Abstract
Many countries have seen a two-wave pattern in reported cases of coronavirus disease-19 during the 2020 pandemic, with a first wave during spring followed by the current second wave in late summer and autumn. Empirical data show that the characteristics of the effects of the virus do vary between the two periods. Differences in age range and severity of the disease have been reported, although the comparative characteristics of the two waves still remain largely unknown. Those characteristics are compared in this study using data from two equal periods of 3 and a half months. The first period, between 15th March and 30th June, corresponding to the entire first wave, and the second, between 1st July and 15th October, corresponding to part of the second wave, still present at the time of writing this article. Two hundred and four patients were hospitalized during the first period, and 264 during the second period. Patients in the second wave were younger and the duration of hospitalization and case fatality rate were lower than those in the first wave. In the second wave, there were more children, and pregnant and post-partum women. The most frequent signs and symptoms in both waves were fever, dyspnea, pneumonia, and cough, and the most relevant comorbidities were cardiovascular diseases, type 2 diabetes mellitus, and chronic neurological diseases. Patients from the second wave more frequently presented renal and gastrointestinal symptoms, were more often treated with non-invasive mechanical ventilation and corticoids, and less often with invasive mechanical ventilation, conventional oxygen therapy and anticoagulants. Several differences in mortality risk factors were also observed. These results might help to understand the characteristics of the second wave and the behaviour and danger of SARS-CoV-2 in the Mediterranean area and in Western Europe. Further studies are needed to confirm our findings.
Introduction
Coronavirus disease-19 (COVID-19), produced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global pandemic, giving rise to a serious health threat globally. Several countries have seen a two-wave pattern of reported cases, with a first wave in spring and a second in late summer and autumn [1–6]. In Spain, the first wave of COVID-19 began in early March 2020, although some isolated cases had been reported in February [7]. As a consequence of the first outbreak, the Spanish Government introduced a series of strict prevention measures, including home confinement, which lasted from 13th March to 4th May, followed by a three-month period of progressively increasing social interaction, work and commercial activity. As of July, life in the country had returned to relative normality, except for the mandatory wearing of a face mask and maintaining a safe social distance. Unfortunately, the number of cases of patients with COVID-19 began to increase towards the end of August and a month later it once again presented numbers similar to those in April. This forced the Government to reintroduce serious restrictive measures, including local and regional lockdowns, closures of bars, restaurants, cultural and sports activities, and a general curfew after 10 pm. The number of cases in Spain has continued to grow since then, with some ups and downs, and at the time of writing this article it seems that it is beginning to stabilize. The second wave of COVID-19 had been predicted months earlier and had already occurred in other countries [4]. The vast majority of Western European countries are currently suffering the consequences of this second wave and are taking similar restrictive measures. However, empirical data would suggest that this second wave differs from the first in such factors as age range and severity of the disease [8]. Indeed, it has been suggested that this second wave in Europe might be linked to the appearance of a new variant of the SARS-CoV-2, termed 20A.EU1, which appears to have originated in Spain, from where it then spread to the rest of Europe through tourists who had spent their summer holidays in that area [9]. The similarities and differences between the characteristics of the two waves remain largely unknown. Population comparison is difficult because the technological and logistical capacity of the countries in detection and diagnosis of asymptomatic patients and those with mild symptoms has improved greatly in the six months since spring, and it is assumed that the incidence of infection in the early months of the pandemic was much higher than had been reported [10]. However, a more accurate comparison of the two waves is feasible through the study of the hospitalized patients for whom disease was confirmed by reverse transcription-polymerase chain reaction (RT-PCR) and severe symptoms.
This study investigated the severity and characteristics of the two waves in hospitalized patients in Reus, Spain. We evaluated age, gender, symptoms, comorbidities, mortality, supportive care, medication, and the outcome for the patient.
Materials and methods
Study design
We conducted a prospective study of all hospitalized cases of SARS-CoV-2 infection in Hospital Universitari de Sant Joan, in Reus, Spain, admitted between 15th March and 15th October 2020. All patients admitted up to 30th June were considered to be in the first wave and all those admitted from 1st July in the second wave, which divided the study period into two equal parts of three and a half months. The only inclusion criterion was to be a hospitalized patient with an analytical diagnosis of SARS-CoV-2. We excluded those with suspected SARS-CoV-2 infection but had no laboratory confirmation and those who came to the hospital with symptoms compatible with COVID-19 but did not require hospitalization. SARS-CoV-2 infection was confirmed by RT-PCR using swab samples from the upper respiratory tract (nasopharyngeal/oropharyngeal exudate), from the lower respiratory tract (sputum/endotracheal aspirate/bronchoalveolar lavage/bronchial aspirate) or from the lower digestive tract (rectal smear). Tests were carried out with the VIASURE SARS-CoV-2 Real Time PCR Detection Kit (CerTest Biotec, Zaragoza, Spain), or with the Procleix® method in a Panther automated extractor and amplifier (Grifols Laboratories, Barcelona, Spain). This study was approved by the Comitè d’Ètica i Investigació en Medicaments (Institutional Review Board) of Hospital Universitari de Sant Joan (Resolution CEIM 040/2018, amended on 16 April 2020). This was a retrospective study of medical records and all data were fully anonymized before the researchers accessed them.
