Abstract
Sarin is a highly toxic nerve agent that was developed for chemical warfare during World War II and is used in present conflicts. Immediate effects of acute sarin exposure are established; however, whether effects persist after initial signs have subsided is debated. The National Toxicology Program (NTP) conducted a systematic review to evaluate the evidence for long-term neurological effects following acute (<24 hour) exposure to sarin. The literature search and screening process identified 32 data sets within the 34 human studies and 47 data sets within the 51 animal studies (from 6,837 potentially relevant references) that met the objective and the inclusion criteria. Four main health effect categories of neurological response were identified as having sufficient data to reach hazard conclusions: (1) cholinesterase levels; (2) visual and ocular effects; (3) effects on learning, memory, and intelligence; and (4) morphology and histopathology in nervous system tissues. NTP concluded that acute sarin exposure is known to be a neurological hazard to humans in the period following exposure up to 7 days and suspected to be a hazard week to years after exposure, given a lower level of evidence in later time periods. Effects included reduced cholinesterase, visual and ocular effects, impaired learning and memory, and altered nervous system morphology. Further mechanistic, targeted animal studies, translational research, and rapid research responses after human exposures may reduce uncertainties on long-term consequences of sarin.
Keywords: sarin, long-term effects, chemical weapon, nerve agent, National Toxicology Program
1. Introduction
Sarin (CAS #: 107-44-8; GB; Isopropylmethylphosphonofluoridate) is a nerve agent developed for chemical warfare during World War II. This highly toxic chemical agent can cause death, seizures, and immediate symptoms caused by acetylcholinesterase (AChE; EC 3.1.1.7) inhibition, an increase in acetylcholine levels, and overstimulation of cholinergic receptors (Hulse et al. 2019). Sarin is sometimes referred to as “sarin nerve gas” even though it is a liquid at ambient temperatures. It is also known as GB, which is a two-character identifier assigned by the North Atlantic Treaty Organization (NATO). Sarin belongs to a chemically diverse group of organophosphorus chemicals that have at least one carbon atom bound to a phosphorous atom. The group includes other chemical weapons and agricultural pesticides such as ethyl parathion (CAS #: 56-38-2; parathion, phosphorothioic acid, O,O-diethyl O-(4-nitrophenyl) ester). This group will collectively be referred to as organophosphorus agents (OPs) in this article. Chemical weapons such as sarin are of immediate concern because of their current use in conflicts in the Middle East, and because of recent assassinations such as the Novichok incident in the United Kingdom, and the poisoning of Kim Jong-nam in Kuala Lumpur. Although prohibited by international treaties, it is likely that sarin continues to be used in conflicts around the world, as reported by the United Nations in Syria in 2013 (Sellström A 2013). Large-scale use of sarin in acts of terrorism are also of major concern, such as the Tokyo subway incident in 1995 when members of the terrorist group Aum Shinrikyo released sarin into rail cars resulting in several fatalities and over a thousand victims needing immediate medical attention (Yanagisawa et al. 2006).
Studies of incidents where sarin attacks and exposure have occurred indicate that survivors of the initial lethal effects of sarin may suffer long-term serious health effects. Neurological effects seem to be the most commonly reported long-term effect, but the data, especially in humans, are sparse and heterogeneous. In partnership with the National Institutes of Health (NIH) Countermeasures Against Chemical Threats (CounterACT) program, the National Toxicology Program (NTP) in the United States (U.S.) conducted a comprehensive systematic review to evaluate the evidence for long-term neurological effects following acute exposure to sarin. This article will summarize the NTP monograph on sarin, which is a an extensive, peer-reviewed systematic review that evaluates the strength of the available data for evidence of potential long-term neurological effects associated with acute sarin exposure. The original protocol, literature search results, and full detailed monograph can be found on the NTP website at https://ntp.niehs.nih.gov/go/sarin (NTP 2019).
The NTP systematic review was initiated because of observations in the literature of long-term neurological effects of sarin poisoning in humans, such as case reports of victims in the Matsumoto and Tokyo subway attacks suffering long-term behavioral abnormalities and altered brain morphology (Murata et al. 1997; Yamasue et al. 2007b), and reports in experimental animal studies of the long-term neurological, behavioral, and neurophysiological effects of sarin (Burchfiel and Duffy 1982; Kassa et al. 2001b). The goal of the review was to critically and transparently evaluate the publicly available evidence and to identify areas of research that would reduce uncertainty in our understanding of long-term neurological effects and potential medical mitigation following acute exposure to sarin. The scientific question being addressed is whether the human, animal, and mechanistic literature support evidence of long-term neurological effects following exposure to sarin. This article and the NTP monograph describe the evaluation and integration of the available human, animal and mechanistic evidence and, as described in the NTP’s systematic review and evidence integration approach (Rooney et al. 2014), the development of hazard identification conclusions as to the evidence of long-term neurological effects in humans.
Several literature reviews of the long-term neurological effects following exposure to sarin have been published previously (Brown and Brix 1998; IOM 2004; White et al. 2016). Many of these reviews, however, have assessed health effects in military personnel during conflicts such as the Gulf War and are confounded by concurrent mixed exposures to other chemicals including other chemical warfare agents. Although other OPs may cause long-term neurological effects through similar mechanisms, there may be differences in health effects, potencies, and durations of effects associated with exposures to different OPs and other toxic chemicals. While results from studies on other OPs may support findings of long-term neurological effects following acute sarin exposure, these data were beyond the scope of this review. The NTP systematic review was developed to focus on a specific data set where sarin is the only suspected exposure. Many of the previous reviews also include chronic exposures to lower levels of various chemical agents. Chronic exposure to nerve agents and pesticides is a serious concern, but the focus of the NTP systematic review was on acute exposures that may occur after a deliberate release or mass causality accident. Prior to this evaluation, a systematic review of the evidence of sarin toxicity has not been performed in which selection criteria were clearly stated and consistently applied; where a broad hierarchy of evidence is considered including all evidence streams (human, animal and mechanistic); where a broad range of human study designs are considered including uncontrolled studies and case-reports or case series; and in which individual studies were assessed for internal validity or risk-of-bias.
The focus of this review is on neurological effects that are observed at any time after initial acute effects have subsided. Such persistent or long-term neurological health effects may be observed several hours, days, weeks, or years after the acute toxicity subsides. In this evaluation, long term is considered any effect occurring more than 24 hours after exposure. The 24-hour time point was selected to reflect the possible variation in time for acute effects to subside due to differing exposure levels and individual responses. For the purpose of characterizing outcomes, the time after exposure was divided into three time periods to better capture effects related to sarin exposure in the days (initial time period, potentially including some acute effects), weeks (intermediate time period, not anticipated to include acute effects), and years (extended time period) after exposure (Table 1). This was done to provide some insight as to the evolution of long-term effects and whether long-term effects resolve or persist.
Table 1.
Definition of time periods after exposure.
| Time period after exposure | Humans and Nonhuman primates | Animals except Nonhuman primates |
|---|---|---|
| Initial time period | >24 hours–7 days | >24 hours–7 days |
| Intermediate time period | 8–364 days | 8 days–90 days (or 3 months) |
| Extended time period | ≥1 year | >90 days |
The research questions for the systematic review were developed and refined through a series of problem formulation steps including: (1) consideration of reports in the literature as to whether long-term neurological effects of OPs in humans are a common occurrence; (2) discussion of these reports and related questions on long-term neurological health effects of OPs at two NIH workshops; and (3) development of the systematic review subcommittee to refine the research question and specific aims, and to develop a draft protocol for conducting the systematic review. The systematic review protocol (https://ntp.niehs.nih.gov/ntp/ohat/sarin/sr_protocol_508.pdf) was peer reviewed and finalized before conducting the review. A summary of the protocol is presented in the Materials and Methods section of this article.
The methodological approach for the systematic review followed that of the NTP Office of Health Assessment and Translation (Program 2015; Program 2019). Literature that assessed long-term neurological health effects following acute exposure to sarin in human, animal, and in vitro/mechanistic studies were first identified (full literature search results available at https://ntp.niehs.nih.gov/ntp/ohat/sarin/literature_results_508.pdf). After selecting articles based on inclusion/exclusion criteria, data were then extracted on potential long-term neurological health effects from relevant studies, and individual studies were assessed for internal validity based on risk-of-bias using predefined criteria. Depending on the extent and nature of the available data, the evidence was synthesized using a narrative approach rather than a meta-analysis, considering limitations on data integration such as the heterogeneity of study design and outcome measures. Confidence in the body of evidence for human and animal studies were rated separately according to risk-of-bias, magnitude of effect, dose-response, and other concerns (Figure 1), and then translated into levels-of-evidence ratings of health effects for human and animal studies separately (see Figure 2 in NTP 2019). Levels-of-evidence ratings for human and animal data were then used to reach one of five possible hazard identification conclusions: Known, Presumed, Suspected, Not Classifiable, or Not Identified to be a Hazard to Humans, after consideration of mechanistic data identified by the literature search (Figure 2).
Fig. 1.
