Abstract
Background
Cough lasting 3–8 and >8 weeks are defined as subacute/prolonged cough and chronic cough (CC), respectively. Studies have revealed that CC negatively impact patients’ quality of life (QoL). In Japan, there is limited data on the impact of CC on health-related quality of life (HRQoL), work productivity and activity impairment (WPAI) and healthcare resource utilisation (HRU) using validated instruments. This study aimed to estimate the burden of CC and to compare the burden among patients with CC between subgroups.
Methods
Data from two cross-sectional online surveys conducted between September and November 2019 were combined for the analysis. Eligible patients with cough were propensity score matched to non-cough respondents. Comparisons of general HRQoL, WPAI, HRU and other symptoms experienced were conducted between matched non-cough respondents and patients with cough. Among patients with CC, subgroup comparisons were performed to understand general HRQoL, WPAI, HRU, cough-related QoL (Leicester Cough Questionnaire and Hull Airway Reflux Questionnaire) between patients with CC of different severities, patients with refractory CC and patients with non-refractory CC and patients with CC whose underlying diseases were unknown and others.
Results
Patients with CC (n=568) in Japan reported significantly poorer HRQoL, increased WPAI, more HRU and higher proportion of psychological and sleep problems, compared with matched non-cough respondents selected from 21 415 non-cough respondents. More patients with severe CC reported significantly poorer HRQoL, increased WPAI and worse cough-related QoL. Patients with refractory CC experienced significantly greater burden measured by cough-related QoL. No significant differences were observed between patients with CC whose underlying diseases were unknown and other patients with CC in terms of general HRQoL and cough-related QoL.
Conclusions
This study showed that patients with CC in Japan experienced significant burden compared with non-cough respondents. Patients with more severe cough and refractory CC experienced worse cough-related QoL. These results highlighted the unmet need for better interventions and treatments to reduce the burden among patients with CC.
Keywords: cough/mechanisms/pharmacology
Key messages.
To understand the disease burden of chronic cough, and the burden among subgroups of the patients with chronic cough in Japan.
There is an unmet need for better interventions and treatments to improve the quality of life, and reduce work productivity and activity impairment, healthcare resource utilisation and experience of anxiety, depression and sleep problems among patients with chronic cough in Japan.
This study provides novel evidence on the burden of chronic cough in Japan through a population-based survey, using validated instruments.
INTRODUCTION
Cough is one of the most commonly reported reasons for visit at outpatient clinics and hospitals in Japan1 2 and one of the most common reasons for seeking primary healthcare worldwide.3 Globally the prevalence of chronic cough (CC) has been estimated to be 9.6%, while a lower prevalence has been estimated in Asia, 4.4%,4 and in Japan, 2.2%.5 Both international and Japanese guidelines have defined CC as cough lasting >8 weeks, while cough lasting 3–8 weeks is defined as subacute/prolonged cough, and acute cough defined as lasting <3 weeks.6–8
Studies in the USA, Europe and Asia have shown that CC has a negative impact on patients’ quality of life (QoL). In the USA, CC was associated with both psychosocial and physical effects on QoL among 39 patients as measured by the Adverse Cough Outcome Survey and Sickness Impact Profile.9 In a European survey, 96% of patients reported that CC had a negative impact on QoL,10 while a Chinese study found that patients with CC had significantly poorer health-related quality of life (HRQoL) represented by lower scores of the Short Form-36 Health Survey compared with healthy volunteers.11 CC has also been associated with increased rates of depression.12
In Japan, one study revealed that patients with CC with cough variant asthma had impaired QoL as measured by the Leicester Cough Questionnaire (LCQ).13 In another Japanese survey from 2012, 65.6% of patients with CC reported being troubled by their cough in daily living.5 However, currently, there is scarcity of data on the impact of CC on the HRQoL of patients in Japan using validated instruments. Furthermore, there is also limited research assessing the impact of CC on patients’ work productivity and healthcare resource utilisation (HRU) in Japan.
Thus, the objective of this study was to estimate the burden of CC in Japan by comparing the HRQoL, work productivity and activity impairment (WPAI), HRU and experience of anxiety/depression/sleep problems among patients with CC and matched controls without subacute cough or CC (non-cough respondents). In addition, among patients with CC in Japan, the secondary objectives were to compare HRQoL, WPAI, HRU and cough-specific QoL in (i) patients with CC with different severity levels, (ii) patients with refractory CC versus patients with non-refractory CC and (iii) patients with CC whose underlying diseases were unknown versus all other patients with CC.
