Abstract
Background
Increasing evidence shows that cognition and gait speed are associated and are important measures of health among older adults. However, previous studies have used different methods to assess these 2 outcomes and lack sufficient sample size to examine heterogeneity among subgroups. This study examined how the relationship between global cognitive function and gait speed are influenced by age, gender, and race utilizing an integrated data analysis approach.
Method
Data on cognition (Montreal Cognitive Assessment [MoCA], Mini-Mental Status Examination [MMSE], and Modified Mini-Mental State Examination [3MSE]) and gait speed (range: 4–400 m) were acquired and harmonized from 25 research studies (n = 2802) of adults aged 50+ from the Wake Forest Older American Independence Center. Multilevel regression models examined the relationship between predicted values of global cognitive function (MoCA) and gait speed (4-m walk), including heterogeneity by age, race, and gender.
Results
Global cognitive function and gait speed exhibited a consistent positive relationship among whites with increasing age, while this was less consistent for African Americans. That is, there was a low correlation between global cognitive function and gait speed among African Americans aged 50–59, a positive correlation in their 60s and 70s, then a negative correlation thereafter.
Conclusion
Global cognition and gait speed exhibited a curvilinear U-shaped relationship among whites; however, the association becomes inverse in African Americans. More research is needed to understand this racial divergence and could aid in identifying interventions to maintain cognitive and gait abilities across subgroups.
Keywords: Cognitive test, Montreal Cognitive Assessment, Short Physical Performance Battery
Although emerging research in aging has suggested a direct relationship between cognition and physical function, evidence is mixed. In an effort to clarify these findings, 2 meta-analyses have recently been published. The first, involving 12 published studies, reported a positive association between physical function and global cognition, although the effect size was only 0.12 (95% confidence interval [CI] = 0.09–0.15, p < .001), suggesting that individuals with faster gait performed better on measures of global cognition (1). In a second meta-analysis, Peel et al. (2) compared the gait speed of patients with cognitive impairment, mild dementia, or moderate dementia to normal controls. They found that there were clinically meaningful, graded reductions in gait speed that ranged from 0.11 m/s in those with cognitive impairment, to 0.20 m/s in those with mild dementia, and to 0.41 m/s in those with moderate dementia.
Despite the advantages that these meta-analyses have provided through pooling of data across studies, they have significant limitations. First, studies reviewed by Demnitz et al. (1) aggregated results from studies involving different populations that employed varied approaches to the assessment of global cognition and gait speed. Yet, it is well known that measures of both cognition and gait speed vary in their sensitivity to change based on the measure and the population under investigation. And second, neither meta-analysis was able to examine potential heterogeneity in the associations related to age, gender, or race, even though individual studies within the meta-analysis may have done so.
Applying integrative data analysis (IDA), the current study leverages the unique resources at the Wake Forest Older American Independence Center (OAIC) to harmonize measures of cognition and physical function across studies conducted within the OAIC. We then investigate the predictive association between cognitive and physical function accounting for differences in key demographic characteristics.
Unlike meta-analysis, which combines summary statistics from different studies, IDA pools together raw data sets to form an integrated database. Integrative data analysis refers to a set of strategies in which 2 or more independent data sets, with measures that capture similar domains but use different measurement instruments, are pooled or combined and then statistically analyze (3). Pooling raw data from multiple studies has advantages, as well as challenges (4–6). An obvious advantage of IDA is the creation of a larger sample size, increasing power. In what is described as a “crisis of reproducibility” of research findings in the behavioral sciences, investigators have argued that “empirical study of individual behavior is awash in small effect sizes and low statistical power that are naturally difficult to reproduce.” (7,8). The larger sample size from pooling studies offers more robust and reproducible results and improves accuracy for assessing subgroups, such as differences by age group, gender, and race.
In aging research, the advantages of IDA go beyond power and accuracy. First, IDA allows the study of heterogeneity in older adults, which is important for designing targeted interventions for different subgroups. For example, if study A only contains whites and study B only African Americans (AA), IDA allows the study of race heterogeneity that is otherwise not possible from separate individual studies. Second, IDA allows broader assessment of a theoretical construct (eg, frailty or cognitive function) by creating commensurate measures across multiple studies through the application of modern psychometric methods. By placing similar measures, such as Montreal Cognitive Assessment (MoCA) in one study and Mini-Mental Status Examination (MMSE) in another on a common scale, IDA permits direct comparison of results across contributing studies and the investigation of moderating and mediating variables. The same can be done with different measurements of gait speed which often vary between research studies often ranging from 4 to 400 m or vary by time limit. Third, IDA can be used to study changes over the life span by integrating cohorts with overlapping ages (9).
