Abstract
Anabolic resistance to a mechanical stimulus may contribute to the loss of skeletal muscle mass observed with age. In this study, young and aged mice were injected with saline or human LM-111 (1 mg/kg). One week later, the myotendinous junction of the gastrocnemius muscle was removed via myotenectomy (MTE), thus placing a chronic mechanical stimulus on the remaining plantaris muscle for 2 weeks. LM-111 increased α7B integrin protein expression and clustering of the α7B integrin near DAPI+ nuclei in aged muscle in response to MTE. LM-111 reduced CD11b+ immune cells, enhanced repair, and improved the growth response to loading in aged plantaris muscle. These results suggest that LM-111 may represent a novel therapeutic approach to prevent and/or treat sarcopenia.
Keywords: Aging, Chronic mechanical loading, Extracellular matrix, Hypertrophy, Integrin
Today, it is estimated that up to 30% of community-dwelling older adults are diagnosed with sarcopenia, defined as the age-related loss of muscle mass and function (1). The primary mechanisms that underlie sarcopenia are complex and not well understood but may include stem cell dysfunction and anabolic resistance to a growth stimulus (2–6). Due to insufficient information regarding mechanism, effective therapeutic strategies to combat the age-associated decline in sensitivity to mechanical loading have not been established.
At the costamere, the α7β1 integrin specifically links laminin in the surrounding basal lamina to the actin cytoskeleton, therefore transmitting force to the ultrastructure and relevant signaling pathways activated by contraction (7). Muscle-specific transgenic expression of the α7β1 integrin can protect against sarcolemmal disruption, reduce inflammation, increase satellite cell accumulation, and accelerate myofiber growth in young mice in response to acute or repeated bouts of eccentric exercise (8–11). Strikingly, no information exists regarding α7β1 integrin expression, localization, and function in the context of natural aging.
The laminin isoform LM-111 (α1β1γ1) is one of the first extracellular matrix (ECM) proteins expressed during embryogenesis and is absent in adult tissues (12). LM-111 can coordinate a variety of biological activities, including stem cell migration, nerve growth, angiogenesis, and matrix remodeling (13–15). Exogenous administration of LM-111 can offer protection against pathology in dystrophic mice (16) and enhance muscle repair following eccentric exercise (17) or cardiotoxin injury (18). These benefits may occur in part due to the ability for LM-111 to concomitantly bind and activate the α7β1 integrin within myofibers, as well as muscle stem/stromal cells outside the fiber (16).
In this study, we investigated the ability for LM-111 administration to recover the hypertrophic response to chronic mechanical loading in aged muscles.
Method
Animals
Mice overexpressing the rat α7 integrin subunit (MCK:α7BX2; SJ6/C57BL/6 strain) and littermate controls were bred and aged to 6 (adult), 16 (middle aged), and 24 (aged) months (n = 7–14) (11). The MHCK7:α7BX2 integrin transgenic mice were developed based on Salva et al. (19), bred and aged to 6–12 (adult) or 24 months (aged) (n = 3–5). Each age group included at least 1 male and 1 female based on availability from litters born.
For the laminin study, young adult (3 months old) and aged (22 months old) WT C57BL/6 mice were acquired from the National Institute of Aging (NIA) colonies at Jackson Laboratory (n = 4–8). Each group included 40%–50% females. These animals received a single intraperitoneal (i.p.) injection of saline or human LM-111 (1 mg/kg) (Sigma-Aldrich, St. Louis, MO). One week postinjection, the myotendinous junction and the base of the gastrocnemius muscle from each limb were removed (myotenectomy, or MTE), to overload soleus and plantaris muscles, as previously described (20). Two weeks postsurgery, mice were euthanized, and plantaris muscles were preserved.
Western Blotting
Western blotting was performed using 25 µg of muscle lysate and an α7B integrin-specific antibody (in-house antibody CDB 347, 1:1000) that detects both endogenous and transgenic α7B integrin.
Immunohistochemistry
Transverse muscle cryosections (10 µm) were immunostained using anti-myosin heavy chain 1, 2X, 2A, and 2B antibodies (Developmental Studies Hybridoma Bank) and dystrophin (Abcam) antibodies. Cryosections were also stained with anti-collagen (ab21286, Abcam, 1:100), anti-CD11b (BD Biosciences, 1:100), anti-LAMA1 primary antibody (HPA032110, Sigma-Aldrich), and α7B integrin subunit antibody.
