Abstract
Gulf War Illness (GWI), a chronic multisymptom health problem, afflicts ~30% of veterans served in the first GW. Impaired brain function is among the most significant symptoms of GWI, which is typified by persistent cognitive and mood impairments, concentration problems, headaches, chronic fatigue, and musculoskeletal pain. This review aims to discuss findings from animal prototypes and veterans with GWI on mechanisms underlying its pathophysiology and emerging therapeutic strategies for alleviating brain dysfunction in GWI. Animal model studies have linked brain impairments to incessantly elevated oxidative stress, chronic inflammation, inhibitory interneuron loss, altered lipid metabolism and peroxisomes, mitochondrial dysfunction, modified expression of genes relevant to cognitive function, and waned hippocampal neurogenesis. Furthermore, the involvement of systemic alterations such as the increased intensity of reactive oxygen species and proinflammatory cytokines in the blood, transformed gut microbiome, and activation of the adaptive immune response have received consideration. Investigations in veterans have suggested that brain dysfunction in GWI is linked to chronic activation of the executive control network, impaired functional connectivity, altered blood flow, persistent inflammation, and changes in miRNA levels. Lack of protective alleles from Class II HLA genes, the altered concentration of phospholipid species and proinflammatory factors in the circulating blood have also been suggested as other aiding factors. While some drugs or combination therapies have shown promise for alleviating symptoms in clinical trials, larger double-blind, placebo-controlled trials are needed to validate such findings. Based on improvements seen in animal models of GWI, several antioxidants and anti-inflammatory compounds are currently being tested in clinical trials. However, reliable blood biomarkers that facilitate an appropriate screening of veterans for brain pathology need to be discovered. A liquid biopsy approach involving analysis of brain-derived extracellular vesicles in the blood appears efficient for discerning the extent of neuropathology both before and during clinical trials.
Keywords: Chemical exposures, Cognitive dysfunction, Coenzyme Q10, Curcumin, DNA methylation, Depression, Microbiota, Mitochondrial dysfunction, Neuroinflammation, Oxidative Stress
1. Introduction
Approximately 30% of 700,000 United States service personnel of the 1991 Gulf War (GW) continue to experience multiple health problems, including fatigue, headaches, cognitive dysfunction, difficulties in concentration, musculoskeletal pain, respiratory, gastrointestinal, and dermatologic complaints (White et al., 2016; National Academies of Sciences, Engineering, and Medicine, 2018). There are multiple case definitions used in Gulf War Veteran studies, causing difficulty in recognizing chronic multisymptom illness (CMI). A large epidemiological study of Kansas Gulf War veterans outlined the GWI case definition (Steele, 2000). As per this definition, GWI cases must have multiple and/or moderate-to-severe chronic symptoms in at least three of six defined symptom domains. Also, qualifying symptoms must have first been a problem during or after the Gulf War (i.e., symptom onset after the year 1990) and persisted over the 6 months preceding the study. The symptom domains include a) fatigue/sleep problems, b) somatic pain, c) neurologic/cognitive/mood symptoms, d) gastrointestinal symptoms, e) respiratory symptoms, and f ) skin abnormalities. Kansas GWI case criteria also exclude veterans who report being diagnosed or treated for medical or psychiatric conditions such as cancer, diabetes, heart disease, chronic infectious disease, lupus, multiple sclerosis, stroke, or any severe psychiatric illness.
It has been proposed that multiple deployment-related chemical exposures are common etiologies (Institute of Medicine, 2001, 2013, 2014; Gulf War and Health, 2017; National Academies of Sciences, Engineering, and Medicine, 2018). Deployment-related exposures include war-related stress, pesticides employed against the insect and rodent populations, nerve gas prophylactic drug pyridostigmine bromide, chemical warfare agents (sarin, and mustard gas), vaccines (including those against anthrax and botulinum), depleted uranium, and hexavalent chromium. More than 35 types of pesticides were administered, including organophosphates, carbamates, pyrethroids, lindane, and N, N-Diethyl-meta-toluamide (DEET). Combustion products comprised particulate matter, polycyclic aromatic hydrocarbons, polychlorinated dibenzo-p-dioxins, dibenzofurans, and fuels from over 600 burning oil wells in Kuwait. Solvents such as benzene, toluene, xylene, trichloroethylene, and tetrachloroethylene were also employed to maintain vehicles and equipment, cleaning, and degreasing. In addition, exposure to the depleted uranium has been suggested as one of the causes of GWI (Bjorklund et al., 2020).
Gulf War veterans endure an array of long-term medically unexplained symptoms known as chronic multisymptom illness (CMI). However, CMI varies from person to person. Many veterans who served in the first GW believe their illness’s legitimacy is often a subject of intense scrutiny because there is no signature etiology for GWI. As per the centers for disease control and prevention (CDC), CMI in veterans of the 1990–1991 GW is defined as having ≥1 symptom lasting ≥6 months in at least two of three categories: fatigue, depressed mood, and altered cognition, and musculoskeletal pain (Fukuda et al., 1998). The Institute of Medicine report in 2013 defined CMI as the occurrence of a spectrum of chronic symptoms experienced for 6 months or longer in at least two of the following six categories: fatigue, mood dysfunction, cognitive impairments, musculoskeletal pain, gastrointestinal problems, respiratory issues, or neurological conditions (Institute of Medicine, 2013, 2014). The report also mentioned that the symptoms of GWI may overlap with but are not fully captured by known syndromes such as chronic fatigue syndrome, fibromyalgia, irritable bowel syndrome, or other diagnoses. A study in 2019 at Ft. Devens measured the incidence of nine enduring medical ailments in GW veterans and compared with the population-based 2013–2014 National Health and Nutrition Examination Survey cohort. The study reported that GW veterans in the population-based Ft. Devens Cohort are at an elevated risk for developing chronic conditions than the general population. These risks are associated with self-reported toxicant exposures (Zundel et al., 2019). Another study comparing the prevalence of CMI in 9,110 GWI veterans with 36,019 era personnel and 31,446 non-era personnel reported that GW veterans exhibit the highest incidence of CMI over time (Porter, Long, Rull, Dursa, & Millennium Cohort Study, 2020). A recent study, controlling for age, race, GW deployment, the branch of service, and smoking status in logistic regression analyses, has reported that GW-deployed women were significantly more likely to report 7 out of 34 symptoms related to cognitive, neurological, and mood problems and respiratory complaints in comparison to GW-era women (Sullivan et al., 2020).
Regarding the pathophysiology of GWI, studies in animal models have shown that exposure to organophosphate (OP) and pesticides, alone or in combination with other GW agents, leads to persistent cognitive and mood dysfunction. Furthermore, such brain impairments were associated with incessantly elevated oxidative stress, mitochondrial dysfunction, astrocyte hypertrophy, microglial activation, chronic neuroinflammation, leaky blood-brain barrier (BBB), altered lipid metabolism and peroxisomes, loss of inhibitory interneurons and waned hippocampal neurogenesis in rodent models of GWI (Abdel-Rahman, Abou-Donia, El-Masry, Shetty, & Abou-Donia, 2004; Abdel-Rahman, Shetty, & Abou-Donia, 2001, 2002; Abdullah et al., 2011, 2012; Hattiangady et al., 2014; Kodali et al., 2018; Madhu et al., 2019; Megahed, Hattiangady, Shuai, & Shetty, 2015; Ojo et al., 2014; Parihar, Hattiangady, Shuai, & Shetty, 2013; Shetty et al., 2017; Shetty et al., 2020; Zakirova et al., 2016). Studies on models that utilized OP exposure alone have also suggested abnormalities in cytoskeletal axonal transport motor proteins, neurotrophins, and their receptors, and mitochondria (Speed et al., 2012; Terry Jr., 2012; Zhu, Hawkins, Phillips, & Deshpande, 2020). Moreover, elevated levels of reactive oxygen species (ROS) and proinflammatory cytokines in the circulating blood paralleling chronic changes in the brain have suggested the likely participation of systemic inflammation in brain dysfunction (Shetty et al., 2017; Shetty et al., 2020). Cognitive and mood dysfunction is also seen consistently in veterans with GWI (Engdahl et al., 2018; Janulewicz et al., 2017; Jeffrey et al., 2019; White et al., 2016; Wylie et al., 2019). In humans, episodic memory and brain function measured by a face-name memory task and functional magnetic resonance imaging (fMRI) demonstrated three major GWI syndrome types (“impaired cognition,” “confusion ataxia,” and “central pain”). Investigations using fMRI demonstrate that memory impairments in veterans with GWI are linked to functional neurobiological chan changes and provide evidence of the role of deployment in the war on brain dysfunction (Cooper et al., 2016). A study on cognitive function found diminished performance in three functional domains: attention and executive function, visuospatial skills, and learning/memory (Janulewicz et al., 2017). Another study reported that GWI varies in severity in a continuum that leads, at the higher end, to a diagnosable mental health disorder (Engdahl et al., 2018).
This review article aims to deliberate critical findings from animal prototypes of GWI and veterans with GWI on mechanisms underlying its pathophysiology, and therapeutic strategies that showed promise for alleviating brain dysfunction in GWI. The review discusses neurobehavioral alterations and cellular and molecular pathogenesis in animal models and veterans with GWI to provide insights into the mechanisms underlying persistent GWI. Broad pathophysiology categories are covered, including elevation of ROS, glial activation, proinflammatory mediators, mitochondrial dysfunction, altered blood flow, and functional connectivity, loss of interneurons, and waning of hippocampal neurogenesis. Furthermore, chronic changes at the systemic level, such as altered microbiome, adaptive immune response, and the role of human leukocyte antigen (HLA) and epigenetic alterations are conferred. Besides, the potential screening targets in veterans to discern the occurrence and severity of GWI are suggested. The efficacy of several treatments is reviewed in animal models as well as veterans. The final section highlights important advances made in understanding the pathophysiology of GWI, limitations of clinical trials performed hitherto, and promising therapeutic approaches that need further consideration in more extensive clinical trials.
2. Mechanisms of brain dysfunction in animal models of GWI
2.1. Functional impairments and associated pathology in rat models of GWI
2.1.1. Cognitive and mood dysfunction
Many studies using a rat model employed combined exposure to lower doses of GWI-related (GWIR) chemicals, including the nerve gas prophylactic drug pyridostigmine bromide (PB, 1.3–2.0 mg/Kg, oral), an insect repellant DEET (40–60 mg/Kg, dermal) and the insecticide permethrin (PER, 0.13–0.2 mg/Kg, dermal) with or without restraint stress (5–15 min). The model simulated the type of exposure experienced by a vast majority of veterans during the GW, and such exposures have previously been shown to cause BBB dysfunction and decreased acetycholinesterase (AChE) activity (Abdel-Rahman et al., 2002). Analyses of cognitive function using a water maze test 2 months after exposure revealed hippocampus-dependent learning impairments and the inability for retrieving memory in a probe test conducted 24 hours after the last learning session (Parihar et al., 2013). Furthermore, brain dysfunction was also manifested by increased depressive- and anxiety-like behavior, when examined with a forced swim test and an elevated plus-maze test. In addition, mild stress (5 min of restraint stress) in the period of exposure to chemicals exacerbated the extent of mood and cognitive dysfunction (Parihar et al., 2013).
Follow-up studies using a larger cohort of GWI rats and a variety of cognitive tests confirmed the occurrence of lasting memory and mood impairments following exposure to GWIR chemicals and mild stress (Hattiangady et al., 2014). Three months following the exposure, the occurrence of cognitive dysfunction was typified by the inability of animals to discern minor changes in an object location test, a task that largely depends on the integrity of the hippocampus (Barker & Warburton, 2011), and an impaired recognition memory, a task that requires the integrity of the perirhinal cortex, hippocampus and the prefrontal cortex (Langston & Wood, 2010). Mood dysfunction was evident from a lack of motivation to run in a voluntary physical exercise paradigm or to eat food following 24-hour food deprivation in a novelty suppressed feeding test (Hattiangady et al., 2014). Behavioral studies performed at 5 or 10 months post-exposure revealed that cognitive and mood impairments in the above-described rat model of GWI persist for prolonged periods (Madhu et al., 2019; Shetty et al., 2020). These include impairments in simple recognition memory in a novel object recognition test, associative recognition memory in an object-in-place test, the location memory in an object location test, and pattern separation in a pattern separation test (Madhu et al., 2019; Shetty et al., 2020). Memory impairment observed at 10 months post-exposure in rats is equivalent to ~29 years after exposure in humans (Sengupta, 2013). Such persistent cognitive impairment is comparable to the type of brain dysfunction seen in veterans affected with chronic GWI even 29 years after the exposure during the GW (Cooper et al., 2016; Janulewicz et al., 2017). Thus, exposure to low doses of GWIR chemicals with mild to moderate stress was sufficient to cause significant and lasting cognitive and mood impairments.
2.1.2. Alterations in hippocampal volume, neurons, glia, and neurogenesis
Four weeks of exposure to GWIR chemicals (PB, DEET, and PER) with or without mild stress in rats resulted in reduced hippocampal volume and neuron loss. The overall volume of the hippocampus was reduced by ~10%, with 19–31% loss of neurons in the dentate granule cell layer, the dentate hilus, and the CA1 pyramidal cell layer of the hippocampus at 3 months post-exposure (Parihar et al., 2013). Furthermore, another study showed significant loss of subclasses of GABA-ergic inhibitory interneurons expressing the calcium-binding protein parvalbumin (PV) and the neuropeptide Y (NPY) (Megahed, Hattiangady, Shuai, & Shetty, 2015). The neuronal loss could be due to both direct and indirect effects of chemicals. For example, PER can induce neurodegeneration via prolonged activation of voltage-gated sodium channels and hyperexcitability in neurons (Ray & Fry, 2006) whereas, DEET can exacerbate the adverse effects of other AChE inhibitors such as PB (Corbel et al., 2009). Neurodegeneration could also be due to altered glutamatergic neurotransmission in the brain following exposure to GWIR chemicals (Joyce & Holton, 2020). Furthermore, the hippocampus exhibited neuroinflammation typified by the sporadic activation of microglia and the hypertrophy of astrocytes. A study in a rat model of GWI, employing diisopropyl fluorophosphate (DFP, an irreversible AChE inhibitor and a surrogate of the organophosphate sarin) and corticosterone (CORT) exposure, revealed varied alterations in oligodendrocytes (Belgrad et al., 2019). In the prefrontal cortex, DFP and CORT exposure increased the proliferation of oligodendrocytes with decreases in differentiation. Whereas, in the corpus callosum, DFP and CORT exposure decreased the proliferation of oligodendrocytes with increases in their differentiation. Increased differentiation of oligodendrocytes in the corpus callosum also resulted in increased myelin basic protein in the subcortical white matter (Belgrad et al., 2019). The study suggested that GW veterans would likely have altered white matter integrity varying by brain region, consistent with the findings from imaging studies of GW veterans (Bierer et al., 2015; Rayhan et al., 2013; Van Riper et al., 2017). Additional studies on oligodendrocytes in other chronic models of GWI will help understand the long-term implications of alterations in oligodendrocyte numbers in different brain regions in GWI.
