Abstract
Background
Weakness is a risk factor for physical limitations and death in older adults (OAs). We sought to determine whether OAs with clinically meaningful leg extensor weakness exhibit differences in voluntary inactivation (VIA) and measures of corticospinal excitability when compared to young adults (YAs) and OAs without clinically meaningful weakness. We also sought to estimate the relative contribution of indices of neural excitability and thigh lean mass in explaining the between-subject variability in OAs leg extensor strength.
Methods
In 66 OAs (75.1 ± 7.0 years) and 20 YAs (22.0 ± 1.9 years), we quantified leg extensor strength, thigh lean mass, VIA, and motor evoked potential (MEP) amplitude and silent period (SP) duration. OAs were classified into weakness groups based on previously established strength/body weight (BW) cut points (Weak, Modestly Weak, or Not Weak).
Results
The OAs had 63% less strength/BW when compared to YAs. Weak OAs exhibited higher levels of leg extensor VIA than Not Weak OAs (14.2 ± 7.5% vs 6.1 ± 7.5%). Weak OAs exhibited 24% longer SPs compared to Not Weak OAs, although this difference was insignificant (p = .06). The Weak OAs MEPs were half the amplitude of the Not Weak OAs. Regression analysis indicated that MEP amplitude, SP duration, and thigh lean mass explained ~62% of the variance in strength, with the neural excitability variables explaining ~33% of the variance and thigh lean mass explaining ~29%.
Conclusion
These findings suggest that neurotherapeutic interventions targeting excitability could be a viable approach to increase muscle strength in order to reduce the risk of physical impairments in late life.
Keywords: Dynapenia, Mobility, Muscle, Sarcopenia
Over 40% of older adults (OAs) in the United States have at least one physical limitation affecting daily tasks essential for independence (1). Preserving physical function drastically reduces health care costs and improves quality of life (2). Muscle weakness is a major risk factor for physical limitations, physical disability, and early death in OAs (3–5). Thus, understanding the causes of muscle weakness is essential in order to guide the development of targeted interventions to enhance strength and function.
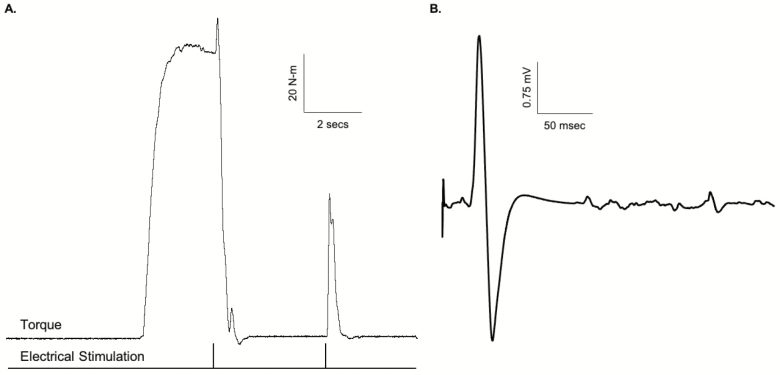
Age-related weakness has long been attributed, principally, to loss of muscle mass (ie, sarcopenia) (6). However, more recent findings clearly indicate that the loss of strength is only modestly associated with loss of mass in OAs (see (7–10) for review). The mechanisms of muscle strength, however, are multifaceted and determined by a combination of both neurological and muscular factors (see (9) for review). Degradation in nervous system function is one potential contributor. Voluntary contraction of a muscle comprises the recruitment of motor neurons, and hence muscle fibers, by increased descending drive. With an increased force of contraction, there is increased activation of neurons in the primary motor cortex resulting in increased firing of corticospinal neurons (11). The larger this descending drive, the greater the number of motor units recruited in the spinal cord, and the faster they fire. When a motor unit fires sufficiently fast, its muscle fibers produce a fused contraction. There are many influences on motor neurons during voluntary contractions that determine the timing and strength of voluntary contractions, such as excitatory and inhibitory sensory feedback, alterations in motor neuron properties that may make them more or less responsive to synaptic input, and descending drive from the motor cortex (12). The ability of the nervous system to fully activate muscle is commonly assessed using the interpolated twitch method or a derivative thereof (eg, interpolated doublet, central activation ratio) (13,14). Here, the motor nerve (or muscle) is electrically stimulated during a maximal voluntary effort and any increment in force evoked by a stimulus indicates an impairment, or deficit, in neural activation. That is, some motor units are not recruited or are not firing fast enough to produce fused contractions (15). The “added force” evoked by stimulation during contraction can be quantified by comparison to the force produced by the whole muscle when it is electrically stimulated (Figure 1A). Thus, it provides an index of the proportion of maximal possible force that is produced voluntarily. Numerous studies have used this technique, as well as others, to examine the effects of aging on neural activation capacity, with discrepant findings reported (see (16) for review). These discrepant findings are likely due to the muscle group investigated as well as the inherent heterogeneity of aging (17). For instance, we have reported data suggesting that impairments (or deficits) in neuromuscular activation are most pronounced in weaker OAs (18), which is consistent with reports in mobility- limited OAs (19). In this study, we sought to extend the prior work to examine the role of neuromuscular activation impairments in clinically meaningful, age-related muscle weakness. Additionally, we sought to examine the mechanistic role of corticospinal neural hypoexcitability in age-related weakness.
Figure 1.
(A) Example trace illustrating the calculation of voluntary inactivation (VIA). Here, VIA (%) = [Electrically evoked doublet torque during MVC/Electrically evoked doublet torque at rest following MVC] × 100. (B) Example trace of the evoked responses using transcranial magnetic stimulation (TMS). Note the motor evoked potential (MEP) followed by period of motor cortical quiescence or silent period (SP) after delivery of a supramaximal TMS pulse of 130% of active motor threshold to the motor cortex while the participant is performing a muscle contraction.
We, and others, have previously suggested that muscle weakness associated with aging, as well as a myriad of other disorders and conditions (eg, disuse, injury, and sepsis), may be due, in part, to neural hypoexcitability (20–22). Neural excitability can be defined, depending on the level of detail, as the readiness of a nerve cell or a neural circuit to respond to a stimulus (23–25). The response is typically in the form of an action potential, a transient change of electrical charge (polarization) of the neuronal membrane. The action potential can be measured either individually, at the level of an individual nerve cell, or as sum of action potentials in the form of a compound action potential (evoked potential), at the level of a group of neurons or neural circuits (23,24). In this study, we assessed corticospinal excitability using single-pulse transcranial magnetic stimulation (TMS). The amplitude of a motor evoked potential (MEP) elicited by a single suprathreshold TMS pulse to the motor cortex provides a composite index of excitability of the entire voluntary motor pathway because the size of the response depends upon both cortical and spinal excitability (14,26). Similarly, when evoked during a voluntary contraction, the MEP is followed by a silent period (SP), observed as a transient cessation of ongoing electromyographic (EMG) activity consistent with an interruption in volitional drive, and hence, withdrawal of descending input to the spinal motor neurons (27). While there is a theoretical basis for neural hypoexcitability serving as a key contributor to weakness (eg, a neuron with low excitability will, conceptually, have a lower maximal steady-state firing frequency (25), prior work has not linked indices of hypoexcitability to clinically meaningful, age-related weakness. This knowledge gap is a major barrier to pursuing the development of neurotherapeutic treatment strategies to enhance muscle strength and physical function in weak OAs.
Accordingly, in the present study, we first sought to determine whether, and to what extent, OAs with clinically meaningful leg extensor muscle weakness exhibit differences in voluntary (neural) inactivation (VIA) and measures of motor corticospinal hypoexcitability when compared to young adults (YAs) and OAs without clinically meaningful weakness. We operationally defined clinically meaningful levels of leg extensor muscle weakness based on isokinetic strength/body weight (BW) thresholds that have been shown to predispose nondisabled OAs to the future development of severe mobility limitations (3). We also sought, as a secondary goal, to estimate the relative contribution of indices of neural (corticospinal) excitability and upper thigh lean mass (assessed via dual-energy x-ray absorptiometry [DEXA]) in explaining the between-subject variability in isokinetic leg extension strength in community-dwelling OAs. A portion of these data have previously been published as a brief report (28).
