Abstract
Aim
To compare the incidence of pulmonary embolism (PE) in COVID-19 pneumonia and non-COVID-19-related community-acquired pneumonia (CAP) in hospitalised patients.
Materials and methods
A retrospective case–control study was conducted. This included patients hospitalised with pneumonia and investigated for suspected PE with computed tomography pulmonary angiogram (CTPA). Cases were defined as patients with COVID-19 pneumonia from 1 March 2020 to 17 May 2020; controls were patients with CAP from 5 July 2019 to 31 January 2020. The primary outcome was to determine the risk of developing PE in both groups. Multivariable logistic regression was used to calculate the adjusted odds ratio for PE.
Results
One hundred and forty-four patients were included; 72 cases (47% male; mean age 59 (±15) years), and 72 controls (56% male; mean age 58 (±20) years). PE was diagnosed in 23.6% of the cases versus 6.9% of the controls. The adjusted odds ratio for PE in hospitalised patients with COVID-19 pneumonia compared with those with CAP was 3.23 (95% confidence interval [CI] 1.04–10.04, p=0.04).
Conclusion
The odds of developing PE in hospitalised patients with COVID-19 pneumonia are three-times higher than in those with CAP. The results provide a quantitative assessment of the risk of PE in COVID-19 pneumonia, a condition new to healthcare, compared to other forms of pneumonia with a well-established scientific basis.
Introduction
Pulmonary embolism (PE) is a major health problem.1 It is one of the most commonly missed diagnoses in clinical practice,2 and is the greatest preventable cause of hospital mortality.3 , 4 The annual incidence of PE is estimated at 39–115 cases per 100,000 population with a mortality rate of 30–35% if untreated.5 , 6
There is mounting evidence in the literature of an increased incidence of PE in hospitalised patients with SARS-CoV-2 infection.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 This novel coronavirus can cause pneumonia of varying degrees of severity; however, studies prior to the COVID-19 pandemic also suggested an increased incidence of PE in hospitalised patients with community-acquired pneumonia (CAP).19, 20, 21
Since pneumonia itself is a risk factor for PE,22 , 23 the present study investigated the risk of PE in COVID-19 pneumonia versus CAP in hospitalised patients to determine whether the reported increased PE incidence in SARS-CoV-2 infection is above that seen in other forms of pneumonia.
Materials and methods
Study design
A retrospective case–control study was undertaken to compare the incidence of PE in COVID-19 pneumonia and CAP in the setting of hospitalised patients investigated with a computed tomography (CT) pulmonary angiogram (CTPA). The primary outcome was to detect the risk of developing PE in both groups.
The current literature suggests an incidence of PE in 20.6–40% and 3.5–5% of COVID-19 pneumonia patients and CAP patients, respectively, when investigated with CTPA.9 , 10 , 16, 17, 18, 19, 20, 21 Sample size was powered to detect a 2% effect size (power = 0.8, alpha = 0.05). This resulted in a total sample size of at least 96 patients across both groups.
Study sample and data collection
The study was performed at two general hospitals. Ethics approval was obtained from the UK Health Research Authority (REC reference: 20/HRA/2259) and the institutional Research and Development department. The need for written informed consent was waived due to the observational nature of the study. The authors declare no conflicts of interest.
