Abstract
Purpose of the review
The adoptive transfer of alloantigen-specific regulatory T cells (Tregs) following organ transplantation is an emerging treatment paradigm that may induce tolerance and reduce the risk for graft rejection. In particular, redirecting Treg specificity via expression of synthetic chimeric antigen receptors (CARs) has demonstrated therapeutic promise in several preclinical studies. In this review, we highlight recent progress and remaining barriers to the clinical translation of CAR-Treg therapies.
Recent findings
CAR Tregs targeting human leukocyte antigen (HLA)-A2 showed antigen-specific in vitro activation and superior in vivo protective function relative to polyclonal Tregs. Adoptively transferred anti-HLA-A2 CAR Tregs prolonged the survival of HLA-A2–positive grafts in humanized mouse models.
Summary
Donor HLA molecules are attractive candidate antigens to target with CAR Tregs in transplantation due to mismatched HLA only expressed on the transplanted organ. The feasibility of this approach has been demonstrated by several independent groups in recent years. However, substantial challenges in CAR design and preclinical modeling must be more extensively addressed prior to clinical application.
Keywords: Tregs, CAR, tolerance, transplantation, HLA
Introduction
Solid organ transplantation remains an important therapy for patients with end-stage organ dysfunction but comes with the need for life-long immunosuppression to prevent allograft rejection. Immunosuppression increases risks for infections and cancer development and has direct toxicity for various organs. Thus, promoting immune tolerance to prevent allograft rejection while minimizing or stopping generalized immunosuppression has been an important topic of research in recent years.
Regulatory T cells (Tregs) are a key player in controlling immune responses. Extensive preclinical research has shown that Treg therapy can induce tolerance in transplantation models [1] and alloantigen-reactive Tregs are more potent than polyclonal Tregs [2–6]. One way of achieving alloantigen-specificity is the use of a synthetic chimeric antigen receptor (CAR) targeted toward donor alloantigen. In theory, the human leukocyte antigens (HLA) that are present on allografts and absent in recipients can be used as CAR targets for directing Treg specificity for organ transplantation. Indeed, preclinical and translational efforts are ongoing to develop anti-HLA CAR Tregs for clinical applications. Here we summarize current efforts in developing CAR Treg therapy for organ transplantation and provide our perspective on its promises and issues needing to be addressed before its clinical application.
Background
Organ transplantation and rejection
After organ transplantation, the patient’s immune system recognizes the transplanted organ as non-self and without immunosuppression, this immune reaction would lead to destruction and rejection of the organ. T cells are the principal driver of alloimmune responses, and they are activated by alloantigens, chiefly the polymorphic major histocompatibility complex (MHC) molecules that are distinct between the graft and the recipient.
The MHC molecules in humans are also referred to as HLA. MHC or HLA are divided into class I and class II. HLA-A, HLA-B and HLA-C are class I MHC and HLA-DP, HLA-DQ, and HLA-DR are class II MHC molecules. Class I MHC are expressed on almost all cell types whereas MHC class II are primarily restricted to antigen-presenting cells (APC) and a few other cell types, such as vascular endothelial cells in humans. CD8+ T cells recognize class I MHC and CD4+ T cells recognize class II MHC.
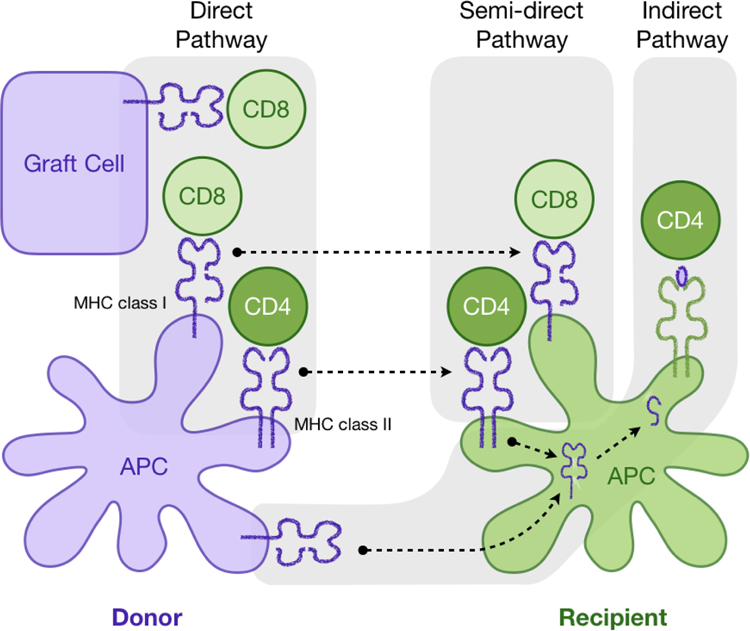
Alloreactive T cells recognize foreign MHC via direct, semi-direct, or indirect pathways ([7], Figure 1). Direct allorecognition is a unique feature of transplantation and it refers to the engagement and activation of T cell receptors (TCR) by intact donor MHC molecules displayed on donor cells. Additionally, recipient dendritic cells can also acquire and display intact donor MHC molecules, thus forming the semi-direct pathway of allorecognition [8]. In this pathway, microvesicles/exosomes containing intact donor class I and II MHC are released from graft-derived cells and travel to the secondary lymphoid organs, where the MHC molecule is acquired and presented by recipient dendritic cells [9, 10]. Canonical immune recognition, however, involves processing of foreign proteins into shorter peptides and then presenting the peptides in the MHC expressed by the host cells. In the context of transplantation, this pathway of host APC processing and presenting donor MHC-derived peptides is referred to as indirect allorecognition. It is worth noting that alloantigen-reactive Tregs, as CD4+ T cells, naturally recognize class II MHCs. Thus, they are activated by intact donor class II MHC directly or semi-directly or by donor class I and class II MHC peptides indirectly presented by recipient class II MHC on recipient APCs. Hence, Tregs primarily, if not exclusively, interact with a limited range of cell types that express class II MHC.