Calculation of sample size
Accepting an alpha risk of 0.05 and a beta risk of less than 0.2 in a bilateral contrast, it takes 137 subjects in the first wave and 105 in the second wave to detect a difference equal to or greater than 8 years in the variable age. The common standard deviation is assumed to be 22. For the study of differences in case fatality rate, a minimum number of 221 cases has been calculated in the first wave, and 107 in the second wave. The ARCSINUS approach has been used. A follow-up loss rate of 0% was estimated.
Statistical analyses
Data is given as numbers and percentages or means and standard deviations. Statistical comparisons between two groups were made using the χ2 test (categorical variables) or the Student’s t test. Logistic regression models were fitted to investigate the combined effect of selected variables on mortality. Statistical significance was set at p ≤0.05. All calculations were made using the SPSS 25.0 statistical package (SPSS Inc., Chicago, IL, USA).
Results
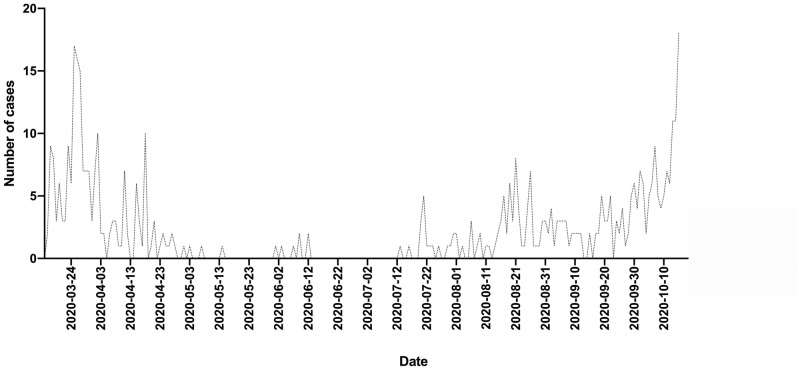
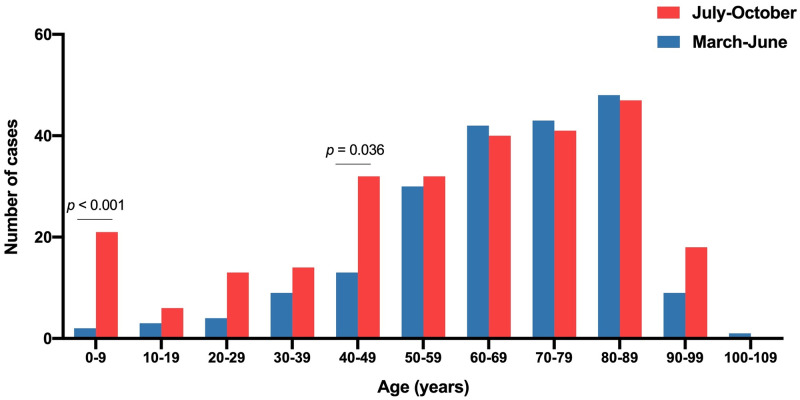
The raw data of this study are as (S1 File). During the study period, 468 patients with SARS-Co-V2 infection, confirmed by RT-PCR, were admitted to the hospital. The seasonal distribution of hospital admissions is shown in Fig 1. The first wave peaked at the end of March and was followed by a progressive decrease with very few patients being admitted in May and June. The number of cases fluctuated upward from mid-July until a sharp increase in mid-October. The number of patients admitted was 204 in the first wave and 264 in the second one. Those in the second wave were significantly younger (58 ± 26 vs. 67 ± 18 years; p <0.001). A noteworthy feature of the second wave was the high number of children between 0 and 9 years of age (n = 21), 12 of them being babies under 1 year (Fig 2). The department to which the patients were admitted is shown in Table 1. The second wave caused a significantly higher number of admissions to Gynecology, Pediatrics and Emergency Departments and fewer to Internal Medicine and ICU. The duration of hospitalization was significantly shorter in the second wave (14 ± 19 vs. 22 ± 25 days; p < 0.001). A total of 49 deaths occurred during the first wave and 35 during the second wave, so the case fatality rate decreased from 24.0% to 13.2%. The patients who died were significantly older than the survivors and those who died in the second wave were older than those in the first wave (83 ± 10 vs. 78 ± 13 years; p = 0.042).