With these advantages in mind, the current study employs IDA to (a) investigate the relationship between global cognitive function and gait speed across 25 studies (n = 2802) collected on older adults (50 years of age and older) from the Wake Forest OIAC; (b) harmonize different measurements of global cognitive function (MoCA, MMSE, and Modified Mini-Mental State Examination [3MSE]) and gait speed (ranging from 4 to 400 m) to compare them on the same scale; and (c) examine how these relationships are influenced by age, gender, and race.
Method
Data Source
Data were collected at the Wake Forest Claude D. Pepper OAIC. The Pepper OAIC program was established as centers of excellence in geriatrics research and research career development to increase scientific knowledge leading to better ways to maintain or restore independence in older persons. Each center has a center-specific theme in aging. At the Wake Forest Pepper OAIC, the theme is physical function and mobility in older adults. A total of 51 small and medium-sized studies over a period of 15 years at Wake Forest OAIC were considered for inclusion into the current study. These data sets were made available to all OAIC investigators (and the public at large with restriction) through the Pepper OAIC data coordinating center, which is also housed at the Wake Forest School of Medicine.
Integrative Data Approach
To date, 51 research studies have been conducted through the Wake Forest OAIC, but not all of them collected both physical and cognitive measures. Studies that did not collect either physical or cognitive measurements were excluded from the current study. As a result, a total of 25 studies were included, with a total sample size of n = 2802. The average sample size is 116, and the range is 14–497. The full sample sizes of the studies and a brief description of the study aims are provided in Supplementary Material.
Measures
Cognitive and gait measures
Because different measures were used for assessing cognitive function, we decided to use an “anchoring measure” such that all other measures would be mapped to the anchoring measure. This measure harmonization targeted the 3 cognitive measures that existed within the data sets—the MoCA (10), the MMSE (11), and the 3MSE (12). MoCA was chosen to be the anchoring measure because it was the most prevalent measure across the included studies and demonstrated some advantages, such as being more predictive of clinical diagnosis of impairment, over other measures (13). We used validated cross-walk conversion tables for this purpose—that is, MMSE mapped to MoCA (14), and 3MSE mapped to MoCA (15). Cross-walk tables link one instrument to another instrument that both measure the same construct or conceptually similar constructs. Typically, cross-walk tables are derived from applying psychometric methods such as equipercentile equating (16) to empirical data. Consider harmonizing the MMSE and MoCA as an example. Briefly, the equipercentile method first ranks data points in A and B, respectively, and then maps the value of MMSE at a given percentile point, say the 10th percentile, to the value of MoCA at the 10th percentile. When the scores are recorded as integer, equipercentile methods may produce decimals in the cross-walk table so smoothing and rounding are necessary (17). This information has been previously published (14,15); therefore, we used those established cross-walk tables to harmonize global cognition to represent a predicted MoCA value.
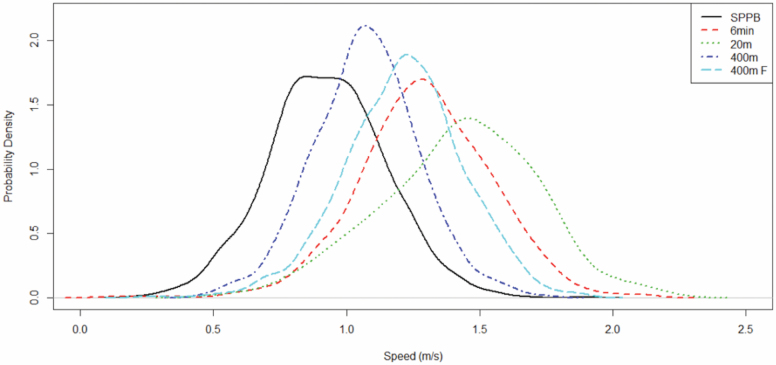
Measure harmonization was also needed for gait measure. The following measures were present across the studies: 4-m walk, 6-min walk, 20-m walk, 20-m fast walk, 400-m walk, and 400-m fast walk. The 4-m walk is a component in the Short Physical Performance Battery (SPPB) (18). Because 20-m fast walk was only used in one small study and the study did not measure SPPB, it was not included for harmonization. Although one could directly use gait speed calculated from different modes of gait measure as a common metric, that may lead to bias because speed was derived from the different modes of measurement (19). Figure 1 shows the various distributions of gait speed by mode of measurement. It can be seen that there are substantial discrepancies in the distributions—including means and variances, between modes of gait measurement.