Figure 1. Pathways of allorecognition.
After organ transplantation recipient’s CD4 and CD8 T cells (green) recognize the antigen presented by MHC class II or class I via the direct pathway, indirect or semi-direct pathways of allorecognition.
Tregs as immune modulators
Tregs are a subset of CD4+ T cells that are specialized in maintaining immune homeostasis and prevention of autoimmune diseases by regulating the activation of other immune cells (reviewed in [11]). They are characterized by the expression of CD4, CD25, and the transcription factor FOXP3 (forkhead box P3). Tregs can suppress the function and proliferation of other CD4+ T cells, CD8+ T cells, B cells, NK cells, and myeloid cells. In the setting of transplantation, a variety of preclinical studies have shown that the infusion of Tregs can control alloimmune responses and promote immune tolerance to allografts [2, 4, 5, 12]. Most of these studies show a clear advantage of donor alloantigen-reactive Tregs over polyclonal Tregs by significantly decreasing the number of Tregs needed to induce graft tolerance. In a murine islet transplantation model in which 80% of alloantigen-reactive endogenous T cells were depleted, infusion of 5×106 alloantigen-reactive Tregs was sufficient to prevent graft rejection, whereas 25–30×106 polyclonal Tregs were necessary to induce graft tolerance [12]. In humans, it has been estimated that 1.5×108 to 1×109 alloantigen-reactive Tregs, instead of 5×109 polyclonal Tregs, may be sufficient to induce graft tolerance when combined with T cell depletion agents to delete 90% of endogenous T cells [13]. To date, there are multiple clinical trials testing the application of polyclonal Tregs in transplantation (summarized in [14]).
The endogenous T cell population is armed with a diverse repertoire of TCRs recognizing a wide variety of distinct antigens. In healthy individuals, 0.1 to 10% of conventional and regulatory T cells have alloreactivity [15–17]. To generate a sufficient number of donor alloantigen-reactive Tregs estimated to be effective for humans, in vitro selective expansion of Tregs using donor APC stimulation can be an effective strategy [18]. A limitation of this approach is the requirement for donor material to be collected under Good Manufacturing Process (GMP)-compliant conditions. An approach using banked B cells has been proposed to overcome this challenge [19]. Alternatively, synthetic antigen-reactive receptors can be used for off-the-shelf engineering of alloantigen-specific Tregs.
Chimeric antigen receptors
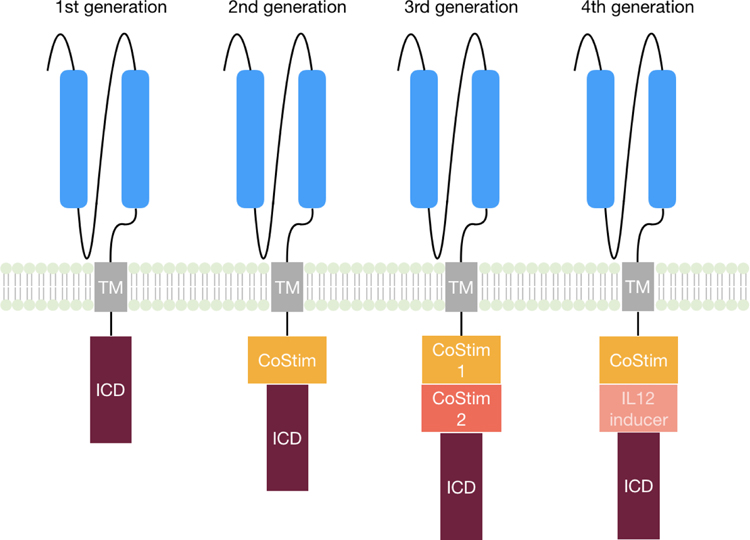
Chimeric antigen receptors (CAR) are synthetic fusion proteins composed of an extracellular antigen recognition domain and an intracellular signal transduction domain connected via a transmembrane domain. The extracellular antigen-binding domain is often derived from an antibody-derived single-chain variable fragment (scFv). Four generations of CARs have been developed, with increasing designed signaling capability (Figure 2). First-generation CARs, consisting of solely a CD3ζ intracellular domain, were first reported in the late 1980s as a research tool [20]. However, CD3ζ stimulation alone is not sufficient for expansion and persistence of CAR-expressing T cells [21, 22]. The necessity of co-stimulation for productive T-cell activation became clear in the 1990s; thus, second-generation CARs were developed by inclusion of a co-stimulatory domain from CD28 [22, 23] or 4–1BB [24, 25]. The expansion and function of CAR-expressing T cells could be further enhanced by incorporating additional intracellular co-stimulatory domains, such as CD28 and OX-40 [26] or 4–1BB and ICOS [27], which led to the third-generation CAR constructs. Other studies, however, could not confirm the superior effect of third-generation CARs over second-generation CARs [28]. Most recently, a cytokine induction cassette was further added to generate fourth-generation CAR-T cells, which also release proinflammatory cytokines such as IL-12 upon CAR T cell activation [29, 30]. The addition of an IL-12 inducer to the third generation CAR led to macrophage infiltration into the tumor and thus enhanced the anti-tumor response [31]. The application of CAR T cells in cancer therapy was initiated in the 2000s and the first report of successful anti-leukemia therapy anti-CD19 CAR T cells in patients was published in 2011 [32]. Since then, a variety of clinical trials have been published demonstrating remarkable efficacy of CAR T cells in their ability to induce remission in otherwise incurable cancers in the blood. Two CD19-targeting CAR-T cell therapies are the first FDA-approved gene-modified cellular therapies.