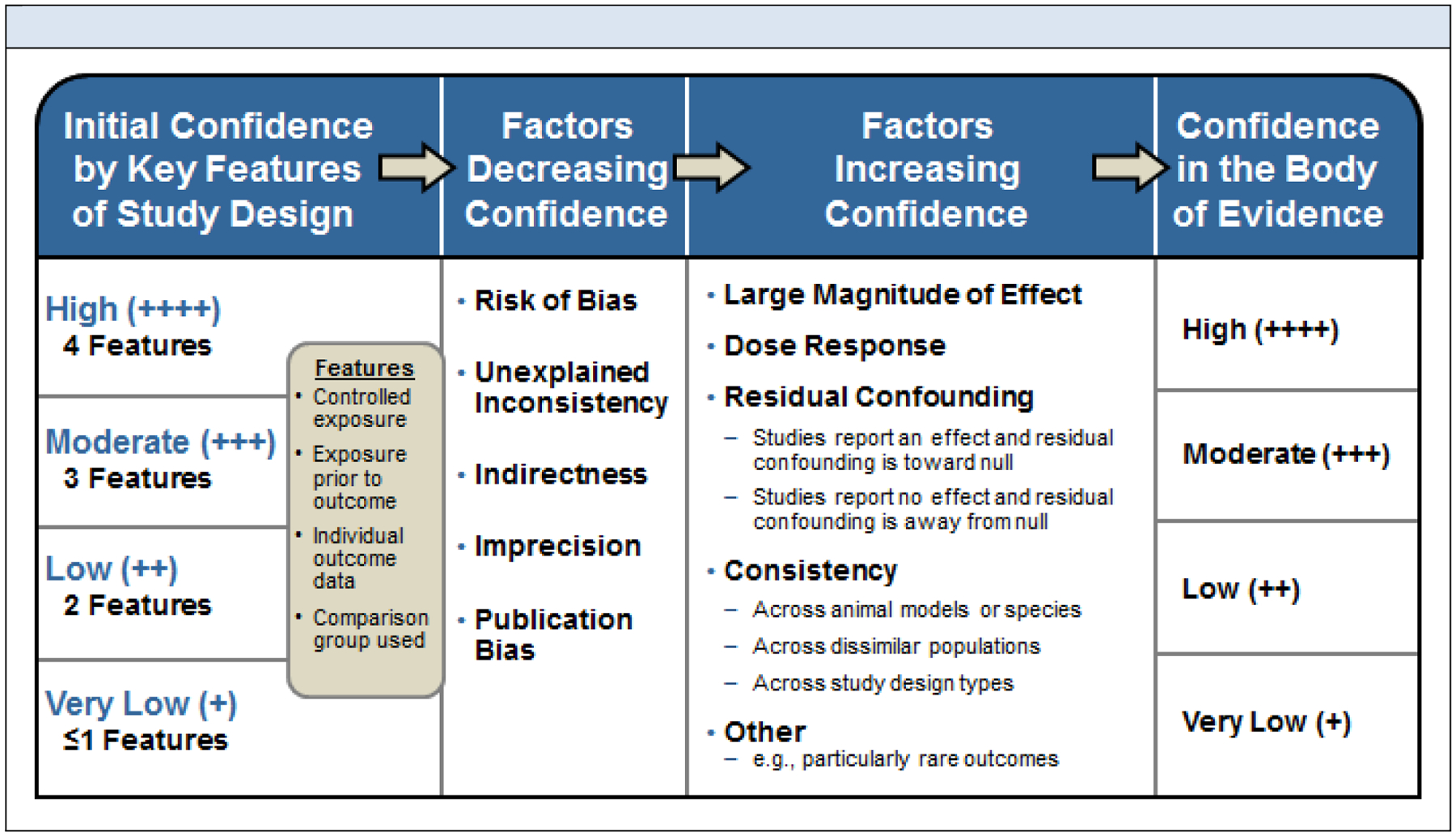
The process for assessing confidence in the body of evidence. Initial confidence is assessed by key factors of study design. Factors are then considered that decrease and increase confidence before determination of confidence in the body of evidence.
Fig. 2.
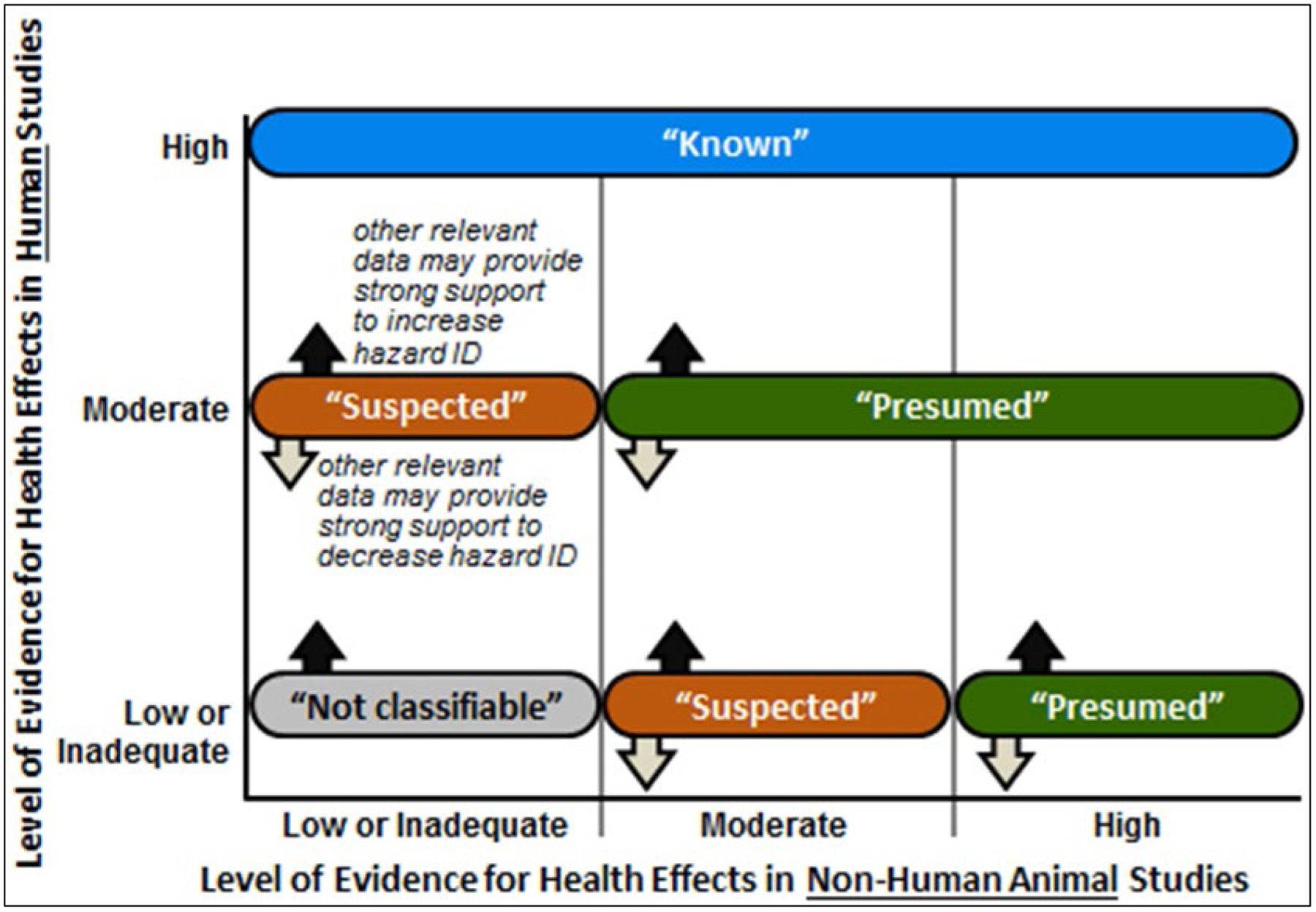
Hazard identification scheme for long-term neurological effects of acute sarin exposure. Initial hazard identification conclusions were determined by integrating the highest level-of-evidence conclusion for long-term neurological health effect(s) on an outcome basis for the human (y-axis) and the animal (x-axis) evidence streams. The level of evidence conclusion for human data for that health outcome was considered together with the level of evidence for animal data to reach one of four initial hazard identification conclusions. When either the human or animal evidence stream was characterized as inadequate evidence for a particular health effect, then conclusions were based on the remaining evidence stream alone.
2. Material and Methods
2.2. Protocol development and literature search
The full protocol for the systematic review was peer reviewed and finalized prior to conducting the literature search and analysis, and it can be found at https://ntp.niehs.nih.gov/ntp/ohat/sarin/sr_protocol_508.pdf. A brief summary of the methods is presented below, and it is also presented in the full monograph (NTP 2019). Using the population, exposure, comparator, and outcome (PECO) statements (Tables 1–3 in NTP 2019), the evaluation searched for evidence of long-term neurological effects associated with acute sarin exposure from human studies across a broad range of study design types along with controlled exposure animal studies and mechanistic/in vitro studies. Search terms were developed to identify all relevant published evidence that addresses the research question on long-term neurological health effects potentially associated with acute, sublethal exposure to sarin in humans and animals. This was accomplished by (1) using the search term “sarin” and related synonyms “GB” or sarin’s IUPAC ID “(RS)-propan-2-yl methylphosphonofluoridate” and (2) without restriction by health outcome or key words to identify long-term neurological effects. A test set of relevant studies was used to ensure that the search terms retrieved 100% of the test set. Eight electronic databases were searched using a search strategy tailored for each database (Cochrane Library, DTIC, EMBASE, NIOSHTIC, PubMed, Scopus, Toxline, Web of Science). The specific search terms used and the search strategy for the databases are available in the protocol (https://ntp.niehs.nih.gov/ntp/ohat/sarin/sr_protocol_508.pdf). No language restrictions or publication year limits were imposed, and the databases were searched in April 2016, with several updated searches and a final search conducted on October 25, 2018. The reference lists of all included studies, relevant reviews or reports, commentaries or letters on specific studies, and other non-research articles were manually searched for additional relevant publications. In addition, the monograph went through peer-review and public comment periods. Any studies identified during that time that met PECO were included.
Given that incidents of human exposure to sarin include terrorist attacks and military personnel, the search was conducted to identify the anticipated range of evidence for human studies. Original papers may include non-peer-reviewed studies, for example, reports from U.S. military observational studies, as well as uncontrolled studies, case series, or case reports. In all instances, the paper had to: (1) document exposure to sarin; and confirm both (2) acute symptoms, i.e., cholinergic crisis; and (3) assess and report some long-term neurological health effects from the exposure. Unpublished data were eligible for inclusion provided the owner of the data was willing to have the data made public and peer reviewed. In order to be eligible for inclusion, studies had to comply with the type of evidence specified by the PECO statements (Tables 1, 2, and 3 in NTP 2019). The following additional exclusion criteria were applied:
Human or animal studies with an exposure duration ≥24 hours, except repeat dose studies in which the outcome is first measured at least 24 hours after the first dose but before any subsequent exposure after 24 hours;
Human controlled studies in which the purpose was only to apply treatment for acute sarin effects;
Human or animal studies with acute exposures to several different chemicals;
Animal treatment/recovery studies that administer sarin and a treatment, unless there is a sarin-only control group;
Human studies with no assessment of health effect outcomes after the cholinergic crisis has subsided;
Animal studies with neurological effects only measured within 24 hours after exposure;
Articles without original data (e.g., editorials or reviews); and
Studies published in abstract form only (grant awards and conference abstracts).
2.3. Screening process and data extraction
References retrieved from the literature search were screened for relevance and eligibility using DistillerSR® by Evidence Partners, a web-based, systematic-review software program with structured forms and procedures to ensure standardization of the process. Search results were first consolidated in Endnote reference management software and duplicate articles were removed prior to uploading the references into DistillerSR®. Screeners from the evaluation team were trained with an initial pilot phase to improve clarity of the evidence selection criteria and to improve accuracy and consistency among screeners. All references were independently screened by two trained screeners at the title and abstract level to determine whether a reference met the evidence selection criteria. Studies that were not excluded by reviewing the title and abstract were screened with a full-text review. Screening conflicts were resolved through discussion. Following full-text review, the remaining studies were included and used for the evaluation.