In Vitro Assessment of Myotube Growth
Untreated Silicon 6-well BioFlex culture dishes (Flexcell International, Burlington, NC) were cross-linked with mouse LM-111 (12.5 μg/mL), or LM-111/Collagen 1 (12.5/50 μg/mL) using EDC (Sigma-Aldrich, 0.002 g/mL). C2C12 myoblasts were subjected to low-magnitude uniaxial strain (10%, 1 Hz) (FX-4000 Tension System, Flexcell International) for 2 days to promote alignment on LM-111 alone or LM-111/Collagen 1 (21). Following 3 days of rest, myotubes underwent 20 min of high-magnitude uniaxial strain in the perpendicular direction (18%, 1 Hz). Myotube number and diameter were quantified.
Statistical Analysis
All data are presented as means ± SEM. Two-way ANOVA was performed to detect significant interactions or main effects. For fiber or myotube size distribution and fiber type-specific data, statistical analysis was confined to each respective category. Post hoc comparisons were conducted when appropriate. Differences were considered significant at p ≤.05. GraphPad Prism 8 was used to complete all statistical analyses.
Results
Overexpression of the α7B integrin subunit in aged skeletal muscle does not recover the growth response to chronic loading
MCK:α7BX2 integrin transgenic mice were aged to 24 months to determine the extent to which overexpression of the integrin could enhance mechanosensing and the anabolic response to chronic loading. α7B integrin protein expression was unexpectedly reduced with age and significant overexpression was no longer observed at 24 months in MCK:α7BX2 mice (~3.5-fold at 6 months vs 1.5-fold at 24 months; Genotype × Age interaction, p < .001) (Supplementary Figure S1A). To overcome this limitation, a new transgenic mouse model was created (MHCK7:α7BX2) to sustain overexpression ~3.5-fold compared to WT (Supplementary Figure S1D). Whereas significant increases in plantaris weight were detected in adult WT and MHCK7:α7BX2 mice with chronic loading as expected (Supplementary Figure S1C and E), no increase in plantaris muscle weight was observed in aged WT mice and overexpression of α7BX2 integrin subunit did not rescue this effect (MTE × Age interaction, p < .001) (Supplementary Figure S1E).
LM-111 Administration Increases α7B Integrin Protein Expression in Aged WT Muscle
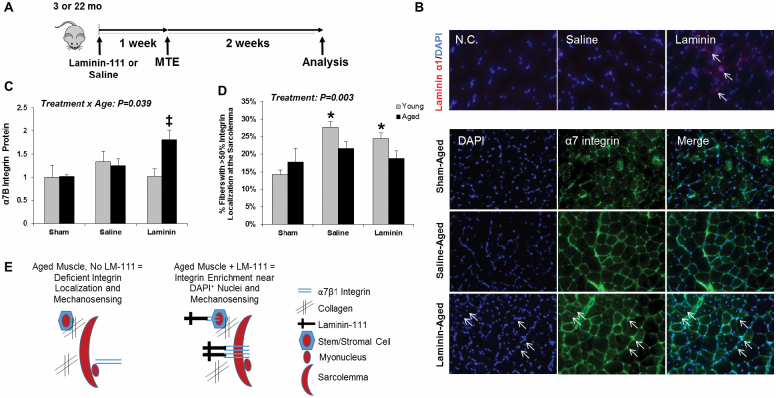
This finding prompted us to consider factors outside the muscle that might influence integrin activation with age. Specifically, we hypothesized that collagen accumulation, a hallmark of aging, prevented the physical interaction between laminin and the α7β integrin (Figure 1B). Thus, a study was conducted to examine the potential for LM-111 to improve the anabolic response to loading in aged mice (Figure 1A). The presence of human laminin-α1 protein in WT mouse skeletal muscle 3 days following i.p. injection was confirmed and a punctate pattern was observed around fibers (Figure 1B, top panel). MTE increased α7B integrin subunit protein expression and the response differed by age (Treatment × Age interaction, p = .039), with a significant increase observed in aged muscle in response to MTE compared to age-matched controls (Figure 1C). MTE induced translocation of the α7B integrin localization to the sarcolemma in young muscle post-MTE, yet this event was not observed in aged muscle in the absence or presence of LM-111 (Figure 1D). In contrast, LM-111 evoked enrichment of α7B integrin in a punctate pattern in close proximity to DAPI+ nuclei (outside and inside fibers) in aged muscle post-MTE (Figure 1B, bottom panel). These observations suggest that LM-111 may restore anchoring of the sarcolemma or stem/stromal cells to the microenvironment via integrin clustering (Figure 1E).
