Abstract
Objective
To identify barriers to appropriate referral and treatment for patients with spasticity and present solutions that address these in a pragmatic way.
Methods
Using the findings of interviews conducted with UK healthcare professionals on the management of post-stroke spasticity, a consensus meeting was held involving 7 UK spasticity experts. The panel identified barriers to timely identification and referral of patients in the acute and post-acute care settings. Barriers were prioritized using a consensus framework based on impact and resolvability and a series of final recommendations were agreed.
Results
High-priority barriers broadly related to: insufficient awareness of spasticity symptoms and benefits of treatment, limited access to spasticity services and lack of standardized pathways for post-stroke spasticity identification. Potential solutions included the appointment of an experienced member of the acute team to gain expertise in spasticity identification, patient education of spasticity symptoms and a greater utilization of training resources for healthcare professionals.
Conclusion
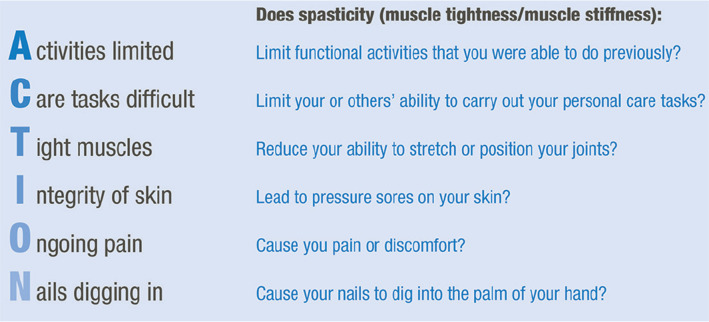
To address the barriers identified, we provide a series of consensus recommendations. As a key recommendation, we propose a set of indicators for the identification of stroke patients requiring specialist assessment and the use of the associated acronym “ACTION”.
LAY ABSTRACT
People who have a stroke may suffer from complications such as spasticity (stiffness in muscles due to muscle contraction). Spasticity can cause difficulty with day-to-day activities and may also cause pain. People with stroke may have spasticity without it being diagnosed, and people who need treatment may not be seen by healthcare professionals. Through formal consultation and analysis with expert doctors, physiotherapists and occupational therapists a range of simple and practical recommendations to help improve treatment is proposed. An acronym ‘ACTION’ was developed, referring to limitation of Activity, difficulty with Care tasks, Tight muscles, Integrity of the skin, Ongoing pain and Nails digging in (to the palm of the hand). The acronym was designed to help clinicians identify spasticity following a stroke that might cause problems. The recommendations from the expert group are focused on clinical practice in the UK but are applicable to other countries and healthcare systems.
Key words: stroke, post-stroke spasticity, rehabilitation, health services, patient, physiotherapy
Spasticity is a common feature of many neurological disorders, such as stroke. It is part of the upper motor neurone syndrome and it manifests as increased muscle tone associated with spasms and/or clonus (1). First described as “a velocity-dependent resistance to passive
movement with exaggerated tendon jerks resulting from hyperexcitability of the stretch reflexes” (2), it has more recently been defined as “disordered sensorimotor control, resulting from an upper motor neurone lesion, presenting as intermittent or sustained involuntary activation of muscles” (3), which more accurately reflects the clinical presentation and is the definition used in this paper.
Epidemiological studies have shown that up to 38% of stroke patients are affected by spasticity (4), which equates to more than 40,000 newly affected patients with post-stroke spasticity (PSS) per year in England alone (5). Onset of spasticity can occur at any time after stroke (6). The estimated prevalence of PSS is 21% in the first week (7), 19% at 3 months, 22% at 4 months, 43% at 6 months (8) and 17-46% at 12 months post-stroke (9-11). Sensorimotor function has been found to be the most important predictor for any, or severe, spasticity (12). Spasticity could be predicted with 85% sensitivity and 90% specificity 10 days post stroke using a prediction model (12).
Spasticity has a negative impact on post-stroke physical and mental wellbeing, as it contributes to functional limitations, including difficulties with personal hygiene and mobility, with subsequent implications for societal participation and quality of life. As a result, patients can experience depression, anxiety and poor self-esteem (13, 14), while their caregivers may also be affected by depression and anxiety due to the considerable burden of care placed upon them (15).
Patients with spasticity are at risk of developing secondary complications, including contractures and pressure sores (16). Complications can emerge as early as 4 weeks post-stroke (13), so it is essential that spasticity is recognized early and an appropriate management plan put in place. Early intervention may help to prevent complications that impede patient rehabilitation and may facilitate more functional outcomes in some individuals (13). Effective spasticity management requires a multidisciplinary approach that combines physical rehabilitation (including postural management) with, if necessary, pharmacological interventions (16).