Materials and Methods
General Overview of Study Design
A group of YAs and OAs participated in a lower extremity testing session in which the neuromuscular function of the nondominant leg was assessed. Maximal voluntary isokinetic and isometric leg extensor strength were initially measured. We then employed electrical stimulation techniques to measure VIA of the leg extensors, and single-pulse TMS to quantify active motor threshold (AMT), MEP amplitude, and SP duration during 5%, 20%, and 40% contractions. We compared YAs and OAs, and we classified the OAs as being “Not Weak,” “Modestly Weak,” or “Weak” based on previously published thresholds of clinically meaningful muscle strength that are predictive of the risk of OAs developing severe mobility limitations, outright disability, and mortality (3).
Study Participants
Twenty YAs (22.0 ± 1.9 years; 13 women and 7 men) and sixty-six OAs (75.1 ± 7.0 years; 45 women and 21 men) participated in the study. To be considered for the study, participants had to be either 18–25 years old or ≥60 years of age and have a body mass index between 18 and 40 kg/m2. Study participants had to be living independently, free of major musculoskeletal, neurological, cardiac, pulmonary, renal, psychiatric, and cognitive disease or disorders and able to perform traditional activities of daily living (eg, toileting, showering). All participants had to be willing to undergo DEXA scan, could not be taking medications that alter the primary TMS outcomes (29), and could not have metal implants or implanted electrical devices which would preclude them from participating in the TMS procedure (see Supplementary Table 1 for a complete list of inclusion and exclusion criteria). Participants were instructed to abstain from drinking caffeinated beverages for 4 hours prior and alcohol for 24 hours prior to the testing session. The Ohio University Institutional Review Board approved this study, and all study participants provided informed written consent.
To characterize the study participants, we measured: 6-minute walk gait speed (on a 30-m walkway with a left hand turn around a cone), short physical performance battery score (30), body composition (including estimates of appendicular and thigh lean mass) using DEXA (31), moderate-to-vigorous intensity physical activity via accelerometry (32), neuropsychological status via the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (33), comorbidities via the Charlson Comorbidity Index (34), depression via the Center for Epidemiological Studies-Depression (CES-D) scale (35), participant-reported knee pain and function via the Knee Injury and Osteoarthritis Outcome Score (KOOS) (36), and self-reported symptoms and other health conditions via the Neuro-QOL surveys (ie, fatigue, cognitive function, sleep disturbance, lower extremity function, upper extremity function, and satisfaction with social roles and activities).
Assessment of Leg Extensor Muscle Strength
Leg extension maximal voluntary isometric and isokinetic strength measures were recorded utilizing a Biodex System 4 Dynamometer (Biodex Medical Systems Inc., Shirley, NY). For quantification of isometric MVC strength measures, participants were seated with their nondominant knee at 90° flexion and the knee axis of rotation in alignment with the rotational axis of the Biodex torque motor. A lap belt was applied to prevent movement at the hip, and the participants’ nondominant lower extremities were affixed to a lower extremity lever arm, which was attached to the Biodex torque motor. We chose to control for leg dominance as there is evidence to suggest that there are slight differences in strength capacity between the dominant and nondominant limbs (37). We selected to test the nondominant limb because there is radiographic evidence suggestive of a higher incidence of knee osteoarthritis in the dominant leg (38). The torque signal was scaled to maximize its resolution (208.7 mV/Nm; Biodex Researchers Tool Kit Software) and sampled at 625 Hz (MP150 Biopac Systems). Participants received visual feedback of torque on a monitor located 1 m in front of them. Provided with strong verbal encouragement, participants performed three isometric Maximum voluntary contractions (MVCs) with 1 minute of rest between each effort, and the peak value of these three trials was utilized as the isometric MVC for the analysis.
For maximal voluntary isokinetic strength, the nondominant knee extension strength was measured concentrically at 60° per second in isokinetic mode. Six trials were performed with 30 seconds rest between bouts, and the maximum voluntary isokinetic strength was calculated as the mean of the highest three values of maximal isokinetic torque (Newton-meters, N-m) produced between 90° and 30° of knee flexion. This testing protocol is consistent with the isokinetic leg extension strength measurement protocol utilized by Manini et al. (2007) when deriving the sex-specific knee extension strength cut points for maintaining mobility (3). We also quantified isometric strength. Here participants were asked to perform five trials of a maximal isometric voluntary contraction with the knee positioned at 90°. During two of the trials, they received electrical stimulation (described below in the “Assessment of Leg Extensor Voluntary Inactivation” section). The highest isometric value was deemed their isometric strength.
Assessment of Leg Extensor Voluntary Inactivation
Voluntary inactivation (VIA) was assessed utilizing a doublet interpolation technique similar to our previous description (39). Here, we applied large (eg, 3 × 4 or 4 × 5 inch depending on the size of the quadriceps being tested) self-adhesive electrodes over the motor points of the rectus femoris and vastus medialis portions of the quadriceps muscles. While the participants were resting with the knee positioned at 90°, we applied single pulses of electrical stimuli (200-μs duration) at incrementally increasing current and constant voltage (400 V) until a plateau was reached in the evoked force output (DS7AH; Digitimer, Hertfordshire, United Kingdom). Next, study participants were asked to perform two 4- to 5-second isometric MVCs, and during these MVCs, a 100-Hz supramaximal doublet was delivered followed by a second doublet delivered to the resting muscle (Figure 1A). The increase in force immediately following the stimulation was expressed relative to a potentiated response evoked by the same doublet with the muscle at rest 1–2 seconds after the isometric MVC. Voluntary inactivation values were calculated as follows:
Here, a value of zero is indicative of complete muscle activation.
Transcranial Magnetic Stimulation
We obtained TMS data on 53 of the 66 OAs (age range: 63–92; mean age 76.1 ± 7.2 years; 32 women and 21 men), and on all 20 of the YAs. Study participants were given the opportunity to “opt in” or “opt out” of the brain stimulation and some of the OAs chose to “opt out” due to a variety of reasons (eg, uncomfortable with the concept of brain stimulation, discomfort associated with lower extremity brain stimulation). Transcranial magnetic stimulation is a noninvasive form of brain stimulation in which a rapidly changing magnetic field is introduced superficially and perpendicularly over the primary motor cortex in order to generate a skeletal muscle contraction in a directed region via electromagnetic induction. In this study, we utilized single-pulse TMS to assess corticospinal excitability by inducing MEPs and corticospinal silent SPs in the motor cortex contralateral to the nondominant leg extensor group.
During the single-pulse TMS, EMG was recorded from the vastus lateralis muscle of the nondominant leg using bipolar surface electrodes (8-mm diameter Ag/AgCl electrodes with a 35-mm interelectrode distance; Trace 1, Nikomed USA, Inc.) placed longitudinally along the distal end of the muscle over shaved and abraded skin. A reference electrode was placed over the dominant patella. The EMG signals were amplified 1,000×, band-pass filtered (10–500 Hz), and sampled at 5,000 Hz (MP150, Biopac Systems Inc., Goleta, CA). Single-pulse, monophasic waveform magnetic stimuli were delivered using a Magstim 2002 (The Magstim Co. Ltd, Whitland, United Kingdom) magnetic stimulator with a 110-mm double cone coil. Using a posterior-anterior orientation of the coil, the optimal point of stimulation was discovered for each participant by identifying the coil position that produced the greatest MEP amplitude consistently while the participants performed isometric leg extensions at 5% MVC. A small mark was placed on the participants’ scalps for reference for subsequent bouts of stimulation.
The AMT stimulation was then determined by finding the minimum stimulation intensity required to produce 4 of 8 MEPs with peak-to-peak amplitudes two times greater than the interference EMG signal observed while the participant performed an isometric leg extensor muscle contraction at 5% MVC (40,41). The AMT is thought to be indicative of the magnitude of voluntary motor drive to the corticomuscular pathway (42). Motor evoked potential amplitude and SP duration were quantified using single-pulse TMS with the stimulation intensity set to 130% AMT while the participant performed isometric leg extension contractions (~5-second duration) at 5%, 20%, and 40% MVC (six trials at each contraction intensity) (40). An example trace is illustrated in Figure 1B.
