Coronavirus disease (COVID)-19 caused by severe acute respiratory syndrome coronarvirus (SARS-COV)-2 infection has been demonstrated to be associated with cardiac injury [1], [2], [3]. Cases of acute myocarditis have been reported, even in patients with COVID-19 in the absence of significant lung involvement, suggesting a viral triggered immune-mediated injury [4]. The modified RNA vaccines, the BNT162b2 and mRNA-1273, that encode the prefusion SARS-COV-2 spike glycoprotein, have shown to confer 94–95% protection against COVID-19 with a safe profile [5], [6]. Although these vaccines can counteract the COVID-19 pandemic, there is apprehension for patients who experienced previous SARS-COV-2 infection, as these subjects have not been tested in the trials [5]. Systemic reactogenicity, leading to systemic adverse events often occurred after dose 2 and within 2 days after vaccination [5]. The present report describes a case of cardiac involvement in a patient with previous SARS-COV-2 infection within days of the second dose of BNT162b2 mRNA vaccine.
An otherwise healthy 56-year-old man presented to the emergency department complaining of acute onset of chest pain 3 days after the second dose of BNT162b2 mRNA COVID-19 vaccine. He did not report fever, systemic symptoms or cutaneous rash after the first and second dose of the vaccine. He had no history of allergy. Nine months earlier he experienced mild signs of COVID-19 infection with fever lasting for 3 days and cough for 1 week, but he did not complain of chest pain or dyspnea. He was not hospitalized, and he took only acetaminophen. Nasopharyngeal swabs by real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay, had been persistently positive for 1 month while he did not undergo any blood tests during that period. One month later, anti-SARS-COV-2 serology demonstrated presence of IgG anti S1 and S2 proteins (titer of 60 AU/mL with positive threshold above 15).
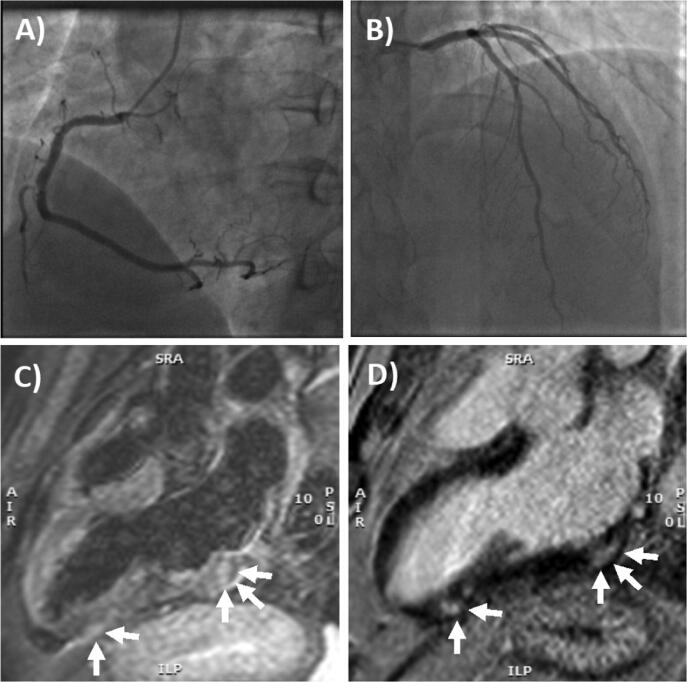
On arrival at the emergency department arterial blood pressure was 165/95 mmHg, heart rate 81 beats per minute, oxygen saturation 99% while breathing ambient air and body temperature 36.2 °C. Electrocardiogram (ECG) showed sinus rhythm, and minimal ST elevation on precordial leads, with peaked T waves. The chest x-ray was unremarkable (Supplemental Fig. 1A-B). Laboratory tests revealed elevated levels of biomarkers of myocardial necrosis, i.e. high-sensitivity (hs) troponin T 289 ng/L, and C-reactive protein 2.9 mg/L with normal blood cell counts, without evidence of peripheral eosinophilia (Table 1). Urgent coronary angiography carried out to rule out an acute coronary syndrome (Fig. 1A-B). Cardiac ventriculography showed preserved global left ventricular function. Chest pain resolved spontaneously within 4 h of admission. The patient was therefore transferred to the cardiology ward with a diagnosis of suspected acute myocarditis. He underwent nasopharyngeal swabbing and the specimens were tested for common respiratory viruses by RT-PCR and resulted all negative. As expected, anti-SARS-COV-2 serology revealed a high-titer of IgG anti S1 and S2 proteins (titer > 400 AU/mL), and positive anti-nucleocapsid antibodies due to previous exposure. Hs-troponin T and CK-MB peaked on day 1 with values of 515 ng/L and 27 µg/L, respectively. No specific anti-inflammatory or steroidal therapy was administered, and CK-MB normalized at day 5 while hs-troponin T normalized within 7 days. Cardiac MRI showed non-dilated ventricles with preserved left (63%) and right ejection fraction (60%). There was focal subepicardial-intramyocardial (non-ischemic pattern) late gadolinium enhancement (LGE) involving the basal and apical segments of the infero-lateral wall, colocalized with signs suggestive for edema on T2 weighted images (Fig. 1C-1D), consistent with the diagnosis of acute myocarditis [7]. No pericardial effusion was observed. During hospital stay no further episodes of chest pain and no arrhythmias were observed on ECG monitoring. Due to increased levels of d-dimer, a CT angiography of the chest was performed and ruled out pulmonary embolism. The patient was discharged on day 7.