Recent analyses have revealed significant underrecording of PSS in primary care data, reflecting likely under-diagnosis or under-reporting of the condition (17).
Notably, in contrast to other common post-stroke complications, the UK Sentinel Stroke National Audit Programme (SSNAP) does not specifically monitor spasticity at any point in the stroke patient journey. Furthermore, there is no nationally agreed pathway for PSS, and a general lack of formalized protocols for the identification, monitoring and referral of patients with PSS or at high risk of developing the condition. As a result, patients with PSS are often only referred for spasticity treatment once secondary complications have arisen. Moreover, an unknown number of patients with problematic spasticity may receive no treatment at all, because their spasticity has not been identified.
In view of these challenges, an expert consensus panel was convened to: (i) assess the barriers to timely identification and referral of patients; and (ii) identify potential solutions to optimize management of PSS.
METHODS
A consensus meeting was held involving a multidisciplinary panel comprising 7 UK expert spasticity practitioners, drawn from acute stroke, secondary and tertiary rehabilitation, intermediate and community care (2 consultant neurologists, 2 consultant physiotherapists, 1 consultant in stroke medicine, geriatrics and general medicine, 1 consultant in rehabilitation medicine, 1 advanced occupational therapist). The aim of the meeting was to discuss barriers to the identification and referral of patients with PSS and propose solutions to overcome them.
The panel used findings gathered from in-depth 1-h telephone qualitative interviews with 12 healthcare professionals (HCPs) (from across the UK) selected based on their knowledge, skills and working in the field of spasticity. Structured interviews were carried out to gain insights into PSS referral and management practices in the UK and were conducted by 2 interviewers from a medical communications agency. Ten of the interviewees were based in acute care or hospital-based rehabilitation settings (2 consultant physiotherapists, 6 consultants in rehabilitation medicine, 1 consultant in neuro-rehabilitation and 1 consultant in geriatrics and stroke medicine), while 2 were based in the community rehabilitation setting (2 community physiotherapists). One of the interviewees was subsequently invited to become a member of the consensus panel. All interviewees were asked a set of questions based on the following topics: sources of patient referral, screening and assessment of patients, treatment goals, factors influencing referral and barriers to timely referral and treatment. Thematic analysis was undertaken by the interviewers and a report documenting the interview themes was compiled.
The consensus meeting was conducted as follows: (i) A roundtable discussion was held based on the interview outcomes. (ii) The consensus panel was split into 2 breakout groups, focusing on acute and post-acute care. (iii) The 2 groups discussed and proposed barriers that hinder the timely identification and referral of patients with PSS in their setting. (iv) Each group prioritized the identified barriers diagrammatically based on impact and resolvability using implementation matrices. (v) The groups reconvened to present, discuss and refine the identified barriers. (vi) The panel split again into “acute” and “post-acute care” breakout groups to identify practical, implementable solutions to address those barriers deemed likely to have the greatest impact on patients and to be surmountable in the short term. (vii) The 2 groups reconvened and presented the proposed solutions to the full panel and the final recommendations to overcome the prioritized barriers were discussed.
All breakout and panel discussion sessions were facilitated by external moderators. Proceedings were audio recorded and the outcomes following the consensus meeting were consolidated into a draft paper.
RESULTS
In-depth interviews with UK healthcare professionals
Key findings from in-depth telephone interviews with 12 UK HCPs involved in spasticity management are summarized in Table I. Barriers to timely patient identification and referral identified during the interviews included: lack of awareness on the signs of spasticity and benefits of treatment among HCPs, patients and carers; limited capacity of services; inaccessibility or absence of services; and lack of adequate patient follow-up.
Table I.
Summary of findings from in-depth interviews with UK healthcare professionals
| Topic areas | Themes from interviews |
|---|---|
| Sources of patient referral |
|
| Screening and assessment of patients |
|
| Treatment goals |
|
| Barriers to timely identification and treatment of patients with PSS |
Level of awareness
Lack of capacity
Other barriers
|
BoNT-A: botulinum toxin A; GP: general practitioner; PSS: post-stroke spasticity.