Figure 2. Different CAR generations.
(TM = transmembrane domain, ICD = intracellular domain, CoStim = costimulatory domain
CAR Tregs
The success of CAR-T cells in cancer treatment has inspired to the consideration of using these receptors to generate antigen-specific Tregs to suppress unwanted immune responses. The first CAR-Treg report was published in 2008, in which Tregs expressing a CAR specific for 2,4,6-trinitrophenol ameliorated the severity of 2,4,6-trinitrobenzene sulphonic acid-induced colitis in an animal model [33]. Later, the same group reported using Tregs expressing an anti-carcinoembryonic antigen CAR to suppress dextran sodium sulfate-induced colitis [34]. In experimental autoimmune encephalitis (EAE), a widely used mouse model for multiple sclerosis, Tregs expressing a CAR with an scFv for myelin oligodendrocyte glycoprotein localized to the brain and were able to suppress active EAE [35].
In the case of organ transplantation, Tregs are needed in the proximity of the transplanted organ to control rejection. Since donor and recipient HLA receptor mismatch is the main cause of graft rejection, these molecules are attractive targets for re-directing Tregs to protect the transplanted organ graft. The most common HLA mismatch is HLA-A2 since 50% of human population is HLA-A2 positive. Thus, an HLA-A2 CAR Treg could be applied in about 25 % of transplantations, in which the donor is HLA-A2 positive and the recipient is HLA-A2 negative [36].
Several research groups have designed anti-HLA-A2 CARs (A2-CARs) for Tregs and evaluated human A2-CAR Tregs in vitro and in vivo [37–41]. Two different approaches in generating the A2-CAR were taken. The Levings group generated the A2-CAR by using the scFv from the mouse anti-HLA-A2 BB7.2 clone [37], which was later used by a different research group [41]; whereas the Lombardi and Jaeckel groups used an scFv from a patient-derived anti-HLA-A2 clone that was published previously [38, 39]. All of these studies focused on human Tregs, including one study on unconventional CD8+FOXP3+ Tregs [41]. The A2-CAR was transduced using a lentivirus and all studies showed that human A2-CAR Tregs remained phenotypically unchanged, as assessed by the expression of FOXP3, CD25, CD39, HELIOS, and CTLA-4. Furthermore, CAR Tregs maintained their suppressive function in vitro when stimulated via the endogenous TCR [37–40]. Importantly, A2-CAR Tregs showed superior in vitro suppression upon stimulation by HLA-A2 antigen compared to polyclonal Tregs [37–41]. The activation of Tregs via the CAR compared to the TCR led to an increased upregulation of proteins important for Treg function, including CTLA-4, LAP and GARP [37]. Using epithelial monolayers, Boardman et al. showed faster migration of A2-CAR Tregs through HLA-A2 negative than HLA-A2 positive monolayers, demonstrating a preferential accumulation within HLA-A2 positive tissue in vitro [38]. All in vivo studies involving A2-CAR Tregs were conducted with humanized mouse models, either using a Graft-versus-Host-Disease (GvHD) or a skin transplantation model with the donor tissue expressing HLA-A2. Two separate studies showed a preferential migration to and persistence of A2-CAR Tregs in the HLA-A2+ skin grafts, confirming the in vitro findings from the Boardman study [39, 40]. Lastly, all studies reported superior function of A2-CAR Tregs in preventing the rejection of allogeneic skin grafts [38–41] or xenogeneic GvHD [37, 40, 41], relative to unmodified Tregs. Together, these studies establish the viability of directing CAR Tregs to alloantigens by using scFvs targeting donor-recipient mismatched HLAs.