Figure 1.
LM-111 treatment increases α7B integrin subunit protein expression in aged plantaris muscle of wild-type mice following chronic mechanical loading. Laminin-111 (LM-111) or saline was administered by intraperitoneal (i.p.) injection to 3- or 22-mo-old wild-type mice 1 wk prior to initiation of chronic mechanical loading induced by myotenectomy (MTE) and muscle was evaluated 2 wk post-MTE (A). LM-111 is present in skeletal muscle 3 d following i.p. injection (top panel). α7B integrin protein demonstrated a punctate pattern of expression in close proximity to DAPI+ nuclei (bottom panel, arrows indicate colocalization of integrin expression with DAPI-stained nuclei around in inside the fiber) (B). Total α7B integrin subunit protein expression in aged plantaris was increased with LM-111 treatment compared to age-matched sham and saline-treated controls (C). The percentage of fibers with >50% integrin localization at the sarcolemma was increased in young muscle post-MTE, but not aged muscle. (D) Graphical depiction of integrin enrichment near DAPI+ nuclei outside and inside muscle fibers with LM-111 treatment and speculative changes in mechanosensing. n = 4–7/group. ‡ vs age-matched sham and saline-treated groups. All values are mean ± SEM. * vs age-matched sham group. Data are expressed relative to young sham for each muscle.
LM-111 Administration Improves the Growth Response to a Mechanical Stimulus in Aged WT Muscle
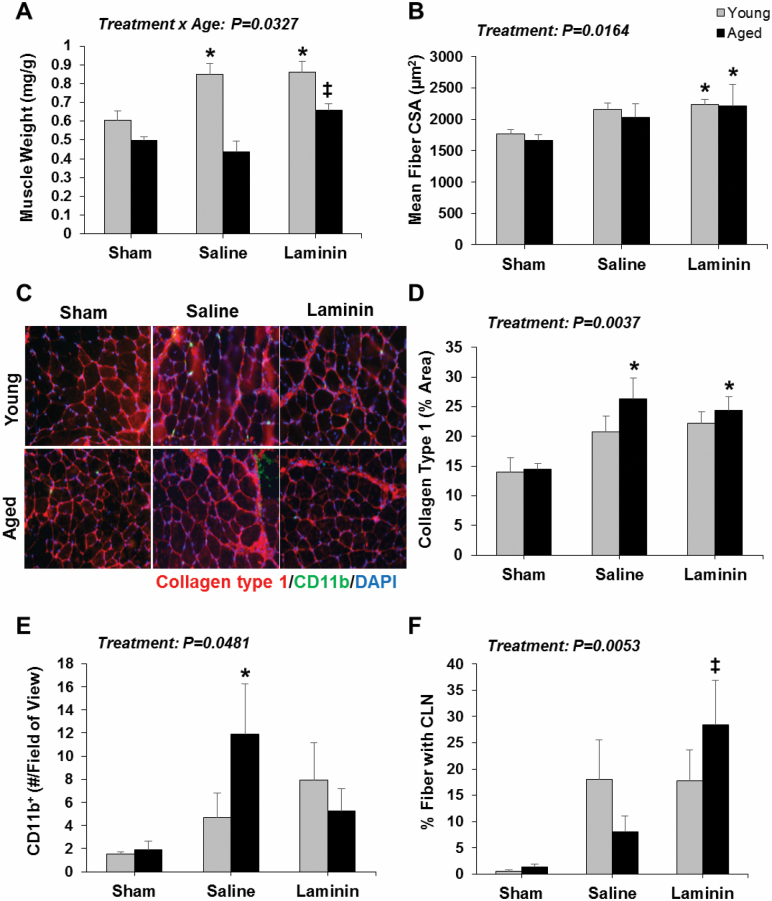
An increase in plantaris muscle wet weight was observed in young, but not aged, WT mice with saline treatment post-MTE (Treatment × Age interaction, p = .0327) (Figure 2A). LM-111 treatment significantly improved the growth response to MTE in aged WT mice compared to age-matched sham and saline-treated groups (Figure 2A). The mean fiber cross-sectional area (CSA) was significantly higher in young and aged muscle post-MTE (treatment effect, p = .0164) (Figure 2B). LM-111 treatment increased myofiber CSA in young and aged muscle post-MTE compared to age-matched shams (Figure 2B).