Fig 1. Number of patients with COVID-19 admitted per day over the entire study period.
Fig 2. Distribution by age intervals of the patients admitted for COVID-19 during the first and second waves.
The p values were calculated using the χ2 test.
Table 1. Distribution of the hospitalized patients in the first and second waves.
| Department | First wave | Second wave | p-value |
|---|---|---|---|
| (n = 204) | (n = 264) | ||
| Internal Medicine | 124 (60.8) | 123 (46.6) | 0.004 |
| Intermediate Care Unit | 42 (20.6) | 47 (17.8) | 0.596 |
| Intensive Care Unit | 35 (17.1) | 19 (7.2) | 0.029 |
| Emergency Unit | 0 (0.0) | 33 (12.5) | N.A. |
| Pediatrics | 0 (0.0) | 22 (8.3) | N.A. |
| Gynecology | 0 (0.0) | 10 (3.8) | N.A. |
| Surgery | 1 (0.5) | 5 (1.9) | 0.102 |
| Oncology | 1 (0.5) | 3 (1.1) | 0.317 |
| Traumatology | 1 (0.5) | 2 (0.8) | 0.564 |
Statistical analysis was performed by the χ2 test. Results are shown as number of cases and percentages (in parenthesis). N.A.: Not applicable. The statistical test cannot be performed when one of the variables is equal to 0.
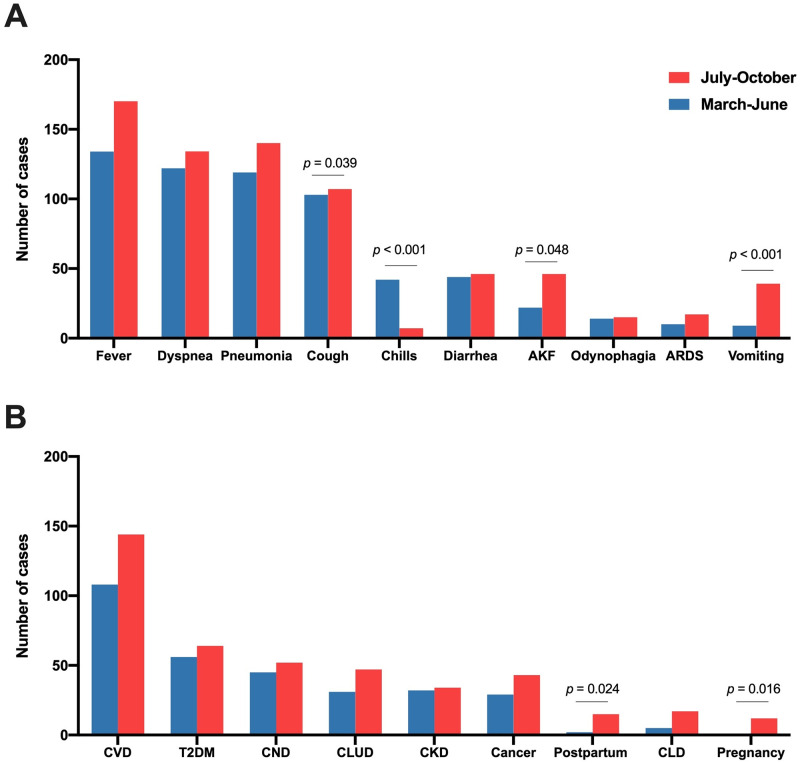
The relationships between COVID-19 and the clinical and epidemiological variables are shown in Fig 3 and Table 2. The most frequent signs and symptoms in both waves were fever, dyspnea, pneumonia, and cough (Fig 3A). The most relevant comorbidities were cardiovascular diseases, type 2 diabetes mellitus, and chronic neurological diseases (Fig 3B). Patients from the second wave differed from those of the first wave in that they more frequently presented a higher frequency of vomiting, astenia, abdominal pain, rhinorrhea, or acute kidney failure, and less frequently a cough or chills. There was no significant difference in the frequency of concomitant chronic diseases. One result that we consider noteworthy is the considerably higher frequency in the second wave of pregnant women who went to the hospital to give birth and post-partum women.