In addition to target specificity, intracellular domains will also likely impact the function of CAR Tregs, as shown for conventional T cells in the cancer setting. While most studies on CAR Tregs used CD28 and CD3ζ as intracellular signalling domains, three studies directly compared CD28 and 4–1BB as CAR co-stimulation domains along with CD3ζ in Tregs [42–44]. Both domains maintained FOXP3 expression in Tregs. However, the CAR with the CD28 co-stimulatory domain induced increased surface expression of CTLA-4 and LAP. Moreover, 4–1BB-expressing CAR Tregs had a decreased suppressive capacity via the CAR compared to CD28-expressing CAR Tregs. This was confirmed in vivo showing that 4–1BB-expressing CAR Tregs could not prevent the skin graft rejection by effector T cells in a humanized mouse model compared to CD28-expressing CAR Tregs [43]. Similarly, the Levings group showed a superior function of CD28-expressing CAR Tregs over 4–1BB-expressing CAR Tregs in preventing GvHD in vivo [44]. Koristka et al., however, observed no difference in Treg suppressive capacity when comparing 41BB- to CD28-CAR Tregs using a ‘universal’ CAR (the scFv binds to a targeting molecule, which then binds to the antigen) [42]. The studies comparing the co-stimulatory domains (reviewed in [45]), however, were conducted in different models and with different antigens targeted by the CAR, so it remains unclear which intracellular domain is the most efficacious in inducing and/or maintaining Treg function.
Unresolved issues in CAR Treg therapy
Function of Tregs in restraining immune responses including that toward alloantigen is a firmly established immunological principal backed by decades of rigorous research. The therapeutic efficacy of Tregs in autoimmune diseases and transplantation has been demonstrated in various preclinical models from various labs around the world. As clinical trials involving Treg therapy continue to expand, there is increasing interest in applying synthetic biology tools to transform Tregs into an indefectible therapy for unwanted immune responses and inflammation. By providing a clearly defined antigen target and duration of antigen exposure, transplantation is considered an ideal starting point to explore the clinical utility of CAR Tregs. So far, a handful of papers have provided the proof of principal data to demonstrate the feasibility of this approach. However, there are a number of key issues that need to be addressed before its clinical translation.
Safety
Nearly all CAR-Treg reports have thus far focused on the efficacy of the strategy, and as the field moves toward clinical translation, safety will become increasingly paramount. While Treg cell therapy has largely been considered safe with clinical experiences thus far supporting this notion [1, 46–48], the same cannot be assumed for CAR Tregs which have been endowed with synthetic features.
There are a number of ways CAR Tregs can be potentially toxic. One of the many suppressive mechanisms that Tregs employ is direct cytotoxicity toward the cell they interact with, mediated by perforin and granzyme B [49]. As mentioned above, Tregs naturally engage APCs expressing class II MHC and killing of APCs leads to reduced antigen presentation, and subsequent priming and activation of effector T cells. It is unclear if re-directing CAR Tregs against parenchymal cells expressing class I MHC, such as A2-CAR Tregs to transplanted liver, would also lead to killing of the cells the CAR Tregs are designed to protect. Encouragingly, short-term co-culture of A2-CAR Tregs with HLA-A2-positive K562 cells (for 24h) [37] and HLA-A2-positive primary epithelial cells (for 3h) [41] did not lead to obvious death of target cells even when a high ratio of A2-CAR Tregs to HLA-A2-positive cells was used. On the other hand, it has been observed that anti-CD19-CAR Tregs led to 50% specific lysis of CD19+ target cells in vitro [43]. It is not immediately clear what may underlie the discrepant findings among these different studies. In the anti-CD19 CAR study, similar levels of target-cell lysis were observed with different intracellular signalling domains of CD3ζ-only, CD28-CD3ζ, and 41BB-CD3ζ, but not with an anti-CD19 CAR devoid of any intracellular signalling domains, suggesting the difference is not likely due to co-stimulatory domains in the CARs. Moreover, the molecular mechanism of CAR Treg-mediated target-cell lysis is not clear. Although anti-CD19 CAR Tregs expressed granzyme B and degranulated upon target engagement, the requirement for granzyme B or other cytolysis machinery for CAR Treg-mediated target-cell killing has not been established [43]. Thorough and mechanistic investigation of this phenomenon is needed to clarify this issue.
Another safety concern of cell-based therapy involves the cell lineage and phenotypic stability of the therapeutic cells. While functional stability is necessary for lasting therapeutic efficacy, lineage stability is particularly important in the setting of Treg therapy, since destabilization could result in an effector T-cell population that potentiates instead of suppresses the immune response [50]. This particular safety concern is even more acute for antigen-specific Tregs targeted against tissue-specific antigens. Treg lineage and suppressive function depend on the stable expression of FOXP3, the master transcription factor for Treg identity [51–53]. Heritable FOXP3 expression during cell proliferation is safeguarded by an epigenetic program that maintains Treg gene expression in progenies [54]. However, it has been shown that repetitive in vitro stimulation of human Tregs with anti-CD3 and anti-CD28 coated beads leads to loss of FOXP3 expression [55–57]. It is unclear if Treg destabilization due to overstimulation also occurs in vivo. Under steady state conditions in vivo, Tregs undergo more frequent TCR stimulation than conventional T-cells, evidenced by higher expression of TCR-induced Nur77 [58, 59], as well as a greater proportion of cells in active cell cycle [60–62]. Despite this, Treg destabilization is rare [63, 64], indicating that the majority of Tregs are not destabilized by repeated stimulation through their TCR in vivo. The effect of repeated CAR stimulation on Tregs has not been reported and is worth in-depth examination considering the supraphysiological signaling through a CAR.