Methods
This is a population-based cross-sectional study comprising the 2019 Japan National Health and Wellness Survey (NHWS) and a CC survey. The Japan NHWS and CC survey were conducted between September and November 2019.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Study population
Potential respondents to the NHWS were recruited through an existing, general-purpose (ie, not healthcare-specific) web-based consumer panel. The consumer panel recruits its panel members through opt-in emails, co-registration with panel partners, e-newsletter campaigns, banner placements and affiliation networks. All panellists explicitly agree to be a panel member. A stratified random sampling procedure, with strata by sex and age, was implemented to ensure that the demographic composition of the NHWS sample is representative of the Japanese adult population.14 15
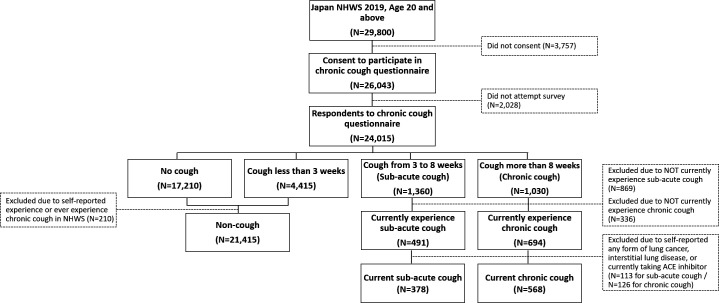
All respondents who completed the Japan NHWS 2019 were screened for participation in the CC survey if they were 20 years or older and self-reported coughing daily for at least 3 weeks at any time in the past 12 months. Respondents were excluded from further analyses if: (i) self-report in NHWS or the CC survey of any form of lung cancer, interstitial lung disease or were currently taking an ACE inhibitor or (ii) reported experiencing CC in the NHWS but not in the CC survey. Patients with lung cancer and interstitial lung disease were excluded as this study focused on patients where cough may persist despite comprehensive diagnostic process and treatment of underlying conditions, and due to the expected substantial impairment on their QoL due to the severe diseases irrespective of coughing.
Based on responses to the CC survey, patients with subacute cough and patients with CC were defined as respondents self-reporting currently coughing for 3–8 weeks and for >8 weeks at the time of survey, respectively. Respondents who did not self-report coughing for at least 3 weeks at any time in the past 12 months were classified as non-cough respondents. This group included respondents having no cough and coughing for <3 weeks in the past 12 months.
Among patients with CC, cough severity over the past 2 weeks were assessed by a visual analogue scale (VAS) ranging from 0 mm (no cough) to 100 mm (extremely severe cough). Patients with CC were categorised into two severity levels—VAS ≤40 mm and VAS >40 mm.16
Among patients with CC who have been told by a physician of any conditions or behaviours that were related to CC, if the medication specific to the underlying conditions did not treat CC adequately (not answered ‘a great deal’ to question “How well did each medication help to treat your CC”), they were defined as patients with refractory CC.
On the other hand, patients with CC who reported that they had not been informed by a physician of any of the conditions or behaviours were related to CC or reported ‘do not know’, despite having seen healthcare professionals (HCPs) since experiencing CC and having spoken with a physician for CC, were identified as ‘patients with CC whose underlying diseases were unknown’.
Covariates and outcomes collected from the NHWS
Demographic characteristics included age, gender and employment status of all respondents. General health characteristics included body mass index (BMI), smoking status, alcohol use, exercise behaviour and Charlson Comorbidity Index (CCI). CCI weights the presence and seriousness of chronic comorbid diseases. An adapted version of the CCI was created excluding chronic pulmonary disease from the index score. Higher index indicates greater comorbid burden.17–19
HRQoL was assessed by 12-Item Short Form Survey Instrument (SF-12v2), a multipurpose, generic HRQoL instrument comprising 12 questions.20 The instrument reports eight health concepts (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) and two summary scores: physical component summary (PCS) and mental component summary (MCS). Summary scores were calculated using a norm-based scoring algorithm and higher scores indicate better QoL.
Health state utilities were generated from SF-12v2 through application of the SF-6D algorithm.21 The SF-6D is a preference-based single index measure for health using general population values and yields summary scores on a theoretical 0–1 scale. Higher scores indicate better QoL.
EuroQol- 5 Dimension (EQ-5D) index was derived from the EQ-5D-5L instrument, which is a standardised measure of health status to provide a simple, generic measure of health.22 Japanese tariff was used for the scoring and higher scores indicate better health status.23
The WPAI questionnaire was used to measure the impact of health on employment-related activities.24 This six-item validated instrument consists of four metrics: absenteeism (the percentage of work time missed because of one’s health in the past 7 days), presenteeism (the percentage of impairment experienced because of one’s health while at work in the past 7 days), total work productivity loss (an overall impairment estimate that is a combination of absenteeism and presenteeism) and activity impairment (the percentage of impairment in daily activities because of one’s health in the past 7 days).24 These four subscales are generated in the form of percentages, with higher percentage values indicate greater impairment. Only respondents who reported being full-time, part-time or self-employed provided data for absenteeism, presenteeism and total work impairment. All respondents provided data for activity impairment.
HRU was quantified by the total number of visits to HCPs (including general practitioner, nurse practitioner/physician assistant, other traditional healthcare provider visits) and emergency rooms (ERs) in the past 6 months.
Experience of other symptoms including experience of anxiety, depression and sleep problems in the past 12 months were self-reported by the respondents.
Covariates and outcomes collected from chronic cough questionnaire
Underlying diseases related to CC, medication usage and satisfaction with the treatment, as well as cough severity over the past 2 weeks were self-reported by patients with CC. Patients with CC also self-reported if they have seen any HCP or if they have spoken with a physician for their CC.
Cough-related QoL was measured by LCQ and Hull Airway Reflux Questionnaire (HARQ).
The LCQ25 is one of the most widely used cough-specific HRQoL questionnaires. LCQ is a 19-item, self-administered questionnaire that comprises three main health domains/subscales related to HRQoL measures of CC: physical, psychological and social. All items are scored on a 7-point Likert response scale (1=‘all of the time’ to 7=‘none of the time’). The LCQ is well-validated and has good internal reliability, repeatability and responsiveness.25 The Japanese language version of the LCQ has been validated previously.13 The minimal important difference for the LCQ is a total score of 1.3, and the total score has a range of 3–21 points.26 The minimal important difference for each subscale was 0.20 for physical, 0.20 for social and 0.80 for psychological.26 Each subscale has a range between 1 and 7 points.