Statistical Analysis
We first describe the statistical analyses associated with the primary goal of the study, which was to determine whether, and to what extent, OAs with clinically meaningful leg extensor muscle weakness exhibit differences in VIA and measures of motor corticospinal excitability when compared to YAs and OAs without clinically meaningful weakness. Here, analysis of covariance (ANCOVA) procedures were used to examine differences between the YAs and OAs for the respective dependent variables when covarying for sex. In addition to the YA versus OA analysis described above, we also used ANCOVA procedures to examine whether there were group differences between OAs with varying levels of clinically meaningful muscle weakness (Weakness Group Analysis) when covarying for sex and age. Here, we classified the OAs as either being “Weak,” “Modestly Weak,” or “Not Weak” based on previously published work that describes cut points of maximal isokinetic (60°/s) knee extension relative strength that predispose nondisabled OAs to severe mobility limitations (3). Two sex-specific knee extension strength cut points were found, with high and low risk of severe mobility limitations corresponding to ≤1.12 Nm/kg (first decile) and ≥1.72 Nm/kg (sixth decile) in men and ≤1.00 Nm/kg (third decile) and ≥1.35 Nm/kg (seventh decile) in women, respectively. Moderate risk was defined as being between the low- and high-risk cut points. For the TMS-based data, contraction intensity (5%, 20%, and 40%) was added as a within-subject factor to the models. Sidak post hoc tests were used to follow-up on any observed significant main effects of interactions.
For the second goal of the study, we sought to estimate the relative contribution of indices of neural (corticospinal) excitability and upper thigh lean mass in explaining the between-subject variability in isokinetic leg extension strength in community-dwelling OAs. Here, we used blocked multiple regression analysis where absolute values for isokinetic leg extensor strength served as the dependent variable, and the neural excitability variables (MEP amplitude and SP duration during the highest contraction intensity [40% of maximal strength] task) were entered into the first block (model 1). The muscle variable of nondominant leg thigh lean mass was entered into the second block in addition to the neural excitability measures (model 2). From this analysis, the coefficient of determination (R2) for model 1 was calculated. This value represents the percentage of between-subject variance in strength that indices of neural excitability explain. The increment of R2 in model 2 was also determined and represented the variance explained due to thigh lean mass. To evaluate the independent contribution of each predictor, the semipartial r2 (sp-r2) values from model 2 were calculated. The sp-r2 value is interpreted as the percentage of variance in isokinetic strength (the dependent variable) uniquely attributable to the given independent variable by factoring out shared variance contributions with other predictors. We should note that we also entered age as well as sex into the model, but they were not significant (p = .62 and .24, respectively), and, as such, they were removed and not included in the final model. A preset α-level of significance ≤ 0.05 (two-sided) was required for significance. The SPSS statistical package (version 19.0 for Mac, Chicago, IL) was used for data analysis. Eta2 (η 2) effect sizes are presented to aid in interpretation. Data are presented as means ± SD in the text, and figures are presented as means ± SE to improve clarity.
Results
Older Adult Versus Young Adult Analysis
Strength and VIA
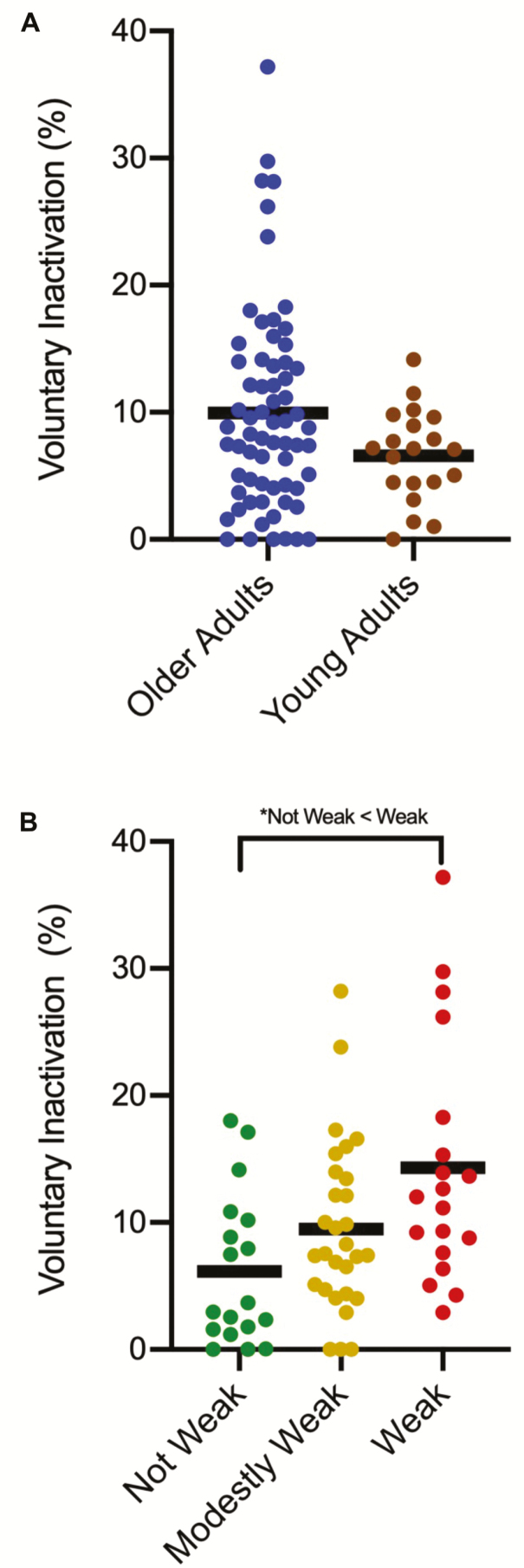
Descriptive statistics are illustrated in Table 1. For the dependent variable of isokinetic torque/BW, we observed a group main effect when adjusting for sex (p < .01; η 2 = 0.60). Here, the estimated marginal means (EMM) of the OAs were 63% weaker than the YAs (1.23 ± 0.41 N-m/kg vs 2.38 ± 0.40 N-m/kg). For the dependent variable of VIA, we did not observe an age group main effect when controlling for sex (p = .07, η 2 = 0.04) (YAs EMM: 6.54 ± 7.24% vs OAs EMM: 9.98 ± 7.23; Figure 2A).
Table 1.