Efficacy
So far, the efficacy of human A2-CAR Tregs has been shown in vitro and in vivo in humanized mouse models [37–42]. All studies reported efficacy of the CAR Tregs in vivo in prevention of human against mouse, i.e. xenogeneic GvHD, or in controlling alloimmune-mediated rejection of HLA-A2+ human skin grafts. In the GvHD model, HLA-A2+ PBMC were used as effector cells to mediate the disease and also to stimulate the co-transferred A2-CAR Tregs. Though efficient, the experimental system has a complication that the effector PBMCs and the Tregs are allogeneic to each other and can react and potentially reject each other, in addition to (or instead of) rejecting the mouse host as the model intended to. Indeed, no human immune cell engraftment, PBMC or Tregs, were detected beyond the short window immediately after cell injection [37, 40]. Thus, this model is limited in the ability to evaluate A2-CAR Treg-mediated control of GvHD since the cells responsible for mediating GvHD failed to persist.
The human skin model is an established approach for measuring alloimmune response of human cells in vivo. For evaluating A2-CAR Tregs, HLA-A2+ human skin grafts are transplanted into NSG mice. After skin engraftment, HLA-A2− PMBCs and autologous A2-CAR Tregs are co-injected. Results in all four reports using this model show efficacy in terms of reduction of graft inflammation or control of rejection. However, this model cannot examine long-term impacts of CAR Tregs on the grafts because the experimental duration is limited by GvHD induced by human T cells against the xenogeneic mouse host. Long-term follow up is important for CAR Treg therapy considering potential Treg overstimulation due to the abundance of HLA-A2 antigen on all graft cells and the potent signalling by CARs. Aside from the concern of overstimulation-induced CAR Treg destabilization discussed above, overstimulation through a CAR may also lead to Treg exhaustion and apoptosis, both resulting in limitation of therapeutic efficacy. In this regard, a human skin graft in a mouse impose a relatively small antigenic load. The likelihood of A2-CAR Treg overstimulation that might promote destabilization or exhaustion/apoptosis, is much higher with a large organ such as a transplanted liver.
In addition to limited follow-up duration, all five studies of A2-CAR Tregs studies have only very limited quantitative data. As one main advantage of alloantigen-reactive Tregs is the reduced number of Tregs needed to achieve tolerance, Treg dose titration is helpful to demonstrate quantitative gain of Treg efficacy using an A2-CAR. Three studies compared A2-CAR to control-CAR Tregs using a single ratio of Tregs to PBMCs [38–40], whereas the other studies compared two ratios, 1:1 and 2:1 [37] or 3:1 PBMC to Tregs [41]. The comparison of a 1:1 and 2:1 PBMC to Treg ratios showed better efficacy over control CARs. However, a noticeable decrease in efficacy was seen at 2:1 ratio when compared to 1:1 ratio of PBMC to Tregs [37]. In the other study, graft survival in the reduced A2-CAR Treg group (3:1 ratio) was better than in the no Treg group but not worse than the 1:1 PBMC to Treg group [41]. These results suggest that the gain of efficacy with A2-CAR may not be as high as hoped and large dose of A2-CAR Tregs would be needed to effectively control graft rejection.
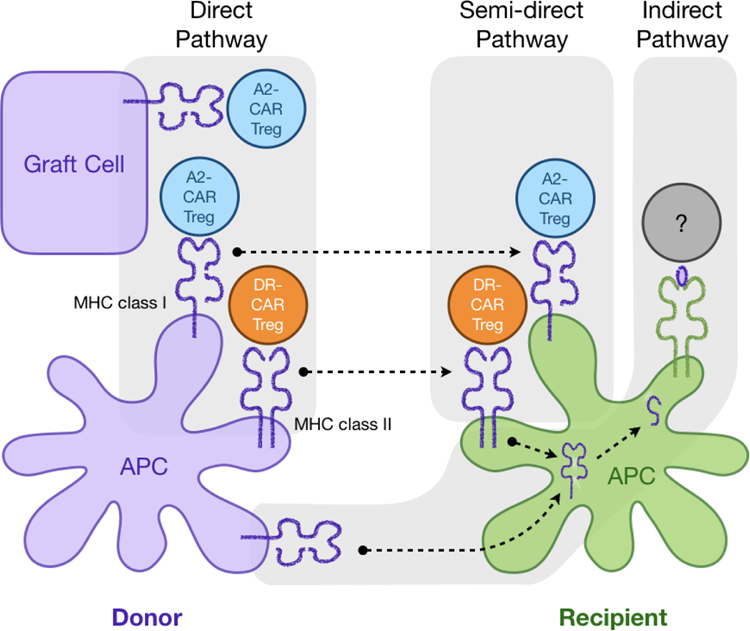
As discussed above in the background section, Tregs normally recognize class II MHC expressed on APCs; thus, it would be more physiological directing CAR Tregs to HLA-DR, DP and DQ. In addition, this will avoid direct engagement of CAR Tregs with parenchymal cells of the graft and potential direct toxicity (Figure 3). A challenge of targeting class II MHC is that they are expressed by a relative rare population of APCs in the grafts and these cells are mostly short-lived. It is not clear how well and how long a donor HLA-DR-targeted CAR Treg would need to be stimulated to exert sustained suppressive function. In mouse and humanized mouse models, Tregs with direct alloreactivity against donor derived APCs can indeed control alloimmune responses and support tolerance induction [3, 6, 12]. It is hypothesized that infusion of Tregs with direct alloreactivity creates a tolerogenic tissue microenvironment so that endogenous Tregs with indirect alloreactivity can expand and dominate over effector cells to maintain tolerance in the long run. Indeed, combining Tregs with direct and indirect alloreactivity can further improve the efficacy of Treg therapy [4]. It would be of interest to directly compare the efficacy of class I targeting CAR Tregs (e.g. A2-CAR) and class II targeting CAR Tregs with direct and indirect alloreactivity. Currently, there has been no report of class II targeting CAR Tregs. Moreover, a strategy for efficient transfer of indirect alloreactivity to Tregs using CAR or other technologies remains to be developed.