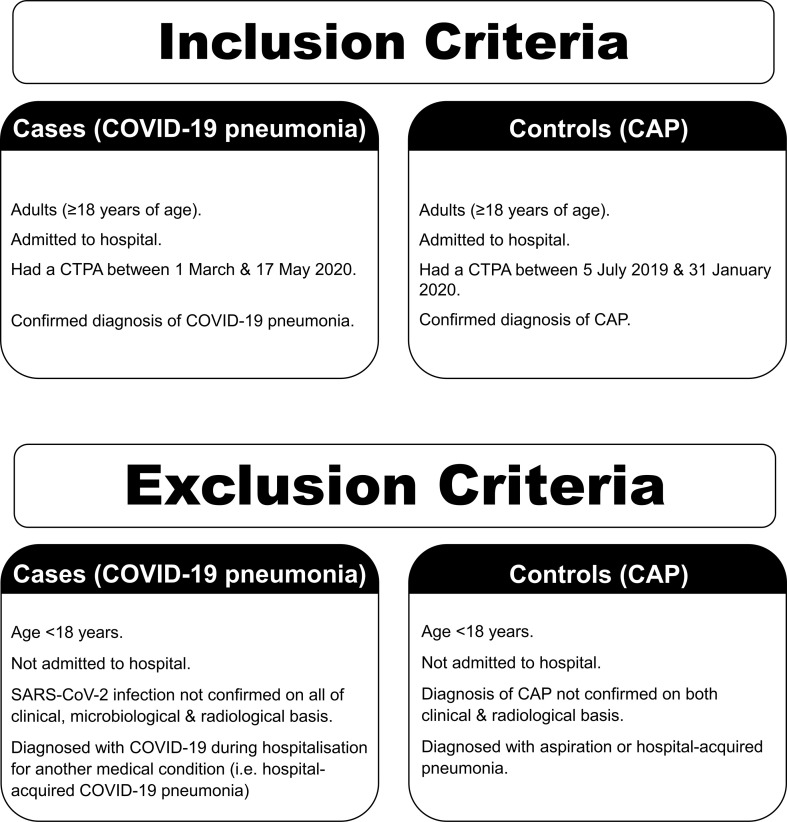
Two groups of patients were investigated: patients with COVID-19 pneumonia (cases), and patients with CAP (controls). The inclusion and exclusion criteria for both groups are detailed in Fig 1 .
Figure 1.
Details of the selection criteria in both study groups.
Cases were identified by performing a keyword search on the computerised radiology information system (CRIS; Healthcare Software Solutions, Mansfield, UK) for CTPA scans performed between 1 March and 17 May 2020 with reports containing the keywords “COVID”, “pneumonia”, “infection”, “ground glass”, “consolidation”, “airspace”, “opacity”, “opacities”, OR “opacification”. All patients who met the eligibility criteria for the COVID-19 pneumonia group were included. This resulted in 72 eligible patients.
Controls were identified using the exact same keyword search criteria, except for “COVID”. The first 72 eligible patients in reverse chronological order prior to 1 February 2020 were included. This sufficient sample size was achieved between 5 July 2019 and 31 January 2020. It is unlikely that this sample included any patients with SARS-CoV-2 infection as the UK declared its first COVID-19 patients on 30 January 2020.24 The selected time periods for both groups were sufficiently close to minimise differences in healthcare teams, management practices, and equipment that they would have been exposed to. The censoring date was 22 June 2020. This ensured that all patients had at least 5 weeks of follow-up to ensure satisfactory data collection.
Variables collected were patient demographics (age, sex, and ethnicity), co-morbidities (cardiovascular diseases, respiratory diseases, chronic kidney disease [CKD], diabetes mellitus [DM], arteriopathy [defined as cerebrovascular and/or peripheral vascular disease], previous malignancy, active malignancy, previous venous thromboembolism [VTE], dementia, mental health issues, and human immunodeficiency virus [HIV]), other PE risk factors (immobility, obesity [defined as body mass index {BMI} ≥30 kg/m2], length of hospitalisation prior to CTPA), VTE prophylaxis, D-dimer levels, and clinical outcomes (presence or absence of PE on CTPA, level of PE [if present], critical care admission, and death versus discharge).
Laboratory analysis
D-dimer levels were analysed in ACL TOP 350 CTS system (Werfen, Barcelona, Spain) using standard commercial reagents. All patients in the COVID-19 pneumonia group tested positive for SARS-CoV-2 using reverse transcriptase polymerase chain reaction (RT-PCR) using either Cepheid GeneXpert Xpress SARS-CoV-2 assay (Cepheid, Sunnyvale, CA, USA) or Roche Cobas SARS-CoV-2 assay (Roche Molecular Systems, Branchburg, NJ, USA).
Radiological analysis
CTPA was performed using one of the following scanners: 128-section SOMATOM Definition Flash, 128-section SOMATOM Definition Drive, 64-section SOMATOM Definition AS, or 16-section SOMATOM Perspective scanner (Siemens Healthcare, Erlangen, Germany). The majority of the scans in both groups (97% in the COVID-19 pneumonia group and 76% in the CAP group) were acquired on the 128- or the 64-section scanners. Images were reconstructed with section thicknesses of 1, 2, and 5 mm in the axial plane. Image analyses were performed using PACS workstations (MergePACS V.7.0; Merge Healthcare, Hartland, WI, USA). All the examinations were reviewed by two reporters: a consultant radiologist with >2 years of experience in general radiology and a senior radiology registrar in training. Each reporter used a dedicated workstation and reviewed the images independently. The images were reviewed for quality to confirm that they were diagnostic for PE. The images were also reviewed for confirmation of pneumonia findings and presence or absence of PE. In cases with a positive finding of PE, the location of the clot was recorded as central, lobar, segmental, or subsegmental, according to the site of the most proximal filling defect. When there was a discrepancy, it was resolved in consensus between the reporters.