The HARQ27 is a 14-item, self-administered instrument that measures symptom severity related to cough hypersensitivity. All items are scored on a 0–5 scale (0=‘no problems’ to 5=‘severe/frequent problems’), with the total score ranging from 0 to 70). The average score is 4 among normal people and the upper limit of normal is 13.27 28
Statistical analysis
Demographic and general health characteristics were reported using counts and percentages for categorical variables and means and standard deviations (SDs) for continuous variables. Bivariate comparison between non-cough respondents and patients with CC were performed using χ2 tests for categorical variables and one-way analysis of variance (ANOVA) for continuous variables, for demographic and general health characteristics, to understand the baseline differences between the two groups.
Propensity score matching was conducted to address differences in patient characteristics as well as unequal sample sizes between the non-cough respondents and patients living with CC or subacute cough. Patients with CC or subacute cough were 1:4 propensity score matched to non-cough respondents. Demographic and health characteristic that were different at bivariate level with p<0.25 and those with theoretical significance were used for propensity score matching. Age, gender, employment status, household income, adapted CCI, BMI, smoking status and use of alcohol were used in the matching. Postmatching bivariate analyses were conducted to assess the balance of matching. Comparisons of general HRQoL, WPAI, HRU and other symptoms experienced were conducted between matched non-cough respondents versus patients with CC, and between matched non-cough respondents versus patients with subacute cough, respectively.
Among patients with CC, subgroup comparisons were performed for comparison of general HRQoL, WPAI, HRU and cough-related QoL (LCQ and HARQ) between patients with CC with VAS ≤40 mm versus VAS >40 mm, and between patients with non-refractory CC versus patients with refractory CC, using generalised linear models (GLMs). GLMs specifying normal, negative binomial and binomial distributions and identity, log and logit link functions were used for predicting normally distributed outcomes (eg, SF-12v2 metrics), discrete, count-like outcomes with skewed distribution (eg, WPAI and HRU) and binary outcomes (eg, anxiety), respectively.
Health outcomes of patients with CC whose underlying diseases were unknown and all other patients with CC were compared using χ2 tests and ANOVA. GLMs were not conducted for this subgroup comparison due to the exploratory nature of this comparison.
Additionally, the correlation between cough-related QoL (LCQ and HARQ) and severity (VAS) among patients with CC was assessed by Pearson’s correlation.
All statistical analyses were performed using IBM SPSS Statistics V.2229 or R V.3.4.4.30 No formal sample size calculation was conducted given the fixed number of respondents from the annual NHWS survey. No correction for multiple testing was conducted as no formal hypothesis testing was planned for this study. P values were provided as a measure of group differences and p values <0.05 were considered to be statistically significant.
Results
Participants
Among the 29 800 adult respondents to the NHWS 2019 who were 20 years and older, 26 043 adults provided informed consent to participate in the CC survey, whereof 24 015 attempted at least one question. Out of the total 24 015 respondents to the CC survey, 21 415 were classified as non-cough respondents, 568 reported currently suffering from CC and 378 from subacute cough (figure 1).
Figure 1.
Respondent flow chart. NHWS, National Health and Wellness Survey.
Among patients with CC, 312 reported VAS ≤40 mm and 255 reported VAS >40 mm, 91 were categorised as patients with refractory CC and 54 were patients with CC whose underlying diseases were unknown.
Demographic and health characteristics in non-cough respondents versus patients with CC
Bivariate comparisons of non-cough respondents and patients with CC showed that patients with CC were significantly older (56.01 vs 52.48, p<0.001), more often male (61.1% vs 54.6%), currently smoking (29.4% vs 18.6%) and had significantly higher CCI (0.33 vs 0.14) and adapted CCI (0.23 vs 0.13) than non-cough respondents (table 1).
Table 1.