Figure 3. Using CARs to re-direct Treg response.
Example of an application of CAR Tregs in transplantation of HLA mismatched donor and recipients: HLA-A2 CAR Tregs can recognize the HLA-A2, which is expressed on donor APCs as well as donor graft cells or epithelial cells via the direct pathway of allorecognition. If the HLA-A2 is integrated into recipient APCs, the CAR Tregs can recognize the antigen as well (semi-direct pathway). Similarly, the application of a CAR targeting a class II MHC (DR Treg) directs the Treg to the antigen via the direct and indirect pathways: CAR Tregs recognize the antigen on donor APCs and endothelial cells and recipient APCs.
Preclinical models for cellular therapy
Preclinical modelling is a major roadblock in the development of cellular therapy for humans including CAR Tregs due to the species incompatibility of human cell therapy products and animal hosts. Researchers in the field face the dilemma of working with a directly clinically relevant cellular product, (i.e., human CAR Tregs), without robust in vivo models to evaluate the product’s efficacy or safety. It is reasonable and even necessary to work directly with the human cellular product to enable clinical translation, which is probably why all A2-CAR studies have focused on humanized mouse models. However, the currently available humanized mouse models are all severely immunodeficient. While human T cells do engraft, human NK, B cells, and myeloid cells are severely impaired [65]. Moreover, even the engrafted T cells fail to retain full functionality in the xenogeneic environment of the mouse. For example, it takes 40 to 50 days to reject allogeneic human skin in this system and in some cases the skin is never rejected. In comparison, allogeneic skin grafts are universally rejected within two weeks in immune replete mouse models. Moreover, successful human T-cell engraftment results in xenogeneic GvHD within 4–6 weeks, which complicates interpretation of the results and limits experimental duration. Lastly, an issue specific for Treg cellular products is their reliance on IL-2 from other cells for their lineage stability and survival. Mouse IL-2 does not bind to the human IL-2 receptor [66]. Thus, solely transferring Tregs to assess potential toxicity of the cellular product, for example, will not provide interpretable data.
Evaluating murine CAR Tregs targeting antigen homologs expressed on mouse cells within immune-competent mice would obviate most of the problems with humanized models outlined above, but with the major drawback that the cells and the CAR construct studied in these models will not ultimately be developed into a clinical product. Not only will the cells need to be of syngeneic mouse origin, the CAR must be constructed from murine domains to ensure proper function in the mouse cell context. Taking the research of CAR Treg therapy from humanized mouse models back to murine models may seem a regress when momentum is building to drive the technology to the clinic. Thoroughly, systematic investigation of CAR function in more physiologically and clinically relevant models will provide valuable biological insights and ultimately yield safer and efficacious CAR Tregs with therapeutic application.