Figure 2.
LM-111 treatment improves the muscle growth response to chronic mechanical loading in aged wild-type mice. Muscle wet weight (A) and mean fiber CSA (B) were assessed in plantaris muscle by immunofluorescence analyses following saline or LM-111 treatment prior to 2 wk of chronic mechanical loading induced by myotenectomy (MTE). Laminin treatment positively impacted the muscle growth response to MTE in aged muscle compared to age-matched shams and/or saline-treated controls. n = 4–7/group. Representative images of collagen type 1 and CD11b+ immune cells as detected by immunofluorescence analysis (C). Collagen type 1 content (D), CD11b+ immune cell content (E), and % of CLN+ myofibers (F) were assessed in plantaris muscle by immunofluorescence analyses following saline or LM-111 treatment prior to 2 weeks of chronic mechanical loading induced by MTE. Laminin treatment positively impacted indices of inflammation and repair in aged muscle post-MTE compared to age-matched saline-treated controls. n = 4–6/group. * vs age-matched sham group. ‡ vs age-matched sham and saline-treated groups. All values are mean ± SEM. Data are expressed relative to young sham for each muscle.
LM-111 Administration Alters Macrophage Content and Myofiber Repair in Aged WT Muscle
The percentage area occupied by collagen type 1 increased post-MTE (treatment effect, p = .0037), and was significantly elevated in aged muscle post-MTE compared to the age-matched sham group (Figure 2C and D). CD11b+ cell quantity significantly increased post-MTE (treatment effect, p = .0481), and was significantly enhanced in aged muscle post-MTE with saline treatment compared to both age-matched sham and LM-111-treated groups (Figure 2C and E). Finally, the percentage of fibers exhibiting centrally located nuclei (CLN) was increased post-MTE (treatment effect, p = .0053), and was significantly improved in aged muscle with LM-111 treatment compared to saline (Figure 2F).
Impact of Mechanical Loading and LM-111 Treatment on Fiber Type-Specific CSA and Frequency
Fiber type CSA and distribution data are reported in Supplementary Figure S2. Type 2A fiber CSA trended towards an increase in young muscle post-MTE with LM-111 treatment compared to age-matched sham (p = .0547) and significantly increased in aged muscle post-MTE with both saline and LM-111 treatment compared to age-matched sham groups (treatment effect, p = .0109). Type 2X fiber CSA was variable in aged muscle post-load with LM-111 treatment (treatment effect, p = .1247), which mirrored the results for the percentage of large caliber fibers in this group (mean fiber CSA > 2000 µm2; p = .0827) (Supplementary Figure S2D).