Key barriers and potential solutions identified by the consensus panel
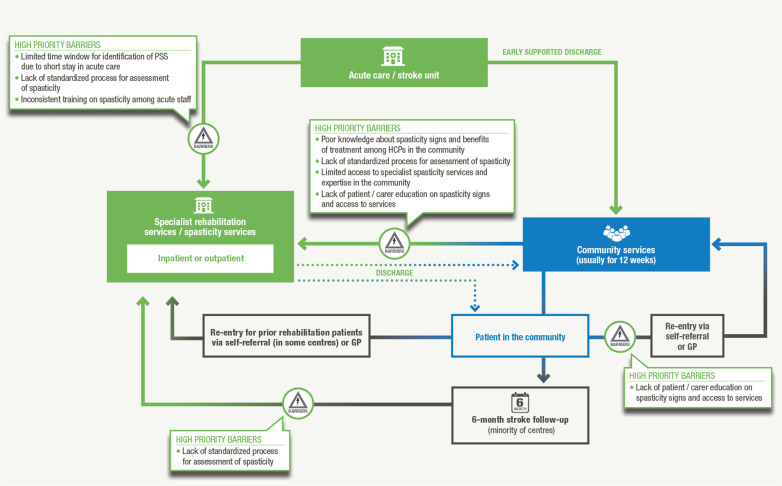
The barriers to timely referral and treatment of patients with PSS identified by the acute and post-acute care breakout groups are presented in Table II. Key barriers (existing throughout the patient journey) prioritized by the panel broadly related to lack of awareness and knowledge of spasticity, insufficient access to spasticity services and a lack of standardized processes/pathways (Fig. 1). Lack of awareness of spasticity among HCPs, and particularly how to identify it against a background of other musculoskeletal and biomechanical changes, was identified as a key barrier at all stages of the patient journey. The potential solutions identified by each breakout group to address high-priority barriers are also listed in Table II.
Table II.
Barriers and solutions proposed during the consensus meeting
| Barriers | Potential solutions to high priority* barriers | ||||
|---|---|---|---|---|---|
| Barriers identified by acute care breakout group | Potential solutions identified by acute care breakout group | ||||
| High priority* |
|
|
|||
| Low priority |
|
||||
| Barriers identified by post-acute care breakout group | Potential solutions identified by post-acute care breakout group | ||||
| High priority* |
|
|
|||
| Low priority |
|
||||
| High priority* barriers agreed by panel |
|
||||
High-priority barriers are those considered by the panel to have a high impact on patients and to be resolvable in the short term within the current UK healthcare framework; all other barriers were considered low priority for the purposes of the consensus process.
HCP: healthcare professional; PGD: patient group direction; PSS: post-stroke spasticity.
Fig. 1.
Key barriers identified and prioritized during the consensus meeting and their positions within the patient journey.
DISCUSSION
Insufficient awareness of the signs of spasticity among some acute care staff means that patients who develop signs of spasticity in the acute setting are often discharged without a spasticity management plan in place.
Once discharged into the community, patient access to spasticity services and expertise can be limited by the highly variable nature of spasticity service provision across the UK. Moreover, lack of pa- A tient education on spasticity means that patients experiencing spasticity onset following discharge are not empowered to seek help. Poor communication and insufficient integration between primary and secondary/tertiary care services in some areas further hinders access to spasticity services.
With the aim of improving clinical outcomes and quality of life for patients with PSS, we identified barriers that hinder the identification and referral of patients with PSS in the UK and are surmountable in the short term. We propose a set of practical recommendations which were agreed following extensive discussions and are shown in Table III and discussed further below.
Table III.
Consensus recommendations to improve timely identification and referral of post-stroke spasticity patients
| Implementation setting | Recommendation |
|---|---|
| All settings |
|
| Acute care |
|
| Community care |
|
| Specialist spasticity services |
|
HCP: healthcare professional; PSS: post-stroke spasticity.
To improve identification and management of PSS at all stages of the patient journey, we propose that HCPs use a set of “indicators for specific review and possible treatment” (Fig. 2). The simple nature of the proposed criteria should enable their inclusion in patient information leaflets and pre-clinic questionnaires to increase the likelihood of timely PSS following discharge. To assist HCPs in applying these criteria, we suggest the use of the acronym “ACTION” (Fig. 2).
Fig. 2.
Indicators for specific review and possible treatment of post-stroke spasticity (PSS) and associated acronym.
As spasticity onset occurs more frequently after discharge from acute care, all stroke patients should be assessed on the acute ward for their risk of future spasticity and high-risk patients should be “flagged” to specialist rehabilitation teams for close monitoring after discharge. Therefore, we recommend that an experienced member of the acute multidisciplinary team (MDT) should liaise between the acute, specialist rehabilitation and community teams to gain not only increased expertise in PSS identification, but also to take responsibility in flagging high-risk patients.