Fig 3. Distribution of symptoms and diseases associated with SARS-CoV-2 infection (A) and comorbidities and gestational variables (B) in patients admitted for COVID-19 during the first and second waves.
The p values were calculated using the χ2 test. AKF, acute kidney failure; ARDS, acute respiratory distress syndrome; CKD, chronic kidney disease; CLD, chronic liver disease; CLUD, chronic lung disease; CND, chronic neurological disease; CVD, cardiovascular disease; T2DM, type 2 diabetes mellitus.
Table 2. Clinical and epidemiological characteristics of patients with COVID-19 infection.
| Feature | First wave | Second wave | p-value |
|---|---|---|---|
| (n = 204) | (n = 264) | ||
| Epidemiological characteristics | |||
| Age | 67 ± 18 | 58 ± 26 | < 0.001 |
| Gender, male | 114 (55.9) | 144 (54.5) | 0.423 |
| Smoking habit | 10 (4.9) | 27 (13.2) | < 0.001 |
| Alcohol consumption | 10 (4.9) | 15 (7.3) | 0.421 |
| Signs and symptoms | |||
| Fever | 134 (65.6) | 170 (64.3) | 0.845 |
| Dyspnea | 122 (59.8) | 134 (50.7) | 0.061 |
| Pneumonia | 119 (58.3) | 140 (53.8) | 0.262 |
| Cough | 103 (50.5) | 107 (40.5) | 0.039 |
| Diarrhea | 44 (21.5) | 46 (17.4) | 0.288 |
| Chills | 42 (20.5) | 7 (2.6) | < 0.001 |
| Acute kidney failure | 22 (10.2) | 46 (17.4) | 0.048 |
| Odynophagia | 14 (6.8) | 15 (5.6) | 0.700 |
| Acute respiratory distress syndrome | 10 (4.9) | 17 (6.4) | 0.552 |
| Vomiting | 9 (4.4) | 39 (14.7) | < 0.001 |
| Other symptoms1 | 12 (5.8) | 69 (26.1) | < 0.001 |
| Comorbidities and gestational variables | |||
| Cardiovascular disease (including hypertension) | 108 (52.9) | 144 (54.5) | 0.502 |
| Type 2 diabetes mellitus | 56 (27.4) | 64 (24.2) | 0.456 |
| Chronic neurological disease | 45 (22.0) | 52 (19.7) | 0.429 |
| Chronic kidney disease | 32 (15.6) | 34 (12.9) | 0.359 |
| Chronic lung disease | 31 (15.2) | 47 (17.8) | 0.401 |
| Cancer | 29 (14.2) | 43 (16.3) | 0.816 |
| Other infectious diseases | 6 (2.9) | 10 (3.8) | 0.464 |
| Chronic liver disease | 5 (2.4) | 17 (6.4) | 0.069 |
| Postpartum (< 6 weeks) | 2 (0.9) | 15 (5.7) | 0.024 |
| Pregnancy | 1 (0.4) | 12 (4.5) | 0.016 |
Statistical analysis was performed by the χ2 test (categorical variables) or the Student’s t test (quantitative variables). Results are shown as number of cases and percentages (in parenthesis) or as means ± standard deviations.
1 Asthenia, rhinorrhea or abdominal pain.
We also evaluated the differences in treatments between the two groups of patients. Subjects from the second wave were treated more often with non-invasive mechanical ventilation and corticoids, and less often with invasive mechanical ventilation, conventional oxygen therapy and anticoagulants (Table 3). Regarding other treatments, patients in the first wave received lopinavir, ritonavir and hydroxychloroquine, while those in the second wave received remdesivir and tocilizumab.
Table 3. Main treatments of patients with COVID-19 infection.
| Treatment | First wave | Second wave | p-value |
|---|---|---|---|
| (n = 204) | (n = 264) | ||
| Noninvasive mechanical ventilation | 7 (3.4) | 25 (9.5) | 0.007 |
| Invasive mechanical ventilation | 27 (13.2) | 11 (4.2) | < 0.001 |
| High-flow oxygen therapy | 18 (8.8) | 28 (10.6) | 0.315 |
| Conventional oxygen therapy | 155 (76.0) | 156 (59.1) | < 0.001 |
| Anticoagulants | 184 (90.2) | 188 (71.2) | < 0.001 |
| Corticosteroids | 86 (42.2) | 156 (59.1) | < 0.001 |
Statistical analysis was performed by the χ2 test. Results are shown as number of cases and percentages (in parenthesis).