Collagen Is Inhibitory to Myotube Formation and Mechanical Strain-Induced Growth In Vitro
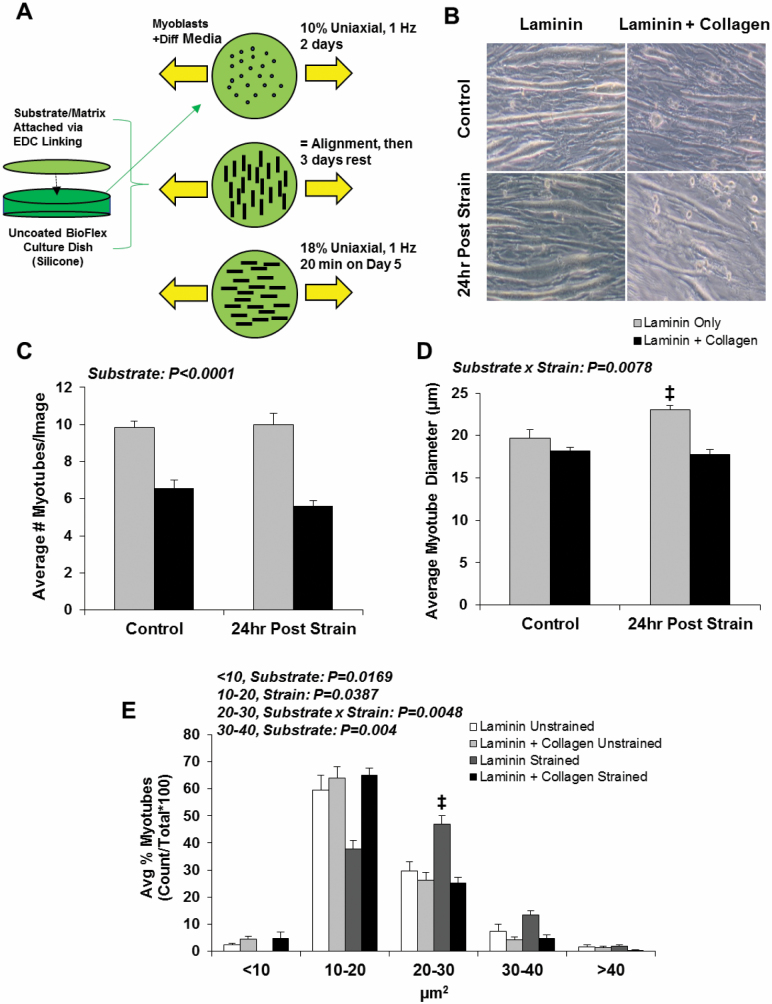
An in vitro experiment was conducted to test the hypothesis that collagen accumulation may directly interfere with mechanical strain-induced muscle growth (Figure 3A). LM-111 alone or LM-111 with the addition of collagen was attached as substrate to silicone membranes prior to addition of C2C12 myoblasts and initiation of strain. Incorporation of collagen in the substrate reduced myotube formation (substrate effect, p < .0001) (Figure 3C) and decreased myotube growth in response to strain (Substrate × Strain interaction, p = .0078) (Figure 3D). A shift in myotube diameter was observed with the addition of collagen (<10 µm: substrate effect, p = .0169; 10–20 µm: strain effect, p = .0387; 20–30 µm: Substrate × Strain interaction, p = .048; 30–40 µm: substrate effect, p = .004) (Figure 3E).
Figure 3.
Collagen is inhibitory to myoblast fusion and mechanical strain-induced growth in vitro. Experimental design for in vitro mechanical stimulation of myotubes with modification of substrate (A). After 2 d of culture, C2C12 myoblasts were differentiated for 2 d in the presence of LM-111 only or a LM-111-collagen mixture in the presence of low-magnitude uniaxial strain (10%) to induce myotube alignment (perpendicular to strain). After 3 days of rest, aligned myotubes were rotated 90° and exposed to 20 min of high-magnitude uniaxial strain (18%) (Post Strain Group) or remained unstrained (Control), then evaluated 24 h later. Myoblast tube development was reduced with the addition of collagen (B, C), and myotube diameter was suppressed in response to strain with the addition of collagen (D). The number of myotubes in the range of 20–30 µm were robustly increased in the presence of laminin only, but suppressed with the addition of collagen (E). n = 6/group. ‡ vs all groups. All values are mean ± SEM. Data are expressed relative to laminin only control.
Discussion
In this study, we report that a single i.p. injection of LM-111 effectively decreased CD11b+ cell quantity, enhanced the capacity for repair, and improved the growth response to chronic load in aged mouse skeletal muscle. The precise mechanism of LM-111 action remains unknown, but our in vitro experiment suggests that the composition of the ECM can significantly impact mechanosensing. We speculate that laminin, the preferential ligand for the α7β1 integrin, is an important component to load-induced growth and that collagen accumulation contributes to anabolic resistance with age.