Concluding remarks
The application of Tregs in transplantation is a promising therapy to minimize or eliminate the chronic use of immunosuppression post transplantation. Alloantigen-reactive Tregs are more potent than polyclonal Tregs. Synthetic biology to engineer a receptor tailored to be alloantigen reactive is a useful tool to re-direct Tregs to the transplanted organ. To date, anti-HLA-A2 CAR Tregs have been generated and tested in vitro and in vivo in humanized mouse models and have shown the viability of directing Tregs to alloantigens using mismatched HLA as an antigen target. However, data on safety and efficacy thus far is still limited. Alloantigen-reactive CAR Tregs losing their ability to regulate alloimmune responses can not only limit the efficacy of the therapy but could potentiate instead of suppressing immune responses in an antigen-specific manner and thus harm the transplanted organ. Furthermore, so far only A2-CAR Tregs have been generated and tested in the setting of transplantation, which re-directs Tregs unnaturally to class I HLA. Engineering CAR Tregs targeting class II HLA could be a more physiological way of generating alloantigen-reactivity, however, this has yet to be established and compared to the existing A2-CAR Tregs. Overall, research in CAR Tregs for transplantation has had a promising start. Further research to address crucial unresolved issues is needed for considering clinical translation of this strategy.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: Moving to the clinic. Cold Spring Harb Perspect Med 2013. 10.1101/cshperspect.a015552.•• Comprehensive review about Treg therapy, the transfer into the clinic and the application in transplantation
- 2.Brennan TV, Tang Q, Liu F-C, Hoang V, Bi M, Bluestone JA, Kang S-M. Requirements for prolongation of allograft survival with regulatory T cell infusion in lymphosufficient hosts. J Surg Res. 2011;169:e69–75. 10.1016/j.jss.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang JY-S, Tanriver Y, Jiang S, Xue S-A, Ratnasothy K, Chen D, et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J Clin Invest. 2008;118:3619–28. 10.1172/JCI33185.• First report of engineered Tregs using TCR for conferring indirect allo-reactivity in a mouse model of transplantation.
- 5.Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JPM. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88–92. 10.1038/nm1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood. 2007;109:827–35. 10.1182/blood-2006-05-025460. [DOI] [PubMed] [Google Scholar]
- 7.Ali JM, Bolton EM, Bradley JA, Pettigrew GJ. Allorecognition pathways in transplant rejection and tolerance. Transplantation. 2013;96:681–8. 10.1097/TP.0b013e31829853ce. [DOI] [PubMed] [Google Scholar]
- 8.Siu JHY, Surendrakumar V, Richards JA, Pettigrew GJ. T cell Allorecognition Pathways in Solid Organ Transplantation. Front Immunol. 2018;9:2548. 10.3389/fimmu.2018.02548.• This review provides an extensive overview on allorecognition and rejection in organ transplantation.
- 9.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest. 2016;126:2805–20. 10.1172/JCI84577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol 2016. 10.1126/sciimmunol.aaf8759. [DOI] [PMC free article] [PubMed]
- 11.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Nguyen V, Lee K-M, Kang S-M, Tang Q. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q, Lee K. Regulatory T-cell therapy for transplantation: How many cells do we need? Curr Opin Organ Transplant. 2012;17:349–54. 10.1097/MOT.0b013e328355a992.• Quantitative estimate of the effective dose of Tregs for controlling transplant rejection in humans.
- 14.Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front Immunol. 2019;10:43. 10.3389/fimmu.2019.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: New answers to an old question. J Immunol. 2001;166:973–81. 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 16.Veerapathran A, Pidala J, Beato F, Yu X-Z, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–80. 10.1182/blood-2011-02-337097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juvet SC, Sanderson S, Hester J, Wood KJ, Bushell A. Quantification of CD4(+) T Cell Alloreactivity and Its Control by Regulatory T Cells Using Time-Lapse Microscopy and Immune Synapse Detection. Am J Transplant. 2016;16:1394–407. 10.1111/ajt.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q, Vincenti F. Transplant trials with Tregs: Perils and promises. J Clin Invest. 2017;127:2505–12. 10.1172/JCI90598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landwehr-Kenzel S, Issa F, Luu S-H, Schmück M, Lei H, Zobel A, et al. Novel GMP-compatible protocol employing an allogeneic B cell bank for clonal expansion of allospecific natural regulatory T cells. Am J Transplant. 2014;14:594–606. 10.1111/ajt.12629. [DOI] [PubMed] [Google Scholar]
- 20.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. 10.1016/0092-8674(91)90314-O. [DOI] [PubMed] [Google Scholar]
- 21.Roselli E, Frieling JS, Thorner K, Ramello MC, Lynch CC, Abate-Daga D. CAR-T Engineering: Optimizing Signal Transduction and Effector Mechanisms. BioDrugs. 2019;33:647–59. 10.1007/s40259-019-00384-z. [DOI] [PubMed] [Google Scholar]
- 22.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70–5. 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 24.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui C-H, Geiger TL, Campana D. Chimeric receptors with 4–1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 25.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41. 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Guedan S, Posey AD, Shaw C, Wing A, Da T, Patel PR, et al. Enhancing CAR T cell persistence through ICOS and 4–1BB costimulation. JCI Insight 2018. 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed]
- 28.Abate-Daga D, Lagisetty KH, Tran E, Zheng Z, Gattinoni L, Yu Z, et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum Gene Ther. 2014;25:1003–12. 10.1089/hum.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holzinger A, Abken H. CAR T Cells: A Snapshot on the Growing Options to Design a CAR. Hemasphere. 2019;3:e172. 10.1097/HS9.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokarew N, Ogonek J, Endres S, Bergwelt-Baildon Mv, Kobold S. Teaching an old dog new tricks: Next-generation CAR T cells. Br J Cancer. 2019;120:26–37. 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–706. 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 32.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–24. 10.1053/j.gastro.2008.02.060.• This study is the first report of CAR Tregs and their function in a mouse model of colitis.