Data were extracted from included studies by one member of the evaluation team and checked by a second member for completeness and accuracy. Any discrepancies in data extraction were resolved by discussion or consultation with a third member of the evaluation team. Data were extracted as presented in the publications, including reported levels of statistical significance. NTP did not conduct independent statistical analyses to confirm levels of statistical significance reported in the publications nor did they determine statistical significance when study authors did not conduct statistical analyses. Data extraction was completed using the Health Assessment Workspace Collaborative (HAWC), an open-source and freely available web-based interface application (https://hawcproject.org/). Data extraction elements are listed separately for human, animal, and in vitro studies in the protocol (https://ntp.niehs.nih.gov/ntp/ohat/sarin/sr_protocol_508.pdf). The data extraction results for studies that were included are publicly available and can be downloaded in Excel format through HAWC (https://hawcproject.org/assessment/302/).
2.4. Quality assessment of individual studies
Risk-of-bias was assessed for individual studies using a tool developed by the NTP Office of Health Assessment and Translation (OHAT) that outlines a parallel approach to evaluating risk-of-bias from human, animal, and mechanistic studies to facilitate consideration of risk-of-bias across evidence streams with common terms and categories. The risk-of-bias tool comprises a common set of 11 questions that are answered based on the specific details of individual studies to develop risk-of-bias ratings for each question (Table 2). Study design determines the subset of questions used to assess risk-of-bias for an individual study. In the OHAT approach, some risk-of-bias questions or elements are considered potentially more important when assessing studies because there is more empirical evidence that these areas of bias have a greater impact on estimates of the effect size or because these issues are generally considered to have a greater effect on the credibility of study results in environmental health studies (Rooney et al. 2016).
Table 2.
Questions in risk -of-bias assessment and applicability to study design.
| Questions to Determine Risk-of-Bias | Experimental Animal | Human Controlled Trials | Cohort | Case-Control | Cross-Sectional | Case Series |
|---|---|---|---|---|---|---|
| 1. Was administered dose or exposure level adequately randomized? | X | X | ||||
| 2. Was allocation to study groups adequately concealed? | X | X | ||||
| 3. Did selection of study participants result in the appropriate comparison groups? | X | X | X | |||
| 4. Did study design or analysis account for important confounding and modifying variables? | X | X | X | X | ||
| 5. Were experimental conditions identical across study groups? | X | |||||
| 6. Were research personnel blinded to the study group during the study? | X | X | ||||
| 7. Were outcome data complete without attrition or exclusion from analysis? | X | X | X | X | X | X |
| 8. Can we be confident in the exposure characterization? | X | X | X | X | X | X |
| 9. Can we be confident in the outcome assessment (including blinding of outcome assessors)? | X | X | X | X | X | X |
| 10. Were all measured outcomes reported? | X | X | X | X | X | X |
| 11. Were there no other potential threats to internal validity? | X | X | X | X | X | X |
There were three key questions for observational human studies: confounding, exposure characterization, and outcome assessment. There were also three key questions for experimental animal studies: randomization, exposure characterization, and outcome assessment. When there was insufficient information to assess the potential bias for a risk-of-bias question and authors did not respond to an inquiry for further information, a conservative approach was followed, and the studies were rated as “probably high” risk-of-bias for that question. Assessors were trained with an initial pilot phase undertaken to improve clarity of rating criteria and to improve consistency among assessors. Studies were independently evaluated by two trained assessors who answered all applicable risk-of-bias questions with one of four options following pre-specified criteria detailed in the protocol (Table 5 in NTP 2019). The criteria describe aspects of study design, conduct, and reporting required to reach risk-of-bias ratings for each question and specify factors that can distinguish among ratings (e.g., what separates “definitely low” from “probably low” risk-of-bias).
2.5. Assessment of body of evidence and confidence rating
The quality of evidence within groups of neurological effects was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system for rating the confidence in the body of evidence (Guyatt et al. 2011; Rooney et al. 2014). More detailed guidance on reaching confidence ratings in the body of evidence as “high,” “moderate,” “low,” or “very low” is provided in the OHAT Handbook for Conducting a Literature-Based Health Assessment (Program 2019). In brief, available human and animal studies on a particular health outcome were initially grouped by key study design features, and each grouping of studies was given an initial confidence rating by those features (Figure 1). Starting at this initial rating (column 1 of Figure 1), potential downgrading of the confidence rating was considered for factors that decrease confidence in the results (column 2 of Figure 1); and potential upgrading of the confidence rating was considered for factors that increase confidence in the results (column 3 of Figure 1). Consideration of consistency across study designs, human populations, or animal species is not included in the GRADE guidance (Guyatt et al. 2011); however, it is considered in the modified version of GRADE used by OHAT (Program 2019). Confidence ratings were independently assessed by the NTP evaluation team, NIH CounterACT personnel, and other NTP analysts for accuracy and consistency, and discrepancies were resolved by consensus and consultation with technical advisors as needed (see NTP 2019 for personnel rosters). Confidence ratings for the primary outcomes are summarized in the monograph (NTP 2019) with evidence profile tables for each outcome.
2.6. Integration of evidence to develop hazard identification conclusions
The confidence ratings were translated into level of evidence of health effects for each type of health outcome separately according to one of four statements: (1) High, (2) Moderate, (3) Low, or (4) Inadequate (Figure 2 in NTP 2019). The descriptor “evidence of no health effect” is used to indicate confidence that the substance is not associated with a health effect. Because of the inherent difficulty in proving a negative, the conclusion “evidence of no health effect” is only reached when there is high confidence in the body of evidence. Finally, the levels of evidence ratings for human and animal data were integrated with consideration of in vitro/mechanistic data to reach one of five possible categories of evidence of long-term neurological health effect: (1) Known, (2) Presumed, (3) Suspected, (4) Not Classifiable, or (5) Not Identified to be a Long-term Neurological Effect in Humans (Figure 2). Initial hazard identification conclusions were attempted by integrating the highest level-of-evidence conclusion for long-term neurological health effect(s) on an outcome basis for the human and the animal evidence streams. The level of evidence conclusion for human data was considered together with the level of evidence for animal data to reach one of the four initial hazard identification conclusions as to the evidence of long-term neurological effects in humans. When either the human or animal evidence stream was characterized as inadequate evidence for a particular health effect, then conclusions were based on the remaining evidence stream alone, which is equivalent to treating the missing evidence stream as “Low”.
An evaluation of biological plausibility of the health effect evidence, including the consideration of how relevant mechanistic data may impact the certainty in the evidence, is important for integrating human, animal, and in vitro data to reach hazard identification conclusions. However, the absence of clear mechanistic or mode of action data on how acute sarin exposure leads to long-term neurological health effects does not preclude reaching conclusions. During synthesis, this and other types of relevant supporting evidence may be used to raise (or lower) the category of the hazard identification conclusion. This source of experimental data includes in vitro and in vivo laboratory studies directed at cellular, biochemical, genetic, and molecular mechanisms that explain how a chemical produces particular adverse effects. For the evaluation of long-term neurological health effects associated with acute exposure to sarin, NTP was interested in mechanistic or in vitro measures that may support the biological plausibility of corresponding neurological outcomes reported from in vivo studies in animals or humans. The PECO statement in Table 3 of NTP 2019 provides the specific endpoints considered, mainly including survival and morphology of neurons or glia. For this assessment, no in vitro studies following these criteria were identified for any time period following exposure (including <24 hours).
3. Results
3.1. Literature search results in human and animal evidence
Population, exposure, comparator, and outcome (PECO) statements were developed as an aid to identify search terms and inclusion/exclusion criteria as appropriate for addressing the overall research question of long-term neurological effects of acute sarin exposure (Tables 1, 2, and 3 in NTP 2019). The PECO inclusion statements included humans and animals of any age, single acute dose of sarin, comparator subjects not exposed to sarin, and outcomes related to neurological disease. Most of the available human data were case reports or series, mostly from the sarin attacks in Japan. Publicly available data from other attacks including Syria were not identified for this review. The electronic database searches retrieved 6,837 references. The screening results and study selection diagram with reasons for exclusion documented at the full text review stage are outlined in Figure 3. All 6,837 references were manually reviewed or screened for relevance and eligibility at the title and abstract level by two reviewers. A total of 497 of these studies were further screened at the full text level to identify the 85 studies that were included in the review (i.e., were relevant, published in English, contained original data, etc.). Of the total references retrieved, 93% (6,340) were excluded during the title and abstract screening and 412 references were excluded during the full text review for not satisfying the PECO criteria. After full text review, 85 studies were considered relevant, which included 34 human studies and 51 animal studies. Some studies were excluded because of other factors described in the NTP monograph (NTP 2019), and these included multiple publications presenting the same data, unpublished data without a clear peer-review process (although these data were reviewed and determined not to have any data that would change the hazard conclusions), or publications in a language other than English. The titles, abstracts (if in English), data tables and figures, and study designs for these non-English studies were reviewed and determined that it was unlikely to have data that would change the hazard conclusions. Therefore, these non-English studies were not translated or included in the assessment.
Fig. 3.
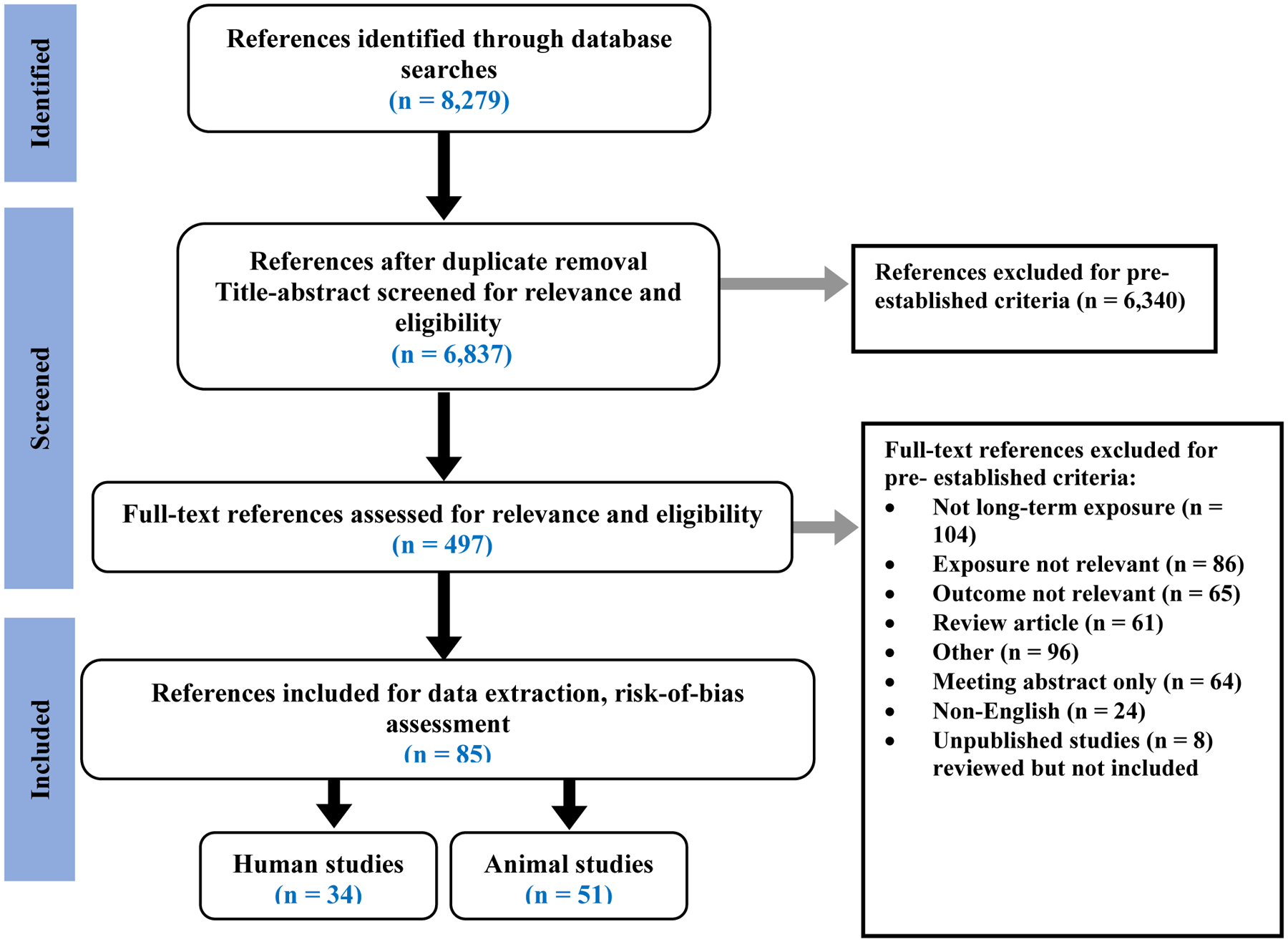
Study selection diagram including articles related to human and animal data on the long-term effects of acute exposure to sarin. Articles were selected based on predetermined eligibility and exclusion/inclusion criteria. A total of 1,442 articles were removed from the original 8,279 because they were duplicates, leaving 6,837 that were screened for relevance and eligibility.