The α7β1 integrin is highly expressed in skeletal muscle and can positively impact the growth response to mechanical loading (8,9). In the current study, we did not observe any difference in the baseline level of integrin expression with age (Supplementary Figure S1A), yet anabolic resistance to chronic loading was present in aged WT mice as expected. Transgenic mice were created to determine the extent to which an abundance of α7 integrin in aged muscle could overcome this. Despite confirmation of elevated protein expression in 24-month-old transgenic mice compared to WT (~3.5-fold), we did not observe any increase in muscle wet weight with loading (Supplementary Figure S1C), suggesting that simply overexpressing the integrin subunit protein was not sufficient to overcome anabolic resistance. These results were unexpected and prompted us to consider factors in the microenvironment that might disrupt integrin activation.
Collagen does not possess the capacity to bind the α7β1 integrin heterodimer, and its excessive deposition has the potential to interfere with laminin-α7B integrin interaction at the sarcolemma. Therefore, we hypothesized that exogenously administered laminin, specifically LM-111, might compete with collagen and restore the capacity for the integrin to serve as a mechanosensor. In the current study, LM-111 stimulated punctate patterning of α7B integrin expression around the fiber that mirrored LM-111 localization and suggested evidence for clustering and anchoring. These results align with the findings by Rooney et al. (16), which reported increased interstitial and extrajunctional localization of α7B integrin expression with LM-111 treatment in mdx mice.
Exogenous LM-111 did not impact any phenotype associated with chronic loading in young mice, yet significantly impacted aged muscle (Figure 2). Importantly, muscle wet weight was significantly improved in aged mice 2 weeks following the onset of chronic loading (Figure 2A). The mean CSA of large caliber fibers (>2000 µm2), including Type 2X fibers, visually appear to be recovered with LM-111 treatment, yet we did not detect a significant difference (Supplementary Figure S2B and D). LM-111 also significantly decreased CD11b+ cell quantity and enhanced the percentage of fibers with a CLN in aged muscle post-MTE. These changes likely reflect the capacity for LM-111 to interact with the α7B integrin present within the sarcolemma, as well as stem and stromal cells (22). Our in vitro experiment provides evidence that LM-111 can promote strain-induced muscle growth in the absence of stem cells, and also suggests that LM-111 and collagen differentially impact mechanosensing. Additional in vitro experiments will elucidate niche regulation of mechanosensing and muscle growth via the α7β1 integrin.
This study has limitations. First, only one time-point was selected for investigation. Future studies will explore if LM-111 administration can support long-term adaptations in aged muscle. Second, muscle weight can be impacted by swelling, inflammation, fat, or fibrosis and provides a nonspecific measure of muscle mass. Finally, the study was underpowered to detect significant differences in some measurements due to low sample sizes for some groups.
In conclusion, we provide the first demonstration that a single systemic injection of LM-111 can successfully improve repair and growth following chronic mechanical loading in aged WT mouse muscle. Thus, LM-111 represents a potential therapeutic solution for sarcopenia.
Funding
This work was supported by the National Institutes of Health (NIH NIAMS R21 AR065578 to M.D.B.; NIH U54 AR065139 to S.D.H.) and the Muscular Dystrophy Association (to S.D.H.). Postdoctoral fellowship from the Ruth L. Kirschstein National Research Service Award NIH 1T32HL139451 was awarded to Z.S.M.
Supplementary Material
Acknowledgments
We would like to thank Dr. Fuming Pan and the Transgenic Mouse Facility at UIUC for assistance in the creation of the MHCK7:α7BX2 integrin transgenic mice.
Conflict of Interest
The University of Nevada, Reno, has been issued patents on the therapeutic use of Laminin-111 and its derivatives. These patents have been licensed to Prothelia Inc., Milford, MA. The University of Nevada, Reno has a small equity share in this company. No other conflicts to report.
Author Contributions
Designed experiments: K.G., Z.S.M., S.D., M.C.V., H.D.H., S.D.H., D.J.B., and M.D.B. Performed experiments: K.G., Z.S.M., S.D., M.C.V., H.D.H., S.L., and Y.-F.W. Data analysis: K.G., Z.S.M., S.D., H.D.H., S.L., Y.-F.W., and M.D.B. Manuscript writing: K.G., Z.S.M., and M.D.B. Manuscript revisions: K.G., Z.S.M., S.D., M.C.V., H.D.H., S.L., Y.-F.W., S.D.H., D.J.B., and M.D.B.