Moreover, analysis of neurogenesis immediately after the exposure or at 3 months post-exposure revealed reductions in the daily production of new neurons and the integration of newly generated neurons in the hippocampus (Parihar et al., 2013). While the adverse effects on hippocampal neurogenesis were seen in animals exposed to GWIR chemicals with or without mild stress, the overall effects were more significant in animals exposed to GWIR chemicals with mild stress. Evaluation of neural stem cells (NSCs) in the subgranular zone of the hippocampus through the NSC marker Sox-2 and the cell proliferative marker Ki-67 revealed that exposure to GWIR chemicals did not kill NSCs but significantly impaired their proliferative behavior (Parihar et al., 2013). Furthermore, studies performed at 6 or 10 months post-exposure revealed that activation of microglia and astrocytes and the decline in neurogenesis in rats exposed to GWIR chemicals and stress persist for extended periods after the exposure (Madhu et al., 2019; Shetty et al., 2020).
2.1.3. Altered calcium levels in neurons
Phillips and Deshpande developed a rat model of GWI by employing repeated low-dose exposure to organophosphate agent DFP over 5 days to match the duration and level of sarin exposure during the GW (Phillips & Deshpande, 2016). Studies in this model are supported by epidemiological, meteorological, and intelligence reports suggesting that some service personnel were exposed to sarin and cyclosarin released from devastations of the ammunition dump at Khamisiyah, Iraq (Couzin, 2004; Haley & Tuite, 2013; Tuite & Haley, 2013). Behavioral tests conducted 3 months after exposure to DFP revealed the occurrence of chronic memory dysfunction and increased depressive- and anxiety-like behavior in GWI rats. Such behavioral deficits were also associated with some sporadic loss of neurons in the hippocampus. A follow-up study at 6 months post-exposure revealed the persistence of memory and mood dysfunction in GWI rats (Phillips & Deshpande, 2018). Moreover, analysis of Fura-2-acetoxymethyl ester loaded acutely isolated hippocampal neurons revealed elevations in intracellular calcium ([Ca2+]i), implying the activation of multiple signaling cascades and altered expression of genes encoding proteins involved in synaptic plasticity in neurons of GWI rats. An additional study showed that pharmacological blockade of Ca2+-induced Ca2+-release mechanisms significantly lowered elevated [Ca2+]i in neurons (Phillips, Santos, Blair, & Deshpande, 2019), suggesting that drugs proficient for blocking intracellular Ca2+ release could be useful for treating neurological symptoms of GWI.
2.1.4. Changes in brain-derived neurotrophic factor and axonal transport
Neurotrophins such as the brain-derived neurotrophic factor (BDNF), critical for synaptic transmission, neurogenesis, neuroplasticity, and cognitive function, also seem to be affected in GWI. A rat prototype of GWI comprising exposure to PB and repeated stress demonstrated increased expression of genes encoding proteins implicated in memory formation (Barbier et al., 2009). The genes include BDNF, its receptor tyrosine kinase B (TrkB), and calcium/calmodulin-dependent protein kinase type II subunit alpha (CamKIIalpha). Another recent study reported that chronic GWI is associated with NLR Family Pyrin Domain Containing 3 (NLRP3) mediated neuroinflammation and decreased BDNF levels (Kimono et al., 2020). Interestingly, mice lacking the NLRP3 gene displayed decreased inflammation and a higher level of BDNF in the frontal cortex following exposure to PB and PER, implying the role of inflammasomes in downregulating BDNF expression in GWI (Kimono et al., 2020).
A study in another model of GWI that employed 14 days of repeated exposure to chlorpyrifos also reported impairments in axonal transport (Hernandez et al., 2015). Such impairments persisted even after a 30-day washout period. Similar axonal transport impairments were seen when animals were exposed to low doses (0.125–0.5 mg/Kg) of DFP, another model of GWI simulating low-level exposure to the nerve gas sarin (Naughton et al., 2018). Such exposure did not cause changes in brain regions’ volume but induced decompactions of the myelinated axons in the white matter areas and axonal transport impairments that persisted at 30 days after the washout period. These observations imply that axonal transport impairments could be one of the issues contributing to brain dysfunction in GWI (Terry Jr., 2012).
2.1.5. Elevated oxidative stress, and neuroinflammation
An investigation evaluated chronic changes in the expression of genes related to increased oxidative stress, mitochondrial dysfunction, and inflammation in the hippocampus 6 months after exposure to GWIR chemicals and stress (Shetty et al., 2017). In addition, the study evaluated the expression of nuclear factor erythroid 2-related factor 2 (NRF2), a master regulator of the antioxidant response, in the hippocampus, as well as the possible inflammation and enhanced oxidative stress at the systemic level. The study revealed that 4 weeks of exposure to GWIR chemicals and stress is adequate for inducing chronically elevated oxidative stress in the hippocampus. Elevated oxidative stress was evident from changes in the expression of genes involved in oxidative stress-response (n = 27), ROS metabolism (n = 6), oxygen transport (n = 4) and antioxidant activity (n = 20). Out of these, 8 genes displayed a 1.5-fold or greater increase in their expression. These include flavin-containing monooxygenase 2 (Fmo2), heme oxygenase 1 (Hmox1), intraflagellar transport 172 (Ift172), Selenoprotein P Plasma 1(Sepp1), solute carrier family 38 member 1 (Slc38a1), superoxide dismutase 3 (Sod3), sulfiredoxin 1 (Srxn1), and uncoupling protein 2 (Ucp2). Significantly elevated expression of these genes likely suggests a compensatory reaction against oxidative stress in the hippocampus of GWI rats as proteins encoding these genes have roles in ROS regulation (Fmo2 and Slc38a1, Mao, Green, Mekonnen, Lindsey, & Gridley, 2010; Ogura et al., 2011), oxidative cleavage of pro-oxidant damaging molecule heme (Hmox1, Xu, Song, Wang, Jiang, & Xie, 2016), oxygen transport (Ift172, Gorivodsky et al., 2009), extracellular antioxidant activity (Sepp1, Sod3, Huang, Ren, Jiang, Xiao, & Lei, 2015; Davis et al., 2017), oxidoreductase activity (Srxn1, Zhou et al., 2015), and uncoupling of oxidative phosphorylation to diminish mitochondrial ROS (Ucp2, Graw et al., 2015).
Elevated oxidative stress in the brain at 6 months post-exposure could also be discerned from increased levels of oxidative stress markers such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and protein carbonyls and the antioxidant superoxide dismutase-2 (Shetty et al., 2020), and NRF2 (Shetty et al., 2017; Shetty et al., 2020), a transcription factor involved in regulating redox homeostasis and curbing inflammation (Innamorato, Lastres-Becker, & Cuadrado, 2009; Lastres-Becker et al., 2014). NRF2 activation in chronic GWI is likely an innate response to reduce the incessantly elevated oxidative stress in the hippocampus. The occurrence of chronically elevated oxidative stress in the hippocampus was associated with alterations in the expression of genes encoding proteins involved in the function of mitochondrial complexes (I, II, IV, or V) of the electron transport chain (ETC) or relevant to mitochondrial/other organelle function (Shetty et al., 2017). Such elevated expression of genes implied an overactive mitochondrial ETC, which may facilitate the synthesis of proteins participating in the ETC at more significant levels to offset the abnormal ETC of mitochondria (Manczak, Jung, Park, Partovi, & Reddy, 2005). The changes in mitochondrial complex V genes, in addition, implied increased energy metabolism, altered energy requirement, or a stress-protection strategy against elevated oxidative stress (Henningsen et al., 2012; Pecorelli et al., 2013). The signs of neuroinflammation in the hippocampus gleaned from the activation of microglia and astrocytes in the previous study at 3 months post-exposure (Parihar et al., 2013) could be confirmed from the increased expression of proinflammatory and decreased expression of antiinflammatory genes at 6 months post-exposure (Shetty et al., 2017). The proinflammatory genes include nuclear factor kappa B subunit 1 (Nfkb1), interleukin-1 alpha (IL1α), colony-stimulating factor 2 (Csf2), B cell lymphoma 6 (Bcl6), and IL6. The antiinflammatory genes comprise IL4 and IL10. Such a gene expression pattern at 6 months post-exposure to GWIR chemicals and stress points to significant inflammatory activity in the hippocampus.
A recent study revealed that memory impairments and neuroinflammation persist at 10 months after exposure to GWIR chemicals and stress (Madhu et al., 2019). Interestingly, chronic neuroinflammation, typified by activated microglia, reactive astrocytes, and increased concentration of TNF-α and IL-1β in the brain at a protracted timepoint, was linked with elevated levels of extracellular high mobility group box-1 (HMGB1) and complement activation in the brain. The animals with chronic GWI exhibited leakage of HMGB1 into the cytoplasm of soma and dendrites of neurons as well as into the extracellular space. Leaked HMGB1 can act as a proinflammatory molecule and trigger an inflammatory response through the activation of microglia by signaling through receptors for advanced glycation end products expressed on microglia (Abraham, Arcaroli, Carmody, Wang, & Tracey, 2000; Bonaldi et al., 2003; Ugrinova & Pasheva, 2017). Continued activation of microglia results in elevated levels of proinflammatory cytokines and complement component 1q, which can modify normal astrocytes into neurotoxic type 1 astrocytes (Arranz & De Strooper, 2019; Liddelow et al., 2017; McQuade & Blurton-Jones, 2019). Additionally, A1 reactive astrocytes can increase the concentration of complement C3 and an unidentified neurotoxin that kills neurons and oligodendrocytes (Arranz & De Strooper, 2019). HMGB1 also activates the classical pathway of the complement system (Kim et al., 2018). The study showed that elevated HMGB1 in the brain paralleled higher concentrations of C3 and TccC5b-9 (a membrane attack complex induced by complement activation) in the brain, suggesting a steady-state complement activation in chronic GWI (Madhu et al., 2019). Thus, modulation of HMGB1 and complement system might be therapeutic in chronic GWI.
The study by Madhu and colleagues also showed that that chronic neuroinflammation markers TNF-α, IL-1β, and HMGB1 could be tracked through the examination of neuron-derived extracellular vesicles (NDEVs) and complement activation related markers C3 and TccC5b-9 by astrocyte-derived extracellular vesicles (ADEVs) circulating in the blood. The findings suggested that the possible occurrence of chronic neuroinflammation and complement activation in the brain of GWI veterans could be determined through analyses of the composition of NDEVs and ADEVs in the circulating blood (Madhu et al., 2019). Comprehending these underlying pathophysiological mechanisms would help in developing a suitable therapeutic approach to tackle chronic GWI.
2.1.6. Systemic inflammation
Altered brain function in chronic GWI was accompanied by increased oxidative stress and inflammation in the circulating blood (Shetty et al., 2017; Shetty et al., 2020). Such changes were evident from elevated levels of MDA and protein carbonyls, and multiple proinflammatory cytokines in the serum of GWI rats. The proinflammatory cytokines include the tumor necrosis factor-alpha (TNF-α), IL-1β, IL-1α, transforming growth factor-beta (TGF-β), macrophage inflammatory protein 1 alpha (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), IL-6, interferon gamma-induced protein 10 (IP10), stem cell factor (SCF), vascular endothelial growth factor (VEGF), fibroblast growth factor-beta (FGF-β), IL-5, and IL-15 (Shetty et al., 2017, Shetty et al., 2020). These findings suggested that chronic neuroinflammation in GWI is associated with significant systemic inflammation.
In a global inflammatory state, other organs are affected as well, which may contribute to the maintenance of chronic systemic illness in GWI. By employing the rat model described in previous studies (Madhu et al., 2019; Parihar et al., 2013), Petrescu and associates showed that exposure to GWIR chemicals and stress increased the proinflammatory cytokine IL-6, and proinflammatory (CD11b+F4/80−) macrophages in the liver, with no changes in biliary mass or hepatic fibrosis when examined 10 weeks post-exposure (Petrescu et al., 2018). However, bile duct ligation (BDL) surgery in animals with GWI exacerbated biliary hyperplasia, fibrogenesis, and inflammation. These changes in GWI rats were typified by enhanced proliferation and reduced apoptosis of cholangiocytes and higher levels of IL-1β, IL6, and TNF-α in comparison to naïve rats undergoing the same BDL surgery. Thus, exposure to GWIR chemicals and stress leads to low-level inflammation in the liver. Such changes appear to contribute to an exacerbated inflammatory reaction typified by higher M1 macrophages, activation of hepatic stellate cells, and increased cholangiocyte proliferation and fibrosis in the liver after cholestasis challenge (Petrescu et al., 2018). These findings imply that GWI patients are more likely to develop worse liver pathology when afflicted with liver diseases.
Another study suggested an altered immune response in a rat prototype of GWI generated with exposure to PB alone for 14 days (Macht et al., 2019). When challenged with a submaximal dose of lipopolysaccharide (LPS), animals exposed to PB displayed an attenuated peripheral immune response, which was evident from decreased IL-6 and TNFα levels in the blood compared to animals exposed to the vehicle. However, LPS treatment considerably enhanced C-reactive protein (CRP) concentration in animals exposed to PB relative to animals exposed to the vehicle. Thus, PB treatment alters the peripheral inflammatory response to subsequent immune challenge. The study also demonstrated that PB treated animals exhibited decreased acetycholine response in the brain to LPS and acute immobilization stress. Decreased cholinergic responses were also associated with contextual and cue-based learning impairments. Overall, it appears that dysregulated peripheral immune response is one of the features of GWI. However, how such changes contribute to altered stimulus-evoked acetylcholine release in the brain remains to be investigated. Also, it will be interesting to examine whether similar responses are seen in other models of GWI.
2.2. Functional impairments and associated pathology in mouse models of GWI
2.2.1. Cognitive and mood dyfunction
Many studies using a mouse prototype employed intraperitoneal injections of a chemical cocktail containing a significant dose PB (2 mg/Kg) and a very high dose of PER (200 mg/Kg) dissolved in dimethyl sulfoxide for 10 days (Abdullah et al., 2011, 2013; Joshi et al., 2018, 2020; Zakirova et al., 2015; Zakirova et al., 2016). The rationale underlying such high dose exposures is based on the significant intake of PB pills as well as the overuse of PER by the veterans during the GW (Hilborne et al., 2005). Moreover, veterans experiencing higher exposure to pesticides were found to have a greater risk of developing GWI than those with limited use (Binns et al., 2008), and high PB intake was coupled with higher exposure to pesticides in a significant percentage of veterans (Hilborne et al., 2005). In this model, motor function was unaffected immediately after the exposure (i.e., post-exposure days 1–7), and anxiety-like behavior was minimal on post-exposure days 15 and 30 (Adbullah et al., 2011). However, the spatial learning ability of animals was inferior to control mice on post-exposure days 43–51 in a water maze test. Furthermore, spatial memory retrieval dysfunction was seen at an extended time point after spatial learning (i.e., on post-exposure day 115), implying that long-term memory recall is affected in this mouse prototype of GWI (Abdullah et al., 2011). A follow-up study using the same mouse model examined cognitive function using a Barnes maze test (Zakirova et al., 2015). The animals with GWI did not show impairments in acquisition trials on post-exposure days 11–14. Multiple probe trials performed at various intervals after the acquisition trials revealed no impairments in memory retrieval on post-exposure days 15, 56, or 77. However, spatial memory retrieval function was impaired at post-exposure day 106, suggesting memory recall dysfunction at an extended time-point after the memory formation (Zakirova et al., 2015).