Statistical analysis
Continuous variables are presented as mean (±standard deviation [SD]) when data were normally distributed, or as medians (interquartile range [IQR]) when data were not normally distributed. Categorical variables are presented as numbers and percentages. Missing data were not imputed.
For continuous variables, Student's t-test was used for normally distributed variables and the Mann–Whitney test for non-normally distributed variables. The chi-square test was used for categorical variables. Statistical significance was defined as p<0.05.
Odds ratios for PE were computed by logistic regression and adjusted for confounding variables. Analysis was initially performed with univariable logistic regression. Variables with a p-value of <0.2 were then entered into a multivariable logistic regression model. Goodness-of-fit using Hosmer–Lemeshow chi-square analysis was performed to assess calibration of the models. Receiver operating characteristic (ROC) curve analysis was performed to establish the optimal D-dimer level to exclude PE. Statistical analysis was performed using IBM SPSS, version 26 (IBM, Armonk, NY, USA).
Results
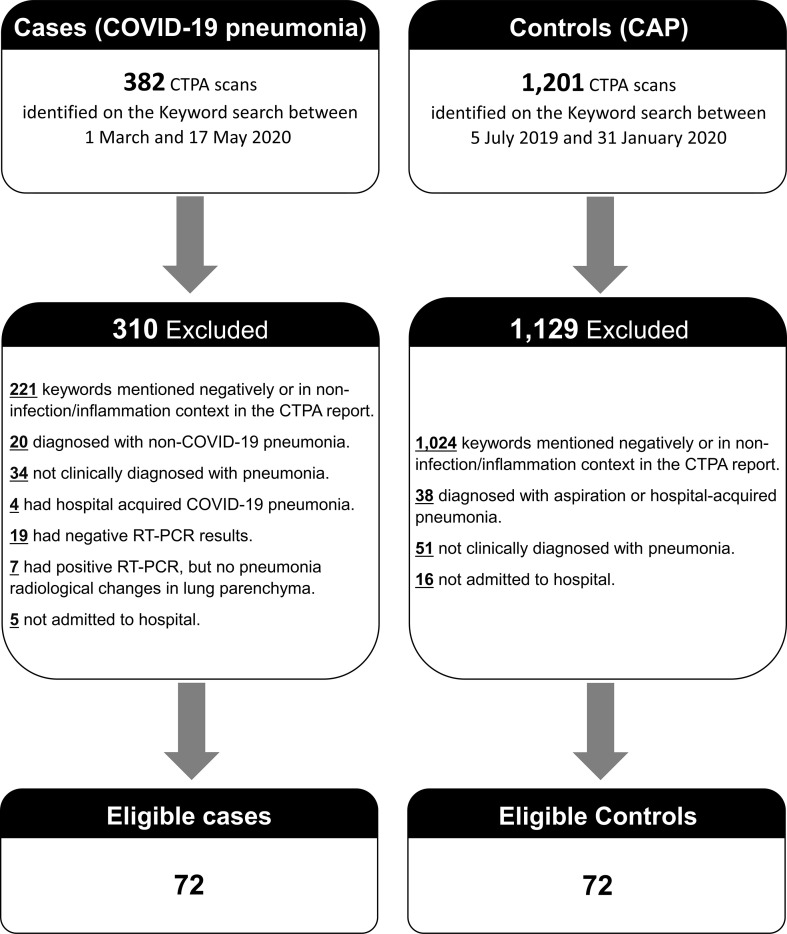
For the COVID-19 pneumonia group, the CRIS keyword search identified 382 potential patients during the period between 1 March 2020 and 17 May 2020. Of these, 310 (81%) were excluded as they did not meet the study's eligibility criteria (Fig 2 ). This resulted in 72 eligible patients in the COVID-19 pneumonia group. For the control group, the CRIS keyword search yielded 1,201 potential patients during the period between 5 July 2019 and 31 January 2020. Of these, 1,129 patients (94%) were excluded as they did not meet the study's eligibility criteria (Fig 2). Eventually, 72 consecutive eligible patients were identified and included in the CAP group.