Descriptive Characteristics of the Study Participants (means ± SD unless otherwise stated)
| Older vs. Younger Adult Analysis | Weakness Group Analysis | ||||
|---|---|---|---|---|---|
| Characteristic | Older Adults N = 66 | Young Adults N = 20 | Not Weak N = 18 | Modestly Weak N = 29 | Weak N = 19 |
| Age (y) | 75.1 ± 7.0 | 22.0 ± 1.9* | 72.0 ± 5.0 | 74.9 ± 7.3 | 78.4 ± 7.1‡ |
| Women (%) | 68.2 | 65 | 66.7 | 72.4 | 63.2 |
| Isokinetic torque/body weight (N-m/kg) | |||||
| Mean values when sex is not covaried for | 1.23 ± 0.42 | 2.39 ± 0.50* | 1.71 ± 0.32 | 1.22 ± 0.14|| | 0.78 ± 0.23‡,§ |
| Isokinetic torque/body weight (N-m/kg) | |||||
| Estimated marginal means when sex is covaried for | 1.23 ± 0.22 | 2.38 ± 0.73* | 1.71 ± 0.17 | 1.23 ± 0.14|| | 0.76 ± 0.17‡,§ |
| Isometric torque/body weight (N-m/kg) | |||||
| Mean values when sex is not covaried for | 1.13 ± 0.41 | 2.02 ± 0.56* | 1.58 ± 0.38 | 1.06 ± 0.24|| | 0.83 ± 0.28‡,§ |
| Isometric torque/body weight (N-m/kg) | |||||
| Estimated marginal means when sex is covaried for | 1.14 ± 0.37 | 2.00 ± 0.38* | 1.57 ± 0.21 | 1.09 ± 0.2|| | 0.81 ± 0.21‡,§ |
| Height (cm) | 164.1 ± 10.1 | 170.9 ± 9.0* | 165.7 ± 9.8 | 164.6±8.2 | 161.7 ± 12.7 |
| Weight (kg) | 72.5 ± 14.9 | 70.7 ± 12.9 | 65.2 ± 11.7 | 73.3 ± 15.6 | 78.3 ± 14.3‡ |
| BMI (kg/m2) | 26.9 ± 4.7 | 24.0 ± 3.0† | 23.7 ± 3.4 | 26.9 ± 4.3|| | 29.9 ± 4.6‡ |
| BMI ≥ 35 (%) | 4.5 | 0 | 0 | 3.4 | 10.5 |
| Appendicular lean mass (kg)/height2 | 6.6 ± 1.2 | 6.8 ± 2.0 | 6.6 ± 1.2 | 6.5 ± 1.1 | 6.9 ± 1.2 |
| Lean thigh mass (kg) | 4.7 ± 1.0 | 5.7 ± 1.4† | 4.7 ± 1.0 | 4.6 ± 1.1 | 4.8 ± 0.9 |
| SPPB score | 11.1 ± 1.3 | Not tested | 11.8 ± 0.5 | 11.2 ± 0.9|| | 10.2 ± 1.2‡ |
| Six-minute walk gait speed (m/s) | 1.3 ± 0.3 | Not tested | 1.6 ± 0.2 | 1.4 ± 0.2|| | 1.1 ± 0.3‡,§ |
| Accelerometry min/wk of moderate–vigorous activity | 107.1 ± 53.4 | 130.2 ± 118.4 | 132.4 ± 41.7 | 104.7 ± 56.0 | 88.1 ± 52.2‡ |
| RBANS Score | 106.7 ± 11.9 | 101 ± 10.6† | 107 ± 10.5 | 109.8 ± 12.6 | 101.9 ± 11.2 |
| Charlson Comorbidity Index (% 10-y survival) | 4.0 ± 1.0 | Not tested | 3.8 ± 0.8 | 4.0 ± 1.2 | 4.3 ± 0.7 |
| Center for Epidemiological Studies-Depression score | 7.0 ± 5.9 | 15.1 ± 3.3* | 6.4 ± 3.8 | 6.6 ± 5.9 | 8.0 ± 7.4 |
| Knee Injury and Osteoarthritis Outcome Score | 88.0 ± 14.4 | Not tested | 89.3 ± 12.0 | 91.7 ± 10.7 | 80.5 ± 19.2§ |
| Neuro-QOL: fatigue | 30.2 ± 10.4 | Not tested | 28.4 ± 7.5 | 28.0 ± 9.0 | 35.3 ± 13.5 |
| Neuro-QOL: cognitive function | 117.6 ± 16.3 | Not tested | 120.0 ± 12.9 | 115.2 ± 21.2 | 118.3 ± 10.5 |
| Neuro-QOL: sleep disturbance | 14.3 ± 4.3 | 13.7 ± 3.4 | 14.8 ± 3.9 | 13.1 ± 3.9 | 15.4 ± 5.0 |
| Neuro-QOL: lower extremity function | 87.6 ± 10.2 | Not tested | 92.1 ± 3.4 | 89.3 ± 7.5 | 79.9 ± 14.6‡,§ |
| Neuro-QOL: upper extremity function | 97.0 ± 7.8 | Not tested | 99.2 ± 1.2 | 98.0 ± 2.2 | 93.3 ± 13.6‡ |
| Neuro-QOL: satisfaction with social roles and activities | 36.6 ± 4.8 | Not tested | 37.1 ± 4.6 | 37.3 ± 4.2 | 35.2 ± 5.9 |
Notes: BMI = body mass index; Neuro-QOL = quality of life in neurological disorders; OAs = older adults; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; SPPB = short physical performance battery; YAs = young adults.
*YAs different from OAs (p ≤ .01).
†YAs different from OAs (p ≤ .05).
‡Weak different from Not Weak (p ≤ .05).
§Weak different from Modestly Weak (p ≤ .05).
||Modestly Weak different from Not Weak (p ≤ .05).
Figure 2.
(A) No differences between older adults (OAs) and young adults (YAs) were observed for voluntary inactivation (VIA) (p = .07). (B) The Weak OAs demonstrated significantly higher levels of leg extensor VIA than the Not Weak OAs (*p < .05).
TMS-based outcomes
For the dependent variable of AMT, we did not observe a group main effect when controlling for sex (p = .34, η 2 < 0.01) (YAs EMM: 39.0 ± 4.4 vs OAs EMM: 40.9 ± 11.9).
For the dependent variable of MEP amplitude, we did not observe a group × contraction intensity interaction when controlling for sex (p = .57, η 2 < 0.01). We did observe a group main effect (p = .04, η 2 = 0.06). Here, the OAs exhibited 33% larger MEPs in comparison to YAs when the data were averaged across contraction intensity (YAs EMM: 1.91 ± 1.34 mV vs OAs EMM: 2.66 ± 1.37 mV).
For the dependent variable of SP duration, we did not observe a group × contraction intensity interaction when controlling for sex (p = .46, η 2 = 0.01). We did observe a group main effect (p = .02, η 2 = 0.09). Here, the OAs exhibited 16% longer SPs when compared to the YAs when the data were collapsed across contraction intensity (YAs EMM: 123.1 ± 33.5 ms vs OAs EMM: 145.3 ± 33.9 ms).
Weakness Group Analysis
Physical function, strength, and VIA
Descriptive statistics are illustrated in Table 1. With regard to isokinetic strength/BW, there was a group main effect when controlling for sex (p < .01, η 2 = 0.81). The Weak OAs were 33% and 84% weaker (strength/BW) than the Modestly Weak and Not Weak OAs, respectively (Table 1). On average, the Weak OAs presented with significantly higher age, greater weight and body mass index, decreased mobility, function, and physical activity than their Modestly Weak and Not Weak peers (Table 1). The Modestly Weak OAs demonstrated significantly lower mobility, physical function, and lower extremity relative strength than the Not Weak OAs (Table 1).
With regard to leg extensor VIA, we observed a group main effect when controlling for sex (p < .01, η 2 = 0.15). Here, the Weak OAs demonstrated significantly higher levels of leg extensor VIA than their Not Weak counterparts (p < .01) (Not Weak EMM: 6.14 ± 7.51% vs Modestly Weak EMM: 9.53 ± 7.49% vs Weak EMM: 14.24 ± 7.50%; Figure 2B). This group main effect was still present when controlling for both sex and age (p = .01, η 2 = 0.14).
TMS-based outcomes
For the dependent variable of AMT, we did not observe a group main effect when controlling for sex (p = .90, η 2 < 0.01) (Not Weak EMM: 38.7 ± 7.3 vs Modestly Weak EMM: 39.5 ± 7.3 vs Weak EMM: 38.6 ± 7.2). This group main effect remained nonsignificant when we controlled for both sex and age (p = .45, η 2 < 0.01).