Another longitudinal study using a battery of behavioral tests in the same mouse model reported that neurobehavioral deficits are more pronounced at 13 months post-exposure (Zakirova et al., 2016). The impairments included disinhibition behavior typified by the increased exploration of open arms in an elevated plus-maze test, which implied dysfunction of the prefrontal cortex, an area of the brain vital for executive function and decision making (Gruber et al., 2010). The study suggested that such disinhibition behavior in GWI mice is consistent with cognition and commission errors found in a cohort of GW veterans in an attention and memory task (Vasterling, Brailey, Constans, & Sutker, 1998). The other impairments in GWI mice comprised a lack of social preference in a three-chamber test and working and reference memory errors in a radial arm water maze test. Another study using the Barnes maze test showed learning and memory impairments in GWI mice at 15–16 months post-exposure (Abdullah et al., 2016). Thus, the PB+PER mouse model of GWI showed significant cognitive and mood impairments when examined at extended time points after exposure.
2.2.2. Activation of astrocytes and microglia
At ~5 months post-exposure, the mouse model of GWI displayed increased glial fibrillary acidic protein (GFAP) immunoreactivity in the cerebral cortex but not in the hippocampus (Abdullah et al., 2011). Increased GFAP immunoreactivity in the cerebral cortex persisted at 22.5 months post-exposure (Zakirova et al., 2016). Another study at 16 months post-exposure also demonstrated increased GFAP immunoreactivity and activated microglia in the dentate gyrus of GWI mice (Abdullah et al., 2016).
2.2.3. Proteomic, lipidomic and metabolomic changes in the brain
Proteomic analysis performed at 150 days post-exposure revealed differential expression of 31 proteins in mice with GWI, particularly proteins relevant to lipid metabolism, molecular transport, and endocrine and immune systems (Abdullah et al., 2011). The study revealed a reduction in fatty acid-binding protein 3 (FABP3) in GWI mice, which implied the dysregulation of lipid metabolism in GWI. Such reduction could be one of the factors underlying cognitive dysfunction in GWI mice, as previous studies have shown that neurocognitive disorders are associated with decreased FABP3 levels (Chiasserini et al., 2010; Wada-Isoe, Imamura, Kitamaya, Kowa, & Nakashima, 2008). Lipidomic analysis at 150 days post-exposure demonstrated elevated levels of phosphatidylcholine (PC) and sphingomyelin (SM), the reservoirs of phosphocholine necessary for acetylcholine (ACh) synthesis, in the brain of GWI mice (Abdullah et al., 2013). Moreover, levels of ether PC (ePC) and catalase (a marker of peroxisomes) were increased, but the concentration of lyso-platelet-activating factors (lyso-PAFs) was decreased. Accumulation of PC and SM resulting from changes in their synthesis or metabolism could induce adverse effects on organelles regulating cellular bioenergetics, and increased levels of catalase and ePC imply increased peroxisome content in cells (Abdullah et al., 2013). Furthermore, increased ePC and catalase with a concurrent decrease in lyso-PAF imply dysfunction of peroxisomes, and accumulation of functionally impaired peroxisomes in the cytoplasm is suggestive of lysosomal dysfunction (Abdullah et al., 2013; Till, Lakhani, Burnett, & Subramani, 2012; Vasko & Goligorsky, 2013). Collectively, the above findings implied peroxisomal and lysosomal dysfunction in the brain of mice afflicted with chronic GWI. A proteomic-lipidomic-metabolomic study on GWI mice at 16 months post-exposure revealed disruption of pathways that regulate mitochondrial bioenergetics, decreases in mitochondria-specific lipids, and alterations in mitochondrial metabolites linked to the corresponding pathways (Abdullah et al., 2016). Thus, proteomic, lipidomic, and metabolomic studies in GWI mice suggest adverse alterations in lipid metabolism, cellular bioenergetics, peroxisomes, lysosomes, and mitochondria.
2.2.4. Changes in microbiota impacting brain function
The gut microbiome harbors a complex array of bacteria, viruses, protozoa, fungi, and other organisms that must maintain a balanced, homeostatic interaction to preserve a healthy host immune response. Alhasson and colleagues examined whether exposures to GWIR chemicals induce dysbiosis in the gut (Alhasson et al., 2017). In the PB+PER mouse model of GWI, next-generation sequencing of microbiome revealed increased levels of phylum firmicutes and tenericutes and gram-negative bacterial genera and decreased abundance of bacteroidetes. These changes in the microbiome resulted in decreased expression of tight junction protein occludin and increased expression of claudin-2 (a protein expression in cation leaky epithelia) implying the occurrence of leaky gut in GWI. Additional analyses revealed that altered microbiome resulted in portal endotoxemia and the upregulation of toll-like receptor 4 (TLR4) signaling in the small intestine as well as the brain. Such changes were expected as some of the enriched bacteria found in GWI mice are known to be associated with intestinal inflammation and a leaky gut (e.g., Allobaculum species; Lee, Han, & Yim, 2015), neuroinflammation and memory dysfunction (e.g., ruminoccoccaceae; Beilharz, Kaakoush, Maniam, & Morris, 2016). TLR4 activation is typically associated with elevated levels of proinflammatory cytokines in conditions such as systemic inflammation (Buchholz & Bauer, 2010). Indeed, GWI mice exhibited increased levels of IL-1β and MCP-1 in both the small intestine and the brain. Another recent study suggested that the taxonomic structure of the gut microbiome gets significantly modified in the mouse model of GWI with a high-fat diet with most alterations occurring at the level of phylum in Firmicutes and Bacteroidetes (Angoa-Perez et al., 2020). This finding has significance because the majority of GW Veterans are overweight.
Additional studies demonstrated that butyrate or TLR4 inhibitor treatment could restore gut homeostasis through decreased expression of claudin-2, and reduced activation of TLR4 and enteric glial cells (Kimono et al., 2019; Seth et al., 2018). Another study showed that antibiotic or antiviral treatment could positively modulate the gut microbiome, improve the tight junction protein and reduce the systemic- and neuroinflammation (Seth et al., 2019; Kimono et al., 2019). Overall, the results implied that the modulation of gut microbiome not only repairs the leaky gut in GWI but also leads to reductions in systemic- and neuroinflammation, which could potentially improve brain function. However, it remains to be addressed whether positive modulation of the gut microbiome would improve cognitive and mood function in GWI.
2.2.5. Activation of adaptive immune response
A study by Joshi and associates examined whether exposure to PB+PER in a mouse model activates peripheral and CNS adaptive immune responses (Joshi et al., 2019). 3-phenoxybenzoic acid (3-PBA), a PER metabolite that forms adducts with endogenous proteins, was found in the brain, blood, and liver of mice at acute and chronic time points after exposure to PB and PER. Additional analyses revealed autoantibodies against 3-PBA-haptenated albumin (3-PBA-albumin) in the plasma of PB+PER treated mice and veterans with chronic GWI. Furthermore, in vitro studies revealed that exposure of blood to 3-PBA-albumin caused activation of B- and T-helper lymphocytes, a feature also observed in the blood of PB+PER treated mice and veterans with chronic GWI. These changes correlated with enhanced infiltration of monocytes, changes in BBB markers and brain macrophages, and the extent of inflammation in the brain. The results suggested that the exposure to PER during the GW caused activation of the peripheral adaptive immune response through haptenated metabolite of PER. Such peripheral immune response resulted in the migration of peripheral immune cells into the brain as pesticide exposure also caused BBB disruption (Abdel-Rahman et al., 2002). Thus, neuroinflammation and brain dysfunction could also result from the persistence of haptenated pesticide metabolites. However, it remains to be confirmed whether such adaptive immune response emanating from PER exposure is widespread among GW veterans.
2.2.6. Mechanisms of neuroinflammation in additional mouse models of GWI
O’Callaghan and colleagues employed a model of GWI where mice were exposed to DFP and/or the pesticide chlorpyrifos (CPF) (Locker et al., 2017; O’Callaghan, Kelly, Locker, Miller, & Lasley, 2015). The rationale for using these chemicals to model GWI stems from the fact that a significant percentage of veterans were exposed to low levels of sarin released into the atmosphere and the CPF was used around tents during the GW (O’Callaghan et al., 2015). This prototype also exhibited significant neuroinflammation. Interestingly, when exposures to these chemicals were combined with a four-day pre-exposure to exogenous CORT to mimic the war-related stress, neuroinflammation was exacerbated. Such response was evident from increased levels of TNFα, IL-6, MCP-1, and IL-1β, leukemia inhibitor factor (LIF), and oncostatin M in the brain (Locker et al., 2017; O’Callaghan et al., 2015). Additional studies revealed that CORT and DFP exposure could induce neuroinflammation without causing persistent systemic inflammation (Michalovicz et al., 2019). In this study, while DFP exposure alone caused the elevated expression of several proinflammatory cytokines in the liver and the circulating blood, exposure to CORT before the DFP exposure did not increase the concentration systemic proinflammatory cytokines. These results are in sharp contrast to the exacerbated neuroinflammation observed after CORT-primed exposure to DFP (Locker et al., 2017; O’Callaghan et al., 2015). Another study in the CORT-DFP mouse model of GWI showed that CORT pretreatment alleviated the DFP-induced ACh increase in the hippocampus and the striatum, which implied that the exacerbated neuroinflammatory response in the CORT-DFP model of GWI is not linked to the accumulation of ACh in different brain regions (Miller et al., 2018). Another study examining the genome-wide transcriptome architecture in a mouse model revealed significant genetic effects following CORT and DFP exposure (Xu et al., 2020). Notably, the study suggested the activation of cytokine-cytokine receptor interaction and TNF signaling pathways and decreased number of myelinating oligodendrocytes after CORT and DFP exposure. The study also identified activation of Adamts9, a gene linked to inflammation and cognitive aging (Garcia-Faroldi et al., 2013; Lin et al., 2017), as the trigger for the inflammatory response following CORT and DFP exposure. These studies suggested that GWI stemming from war-related stress and low-level exposure to sarin is likely a neuroimmune disorder rather than persistent systemic inflammation or ACh accumulation, causing neuroinflammation and brain dysfunction (Michalovicz, Kelly, Sullivan, & O’Callaghan, 2020).
2.3. Insights on brain dysfunction in GWI gleaned from animal model studies
The rat models of chronic GWI demonstrated that continual cognitive and mood impairments were concomitant with incessantly raised oxidative stress, inflammation, loss of inhibitory neurons in the brain, and significant systemic inflammation. The various alterations contributing to the persistent cognitive and mood dysfunction are summarized in Figure 1. Heightened oxidative stress in the brain was manifested through higher levels of MDA, 4-HNE, protein carbonyls and NRF2, and alterations in the expression of multiple genes that encode proteins involved in oxidative stress-response, ROS metabolism, oxygen transport, antioxidant activity, and the function of mitochondrial complexes and the ETC (Kodali et al., 2018;Shetty et al., 2017; Shetty et al., 2020). Brain inflammation in rats with chronic GWI was typified by the presence of activated microglia, reactive astrocytes, higher expression of multiple genes that encode proinflammatory proteins, increased concentrations of inflammatory mediators HMGB1, TNF-α, IL-1β, C3 and TccC5b-9 (Kodali et al., 2018; Madhu et al., 2019; Parihar et al., 2013; Shetty et al., 2017; Shetty et al., 2020). The hippocampus of GWI rats also displayed a loss of inhibitory interneurons and decreased neurogenesis (Megahed, Hattiangady, Shuai, & Shetty, 2015; Parihar et al., 2013; Shetty et al., 2020). The occurrence of systemic inflammation in the rat model was evident from the elevated levels of oxidative stress markers MDA and protein carbonyls and proinflammatory cytokines TNF-α, IL-1β, IL-1α, TGF-β, MIP-1α, IL-6, IP10, SCF, VEGF, FGF-β, IL5, and IL15 in the circulating blood (Shetty et al., 2017; Shetty et al., 2020). The mouse models of chronic GWI also revealed possible links of cognitive and mood dysfunction with neuro- and systemic inflammation. Neuroinflammation in the mouse model was evident by the presence of reactive astrocytes and activated microglia (Abdullah et al., 2016; Zakirova et al., 2015; Zakirova et al., 2016) and increased levels of proinflammatory cytokines TNFα, IL-1β, MCP-1, IL-6, LIF, and oncostatin M (Locker et al., 2017; O’Callaghan et al., 2015). Additional changes in the brain in a mouse model of chronic GWI include adverse alterations in lipid metabolism, cellular bioenergetics, peroxisomes, lysosomes, and mitochondria (Abdullah et al., 2011, 2013; Abdullah et al., 2016). The occurrence of systemic inflammation in mouse models was ostensible from the activation of the peripheral adaptive immune response through haptenated metabolite of PER (Joshi et al., 2018), the altered microbiome in the gut causing portal endotoxemia and activation of TLR4 signaling in the small intestine (Alhasson et al., 2017; Seth et al., 2019; Kimono et al., 2019).
Fig. 1.
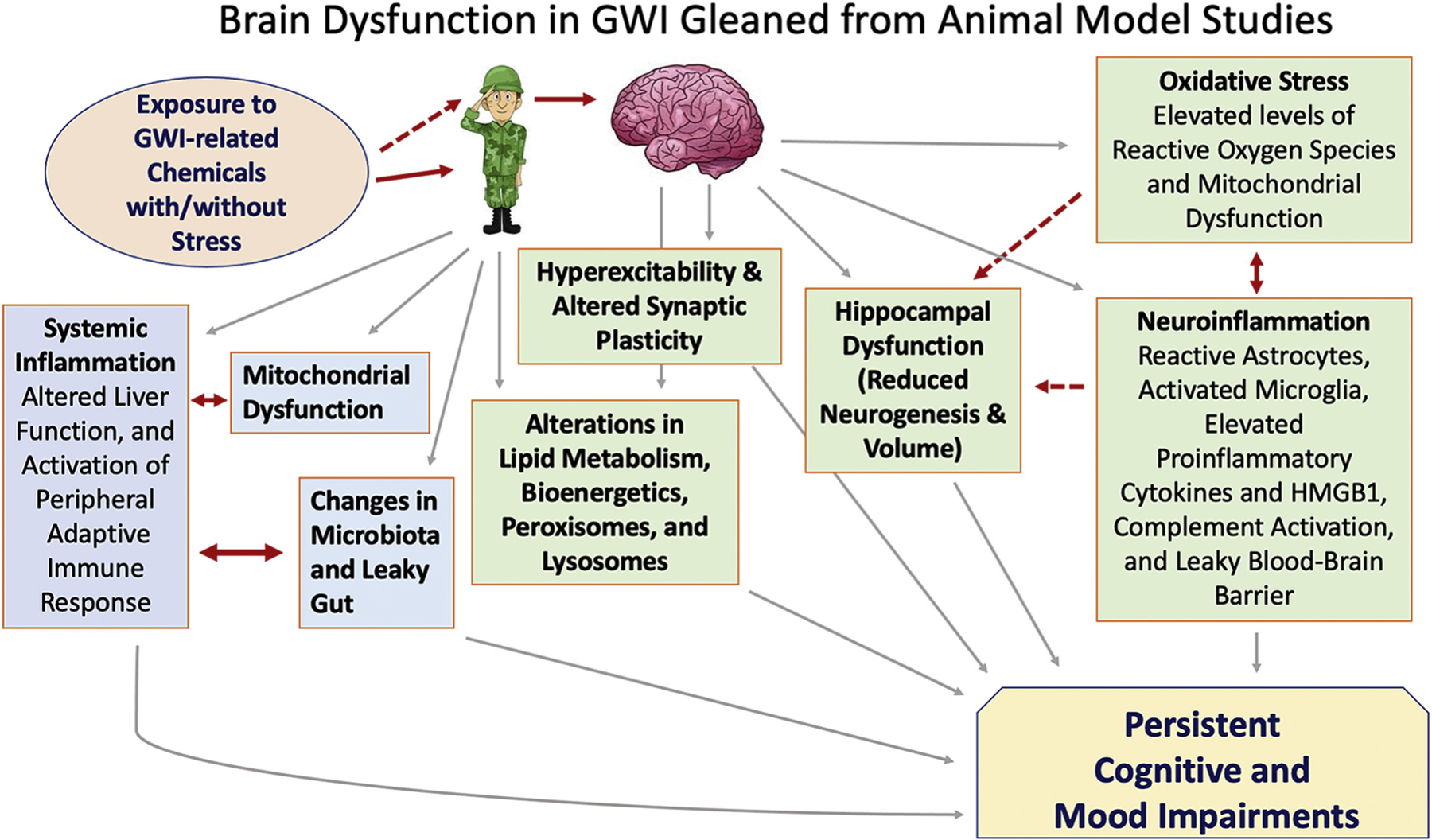
A schematic illustrating the potential mechanisms causing brain dysfunction in Gulf War Illness (GWI) as gleaned from animal model studies. Systemic changes caused by the exposure to GWI-related chemicals have been mostly correlated between animal models and GWI patients (solid red line). These include activation of the peripheral adaptive immune response and alterations in lipid metabolism, bioenergetics, peroxisomes, lysosomes, liver function, mitochondrial function, microbiome, and leaky gut. Animal model studies also suggested that such exposures could directly affect the brain of veterans (indicated by a dotted red line), which may result in neuronal hyperexcitability and altered synaptic plasticity. Furthermore, both direct and indirect effects of exposures likely underlie persistently increased oxidative stress and inflammation in the brain, which could lead to a leaky blood-brain barrier, reduced hippocampal neurogenesis, and cognitive and mood impairments.HMGB1, high mobility group box-1.