Figure 2.
Detailed flowchart of included and excluded patients.
Twelve of the 72 (17%) scans in the COVID-19 pneumonia group and 10/72 (14%) scans in the CAP group showed artefacts at the subsegmental level due to suboptimal contrast opacification and/or respiratory motion artefact. These were included in the study, as there was either a positive diagnosis of PE proximal to the subsegmental level or when the scan was negative for PE, there was no ongoing clinical concern of or a proven PE on the patients' follow-up.
The baseline characteristics of both groups are summarised in Table 1 . There was no significant difference in the age and sex between the groups (p=0.62 and 0.40, respectively). The COVID-19 pneumonia group showed a more diverse range of ethnicities with black, Asian, and minority ethnic (BAME) groups comprising 50%, compared to 28% of the CAP group (p=0.02). There was no significant difference in most of the co-morbidities between the groups, except for cardiovascular diseases, which were more prevalent with COVID-19 pneumonia (p=0.04). Moreover, patients with COVID-19 pneumonia had more risk factors for PE, specifically a higher prevalence of obesity (p=0.04), and longer period of hospitalisation prior to acquiring the CTPA (p=0.001). In both groups, VTE prophylaxis was given appropriately to all the patients who were later confirmed to have PE. There were no pregnant patients in the cohort who would tend to be investigated with ventilation–perfusion single-photo-emission computed tomography (VQ SPECT) using krypton-81m as the ventilation agent.
Table 1.
Baseline characteristics of COVID-19 pneumonia and community-acquired pneumonia (CAP) patients.
| COVID-19 pneumonia group (n=72) |
CAP group (n=72) |
p-Value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 59 (±15) | 58 (±20) | 0.62 |
| Sex, male | 34 (47%) | 40 (56%) | 0.40 |
| Ethnicity | 0.02 | ||
| White | 36 (50%) | 52 (72%) | 0.006 |
| Black | 9 (12.5%) | 5 (7%) | 0.26 |
| Asian | 17 (23.6%) | 13 (18%) | 0.41 |
| Other | 10 (13.9%) | 2 (3%) | 0.02 |
| Co-morbidities | |||
| DM | 21 (29%) | 16 (22%) | 0.45 |
| Respiratory diseasesa | 18 (25%) | 29 (40%) | 0.07 |
| Cardiovascular diseasesb | 33 (46%) | 20 (28%) | 0.04 |
| Arteriopathy | 5 (6.9%) | 6 (8.3%) | NS |
| Moderate–severe CKD | 10 (14%) | 10 (14%) | NS |
| Previous malignancy | 4 (5.6%) | 6 (8.3%) | 0.74 |
| Active malignancy | 1 (1.4%) | 4 (5.6%) | 0.37 |
| Previous VTE | 4 (5.6%) | 6 (8.3%) | 0.74 |
| Dementia | 2 (2.8%) | 1 (1.4%) | NS |
| Mental health issues | 6 (8.3%) | 3 (4.2%) | 0.49 |
| HIV | 0 (0%) | 2 (2.8%) | 0.50 |
| Other PE risk factors | |||
| Immobility | 4 (5.6%) | 3 (4.2%) | NS |
| Obesity | 33 (46%) | 20 (28%) | 0.04 |
| Length of hospitalisation prior to CTPA (days) | 5.1 (±7) | 1.9 (±3.5) | 0.001 |
| VTE prophylaxis administered | |||
| Total | 67 (93%) | 53 (74%) | 0.003 |
| PE positive groupc | 17/17 (100%) | 5/5 (100%) | NS |
| PE negative groupc | 50/55 (91%) | 48/67 (72%) | 0.008 |
Data are n (%) or mean (±SD).
DM, diabetes mellitus; NS, indicates non-significant, CKD, chronic kidney disease; VTE, venous thromboembolism; HIV, human immunodeficiency virus; PE, pulmonary embolism; CTPA, computed tomography pulmonary angiogram.
Respiratory diseases included: chronic obstructive pulmonary disease (COPD), asthma, and interstitial lung disease.