Table 1.
Clinical Laboratory findings.
| Measure | Reference Range | Emergency Department | Hospital Day 1 | Hospital Day 2 | Hospital Day 3 | Hospital Day 4 | Hospital Day 5 | Hospital Day 6 | Hospital Day 7 |
|---|---|---|---|---|---|---|---|---|---|
| Cardiac biomarkers: | |||||||||
| hs-Troponin T (ng/L) | 0–14 | 289* | 515* | 413* | 457* | – | 19.7* | – | 12.8 |
| CK-MB (µg/L) | <4.9 | – | 27.4* | 21.0* | 5.2* | – | 1.7 | – | 1.6 |
| NT-pro-BNP (ng/L) | 0–210 | – | – | – | – | – | – | – | 20 |
| Inflammatory biomarkers: | |||||||||
| C-reactive protein (mg/dL) | <0.5 | 2.9* | – | 2.1* | – | – | – | – | 0.4 |
| ESR (mm/h) | 2–10 | – | – | – | – | – | – | – | 16* |
| Ferritin (ng/mL) | 30–400 | – | – | 370 | – | – | – | – | 457* |
| Whole blood count: | |||||||||
| White-cell count (per µl) | 4,000–10,000 | 5,500 | – | 7,700 | 5,870 | – | – | – | 7,120 |
| Lymphocytes (absolute count per µL) | 900–4,000 | 1,330 | – | 1,460 | 1,830 | – | – | – | 1,760 |
| Lymphocytes (%) | 20.0–50.0 | 24.2 | – | 18.8 | 31.2 | – | – | – | 24.7 |
| Eosinophilis (absolute count per µL) | 0–450 | 60 | – | 80 | 120 | – | – | – | 150 |
| Eosinophilis (%) | 0–4.5 | 1.1 | – | 1.0 | 2.0 | – | – | – | 2.1 |
| Hemoglobin (g/dl) | 14.0–18.0 | 16.6 | – | 15.2 | 15.7 | – | – | – | 15.2 |
| Hematocrit (%) | 32.0–52.0 | 47.9 | – | 45.3 | 47.4 | – | – | – | 44.5 |
| Platelet count (per µl) | 140,000–440,000 | 249,000 | – | 207,000 | 237,000 | – | – | – | 288,000 |
| Hepatic biomarkers: | |||||||||
| ALT (U/L) | 3–45 | 35 | – | 33 | – | – | – | – | 39 |
| GGT (U/L) | 2–50 | – | – | 41 | – | – | – | – | 35 |
| Bilirubin (mg/dL) | 0.25–1 | 0.34 | – | 0.86 | – | – | – | – | 0.57 |
| Proteins (g/dL) | 6.4–8.5 | – | – | 6.5 | – | – | – | – | 6.5 |
| Total cholesterol (mg/dL) | – | – | 203 | – | – | – | – | 154 | |
| Triglycerides (mg/dL) | – | – | 126 | – | – | – | – | 82 | |
| INR | 0.86–1.13 | 0.98 | – | – | – | – | – | – | 0.98 |
| Renal function: | |||||||||
| Creatinine (mg/dL) † | 0.67–1.17 | 0.90 | – | 0.82 | 0.84 | – | – | – | 0.99 |
| Others: | |||||||||
| LDH (U/L) | 135–225 | – | – | – | – | – | – | – | 186 |
| CK (U/L) | 30–200 | 235 | 356* | – | – | – | – | – | 78 |
| Amilase (U/L) | 28–100 | – | – | 43 | – | – | – | – | 53 |
| Glucose (mg/dL) | 70–100 | 99 | – | 90 | – | – | – | – | 85 |
| Sodium (mmol/L) | 132–143 | 140 | – | 141 | 142 | – | – | – | 139 |
| Potassium (mmol/L) | 3.4–5.2 | 3.78 | – | 4.12 | 4.29 | – | – | – | 5.1 |
| D-dimer (µg/mL) | 0.0–0.57 | – | – | – | – | – | – | – | 1.99* |
| TSH (µg/mL) | 0.27–4.20 | – | – | – | – | – | – | – | 0.30 |
* The value in the patient was above normal.
† To convert the values for creatinine to micromoles per liter, multiply by 88.4.