From multiple studies in rat and mouse models of GWI, it appears that cognitive and mood dysfunction in GW veterans is likely due to a combination of factors. First, the persistent oxidative stress and inflammation in the brain could considerably interfere with the cognitive and mood function (Krishnadas & Cavanagh, 2012; Navakkode, Liu, & Soong, 2018; McGrattan et al., 2019; Carlessi, Borba, Zugno, Quevedo, & Reus, 2019). While the inflammatory response of an organism is a defense mechanism in a state of redox balance, dysregulation of the immune response occurs with increased oxidative stress (Solleiro-Villavicencio & Rivas-Arancibia, 2018). Mitochondrial dysfunction associated with elevated oxidative stress could also contribute to cognitive impairment (Fernandez et al., 2019; Netto et al., 2018). Neuroinflammation, depending on the severity, can directly worsen cognitive and mood function or indirectly affect cognition and mood by reducing hippocampal neurogenesis (Kaltschmidt & Kaltschmidt, 2015; McAvoy & Sahay, 2017). Chronic neuroinflammation typically leads to degradation and reduction of neural tissue volume and induces a leaky BBB, which allows the passage of proinflammatory cytokines into the circulating blood and result in immune modulation (Finneran & Nash, 2019; Heneka et al., 2015). Furthermore, reduced numbers of inhibitory interneurons observed in animals with GWI (Megahed, Hattiangady, Shuai, & Shetty, 2015) could contribute to cognitive dysfunction because interneuron populations play roles in the maintenance of cognitive function by controlling the activity of principal neurons in the hippocampus. For example, reduced numbers of PV+ and NPY+ interneurons affect network properties, which can result in hippocampal hyperexcitability (Andrioli, Alonso-Nanclares, Arellano, & DeFelipe, 2007; Schwaller et al., 2004; Sperk, Hamilton, & Colmers, 2007) and cognitive dysfunction (Korotkova, Fuchs, Ponomarenko, von Engelhardt, & Monyer, 2010; Spiegel, Koh, Vogt, Rapp, & Gallagher, 2013; Zaben & Gray, 2013).
Reduced hippocampal neurogenesis seen in animals with chronic GWI is another factor underlying cognitive and mood dysfunction because of its role in maintaining such functions (Kempermann, Gast, & Gage, 2002; Santarelli et al., 2003; Deng, Aimone, & Gage, 2010; Snyder, Soumier, Brewer, Pickel, & Cameron, 2011; Parihar et al., 2011; Eisch & Petrik, 2012; Fotuhi, Do, & Jack, 2012). In addition, significant systemic inflammation observed in animals with chronic GWI might be contributing to persistent cognitive and mood dysfunction in GWI because studies have shown that significant systemic inflammation could lead to cognitive and mood impairments (Di Filippo et al., 2013; Iwashyna, Ely, Smith, & Langa, 2010; Yamanaka et al., 2017). Moreover, cognitive and mood dysfunction can also occur through increased expression of genes linked to neurodegenerative disorders. Indeed, animals with chronic GWI exhibited increased expression of genes Mapk1 and Mapk3 (Shetty et al., 2017) that are implicated in Alzheimer’s dementia (Gerschutz et al., 2014). Thus, from studies in rat and mouse models of chronic GWI, it appears that incessantly elevated oxidative stress, altered mitochondrial function, inflammation, loss of inhibitory interneurons, reduced hippocampal neurogenesis in the brain, and increased oxidative stress and inflammation at the systemic level associated with altered gut microbiome underlie cognitive and mood dysfunction in GWI. However, it is unknown whether the various alterations in the CNS precede the systemic inflammatory changes, or both CNS and systemic changes occur concurrently. Nonetheless, considering both neuro- and systemic inflammation in animal models of GWI, several studies focused on developing therapeutic strategies are fittingly examining the efficacy of systemic administration of various antioxidant and/or antiinflammatory compounds in animal models of GWI.
3. Brain Dysfunction in Veterans with GWI – Extent and Potential Mechanisms
3.1. Extent of cognitive and mood dysfunction
Cognitive impairment or cognitive fatigue is one of the most conspicuous features of GWI in veterans (Cooper et al., 2016; White et al., 2016). The hippocampus-dependent memory dysfunction seen in animal models of GWI correlated well with the hippocampus-related memory impairment observed in veterans with GWI. Hippocampus dysfunction could be seen from both magnetic resonance spectroscopy and single-photon emission computed tomography studies (Haley et al., 2000,Haley et al., 2000, Haley et al., 2009; Menon, Nasrallah, Reeves, & Ali, 2004) in veterans with GWI. Arterial spin labeling comprising intravenous infusions of physostigmine and measurement of the regional cerebral blood flow with left and right hippocampal function measures also confirmed such impairment in GWI (Li et al., 2011). Furthermore, studies employing a face-name associative recall test, a measure directly linked to the hippocampus function, and assessments by functional MRI (fMRI) demonstrated memory impairment in veterans with GWI (Cooper et al., 2016; Odegard et al., 2013). A meta-analysis by Janulewicz and colleagues showed that GW veterans, in general, displayed poorer visuospatial, attention, executive, learning, and memory functions, without showing any motor impairment (Janulewicz et al., 2017). Moreover, MRI studies reported significant reductions in the volume of CA2, CA3 and dentate gyrus regions, and the overall hippocampus in veterans exposed to sarin during the GW (Chao, Kriger, Buckley, Ng, & Mueller, 2014; Chao, Rothlind, Cardenas, Meyerhoff, & Weiner, 2010; Toomey et al., 2009). Thus, veterans with GWI display persistent cognitive dysfunction, which is linked to both hippocampus and the cerebral cortex, particularly the medial prefrontal cortex.
Mood dysfunction is another symptom seen in a significant percentage of GW veterans (Smith et al., 2013). Engdahl and associates examined the extent of mood dysfunction in veterans with GWI (Engdahl et al., 2018). By examining both objective (neural) and subjective (symptoms) indices, the study implied that GWI is distinct from the healthy population and the severity of mood dysfunction varies in a continuum that leads, at the higher end, to a diagnosable mental health illness (Engdahl et al., 2018). The severity of GWI symptoms varied considerably between GWI without mood dysfunction vis-à-vis GWI with mood dysfunction. Interestingly, synchronous neural interactions (SNI) derived from the magnetoencephalography (MEG) recordings revealed that GWI alone involved the left cerebral hemisphere predominantly, whereas GWI with mental illness involved both left and right cerebral hemispheres. The study also showed that both GWI symptom severity and SNI distribution measures could predict mental illness occurrence in veterans with GWI (Engdahl et al., 2018). The pathophysiology gleaned from studies on the brain, cerebrospinal fluid, and blood from veterans with GWI are discussed below and summarized in Figure 2.
Fig. 2.
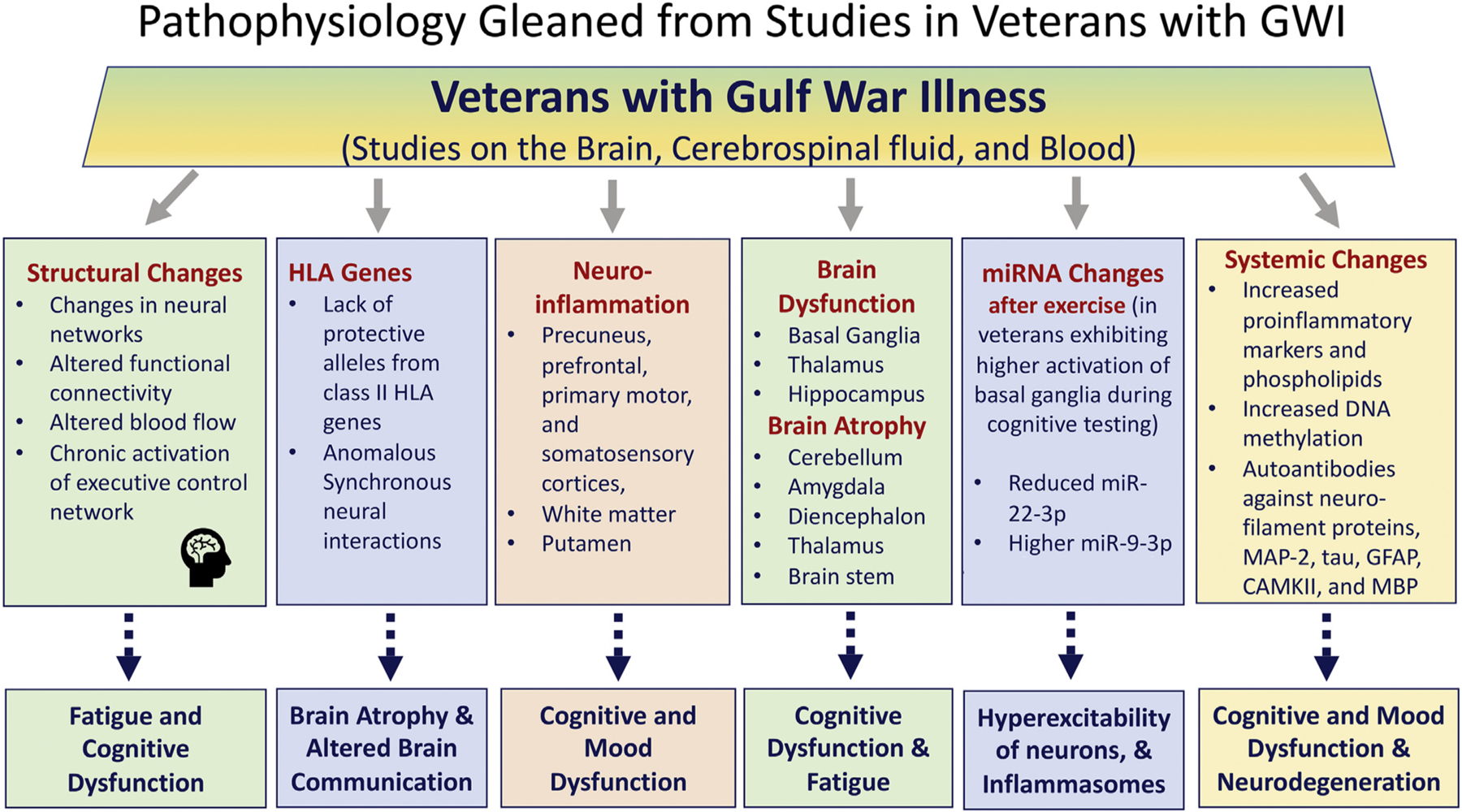
A flowchart illustrating the potential mechanisms underlying cognitive and mood dysfunction, fatigue, neuronal hyperexcitability, and neuroinflammation in Gulf War Illness (GWI) as observed from studies on the brain, cerebrospinal fluid, and the blood from veterans with GWI. CAMKII, calcium-calmodulin kinase II; GFAP, glial fibrillary acidic protein; HLA, human leukocyte antigen; MAP2, microtubule-associated protein-2; MBP, myelin basic protein; tau, microtubule-associated tau (T) proteins.
3.2. Alterations in neural networks and functional connectivity
A recent study by Wylie and colleagues investigated the neural networks associated with cognitive fatigue in GWI by asking veter veterans to perform a series of fatiguing tasks while in the MRI scanner (Wylie et al., 2019). This investigation measured the neural networks linked to state fatigue, a weariness that transpires as the task is accomplished and connected to brain areas implicated in memory tasks, and trait fatigue, a type of tiredness that occurs over several weeks and couples to key areas of the fronto-striatal-thalamic circuit. The results showed that, in healthy controls, the caudate nucleus of the striatum displayed little activation when performing a less difficult task, but significant activation during a more difficult task, implying that the performance of a difficult task required increased motivation or is more rewarding than an easier task. Contrastingly, in veterans with GWI, the caudate nucleus displayed considerable activation while performing a simple task but showed less activation when performing a complex task, suggesting that veterans in the GWI group cannot modulate the motivation and reward circuitry as the demands of the task change (Wylie et al., 2019). The findings suggested that veterans with GWI required the expenditure of substantial cognitive resources for performing even simple tasks, which interfered with the activation of the fronto-striatalthalamic circuit. Such a conclusion was supported by observations that the GWI group showed persistently high activation in the executive control network, comprising the parietal and inferior frontal cortical areas and cerebellar areas (D’Esposito & Postle, 2015). Thus, chronic activation of the executive control network underlies cognitive fatigue in GWI, which likely reflects a chronic sense of fatigue and the increased impact of physical and mental work on fatigue experienced by veterans with GWI (Wylie et al., 2019).
Another study suggested that functional connectivity impairments in the brain underlie brain dysfunction in GWI (Gopinath, Sakoglu, Crosson, & Haley, 2019). The study employed the resting-state fMRI from 60 veterans with GWI and 30 age-matched military controls, assessed functional connectivity networks, and mapped impaired brain function in GWI with advanced network analysis. The findings suggested impaired functional connectivity in veterans with GWI for language, sensory input and motor output networks. Furthermore, GWI veterans also exhibited impaired functional connectivity between different sensory perception and motor networks, and between different networks in the sensorimotor domain (Gopinath et al., 2019).