Cardiovascular diseases included: ischaemic heart disease, congestive heart failure, atrial fibrillation, and hypertension.
Sample number is different to the total; detailed in each cell.
Risk of PE
PE was confirmed in 17/72 (23.6%) of the cases and in 5/72 (6.9%) of the controls (p=0.005). The crude odds ratio for PE in the hospitalised patients with COVID-19 pneumonia compared with those with CAP was 4.14 (95% CI 1.44–11.94, p=0.009). After adjustment for potential confounding factors, the odds ratio among hospitalised patients with COVID-19 pneumonia was 3.23 (95% CI 1.04–10.04, p=0.04). The goodness-of-fit test Hosmer–Lemeshow of the multivariable logistic regression model was 0.97. The other confounding variables did not contribute significantly to the logistic regression model, which showed that COVID-19 pneumonia was an independent risk factor for PE (Table 2 ).
Table 2.
Crude and adjusted odds ratio (OR) and 95% confidence interval (CI) of pulmonary embolism (PE) associated with pneumonia type and other confounding variables in logistic regression model.
| Crude ORa (95% CI) |
p-Value | Adjusted ORa (95% CI) |
p-Value | |
|---|---|---|---|---|
| Pneumonia (COVID-19 pneumonia) | 4.14 (1.44, 11.94) | 0.009 | 3.23 (1.04, 10.04) | 0.04 |
| Demographics | ||||
| Age | 0.99 (0.96,1.02) | 0.42 | ||
| Sex (male) | 0.48 (0.19, 1.24) | 0.13 | 0.37 (0.13, 1.07) | 0.07 |
| Ethnicity (constant is white) | 0.72 | |||
| Asian | 0.97 (0.32, 2.95) | |||
| Black | 0.37 (0.05, 3.08) | |||
| Other | 0.44 (0.05, 3.69) | |||
| Co-morbidities | ||||
| DM | 0.83 (0.28, 2.43) | 0.73 | ||
| Respiratory diseases | 0.74 (0.27, 2.04) | 0.56 | ||
| Cardiovascular diseases | 1.90 (0.76, 4.76) | 0.17 | 1.32 (0.47, 3.75) | 0.60 |
| Arteriopathy | 0.53 (0.06, 4.39) | 0.56 | ||
| Moderate–severe CKD | 0.98 (0.26, 3.65) | 0.97 | ||
| Previous malignancy | 2.59 (0.62, 10.92) | 0.19 | 3.50 (0.62, 19.89) | 0.16 |
| Active malignancy | 0.00 (0.00, 4.45E+198) | 0.96 | ||
| Previous VTE | 1.43 (0.28, 7.21) | 0.67 | ||
| Dementia | 0.00 (0.00, 9.22E+257) | 0.97 | ||
| Mental health issues | 0.00 (0.00, 7.06E+244) | 0.97 | ||
| HIV | 0.00 (0.00, 5.49E+190) | 0.96 | ||
| Other PE risk factors | ||||
| Obesity | 1.53 (0.61, 3.83) | 0.36 | ||
| Immobility | 0.00 (0.00, 2.47E+278) | 0.97 | ||
| Length of hospitalisation prior to CTPA | 1.07 (1.01, 1.15) | 0.03 | 1.06 (0.99–1.41) | 0.11 |
| Not on VTE prophylaxis | 0.00 (0.00, 5.82E+246) | 0.96 | ||
DM, diabetes mellitus; CKD, chronic kidney disease; VTE, venous thromboembolism; HIV, human immunodeficiency virus; CTPA, computed tomography pulmonary angiogram.
OR indicates odds ratio.
Clinical outcomes
Regarding patients who required higher levels of care, there was no significant difference in the proportion of patients who required critical care management in both groups (p=0.16), even amongst those diagnosed with PE (p=0.61); however, within the COVID-19 pneumonia group, critical care management was required for a significantly greater proportion of the patients with PE than those without PE (p=0.004; Table 3 ).
Table 3.