Finally, we wanted to identify which factors were the most important determinants of death in the two groups of patients. Logistic regression analyses highlighted the importance of age, fever, dyspnea, acute respiratory distress syndrome, type 2 diabetes mellitus, and cancer in the first wave (Table 4), and of age, gender, smoking habit, acute respiratory distress syndrome, and chronic neurological diseases in the second wave (Table 5).
Table 4. Logistic regression analysis on the relationships of comorbidities with deaths for patients from the first wave of COVID-19.
| Variable | B | SE | Exp (B) | p-value |
|---|---|---|---|---|
| Age | 0.096 | 0.024 | 1.101 | < 0.001 |
| Gender | 0.365 | 0.517 | 1.441 | 0.480 |
| Smoking habit | 0.060 | 0.352 | 1.062 | 0.865 |
| Alcohol consumption | -0.570 | 0.468 | 0.565 | 0.223 |
| Fever | 2.138 | 0.658 | 8.481 | 0.001 |
| Cough | 0.238 | 0.581 | 1.269 | 0.682 |
| Pneumonia | -1.139 | 0.651 | 0.320 | 0.080 |
| Odynophagia | -2.107 | 1.148 | 0.122 | 0.067 |
| Chills | -1.288 | 0.760 | 0.276 | 0.090 |
| Dyspnea | 1.365 | 0.628 | 3.915 | 0.030 |
| Vomiting | -1.132 | 1.481 | 0.322 | 0.445 |
| Diarrhea | -0.846 | 0.657 | 0.429 | 0.198 |
| Acute respiratory distress syndrome | 3.606 | 1.185 | 36.828 | 0.002 |
| Acute kidney failure | 0.442 | 0.769 | 1.556 | 0.565 |
| Other symptoms1 | 0.192 | 0.964 | 1.211 | 0.843 |
| Type 2 diabetes mellitus | 1.298 | 0.505 | 3.662 | 0.010 |
| Cardiovascular diseases | 0.114 | 0.559 | 1.121 | 0.839 |
| Chronic liver diseases | 0.122 | 1.371 | 1.130 | 0.929 |
| Chronic lung diseases | -0.458 | 0.682 | 0.632 | 0.502 |
| Chronic kidney diseases | -0.256 | 0.665 | 0.774 | 0.701 |
| Chronic neurological diseases | -0.547 | 0.598 | 0.579 | 0.360 |
| Other infectious diseases | 0.476 | 1.705 | 1.610 | 0.780 |
| Cancer | 1.518 | 0.595 | 4.565 | 0.011 |
| Pregnancy | -31.735 | 42695.071 | 0.000 | 0.999 |
| Postpartum | 20.726 | 40192.969 | 0.1 x 109 | 1.000 |
| Constant | -10.394 | 2.044 | 0.000 | < 0.001 |
Model summary: log-likelihood(-2) = 136.623; r2 Cox & Snell = 0.343; r2 Nagelkerke = 0.515; p <0.001. B: Non-standardized β coefficient. SE: Standard error of B.
1 Asthenia, rinorrhea or abdominal pain.
Table 5. Logistic regression analysis on the relationships of comorbidities with deaths for patients from the second wave of COVID-19.
| Variable | B | SE | Exp (B) | p-value |
|---|---|---|---|---|
| Age | 0.094 | 0.030 | 1.098 | 0.002 |
| Gender | 1.755 | 0.716 | 5.782 | 0.014 |
| Smoking habit | -2.874 | 1.446 | 0.056 | 0.047 |
| Alcohol consumption | 0.558 | 0.789 | 1.747 | 0.479 |
| Fever | -0.583 | 0.756 | 0.558 | 0.441 |
| Cough | -0.173 | 0.641 | 0.841 | 0.787 |
| Pneumonia | 0.186 | 0.744 | 1.204 | 0.803 |
| Odynophagia | -16.683 | 8820.456 | 0.000 | 0.998 |
| Chills | -18.312 | 12533.763 | 0.000 | 0.999 |
| Dyspnea | -0.305 | 0.708 | 0.737 | 0.666 |
| Vomiting | -1.544 | 1.335 | 0.214 | 0.247 |
| Diarrhea | -1.329 | 1.319 | 0.265 | 0.313 |
| Acute respiratory distress syndrome | 2.242 | 0.988 | 9.410 | 0.023 |
| Acute kidney failure | 0.195 | 0.765 | 1.216 | 0.799 |
| Other symptoms1 | 0.485 | 0.605 | 1.624 | 0.423 |
| Type 2 diabetes mellitus | 0.183 | 0.599 | 1.201 | 0.759 |
| Cardiovascular diseases | 0.276 | 0.832 | 1.318 | 0.740 |
| Chronic liver diseases | 2.419 | 1.249 | 11.234 | 0.053 |
| Chronic lung diseases | 0.178 | 0.697 | 1.195 | 0.799 |
| Chronic kidney diseases | 0.234 | 0.835 | 1.264 | 0.779 |
| Chronic neurological diseases | 1.945 | 0.723 | 6.993 | 0.007 |
| Other infectious diseases | 2.042 | 1.451 | 7.704 | 0.160 |
| Cancer | 0.289 | 0.626 | 1.335 | 0.644 |
| Pregnancy | -11.766 | 10235.783 | 0.000 | 0.999 |
| Postpartum | -0.555 | 0.542 | 0.574 | 0.306 |
| Constant | -10.590 | 2.789 | 0.000 | < 0.001 |
Model summary: log-likelihood(-2) = 98.286; r2 Cox & Snell = 0.318; r2 Nagelkerke = 0.597; p <0.001. B: Non-standardized β coefficient. SE: Standard error of B.