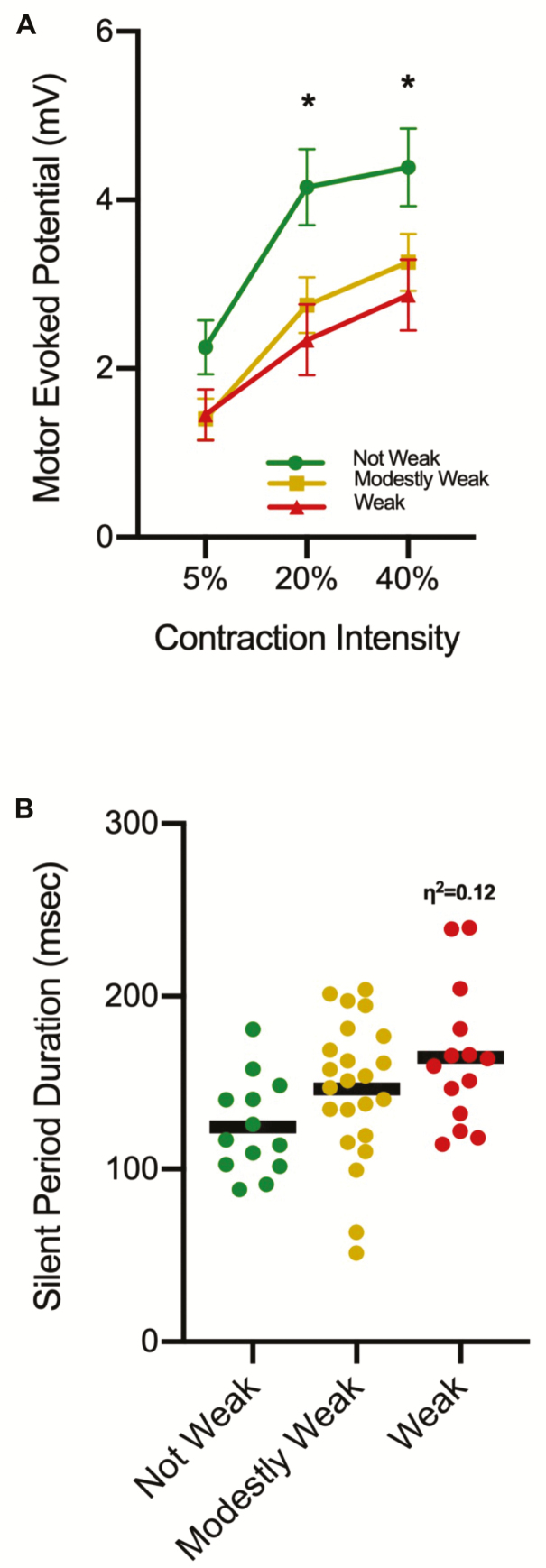
For the dependent variable of MEP amplitude, we observed a group × contraction intensity interaction when controlling for sex (p = .05, η 2 = 0.09) (Figure 3A). Follow-up testing indicating that there were no group differences at the 5% contraction intensity (p = .10) (Not Weak EMM: 2.25 ± 1.15 mV vs Modestly Weak EMM: 1.40 ± 1.18 mV vs Weak EMM: 1.45 ± 1.16). However, at the 20% and 40% contraction intensities, the Weak exhibited 42 and 55% smaller MEPs than the Not Weak OAs (p = .01 and .05, respectively) (20% contraction intensity: Not Weak EMM: 4.15 ± 1.62 mV vs Modestly Weak EMM: 2.75 ± 1.62 mV vs Weak EMM: 2.34 ± 1.63; 40% contraction intensity: Not Weak EMM: 4.39 ± 1.66 mV vs Modestly Weak EMM: 3.26 ± 1.67 mV vs Weak EMM: 2.87 ± 1.63). The Not Weak group exhibited larger MEPs at the 20% and 40% contraction intensity when compared to 5% (p < .01), but the 20% and 40% contraction intensities were not significantly different (p = .43) (5% EMM: 2.26 ± 1.77 mV vs 20% EMM: 4.23 ± 2.34 mV vs 40% EMM: 4.50 ± 2.06 mV). The Modestly Weak group exhibited a progressive increase between all contraction intensities (p < .01) (5% EMM: 1.39 ± 0.78 mV vs 20% EMM: 2.65 ± 1.27 mV vs 40% EMM: 3.12 ± 1.42 mV). The Weak group exhibited larger MEPs at the 20% and 40% contraction intensity when compared to 5% (p < .01), and the mean differences between the 20% and 40% contraction intensities was notable although it did not reach statistical significance (p = .06) (5% EMM: 1.46 ± 1.01 mV vs 20% EMM: 2.43 ± 1.21 mV vs 40% EMM: 2.99 ± 1.59 mV). When we adjusted for sex and age, neither the group × contraction intensity interaction (p = .35, η 2 = 0.05) or the group main effect were significant (p = .09, η 2 = 0.10).
Figure 3.
(A) A group × contraction intensity interaction was observed for motor evoked potential (MEP) amplitude. Subsequent analysis indicated the Weak older adults (OAs) had smaller MEPs in comparison to the Not Weak OAs at the 20% and 40% contraction intensities (*p < .05). Also, while not noted on the figure for the sake of clarity, the Not Weak OAs and the Weak OAs exhibited larger MEPs at the 20% and 40% contraction intensity when compared to 5% (p < .01), but the 20% and 40% contraction intensities were not significantly different. The Modestly Weak group exhibited a progressive increase between all contraction intensities (p < .01). (B) The Weak OAs had, on average, ~24% longer silent periods (SPs) than the Not Weak OAs when the data were averaged across contraction intensity. While this difference was not statistically significant (p = .06) a moderate effect size was observed and is noted (η 2 = 0.12).
For the dependent variable of SP duration, we did not observe a group × contraction intensity interaction when controlling for sex (p = .99, η 2 < 0.01). We also did not observe a group main effect (p = .06), although we did observe a moderate effect size (η 2 = 0.12) as the Weak OAs had 24% longer SPs than the Not Weak (Not Weak: 127.6 ± 36.8 ms vs Modestly Weak: 143.5 ± 37.1 ms vs Weak: 162.9 ± 36.7 ms; Figure 3B). Controlling for sex and age also resulted in a nonsignificant group × contraction intensity interaction (p = .99, η 2 < 0.01) as well as a group main effect (p = .22, η 2 < 0.07).
Relative Contribution of Neural Excitability and Lean Mass in Between-Subject Variance in Muscle Strength
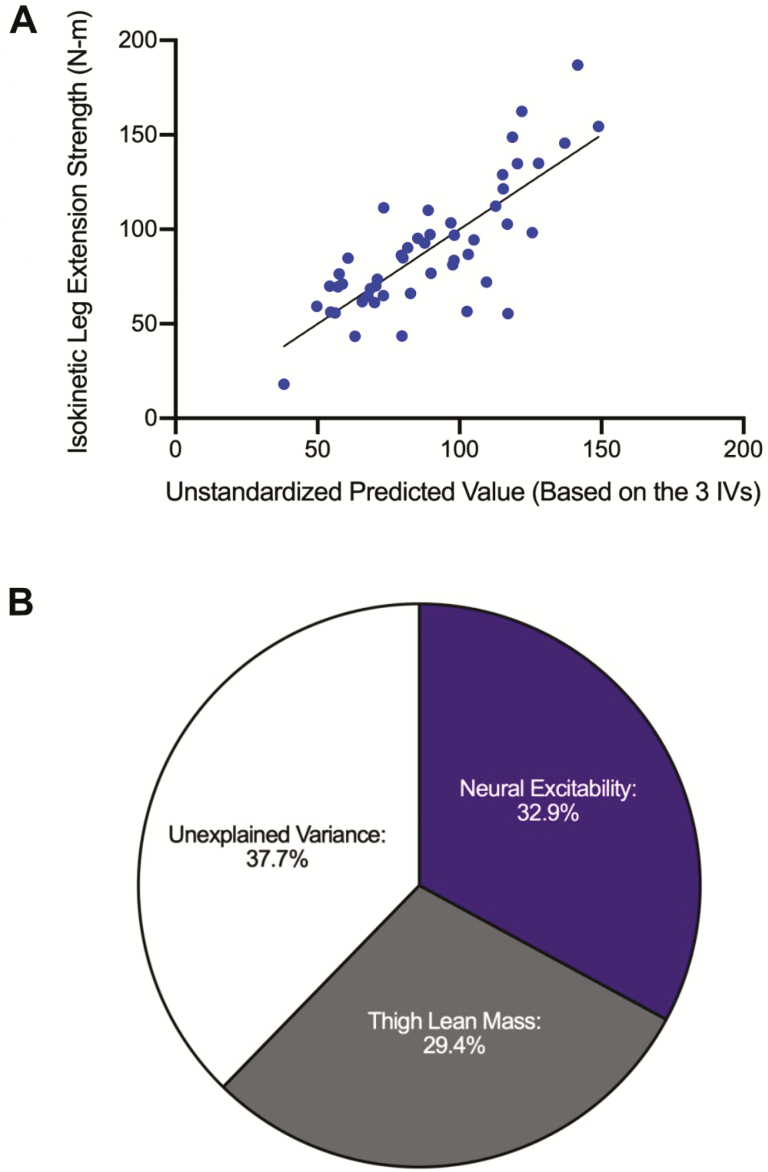
Collectively, the three predictor variables (MEP amplitude, SP duration, and thigh lean mass) in our multiple regression analysis explained ~62% of the between-subject variability in OA leg extensor isokinetic strength (R2 = 0.623, adjusted R2 = 0.598, p < .001). Figure 4A illustrates the multiple linear regression scatterplot comparing the unstandardized predicted values of the combined independent variables (neural excitability and thigh lean mass) and the dependent variable (isokinetic leg extension strength). Within this analysis, the neural excitability variables explained ~33% of the variation in strength (R2 = 0.329, p < .001), whereas thigh lean mass explained ~29% (R2 = 0.294, p < .001) (Figure 4B). To evaluate the contribution of each individual predictor variable, the sp-r2 values (a measure of the independent variance contribution that each predictor contributes to the model after factoring out shared covariance contributions with other predictors) indicated that MEP amplitude uniquely explained ~12% of the variance (sp-r2 = 0.123, p < .001), SP duration uniquely explained ~6% of the variance (sp-r2 = 0.060, p = .01), and thigh lean mass uniquely explained ~29% of the variance (sp-r2 = 0.293, p < .001). We should note that the association direction for MEP amplitude and thigh lean mass were positive (ie, greater values were predictive of greater strength), whereas for SP duration the direction was negative (ie, greater values were predictive of less strength).