Clinical outcomes in COVID-19 pneumonia and community-acquired pneumonia (CAP) patients.
| COVID-19 pneumonia group | CAP group | p-Value | |
|---|---|---|---|
| Critical care management | |||
| Total | 19/72 (26%) | 12/72 (17%) | 0.16 |
| PE positive group | 9/17 (53%) | 2/5 (40%) | 0.61 |
| PE negative group | 10/55 (18%) | 10/67 (15%) | 0.63 |
| Death | |||
| Total | 14/72 (19%) | 8/72 (11%) | 0.17 |
| PE positive group | 4/17 (24%) | 1/5 (20%) | 0.87 |
| PE negative group | 10/55 (18%) | 7/67 (10%) | 0.22 |
| Level of PE in confirmed cases | |||
| Central | 3/17 (17.7%) | 0/5 (0%) | 0.31 |
| Lobar | 9/17 (52.9%) | 3/5 (60%) | 0.78 |
| Segmental | 5/17 (29.4%) | 2/5 (40%) | 0.66 |
| Subsegmental | 0/17 (0%) | 0/17 (0%) | NS |
Data are n (%).
PE, pulmonary embolism; NS indicates non-significant.
By the end of the data collection period on 22 June 2020, all the patients in the study had either been discharged from hospital alive or died. The mortality rate was higher in the COVID-19 pneumonia group (19%) compared to the CAP group (11%); however, this did not reach statistical significance (p=0.17). There was no significant difference at all in the mortality rate between those with confirmed PE between the groups (p=0.87). Furthermore, there was no significant difference in the mortality rates amongst patients with COVID-19 pneumonia whether they had PE or not (p=0.63; Table 3).
When there was a confirmed diagnosis of PE, the commonest locations of the most proximal filling defect in both groups were in the lobar vessels, followed by the segmental vessels. Only 17.7% of the positive scans in patients with COVID-19 pneumonia showed PE in the central vessels, whilst none of the patients with CAP developd central PE; this difference was not statistically significant (p=0.31; Table 3).
D-dimer cut-off level
The median D-dimer level was approximately 500 μg/l higher in the COVID-19 pneumonia group (p=0.02; Table 4 ). ROC curve was derived for the entire study sample. A D-dimer level above 1,640 μg/l correctly predicted all patients who later had confirmed PE on CTPA (area under the curve [AUC] of 0.88; p<0.001, 95% CI 0.80, 0.96). This level had 100% sensitivity, 62% specificity, 18% positive predictive value (PPV), and 100% negative predictive value (NPV).
Table 4.
Analysis of D-dimer levels in COVID-19 pneumonia and community-acquired pneumonia (CAP) patients.
| COVID-19 pneumonia group (n=42)a | CAP group (n=38)b | p-Value | |
|---|---|---|---|
| D-dimer, μg/l (median, IQR) | 1,835 (993–4,323) | 1,330 (703–2,358) | 0.02 |
| Performance (sensitivity, specificity, PPV, and NPV) of D-dimer cut-offs | |||
| Standard D-dimer cut-off | 100%, 3%, 29%, 100% | 100%, 11%, 6%, 100% | 0.29 |
| Age-adjusted D-dimer cut-off | 100%, 7%, 30%, 100% | 100%, 19%, 7%, 100% | 0.14 |
Forty-two of the 72 cases had D-dimer levels available.
Thirty-eight of the 72 controls had D-dimer levels available.
Sandwell and West Birmingham Hospitals currently employs a standard D-dimer cut-off level of 500 μg/l for all age groups rather than an age-adjusted cut-off in patients >50 years of age. The sensitivity, specificity, PPV, and NPV for both the standard and age-adjusted D-dimer cut-offs were calculated (Table 4). The analysis showed that adopting an age-adjusted D-dimer cut-off improved the specificity and PPV in both groups without compromising the sensitivity or the NPV. Implementing this approach would have avoided unnecessary CTPA in 1/72 patients with COVID-19 pneumonia (1.4%) and in 3/72 patients with CAP (4.2%).
Discussion
Pneumonia is a known risk factor for PE, even before the COVID-19 pandemic.22 , 23 A high incidence of PE in patients with SARS-CoV-2 infection has been recently reported in the literature7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18; however, given the excessive number of infected cases during the pandemic, comparison with a control group of patients with classical pneumonia would provide more confirmation as to whether the perceived high incidence is true. This study shows that the odds of developing PE in hospitalised patients with COVID-19 pneumonia are three-times higher than in those hospitalised with CAP. The study results may guide the clinical teams to gauge the risk of PE in COVID-19 pneumonia in comparison with the risk of PE in the classical CAP.