Risk-of-bias ratings for the individual studies for all questions were determined. The key risk-of-bias questions of confounding, exposure characterization, and outcome assessment are discussed in the monograph (NTP 2019) within the consideration of the body of evidence for each health effect. Confidence in the bodies of evidence was downgraded twice if studies were consistently rated probably high or definitely high risk-of-bias for all three key questions. It is usually impossible to know to what extent biases have affected the results of a given study, although there is good empirical evidence that particular flaws in the design, conduct, and analysis of randomized studies lead to bias (Program 2019). Based on these factors, it is important that all studies are evaluated for risk of bias (Program 2019). Below, each section is organized to present an introduction and then a brief summary of the rating of confidence in the body of evidence that sarin exposure is associated with the health effect described in that section for human and animal data separately. Data integration and hazard conclusions conducted according to the OHAT protocol (Figure 3) for that health effect will then be presented. There were four main categories of neurological effects identified based on the availability of data: 1) cholinesterase (ChE) levels; 2) visual and ocular effects; 3) effects on learning, memory, and intelligence; and 4) morphology and histopathology. Confidence ratings and hazard conclusions are presented in italics to signify a final NTP designation for each of the 4 main categories. The level of evidence ratings and final hazard conclusions for all 4 categories are provided in Table 3. At the end of each introduction to the category is a reference to the detailed description of the data in the monograph (NTP 2019). Other neurological effects were analyzed but were not included in the full hazard assessment because of limited data; these included activity and strength, anxiety and fear, avoidance and depression, electroencephalogram (EEG) activity, sleep disruption, other neurological symptoms, and other sensory effects (page 125 in NTP 2019).
Table 3.
NTP Final hazard conclusions by time period. The four possible level of evidence ratings are: High, Moderate, Low, or Inadequate. The five possible NTP final hazard identification conclusions are: Known, Presumed, Suspected, Not Classifiable, or Not Identified to be a Hazard to Humans.
| Outcome | Time Period | Level of Evidence Rating (Human/Animal) | NTP Final Hazard Conclusion |
|---|---|---|---|
| Cholinesterase | Initial | High/Moderate | Known |
| Intermediate | Low/Moderate | Suspected | |
| Extended | Inadequate/Inadequate | Not Classifiable | |
| Vision & Ocular Effects | Initial | Moderate/Inadequate | Suspected |
| Intermediate | Moderate/Inadequate | Suspected | |
| Extended | Low/Inadequate | Not Classifiable | |
| Learning, Memory, & Intelligence | Initial | Inadequate/Low | Not Classifiable |
| Intermediate | Low/Low | Not Classifiable | |
| Extended | Moderate/Low | Suspected | |
| Morphological & Histological Effects | Initial | Inadequate/Moderate | Suspected |
| Intermediate | Inadequate/Moderate | Suspected | |
| Extended | Moderate/Inadequate | Suspected |
3.2. Cholinesterase inhibition effects
Sarin is an OP that inhibits AChE, which leads to an increase in acetylcholine and cholinergic receptor hyperstimulation in the central and peripheral nervous systems. Direct measurements of AChE activity can be made in nervous system tissues, or in erythrocytes. Some studies in this review measured butyrylcholinesterase (BChE) in plasma as a surrogate of AChE activity, and thus the more general term “cholinesterase (ChE)” will be used in the following description. It is noted that BChE has no known role in the toxicity of OPs but it is abundant in human plasma and can be inhibited by OPs at levels that do not cause toxicity or inhibition of RBC AChE (Chen et al. 1999). Therefore except for a potential role in the sequestration of circulating sarin, plasma BChE has limitations as a true surrogate for tissue and RBC AChE. It is well-established that AChE inhibition is biomarker for exposure to OPs, but it is also an effect of OP that mediates the signs and symptoms of acute OP intoxication (Hulse et al. 2019). The signs and symptoms of acute exposure are generally referred to as the cholinergic crisis (e.g., miosis, salivation, lacrimation, rhinorrhea, difficulty breathing, convulsions, seizures, diarrhea). The duration of the cholinergic crisis depends upon the dose and genetic differences in OP toxicokinetics, but it generally subsides in a few days. While it is acknowledged that AChE inhibition may persist for many days after exposure depending on the dose and the amount of time required for the de novo synthesis of AChE to replace AChE inhibited by sarin, for the purposes of this review, the inhibition of AChE is considered a long-term neurological health effect of sarin if it persists for more than 24 hr after exposure. There is evidence from animal studies that control of seizures caused by sarin-induced AChE inhibition is associated with protection against brain pathology (Shih et al. 2003), suggesting that other neurological effects may be secondary or tertiary to AChE inhibition. There was insufficient data in this review to determine if the inhibition of AChE or ChE is responsible for other neurological effects, or if other noncholinergic mechanisms (Schuh et al. 2002; Tuin et al. 2009) are involved. For more details on human and animal evidence on cholinesterase inhibition see pages 24–38 in NTP 2019.
3.2.1. Human evidence for cholinesterase effects
There is high confidence in the body of evidence that acute sarin exposure suppresses ChE blood levels in humans over the initial period of 1–7 days following initial exposure, and low confidence that suppression continues over a period of months after exposure (Table 3). The studies that provide data on ChE response in blood for a period of days, including two nonrandomized controlled trials and two case reports, reported consistent evidence of ChE inhibition following acute sarin exposure (Figure D1 in NTP 2019). Similarly, the studies that provide data on ChE response for a period of weeks to months (intermediate period), including six case reports, showed consistent lowering of blood levels of ChE following acute sarin exposure (figs. D2 and D3 in NTP 2019). Although results show consistent lowering of blood levels of ChE for a period of days to months following acute sarin exposure, there are limitations in the body of evidence including small sample sizes (n = 8–10 for the controlled exposure studies), risk-of-bias concerns, and uncertainties related to study design for the case reports. One cross-sectional study that evaluated ChE blood levels 5 years after exposure did not observe a difference in ChE compared to controls (Tochigi et al. 2002) and there was low confidence in the body of evidence (Table 3).
3.2.2. Animal evidence for cholinesterase effects
There is moderate confidence in the body of evidence that acute sarin exposure suppressed ChE blood and brain levels in animals over a period of days to months after the initial exposure (initial and moderate time periods). The results show a consistent lowering of ChE blood levels following acute sarin exposure across multiple studies and at different time periods following exposure (figs. D4 and D5 in NTP 2019). However, there are limitations in the body of evidence, including small sample sizes (n = 2–6 for several studies) and risk-of-bias concerns. The consistent evidence supports suppression of ChE within days following acute sarin exposure, but the length of the suppression varied by study, and there was less evidence for suppression 1 week to 90 days, which is considered relevant for humans 1 week to 12 months after exposure. Downgrades by one or two levels were considered for the probably high risk-of-bias ratings on one key question as well as other questions. Upgrades were considered for several factors: large magnitude of effect (10–85% suppression of ChE), evidence for dose response in some studies, and consistency of effect across species including rodents and nonhuman primates. Considering these opposing factors, the serious risk-of-bias concerns resulted in a downgrade of one level, and no upgrades were applied given the extent of the risk-of-bias concern. Therefore, confidence in the body of evidence for the animal studies was downgraded for both the initial period and intermediate period (up to 90 days) from an initial high confidence to support the final confidence rating of moderate. There is no animal evidence to evaluate the potential association between sarin exposure and effects greater than 90 days after exposure in rodents or 1 year in nonhuman primates.
3.2.3. Data integration and hazard conclusions for cholinesterase inhibition
There is consistent evidence that ChE levels are reduced in humans and animals after acute exposure to sarin, however, the evidence varies depending on the length of time after exposure. There is high confidence in the human data in the initial period after exposure, but lower confidence in the intermediate period after exposure based on limitations largely due to the relative paucity of clinical studies other than case reports. The data in the extended period after exposure are inadequate to evaluate potential effects based on the limited number of studies and the limitations in the one study that was available. There is moderate confidence in the animal data for both the initial period and intermediate period with no data for the extended period after exposure. The uncertainty in the animal evidence is mainly due to lack of reporting information necessary to evaluate risk-of-bias concerns, and the heterogeneity of the data concerning the outcomes when measured, the species or strain used, and the method for administering sarin. Taken together, the human and animal bodies of evidence provide a consistent pattern of findings in the initial period after exposure that acute sarin exposure is associated with decreased ChE levels. The final hazard conclusions are Known to be a Neurological Hazard in humans in the initial time period and Suspected to be a Neurological Hazard in humans in the intermediate time period (Table 3). The body of evidence is inadequate in the extended period due to only a single study in humans, which did not observe an effect at 5 years after exposure.