Figure 4.
(A) Multiple linear regression scatterplot comparing the unstandardized predicted values of the combined independent variables (neural excitability and thigh lean mass) and the dependent variable (isokinetic leg extension strength). The R2 value (0.62) is significant (p < .001). (B) Relative contribution of the constructs of neural excitability (R2 = 0.329, p < .001) and thigh lean mass (R2 = 0.294, p < .001) from the multiple regression analysis.
Discussion
Weakness in OAs is conceptualized by many as a disorder of skeletal muscle. This work presents evidence for two key notable findings indicating that nervous system impairments are, in part, responsible for clinically meaningful, age-related muscle weakness. First, we observed that weak OAs have significant deficits in their nervous systems’ ability to fully activate their leg extensor muscles. Second, we noted that the relative contribution of indices of neural excitability explained ~33% of the between-subject variability in OAs leg extensor strength, which was roughly equal to the amount explained by thigh lean mass. These data suggest that treatments targeting the nervous system could enhance muscle strength, thereby mitigating future health risks in weak OAs. It should be noted that we did not observe overwhelming evidence for systematically compromised neural function in OAs per se (ie, in many instances there were no differences between YAs and OAs). However, what we did observe was solid evidence for compromised neural function in the weak OA phenotype, which is arguably the phenotype that, from a clinical care and treatment perspective, is of highest interest. We further discuss our findings below.
We found that weak OAs displayed significantly greater VIA in comparison to their stronger counterparts. While a number of investigations have compared muscle activation between YAs and OAs, with discrepant findings (see (16) for review), few studies have examined whether or not differences in muscle activation map to the phenotype of physically impaired OAs (eg, weak, frail, sarcopenic) (18,43,44). Our findings are in agreement with our prior work where we stratified OAs into tertiles based on their relative wrist flexor strength and compared VIA between stronger OAs and weaker OAs (18). Here, the weaker OAs exhibited greater VIA than their stronger counterparts. In our prior work, however, we were unable to determine whether the weaker OAs actually had clinically meaningful weakness as wrist flexion strength is not a common measure. Moreover, our prior work was not in a muscle group that has functional relevance to mobility, which limited the impact of these findings. We should note that the cut points used to classify the study participants into the weakness groups in the present study were based on isokinetic strength cut points developed to identify OAs at high and low risk of developing severe mobility limitations (defined as two consecutive reports of difficulty or inability to walk one-quarter of a mile or climb 10 steps). As such, this likely explains why, on average, the participants that were classified as “weak” were still relatively robust and exhibited reasonably preserved physical function and mobility. This nuance does raise the question of whether even greater degrees of neural impairments would be observed in frail OAs. Our findings are also consistent with Harridge and colleagues who reported that 11 very old adults requiring some form of assistance of with everyday activities, showed evidence of incomplete activation of the leg extensors during maximal voluntary efforts (43). Conversely, McPhee and colleagues (44) measured leg extensor voluntary activation in 40 OAs who they deemed as “sarcopenic” and observed average VIA levels of ~9% for women and 11% for men, which is similar to the mean values we observed when we averaged across all of our OA participants. There was no significant difference between YAs and OAs in their study, nor did VIA explain the between-subject variance in muscle strength. Close examination of this report, however, indicates that many of their OAs were not weak, as the men and women both, on average, exhibited ~10 kg (or ~50%) higher grip strength values than what is commonly deemed to be indicative of weakness (45). In a 5-year longitudinal follow-up of their OA participants, they observed that VIA increased and that this impairment was significantly associated with the between-subject variability in strength loss over time (44). Thus, our findings are in general, if not exact, agreement with their conclusions. We should also note that our “not weak” OAs and YAs both exhibited VIA measures, on average, of ~6%. This finding suggests that aging is not inherently associated with impairments in voluntary (neural) activation, which highlights the heterogeneity of aging and raises the possibility that reduced physical activity (as opposed to age per se) leads to impaired activation.
The association of our outcomes of reduced motor cortical excitability (eg, smaller MEP amplitudes and longer SP durations) and decreased relative strength is notable as it theoretically raises the possibility that increasing neuronal excitability in weak OAs could enhance interventions to increase muscle strength in this population. When TMS is applied to the motor cortex at an intensity above motor threshold, high-frequency indirect waves (I waves) are elicited in the corticospinal tract (46), which are modifiable by many mechanisms. These include neurotransmitters (ie, glutamate, GABA), modulators of neurotransmission (ie, acetylcholine, norepinephrine, and dopamine) (29), and interneurons contacted by corticospinal tract cells (47) with the actual efficacy of the corticomotoneuronal synapse itself demonstrating some activity-dependent changes (48). Ultimately, all of these factors function to influence the amplitude of the MEP. Thus, the amplitude of an MEP evoked by a single suprathreshold TMS pulse to the motor cortex provides a composite index of excitability of the entire voluntary motor pathway because the size of the response depends upon both cortical and spinal excitability (14,26). With increasing contraction intensities in the low-to-moderate force range (as performed herein), the MEP amplitude has been shown to increase (49,50). This increased response has been attributed to (i) enhanced excitability of cortical and spinal neurons through increased voluntary drive (both to and from the motor cortex), and (ii) consequent increased descending drive to recruit motor neurons in order to increase muscle activation (51,52). Similarly, when evoked during a voluntary contraction, the MEP is followed by a SP, observed as a transient cessation of ongoing EMG activity consistent with an interruption in volitional drive, and hence, withdrawal of descending input to the spinal motor neurons (27). There are several mechanisms thought to contribute to the SP, with spinal inhibitory mechanisms thought to be active in the early part and the latter part being specifically cortical in its origin and most likely mediated by GABAergic and dopaminergic cortical inhibitory mechanisms (53). Thus, MEP amplitude and SP duration both provide insight into corticospinal excitability and contain both shared and independent neurophysiologic cell and molecular mechanistic influences.