Demographics and health characteristics ofpatients with chronic cough, non-cough prematching and matched non-cough (1:4) respondents
| Patients with CC (n=568) |
No cough (n=21 415) |
Matched non-cough (n=2272) |
||||||
| Mean | SD | Mean | SD | No cough vs CC p value |
Mean | SD | Matched non-cough vs CC p value |
|
| Age | 56.01 | 15.15 | 52.48 | 16.37 | <0.001 | 56.18 | 15.02 | 0.802 |
| Charlson Comorbidity Index | 0.33 | 0.67 | 0.14 | 0.45 | <0.001 | 0.23 | 0.66 | 0.003 |
| Adapted Charlson Comorbidity Index | 0.23 | 0.55 | 0.13 | 0.43 | <0.001 | 0.22 | 0.64 | 0.810 |
| Gender | % | Count | % | Count | P value | % | Count | P value |
| Male | 61.1% | 347 | 54.6% | 11 698 | 0.002 | 65.0% | 1477 | 0.082 |
| Female | 38.9% | 221 | 45.4% | 9717 | 35.0% | 795 | ||
| Currently employed | ||||||||
| No | 44.4% | 252 | 41.6% | 8915 | 0.192 | 43.2% | 981 | 0.609 |
| Yes | 55.6% | 316 | 58.4% | 12 500 | 56.8% | 1291 | ||
| BMI category | ||||||||
| Underweight (BMI <18.5) | 9.5% | 54 | 11.2% | 2404 | 0.003 | 7.7% | 174 | 0.163 |
| Normal weight (18.5≤BMI<23) | 65.7% | 373 | 66.2% | 14 183 | 70.7% | 1606 | ||
| Overweight (23≤BMI<25) | 16.5% | 94 | 15.5% | 3318 | 15.2% | 346 | ||
| Obese (BMI ≥25) | 5.5% | 31 | 2.9% | 626 | 4.0% | 92 | ||
| Decline to answer | 2.8% | 16 | 4.1% | 884 | 2.4% | 54 | ||
| Smoking status | ||||||||
| Never | 47.0% | 267 | 57.8% | 12 385 | <0.001 | 45.8% | 1040 | 0.862 |
| Former | 23.6% | 134 | 23.5% | 5040 | 24.4% | 554 | ||
| Current | 29.4% | 167 | 18.6% | 3990 | 29.8% | 678 | ||
| Use of alcohol | ||||||||
| Abstain | 33.6% | 191 | 36.2% | 7750 | 0.209 | 30.4% | 690 | 0.133 |
| Currently consume alcohol | 66.4% | 377 | 63.8% | 13 665 | 69.6% | 1582 | ||
| Vigorous exercise in past 30 days | ||||||||
| No | 53.7% | 305 | 53.2% | 11 394 | 0.817 | 51.0% | 1159 | 0.252 |
| Yes | 46.3% | 263 | 46.8% | 10 021 | 49.0% | 1113 | ||
BMI, body mass index; CC, chronic cough.
After matching, no significant differences were found between matched non-cough respondents (n=2272) and patients with CC (n=568) for all baseline characteristics, except for CCI which was not included as a covariate in the propensity score matching (table 1).
Among all patients with CC, allergic rhinitis, asthma and cough variant asthma were the most common underlying diseases told by the physicians, but more than half of the patients were not certain about the underlying causes by answering ‘do not know’ or ‘none’.
Outcomes assessment in matched non-cough respondents versus patients with CC
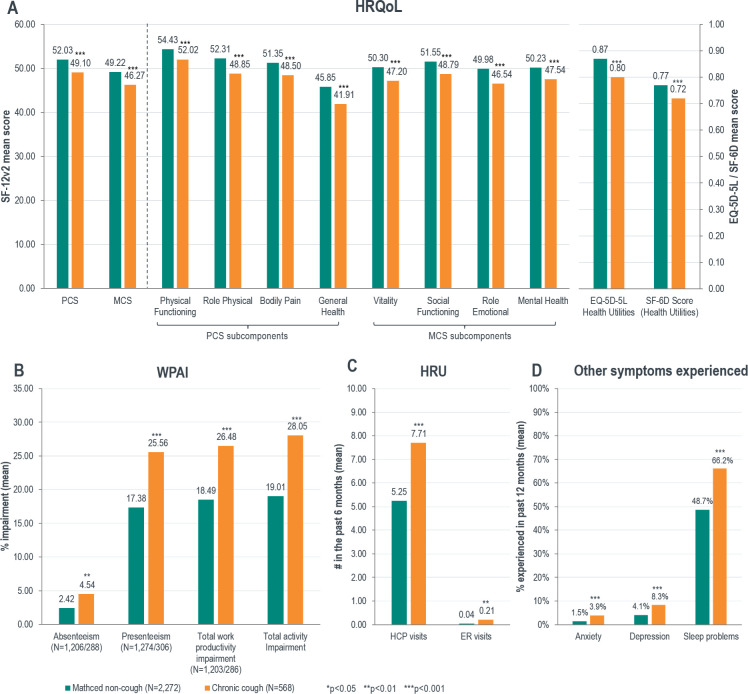
After propensity score matching, patients with CC (n=568) reported significantly poorer HRQoL relative to matched non-cough respondents (n=2272), in terms of PCS (49.10 vs 52.03, p<0.001), MCS (46.27 vs 49.22, p<0.001), SF-6D (0.72 vs 0.77, p<0.001) and EQ-5D-5L (0.80 vs 0.87, p<0.001) health utilities scores and all subcomponents of the SF-12v2 (figure 2A).
Figure 2.
Comparison of health-related quality of life (HRQoL), work productivity and activity impairment (WPAI), healthcare resource utilisation (HRU) and other symptoms experienced in matched non-cough respondents and patients with chronic cough (CC). ER, emergency room; HCP, healthcare professional; MCS, mental component summary; PCS, physical component summary; SF-12v2, 12-Item Short Form Survey Instrument.
Patients with CC reported significantly increased absenteeism (4.54 vs 2.42, p=0.004), presenteeism (25.56 vs 17.38, p<0.001), total work productivity impairment (26.48 vs 18.49, p<0.001) and total activity impairment (28.05 vs 19.01, p<0.001), compared with matched non-cough respondents (figure 2B).
Compared with matched non-cough respondents, patients with CC had significantly more visits to the HCP (7.71 vs 5.25, p<0.001) and the ER (0.21 vs 0.04, p=0.009) (figure 2C) and reported more anxiety (3.9% vs 1.5%, p<0.001), depressive symptoms (8.3% vs 4.1%, p<0.001) and sleep problems and symptoms (66.2% vs 48.7%, p<0.001) (figure 2D).