Figure 1.
Distributions of gait speed from different modes of measurement. 400 m F = 400-m fast walk.
Consistent with the treatment of cognitive measures, a decision was made to apply the same equipercentile method to gait measure. To determine the anchoring measure, we considered prevalence of a measure and its overlap with other gait measures (ie, whether the 2 gait measures were administered within the same study). Because the SPPB, which includes the 4-m habitual walk component, was administered in a high proportion of the studies and had overlap with other gait measures in at least one or more studies, the 4-m walk was selected as the anchoring measure. As far as we know, there are no published cross-walk tables for gait measures. Table 1 shows the cross-walk table for gait measures, which is derived from studies that had overlapping gait measures in 4-m walk and another gait measure. Participants that completed the 4-m habitual walk were instructed to start and stop at designated tape markings on the ground and were asked to walk at their usual walking pace. The walk distance included a 1-m acceleration phase and 1-m deceleration phase not included in the recorded time. The value of 4-m walk was obtained either from the observed value of the 4-m habitual walk if that was present, or set to the corresponding mapped value of 4-m walk in the cross-walk table if another gait measure was observed. In the latter case, the mapped value was adjusted for gender, race, and age using results from regression models locally fitted to discretized values of gait measure. Equating was conducted using the R program kequate (17).
Table 1.
Conversion Table for Gait Measures to Short Physical Performance Battery (SPPB) 4-m Walk
| 6 min | SPPB | 20 m | SPPB | 400 m | SPPB | 400 m F | SPPB |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 6 | 3 | 5 | 4 | 7 | 5 |
| 2 | 2 | 7 | 3 | 6 | 5 | 8 | 6 |
| 3 | 3 | 8 | 4 | 7 | 6 | 9 | 7 |
| 4 | 3 | 9 | 5 | 8 | 7 | 10 | 8 |
| 5 | 4 | 10 | 5 | 9 | 8 | 11 | 9 |
| 6 | 5 | 11 | 6 | 10 | 9 | 12 | 10 |
| 7 | 5 | 12 | 6 | 11 | 10 | 13 | 11 |
| 8 | 6 | 13 | 7 | 12 | 11 | 14 | 11 |
| 9 | 7 | 14 | 8 | 13 | 12 | 15 | 12 |
| 10 | 8 | 15 | 8 | 14 | 13 | 16 | 13 |
| 11 | 9 | 16 | 9 | 15 | 14 | 17 | 14 |
| 12 | 9 | 17 | 9 | 16 | 15 | NA | NA |
| 13 | 10 | 18 | 10 | NA | NA | NA | NA |
| 14 | 11 | 19 | 10 | NA | NA | NA | NA |
| 15 | 12 | 20 | 11 | NA | NA | NA | NA |
| 16 | 12 | 21 | 12 | NA | NA | NA | NA |
| 17 | 13 | NA | NA | NA | NA | NA | NA |
| 18 | 14 | NA | NA | NA | NA | NA | NA |
| 19 | 15 | NA | NA | NA | NA | NA | NA |
| 20 | 16 | NA | NA | NA | NA | NA | NA |
| 21 | 16 | NA | NA | NA | NA | NA | NA |
Note: The unit is 0.1 m/s. For example, SPPB = 10 refers to the converted gait speed of 1 m/s. SPPB = 4-m walk in SPPB; 400 m F = 400-m fast walk; NA = no data are available.
Factors for examining heterogeneity
The following factors were considered in examining heterogeneity in the association between cognitive and gait: race, age, gender, and body mass index (BMI). Besides the harmonization of the cognitive function and gait measure, other variables also needed to be aligned. For example, race/ethnicity across different studies might not be uniformly defined. We defined 2 broad categories of race/ethnicity—white and AA, which could be successfully derived from all included studies. The other race/ethnicity categories only constituted an extremely small proportion (n = 161 in overall sample and n = 0 in the final sample). Because different studies had different designs (eg, intervention vs observational), different follow-up schedules, and different duration, we only used baseline data from all of the studies in the current IDA.