- 34.Blat D, Zigmond E, Alteber Z, Waks T, Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther. 2014;22:1018–28. 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fransson M, Piras E, Burman J, Nilsson B, Essand M, Lu B, et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation. 2012;9:112. 10.1186/1742-2094-9-112.• This study shows a successful application of CAR Tregs to decrease disease severity in EAE.
- 36.González-Galarza FF, Takeshita LYC, Santos EJM, Kempson F, Maia MHT, da Silva ALS, et al. Allele frequency net 2015 update: New features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43:D784–8. 10.1093/nar/gku1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacDonald KG, Hoeppli RE, Huang Q, Gillies J, Luciani DS, Orban PC, et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest. 2016;126:1413–24. 10.1172/JCI82771.•• Seminal study and first report of anti-HLA-A2 human Tregs.
- 38.Boardman DA, Philippeos C, Fruhwirth GO, Ibrahim MAA, Hannen RF, Cooper D, et al. Expression of a Chimeric Antigen Receptor Specific for Donor HLA Class I Enhances the Potency of Human Regulatory T Cells in Preventing Human Skin Transplant Rejection. Am J Transplant. 2017;17:931–43. 10.1111/ajt.14185.•• Seminal study using anti-HLA-A2 CAR human Tregs to prevent alloimmune-mediated human skin rejection in a humanized mouse model.
- 39.Noyan F, Zimmermann K, Hardtke-Wolenski M, Knoefel A, Schulde E, Geffers R, et al. Prevention of Allograft Rejection by Use of Regulatory T Cells With an MHC-Specific Chimeric Antigen Receptor. Am J Transplant. 2017;17:917–30. 10.1111/ajt.14175.•• Seminal study showing a superior protective effect of anti-HLA-A2 CAR human Tregs in a humanized mouse model of allogeneic human skin transplantation.
- 40.Dawson NA, Lamarche C, Hoeppli RE, Bergqvist P, Fung VC, McIver E, et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 2019. 10.1172/jci.insight.123672.•• Seminal study testing different anti-HLA-A2 human CARs for cross-reactivity and in vivo functionality in humanized mouse models.
- 41.Bézie S, Charreau B, Vimond N, Lasselin J, Gérard N, Nerrière-Daguin V, et al. Human CD8+ Tregs expressing a MHC-specific CAR display enhanced suppression of human skin rejection and GVHD in NSG mice. Blood Adv. 2019;3:3522–38. 10.1182/bloodadvances.2019000411.•• Seminal study using anti-HLA-A2-CAR CD8+ human Tregs to decrease the risk for xenogenic GvHD and human skin transplant rejection in a humanized mouse model.
- 42.Koristka S, Kegler A, Bergmann R, Arndt C, Feldmann A, Albert S, et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun. 2018;90:116–31. 10.1016/j.jaut.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Boroughs AC, Larson RC, Choi BD, Bouffard AA, Riley LS, Schiferle E, et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 2019. 10.1172/jci.insight.126194. [DOI] [PMC free article] [PubMed]
- 44.Dawson NAJ, Rosado-Sánchez I, Novakovsky GE, Fung VCW, Huang Q, McIver E, et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human Tregs. bioRxiv. 2019:749721. 10.1101/749721. [DOI] [PubMed]
- 45.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18:749–69. 10.1038/s41573-019-0041-4.•• Comprehensive review of current Treg therapy and the future directions in autoimmunity and transplantation.
- 46.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–70. 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esensten JH, Muller YD, Bluestone JA, Tang Q. Regulatory T-cell therapy for autoimmune and autoinflammatory diseases: The next frontier. J Allergy Clin Immunol. 2018;142:1710–8. 10.1016/j.jaci.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt A, Oberle N, Krammer PH. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front Immunol 2012. 10.3389/fimmu.2012.00051. [DOI] [PMC free article] [PubMed]
- 50.Barbi J, Pardoll DM, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259:115–39. 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 52.Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 53.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 54.Kitagawa Y, Ohkura N, Sakaguchi S. Molecular Determinants of Regulatory T Cell Development: The Essential Roles of Epigenetic Changes. Front Immunol 2013. 10.3389/fimmu.2013.00106. [DOI] [PMC free article] [PubMed]
- 55.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–97. 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 56.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dijke IE, Hoeppli RE, Ellis T, Pearcey J, Huang Q, McMurchy AN, et al. Discarded Human Thymus Is a Novel Source of Stable and Long-Lived Therapeutic Regulatory T Cells. Am J Transplant. 2016;16:58–71. 10.1111/ajt.13456. [DOI] [PubMed] [Google Scholar]
- 58.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–89. 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–4. 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 61.Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–46. 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thome JJC, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72–7. 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–5. 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory T cells. J Clin Invest. 2013;123:939–44. 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh NC, Kenney LL, Jangalwe S, Aryee K-E, Greiner DL, Brehm MA, Shultz LD. Humanized Mouse Models of Clinical Disease. Annu Rev Pathol. 2017;12:187–215. 10.1146/annurev-pathol-052016-100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins MK. Species specificity of interleukin 2 binding to individual receptor components. Eur J Immunol. 1989;19:1517–20. 10.1002/eji.1830190828. [DOI] [PubMed] [Google Scholar]