3.3. Altered cerebral blood flow
A study evaluated changes in the cerebral blood flow (CBF) velocity in response to orthostatic stress, such as sit-to-stand maneuver and increased fractional concentration of carbon dioxide in veterans with GWI (Falvo, Lindheimer, & Serrador, 2018). The findings revealed diminished dynamic autoregulation of CBF and declined CBF velocity at the nadir after standing and steady-state standing in veterans with GWI. Such changes in CBF has been proposed as one of the contributing factors for cognitive dysfunction in GWI because cognitive impairment in older adults with or without dementia is typically associated with cerebral hypoperfusion (Bertsch et al., 2009; Binnewijzend et al., 2016; Marshall, 2012) and transient occlusion of CBF leads to reversible cognitive impairment in patients with cardiovascular disease (Marshall et al., 2001).
3.4. Neuroinflammation and atrophy of brain regions
A recent study has measured neuroinflammation in veterans with GWI through a positron emission tomography (PET) using [11C]PBR28, which binds to the 18 kDa translocator protein (TSPO) (Alshelh et al., 2020). Since TSPO is upregulated in activated microglia and astrocytes, the approach provides a measure of neuroinflammation in the brain. In comparison to the healthy controls or the healthy veterans, veterans with GWI (typified by higher Kansas GWI score, fibromyalgia, fatigue, pain, and depression) displayed widespread cortical elevations in [11C] PBR28 PET signal in several cerebral cortical areas, which include precuneus, prefrontal, primary motor, and somatosensory cortices and the underlying white matter and the putamen. Statistically, the plasma levels of the inflammatory cytokines measured in the study did not reveal significant group differences. The study also reported no significant correlations between [11C]PBR28 PET signal and circulating proinflammatory cytokines. Nonetheless, in comparison to all healthy controls (n = 33), the GWI group (n = 15) appeared to have higher levels of IL-6, TNF-α, and IL-1β in the circulating blood. The overall increases were 5.8 folds for IL-6, 1.9 folds for TNF-α, and 3.3 folds for IL-1β. The increased concentration of proinflammatory cytokines in the circulating blood is consistent with studies in animal models of GWI (Shetty et al., 2017; Shetty et al., 2020). Overall, this study provided an in vivo evidence of neuroinflammation in veterans with GWI, which is consistent with incessant neuroinflammation observed in animal models of GWI (Abdullah et al., 2016; Madhu et al., 2019; Parihar et al., 2013; Shetty et al., 2017; Shetty et al., 2020; Zakirova et al., 2016). The findings also support the application of therapeutic strategies that can potentially alleviate neuroinflammation as a means to improve brain function in GWI.
Functional imaging studies have shown abnormal function in the basal ganglia (Haley, Marshall, et al., 2000), thalamus (Liu et al., 2011), and hippocampus (Li et al., 2011) of veterans with GWI. A study by Christova and associates reported that GWI is associated with the atrophy of the cerebellum, amygdala, and diencephalon (Christova et al., 2017). A recent study in 111 veterans with GWI and 59 healthy controls examined regional brain volumes using structural MRI (Zhang et al., 2020). The study reported significant subcortical atrophy in veterans with GWI, with no alterations in the cerebral cortex’s volume or the cerebellum. Significant atrophy was seen in the brainstem, ventral diencephalon, and thalamus. These results are consistent with the reduced brainstem metabolism evident from MR spectroscopy for N-acetyl aspartate in veterans with GWI (Haley, Marshall, et al., 2000). Since the major autonomic and neurotransmitter pathways and nuclei that regulate the sympathetic and parasympathetic nervous systems are located in the brainstem, reduced brainstem volume could affect functions such as sensation, motion, cardiovascular system control, respiratory control, pain sensitivity control, alertness, awareness, and consciousness (Simic et al., 2017). From these perspectives, autonomic dysfunction and dysregulated neurotransmission in the brainstem could be one of the reasons underlying several symptoms of GWI (Zhang et al., 2020). Furthermore, the study suggested that a diminished volume of the pons was linked with the intensity and frequency of chronic fatigue. Also, there was a positive correlation between brainstem atrophy and memory loss and depression severity, implying that brainstem structural changes could be among the underlying causes of memory and mood dysfunction in GWI (Zhang et al., 2020). It is interesting to note that the study did not find atrophy of the hippocampus, which is somewhat consistent with only 10% reduction in hippocampus volume observed in an animal model of GWI (Parihar et al., 2013). Perhaps, the increased activity of astrocytes and microglia seen in animals with chronic GWI (Madhu et al., 2019) maintain the volume of the hippocampus despite some neuron loss occurring in different subregions of the hippocampus.
3.5. Changes in miRNA levels
Veterans with GWI displayed post-exertional malaise, akin to that observed in patients with chronic fatigue syndrome (CFS) (Rayhan, Raksit, et al., 2013, Rayhan et al., 2013). Following an exercise regimen, veterans with GWI displayed either stress test activated reversible tachycardia (START) or stress test originated phantom perception (STOPP). The START group showed increased blood oxygenation level-dependent (BOLD) signal in the cerebellar vermis during a cognitive task before exercise, lower BOLD signals during a working memory task after exercise, and diminished brainstem volume (Rayhan, Stevens, et al., 2013; Washington et al., 2020). In contrast, the STOPP group exhibited higher BOLD activation of basal ganglia and anterior insula during cognitive testing than sedentary controls and the START group (Rayhan, Stevens, et al., 2013), a pattern that mimicked phantom limb pain (Liaw, You, Cheng, Kao, & Wong, 1998). The occurrence of two distinct phenotypes following exercise likely suggests two distinct mechanisms of CNS damage following exposure to GWIR chemicals or variable progression of GWI. Since such changes imply adverse alterations in distinct regions of the CNS following exercise, a study investigated miRNA levels in veterans’ cerebrospinal fluid following either an overnight rest (nonexercised) or submaximal bicycle exercise (Baraniuk & Shivapurkar, 2017). The levels of various miRNAs measured were comparable between nonexercised GWI, CFS, and control subjects. However, after exercise, the START group displayed lower miR-22–3p than control and STOPP groups. The START group also exhibited higher miR-9–3p than the STOPP group. Reduced miR-22–3p in the START group might increase the vulnerability for hyperexcitability of neurons, as this miRNA acts as an endogenous antiepileptogenic agent (Jimenez-Mateos et al., 2015). Moreover, one of the targets of miR-22 is P2X7 expressed on immune cells, which mediates the activation of NLRP3 inflammasomes and the release of proinflammatory cytokines and chemokines (Di Virgilio, Dal Ben, Sarti, Giuliani, & Falzoni, 2017). Therefore, it will be interesting to investigate in the future whether the START group has higher CNS inflammation levels than the STOPP and control groups. On the other hand, higher expression of miR-9–3p in the START group likely beneficial, as this miRNA is involved in facilitating long-term potentiation, synaptic plasticity, and memory formation (Sim et al., 2016).
3.6. Autoantibodies
A study by Abou-Donia and colleagues investigated the serum of veterans with GWI (n = 20, collected from 2010 to 2012) and non-veteran symptomatic controls (n = 10) for the presence of autoantibodies against neuron- and glia-specific proteins (Abou-Donia et al., 2017). The proteins analyzed included neurofilament triplet proteins, tubulin, microtubule-associated tau proteins, microtubule-associated protein-2, myelin basic protein (MBP), myelin-associated glycoprotein, GFAP, calcium-calmodulin kinase II (CaMKII) and glial S-100B protein. Remarkably, veterans with GWI displayed significantly elevated levels of autoantibody reactivity to all proteins except S-100B. Compared to controls, the highest increases were observed for CaMKII (~9 fold), GFAP (~7 fold), and neurofilaments (~2.5 fold) in GWI patients. Such results implied that the continuation of neurodegenerative processes and reactive changes in astrocytes and oligodendrocytes in veterans with GWI even ~25 years after the GW (Abou-Donia et al., 2017). Such serum autoantibodies are likely useful as biomarkers of GWI but need confirmation in a larger cohort of veterans with GWI.
3.7. Other issues potentially impacting brain function in GWI
3.7.1. Human leukocyte antigen
Several studies suggested that the brain atrophy and altered brain communication in GWI are due to immune-related processes (Christova et al., 2017; Georgopoulos et al., 2017; James, Engdahl, Leuthold, & Georgopoulos, 2016). Specifically, the lack of six protective alleles from Class II HLA genes has been proposed as one of the reasons underlying functional brain abnormalities in veterans with GWI (Georgopoulos et al., 2015). HLA genes are positioned in the major histocompatibility complex of chromosome 6, which has a significant role in immune recognition (Meuer et al., 1982). HLA genes mediate immune responses to intracellular and extracellular substances (Blum, Csurhes, Reddel, Spies, & McCombe, 2014). Protection against GWI was seen when six Class II HLA alleles were present, which include DRB1*01:01, DRB1*08:11, DRB1*13:02, DQB1*02:02, DPB*01:01, and DPB1*06:01. Because each person has 2 copies of Class II HLA genes and HLA genes are highly polymorphic, the number of copies, collectively, of the 6 protective alleles that a person can have is zero, one or two. Interestingly, the overall GWI symptom severity diminished linearly with the increased number of copies of the 6 protective alleles (Georgopoulos et al., 2016). HLA protection affected the severity of cognitive and mood dysfunction, pain, and fatigue. In a follow-up study, the same research group examined neural communication patterns by assessing the brain’s synchronous neural interactions using MEG recordings, as the synchronous neural activity is central to several cognitive functions, including attention, memory, and sensory-motor integration (James et al., 2016). It is also known from previous studies that cognitively healthy individuals display remarkably similar patterns of neural synchronicity (Langheim, Leuthold, & Georgopoulos, 2006), whereas neuropsychiatric conditions display altered neural synchrony (Engdahl et al., 2010; Georgopoulos et al., 2007; Georgopoulos et al., 2010; James, Engdahl, Leuthold, Krueger, & Georgopoulos, 2015). In veterans with GWI, anomalous synchronicity affecting cortical areas, and the cerebellum was found, however (Engdahl et al., 2016).
In a subsequent study, James and associates investigated the protective role of HLA allele DRB1*13:02 in GW veterans against brain atrophy (James et al., 2017), as this allele has been found earlier to have a protective role in a broad range of autoimmune diseases (Furukawa et al., 2017). The findings revealed that DRB1*13:02 spared subcortical brain atrophy in GW veterans. The authors proposed that the protective role of DRB1*13:02 is due to its ab ability to eliminate external antigens to which GW veterans were exposed. Such antigens would otherwise have caused persistent low-grade inflammation and autoimmunity (James et al., 2017). Thus, the severity of cognitive dysfunction in GWI can vary considerably depending on the number of copies of the 6 protective alleles of Class II HLA the veteran has.
3.7.2. Systemic inflammation and metabolic changes
Studies have reported that veterans with GWI exhibited chronic inflammation and altered cytokine levels in the circulating blood (Broderick et al., 2011). The cytokines include TNF-α, IL-4, IL-7, IL-13, and IL-17F (Khaiboullina et al., 2015; Parkitny, Middleton, Baker, & Younger, 2015). Furthermore, a study comparing total phospholipid (PL) classes in the blood of GWI patients with GW deployed controls demonstrated no differences for total PC, phosphatidylethanolamine (PE), lysophosphatidylethanolamine (LPE) and phosphatidylinositol (PI). However, the total lysophosphatidylcholine (LPC) was increased by 15% in GWI patients (Emmerich et al., 2017). Analysis of the extent of unsaturation showed no differences for PE and PI, but saturated fatty acid (SFA) containing PC species were reduced in GWI patients with no changes in monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA) containing PC species. For LPE, PUFA containing species showed a 50% increase in GWI patients. Furthermore, for LPC, SFA, MUFA, and PUFA were elevated in GWI patients. Also, the levels of ePC, ether lysophosphatidylethanolamine (eLPE), and ether phosphatidylethanolamine were unchanged in GWI subjects, but eLPE levels were increased (Emmerich et al., 2017). Additionally, ω−6 arachidonic acid (AA) and ω−3 docosahexaenoic acid (DHA) containing species within LPC and LPE were increased in GWI patients. This study also demonstrated that some of the lipid biomarkers co-occurred in the plasma of rodent models of GWI and GWI patients (Emmerich et al., 2017). Thus, multiple PL species are altered in the circulating blood of patients with GWI. These results have implications because the metabolism of DHA and AA could generate bioactive lipids, which can influence the inflammatory response in GWI. DHA is a precursor of resolvins and neuroprotectins involved in mediating antiinflammatory activity, whereas AA is a precursor of proinflammatory lipid mediators, such as prostaglandins and leukotrienes (Emmerich et al., 2017; Fredman & Serhan, 2011; Serhan, Yacoubian, & Yang, 2008). Consistent with the concept of persistent systemic inflammation underlying GWI, another study on plasma samples demonstrated a positive correlation between IL-6 and C-reactive protein (CRP) levels in veterans with GWI (Butterick et al., 2019). A metabolomic study on the serum showed that veterans with GWI displayed abnormalities in 8 out of 46 biochemical pathways examined, with lipid abnormalities accounting for 78% of the metabolic change (Naviaux et al., 2019). The study showed increases in 15 ceramides and sphingomyelins, and 4 phosphatidylcholine lipids. Additional analysis of the plasma from veterans with GWI suggested decreased prostaglandin F2 alpha (pgf2α) and leukotriene B4 (Golomb et al., 2019). The study suggested that exposures to fuel-solvents, radioactive chemicals, and vaccines were linked to lower pgf2α. Also, the lower pgf2α correlated with GWI “Kansas criteria” domains of pain and respiratory symptoms (Golomb et al., 2019). Furthermore, a study comparing the multiple metabolic features observed in the peripheral blood between 20 veterans with GWI and 20 nonveteran control subjects reported abnormalities in several biochemical pathways in GWI (Naviaux et al., 2019). Lipid abnormalities, notably increased levels of ceramides and sphingomyelins and phosphatidylcholine lipids, were seen. Interestingly, key pathways typically downregulated in myalgic encephalitis/chronic fatigue syndrome were upregulated in GWI (Naviaux et al., 2019). The results suggested that veterans with GWI display a distinct metabolic phenotype compared to control subjects.
3.7.3. DNA methylation changes and chronic inflammation
Alterations in epigenetic changes such as DNA methylation could also contribute to inflammatory conditions, and disruption of DNA methylation pattern has been observed in various immune and neurological diseases (Delgado-Morales, Agis-Balboa, Esteller, & Berdasco, 2017; Landgrave-Gomez, Mercado-Gomez, & Guevara-Guzman, 2015). DNA methylation, a biological process involving the covalent binding of a methyl group to a Cytosine-5 at a C-phosphate-G (CpG) site yielding 5-methylcytosine, could change the activity of a DNA segment without altering its sequence (Long, Smiraglia, & Campbell, 2017). Increased methylation of DNA in gene promoters results in repression of genes, and such changes could play a vital role in the interaction between environmental and gene expression factors (Long et al., 2017). Therefore, a recent study examined the DNA methylation patterns in peripheral blood mononuclear cells from GWI patients compared to control subjects (Trivedi et al., 2019). The study did not find global alterations in DNA methylation in GWI patients. However, 10,767 differentially methylated CpG sites were found across gene regulatory elements and within coding regions, with 88% of them hypermethylated with 776 differentially methylated gene promoters (DMP) in GWI patients. Interestingly, CpG islands in promoters of 16 different T cell receptor joining genes were hypermethylated, which could impact T cell receptors’ recombination and function of helper T cells (Trivedi et al., 2019). The study also found that the CpG islands in promoters of immunoglobulins and interleukin 1 alpha and interleukin 1 receptor, were hypermethylated.