Increased education on spasticity could empower patients developing signs of PSS to seek help, while a “patient passport” containing details of the patient’s stroke and any spasticity (and treatment(s)) could help to improve patient management following discharge into the community.
The introduction of telephone or email triage services would not only facilitate patient follow-up, but would also allow community HCPs to seek advice on patients with PSS or suspected PSS. These services, together with enablement of specialist spasticity clinic practitioners to visit patients in the community, could contribute significantly to improving access to specialist spasticity services.
Improving knowledge of spasticity among community teams with the use of existing training resources, particularly easily accessible online resources, should also serve to increase access to appropriate care.
Limitations of our methodology
A limitation of this work included the relatively small number of respondents chosen for the in-depth interviews ahead of the consensus meeting.
Another limitation consisted in their selection process. Although respondents were chosen based on their expertise in PSS, further qualifying criteria, such as geographical representation or representation of all care settings and professional disciplines involved in the management of PSS management were not strictly applied (though were broadly considered).
Nonetheless, we hope that this paper will serve as a valuable discussion point for stroke services across the clinical pathway and that the recommendations we propose will serve as a useful foundation for improving current PSS services as well as for the development of formalized PSS pathways in areas where none currently exist.
Future perspectives
While the measures recommended in this paper may not all be applicable or implementable in every region, they provide a framework for PSS management improvement, individual aspects of which could be implemented depending on the services currently in place and the resources available. Some of these recommendations, for example telephone consultations, already operate successfully in individual centres, but could be rolled out more widely and established as standard approach.
Moreover, the recommendations herein presented could potentially be adapted and considered internationally.
In conclusion, we focused on barriers that could be realistically addressed in the short-term and on solutions that were practical within the current healthcare setting. A fundamental barrier that is not easy to address is insufficient funding, which limits the capacity and resources of spasticity services. However, earlier intervention in PSS could help to limit the costs associated with poststroke management by reducing secondary complications and improving long-term outcomes. Cost-effectiveness evaluations to demonstrate the long-term benefits of effective spasticity management will be an important step towards the greater prioritization of spasticity treatment among commissioners of clinical services and to improve the management of PSS on a national scale.
Monitoring performance in spasticity management could increase accountability and provide an incentive for greater prioritization of spasticity care. In the UK setting, the creation of a national database to support the evaluation of performance and outcomes related to PSS management would be beneficial to this end. This will, however, require time and funding to establish. Inclusion of spasticity metrics in the SSNAP could help to drive improvements in PSS management. Meanwhile, we encourage HCPs to refer to up-to-date Royal College of Physicians national clinical guidelines (18) for recommendations to optimize patient management.
Acknowledgements
Dr Gerry Christofi, University College London Hospitals NHS Foundation Trust, chaired the meeting that was held at the Hilton London Euston, UK.
The Consensus Group: Gerry Christofi (Chair), Stephen Ashford, Jonathan Birns, Catherine Dalton, Lynsay Duke, Claire Madsen, Sohail Salam.
Medical writing support was provided by Denise Taylor and Licia Genovese at Havas Life Medicom, supported by Merz Pharma GmbH (Frankfurt). All drafts of the paper were reviewed and discussed by the consensus group prior to submission.
Disclosures: The overall content of this paper was reviewed and supported by Different Strokes, a registered charity providing a unique service to younger stroke survivors throughout the UK.
Declaration of interests and funding: The in-depth interviews and consensus meeting were arranged and facilitated by Havas Life Medicom and funded by Merz Pharma GmbH (Frankfurt).
All authors received a fee from Merz Pharma GmbH (Frankfurt) for participation in the consensus meeting and contributed equally to the development of the paper. However, this manuscript in no way discusses or sponsors specific treatments or interventions.
Merz Pharma UK reviewed the final consensus paper for factual accuracy but responsibility for opinions, conclusions and interpretation of clinical evidence, and the submitted draft, lies with the authors.
Footnotes
Gerry Christofi has received educational grants from Allergan. Stephen Ashford has undertaken research sponsored by investigator-led grants from Ipsen Ltd and has received sponsorship from Allergan, Ipsen and Merz to attend conferences and meetings in the UK and overseas, and was the editor of the Royal College of Physicians report on adult spasticity. Jonathan Birns has no conflicts of interests to declare. Catherine Dalton received salary support from Elan (through a grant held by the Institute of Neurology) to analyse MRI data from 2000 to 2003. Lynsay Duke was part of the National Guidelines group involved with the second edition of the RCP Spasticity in Adults: Management Using Botulinum Toxin (2018). She has undertaken research sponsored by an investigator-led grant from Ipsen Ltd and has received sponsorship from Ipsen and Merz to attend conferences and meetings in the UK and overseas. Claire Madsen has received sponsorship from Allergan, Ipsen and Merz to attend conferences and meetings in the UK. Sohail Salam has received sponsorship from Ipsen and Merz to attend conferences and meetings in the UK and overseas. He has also attended advisory meetings for Ipsen and Merz.