Male sex, BAME backgrounds, and multiple co-morbidities are recognised risk factors for developing severe COVID-19 pneumonia.25, 26, 27, 28 This was demonstrated in the present study, which identified that a higher proportion of these patients that required hospitalisation were from BAME communities than in the CAP group. The present data also showed that cardiovascular diseases, obesity, and longer hospitalisation prior to CTPA were more prevalent in the COVID-19 pneumonia group. Despite that, COVID-19 pneumonia remained an independent risk factor for PE in those hospitalised patients.
At Sandwell and West Birmingham Hospitals, all inpatients undergo a risk assessment for VTE on admission and again 24 h later. The agent of choice for VTE prophylaxis is enoxaparin sodium, which is dose-adjusted based on the patient's weight and creatinine clearance. The normal dose is 40 mg once a day (OD). Patients <50 kg or with creatine clearance of 15–30 ml/min receive 20 mg OD. Patients with BMI >35 kg/m2 receive 0.5 mg/kg of body weight (with a maximum dose of 100 mg) OD. In the present study, all the PE events occurred despite the appropriate standard of care VTE prophylaxis. This is in line with the other published data,8 , 11 , 12 , 15 , 17 and supports that COVID-19 pneumonia may be an independent risk factor for PE. It is possible that the standard doses of VTE prophylaxis are insufficient, and that higher dose regimens may be more efficacious.
The present study supports other published data that patients with COVID-19 pneumonia who develop PE are more likely to require critical care management.17 , 18 Therefore, these patients may require close monitoring. An interesting finding in the present data is that in patients with confirmed PE, there was no difference in the mortality rates between the COVID-19 pneumonia and CAP groups. More importantly, there was no difference in the mortality rates amongst patients with COVID-19 pneumonia, whether they had PE or not. As this study only assessed patients in whom a CTPA was performed, it suggests that PE in COVID-19 pneumonia may not adversely impact upon mortality rate, as long as it is promptly recognised and treated. Even though the number of patients in the present study is too small to confirm this finding, it is particularly noteworthy as, unlike in CAP, there is no effective treatment for COVID-19 pneumonia to date. The treatment for PE, however, is effective and so the diagnosis should not be missed.
A D-dimer level of 1,640 μg/l was the optimal cut-off value according to the present data; however, this level is only related to the study sample and is different to the levels suggested elsewhere.16 After assessing the utility of an age-adjusted D-dimer cut-off for suspected PE in patients with COVID-19 pneumonia, the specificity and PPV for PE increased, without compromising the sensitivity or NPV; however the yield was lower in COVID-19 pneumonia compared to CAP, due to the generally higher D-dimer levels in the former. Implementing an age-adjusted D-dimer cut-off could have avoided unnecessary CTPA in 1.4% of the patients with COVID-19 pneumonia in the present sample. Prior to the COVID-19 pandemic, the use of an age-adjusted D-dimer cut-off in suspected PE was established by meta-analysis and recommended by the major guidelines.29, 30, 31, 32 Its usage is recommended for suspected PE in COVID-19 pneumonia patients when appropriate.
The present study had limitations and strengths. Regarding limitations, the study was retrospective. Imaging was only performed in patients with clinical signs and symptoms suggestive of PE; therefore, it is conceivable that patients with subclinical features of PE may not have undergone further imaging. The results of this study may not be generalisable to the community setting as the incidence of PE in non-hospitalised individuals with COVID-19 pneumonia was not investigated; however, the study has many strengths as the diagnoses of COVID-19 pneumonia, CAP, and PE were confirmed objectively. Data were collected from two general hospitals in a major city, which draws from a wide, ethnically diverse population. The results of this study may therefore be transferable to various general population groups within a hospital setting.