There are several notable findings in our data set with respect to these parameters. With respect to the MEP data, the weak OAs exhibited smaller MEPs than the nonweak seniors at the higher intensity contractions. With respect to the SP data, the OAs exhibited longer SPs in comparison to YAs. Also, there was a moderate effect size observed for the weak OAs to exhibit longer SPs in comparison to the nonweak OAs. We interpret these findings to suggest that muscle weakness in OAs is mechanistically due, in part, to motor neuronal hypoexcitability. Unfortunately, because single-pulse TMS responses are mediated at both the cortical and spinal levels, it is difficult to determine the site (eg, cortical vs spinal) at which differences in these parameters are effectuated. Our finding of larger MEP’s in OAs in comparison to YAs should not be viewed as evidence against this notion. Prior studies have also reported that OAs exhibited larger MEPs than YAs when performing muscle contractions (54,55). Moreover, work from Bernard and Seidler observed larger MEP amplitudes in OAs along with more spatially extensive motor cortical representations when compared to YAs, which was associated with longer reaction times (55). They interpreted these findings to suggest greater dedifferentiation (ie, decreased distinctiveness in motor cortical representations) in OAs. Thus, it is likely that our findings of greater MEP amplitudes in OAs is due to greater dedifferentiation, or perhaps, age-related changes in motor unit reorganization, as opposed to actual increases in motor cortical excitability per se.
Our multiple regression model estimating the relative contribution of the abovementioned indices of neural excitability (MEP amplitude and SP duration) and thigh lean mass in explaining the between-subject variability in OAs leg extensor strength is particularly insightful. Here, we observed that neural excitability explained ~33% of the between-subject variability in OAs leg extensor strength, which was roughly equal to the amount explained by thigh lean mass. While this analysis has limitations particularly as it relates to the estimated contribution of lean mass (see limitations paragraph below for further discussion), the salient point indicating that indices of neural excitability explain a large portion of the variance in strength observed in OAs should not be overlooked. The magnitude of this finding (~1/3rd of the variance explained) is sizeable as it suggests that neurotherapeutic interventions targeting excitability could be a viable approach to substantially increase muscle strength in weak OAs. Our model that included two measures of neural excitability and thigh lean mass explained ~62% of the between-subject variance in strength. This finding further highlights that the mechanisms of weakness are multifactorial, and it is likely that other measures of the same constructs (ie, excitability and mass), as well as measures reflective of other constructs (eg, muscle quality and the excitation-contraction coupling process; corticospinal form and function), would increase the percent of explained variance.
Our electrophysiological findings are consistent with prior reports from a myriad of other disorders and conditions associated with muscle weakness (18,20–22,41,56–60). For instance, using a cast-immobilization model to experimentally induce muscle weakness in young healthy adults, we observed that immobilization decreased strength, and increased VIA and SP duration, with significant associations between percent changes in the respective outcomes observed (20). Moreover, elegant studies by Rich and colleagues have demonstrated that reduced motor neuron excitability is an important contributor to weakness in a rat model of sepsis (21), and that administration of a serotonin agonist (lorcaserin) to septic rats greatly improved repetitive firing and motor unit force generation (57). Findings of this nature raise the following question: Could interventional strategies that increase corticospinal excitability enhance muscle strength and physical function in weak OAs? While no work has addressed this question, there is indeed evidence that interventions primarily designed to improve neurological function result in enhancements in muscle strength in OAs (61). Future work is needed to address this question.
There are several limitations of our work that should be noted. First, it is a cross-sectional design, and thus must be interpreted accordingly. Stated explicitly, cross-sectional study designs are appropriate for preliminary evaluations of association, but provide no information with regards to the influence of time (ie, within-subject aging) on the variables measured, and are thus generally less valid for examining cause-and-effect relationships (62). The reader is encouraged to interpret our findings within this context of the general limitation of cross-sectional study designs. Second, we should fully note that our weakness group classifications were based on isokinetic strength cut points developed to identify OAs at future risk of developing physical impairment, outright disability, and mortality (defined as two consecutive reports of notable difficulty or inability to walk one-quarter of a mile or climb 10 steps) (3). This criterion was ideal for our community-dwelling group who had higher 6-minute walk gait speeds than more compromised populations, but may not be appropriate for more geographically and racially diverse populations (63). Thus, it is plausible that lower-functioning and/or weaker OAs could show even higher levels of VIA. Third, we urge caution when interpreting our multiple regression results relating to the relative contribution of thigh lean mass to leg extensor strength. The spatial resolution of the DEXA-derived measures of thigh lean mass cannot be distilled down to the muscle group involved in the strength task (ie, quadriceps femoris). That is, our measure of thigh lean mass reflects not only the quadriceps muscle group, but also those of other muscles in the thigh region (eg, biceps femoris, sartorius, adductors). Additionally, DEXA-derived measures of thigh lean mass not only consist of skeletal muscle mass, but also includes other tissue components (eg, connective tissue). Thus, our findings related to the relative contribution of thigh lean mass to leg extensor strength should be interpreted cautiously.
Conclusion
While weakness in OAs is conceptualized by many as a disorder of skeletal muscle, in recent years it has become increasingly accepted that weakness in OAs is attributable, in part, to degradation in nervous system function. In this study we sought to (i) compare differences in VIA and measures of motor corticospinal excitability in OAs with clinically meaningful muscle weakness compared to YAs and stronger OAs; and (ii) estimate the relative contribution of indices of neural excitability and thigh lean mass in explaining the between-subject variability in OAs leg extensor strength. Herein, we present evidence for two key notable findings indicating that nervous system impairments are, in part, responsible for clinically meaningful, age-related muscle weakness. First, we observed that weak OAs have significant deficits in their nervous systems’ ability to fully activate their leg extensor muscles. Second, we noted that the relative contribution of indices of neural excitability explained ~33% of the between-subject variability in OAs leg extensor strength, which was roughly equal to the amount explained by thigh lean mass. Thus, while we did not observe overwhelming evidence for compromised neural function systematically in OAs per se, we did observe solid evidence for compromised neural function in the weak OA phenotype, which is arguably the phenotype that from a clinical care and treatment perspective is of highest interest. These data suggest that medical and/or behavioral interventions targeting the nervous system, in particular those increasing corticospinal excitability, could have potential for substantially enhancing muscle strength to prevent future health risks in weak OAs.
Funding
This work was supported, in part, by a grant from the National Institute of Health (NIH) (NIA R01AG044424 to B.C.C.).
Conflict of Interest
In the past 5 years, B.C.C. has received research funding from Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., and RTI Health Solutions for contracted studies that involved muscle-related research. In the past 5 years, B.C.C. has received consulting fees from Regeneron Pharmaceuticals, Abbott Laboratories, and the Gerson Lehrman Group for consultation specific to age-related muscle weakness. In the past 5 years, T.M.M. has received research funding from Regeneron Pharmaceuticals and Sanofi Pharmaceuticals for contracted studies that involved muscle-related research. T.M.M. also owns publicly traded stock in Abbott Laboratories and Amgen Inc. who both make muscle health-related products. Purchase of these stocks occurred prior to the beginning of the current research. All other authors declare no conflicts of interest, financial or otherwise.
Author Contributions
The authors contributed to at least one of the key contributions that justify authorship, including (i) conception and design of the study (B.C.C., D.W.R., and T.M.M.), (ii) acquisition and analysis of data (L.A.C., N.P.W., J.E.S., and B.C.C.), or (iii) drafting a significant portion of the manuscript or figures (L.A.C. and B.C.C.).