1 Asthenia, rinorrhea or abdominal pain.
Discussion
We have previously reported the main epidemiological and clinical characteristics and the mortality risk factors of the first wave patients during a month and a half between March and April [11]. In the present investigation we extended the study to mid-October to cover two equal periods of three and a half months. More patients were admitted during the second wave, they were younger and there were fewer deaths, in agreement with results reported by previous research in several countries [2, 3, 12]. The reasons for the clear differences between the two periods are not yet known although it has been suggested that a new variant of SARS-CoV-2 emerged in early summer 2020 in Spain [9], a variant that was linked to outbreaks among young agricultural workers in the north-east of the country. Transmission to the general population in that area was then replicated across the country. Furthermore, poor compliance with social distancing guidelines by young people might have facilitated contagion in young, healthy adults and children [2, 13]. The decrease in the age of the patients then resulted in a decrease in the case fatality rate in that those patients who died were on average 5 years older than the victims of the first wave. Moreover, fewer patients required respiratory assistance via invasive mechanical ventilation methods. This improvement in the results of admitted patients might be linked to the fact that the health system in our country, as in many others, has since become better prepared. We have more experience and better treatment regimens, and we carry out more diagnostic tests, allowing serious cases to be detected early and to receive more effective treatments. In this regard, during the second period, patients were treated more frequently with dexamethasone, as suggested by the RECOVERY study [14], and hydroxychloroquine and loponavir-ritonavir were substituted by remdesivir and tocilizumab, which several studies have reported to be more effective in preventing death and shortening the duration of hospital stays [15–17]. The use of hydroxychloroquine for the treatment of COVID-19 is controversial. Some studies have reported that this drug reduces mortality [18], but others have not confirmed this finding [19]. Our subjective clinical impression is that hydroxychloroquine can be useful in the first days of hospitalization. However, in the second wave, we updated the treatments in accordance with the guidelines of the Department of Health of the Autonomous Government of Catalonia, and we cannot compare its effectiveness in the two periods. Another factor that might have contributed to the decrease in the case fatality rate is the improvement in environmental conditions. For example, warm weather and improved air quality following the city lockdown have been reported to correlate negatively with SARS-CoV-2 transmissibility [20–22].
A new and remarkable characteristic of the incidence of COVID-19 in this second wave in our population is the higher incidence in babies, children and pregnant women who went to the hospital to give birth or in post-partum women. The vast majority of these patients did not present serious symptoms and so did not require hospitalization for more than 4 days. There were no deaths among children up to 9 years of age, pregnant or post-partum women. The predominant symptom presented by the children was fever (19 out of 21 cases, 90.5%), while pregnant and post-partum women (13 and 17 cases, respectively) were asymptomatic and promptly discharged. These results highlight the role of family contact in the transmission of the virus and agree with previous reports that have indicated the generally low severity of the disease in these patients [23–26].
The predominant symptoms of infection (fever, dyspnea, pneumonia cough) were similar in both waves, although the patients in the second wave presented renal (acute kidney failure) and gastrointestinal symptoms (vomiting, abdominal pain) more frequently. Indeed, the Spanish Ministry of Health has already highlighted, in a document updated on 2nd October, the higher incidence of the latter in the second wave [27]. The present study did not find any differences between the frequency of concomitant diseases in the two waves, similar findings to those of our preliminary study [11]. In this respect, we differ from a previous study conducted in Japan that has reported a lower incidence of cardiovascular and cerebrovascular diseases [3], and also from a multicenter study in Italy [28, 29] that identified impaired renal function, but not obesity, cardiovascular disease or cancer, as the major predictors of in-hospital death.