In conclusion, the risk of PE in hospitalised patients with COVID-19 pneumonia is higher than in those with CAP (adjusted odds ratio 3.23). This demonstrates the impact of this widespread illness on the healthcare resources. Furthermore, the results provide a quantitative assessment of the risk of PE in COVID-19 pneumonia, a condition new to healthcare, compared to other forms of pneumonia with a well-established scientific basis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Bĕlohlávek J., Dytrych V., Linhart A. Pulmonary embolism, part I: epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol. 2013;18(2):129–138. [PMC free article] [PubMed] [Google Scholar]
- 2.Schiff G.D., Hasan O., Kim S. Diagnostic error in medicine: analysis of 583 physician-reported errors. Arch Intern Med. 2009;169(20):1881–1887. doi: 10.1001/archinternmed.2009.333. [DOI] [PubMed] [Google Scholar]
- 3.The Lancet Haematology Thromboembolism: an under appreciated cause of death. Lancet Haematol. 2015;2(10) doi: 10.1016/S2352-3026(15)00202-1. [DOI] [PubMed] [Google Scholar]
- 4.Geerts W.H., Pineo G.F., Heit J.A. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(Suppl):338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 5.Wendelboe A.M., Raskob G.E. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 6.Dalen J.E. Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest. 2002;122(4):1440–1456. doi: 10.1378/chest.122.4.1440. [DOI] [PubMed] [Google Scholar]
- 7.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas W., Varley J., Johnston A. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ooi M.W.X., Rajai A., Patel R. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography — prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol. 2020 Oct 6;132:109336. doi: 10.1016/j.ejrad.2020.109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oudkerk M., Büller H.R., Kuijpers D. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of The Netherlands. Radiology. 2020 Oct;297(1):E216–E222. doi: 10.1148/radiol.2020201629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalised patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poissy J., Goutay J., Caplan M. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 14.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bompard F., Monnier H., Saab I. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;56(1):2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard-Lorant I., Delabranche X., Severac F. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poyiadji N., Cormier P., Patel P.Y. Acute pulmonary embolism and COVID-19. Radiology. 2020;297(3):E335–E338. doi: 10.1148/radiol.2020201955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillet F., Behr J., Calame P. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296(3):E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha S.I., Choi K.J., Shin K.M. Clinical characteristics of pulmonary embolism with concomitant pneumonia. Blood Coagul Fibrinol. 2016;27(3):281–286. doi: 10.1097/MBC.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 20.Smith S.B., Hart E., Courtney D.M. The incidence and significance of pulmonary emboli at the time of presentation of community-acquired pneumonia. Am J Respir Crit Care Med. 2020;201:A3327. [Google Scholar]
- 21.Zhang Y., Zhou Q., Zou Y. Risk factors for pulmonary embolism in patients preliminarily diagnosed with community-acquired pneumonia: a prospective cohort study. J Thromb Thrombolysis. 2016;41(4):619–627. doi: 10.1007/s11239-015-1275-6. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro D.D., Lijfering W.M., Van Hylckama Vlieg A. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012;10(6):1179–1182. doi: 10.1111/j.1538–7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 23.Smeeth L., Cook C., Thomas S. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367(9516):1075–1079. doi: 10.1016/S0140-6736(06)68474–2. [DOI] [PubMed] [Google Scholar]
- 24.Lillie P.J., Samson A., Li A. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J Infect. 2020;80(5):578–606. doi: 10.1016/j.jinf.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lusignan S., Dorward J., Correa A. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners Research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. Lancet Respir Med. 2020;8(6):547–548. doi: 10.1016/S2213-2600(20)30228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen N., Zhu Y., Wang X. Characteristics and diagnosis rate of 5630 subjects receiving SARS-CoV-2 nucleic acid tests from Wuhan, China. JCI Insight. 2020;5(10) doi: 10.1172/jci.insight.137662. Published 2020 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinides S.V., Torbicki A., Agnelli G. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2015 Oct 14;36(39):2666. doi: 10.1093/eurheartj/ehu283. [published correction appears in Eur Heart J. 2015 Oct 14;36(39):2642]. Eur Heart J 2014;35(43):3033–3069k. [DOI] [PubMed] [Google Scholar]
- 30.Raja A.S., Greenberg J.O., Qaseem A. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701–711. doi: 10.7326/M14-1772. [DOI] [PubMed] [Google Scholar]
- 31.Kirsch J., Brown R.K.J., Henry T.S. ACR Appropriateness Criteria® acute chest pain—suspected pulmonary embolism. J Am Coll Radiol. 2017;14(5S):S2–S12. doi: 10.1016/j.jacr.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Care Excellence . NICE; 2020. Venous thromboembolic disease: diagnosis, management and thrombophilia testing.https://www.nice.org.uk/guidance/ng158 Guideline 158. [PubMed] [Google Scholar]