References
- 1. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490(7420):355–360. doi: 10.1038/nature11438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJ. Satellite cells in human skeletal muscle; from birth to old age. Age (Dordr). 2014;36(2):545–547. doi: 10.1007/s11357-013-9583-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drummond MJ, Dreyer HC, Pennings B, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985). 2008;104(5):1452–1461. doi: 10.1152/japplphysiol.00021.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwee DT, Bodine SC. Age-related deficit in load-induced skeletal muscle growth. J Gerontol A Biol Sci Med Sci. 2009;64(6):618–628. doi: 10.1093/gerona/glp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fry CS, Drummond MJ, Glynn EL, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1(1):11. doi: 10.1186/2044-5040-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boppart MD, Mahmassani ZS. Integrin signaling: linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2019;317(4):C629–C641. doi: 10.1152/ajpcell.00009.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zou K, Meador BM, Johnson B, et al. The α 7β 1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol (1985). 2011;111(4):1134–1141. doi: 10.1152/japplphysiol.00081.2011 [DOI] [PubMed] [Google Scholar]
- 9. Lueders TN, Zou K, Huntsman HD, et al. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am J Physiol Cell Physiol. 2011;301(4):C938–C946. doi: 10.1152/ajpcell.00515.2010 [DOI] [PubMed] [Google Scholar]
- 10. Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1623–R1630. doi: 10.1152/ajpregu.00089.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol. 2006;290(6):C1660–C1665. doi: 10.1152/ajpcell.00317.2005 [DOI] [PubMed] [Google Scholar]
- 12. Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–1521. doi: 10.1083/jcb.139.6.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dixelius J, Jakobsson L, Genersch E, Bohman S, Ekblom P, Claesson-Welsh L. Laminin-1 promotes angiogenesis in synergy with fibroblast growth factor by distinct regulation of the gene and protein expression profile in endothelial cells. J Biol Chem. 2004;279(22):23766–23772. doi: 10.1074/jbc.M311675200 [DOI] [PubMed] [Google Scholar]
- 14. Ichikawa-Tomikawa N, Ogawa J, Douet V, et al. Laminin α1 is essential for mouse cerebellar development. Matrix Biol. 2012;31(1):17–28. doi: 10.1016/j.matbio.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ning L, Kurihara H, de Vega S, et al. Laminin α1 regulates age-related mesangial cell proliferation and mesangial matrix accumulation through the TGF-β pathway. Am J Pathol. 2014;184(6):1683–1694. doi: 10.1016/j.ajpath.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2009;106(19):7991–7996. doi: 10.1073/pnas.0811599106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zou K, De Lisio M, Huntsman HD, et al. Laminin-111 improves skeletal muscle stem cell quantity and function following eccentric exercise. Stem Cells Transl Med. 2014;3(9):1013–1022. doi: 10.5966/sctm.2014-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Ry PM, Minogue P, Hodges BL, Burkin DJ. Laminin-111 improves muscle repair in a mouse model of merosin-deficient congenital muscular dystrophy. Hum Mol Genet. 2014;23(2):383–396. doi: 10.1093/hmg/ddt428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salva MZ, Himeda CL, Tai PW, et al. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther. 2007;15(2):320–329. doi: 10.1038/sj.mt.6300027 [DOI] [PubMed] [Google Scholar]
- 20. You JS, McNally RM, Jacobs BL, et al. The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J. 2019;33(3):4021–4034. doi: 10.1096/fj.201801653RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pennisi CP, Olesen CG, de Zee M, Rasmussen J, Zachar V. Uniaxial cyclic strain drives assembly and differentiation of skeletal myocytes. Tissue Eng Part A. 2011;17(19-20):2543–2550. doi: 10.1089/ten.TEA.2011.0089 [DOI] [PubMed] [Google Scholar]
- 22. Crawley S, Farrell EM, Wang W, et al. The alpha7beta1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res. 1997;235(1):274–286. doi: 10.1006/excr.1997.3671 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.