Statistical Analysis
The aim of the current IDA is to examine factors that potentially affect the association between cognition and gait. Analysis of data was conducted as a 2-stage process. In the first stage, we used both descriptive statistics and visualization to explore the integrated data set. For example, scatterplots were used to visualize relationship between cognitive and gait measures. Multiple dimensions in the data were depicted using modern interactive visualization tools. Observations from the first stage and insight about trends and patterns were then used to inform the construction of statistical models in the second stage.
In the second stage, multilevel regression models were used to validate observed trends and test hypotheses. Data from an individual study were considered clustered in the multilevel analysis. In other words, the first level was participants within a given study, and the second level was individual studies. The multilevel regression analysis thus took into account within-study clustering effect. The dependent variable was the anchoring measure of MoCA (as harmonized by IDA) and the primary quantities of interest were the coefficients in the independent variable of the anchoring measure of 4-m walk (Gait) and the interaction terms of gait with other factors, which included race, gender, age, and BMI (kg/m2). For example, the following models (error terms not shown) were considered:
Model 1
| (1) |
Model 2
| (2) |
Model 3
| (3) |
and Model 4
| (4) |
Other covariates including gender and BMI were included in the final model (Model 4). The variables MoCA, Gait, Age, and BMI were standardized such that the model parameters especially for moderation could be readily interpreted. In Model 1, the coefficient β 1 quantifies the association between cognitive (MoCA) and gait (4-m walk) measures. In Model 2, age is included in the model. When an interaction term between gait and age was added to the model (Model 3), the interaction term represents the additional (positive or negative) association between MoCA and gait for age, with the presence of other factors in the model. A significant coefficient for the interaction term β 3 therefore would signal measurable moderation effect, or age heterogeneity for the association between MoCA and 4-m gait measure.
In Model 4, we found heterogeneity across multiple variables– in this case Race and Age, which was represented in the model by the presence of a third-order interaction term with gait. If the quantity β 5 is positive, it would mean that higher value of age (older) is associated with higher correlation between MoCA and 4-m walk for the subgroup of AA compared to white.
Intraclass correlation (ICC) was used to indicate the strength of clustering within study. In all of the multilevel models, 2-sided tests at α = .05 were used. We used PROC MIXED in SAS v9.4 for multilevel regression analysis. Interactive visualization was conducted in Tableau v10 (20).
Results
Table 2 shows the sample characteristics of the entire integrated sample. It is noted that the sample has an average MoCA score of 24.4 (SD 3.4; range 7–30). A cutoff score of MoCA score for mild cognitive impairment (MCI) is 25 (21). In the current sample, 45% were below 25.
Table 2.
Sample Characteristics of the Integrated Data Set by Race
| White (N = 2239; 79.9%) | Black (N = 563; 20.1%) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age | 71.9 (6.7) | 70.5 (6.2) |
| BMI | 31.3 (5.2) | 33.2 (5.4) |
| MoCA | 24.7 (3.2) | 22.8 (3.7) |
| 4-m walk speed | 1.0 (0.2) | 0.9 (0.2) |
| N (%) | N (%) | |
| Gender | ||
| Female | 1343 (60.0) | 444 (78.9) |
| Male | 896 (40.0) | 119 (21.4) |
| Age group | ||
| 50–59 | 866 (38.7) | 276 (49.0) |
| 60–69 | 1023 (45.7) | 221 (39.3) |
| 70–79 | 276 (12.3) | 46 (8.2) |
| 80 and above | 74 (3.3) | 20 (3.6) |
Note: BMI = body mass index; MoCA = Montreal Cognitive Assessment.
