London, England). 2013;30(1):419. doi: 10.1007/s12032-012-0419-1. [DOI] [PubMed] [Google Scholar]
- 80.Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J Clin Oncol. 2005;23(27):6639–46. doi: 10.1200/jco.2005.12.633. [DOI] [PubMed] [Google Scholar]
- 81.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol. 2010;28(27):4135–41. doi: 10.1200/jco.2009.27.2427. [DOI] [PubMed] [Google Scholar]
- 82.Reeder-Hayes KE, Meyer AM, Dusetzina SB, Liu H, Wheeler SB. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145(3):743–51. doi: 10.1007/s10549-014-2957-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–44. doi: 10.1200/jco.2013.49.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dunmore C, Plummer P, Regan G, Mattingly D, Jackson S, Millikan R. Re: race and differences in breast cancer survival in a managed care population. Journal of the National Cancer Institute. 2000;92(20):1690–1. [DOI] [PubMed] [Google Scholar]
- 85.Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Carey LA et al. Racial Variation in the Uptake of Oncotype DX Testing for Early-Stage Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(2):130–8. doi: 10.1200/JCO.2015.63.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Troester MA et al. Racial variation in adjuvant chemotherapy initiation among breast cancer patients receiving oncotype DX testing. Breast cancer research and treatment. 2015;153(1):191–200. doi: 10.1007/s10549-015-3518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spencer JC, Reeve BB, Troester MA, Wheeler SB. Factors Associated with Endocrine Therapy Non-Adherence in Breast Cancer Survivors. Psycho-oncology. 2020. doi: 10.1002/pon.5289. [DOI] [PMC free article] [PubMed]
- 88. •.Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L et al. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J Natl Cancer Inst. 2019;111(5):498–508. doi: 10.1093/jnci/djy136.This study assessed endocrine therapy use by race, including behavioral predictors. Black women with HR+ breast cancers were more likely to be nonadherent to ET, but were not more likely to discontinue. Major predictors of nonadherence included differential risk perceptions and a lack of shared treatment decision making.
- 89.Pinheiro LC, Wheeler SB, Reeder-Hayes KE, Samuel CA, Olshan AF, Reeve BB. Investigating Associations Between Health-Related Quality of Life and Endocrine Therapy Underuse in Women With Early-Stage Breast Cancer. Journal of oncology practice. 2017;13(5):e463–e73. doi: 10.1200/jop.2016.018630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wheeler SB, Kohler RE, Reeder-Hayes KE, Goyal RK, Lich KH, Moore A et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. Journal of cancer survivorship : research and practice. 2014;8(4):603–10. doi: 10.1007/s11764-014-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–17. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roberts MC, Wheeler SB, Reeder-Hayes K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015;105 Suppl 3:e4–e15. doi: 10.2105/ajph.2014.302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–57. doi: 10.1007/s10549-007-9675-8. [DOI] [PubMed] [Google Scholar]
- 94.Camacho FT, Tan X, Alcala HE, Shah S, Anderson RT, Balkrishnan R. Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in medicare breast cancer survivors. Medicine. 2017;96(24):e7147. doi: 10.1097/md.0000000000007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Press DJ, Ibraheem A, Dolan ME, Goss KH, Conzen S, Huo D. Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat. 2018;168(1):207–20. doi: 10.1007/s10549-017-4587-8. [DOI] [PubMed] [Google Scholar]
- 96.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute.106(5). 10.1093/jnci/dju055 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76(1):27–36. [DOI] [PubMed] [Google Scholar]
- 98.Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J et al. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Annals of internal medicine. 2006;144(8):541–53. [DOI] [PubMed] [Google Scholar]
- 99.Howlader NNA, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). . SEER Cancer Statistics Review, 1975–2013. National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 100.Chen VW, Correa P, Kurman RJ, Wu XC, Eley JW, Austin D et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3(2):127–35. [PubMed] [Google Scholar]
- 101.Porter PL, Lund MJ, Lin MG, Yuan X, Liff JM, Flagg EW et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–42. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 102.Barnholtz-Sloan JS, Shetty PB, Guan X, Nyante SJ, Luo J, Brennan DJ et al. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis. 2010;31(8):1417–23. doi: 10.1093/carcin/bgq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhattacharya A, Garcia-Closas M, Olshan AF, Perou CM, Troester MA, Love MI. A framework for transcriptome-wide association studies in breast cancer in diverse study populations. Genome biology. 2020;21(1):42. doi: 10.1186/s13059-020-1942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–82. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pinheiro LC, Samuel CA, Reeder-Hayes KE, Wheeler SB, Olshan AF, Reeve BB. Understanding racial differences in health-related quality of life in a population-based cohort of breast cancer survivors. Breast Cancer Res Treat. 2016;159(3):535–43. doi: 10.1007/s10549-016-3965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. •.Pinheiro LC, Tan X, Olshan AF, Wheeler SB, Reeder-Hayes KE, Samuel CA et al. Examining health-related quality of life patterns in women with breast cancer. Qual Life Res. 2017;26(7):1733–43. doi: 10.1007/s11136-017-1533-5.This study identified subgroups of women with breast cancer who experience different health-related quality of life patterns during treatment and survivorship. Findings suggest that age, race, comorbid conditions and modifiable patient-level factors such as smoking and obesity can identify women at risk for experiencing poor health-related quality of life patterns.