Outcomes assessment in matched non-cough respondents versus patients with subacute cough
Similar to patients with CC versus matched non-cough controls, patients with subacute cough (n=378) reported significantly poorer HRQoL (all measures except for physical functioning, p<0.001), significantly greater impairment in WPAI (all measures, p<0.001), more visits to the HCP (7.47 vs 4.57, p<0.001) and more anxiety (5.3% vs 1.7%, p<0.001), and sleep problems and symptoms (65.1% vs 46.2%, p<0.001) than matched non-cough respondents (n=1512). No differences were observed in self-reported depression (online supplemental table 1).
bmjresp-2020-000764supp001.pdf (50KB, pdf)
Outcomes assessment in patients with CC with VAS ≤40 mm versus with VAS >40mm
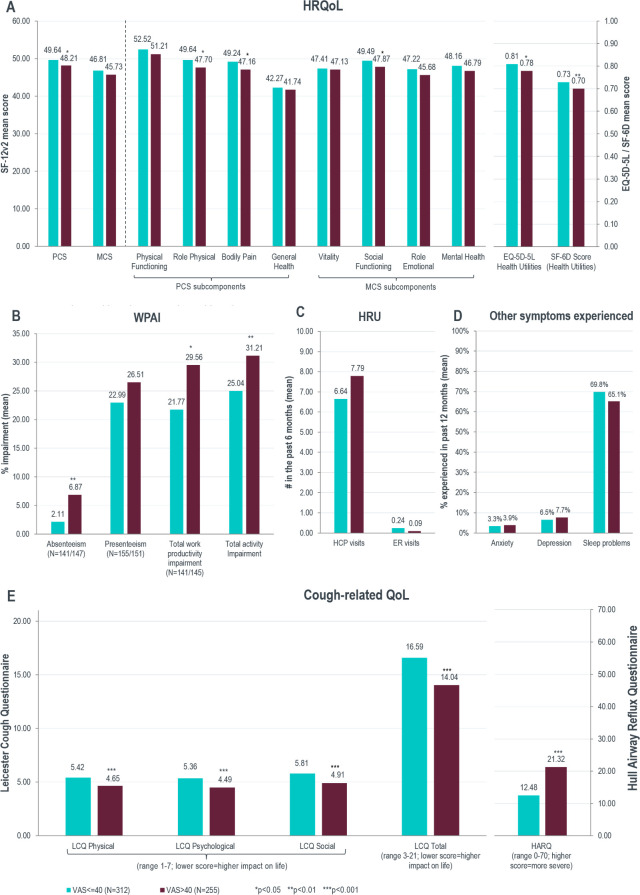
Patients with CC with VAS >40 mm reported significantly poorer HRQoL relative to patients with CC with VAS ≤40 mm, in terms of PCS (48.21 vs 49.64, p=0.037), SF-6D (0.70 vs 0.73, p=0.009) and EQ-5D-5L (0.78 vs 0.81, p=0.041) health utilities scores, and some subcomponents of the SF-12v2 (figure 3A).
Figure 3.
Comparison of health-related quality of life (HRQoL), work productivity and activity impairment (WPAI), healthcare resource utilisation (HRU), other symptoms experienced and cough-related quality of life (QoL) in patients with chronic cough (CC) with visual analogue scale (VAS) ≤40 mm versus with VAS >40 mm. ER, emergency room; HARQ, Hull Airway Reflux Questionnaire; HCP, healthcare professional; LCQ, Leicester Cough Questionnaire; MCS, mental component summary; PCS, physical component summary; SF-12v2, 12-Item Short Form Survey Instrument.
Patients with CC with VAS >40 mm reported significantly increased absenteeism (6.87 vs 2.11, p=0.002), total work productivity impairment (29.56 vs 21.77, p=0.020) and total activity impairment (31.21 vs 25.04, p=0.009), compared with patients with CC with VAS ≤40 mm, but no significant difference was observed in presenteeism (figure 3B). Nor were any significant differences observed in HRU (figure 3C), anxiety, depression and sleep problems (figure 3D).
Compared with patients with CC with VAS ≤40 mm, patients with CC with VAS >40 mm reported significantly greater burden as measured by the LCQ total score (14.04 vs 16.59, p<0.001) and the HARQ score (21.32 vs 12.48, p<0.001) (figure 3E). The correlation between cough-related QoL and cough severity was further analysed among patients with CC. All LCQ subscores and total LCQ score were negatively correlated with cough severity measured by VAS (p<0.001). HARQ score was positively correlated with cough severity (p<0.001) (online supplemental table 2).