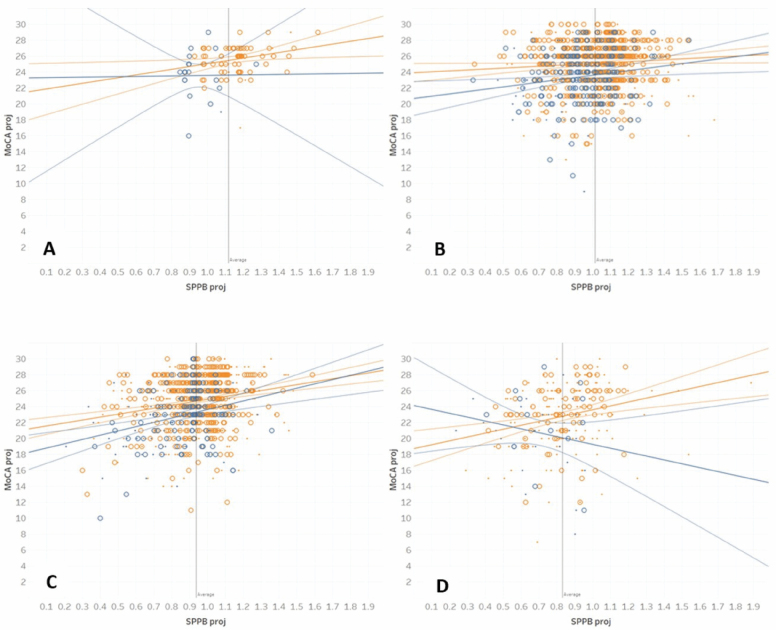
Figure 2A–D shows the scatterplots of predicted MoCA and 4-m walk (both standardized) by age group. For ease of interpretation, the aging categories are labeled 50s (<60 years old; minimum age in sample = 55.0), 60s (60–69), 70s (70–79), and 80s (80 and above). Regression lines for white and AA and 95% confidence limits are shown in orange (light) and blue (dark) colors, respectively. Body mass index, a potentially discriminating factor, is shown as small (BMI < 30) and large dots (BMI ≥ 30).
Figure 2.
Scatterplots of projected MoCA and SPPB 4-m gait speed for participants in the following age groups: (A) 50–59, (B) 60–69, (C) 70–79, and (D) 80 or above. Orange = White, Blue = African American; small dot = BMI < 30, large dot = BMI > =30. Straight lines indicate linear regression and curve lines 95% confidence limits.
The figures show important differentiation in the associations of cognitive and gait measures across whites and AAs. For whites, the positive correlation between cognitive and gait measures is maintained across the 4 age groups, with slightly increasing trends as people age. For AAs, there is a low correlation under the age of 60 (Figure 2A). At the age range 60–69, the correlation becomes positive, and it increases further for the age range 70–79 (Figure 2B and C). Figure 2D shows that this trend begins to reverse for the age range 80+, and the correlation turns negative.
Exploratory analysis using visualization thus suggested differentiation between AAs and whites in terms of association between cognition and gait. The result generated the following hypotheses about the association: (a) there is a general positive trend in association (with subtle differences exist across race and age groups); (b) there is a general increasing trend in the association as people age (subtle differences also exist across race); and (c) older AAs have a general decreasing association compared to white. These hypotheses were examined using a sequence of nested multilevel models represented by eqns (1)–(4).
Table 3 summarizes the results of the multilevel analysis. The association between cognition and gait is positive and significant (p < .001, Model 1) when no differentiation is made between subgroups represented by race, gender, age, and BMI. Age was also statistically significant (p < .001) when it was included into the model (Model 2). Model 3 shows that age, besides having a negative association of its own, also moderates gait. The positive coefficient of the Gait Age term indicates that older adults on average tend to show a higher correlation between cognition and gait measure.
Table 3.
Multilevel Analysis of Association Between Global Cognitive Function and 4-m Walk Gait Measure
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Gait (4-m walk) | 0.24*** | 0.20*** | 0.20*** | 0.14*** |
| Age | −0.13*** | −0.12*** | −0.16*** | |
| Gait × Age | 0.06*** | 0.074*** | ||
| Gait × Race | 0.028 | |||
| Gait × Age × Race | −0.10** | |||
| Gender | 0.10** | |||
| Race | −0.56*** | |||
| BMI | 0.03 |
Notes: Gait × Age represents the change in association between gait and cognition by age, and Gait × Age × Race represents the moderation effect of such change in association by race
**p < .01; ***p < .001.
We tested another intermediate model (full results not shown) that included gait (4-m walk), race, gender, and age into the model as well as all the second-order interaction terms of the latter 3 variables with gait. Race, gender, and age were significant. However, the second-order interaction between gait and gender and race were not significant. In the final model (Model 4), we included a third-order interaction term Gait × Age × Race and followed the statistical convention of also including all second-order interaction terms between age and race with gait. Model 4 shows that the age-related association between MoCA and gait is further moderated by race—AAs as a group tend to have a lower association as they age.