- 107. ••.Wheeler SB, Spencer JC, Pinheiro LC, Carey LA, Olshan AF, Reeder-Hayes KE. Financial Impact of Breast Cancer in Black Versus White Women. J Clin Oncol. 2018;36(17):1695–701. doi: 10.1200/jco.2017.77.6310.This study examined racial variation in the financial impact of cancer. Findings showed that black women with breast cancer experience disproportionate financial strain which may contribute to higher stress, lower treatment compliance, and worse outcomes by race.
- 108.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A et al. Breast cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(6):438–51. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 109.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA: a cancer journal for clinicians. 2016;66(1):43–73. doi: 10.3322/caac.21319. [DOI] [PubMed] [Google Scholar]
- 110.Gotay CC, Pagano IS. Assessment of Survivor Concerns (ASC): a newly proposed brief questionnaire. Health and quality of life outcomes. 2007;5:15. doi: 10.1186/1477-7525-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Lo SK et al. The development and evaluation of a measure to assess cancer survivors’ unmet supportive care needs: the CaSUN (Cancer Survivors’ Unmet Needs measure). Psycho-oncology. 2007;16(9):796–804. doi: 10.1002/pon.1137. [DOI] [PubMed] [Google Scholar]
- 112.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health services research. 2005;40(6 Pt 1):1918–30. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lopez de Maturana E, Pineda S, Brand A, Van Steen K, Malats N. Toward the integration of Omics data in epidemiological studies: still a “long and winding road”. Genetic epidemiology. 2016;40(7):558–69. doi: 10.1002/gepi.21992. [DOI] [PubMed] [Google Scholar]
- 114.Emerson MA, Golightly YM, Tan X, Aiello AE, Reeder-Hayes KE, Olshan AF et al. Integrating access to care and tumor patterns by race and age in the Carolina Breast Cancer Study, 2008–2013. Cancer Causes Control. 2020. doi: 10.1007/s10552-019-01265-0. [DOI] [PMC free article] [PubMed]
- 115.DeSantis C, Jemal A, Ward E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer Causes Control. 2010;21(9):1445–50. doi: 10.1007/s10552-010-9572-z. [DOI] [PubMed] [Google Scholar]
- 116.Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus. 2015;4:508. doi: 10.1186/s40064-015-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sineshaw HM, Gaudet M, Ward EM, Flanders WD, Desantis C, Lin CC et al. Association of race/ethnicity, socioeconomic status, and breast cancer subtypes in the National Cancer Data Base (2010–2011). Breast Cancer Res Treat. 2014;145(3):753–63. doi: 10.1007/s10549-014-2976-9. [DOI] [PubMed] [Google Scholar]
- 118.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–62. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palumbo A, Michael Y, Hyslop T. Latent class model characterization of neighborhood socioeconomic status. Cancer Causes Control. 2016;27(3):445–52. doi: 10.1007/s10552-015-0711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chebli P, Lemus J, Avila C, Pena K, Mariscal B, Merlos S et al. Multilevel determinants of financial toxicity in breast cancer care: perspectives of healthcare professionals and Latina survivors. Support Care Cancer. 2019. doi: 10.1007/s00520-019-05119-y. [DOI] [PMC free article] [PubMed]
- 121.Freedman RA, Revette AC, Hershman DL, Silva K, Sporn NJ, Gagne JJ et al. Understanding Breast Cancer Knowledge and Barriers to Treatment Adherence: A Qualitative Study Among Breast Cancer Survivors. BioResearch open access. 2017;6(1):159–68. doi: 10.1089/biores.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. Journal of community health. 2010;35(4):398–408. doi: 10.1007/s10900-010-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. ••.Cheng AC, Levy MA. Measures of Treatment Workload for Patients With Breast Cancer. JCO clinical cancer informatics. 2019;3:1–10. doi: 10.1200/cci.18.00122.This study developed measures of patient treatment workload derived from electronic health record data. The findings showed that these treatment workload measures, including time spend in clinic, and commuting time and costs, capture an important dimension in the experience of patients with cancer.