Outcomes assessment in patients with refractory CC versus patients with non-refractory CC
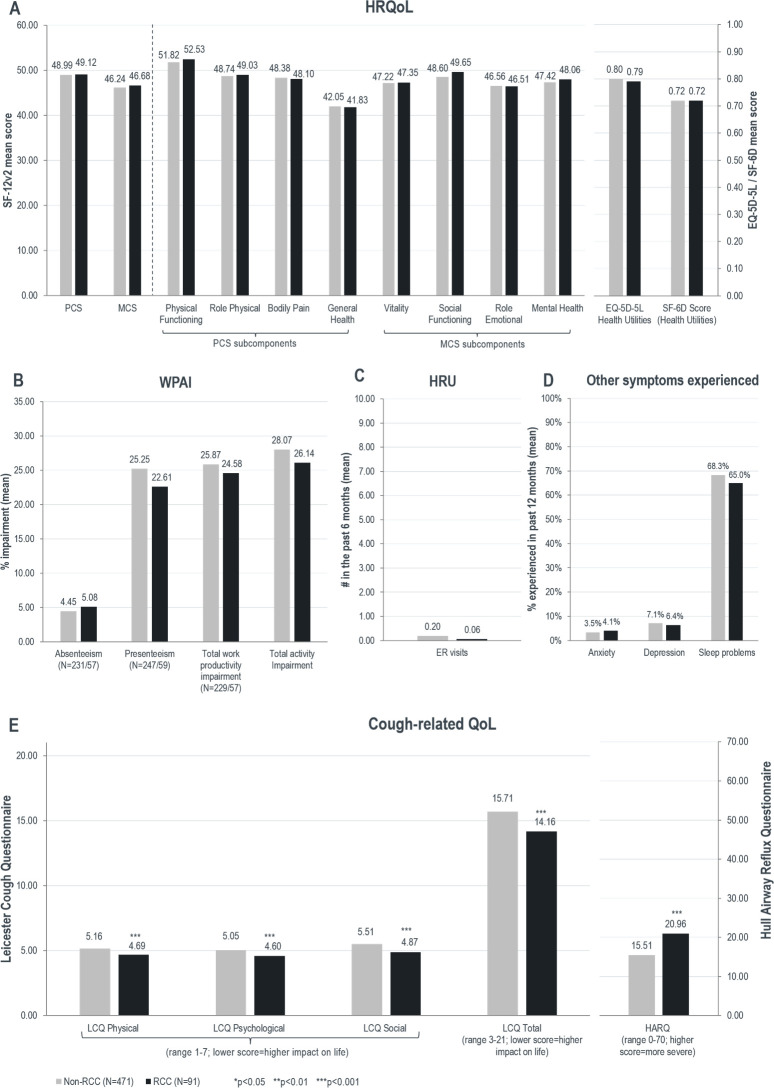
Among patients with CC, 91 met the criteria for refractory CC. When comparing patients with refractory CC and patients with non-refractory CC (n=471), no significant differences were observed in general HRQoL (figure 4A). However, patients with refractory CC reported significantly greater burden as measured by the LCQ total score (14.16 vs 15.71, p<0.001), and the HARQ score (20.96 vs 15.51, p<0.001) relative to patients with non-refractory CC (figure 4E). No significant differences were observed in WPAI, HRU, anxiety, depression and sleep problems between patients with refractory CC and patients with non-refractory CC (figure 4B, C and D).
Figure 4.
Comparison of health-related quality of life (HRQoL), work productivity and activity impairment (WPAI), healthcare resource utilisation (HRU), other symptoms experienced and cough-related quality of life (QoL) in patients with refractory chronic cough (CC) versus patients with non-refractory CC. ER, emergency room; HARQ, Hull Airway Reflux Questionnaire; HCP, healthcare professional; LCQ, Leicester Cough Questionnaire; MCS, mental component summary; PCS, physical component summary; SF-12v2, 12-Item Short Form Survey Instrument.
Outcomes assessment in patients with CC whose underlying diseases were unknown versus all other patients with CC
Among patients with CC, a total of 54 patients who had seen HCPs and had spoken with a physician for CC, had not been informed that they had underlying diseases related to their cough or answered ‘do not know’ about the underlying diseases. When comparing patients with CC whose underlying diseases were unknown (n=54) versus all other patients with CC (n=514), no significant differences were observed in general HRQoL and in cough-related QoL assessed by LCQ and HARQ (online supplemental table 3). Although no significant differences were observed in WPAI, anxiety, depression and sleep problems between the two groups, the number of HCP visits and the number of ER visits were significantly higher in patients with CC whose underlying diseases were unknown compared with all other patients with CC (11.6 vs 7.3 and 1.3 vs 0.1, respectively). No GLMs were conducted for the comparisons due to the exploratory nature of these groups.
Discussion
The results showed that patients with CC had significantly poorer QoL compared with matched non-cough respondents. This was both in terms of poorer physical and mental summary scores (PCS and MCS), and poorer general health scores (SF-6D and EQ-5D-5L). The poorer QoL among patients with CC is in accordance with findings in other studies in the USA, Europe and Asia, where CC was found to be associated with deterioration in QoL of patients.5 9–11 13 31 Another Japanese survey from 2012 similarly showed that CC impacted the daily life of patients, with 7.0% of respondents reporting that cough interfered with daily living and 65.6% found cough troublesome.5 However, to our knowledge this is the first study in Japan using validated HRQoL tools to describe the burden among patients with CC.
In addition to poorer QoL, a significantly higher percentage of patients with CC reported anxiety, depression and sleep problems in the past 12 months. A South Korean study similarly found a significant association between chronic, frequent and nocturnal cough and depression.12 Disturbed sleep, anxiety and depression were also commonly reported among patients with CC in a UK study, in respectively 70%, 69% and 55% of patients with CC.32
In this study, currently employed patients with CC reported a significantly higher impairment of work productivity compared with matched non-cough respondents. Total work productivity impairment was 1.4 times higher among patients with CC compared with matched non-cough respondents, with absenteeism almost twice as high, while presenteeism 1.4 times higher. Patients with CC also reported a 1.5 times higher total activity impairment compared with matched non-cough respondents. These results indicate that CC has a significant impact on patients’ work and daily life. It corroborated a previous Swedish study that patients with CC were significantly affected in their paid employment, social life, hobbies and holidays.31 A UK study also reported >60% of patients with CC felt their daily activities and social life, including housework, mealtimes, shopping and hobbies, were affected due to cough.32
Compared with matched non-cough controls, patients with CC reported two additional visits (1.4 times more visits) to the HCPs in the past 6 months and visited the ER 5 times more often. The increased HRU could possibly explain the increased absenteeism among patients with CC. The incremental burden borne by patients with CC compared with matched non-cough respondents indicated the unmet need to improve the overall health outcomes and reduce HRU among this group of patients.