Functional analysis revealed that most of the DMPs belonged to genes responsible for the metabolism and immune system. Hypermethylation was seen in the promoters of several genes linked to the glutathione-mediated detoxification pathway, and eicosanoid signaling pathway. Furthermore, promoter methylation was impaired in the inflammatory response genes, which include IL-10 and NF-κB signaling, and activation of interferon regulatory factor by cytosolic pattern recognition receptors. Moreover, promoter hypermethylation was seen for NFκB inhibitor alpha (NFKBIA, an inhibitor of inflammation), mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4, a mediator of TNFα pathway signaling), MAP3K7 binding protein 3 (TAB3, a regulator of NF-κB signaling), and interferon-alpha 6 (IFNA6, a regulator of cytokine signaling) (Trivedi et al., 2019). Moreover, 3 genes with DMPs belonged to a core component of the circadian clock, implying a link to sleep problems seen in GWI patients (Chao, Abadjian, Esparza, & Reeb, 2016). Overall, this study uncovered the occurrence of epigenetic dysfunction in GWI patients. However, it remains to be investigated whether such epigenetic changes also occur in the mediators of chronic neuroinflammation in the brain (i.e., astrocytes and microglia), and such changes underlie chronic neuroinflammation in GWI.
3.7.4. Changes in microbiota
A preliminary study on the gut microbiome in veterans with GWI reported altered gut microbiome patterns, particularly in veterans with GWI exhibiting gastrointestinal symptoms (Janulewicz et al., 2019). This subgroup also showed elevated TNF receptor 1 in the plasma and significantly higher levels of chronic pain, fatigue, and sleep problems. Thus, the altered microbiome is likely one of the factors contributing to chronic neuroinflammation and brain dysfunction in GWI.
3.7.5. Mitochondrial dysfunction and mitochondrial DNA damage
Mitochondrial damage or dysfunction is another issue that received esignificant attention in comprehending mechanisms underlying GWI in veterans. Koslik and colleagues measured post-exercise phosphocreatine-recovery time constant (PCr-R) using (31)Phosphorus Magnetic Resonance Spectroscopy ((31)P-MRS) in the calf muscle of GW veterans with GWI, as the quantification of PCr-R provides an index of mitochondrial status (Koslik, Hamilton, & Golomb, 2014). The study employed seven male veterans meeting CDC and Kansal criteria for GWI and seven non-deployed healthy men. The results demonstrated that PCr-R was considerably prolonged in veterans with GWI compared to control subjects. Another study employing 21 veterans with GWI and seven control subjects examined the mononuclear cells from the blood for mitochondrial and nuclear DNA lesion frequency and mitochondrial DNA (mtDNA) copy number (mtDNAcn) (Chen et al., 2017). The results demonstrated elevated mtDNA lesion frequency, mtDNAcn, and nuclear DNA lesions in veterans with GWI compared to controls. While the activity of mitochondrial complex I and IV was similar between veterans with GWI and control subjects, the study found an association between higher mtDNA lesion frequency and reduced activity of complex I and IV in veterans with GWI (Chen et al., 2017). Thus, the occurrence of mitochondrial dysfunction in veterans with GWI could be gleaned from these studies performed in the calf muscle and peripheral blood mononuclear cells. Since mitochondrial pathology can induce multiple adverse down-stream effects, targeted therapies that enhance mitochondrial function are likely useful for improving the overall health of veterans with GWI.
4. Potential therapeutic approaches for improving brain function in GWI
While there is no specific treatment available for GWI, the current treatment recommendations for GWI include cognitive behavioral therapy (CBT) and exercise (Institute of Medicine, 2001). Although these therapies likely mediate beneficial effects, they are not targeted and are therefore have limited efficacy for resolving the underlying causes that maintain CMI. In the following section, we discuss studies that examined different therapeutic approaches for improving brain function in animal models of GWI or veterans with GWI.
4.1. Strategies that improved brain function in animal models of GWI
Because of persistent oxidative stress and inflammation in the brain and systemic inflammation in animal models of GWI, studies have examined the efficacy of several antioxidant and antiinflammatory compounds for improving brain function. Additionally, modulation of calcium and BDNF dysregulation by ketamine has been examined. The studies reporting significant beneficial effects are discussed in detail in the following sections. Figure 3 illustrates the most promising/translatable strategies identified in animal models for treating GWI.
Fig. 3.
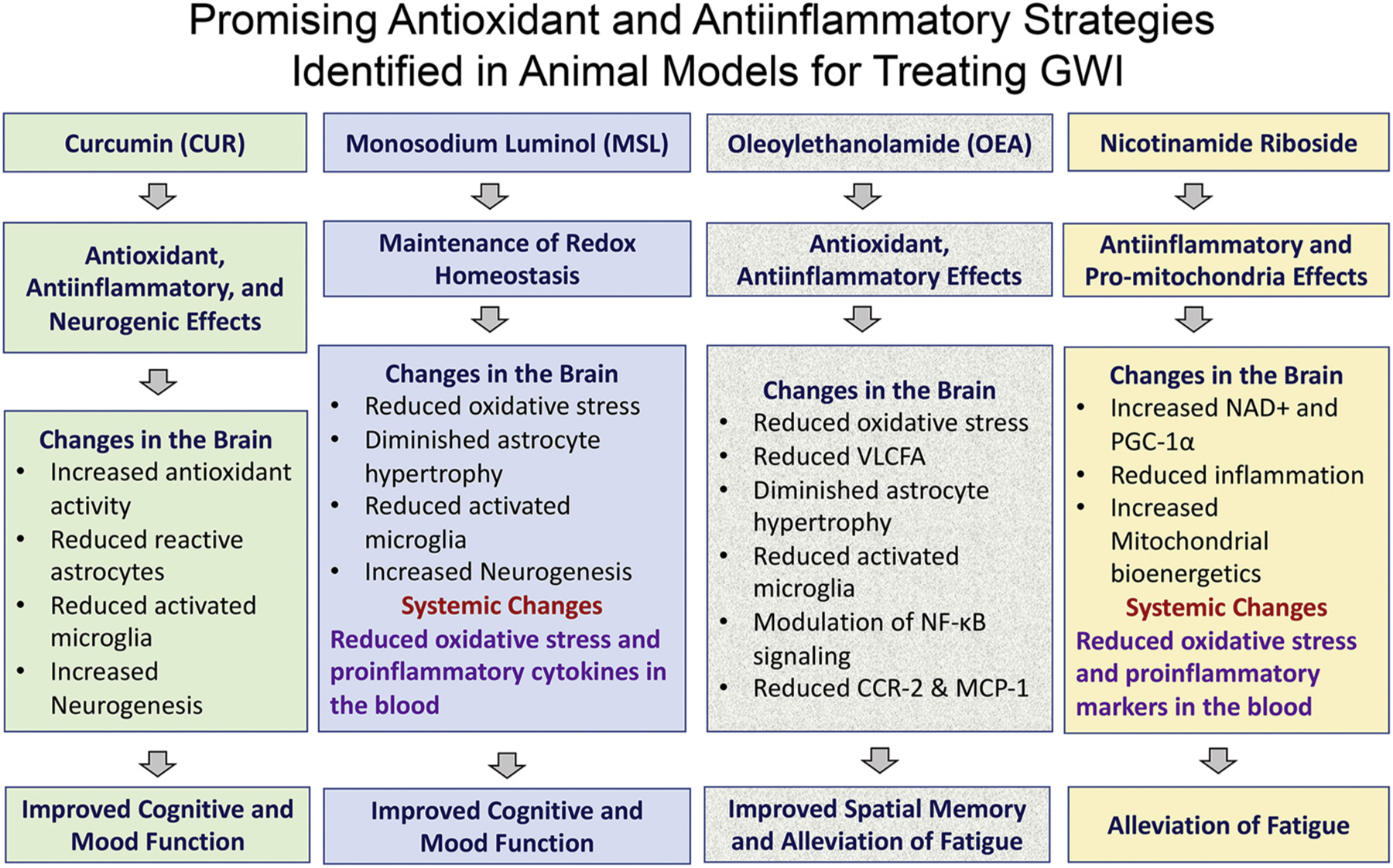
A schematic showing the major results of preclinical studies that examined the efficacy of compounds such as curcumin, monosodium luminol, oleoylethanolamide, and nicotinamide riboside in animal models of Gulf War Illness (GWI). CCR-2, C-C chemokine receptor type 2; MCP-1, monocyte chemoattractant protein-1; NAD+, nicotinamide adenine dinucleotide; NF-kB, nuclear factor kappa B; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; VLCFA, very-long-chain fatty acid.
4.1.1. Curcumin
Curcumin (CUR), a natural phenolic element of turmeric (Curcuma Longa), has antioxidant, antiinflammatory, neuroprotective, neurogenic, and cognitive and mood-enhancing properties (Kim et al., 2008; Samarghandian, Azimi-Nezhad, Farkhondeh, & Samini, 2017; Tiwari et al., 2014). Therefore, a study examined whether CUR administration would maintain better cognitive and mood function in a rat model of GWI (Kodali et al., 2018). In this study, animals exposed to GWI-related chemicals and mild stress (GWI animals, Parihar et al., 2013) received a daily dose of CUR (30 mg/Kg/day) for 30 days and tested for cognitive and mood function 2 months after CUR treatment. The animals were euthanized 3 months after CUR treatment, and changes in the hippocampus were examined through histological and molecular biological approaches. The GWI animals that received CUR displayed better cognitive ability to discern subtle changes in their environment in an object location test, a function dependent on the hippocampal trisynaptic pathway (Warburton, Barker, & Brown, 2013), and improved recognition memory in a novel object recognition test, which is a task that relies on the normal functioning of the perirhinal cortex and the hippocampus (Langston & Wood, 2010). Also, CUR-treated GWI animals displayed reduced anxiety-like behavior in a novelty suppressed feeding test, a task examining the ability to reconcile a struggle between a situation that generates increased anxiety and a drive to move towards an appetitive stimulus (Merali, Levac, & Anisman, 2003).
Improved cognitive and mood function in GWI animals receiving CUR was associated with increased neurogenesis in the hippocampus not only in the last week of treatment but also several months after treatment, consistent with the ability of CUR to increase neurogenesis through activation of canonical Wnt/β-catenin signaling pathway, extracellular signal-regulated kinases and p38 kinases, and multiple genes involved in stem cell proliferation (Kim et al., 2008; Tiwari et al., 2014). Furthermore, CUR treatment reduced astrocyte hypertrophy and activated microglia in the hippocampus, which is not surprising as CUR is well known to mediate antiinflammatory effects in neurodegenerative and neuroinflammatory diseases (Bassani et al., 2017; Ullah et al., 2017) through modulation of distinct transcription factors, inhibition of TLR4-MAPK/NF-κB pathway, and activation of ERK1/2 and p38 signaling pathway (Rahimifard et al., 2017; Shi et al., 2015; Ullah et al., 2017). Moreover, CUR treatment appeared to blunt the increased oxidative stress linked with hyperactive mitochondria in animals with GWI (Shetty et al., 2017). Such effects were evident from the increased expression of multiple genes encoding antioxidant proteins and normalized expression of genes encoding mitochondrial complex proteins, again consistent with the ability of CUR to attenuate oxidative stress in brain tissues via increased antioxidant defense activity and maintaining mitochondrial function (Samarghandian et al., 2017). Overall, the study implied that improved neurogenesis, reduced astrocyte hypertrophy, diminished numbers of activated microglia, increased expression of genes encoding proteins mediating antioxidant activity, and normalized expression of genes encoding proteins related to mitochondrial respiration underlie the beneficial effects of CUR treatment. Thus, CUR treatment has promise for improving cognitive and mood function in veterans with GWI. Clinical trials testing the efficacy of CUR to alleviate the symptoms of GWI in veterans are currently in progress.
4.1.2. Oleoylethanolamide
The drug oleoylethanolamide (OEA), an endogenous peroxisome proliferator-activated receptor alpha agonist, is a naturally occurring ethanolamide lipid that regulates feeding and body weight (Gaetani, Oveisi, & Piomelli, 2003; Lo Verme et al., 2005). OEA can cross the BBB after systemic administration (Gonzalez-Aparicio et al., 2014) and mediate antioxidant and antiinflammatory effects in the brain (Anton et al., 2017) through activation of the nuclear receptor PPARα, which induces changes in inflammation -related genes by repressing the nuclear factors NF-κB and activator protein −1 (Stahel, Smith, Bruchis, & Rabb, 2008). A study investigated whether OEA treatment would be efficacious for improving brain function in a mouse model of GWI (Joshi et al., 2018). The rationale underlying OEA treatment includes chronic inflammation and impaired lipid metabolism with altered peroxisome function in GWI mice (Abdullah et al., 2011, 2016). In this study, animals dosed with GWI chemicals PB and PER (Abdullah et al., 2011) received OEA (10 mg/Kg/day through rodent chow) at 5–11 months post-exposure. At different time-points during OEA treatment, the GWI animals were interrogated with a series of behavioral tests.
The GWI animals that received OEA for a month exhibited better spatial learning ability during the first two days in a Barnes maze test, and improved spatial memory retrieval ability in a probe test conducted a month after learning. Also, GWI mice receiving OEA displayed reduced immobility at 5 months post-OEA treatment in a forced swim test, implying alleviation of fatigue-like behavior. The OEA treated GWI mice also exhibited reduced disinhibition-like behavior in an elevated plus-maze test (Joshi et al., 2018). Furthermore, OEA treatment reduced the levels of very-long-chain fatty acid and MDA in GWI mice, suggesting antioxidant effects. Moreover, GWI mice receiving OEA displayed diminished hypertrophy of astrocytes and activated microglia in the dentate gyrus of the hippocampus, decreased ratio of phosphorylated p65 to total p65, and phosphorylated signal transducer and activator of transcription 3 in the brain, implying that the antiinflammatory effects of OEA were mediated through modulation of NFκB. The antiinflammatory effects were also evident from a reduced concentration of chemokine receptor 2 and MCP-1 in the brain (Joshi et al., 2018). Collectively, the study suggested that OEA treatment could be beneficial to veterans with GWI. A clinical trial testing the effects of OEA treatment in veterans with GWI is currently underway.
4.1.3. Monosodium Luminol
Monosodium luminol (MSL), a drug that acts as a buffer to facilitate the maintenance of redox homeostasis, was found to be effective in reducing oxidative stress and inflammation in several models of human diseases earlier (Jiang et al., 2006; Kim & Wong, 2012; Kuang et al., 2012; Reddy, Lungu, Kuang, Stoica, & Wong, 2010). Because the persistent cognitive and mood impairments in a rat model of GWI are associated with incessantly elevated oxidative stress and inflammation and waned neurogenesis in the hippocampus, a recent study examined the efficacy of 8 weeks of MSL treatment to investigate whether reinstatement of redox balance through MSL treatment would improve brain function (Shetty et al., 2020). Animals were first exposed to GWI-related chemicals PB, DEET, and PER and moderate stress (15 min of restraint stress). Four months later, animals were treated with different doses of MSL (40, 80, or 160 mg/Kg/day) for 8 weeks (5 days/week), and tested for cognitive and mood function using multiple behavioral tests in the last 3 weeks of the treatment period. The animals were euthanized 6 months after exposure to GWIR chemicals and stress, and the various changes in the brain were assessed through immunohistochemical, biochemical, and molecular biological approaches.