3.3. Visual and ocular effects
Initial signs of acute intoxication with OPs include narrowing of the pupil of the eye (miosis). This is considered a sensitive and early presentation of acute exposure (Brown and Brix 1998) and a hallmark for OP toxicity. Miosis is also used as the basis for establishing threshold exposure limits for military occupational exposure and is considered as a sign of possible high-level exposure. This section describes long-term visual or ocular effects that are reported to occur after acute sarin exposure. The data include outcomes from medical evaluations of pupillary response, miosis, pupil diameter, visual evoked potential (VEP) and self-reported symptoms such as blurred vision, dimmed vision, double vision, and ocular pain. For more details on human and animal evidence on visual and ocular effects see pages 39–53 in NTP 2019.
3.3.1. Human evidence for visual/ocular effects
Based on the available studies, there is moderate confidence in the body of evidence that acute sarin exposure is associated with visual or ocular effects in humans over the initial period of 1–7 days following initial exposure, moderate confidence that visual or ocular effects persist over the intermediate period of 8 days to 1 year, and low confidence over the extended period of ≥1 year (figs. D12-D14 and Table 9 in NTP 2019). The studies that provide visual or ocular data 1–7 days after acute exposure to sarin, including five case reports/series, showed consistent effects of miosis and other visual or ocular parameters such as visual-field abnormalities and conjunctival hyperemia. The studies that provide data for a period of weeks to months, including eight case series and two cross-sectional studies, showed consistent evidence that miosis occurred but recovered within the first 1–2 months after exposure, whereas other visual or ocular effects persisted from weeks to months in some of the study subjects, and VEPs were found to be significantly slower 6 to 8 months following exposure. The studies that provide data on visual or ocular effects for a period of years, including four case reports/series and one prospective cohort study, showed evidence that other visual or ocular effects such as ocular pain and blurred vision persisted 1–5 years following exposure (Table 9 in NTP 2019). Moderate confidence in the body of evidence for the initial period following exposure is primarily based on the consistent pattern of findings of miosis from the five case series/reports. Moderate confidence in the body of evidence of visual or ocular effects for the intermediate period following exposure is based on two cross-sectional studies supported by data from eight case series, which also reported visual or ocular effects. Low confidence in the body of evidence for visual or ocular effects for the extended period following exposure is based on one cohort study with support from four case reports/series. The initial confidence of moderate for the cohort study was downgraded once for potential biases in outcome assessment from self-reporting of symptoms via questionnaires and loss of subjects over time; this resulted in a final rating of low confidence in the body of evidence for the extended period.
3.3.2. Animal evidence for visual/ocular effects
There is very low confidence in the body of evidence that acute sarin exposure is associated with visual or ocular effects in animals over the intermediate and extended periods. The animal body of evidence consists of three studies (Figure D14 in NTP 2019) that evaluated pupil diameter over the initial period of 1–7 days and one study (Kassa et al. 2001a) that evaluated visual functional observational battery (FOB) scores 3–12 months after acute sarin exposure. In the initial period of 1–7 days following exposure, two studies did not find statistically significant sarin-related ocular effects. One study did observe a sarin-related effect on pupil diameter in the initial period following exposure; however, the pattern of effect was not consistent with other animal data or the human data (Figure D14 in NTP 2019). In the intermediate and extended periods following exposure, one study explored visual parameters 3–12 months after an acute exposure and reported no effect in visual FOB scores (Kassa et al. 2001b). There are multiple limitations in the body of evidence, including a small number of available studies and risk-of-bias concerns. The judgment was reached to downgrade the bodies of evidence for the intermediate and extended periods by three levels to reflect overall concerns across multiple downgrade factors (i.e., risk-of-bias and inconsistency).
3.3.3. Data integration and hazard conclusions for visual and ocular effects
There is evidence that pupil size is reduced (i.e., miosis) in humans 1–7 days after acute exposure to sarin, VEPs are reduced 6–8 months after acute exposure, and other visual and ocular effects persist in humans during the first week and remain for several months to years after exposure. Although there are limitations in the body of evidence in the initial period largely due to study design, there is moderate confidence in the human data in the initial period following acute sarin exposure based on the consistent pattern of findings that miosis occurs 1–7 days after exposure with data supporting miosis persisting for the first several weeks. There is moderate confidence for sarin-associated reductions in VEPs 6–8 months after acute exposure in humans based on two cross-sectional studies with little potential for bias; however, the two studies are presumed to have reported on the same subjects. There is low confidence in the persistence of other visual or ocular effects in the extended period after exposure based on one perspective cohort and four case reports/series due to risk-of-bias concerns. There is very low confidence in the animal data for the intermediate and extended periods following exposure based on one animal study that evaluated ocular effects (e.g., pupil size, pupillary response to light) and did not find evidence of an effect. Although a decrease in pupil diameter 1–7 days was consistently observed in the human data, this effect was harder to assess in the animal data. Two experimental animal studies found no effect on pupil diameter 48 hours or more after exposure (with no measurement available between 24 and 48 hours after exposure), and one experimental animal study observed an increase in pupil diameter 2–7 days following exposure. Taken together, the human and animal bodies of evidence indicate during the initial and intermediate periods that sarin is Suspected to be a Neurological Hazard to Humans, and the data during the extended period after exposure are not classifiable due to low confidence in the body of evidence including consideration of biological plausibility (Table 3).
3.4. Effects on learning, memory, and intelligence
ACh is among the most studied neurotransmitter in the nervous system and is highly involved in learning, memory, and intelligence (Haam and Yakel 2017). Disruption of cholinergic tone by OPs is known to cause deficits in learning and memory in animal studies (Chen 2012; Guignet et al. 2019a) and in some human studies (Figueiredo et al. 2018). Learning, memory, and intelligence are related cognitive functions and these endpoints are discussed together in this section. Tests in humans that are specific for learning (e.g., California Verbal Learning Test [CVLT]), memory (e.g., digit span, self-reported memory loss, memory function tests), and intelligence (e.g., Wechsler Adult Intelligence Scale [WAIS-III]) are considered relevant. Other tests that include a learning or memory component (e.g., digit symbol test, Thurstone word fluency test, Boston naming test) are also considered. For animals, studies that assess maze performance and discrimination learning activities are included. The differential-reinforcement-of-low-rate (DRL) test measures various components of cognition of which short-term memory is only a small portion. The DRL measurement of cognition also involves vigilance, patience, time estimation, excitability of the animal, etc. This test is not discussed here, although it is recognized that it might provide some supporting data. It is also recognized that lack of attention or concentration is a symptom in humans or animals that could affect learning and memory, but these endpoints are not considered in this section, as they were not specifically evaluated in relation to learning and memory issues. For more details of human and animal evidence of learning, memory, and intelligence effects see pages 54–65 in NTP 2019.
3.4.1. Human evidence of learning, memory, and intelligence effects
There is low confidence in the body of evidence that acute sarin exposure is associated with impairments to learning, memory, and intelligence in humans over the intermediate period of 8 days to 1 year after exposure and moderate confidence in the body of evidence for the extended period of ≥1 year after exposure. The studies that provide memory data for the intermediate period, including one cross-sectional study and two case reports (Table 11 in NTP 2019), demonstrated some effect on memory or cognitive function, but there is no consistency in the endpoints measured across studies. The studies that provide memory data for the extended period, including two case series studies and two cross-sectional studies, report evidence of effects on memory and cognitive function years after sarin exposure using different tests for evaluating memory and cognitive function. Although results show a pattern of findings of impaired learning, memory, and intelligence for a period of weeks to years following acute sarin exposure, there are limitations in the body of evidence including risk-of-bias concerns (figs. D25 and D26 in NTP 2019) and uncertainties related to study design of case reports. There is low confidence in the body of evidence for the intermediate period following acute sarin exposure based on one cross-sectional study with a small sample size (n = 18) and two case report studies. None of the studies were downgraded for risk-of-bias concerns. The final rating of low confidence in the intermediate period was supported by heterogeneity of the endpoints evaluated, small sample sizes, and the small number of available studies. Moderate confidence in the body of evidence for the extended period following acute sarin exposure is primarily based on the two cross-sectional studies, which had an initial and final confidence of moderate with support from two case report studies. The two case reports had an initial low confidence rating, which was downgraded to very low confidence for serious risk-of-bias concerns (i.e., failure to control for confounding, potential biases in outcome assessment from self-reporting of symptoms, and few of the initial subjects responded or were included in the study). For the initial period covering 1–7 days following acute sarin exposure, no studies were available.
3.4.2. Animal evidence of learning, memory, and intelligence effects
There is low confidence in the animal body of evidence that acute sarin exposure affects learning and memory over all three time periods (Tables A5–3 and A5–5 in NTP 2019). In rats, the results show some evidence of impaired learning and memory following acute sarin exposure across multiple studies and at different time periods following exposure. The studies in monkeys showed little to no effect, but in many cases, were of limited utility due to small sample sizes. There are limitations in the body of evidence, including small sample sizes and risk-of-bias concerns for the key risk-of-bias questions regarding randomization, exposure assessment, and outcome assessment (figs. D29 and D30 in NTP 2019). The initial high confidence ratings for the animal body of evidence were downgraded once for all time periods for risk-of-bias concerns. For the initial and intermediate time periods, confidence ratings for the animal body of evidence were also downgraded once for imprecision (due to wide ranges in confidence intervals and large standard deviations in the data) to support a final rating of low confidence. The body of evidence for the animal studies in the extended time period was downgraded for inconsistency to support a final rating of low confidence (Table 12 in NTP 2019). An additional downgrade for indirectness was considered for the animal studies given that the tests used as indicators of learning and memory may not have adequately ruled out the role of impaired motor or sensory function. Multiple downgrade factors (i.e., risk-of-bias, inconsistency, indirectness, and imprecision) were considered for all three time periods, and the judgement was reached to downgrade each body of evidence by two levels to reflect the overall concerns.
3.4.3. Data integration and hazard conclusions for learning, memory, and intelligence
Collectively, the human and animal bodies of evidence provide some evidence that acute exposure to sarin may be associated with long-term issues with learning and memory. The human data are mainly based on cross-sectional studies evaluating subjects from the Tokyo subway attack. The animal data in rats support that an acute sarin exposure may affect memory in the initial, intermediate, and extended periods following exposure. In humans, evidence suggests effects on learning and memory in the intermediate and extended periods after sarin exposure, with low to moderate confidence in the body of evidence, respectively. There is no clear evidence to suggest deficits in intelligence as measured by IQ in humans. In animals, there is low confidence that acute sarin exposure is associated with learning and memory effects across all time periods after exposure, with some evidence of effects in the initial and intermediate periods, and inconsistent results from two studies in the extended time period. Final hazard conclusions for the initial and intermediate periods after consideration of biological plausibility were not possible due to low confidence in the human and animal data. For the effects during the extended period after exposure, the confidence in the human data was higher than the initial and intermediate periods, and final hazard conclusion for this period after consideration of biological plausibility is Suspected to be a Neurological Hazard to humans (Table 3).