Similar to patients with CC, the results showed that patients with subacute cough also had significantly poorer HRQoL and significantly higher WPAI, HRU and level of anxiety and sleep problems compared with matched non-cough controls. This indicates that even cough lasting between 3 and 8 weeks have sizeable impact on patients’ QoL and work productivity, stressing the need for quick diagnosis and effective treatment.
In this study, we found that patients with CC with VAS >40 mm was associated with worse PCS and worse general health measures, but not significantly associated with worse MCS nor higher/lower level of anxiety, depression and sleep problems. This and the previous results could indicate that CC itself has significant impacts on overall QoL and increase in the severity of CC impairs respondents in a physical manner rather than psychological. However, when QoL was measured by the cough-specific instrument LCQ, patients with VAS >40 mm had significantly worse psychological and physical QoL compared with patients with VAS ≤40 mm.
High severity among patients with CC was also significantly associated with higher absenteeism, total work productivity impairment and total activity impairment, but no significant differences were observed for HRU.
Finally, compared with patients with non-refractory CC, refractory CC was also found significantly associated with poorer QoL measured by LCQ and HARQ, while no association was found in general HRQoL measures. This indicates that there is an unmet need for improved QoL, especially for this group of patients within the CC population. Timely identification of the underlying diseases and earlier targeted therapy could resolve or improve the symptoms of patients with refractory CC.33 For patients with refractory CC whose cough still remains after appropriate treatments for the underlying diseases, an emerging therapy was reported to be clinically evaluated.16 Furthermore, as poorer QoL were only detected using the cough-specific measures LCQ and HARQ but not using the general HRQoL measures, it suggested that the utility of cough-specific QoL measures may be more sensitive and more appropriate to evaluate the health outcomes of patients with CC.
Apart from providing novel data on the burden of CC in Japan, one of the strengths of this study was the use of data from NHWS collected from respondents representative of the general population in Japan. Also, QoL and WPAI were measured using validated instruments. Furthermore, the burden of CC was quantified by comparison against a matched non-cough population accounting for differences in demographic and health characteristics. Future studies may investigate variability in cough across regions, which may be due to treatment or cultural differences, such as the documented use in Asian culture of meditation and mindfulness training for control of cough symptoms.34
However, we recognise the limitations of this study. Although NHWS is broadly representative of the Japanese national adult population, it is likely to under-represent people without internet access or comfort with online administration, as well as less healthy elderly people, institutionalised patients and those with severe comorbidities and disabilities. Moreover, respondents to NHWS had the option to voluntarily agree to participate in the cough-specific questionnaire, wherefore the sample within the CC survey may not be representative of the Japanese CC population. All data were self-reported, wherefore recall bias and self-representation bias should be acknowledged. Finally, the definition of refractory CC in this study was based on self-reported medication use and satisfaction with the treatment of cough, and it could not be validated if patients suffered persistent cough despite treatment according to guidelines as is the established definition of refractory cough.35
Conclusion
This study showed that patients with CC in Japan experience a significant burden in terms of poorer HRQoL, higher WPAI and HRU and higher level of self-reported anxiety, depression and sleep problems. CC thereby has a significant impact on patients’ QoL, social life and work life. Patients with VAS >40 mm experience an increased burden compared with patients with VAS ≤40 mm, as severity was associated with worse PCS as well as higher impacted WPAI. Severe cough and refractory CC were also significantly associated with worse QoL in terms of total LCQ and HARQ scores. These results indicate that there is an unmet need for better interventions and treatments to improve QoL, and reduce WPAI, HRU and experience of anxiety, depression and sleep problems among patients with CC in Japan.
Footnotes
Contributors: TK, KT, KO, MK, JS, MA and ST conceptualised and designed the study. YC and KO analysed the data. TK, KT, KO, MK, YC, JS, MA and ST interpreted the results and contributed in the manuscript development. All authors read and approved the final manuscript.
Funding: This study was sponsored by MSD KK, Tokyo, Japan.
Competing interests: TK, KT, KO, MK, MA, ST are employees at MSD KK, Tokyo, Japan. JS is an employee at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. YC is an employee at Kantar, Health Division, Singapore. Kantar received funding from MSD KK, Tokyo, Japan for conduction of the study, analysis and manuscript development.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The National Health and Wellness Survey (NHWS) was approved by the Pearl Pathways Institutional Review Board (Indianapolis, Indiana, USA) and the chronic cough survey was reviewed and approved by Toukeikai Kitamachi Clinic ERB. All respondents provided informed consent prior to participating.