References
- 1.Kheder A, Nair KP.. Spasticity: pathophysiology, evaluation and management. Pract Neurol 2012; 12: 289-298. [DOI] [PubMed] [Google Scholar]
- 2.Lance JW.. Pathophysiology of spasticity and clinical experience with Baclofen. In: Lance JW FR, Young RR and Koella WP,. editor. Spasticity: disordered motor control. Chicago: Yearbook Medical; 1980. p. 185-204. [Google Scholar]
- 3.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al.. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005; 27: 2-6. [DOI] [PubMed] [Google Scholar]
- 4.Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK.. Prevalence of spasticity post stroke. Clin Rehabil 2002; 16: 515-522. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence . Draft scope for the proposed appraisal of botulinum toxin type A (Botox, Dysport and Xeomin) for treating upper and lower limb spasticity associated with stroke [cited 2014]. Available from: https://www.nice.org.uk/guidance/gid-tag499/documents/spasticity-after-stroke-botulinum-toxin-type-a-draft-scope-for-consultation-prereferral-july-20142 (accessed May 2018).
- 6.Thibaut A, Chatelle C, Ziegler E, Bruno MA, Laureys S, Gos-series O.. Spasticity after stroke: physiology, assessment and treatment. Brain Inj 2013; 27: 1093-1105. [DOI] [PubMed] [Google Scholar]
- 7.Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH.. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 2004; 35: 134-139. [DOI] [PubMed] [Google Scholar]
- 8.Urban PP, Wolf T, Uebele M, Marx JJ, Vogt T, Stoeter P, et al.. Occurence and clinical predictors of spasticity after ischemic stroke. Stroke 2010; 41: 2016-2020. [DOI] [PubMed] [Google Scholar]
- 9.Gozum MALP, Rosales RL.. Botulinum toxin A therapy in early post-stroke spasticity: providing a wider treatment avenue. Int J Neurorehabilitation 2016; 3: 1000207. [Google Scholar]
- 10.Lundstrom E, Terent A, Borg J.. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol 2008; 15: 533-539. [DOI] [PubMed] [Google Scholar]
- 11.Opheim A, Danielsson A, Alt Murphy M, Persson HC, Sun-nerhagen KS.. Upper-limb spasticity during the first year after stroke: stroke arm longitudinal study at the University of Gothenburg. Am J Phys Med Rehabil 2014; 93: 884-896. [DOI] [PubMed] [Google Scholar]
- 12.Opheim A, Danielsson A, Alt Murphy M, Persson HC, Sun-nerhagen KS.. Early prediction of long-term upper limb spasticity after stroke: part of the SALGOT study. Neurology 2015; 85: 873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bavikatte G. Spasticity early and ongoing management. In: Bavikatte G,. editor. 1st edn. UK: ASK-GB Ltd; 2017. p. 1-112. [Google Scholar]
- 14.Bhimani R, Anderson L.. Clinical understanding of spasticity: implications for practice. Rehabil Res Pract 2014; 2014: 279175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denno MS, Gillard PJ, Graham GD, DiBonaventura MD, Goren A, Varon SF, et al.. Anxiety and depression associated with caregiver burden in caregivers of stroke survivors with spasticity. Arch Phys Med Rehabil 2013; 94: 1731-1736. [DOI] [PubMed] [Google Scholar]
- 16.Chang E, Ghosh N, Yanni D, Lee S, Alexandru D, Mozaf-far T.. A review of spasticity treatments: pharmacological and interventional approaches. Crit Rev Phys Rehabil Med 2013; 25: 11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox AP, Raluy-Callado M, Wang M, Bakheit AM, Moore AP, Dinet J.. Predictive analysis for identifying potentially undiagnosed post-stroke spasticity patients in United Kingdom. J Biomed Inform 2016; 60: 328-333. [DOI] [PubMed] [Google Scholar]
- 18.Royal College of Physicians . [cited 2018]. National guidelines: Spasticity in adults: management using botulinum toxin. 2nd edn Available from: http://bit.ly/2FInQtV. [Google Scholar]