Supplementary Material
References
- 1. Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National Health And Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100:100–107. doi: 10.2105/AJPH.2008.157388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276:1473–1479. [PubMed] [Google Scholar]
- 3. Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x [DOI] [PubMed] [Google Scholar]
- 4. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 5. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- 6. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829 [DOI] [PubMed] [Google Scholar]
- 7. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manini TM, Gundermann DM, Clark BC. Aging of the muscles and joints. In: Halter JB, Ouslander JG, Studenski S, et al. , eds. Hazzard’s Geriatric Medicine and Gerontology. 7th ed. McGraw-Hill Medical; 2017:1715–1738. [Google Scholar]
- 9. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 11. Ashe J. Force and the motor cortex. Behav Brain Res. 1997;87:255–269. doi: 10.1016/s0166-4328(97)00752-3 [DOI] [PubMed] [Google Scholar]
- 12.Rekling, JC, Funk GD, Bayliss DA, Dong XW, and Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80(2):767–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090 [DOI] [PubMed] [Google Scholar]
- 14. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725 [DOI] [PubMed] [Google Scholar]
- 15. Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–199. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. doi: 10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark BC, Taylor JL, Hong SL, Law TD, Russ DW. Weaker seniors exhibit motor cortex hypoexcitability and impairments in voluntary activation. J Gerontol A Biol Sci Med Sci. 2015;70:1112–1119. doi: 10.1093/gerona/glv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121. doi: 10.1093/gerona/glq153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark BC, Mahato NK, Nakazawa M, Law TD, Thomas JS. The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J Neurophysiol. 2014;112:3219–3226. doi: 10.1152/jn.00386.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nardelli P, Vincent JA, Powers R, Cope TC, Rich MM. Reduced motor neuron excitability is an important contributor to weakness in a rat model of sepsis. Exp Neurol. 2016;282:1–8. doi: 10.1016/j.expneurol.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stefanelli L, Lockyer EJ, Collins BW, et al. Delayed-onset muscle soreness and topical analgesic alter corticospinal excitability of the biceps brachii. Med Sci Sports Exerc. 2019;51:2344–2356. doi: 10.1249/MSS.0000000000002055 [DOI] [PubMed] [Google Scholar]
- 23. Konstantinovic LM, Fliipovic SR. Effects of near-infrared low-level laser stimulation on neuronal excitability. In: Hamblin M, Huang, Y-Y, eds. Photobiomodulation in the Brain. Elsevier; 2019:233–240. [Google Scholar]
- 24. Kandel ER, Schwartz JH, Jessel TM.. Principles of Neural Science. 4th ed. New York: Mc-Graw Hill; 2000. [Google Scholar]
- 25. Schulz DJ, Baines RA, Hempel CM, Li L, Liss B, Misonou H. Cellular excitability and the regulation of functional neuronal identity: from gene expression to neuromodulation. J Neurosci. 2006;26:10362–10367. doi: 10.1523/JNEUROSCI.3194-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1 [DOI] [PubMed] [Google Scholar]
- 27. Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878 [DOI] [PubMed] [Google Scholar]
- 28. Clark BC, Manini TM, Wages NP, Simon JE, Clark LA. Voluntary vs electrically stimulated activation in age-related muscle weakness. JAMA Netw Open. 2019;2:e1912052. doi: 10.1001/jamanetworkopen.2019.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006 [DOI] [PubMed] [Google Scholar]
- 30. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 31. Tavoian D, Ampomah K, Amano S, Law TD, Clark BC. Changes in DXA-derived lean mass and MRI-derived cross-sectional area of the thigh are modestly associated. Sci Rep. 2019;9:10028. doi: 10.1038/s41598-019-46428-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corbett DB, Valiani V, Knaggs JD, Manini TM. Evaluating walking intensity with Hip-Worn accelerometers in elders. Med Sci Sports Exerc. 2016;48:2216–2221. doi: 10.1249/MSS.0000000000001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 34. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277 [DOI] [PubMed] [Google Scholar]
- 36. Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hunter SK, Thompson MW, Adams RD. Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. J Gerontol A Biol Sci Med Sci. 2000;55:B264–B273. doi: 10.1093/gerona/55.6.b264 [DOI] [PubMed] [Google Scholar]
- 38. Neame R, Zhang W, Deighton C, et al. Distribution of radiographic osteoarthritis between the right and left hands, hips, and knees. Arthritis Rheum. 2004;50:1487–1494. doi: 10.1002/art.20162 [DOI] [PubMed] [Google Scholar]
- 39. Russ DW, Clark BC, Krause J, Hagerman FC. Development of a neuromuscular electrical stimulation protocol for sprint training. Med Sci Sports Exerc. 2012;44:1810–1819. doi: 10.1249/MSS.0b013e31825423f1 [DOI] [PubMed] [Google Scholar]
- 40. Damron LA, Dearth DJ, Hoffman RL, Clark BC. Quantification of the corticospinal silent period evoked via transcranial magnetic stimulation. J Neurosci Methods. 2008;173:121–128. doi: 10.1016/j.jneumeth.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 41. Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol (1985). 2008;105:868–878. doi: 10.1152/japplphysiol.90530.2008 [DOI] [PubMed] [Google Scholar]
- 42. Tergau F, Wanschura V, Canelo M, et al. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Exp Brain Res. 1999;124:447–454. doi: 10.1007/s002210050640 [DOI] [PubMed] [Google Scholar]
- 43. Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. doi: [DOI] [PubMed] [Google Scholar]
- 44. McPhee JS, Cameron J, Maden-Wilkinson T, et al. The contributions of fiber atrophy, fiber loss, in situ specific force, and voluntary activation to weakness in sarcopenia. J Gerontol A Biol Sci Med Sci. 2018;73:1287–1294. doi: 10.1093/gerona/gly040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Lazzaro V, Oliviero A, Pilato F, et al. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009 [DOI] [PubMed] [Google Scholar]
- 47. Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. J Physiol. 1992;455:425–446. doi: 10.1113/jphysiol.1992.sp019309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521(Pt 3):749–759. doi: 10.1111/j.1469-7793.1999.00749.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gelli F, Del Santo F, Popa T, Mazzocchio R, Rossi A. Factors influencing the relation between corticospinal output and muscle force during voluntary contractions. Eur J Neurosci. 2007;25:3469–3475. doi: 10.1111/j.1460-9568.2007.05590.x [DOI] [PubMed] [Google Scholar]
- 50. Taylor JL, Allen GM, Butler JE, Gandevia SC. Effect of contraction strength on responses in biceps brachii and adductor pollicis to transcranial magnetic stimulation. Exp Brain Res. 1997;117:472–478. doi: 10.1007/s002210050243 [DOI] [PubMed] [Google Scholar]
- 51. McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol. 2011;589(Pt 14):3533–3544. doi: 10.1113/jphysiol.2011.207191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rothwell JC. The fatigued spinal cord. J Physiol. 2009;587(Pt 23):5517–5518. doi: 10.1113/jphysiol.2009.183475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziemann U, Netz J, Szelényi A, Hömberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v [DOI] [PubMed] [Google Scholar]
- 54. Baudry S, Penzer F, Duchateau J. Input-output characteristics of soleus homonymous Ia afferents and corticospinal pathways during upright standing differ between young and elderly adults. Acta Physiol (Oxf). 2014;210:667–677. doi: 10.1111/apha.12233 [DOI] [PubMed] [Google Scholar]
- 55. Bernard JA, Seidler RD. Evidence for motor cortex dedifferentiation in older adults. Neurobiol Aging. 2012;33:1890–1899. doi: 10.1016/j.neurobiolaging.2011.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clark BC, Taylor JL, Hoffman RL, Dearth DJ, Thomas JS. Cast immobilization increases long-interval intracortical inhibition. Muscle Nerve. 2010;42:363–372. doi: 10.1002/mus.21694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nardelli P, Powers R, Cope TC, Rich MM. Increasing motor neuron excitability to treat weakness in sepsis. Ann Neurol. 2017;82:961–971. doi: 10.1002/ana.25105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weier AT, Pearce AJ, Kidgell DJ. Strength training reduces intracortical inhibition. Acta Physiol (Oxf). 2012;206:109–119. doi: 10.1111/j.1748-1716.2012.02454.x [DOI] [PubMed] [Google Scholar]
- 59. Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee. 2014;21:736–742. doi: 10.1016/j.knee.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 60. Varray A, Alexandre F, Tremey E, Oliver N, Bourgouin D, Heraud N. Motor cortex hypoexcitability and hypoactivation in COPD patients with peripheral muscle weakness. Eur Resp J. 2016;48: OA4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang C, Ranganathan VK, Zhang J, Siemionow V, Yue GH. Motor effort training with low exercise intensity improves muscle strength and descending command in aging. Medicine (Baltimore). 2016;95:e3291. doi: 10.1097/MD.0000000000003291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caruana EJ, Roman M, Hernández-Sánchez J, Solli P. Longitudinal studies. J Thorac Dis. 2015;7:E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manini TM, Beavers DP, Pahor M, et al. Effect of physical activity on self-reported disability in older adults: results from the LIFE study. J Am Geriatr Soc. 2017;65:980–988. doi: 10.1111/jgs.14742 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.