References
- 1.Kajiwara N, Hayashi K, Misago M, et al. First-visit patients without a referral to the Department of internal medicine at a medium-sized acute care hospital in Japan: an observational study. Int J Gen Med 2017;10:335–45. 10.2147/IJGM.S146830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshima T, Kumada M, Mise J, et al. Reasons for encounter and diagnoses of new outpatients at a small community hospital in Japan: an observational study. Int J Gen Med 2014;7:259–69. 10.2147/IJGM.S62384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? systematic review. Can Fam Physician 2018;64:832–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Song W-J, Chang Y-S, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479–81. 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 5.Fujimura M. Frequency of persistent cough and trends in seeking medical care and treatment-results of an Internet survey. Allergol Int 2012;61:573–81. 10.2332/allergolint.11-OA-0368 [DOI] [PubMed] [Google Scholar]
- 6.Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006;129:222S–31. 10.1378/chest.129.1_suppl.222S [DOI] [PubMed] [Google Scholar]
- 7.The committee for The Japanese Respiratory Society guidelines for management of cough . Concept and use of the guidelines. Respirology 2006;11:S135–6. 10.1111/j.1440-1843.2006.00920_1.x [DOI] [PubMed] [Google Scholar]
- 8.The committee for The Japanese Respiratory Society guidelines for management of cough . Prolonged cough and chronic cough. Respirology 2006;11:S141–2. 10.1111/j.1440-1843.2006.00920_4.x [DOI] [PubMed] [Google Scholar]
- 9.French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998;158:1657–61. 10.1001/archinte.158.15.1657 [DOI] [PubMed] [Google Scholar]
- 10.Chamberlain SAF, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015;193:401–8. 10.1007/s00408-015-9701-2 [DOI] [PubMed] [Google Scholar]
- 11.Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009;5:7. 10.1186/1745-9974-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn K-H, Song W-J, Kim S-H, et al. Chronic cough, not asthma, is associated with depression in the elderly: a community-based population analysis in South Korea. Korean J Intern Med 2019;34:1363–71. 10.3904/kjim.2018.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanemitsu Y, Niimi A, Matsumoto H, et al. Gastroesophageal dysmotility is associated with the impairment of cough-specific quality of life in patients with cough variant asthma. Allergol Int 2016;65:320–6. 10.1016/j.alit.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Kantar . National health and wellness survey: patient-reported healthcare evidence, 2020. Available: https://www.kantar.com/expertise/health/da-real-world-data-pros-claims-and-health-records/national-health-and-wellness-survey-nhws
- 15.Dibonaventura MD, Fukuda T, Stankus A. PRM11 evidence for validity of a national patient-reported survey in Japan: the Japan National health and wellness survey. Value in Health 2012;15:A646–7. 10.1016/j.jval.2012.08.262 [DOI] [Google Scholar]
- 16.Smith JA, Kitt MM, Morice AH, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med 2020;8:775–85. 10.1016/S2213-2600(19)30471-0 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008;61:1234–40. 10.1016/j.jclinepi.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 20.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851–9. 10.1097/01.mlr.0000135827.18610.0d [DOI] [PubMed] [Google Scholar]
- 21.Liu GG, DiBonaventura MdaCosta, Yuan Y, et al. The burden of illness for patients with viral hepatitis C: evidence from a national survey in Japan. Value Health 2012;15:S65–71. 10.1016/j.jval.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 22.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 23.Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D. Qual Life Res 2016;25:707–19. 10.1007/s11136-015-1108-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly MC, Gooch KL, Wong RL, et al. Validity, reliability and responsiveness of the work productivity and activity impairment questionnaire in ankylosing spondylitis. Rheumatology 2010;49:812–9. 10.1093/rheumatology/kep457 [DOI] [PubMed] [Google Scholar]
- 25.Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester cough questionnaire (LCQ). Thorax 2003;58:339–43. 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj AA, Pavord DI, Birring SS. Clinical Cough IV:What is the Minimal Important Difference for the Leicester Cough Questionnaire? : Chung KF, Widdicombe J, . Pharmacology and therapeutics of cough. Berlin, Heidelberg: Springer, 2009: 311–20. [DOI] [PubMed] [Google Scholar]
- 27.International Society for the Study of Cough . Hull cough hypersensitivity questionnaire. Available: http://www.issc.info/HullCoughHypersensitivityQuestionnaire.html [Accessed 27 Apr 2020].
- 28.Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011;189:73–9. 10.1007/s00408-010-9272-1 [DOI] [PubMed] [Google Scholar]
- 29.Corp IBM. Ibm SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corp, 2013. [Google Scholar]
- 30.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 31.Ternesten-Hasséus E, Larsson S, Millqvist E. Symptoms induced by environmental irritants and health-related quality of life in patients with chronic cough - A cross-sectional study. Cough 2011;7:6. 10.1186/1745-9974-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everett CF, Kastelik JA, Thompson RH, et al. Chronic persistent cough in the community: a questionnaire survey. Cough 2007;3:5. 10.1186/1745-9974-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Good JT, Rollins DR, Kolakowski CA, et al. New insights in the diagnosis of chronic refractory cough. Respir Med 2018;141:103–10. 10.1016/j.rmed.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Liang D, Gao Y, et al. Mindfulness and asthma symptoms: a study among college students. J Asthma 2018;55:101–5. 10.1080/02770903.2017.1306545 [DOI] [PubMed] [Google Scholar]
- 35.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015;351:h5590. 10.1136/bmj.h5590 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000764supp001.pdf (50KB, pdf)
Data Availability Statement
Data are available on reasonable request.