The findings revealed that MSL treatment (at 160 mg/Kg) was adept for relieving cognitive and mood impairment in a rat model of GWI. Improvements in cognitive function were evident from the enhanced capacity to recognize minimal alterations in the surroundings in an object location test and the proficiency for recollecting analogous but not the same experiences in a non-overlapping pattern in a pattern separation test. Furthermore, MSL treatment in GWI animals abolished anhedonia (inability to feel pleasure in activities that are pleasurable in healthy conditions, a measure of depression) in a sucrose preference test and diminished anxiety-like behavior in a novelty suppressed feeding test (Shetty et al., 2020).
Remarkably, the functional improvements were associated with the re-establishment of redox homeostasis, which was evident from the normalization of multiple genes encoding proteins involved in combating oxidative stress in the hippocampus, and oxidative stress markers MDA and protein carbonyls in the brain and the circulating blood to control levels. MSL treatment normalized the expression of many genes that encode proteins implicated in redox regulation (Prdx6, Srxn1, Txn1), mediating oxidative stress (Txnip), cellular detoxification (Gstp1), protection against ROS (Idh1, Prdx6, Sod2, Txnrd1, Txnrd2), the clearance of mitochondrial ROS (Sod2), and antioxidant effects (Gsr, Prdx1, 2, and 4). The other normalized genes include those involved in the transportation of mitochondrial protons (Ucp2), regulation of the NF-kB activation (Sqstm1), the formation of protein aggregates (Sqstm1, Prnp), proteolytic processing of amyloid precursor protein (Ctsb), cholesterol biosynthesis (Dhcr24), and prostaglandin biosynthesis (Ptgs2) (Shetty et al., 2020). The study also revealed that reinstatement of redox homeostasis could reverse multiple adverse changes seen in GWI because persistently raised ROS oxidize proteins and lipids, damage DNA, and activate astrocytes and microglia (Solleiro-Villavicencio & Rivas-Arancibia, 2018) and can interfere with neurogenesis (Huang, Leu, & Zou, 2015). The beneficial effects include reductions in hypertrophied astrocytes and activated microglia, enhanced NSC proliferation, and higher neurogenesis. Also, MSL treatment reduced the levels of multiple proinflammatory cytokines and chemokines (IL-1α, IL-1β, TNF-α, MCP-1, MIP-1α (Ccl3), TGF-β, VEGF, and FGF-β) in the circulating blood, implying its effects at the systemic level. Reduced systemic inflammation likely reflects the outcome of the reinstatement of redox homeostasis at the systemic level by MSL, which has beneficial implications because persistent systemic inflammation could also contribute to brain dysfunction (Chesnokova, Pechnick, & Wawrowsky, 2016; Di Filippo et al., 2013; Malaeb et al., 2014). Thus, 8 weeks of MSL treatment could improve cognitive and mood function, parallel with the reinstatement of redox homeostasis in the brain and the circulating blood. These findings suggest testing MSL to ease brain dysfunction in veterans with GWI using a double-blind, placebo-controlled, clinical trial.
4.1.4. Ketamine
A study tested the effects of administration of sub-anesthetic doses of ketamine (3, 5 or, 10 mg/Kg) in a DFP model of chronic GWI (Zhu et al., 2020). Ketamine, an FDA-approved drug for treatment-resistant depression (Kaufman, 2019), is effective in patients with major depression and suicidal ideation (DiazGranados et al., 2010). Before ketamine administration, rats with GWI displayed anhedonia in a sucrose preference test, anxiety-like behavior in an elevated plus-maze test, and depressive-like behavior in a forced swim test (FST). GWI rats receiving ketamine treatment did not exhibit changes in baseline locomotor activity in an open field test but showed reductions in immobility time in FST, in a dose-dependent manner. Also, ketamine treatment in GWI animals reversed anhedonia and reduced anxiety-like behavior at early time-points after treatment. KET’s stereoisomers, R-KET, and S-KET, also displayed similar effects in GWI rats, with R-KET inducing more potent effects (Zhu et al., 2020). A follow-up study by the same research group showed that ketamine treatment could diminish calcium elevations ([Ca2+]i) in the hippocampus of GWI rats at 1-hour post-treatment (Ribeiro et al., 2020), and significantly increase the concentration of BDNF at 24 hours post-treatment. Interestingly, the blockade of the BDNF receptor TrkB during ketamine administration blunted the antidepressant effect of ketamine 24 hours after treatment. The results suggested that rapid-onset antidepressant effects of ketamine are linked to inhibition of the NMDAR-Ca2+ whereas, antidepressant effects at 24 hours post-treatment are attributed to the upregulation of the BDNF signaling (Ribeiro et al., 2020). Thus, short-term ketamine treatment might be beneficial for treating severe depression, especially those not responding to traditional antidepressants. However, while many of the beneficial effects of ketamine treatment persisted 24 hours after treatment, the antidepressant effect did not last at 1-week post-treatment, implying that short-term ketamine treatment is insufficient to reverse major depression. Moreover, long-term ketamine treatment might not be feasible because of the possible adverse effects on brain function. While a low single dose (0.5 mg/Kg) of ketamine is not harmful for the cognitive function in patients with treatment-resistant depression (Chen et al., 2018), chronic ketamine users typically display cognitive impairments (Ke et al., 2018).
4.1.5. Nicotinamide riboside
The dietary supplement nicotinamide riboside (NR), a precursor of NAD+ involved in cellular bioenergetics, can cross the BBB following systemic administration (Hou et al., 2018; Lee & Yang, 2019; Spector & Johanson, 2007). Once NR enters the cells, it gets converted into NAD + by nicotinamide riboside kinase (Wang et al., 2018). Furthermore, NAD+ is an essential cofactor in metabolic processes such as glycolysis, fatty acid β-oxidation, and the Krebs cycle. Because the reduced form of NAD+ (i.e., NADH) also takes part in oxidative phosphorylation, increased NAD+ levels could contribute to enhanced levels of adenosine triphosphate as energy (Berger, Ramirez-Hernandez, & Ziegler, 2004; Ruggieri, Orsomando, Sorci, & Raffaelli, 2015). A study by Joshi and associates investigated whether NR treatment would result in improved brain function in a mouse model of GWI (Joshi et al., 2020). The rationale underlying such approach is that NR administration in animal models of neurodegeneration had reduced inflammation, glial activation and cognitive dysfunction through sirtuin-1 (Sirt1)-mediated deacetylation of the NF-κB pathway (Chen et al., 2018; Hou et al., 2018), and both NAD and Sirt1 levels are decreased in the blood of veterans with GWI (Joshi et al., 2020). In this experiment, animals dosed with PB+PER (Abdullah et al., 2011) received NR at 100 μg/kg daily for 2-months via mouse chow, commencing 6 months after exposure to PB +PER. During NR treatment, GWI mice were investigated for fatigue-like behavior using FST. The results showed that NR treatment diminished fatigue-like behavior with increased levels of NAD+ levels in the circulating blood and the brain, and enhanced Sirt1 and Sirt3 levels in the brain (Joshi et al., 2020). Also, NR treated GWI mice displayed deacetylation of NF-κB p65 subunit, increased concentration of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, and reduced astrocyte activity in the brain. Such changes were associated with decreased levels of proinflammatory cytokines (IL-1β, IFN-γ, IL-6) and markers of lipid peroxidation, and increased concentration of mitochondrial bioenergetics in the brain (Joshi et al., 2020). Proinflammatory cytokine levels were also reduced in the circulating blood of GWI mice. Overall, the study suggested that NR dietary supplementation is useful for restoring brain bioenergetics and reducing inflammation in GWI. However, it remains to be examined whether long-term NR supplementation would be adequate for improving cognitive and mood function in GWI.
4.1.6. Lacto-N-fucopentaose III
Lacto-N-ducopentaose III (LNFPIII) is a component of human breast milk with no reported adverse effects (Atochina, Da’dara, Walker, & Harn, 2008; Bhargava et al., 2012). In chronic inflammatory conditions, LNFPIII can mediate antiinflammatory effects. A previous study has shown that LNFPIII utilizes CD14/TLR-4 signaling to activate the ERK-dependent production of antiinflammatory mediators, which tilts the innate immune system’s balance towards an antiinflammatory direction (Tundup, Srivastava, Norberg, Watford, & Harn, 2015). A study conducted by Carpenter and colleagues examined the effects of LNFPIII in two different models of GWI (Carpenter et al., 2020). The exposures comprised PB and PER for 10 days (Model 1), or PER and DEET for 14 days with CORT exposure on days 8–14 and DFP treatment on day 15 (Model 2). LNFPIII treatment was provided during the exposure to chemicals. In the absence of any treatment, both models displayed dyshomeostasis of the serotonergic system in the nucleus accumbens and serotonergic and dopaminergic systems in the hippocampus. The hippocampus also exhibited elevated levels of proinflammatory cytokines. However, LNFPIII treatment prevented changes in monoamine levels as well as reduced the rise of proinflammatory cytokines. Overall, the study demonstrated the promise of LNFPIII to normalize monoamine levels and suppress neuroinflammation in GWI. However, it remains to be determined whether LNFPIII is effective if the treatment is initiated after the establishment of chronic GWI.
4.2. Results of treatment strategies employed in veterans with GWI
Many clinical trials have been completed or are currently underway in veterans with GWI. Table 1 lists the completed clinical trials on veterans with GWI with information such as the study title, objectives, the number of participants, the type of trial, and references to the trial results. Table 2 lists the ongoing clinical trials on veterans with GWI with details on the study title, objectives, the number of participants, and the recruitment status.
Table 1.
Completed clinical trials on Gulf War Illness (GWI) posted at Clinicaltrials.gov.
| Study title | Objective and participants | NCT number | Current status | Trial type/Phase | Results |
|---|---|---|---|---|---|
| Antibiotic Treatment of Gulf War Veterans’ Illnesses | To determine if antibiotic treatment directed against Mycoplasma species (i.e. doxycycline) will improve functioning and symptoms in deployed veterans with GWI (450 participants). | NCT00007735 | Completed | Randomized | Results Published (Donta et al., 2004) |
| Exercise and Behavioral Therapy Trial | To investigate the effects of cognitive behavioral therapy (CBT) and aerobic exercise, aerobic exercise alone, CBT alone or usual and customary care in veterans with GWI (1064 participants). | NCT00007748 | Completed | Phase III, Randomized | Results Published (Donta et al., 2003). |
| A Controlled Trial of Mifepristone in Gulf War Veterans With Chronic Multisymptom Illness | To examine the effects of glucocorticoid receptor antagonist mifepristone for improving physical and mental health and cognitive functioning in veterans with GWI (65 participants). | NCT00691067 | Completed | Phase IV,Randomized | Results Published (Golier et al., 2016). |
| Treatment Study of Carnosine Versus Placebo in Gulf War Illness | To study of the effects of carnosine treatment on cognitive, psychometric, autonomic, and muscle strength outcomes in veterans with GWI (33 participants). | NCT00810368 | Completed | Randomized | Results published (Baraniuk, El-Amin, Corey, Rayhan, & Timbol, 2013) |
| Q10 for Gulf War Veterans | To examine whether giving the supplement coenzyme Q10 improves symptoms and subjective health in GW veterans with chronic, multi-symptom health problems (58 participants). | NCT01011348 | Completed | Randomized | Results published (Golomb et al., 2014). |
| Exercise in Gulf War Illness | To determine if submaximal exercise by bicycle stress tests with pulmonary measurement of VO2MAX plus maximal isometric hand grips on 2 consecutive days causes a higher level of “exertional exhaustion” in veterans with GWI (50 participants). | NCT01291758 | Completed | Observational | Results published (Rayhan, Stevens, et al., 2013) |
| Acupuncture in the Treatment of Gulf War Illness | To test the effects of individualized acupuncture treatments for veterans with GWI (104 participants). | NCT01305811 | Completed | Phase I and II, Randomized | Results published (Conboy et al., 2016). |
| Clinical Benefits of a Novel Sleep-focused Mind-body Program on Gulf War Illness | To investigate clinical benefits of a novel sleep-focused mind-body program on GWI symptoms (75 participants). | NCT01543997 | Completed | Randomized | Results Published (Nakamura et al., 2017). |
| rTMS for the Treatment of Chronic Pain in GW1 Veterans (rTMS) | To evaluate the effect of repetitive transcranial magnetic stimulation (rTMS) in the resolution of chronic pain in veterans from the first GW with chronic pain problems (17 participants). | NCT01608321 | Completed | Phase III, Randomized | Results Posted (but yet to be Published). |
| Gulf War Illness: Evaluation of an Innovative Detoxification Program | To test the efficacy of the Hubbard detoxification program (exercise and sauna therapy together with administration of several dietary supplements), over a period of about 4 weeks in veterans with GWI (32 participants). | NCT01672710 | Completed | Randomized | Results published (Kerr et al., 2019). |
| Gulf War Illness Nasal Irrigation Study | To determine whether nasal irrigation with xylitol or saline are effective in the treatment of chronic rhinosinusitis and fatigue symptoms associated with GWI (40 participants). | NCT01700725 | Completed | Randomized | Results published (Hayer et al., 2015). |
| Scalp Application of LED Therapy to Improve Thinking and Memory in Veterans With Gulf War Illness | To learn if an experimental treatment (light-emitting diodes, LEDs) can help thinking ability, and memory in Veterans with GWI (96 participants). | NCT01782378 | Completed | Randomized | Results Posted (but yet to be published). |
| Research Examining Gulf War Illness in Our Nations Service Members (REGIONS) | To test the efficacy of Duloxetine and Pregabalin for treating veterans with GWI (112 participants). | NCT01846182 | Completed | Randomized | Results not Posted |
| Complementary Neurosteroid Intervention in Gulf War Illnesses | To investigate the effects of pregnenolone treatment for fatigue, musculoskeletal pain, cognitive symptoms in veterans deployed to the GW theatre of operations between 1990 and 1991 (170 participants). | NCT01956279 | Completed | Phase II, Randomized | Results Posted (but yet to be published). |
| Cognitive Rehabilitation for Gulf War Illness | Problem-solving therapy - a treatment approach that teaches patients strategies to address real-life problems (268 participants). | NCT02161133 | Completed | Randomized, double-blind | Results published (Sullivan et al., 2019). |
| Trial of Naltrexone and Dextromethorphan for Gulf War Illness | To examine the effects of Naltrexone and Dextromethorphan treatment on chronic fatigue, memory, and chronic pain, and autonomic dysfunction in GWI (60 participants). | NCT02206490 | Completed | Randomized | Results notPosted |
| Methylphenidate Plus GWI-Nutrient Formula as a Treatment for Patients With Gulf War Illness | To evaluate the safety and efficacy of a currently available medication, methylphenidate (Ritalin), combined with a mitochondrial support nutrient formula (K-PAX Synergy) to treat GWI. (30 participants). | NCT02357030 | Completed | Phase 2A - open label | Results published (Holodniy & Kaiser, 2019). |
| Treating Chronic Pain in Gulf War Illness | To determine whether yoga is effective for the treatment of chronic pain in GWI (75 participants). | NCT02378025 | Completed | Randomized | Results published (Mathersul et al., 2020). |
| Cognitive Behavioral Therapy for Insomnia for Gulf War Illness | To examine whether better sleep with cognitive behavioral therapy would help to alleviate non-sleep symptoms of GWI (165 participants). | NCT02782780 | Completed | Randomized | Results notPosted |
| Vagus Nerve Stimulation: Treatment for Gulf Veterans With Gulf War Illness | To assess the effect of vagus nerve stimulation (VNS) in alleviating migraine headache and pain in veterans with GWI (27 participants). | NCT02791893 | Completed | Randomized | Results notPosted |
| Development of a Polyphenol-rich Dietary Preparation for Treating Veterans With Gulf War Illness | To test the efficacy of flavonoid-rich concord grape juice for alleviating clinical complications in veterans with GWI (36 participants). | NCT02915237 | Completed | Phase 1/2A, Randomized, double-blind, placebo-controlled trial. | Results published (Helmer et al., 2020). |
| Alleviating Headache and Pain in GWI With Neuro-navigation Guided rTMS | To assess the effect of repetitive transcranial magnetic stimulation (rTMS) on GWI-related headaches and pain (90 participants). | NCT03030794 | Completed | Randomized | Results notPosted |
Table 2.