Lastly, regarding the risk factors associated with mortality, we also found differences between the first and second waves. Multiple regression analysis showed that older age and the presence of fever, dyspnea, acute respiratory distress syndrome, diabetes, and cancer were independently associated with higher mortality in the first wave, while age, gender, and the presence of acute respiratory distress syndrome and chronic neurological diseases were associated with mortality in the second. This might be a reflection of a better management of cancer or diabetes patients. On the other hand, the association of neurological diseases with mortality might be due to the higher mean age of those who died in this second wave. The importance of neurological diseases has also been highlighted in other studies [30].
Limitations of the study
A limitation of the present study is the small sample size. This is an unicentric study in a medium size hospital, and that covers a relatively small geographical area. In addition, we are at the limit of statistical significance for the calculation of mortality differences. Therefore, our results must be taken with caution. However, we believe that the results obtained are relevant since they might be representative of many similar centres in the Mediterranean area, and little information is yet available on this issue.
Conclusions
The results of the present study show that hospitalized patients in the second wave were younger, required fewer days of hospitalization, had lower mortality rates and treatments were more effective and less intensive. Although the majority of symptoms were similar in both periods, the higher incidence of gastrointestinal symptoms in the second wave stands out as a difference. Comorbidities were similar, but there were differences between those associated with mortality, highlighting the importance of chronic neurological diseases in this second wave. An important difference was the high incidence of babies, children and pregnant and post-partum women admitted but, in general, these cases were not serious and were resolved promptly and successfully. These results might help to understand the characteristics of this second wave and the behaviour and danger of SARS-CoV-2 in the Mediterranean area and in Western Europe generally.
Future prospects are difficult to predict. We think that COVID-19 will not disappear in the short or medium term. New variants of the virus may appear, the vaccination process can predictably last all year 2021 or more, until a sufficiently high percentage of the population is protected, and the maintenance of strict lockdowns for very long periods is difficult to bear from the economic, social and psychological points of view. Currently, the whole world is in the middle of the second or perhaps the third wave, and the results of our study indicate that the characteristics of the infection may vary over time. We believe that the most important conclusion of our work is that we must remain vigilant in the constant study of the characteristics of the disease, be able to modify treatments quickly, if necessary, and disseminate our results to the scientific community and society as soon as possible for coordinate and global action.
Supporting information
(SAV)
Acknowledgments
The authors are indebted to all the staff of the Hospital Universitari de Sant Joan, doctors, nurses, assistants, cleaning and security personnel, and all the volunteer students, who with their enormous effort are managing to overcome this dramatic situation. Editorial assistance was provided by Phil Hoddy at the Service of Linguistic Resources of the Universitat Rovira i Virgili.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
SI, AHA, JJ, JC, and AC were funded by a grant from Fundació La Marató de TV3 (201807-10), Barcelona, Spain. Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vahidy FS, Drews AL, Masud FN, Schwartz RL, Boom ML, Phillips RA, et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston metropolitan area. JAMA. 2020; 324: 998–1000. 10.1001/jama.2020.15301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2020; 10.1111/tbed.13819 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Saito S, Asai Y, Matsunaga N, Hayakawa K, Terada M, Ohtsu H, et al. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics: Comparison of the two COVID-19 waves in Japan. J Infect. 2020: S0163-4453(20)30693-9. 10.1016/j.jinf.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renardy M, Eisenberg M, Kirschner D. Predicting the second wave of COVID-19 in Washtenaw County, MI. J Theor Biol. 2020; 507: 110461. 10.1016/j.jtbi.2020.110461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looi MK. Covid-19: Is a second wave hitting Europe? BMJ. 2020; 371: m4113. 10.1136/bmj.m4113 [DOI] [PubMed] [Google Scholar]
- 6.Win A. Rapid Rise of COVID-19 second wave in Myanmar and implications for the Western Pacific Region. QJM. 2020; hcaa290. 10.1093/qjmed/hcaa290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballester-Arnal R, Gil-Llario MD. The virus that changed Spain: Impact of COVID-19 on people with HIV. AIDS Behav. 2020; 1–5. 10.1007/s10461-019-02470-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long SW, Olsen RJ, Christensen PA, Bernard DW, Davis JJ, Shukla M, et al. Molecular architecture of early dissemination and massive second wave of the SARS-CoV-2 virus in a major metropolitan area. mBio. 2020; 11: e02707–20. 10.1128/mBio.02707-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodcroft EB, Zuber M, Nadeau S, Comas I, González Candelas F, et al. Emergence and spread of a SARS-CoV-2 variant through Europe in the summer of 2020. medRxiv. 2020.10.25.20219063 [Preprint]. 2020 [cited 2020 November 18].