ESR indicates erythrocyte sedimentation rate.
Furthermore, anti-ANA, -ENA and -nDNA tests were negative, rheumatoid factor, and complement C3 and C4 levels were within the normal ranges.
Fig. 1.
Coronary angiography and cardiac MRI. (A) The right coronary artery only had a mild plaque (<30% luminal diameter) in the mid portion, while (B) left main stem, left anterior descending artery and circumflex artery had no evidence of coronary plaques. (C) T2- weighted 3-chamber view on cardiac MRI, showing focal areas of edema involving the subepicardial-intramyocardial regions of the basal and apical segments of the infero-lateral wall (arrows). (D) Late gadolinium enhancement confirmed the presence of non-ischemic myocardial lesions in the basal and apical segments of the infero-lateral wall (arrows) consistent with acute myocarditis.
This case highlights a potential relation between the second dose of a COVID-19 vaccine and mild cardiac involvement, compatible with acute myocarditis in an otherwise healthy patient with a previous exposure to SARS-COV-2 infection. Pharmacovigilance on cardiac injury is of paramount importance in this initial phase of massive COVID-19 campaigns with the aim to identify specific populations at increased risk and the target organs of adverse events. Even if no causal relationship can be demonstrated in this case report between the second dose of BNT162b2 mRNA COVID-19 vaccination and acute myocarditis, the timing of the onset and the inflammatory nature of the event make the relationship plausible. Of interest, among the 4 related serious events in the BNT162b2 vaccine trial, a paroxysmal ventricular arrhythmia was observed [5]. Ventricular arrhythmias can occur as consequence of an inflammatory cardiac injury [8], [9], and they were frequently observed in patients with COVID19 and cardiac injury [3]. Two deaths were documented during the trial and were judged as unrelated, although in one case a cardiac arrest occurred [5]. No reports of acute myocarditis were observed in the BNT162b2 mRNA and mRNA-1273 trials. Acute myocarditis can be underestimated in clinical trials. Myocarditis must be actively searched as observed in anticancer agent studies involving immune checkpoints inhibitors [10], [11]. The expected long-term prognosis of the patient we reported is good [12]. Acute myocarditis can occur following vaccination [13], and smallpox vaccination has been frequently associated with eosinophilic myocarditis [14]. In the present case, no peripheral eosinophilia and no signs suggesting a hypersensitivity reaction (rush and fever) were observed, thus eosinophilic myocarditis appears unlikely. As a potential explanation of myocarditis, it is possible that molecular mimicry between viral proteins of SARS-CoV-2 and cardiac structures can explain at least partially the high incidence of cardiac injury observed during COVID-19. Thus, in predisposed individuals, also an immune response against the viral spike glycoprotein could confer a risk for immune-mediated organ injury. Alternatively, the cardiac injury could derive from a nonspecific inflammatory response secondary to vaccination [15]. Hypothetically, both mechanisms might explain the observed acute myocarditis. In conclusion, our case report suggests that pharmacovigilance on cardiac injury could be considered, especially with suspected or confirmed previous history of COVID-19 aimed to actively search for acute myocarditis when chest pain or discomfort is reported.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2021.100774.
Contributor Information
Enrico Ammirati, Email: enrico.ammirati@ospedaleniguarda.it.
Marco Metra, Email: metramarco@libero.it.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J. Mol. Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U. Cardiovascular Magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J. Am. Coll. Cardiol. 2018;72(24):3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 8.Peretto G., Sala S., Rizzo S., Palmisano A., Esposito A., De Cobelli F. Ventricular arrhythmias in myocarditis: characterization and relationships with myocardial inflammation. J. Am. Coll. Cardiol. 2020;75(9):1046–1057. doi: 10.1016/j.jacc.2020.01.036. [DOI] [PubMed] [Google Scholar]
- 9.E. Ammirati, M. Frigerio, E.D. Adler, C. Basso, D.H. Birnie, M. Brambatti, et al., Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document, Circ. Heart Fail. 2020:CIRCHEARTFAILURE120007405. [DOI] [PMC free article] [PubMed]
- 10.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonaca M.P., Olenchock B.A., Salem J.E., Wiviott S.D., Ederhy S., Cohen A. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammirati E., Cipriani M., Moro C., Raineri C., Pini D., Sormani P. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis. Circulation. 2018;138(11):1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 13.Mei R., Raschi E., Forcesi E., Diemberger I., De Ponti F., Poluzzi E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int. J. Cardiol. 2018;273:183–186. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 14.Brambatti M., Matassini M.V., Adler E.D., Klingel K., Camici P.G., Ammirati E. Eosinophilic myocarditis: characteristics, treatment, and outcomes. J. Am. Coll. Cardiol. 2017;70(19):2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Segal Y., Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol. Immunol. 2018;15(6):586–594. doi: 10.1038/cmi.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.