To validate the results in Model 4, we computed simple Pearson correlations between cognition and gait by race. Across the age groups of below 60, 60–69, 70–79, and 80 or above, the correlations were 0.27, 0.07, 0.24, and 0.26 for white, and 0.01, 0.15, 0.33, −0.09 for AA, respectively. The trends were distinct—white shows a curvilinear U shape while AA shows an inverted U shape. We note that while Model 4 offers evidence of differentiation between race, the model does not completely capture the 2 distinctly different U-shaped trends. Because of interpretability and estimation issues, we did not include quadratic terms in the final model.
For Model 4, ICC was 0.18, which implies a substantial level of within-study clustering effect.
Discussion
We applied IDA to derive a data set that combined data from 25 small to medium-sized studies and harmonized measures of cognitive function and gait speed among older adults. Compared to the analysis of a single data set, the IDA used a larger sample size and thus better statistical power to examine subgroup differences. In the current study, we delineated heterogeneity about the association between cognitive function and gait.
Our results show heterogeneity in the relationship between global cognitive function and gait speed by race and age once participants entered the seventh decade of their lives. Specifically, as shown by the regression lines in the figure, although there is a consistent (small) positive relationship between global cognitive function and gait speed for whites in the age range of 50–90, the pattern for AAs was markedly different: almost no correlation in their 50s, a positive correlation in their 60s that becomes more pronounced in their 70s, and then a negative correlation in their 80s. This trend among AAs may be attributed to the earlier appearance of poorer cognitive function (in their 60s) in conjunction with low physical function (as measured by gait speed). In other words, compared to whites, there is a higher proportion of AAs in their 60s and 70s that have poorer global cognitive function in combination with compromised gait speed, a pattern that results in the 2 constructs being directly related to one another. The result appears to be consistent with the observation that mobility limitations drop off more precipitously among older AA individuals compared to whites (22) and that differential changes in gait may precede further decline in global cognition function (23,24).
AAs exhibited a positive relationship between gait speed and global cognition in the age range of 70–79. However, this pattern fell apart and trended in a negative direction in the age range of 80–89. While a definitive explanation for this conundrum must await data from a longitudinal study design, a plausible explanation is a survivor effect for AAs in the age range of 80–89. Specifically, cumulative disadvantage theory postulates that a health gap exists between AA and whites due to an accumulation of negative health events and stress across the life span which increases health disparities particularly for AAs. This gap is further widened with increasing age (25). Thus, AAs who survived into their 80s were more resilient than AAs in the age range of 70–79.
There are limitations in the interpretation of these findings. First, the sample size for AAs in their 80s was the smallest of all age groups and may not be representative of the true population. It is important to keep in mind that all participants were community dwelling, ambulatory, and volunteered to participate in a research study. Although the studies were not population-based, all 25 included both white and AA participants (AA participation range 2%–37.5% of study sample). Second, there could be a sampling bias given that older, sicker, and minority individuals are less likely to participate in a research study (26). And third, although education was not included into the reported final model, we conducted additional analyses that included education. Our result showed that (a) education (1–5, 1 = no formal education, 2 = elementary school, 3 = high school, 4 = college, 5 = post graduate; treated as continuous) and race were strongly associated (chi-squared test p < .0001), and (b) when education was included into the model, the effect is 0.26 (p < .01) or approximately 1 point increase from the lowest to the highest education category. The Gait × Age × Race interaction term was no longer statistically significant (p = .14 vs p < .01); however, results for other factors remained unchanged.
In spite of these limitations, there are several noteworthy strengths. To our knowledge, we are the first group to harmonize cognitive and gait measures and to examine heterogeneity in the relationship between these constructs as a function of race, gender, and age. Using the novel and robust approach of IDA we have sufficient power to describe subgroup analyses and most notably our results found differences between AA’s and whites.
Conclusion
There is a growing need for research on the role of racial health disparities particularly in understanding differences in mobility and cognitive function with age. More studies including diverse racial groups with are needed which could help inform researchers in developing targets to preserving function and independence among older adults.
Supplementary Material
Acknowledgment
We acknowledge assistance from June Piece (data extraction), and Kimberly Kennedy (project management).
Funding
This work was (partially) supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center under the 2 grants from the National Institute of Aging at the National Institutes of Health (grant numbers P30-AG21332 and U24 AG059624), and the National Institutes of Health CTSI award (grant number 1UL1TR001420-01).
Conflict of Interest
None declared.