- 10.Yang J, Chen X, Deng X, Chen Z, Gong H, Yan H, et al. Disease burden and clinical severity of the first pandemic wave of COVID-19 in Wuhan, China. Nat Commun. 2020; 11: 5411. 10.1038/s41467-020-19238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iftimie S, López-Azcona AF, Vicente-Miralles M, Descarrega-Reina R, Hernández-Aguilera A, Riu F, et al. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLoS One. 2020; 15: e0234452. 10.1371/journal.pone.0234452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonanno P, Galletta S, Puca M. Spatial dynamics of SARS-CoV-2 and reduced risk of contagion: evidence from the second Italian epidemic wave. medRxiv 2020.11.08.20227934 [Preprint]. 2020 [cited 2020 November 18].
- 13.Aleta A, Moreno Y. Age differential analysis of COVID-19 second wave in Europe reveals highest incidence among young adults. medRxiv 2020.11.11.20230177[Preprint]. 2020 [cited 2020 November 18].
- 14.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020; July 17 10.1056/NEJMoa2021436 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: Recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020; 27: 4536–4541. 10.2174/0929867327666200416131117 [DOI] [PubMed] [Google Scholar]
- 16.RECOVERY Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020; 396: 1345–1352. 10.1016/S0140-6736(20)32013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam S, Lombardi A, Ouanounou A. COVID-19: A review of the proposed pharmacological treatments. Eur J Pharmacol. 2020; 886: 173451. 10.1016/j.ejphar.2020.173451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-19 RISK and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: Findings from the observational multicentre Italian CORIST study. Eur J Intern Med. 2020; 82: 38–47. 10.1016/j.ejim.2020.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ip A, Ahn J, Zhou Y, Goy AH, Hansen E, Pecora AL, et al. Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study. BMC Infect Dis. 2021; 21: 72. 10.1186/s12879-021-05773-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ran J, Zhao S, Han L, Liao G, Wang K, Wang MH, et al. A re-analysis in exploring the association between temperature and COVID-19 transmissibility: an ecological study with 154 Chinese cities. Eur Respir J. 2020; 56: 2001253. 10.1183/13993003.01253-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ran J, Zhao S, Han L, Chen D, Yang Z, Yang L, et al. The ambient ozone and COVID-19 transmissibility in China: A data-driven ecological study of 154 cities. J Infect. 2020; 81: e9–e11. 10.1016/j.jinf.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ran J, Zhao S, Han L, Qiu Y, Cao P, Yang Z, et al. Effects of particulate matter exposure on the transmissibility and case fatality rate of COVID-19: A Nationwide Ecological Study in China. J Travel Med. 2020; 27: taaa133. 10.1093/jtm/taaa133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020; 145: e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 24.Shen KL, Yang YH, Jiang RM, Wang TY, Zhao DC, Jiang Y, et al. Updated diagnosis, treatment and prevention of COVID-19 in children: experts’ consensus statement (condensed version of the second edition). World J Pediatr. 2020; 16: 232–239. 10.1007/s12519-020-00362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: Maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020. March 17. 10.5858/arpa.2020-0901-SA Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020; 395: 760–762. 10.1016/S0140-6736(20)30365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanish Ministry of Health. Questions and answers about the new coronavirus (COVID-19). Updated October 2, 2020. 2020; 1–4.
- 28.Di Castelnuovo A, Bonaccio M, Costanzo S, Gialluisi A, Antinori A, Berselli N, et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020; 30: 1899–1913. 10.1016/j.numecd.2020.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.COVID-19 RISk and Treatments (CORIST) Collaboration. RAAS inhibitors are not associated with mortality in COVID-19 patients: Findings from an observational multicenter study in Italy and a meta-analysis of 19 studies. Vascul Pharmacol. 2020; 135: 106805. 10.1016/j.vph.2020.106805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Gennaro F, Marotta C, Storto M, D’Avanzo C, Foschini N, Maffei L, et al. SARS-CoV-2 transmission and outcome in neuro-rehabilitation patients hospitalized at neuroscience hospital in Italy. Mediterr J Hematol Infect Dis. 2020; 12: e2020063. 10.4084/MJHID.2020.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.