3.5. Effects on morphology and histopathology
Morphological or histological changes in neural tissue are direct measures of neurological damage. It is important to note that many types of pathology observed in the human nervous system can be readily modeled in experimental animals. There is a general paucity of relevant human data, in part because the resources required such as magnetic resonance imaging (MRI) or positron emission tomography (PET) are not routinely available for assessing neurological damage in living individuals, and because histopathological analyses, which can only be performed after death, are difficult to conduct in such a way as to obtain high-quality data. However, some human studies are available that examined morphological and histological changes in nervous system tissues (including brain, spine, and sural nerve) of subjects who were accidently exposed to low levels of sarin during a military operation or during the Tokyo subway attack. Despite the small number of studies, any human data on this endpoint were considered highly informative and therefore have been included and assessed in this report. Changes to muscle tissue are not considered in this section because the focus of the section is neurological effects; however, it should be noted that any muscle effects could also be related to some of the neuromuscular effects observed. For more details of human and animal evidence of effects on morphology and histopathology see pages 66–75 in NTP 2019.
3.5.1. Human evidence of morphology and histopathology effects
There is moderate confidence in the body of evidence that acute sarin exposure is associated with morphological and histological changes in human neurological tissues in the extended period. The human body of evidence consists of two case reports and one cross-sectional study (Tables 13 and 14 in NTP 2019) that evaluate effects months to years after sarin exposure. None of the studies provide data on morphological and histological changes over a period of days or weeks after exposure. A single case report was available that evaluated morphological or histological changes in neurological tissue at 8 months following sarin exposure but found no abnormalities during an MRI examination of the brain and spine (Loh et al. 2010). The studies that provide data on morphological and histological changes in nervous tissue ≥1 year after exposure, including one cross-sectional (Yamasue et al. 2007a) and one case report (Himuro et al. 1998), report evidence of morphological and histological changes to human nervous system components following acute sarin exposure. The moderate confidence in the body of evidence is based mainly on the cross-sectional study with an initial and final confidence of moderate and support from the one case report. While the case report had an initial and final rating of low confidence due to general limitations based mainly on the study design not having a control for comparison, the study assessed damage to the brain against a normal standard, which could potentially increase the confidence in the case reports. There are inadequate data to assess the relationship between sarin and morphological changes in the initial and intermediate time periods after exposure due to the lack of available data. The available epidemiological studies in the human body of evidence that evaluated the association between acute exposure to sarin and morphological and histological changes in the human nervous system tissues evaluated the outcomes months to years after the initial exposure (Table 13 in NTP 2019). There are no studies that specifically evaluated morphological and histological changes in the human nervous system within days or weeks after exposure. Two studies (one cross-sectional and one case report) were conducted in adults who were exposed during the Tokyo subway sarin terrorist attack. The third study was a case report of a military man deployed in Iraq who was exposed while disarming an improvised explosive device (IED) containing sarin.
3.5.2. Animal evidence of morphology and histopathology effects
There is moderate confidence in the body of evidence that acute sarin exposure is associated with morphological and histological changes in neurological tissues in animals over the initial period and intermediate period after exposure (Table 14 in NTP 2019). The results provide consistent evidence of sarin-related effects related to nervous tissue changes within the first 7 days and through 90 days following acute sarin exposure. However, there are limitations in the body of evidence, including serious risk-of-bias concerns (figures D33 and D34 in NTP 2019). In addition, although the staining methods used allowed the authors to detect morphologic changes in nervous tissue, modern techniques that provide a more comprehensive assessment of underlying neuropathology not revealed by classical Nissl/H&E staining were not employed, and therefore the full extent of the morphological changes may not have been detected and reported, suggesting a bias toward the null. Downgrades of one or two levels were considered for the risk-of-bias concerns. Downgrade considerations were also made for the opposing issue of the impact of changes in histological techniques. The decision was reached to downgrade the bodies of evidence one level to reflect the overall concerns. The initial high confidence in the animal body of evidence was downgraded for risk-of-bias concerns related to randomization, exposure assessment, and outcome assessment to support a final rating of moderate confidence for a period of days to months following acute sarin exposure. There were no data available in animals for the extended period.
3.5.3. Data integration and hazard conclusions for morphology and histopathology effects
There is evidence to suggest morphological and histological changes in human and animal nervous tissue following acute exposure to sarin. There is moderate confidence in the human data for sarin-associated nervous tissue effects in the extended period based on one cross-sectional study with support from one case report with little potential for bias. The body of evidence prior to a year (i.e., in the initial and intermediate periods) is inadequate in humans. There is moderate confidence that acute sarin exposure is associated with nervous tissue effects in animals based on the consistency of the findings in rats through 90 days after exposure. These confidence ratings translate directly into level-of-evidence conclusions and support an initial hazard identification conclusion of suspected to be a neurological hazard to humans; and a final hazard conclusion after consideration of biological plausibility is Suspected to be a Neurological Hazard to Humans (Table 3). Similarly, the neurological effects in the intermediate and extended periods after exposure also were consistent with a final hazard conclusion after consideration of biological plausibility to be Suspected to be a Neurological Hazard to Humans. Collectively, the human and animal bodies of evidence provide consistent patterns of findings that acute exposure to sarin is associated with morphological and histological changes in nervous tissue.
4. Discussion
4.1. What is the biological plausibility of long-term neurological effects?
The mechanism(s) by which acute sarin exposure causes long-term effects are suggested by several studies but have yet to be well-defined. It is accepted that OPs, including sarin, trigger hyperstimulation of cholinergic receptors as a consequence of rapid AChE inhibition. This effect leads to respiratory failure via peripheral and central mechanisms (Shaffo et al. 2018) and seizures via central mechanisms (Figueiredo et al. 2018). It is believed that seizures are a prerequisite for the neuropathology observed after exposure to AChE-inhibiting compounds (Chen 2012; Shih et al. 2013). A study of a subset of the data in this systematic review was planned to assess this observation in sarin-exposed humans, but unfortunately, the limited number of studies and heterogeneity of the data did not allow for a subset that would support the analysis. Hyperstimulation of cholinergic drive in the central nervous system (CNS) disrupts the balance of glutamatergic and GABAergic activity, resulting in excitotoxicity (Chen 2012). The initial excitotoxicity is thought to initiate a secondary excitotoxic and neurodegenerative cascade that may last hours, day, weeks or even months beyond the initial cholinergic crisis (Chen 2012; M and PJ 2019). AChE inhibition and other effects may generate hypoxia which contributes to both the initial and sustained excitotoxic responses in the CNS, both of which are typically accompanied by significantly elevated levels of intracellular calcium (Deshpande and DeLorenzo 2019), robust neuroinflammatory responses (Collombet 2011; M and PJ 2019) and oxidative stress (Pearson-Smith and Patel 2019). Studies in animals have linked oxidative stress with acute and chronic exposures to OPs (Pearson-Smith and Patel 2019), and the highly potent OP soman (GD; O-pinacolyl methylphosphonofluoridate) causes changes in brain region oxygenation after sublethal doses that cause seizures (Lee et al. 2018). Hypoxia and oxidative stress are important considerations because they may be effects unrelated to OP-induced seizures, and both hypoxia and oxidative stress have been linked to many neurological diseases and disorders (Carvalho et al. 2017).
The few mechanistic animal studies conducted specifically with sarin are consistent with the above studies of other OPs. For example, rats exposed to sublethal doses of sarin exhibited significant cell death and neurodegeneration in the CNS associated with changes in apoptotic proteins and an early bi-phasic activation of astrocytes (Lazar et al. 2016; Lewine et al. 2018; Lumley et al. 2019), and proteomic studies of sarin-exposed rats are beginning to unmask details of the excitotoxicity and other mechanisms described above (Chaubey et al. 2017). Additional rodent studies have been published regarding potential long-term effects associated with acute sarin exposure. Of note are several publications addressing visual and ocular effects, impaired learning and memory, as well as histopathology in the brain in rat models of acute sarin exposure. Direct exposure of the eye to sarin was reported to cause dose-dependent miosis, reduced visual function, and ocular surface histopathological lesions that persisted days to weeks post-exposure (Egoz et al. 2017; Gore et al. 2019). Acute sarin intoxication also caused significantly impaired performance of rats in the Morris water maze test of learning and memory that persisted for up to 3 months post-exposure (Lewine et al. 2018; Lumley et al. 2019). Impaired learning and memory were significantly correlated to histopathological evidence of neurodegeneration in key brain regions involved in performance in the Morris water maze.
The long-term effects associated with acute sarin exposure that were identified in this review have also been reported as long-term effects associated with acute exposures to other OPs and have been presented in several recent review articles (Guignet et al. 2019a; Pereira et al. 2014). An additional long-term effect not identified in this review is electrographic abnormalities, including spontaneous recurrent seizures. This is increasingly being reported in rodent models of OP-induced status epilepticus (Guignet et al. 2019b; Putra et al. 2019; Shrot et al. 2014). Spontaneous recurrent seizures, which persist for intermediate and extended periods of time following acute OP intoxication, have also recently been reported as a long-term effect in at least two different studies of rats acutely intoxicated with sarin (Lewine et al. 2018; Lumley et al. 2019). There are some reports in the literature suggesting the development of chronic epilepsy in humans following acute OP intoxication (Waheed et al. 2014), and humans who survived acute sarin intoxication in the 1995 Tokyo subway attacks have been reported to exhibit EEG abnormalities years after exposure (Okumura et al. 2005).
4.2. Limitations to the systematic review
Several animal studies discussed in this article were not included in the systematic review process. This was due to two main factors: they were published after October 25, 2018 when the literature search was concluded, or they did not meet the PECO and exclusion/inclusion criteria. The articles that were published after the literature search was concluded, as well as those that did not meet inclusion criteria, generally support the biological plausibility of long-term effects in humans and animals. At this time, these newer studies are unlikely to have impacted the conclusions in the systematic review; however, over time it is possible that additional data may warrant a re-evaluation of conclusions (particularly where uncertainties in the data result in lower confidence ratings).
The available mechanistic data suggest that long-term effects are plausible, however the data were not sufficient at the time of this review to impact the confidence ratings. Although there were a few in vivo studies that reported mechanistic data relevant to the review, they were very limited in number and none of the studies evaluated the same endpoints or potential mechanisms. The NTP literature search was focused on mechanistic data that were clearly relevant for evaluating the biological plausibility of neurological outcomes reported from in vivo studies in animals or humans. The literature search only included in vitro data if the endpoint was directly relevant to survival or morphology of neuronal or glial cells. This focused approach may have missed mechanistic studies of broader mechanistic categories that underly some of the observed effects, such as oxidative stress, neuroinflammation, or other mechanisms separate from the cholinergic pathway. These may inform the overall evaluation of potential neurotoxicity associated with exposure to sarin.