References
- 1. Demnitz N, Esser P, Dawes H, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture. 2016;50:164–174. doi: 10.1016/j.gaitpost.2016.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peel NM, Alapatt LJ, Jones LV, Hubbard RE. The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(6):943–948. doi: 10.1093/gerona/gly140 [DOI] [PubMed] [Google Scholar]
- 3. Integrative Data Analysis. National Cancer Institute. https://cancercontrol.cancer.gov/brp/priority-areas/big-data.html. Accessed September 19, 2020.. [Google Scholar]
- 4. Curran PJ, Hussong AM. Integrative data analysis: the simultaneous analysis of multiple data sets. Psychol Methods. 2009;14(2):81–100. doi: 10.1037/a0015914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychol Methods. 2009;14(2):150–164. doi: 10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bauer DJ, Hussong AM. Psychometric approaches for developing commensurate measures across independent studies: traditional and new models. Psychol Methods. 2009;14(2):101–125. doi: 10.1037/a0015583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maxwell SE. The persistence of underpowered studies in psychological research: causes, consequences, and remedies. Psychol Methods. 2004;9(2):147–163. doi: 10.1037/1082-989X.9.2.147 [DOI] [PubMed] [Google Scholar]
- 8. Maxwell SE, Lau MY, Howard GS. Is psychology suffering from a replication crisis? What does “failure to replicate” really mean? Am Psychol. 2015;70(6):487–498. doi: 10.1037/a0039400 [DOI] [PubMed] [Google Scholar]
- 9. Hansen WB, Chen SH, Saldana S, Ip EH. An algorithm for creating virtual controls using integrated and harmonized longitudinal data. Eval Health Prof. 2018;41(2):183–215. doi: 10.1177/0163278718772882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 12. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 13. Dong Y, Lee WY, Basri NA, et al. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr. 2012;24(11):1749–1755. doi: 10.1017/S1041610212001068 [DOI] [PubMed] [Google Scholar]
- 14. Bergeron D, Flynn K, Verret L, et al. Multicenter validation of an MMSE-MoCA conversion table. J Am Geriatr Soc. 2017;65(5):1067–1072. doi: 10.1111/jgs.14779 [DOI] [PubMed] [Google Scholar]
- 15. Sink KM, Craft S, Smith SC, et al. Montreal Cognitive Assessment and Modified Mini Mental State Examination in African Americans. J Aging Res. 2015;2015:872018. doi: 10.1155/2015/872018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolen MJ, Brennan RL. Test Equating, Scaling, and Linking Methods and Practices. Statistics for Social Science and Public Policy. 3rd ed. Springer; 2014. [Google Scholar]
- 17. Andersson B, Bränberg K, Wiberg M. Performing the kernel method of test equating with the package kequate. J Stat Softw. 2013;55(6):1–25. doi: 10.18637/jss.v055.i06 [DOI] [Google Scholar]
- 18. Guralnik JM, Simonsick EM, Ferrucci L, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 19. Lange-Maia BS, Newman AB, Strotmeyer ES, Harris TB, Caserotti P, Glynn NW. Performance on fast- and usual-paced 400-m walk tests in older adults: are they comparable? Aging Clin Exp Res. 2015;27(3):309–314. doi: 10.1007/s40520-014-0287-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deardorff A. Review of Tableau. Version. 9.1 ed. J Med Lib Assoc. 2016;104(2):182–183. [Google Scholar]
- 21. Milani SA, Marsiske M, Cottler LB, Chen X, Striley CW. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimers Dement (Amst). 2018;10:773–781. doi: 10.1016/j.dadm.2018.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vásquez E, Germain CM, Tang F, Lohman MC, Fortuna KL, Batsis JA. The role of ethnic and racial disparities in mobility and physical function in older adults. J Appl Gerontol. 2020;39(5):502–508. doi: 10.1177/0733464818780631 [DOI] [PubMed] [Google Scholar]
- 23. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dumurgier J, Artaud F, Touraine C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci. 2017;72(5):655–661. doi: 10.1093/gerona/glw110 [DOI] [PubMed] [Google Scholar]
- 25. Taylor M. Timing, accumulation, and the black/white disability gap in later life: A test of weathering. Research on Aging. 2008;30(2):226–50. doi: 10.1177/0164027507311838 [DOI] [Google Scholar]
- 26. Hughes TB, Varma VR, Pettigrew C, Albert MS. African Americans and clinical research: evidence concerning barriers and facilitators to participation and recruitment recommendations. Gerontologist. 2017;57(2):348–358. doi: 10.1093/geront/gnv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.