The NTP systematic review did not consider unpublished data for this review. Publicly available, unpublished data were identified from the literature search. Data from the identified unpublished studies were either subsequently published and therefore included in this review or were from authors who had published several other studies on the topic. Based on a review of the unpublished data identified, it was determined that the inclusion of the unpublished data to the body of evidence would not change any of the hazard conclusions; therefore, unpublished data were not included in the review. However, because sarin is a nerve agent used in chemical warfare, there are likely to be unpublished studies that are not publicly available that might provide additional support for the effects observed and discussed in this review. Therefore, the omission of unpublished data from sources that are not publicly available may be a limitation of the systematic review. It should be noted that the transparency of data sources and the public availability of all the studies used in the NTP systematic review is an important feature of the methodology.
Other limitations include the narrow focus on acute sarin exposure only. Data may have been available from short-term or chronic exposures to sarin that may have relevance to the findings described in this report. In addition, there is a very large literature base on the effects of other OPs including pesticides, and there may be information on these that could support the findings in this review. The evaluation also did not attempt to quantitatively characterize exposure or identify exposure levels of sarin at which long-term neurological effects occur. In general, there was a lack of quantitative exposure data in the human studies identified, and it is likely that there is insufficient data from these studies to identify a threshold exposure level for long-term neurological effects of sarin. After our initial assessment of all the available data, it became apparent that a qualitative approach to the determination of hazard conclusions is appropriate due to heterogeneity of the data including different endpoints, small sample sizes, the small number of studies that meet inclusion/exclusion criteria, and spatial and temporal variability. Finally, it is recognized that some of the effects observed may be caused by factors other than the acute sarin exposure. These could include post-traumatic stress disorder (PTSD), other exposures, and other illnesses. This is especially pertinent to the extended time periods where potential confounding factors accumulate over time.
4.3. The systematic review results suggest specific data gaps and research needs
Mechanistic research.
In order to understand potential therapeutic approaches, research is needed on the underlying mechanisms of toxicity and injury involved in the long-term effects. This includes in vitro and in vivo studies on the mechanisms of excitotoxic, neuropathological, and neuroinflammatory responses to OP exposure, and how these may relate to other effects such as cognitive and sensory dysfunction. A higher level of understanding of the molecular processes involved in the transition from acute sarin sublethal toxicity to the long-term effects observed is needed. These studies are an important precursor to the identification of molecular targets for therapeutic development, as well as a foundation for developing appropriate animal models and the establishment of outcome measures that are relevant to mortality or serious morbidity in humans.
Natural history studies in animal models.
Because of the inability to conduct controlled exposure studies in humans, experimental animal studies are particularly important for addressing research gaps identified by the systematic review, especially for identifying specific effects that could be targeted for medical mitigation. Studies in well-characterized animal models are needed for rigorous experimental assessments of the dose- and time-related factors that influence the long-term neurological effects of sarin. These studies are necessary to identify the relationship between exposure level and long-term effects, as well as relevant toxicity outcomes that could be potentially extrapolated to humans. Longer-term studies with animals would be required to estimate the temporal progression of some of the more common and persistent long-term effects. For all of the animal and human studies, study design and adequate sample sizes will be required, and outcome measures that could be comparable across laboratories and different studies would be highly desirable.
Targeted animal studies.
Other more targeted studies using animal models are also needed. Animal studies indicate differences in inhibition and recovery of ChE activity, as well as histological changes, in different areas of the brain; however, the data are insufficient to determine if these differences correlate with the changes observed in brain regions that are known to be involved in learning, memory, and intelligence. Behavioral studies are needed to separate out learning and memory effects from effects on motor and sensory function. Future research could focus on these effects to help identify potentially vulnerable brain areas and behavior that could be targeted for therapeutic intervention. Research is needed to further characterize the morphological and histological effects of sarin observed in humans and animals to determine their clinical significance and the potential therapeutic approaches that may preclude these effects (e.g., neuroprotectants). Data on other persistent symptoms and neurological effects would also be valuable, as a range of effects have been reported, but the evidence was inadequate to evaluate these health outcomes due to serious limitations in the bodies of evidence including heterogeneity in the endpoints examined, too few studies, small sample sizes, and serious risk-of-bias concerns. More rigorous animal studies are needed to investigate further some of the emerging long-term effects of sarin and other OPs, such as spontaneous recurrent seizures and other abnormal EEG activity, as well as other neurological symptoms and sensory effects identified in the systematic review (see page 125 in NTP 2019).
Translational research.
The systematic review indicates that acute exposure sarin is known or suspected to be a hazard for long-term effects in humans. Therefore, it is imperative to continue translational research efforts to identify therapeutic targets and potential candidate therapeutics for the effects identified, in parallel with efforts to confirm these effects occur in humans and basic research to determine their biological plausibility. These effects include extended ChE inhibition, visual and ocular effects, morphological and histological changes in nervous system tissues, and effects on learning and memory. Although it was attempted to match animal tests to the human symptoms, animal studies that specifically are designed to examine endpoints that directly correspond to commonly self-reported symptoms in humans would strengthen the ability to evaluate human and animal data together. Outcomes in humans that are observed and supported by evidence with high confidence should be used to develop animal models that are more predictive of the human response under an extended post-exposure effects window. Pre-hospital treatments should be amenable to a mass casualty context of use, e.g. rapid administration, appropriate pharmacokinetics and safety profiles. A critical gap in both the human and animal data is the effects of sarin on the developing and aging brain. It currently has not been adequately assessed in pregnant women, the very young, or the very old, even though they are more susceptible populations and there are reports of such exposures occurring in current conflicts (Rosman et al. 2014).
A robust clinical research response after human exposures.
Considering that most humans are typically exposed to sarin during wartime situations and terrorist attacks, researchers are somewhat limited in their opportunities to study human populations acutely exposed to sarin with appropriate control groups, which can make the data gaps identified in this review difficult to address. Although there were two terrorist attacks in Japan, they both occurred more than 20 years ago, so additional studies on any remaining subjects are not likely to provide the additional data needed since age is an important confounder for many of the outcomes detailed in this article. Because these are rare and unpredictable events, there could be value in developing a rapid research response capability that could collect vital human clinical data soon after mass casualty chemical exposures (Miller and Birnbaum 2015). Rigorous epidemiological studies would also add valuable data to the body of evidence that would likely impact conclusions or the confidence in the conclusions reached in the systematic review, given the lack of human data on many endpoints and time periods. Well-designed and well-controlled human cohort studies would be invaluable in characterizing the relationship between neurological effects over time and sarin exposure, including the potential relationship between acute sarin exposure and the development of PTSD. Only one available study (Tochigi et al. 2002) addressed subjects with PTSD symptoms as a subgroup and found evidence of a long-term depression in serum ChE levels in the PTSD subset of patients (n = 8) compared with controls, whereas the same association was not seen in the entire study population compared to controls.
Acknowledgements
We thank the staff at NTP and the National Institutes of Health (NIH) for providing advice for this article, and the evaluation team and peer reviewers for work on the NTP systematic review. We thank A. Livinski for conducting literature searches for the systematic review. We thank all the staff from ICF and i2 Grants Associates, LLC for assistance with the systematic review.
Funding
This article was supported by the NTP and NIH CounterACT program.
Abbreviations and Acronyms
- AChE
acetylcholinesterase
- ChE
cholinesterase
- CNS
central nervous system
- DRL
differential reinforcement of low rates
- EEG
electroencephalogram
- FOB
functional observational battery
- GB
sarin [two-character identifier]
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- HAWC
Health Assessment Workspace Collaborative
- MRI
magnetic resonance imaging
- NIH
National Institutes of Health
- NTP
National Toxicology Program
- OHAT
Office of Health Assessment and Translation
- OPNA
organophosphorus nerve agent
- PECO
population, exposure, comparator, and outcome
- PTSD
post-traumatic stress disorder
- VEP
visual evoked potential
Short biographical notes on all contributors
D.A. Jett: Director of the NIH Countermeasures Against Chemical Threats (CounterACT) Program, a program supported by a specific Congressional appropriation to the NIH for the development of new drugs and diagnostic tools for treating victims of chemical exposures during an emergency. He also serves as Program Director and Scientific Team Leader within the Division of Translational Research at the National Institute of Neurological Disorders and Stroke (NINDS). He holds the position of Professor Adjunct of Chronic Disease and Epidemiology within the Yale School of Public Health.
C.A. Sibrizzi: Lead Health Scientist with a Master of Public Health (M.P.H.) in environmental health science and policy. Mr. Sibrizzi has more than eight years of experience in environmental health science focused on the review and analysis of epidemiological and toxicological data, systematic review, and risk assessment.
R. Blain: Technical Director of Toxicology and Systematic Review at ICF with a doctorate in environmental health sciences and a master’s in public health. Dr. Blain has nearly 25 years of experience reviewing and analyzing public health and mammalian toxicity studies and over 10 years of experience reviewing and analyzing epidemiologic and mechanism studies.
P.A. Hartman: Senior Technical Specialist at ICF with a master’s in environmental management. Ms. Hartman has over 20 years of experience in conducting human health and environmental assessments, with expertise in risk assessment, exposure assessment, toxicology, literature search and review, and visualization development.
P.J. Lein: Professor of Neurotoxicology in the Department of Molecular Biosciences, School of Veterinary Medicine, University of California, Davis. Dr. Lein also holds a faculty appointment in the UC Davis MIND Institute. Her research focuses environmental stressors and their contribute to neural dysfunction and neurological disease, including asthma, neurodevelopmental disorders, intellectual impairment, and Alzheimer’s disease. Her service activities include Director and PI of the NIH-funded training program in environmental health sciences at UC Davis, Director and PI of the NIH-funded UC Davis CounterACT Center of Excellence, and Co-Editor-in-Chief of the journal NeuroToxicology.
K.W. Taylor: Health scientist in the Office of Health Assessment and Translation in the Division of National Toxicology Program at the National Institute of Environmental Health Sciences. Dr. Taylor received her PhD in epidemiology from UNC-Chapel Hill. At NTP, Dr. Taylor is involved in conducting literature-based evaluations, including systematic reviews, on environmental exposures and health effects. She also works on developing methods related to study quality and risk of bias to better address environmental health questions. Her research also includes a consumer product exposure assessment study and the association between personal care product use and breast cancer risk.
A.A. Rooney: Acting Director of the Office of Health Assessment and Translation in the Division of the National Toxicology Program (NTP). Dr. Rooney has over 25 years of experience in toxicology and risk assessment for the protection of public health resulting in contributions to the peer-reviewed literature and government assessments (NTP Monographs, EPA IRIS Toxicological Reviews and WHO Guidelines). He has been actively involved in developing risk assessment methods and guidance throughout his professional career and led the team that developed NTP’s OHAT Approach to Systematic Review and Evidence Integration.
Footnotes
Supplementary Materials
The original protocol, literature search results, and full detailed NTP monograph can be found on the NTP website at https://ntp.niehs.nih.gov/go/sarin.
This article is a summary of material from the National Toxicology Program (NTP) monograph entitled Systematic Review of Long-Term Neurological Effects Following Acute Exposure to Sarin. The authors were part of the evaluation team for the systematic review and wrote parts of the manuscript and reviewed the full draft. David Jett and Andrew Rooney were project co-leaders on the systematic review. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The website for the NTP systematic review containing the NTP monograph and additional information including NTP Board of Scientific Councilors and Peer Review meetings can be found at https://ntp.niehs.nih.gov/go/sarin.
Declaration of Interest
The authors declare no competing financial interest. This report does not represent the official view of the National Institutes of Health (NIH) or any part of the US Federal Government. No official support or endorsement of this article by the NIH is intended or should be inferred.
References
- Brown MA, Brix KA (1998) Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol 18(6):393–408 [DOI] [PubMed] [Google Scholar]
- Burchfiel JL, Duffy FH (1982) Organophosphate neurotoxicity: chronic effects of sarin on the electroencephalogram of monkey and man. Neurobehav Toxicol Teratol 4(6):767–78 [PubMed] [Google Scholar]
- Carvalho AN, Firuzi O, Gama MJ, Horssen JV, Saso L (2017) Oxidative Stress and Antioxidants in Neurological Diseases: Is There Still Hope? Curr Drug Targets 18(6):705–718 doi: 10.2174/1389450117666160401120514 [DOI] [PubMed] [Google Scholar]
- Chaubey K, et al. (2017) From the Cover: Proteome Profile of Different Rat Brain Regions After Sarin Intoxication. Toxicol Sci 160(1):136–149 doi: 10.1093/toxsci/kfx162 [DOI] [PubMed] [Google Scholar]
- Chen WL, Sheets JJ, Nolan RJ, Mattsson JL (1999) Human red blood cell acteylcholinesterase inhibition as the appropriate and conservative surrogate endpoint for establishing chlorpyrifos reference dose. Reg Toxicology Pharm 29(1):15–22 [DOI] [PubMed] [Google Scholar]
- Chen Y (2012) Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology 33(3):391–400 doi: 10.1016/j.neuro.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Collombet JM (2011) Nerve agent intoxication: recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicol Appl Pharmacol 255(3):229–41 doi: 10.1016/j.taap.2011.07.003 [DOI] [PubMed] [Google Scholar]
- Deshpande LS, DeLorenzo RJ (2019) Novel therapeutics for treating organophosphate-induced status epilepticus co-morbidities, based on changes in calcium homeostasis. Neurobiol Dis doi: 10.1016/j.nbd.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egoz I, Nili U, Grauer E, Gore A (2017) Optimization of the Ocular Treatment Following Organophosphate Nerve Agent Insult. Toxicol Sci 159(1):50–63 doi: 10.1093/toxsci/kfx119 [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Apland JP, Braga MFM, Marini AM (2018) Acute and long-term consequences of exposure to organophosphate nerve agents in humans. Epilepsia 59 Suppl 2:92–99 doi: 10.1111/epi.14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore A, et al. (2019) Ocular surface histopathological insult following sarin and VX exposure and potential treatments in the rat model. Toxicol Lett 314:153–163 doi: 10.1016/j.toxlet.2019.08.002 [DOI] [PubMed] [Google Scholar]
- Guignet M, et al. (2019a) Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol Dis doi: 10.1016/j.nbd.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignet M, et al. (2019b) Persistent behavior deficits, neuroinflammation, and oxidative stress in a rat model of acute organophosphate intoxication. Neurobiol Dis:104431 doi: 10.1016/j.nbd.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, et al. (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64(4):383–94 doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Haam J, Yakel JL (2017) Cholinergic modulation of the hippocampal region and memory function. J Neurochem 142 Suppl 2:111–121 doi: 10.1111/jnc.14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himuro K, et al. (1998) Distal sensory axonopathy after sarin intoxication. Neurology 51(4):1195–7 doi: 10.1212/wnl.51.4.1195 [DOI] [PubMed] [Google Scholar]
- Hulse EJ, Haslam JD, Emmett SR, Woolley T (2019) Organophosphorus nerve agent poisoning: managing the poisoned patient. Br J Anaesth 123(4):457–463 doi: 10.1016/j.bja.2019.04.061 [DOI] [PubMed] [Google Scholar]
- IOM (2004) Gulf War and Health: Updated Literature Review of Sarin. Washington, DC: Institute of Medicine [Google Scholar]
- Kassa J, Koupilova M, Vachek J (2001a) The influence of low-level sarin inhalation exposure on spatial memory in rats. Pharmacol Biochem Behav 70(1):175–9 [DOI] [PubMed] [Google Scholar]
- Kassa J, et al. (2001b) Toxic effects of sarin in rats at three months following single or repeated low-level inhalation exposure. Pharmacol Toxicol 88(4):209–12 [DOI] [PubMed] [Google Scholar]
- Lazar S, Egoz I, Brandeis R, Chapman S, Bloch-Shilderman E, Grauer E (2016) Propagation of damage in the rat brain following sarin exposure: Differential progression of early processes. Toxicol Appl Pharmacol 310:87–97 doi: 10.1016/j.taap.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Lee K, et al. (2018) Assessment of brain oxygenation imbalance following soman exposure in rats. Neurotoxicology 65:28–37 doi: 10.1016/j.neuro.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Lewine JD, et al. (2018) Addition of ketamine to standard-of-care countermeasures for acute organophosphate poisoning improves neurobiological outcomes. Neurotoxicology 69:37–46 doi: 10.1016/j.neuro.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Loh Y, Swanberg MM, Ingram MV, Newmark J (2010) Case report: Long-term cognitive sequelae of sarin exposure. Neurotoxicology 31(2):244–6 doi: 10.1016/j.neuro.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Lumley L, et al. (2019) Neurosteroid and benzodiazepine combination therapy reduces status epilepticus and long-term effects of whole-body sarin exposure in rats. Epilepsia Open 4(3):382–396 doi: 10.1002/epi4.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- M G, PJ L (2019) Organophosphates. In: Aschne M, Costa L (eds) Advances in Neurotoxicology: Role of Inflammation in Environmental Neurotoxicity vol 3. Academic Press, Cambridge, MA, p 35–79 [Google Scholar]
- Miller A, Birnbaum L (2015) Preparing for disasters. Science 348(6236):766–7 doi: 10.1126/science.348.6236.766-c [DOI] [PubMed] [Google Scholar]
- Murata K, et al. (1997) Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack. J Neurol 244(10):601–6 [DOI] [PubMed] [Google Scholar]
- NTP (2019) NTP monograph on the systematic review of long-term neurological effects following acute exposure to sarin. Office of Health Assessment and Translation Division of the National Toxicology Program, National Institute of Environmental Health Sciences. https://ntp.niehs.nih.gov/ntp/ohat/sarin/sarin_prepublication20190600_508.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T, et al. (2005) Acute and chronic effects of sarin exposure from the Tokyo subway incident. Environ Toxicol Phar 19(3):447–450 doi: 10.1016/j.etap.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Pearson-Smith JN, Patel M (2019) Antioxidant drug therapy as a neuroprotective countermeasure of nerve agent toxicity. Neurobiol Dis doi: 10.1016/j.nbd.2019.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira EF, et al. (2014) Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther 350(2):313–21 doi: 10.1124/jpet.114.214932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program NT (2015) Handbook for conducting a literature-based health assessment using OHAT approach for systematic review and evidence integration. . National Institute of Environmental Health Sciences, National Toxicology Program, Office of Health Assessment and Translation, RTP, NC [Google Scholar]
- Program NT (2019) Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Office of Health Assessment and Translation. Division of the National Toxicology Program, National Institute of Environmental Health Sciences., RTP NC [Google Scholar]
- Putra M, et al. (2019) Inducible nitric oxide synthase inhibitor, 1400W, mitigates DFP-induced long-term neurotoxicity in the rat model. Neurobiol Dis:104443 doi: 10.1016/j.nbd.2019.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney A, et al. (2016) How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ Int 92–93:617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA (2014) Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122(7):711–8 doi: 10.1289/ehp.1307972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosman Y, et al. (2014) Lessons learned from the Syrian sarin attack: evaluation of a clinical syndrome through social media. Ann Intern Med 160(9):644–8 doi: 10.7326/M13-2799 [DOI] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA (2002) Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol 182(2):176–85 doi: 10.1006/taap.2002.9445 [DOI] [PubMed] [Google Scholar]
- Sellström ACS, Barbeschi M (2013) United nations mission to investigate allegations of the use of chemical weapons in the Syrian Arab Republic Report on the Alleged Use of Chemical Weapons in the Ghouta Area of Damascus on 21 August 2013. vol 41. United Nations, United Nations [Google Scholar]
- Shaffo FC, Grodzki AC, Fryer AD, Lein PJ (2018) Mechanisms of organophosphorus pesticide toxicity in the context of airway hyperreactivity and asthma. Am J Physiol Lung Cell Mol Physiol 315(4):L485–L501 doi: 10.1152/ajplung.00211.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188(2):69–80 doi: 10.1016/s0041-008x(03)00019-x [DOI] [PubMed] [Google Scholar]
- Shrot S, et al. (2014) Prevention of organophosphate-induced chronic epilepsy by early benzodiazepine treatment. Toxicology 323:19–25 doi: 10.1016/j.tox.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Tochigi M, et al. (2002) Serum cholesterol, uric acid and cholinesterase in victims of the Tokyo subway sarin poisoning: A relation with post-traumatic stress disorder. Neurosci Res 44(3):267–272 doi:Pii S0168–0102(02)00146–3 Doi 10.1016/S0168-0102(02)00146-3 [DOI] [PubMed] [Google Scholar]
- Tuin AW, et al. (2009) Activity-Based Protein Profiling Reveals Broad Reactivity of the Nerve Agent Sarin. Chem Res Toxicol 22(4):683–9 doi: 10.1021/tx8004218 [DOI] [PubMed] [Google Scholar]
- Waheed S, Sabeen A, Ullah Khan N (2014) New onset refractory status epilepticus as an unusual presentation of a suspected organophosphate poisoning. Case Rep Emerg Med 2014:676358 doi: 10.1155/2014/676358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, et al. (2016) Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 74:449–75 doi: 10.1016/j.cortex.2015.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, et al. (2007a) Human brain structural change related to acute single exposure to sarin. Ann Neurol 61(1):37–46 doi:DOI 10.1002/ana.21024 [DOI] [PubMed] [Google Scholar]
- Yamasue H, et al. (2007b) Human brain structural change related to acute single exposure to sarin. Ann Neurol 61(1):37–46 doi: 10.1002/ana.21024 [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T (2006) Sarin experiences in Japan: Acute toxicity and long-term effects. J Neurol Sci 249(1):76–85 doi: 10.1016/j.jns.2006.06.007 [DOI] [PubMed] [Google Scholar]