Ongoing clinical trials on Gulf War Illness (GWI) posted at Clinicaltrials.gov.
| Study title | Objective and participants | NCT number | Recruitment status | Trial type/Phase | Results |
|---|---|---|---|---|---|
| Intranasal Insulin: A Novel Treatment for Gulf War Multisymptom Illness | To investigate whether intranasal insulin is an effective, safe, and affordable therapy for veterans with GWI (114 participants). | NCT01802944 | Unknown | Phase II, Randomized | Not Available |
| CAM in Veterans With Gulf War Illnesses | To explore the effectiveness Gulf War Health Education (GWHE) and iRest Yoga Nidra (meditation)/auricular (ear) acupuncture for veterans with GWI (172 participants). | NCT02180243 | Active, not Recruiting | Phase I & II, Randomized | Not Available |
| Gulf War Illness Inflammation Reduction Trial | To determine if treatment with an anti-inflammatory drug (delayed-release prednisone) improves the health-related quality of life (HRQOL) of veterans with Gulf War Illness (100 participants). | NCT02506192 | Recruiting | Randomized | Not Available |
| Novel Interventions for GWVI | To examine the beneficial effects of two novel treatments for GW Veteran’s Illness (Tai Chi and Wellness intervention) and to establish the efficacy of these mind-body approaches to symptom reduction (120 participants). | NCT02661997 | Recruiting | Randomized | Not Available |
| Mitochondrial Cocktail for Gulf War Illness | To develop effect size and variance estimates, to guide successful conduct of a properly-powered clinical trial to assess the benefit of a mitochondrial cocktail in GWI (32 participants). | NCT02804828 | Not yet Recruiting | Randomized | Not Available |
| CoQ10 in Gulf War Illness | To determine if treatment with ubiquinol, a form of coenzyme Q10, improves the physical function of men and women Veterans suffering from GWI (200 participants) | NCT02865460 | Active, not Recruiting | Randomized | Not Available |
| Effect of Diet on Gulf War Illness | To compare a low fermentable oligo-, di and mono-saccharides and polyols FODMAP - modified healthy) diet to a high FODMAP (typical healthy) diet for effect on veterans with irritable bowel syndrome (IBS) and symptoms of GWI (68 participants). |
NCT02881944 | Unknown | Randomized | Not Available |
| Effects of Botanical Microglia Modulators in Gulf War Illness | To test the effects of nine different botanical compounds (supplements) that are known to suppress inflammation (64 participants). | NCT02909686 | Active, not Recruiting | Randomized | Not Available |
| D-cycloserine: A Novel Treatment for Gulf War Illness | To investigate the efficacy of d-cycloserine treatment for Gulf War Illness (56 participants). | NCT02983734 | Recruiting | Phase II Randomized | Not Available |
| Probiotic (Vibiome) for Gulf War Illness | To determine whether VSL #3 will improve intestinal symptoms of IBS and non-intestinal symptoms (fatigue, joint pain, insomnia, general stiffness and headache) associated with IBS (60 participants). | NCT03078530 | Unknown | Phase II & III | Not Available |
| Treatment of Memory Disorders in Gulf War Illness With High Definition Transcranial Direct Cortical Stimulation | To determine if delivery of High definition transcranial direct current stimulation (HD tDCS) over the presupplementary motor area (preSMA) will improve performance in GWI veterans with a verbal retrieval deficit (120 participants). |
NCT03542383 | Recruiting | Randomized | Not Available |
| Transcranial Direct Current Stimulation for Pain Treatment in Gulf War Illness | To investigate long-term modulation of pain pathways leading to a suppression of pain symptoms in GWI patients by applying transcranial direct current stimulation (120 participants). | NCT03547869 | Recruiting | Randomized | Not Available |
| Multimodal Investigation of the Neuroprotective Effects of Resveratrol | To examine the efficacy of resveratrol treatment for improving memory, thinking and mood in veterans with GWI (68 participants). | NCT03665740 | Recruiting | Randomized Double-blind, Placebo controlled | Not Available |
| Repetitive transcranial magnetic stimulation in Alleviating Pain and Co-Morbid Symptoms in Gulf War Veterans Illness | To investigate the effectiveness of using repetitive transcranial magnetic stimulation in relieving pain and other co-morbid symptoms of Gulf War Illness (80 participants). | NCT04046536 | Recruiting | Randomized | Not Available |
| Long Term Efficacy of Neuronavigation Guided tepetitive transcranial magnetic stimulation in Alleviating GWI Related Headaches and Pain Symptoms | To examine the long term efficacy of using repetitive transcranial magnetic stimulation in relieving GWI related headaches and pain (150 participants). | NCT04182659 | Recruiting | Randomized | Not Available |
| Tumor Necrosis Factor and Glucocorticoid Antagonist for GWI-Associated Multi-symptom Disease Homeostasis Reset | To assess the safety and mechanistic efficacy of a sequential etanercept-mifepristone intervention for GWI (20 participants). | NCT04254627 | Recruiting | Randomized | Not Available |
| Understanding GWI: Integrative Modeling | To assess the safety, efficacy, and biomarker response to the therapeutic interventions of Etanercept followed by mifepristone for veterans with GWI (20 participants). | NCT04255498 | Recruiting | Non-Randomized | Not Available |
4.2.1. Cognitive behavioral therapy
A study by Donta and colleagues compared the efficacy of cognitive-behavioral therapy (CBT), aerobic exercise, and the combination of CBT and exercise for alleviating the symptoms of GWI in veterans (Donta et al., 2003). The study enrolled 1092 veterans exhibiting at least two of the three symptoms (fatigue, pain, and cognitive dysfunction) for more than 6 months. The exercise sessions lasted 60 min, and CBT sessions lasted 60–90 min per week, and the sessions were continued for 12 weeks, and the primary endpoint was a 7-point or more significant improvement in the physical component summary scale. The secondary outcomes comprised measures of pain, fatigue, and cognitive and mood dysfunction, and the evaluations were performed at baseline and 3, 6, and 12 months (Donta et al., 2003). The findings suggested modest improvements in the physical component summary scale with CBT or combined CBT and exercise regimen. Exercise alone or exercise plus CBT reduced fatigue, distress, cognitive, and mood symptoms. CBT alone also improved cognitive and mood function. However, none of the treatments alleviated pain. Another recent pilot study in veterans with GWI examined the effects of either yoga or CBT for pain (Mathersul, Eising, DeSouza, Spiegel, & Bayley, 2020). The study reported alleviation of pain with both treatment approaches. The alterations in the biological autonomic nervous system moved in the direction of healthy profiles, especially with the yoga practice (Mathersul et al., 2020). Thus, CBT, exercise, and yoga regimens are useful for lessening several symptoms of GWI.
4.2.2. Doxycyline
One of the hypotheses underlying chronic symptoms such as pain, fatigue, and cognitive dysfunction in GWI includes the presence of mycoplasma species in GW veterans’ blood. Therefore, a randomized, double-blind, and placebo-controlled study investigated whether a 12-month course of doxycycline (a tetracycline antibiotic, 200 mg/day) would alleviate the extent of sickness in GWI (Donta et al., 2004). The study recruited 491 veterans with GWI having detectable levels of mycoplasma in the blood, and improvement in the physical component summary score was utilized as the primary outcome of treatment. The secondary outcomes comprised the alleviation of pain, fatigue, and cognitive function. The study revealed that neither the physical component summary score nor the secondary outcomes improved with doxycycline treatment. Also, veterans receiving doxycycline experienced a higher incidence of nausea and photosensitivity. Overall, the study demonstrated that doxycycline treatment is not effective for alleviating symptoms of GWI.
4.2.3. Coenzyme Q10
A randomized, double-blind, placebo-controlled study examined the effects of two different doses of coenzyme Q10 (CoQ10, 100, or 300 mg/day) treatment for ~3.5 months in 46 veterans meeting Kansas and Centers for Disease Control criteria for GWI (Golomb et al., 2014). The study reported improvements in general self-reported health (GSRH) in ~85% male participants with 100 mg/dayday dose (Q100 group), in comparison to the placebo group or the group receiving 300 mg/day dose (Q300 group). The Q100 group also exhibited better physical function than the placebo group. Improved GSRH in the Q100 group was associated with a rise in CoQ10. Furthermore, the 100 mg/day treatment appeared effective for treating 19 common symptoms of GWI but did not ease sleep disturbances associated with GWI (Golomb et al., 2014). Overall, the study revealed the promise of CoQ10 for alleviating multiple symptoms of GWI. Since the sample size was small in this study, a larger clinical trial is currently underway.
4.2.4. Mifipristone
A clinical trial conducted by Goliier and coworkers examined the effects of mifepristone in veterans with GWI (Golier et al., 2016). Mifepristone, a synthetic steroid that blocks glucocorticoid receptors, has anti-progestin activity and weak anti-androgen activity. Its relative binding affinity at the glucocorticoid receptor is more than ten times that of cortisol (Castinetti, Conte-Devolx, & Brue, 2010). In this randomized, double-blind trial, the participants (n = 32) received 200 mg mifepristone or matched placebo per day with two 6-week treatment phases separated by a month of washout period, and self-reported physical health was the primary measure. The secondary outcomes included neurocognitive function, self-reported depression, and fatigue. The findings demonstrated that mifepristone treatment did not improve physical health or various secondary measures (Golier et al., 2016). Interestingly, veterans receiving mifepristone displayed improvements in verbal learning, which was linked to the magnitude of cortisol change (Golier et al., 2016). Nonetheless, mifepristone treatment did not improve working memory, visual learning, or global composite cognitive measure in veterans with GWI. Thus, the cognitive-enhancing effect of mifepristone was minimal in veterans with GWI, as both primary and secondary outcomes of treatment were found to be negative.
4.2.5. Detoxification through Hubbard regimen
A randomized study examined the feasibility and efficacy of a detoxification procedure for alleviating the symptoms of GWI (Kerr et al., 2019). An intervention that enhanced detoxification called Hubbard regimen was employed in this study. Earlier studies have shown that the Hubbard regimen improved cognitive and long-term memory function in firefighters following polychlorinated biphenyl exposure (Kilburn, Warsaw, & Shields, 1989). Thirty-two eligible participants were randomly assigned to the intervention (n = 22) or 4-week waitlist control (n = 10). The daily protocol for 4–6 weeks comprised exercise, sauna-induced sweating, crystalline nicotinic acid, and other nutrient supplementation to enhance chemical elimination (Hubbard, 1990). The efficacy of treatment was measured through the overall physical component summary scale, quality of life, McGill pain, fatigue questionnaires, and neuropsychological assessments. The results showed that veterans receiving the detox intervention had 6.9 points higher physical component summary scores than veterans in the waitlist control (Kerr et al., 2019). Also, measures of quality of life, pain, and fatigue were improved with no serious adverse events in the intervention group. Follow-up studies demonstrated that most improvements were retained after 3 months. Thus, the Hubbard regimen is a feasible and safe intervention protocol for alleviating the symptoms of GWI. Furthermore, the intervention has the potential to provide benefits with minimal disruption of the lives of individuals.
4.2.6. Methylphenidate hydrochloride with micronutrient formula
A study by Holodniy and Ka Kaiser recently investigated the safety, tolerability, and efficacy of KPAX002 in veterans with GWI (Holodniy & Kaiser, 2019). KPAX002, comprising a low dose methylphenidate hydrochloride (Ritalin®, 2.5–10 mg, twice daily) and a micronutrient formula comprising essential antioxidants, has been shown to support mitochondrial function and induce clinical benefits in patients with mitochondrial dysfunction (Hart et al., 2004; Kaiser et al., 2006). The rationale for testing KPAX002 in veterans with GWI include the evidence of persistently increased oxidative stress and mitochondrial dysfunction in GWI (Golomb et al., 2014; Shetty et al., 2017; Shetty et al., 2020) and that mitochondrial dysfunction is one of the underlying causes of multi-factorial disorders (Niyazov, Kahler, & Frye, 2016). Methylphenidate hydrochloride stimulates CNS neuronal activity by increasing dopamine and norepinephrine levels through inhibition of their reuptake. This study tested the hypothesis that broad-spectrum mitochondrial support treatment would increase the ability of methylphenidate to provide a sustained alleviation of fatigue and cognitive dysfunction in GWI. This open-label trial comprised veterans with GWI meeting the Kansas case definition with 10 GWI patients completing the trial per protocol. The overall symptoms severity, measured through the GWI symptoms assessment tool, decreased by 25% in veterans taking KPAX002 for 12 weeks. The study also reported decreased fatigue and pain and improved cognitive function and sleep.
An indirect measure of mitochondrial function assessed through oxidative stress analysis panel revealed a decreased level of lipid peroxides in the circulating blood, implying reduced oxidative stress in GWI patients receiving the treatment. However, glutathione, glutathione peroxidase, SOD levels were unchanged in the circulating blood of veterans receiving the treatment, which suggested that clinical benefits of KPAX002 treatment in veterans did not involve significant elevation of multiple antioxidants. Overall, the results of this study are promising, but follow-up studies are necessary to determine if the observed benefits could be replicated in a larger randomized, double-blinded, placebo-controlled clinical trial. Furthermore, the mechanisms underlying clinical benefits following KPAX002 treatment need to be investigated through methods that directly assess the function of mitochondria. Also, since all subjects enrolled in the trial were unable to reach the maximum daily methylphenidate dose per protocol because of reported side effects such as headache, hypertension, and dizziness, it will be necessary to determine whether lower doses of methylphenidate would also be efficacious with the micronutrient formula.
4.2.7. Other clinical trials in veterans with GWI
A randomized, double-blind, Phase I/IIa clinical trial examined the effects of Concord grape juice (CGJ, 16 ounces/day for 24 weeks) containing anti-inflammatory flavonoids in 36 veterans with GWI (Helmer et al., 2020). Neurocognitive tests were performed at baseline and after 12 and 24 weeks of CGJ consumption. The study did not find statistically significant differences in cognitive function between CGJ and placebo groups at any of the time points measured in the study (Helmer et al., 2020). The clinical trials that showed promise for treating GWI are highlighted in Figure 4.
Fig. 4.
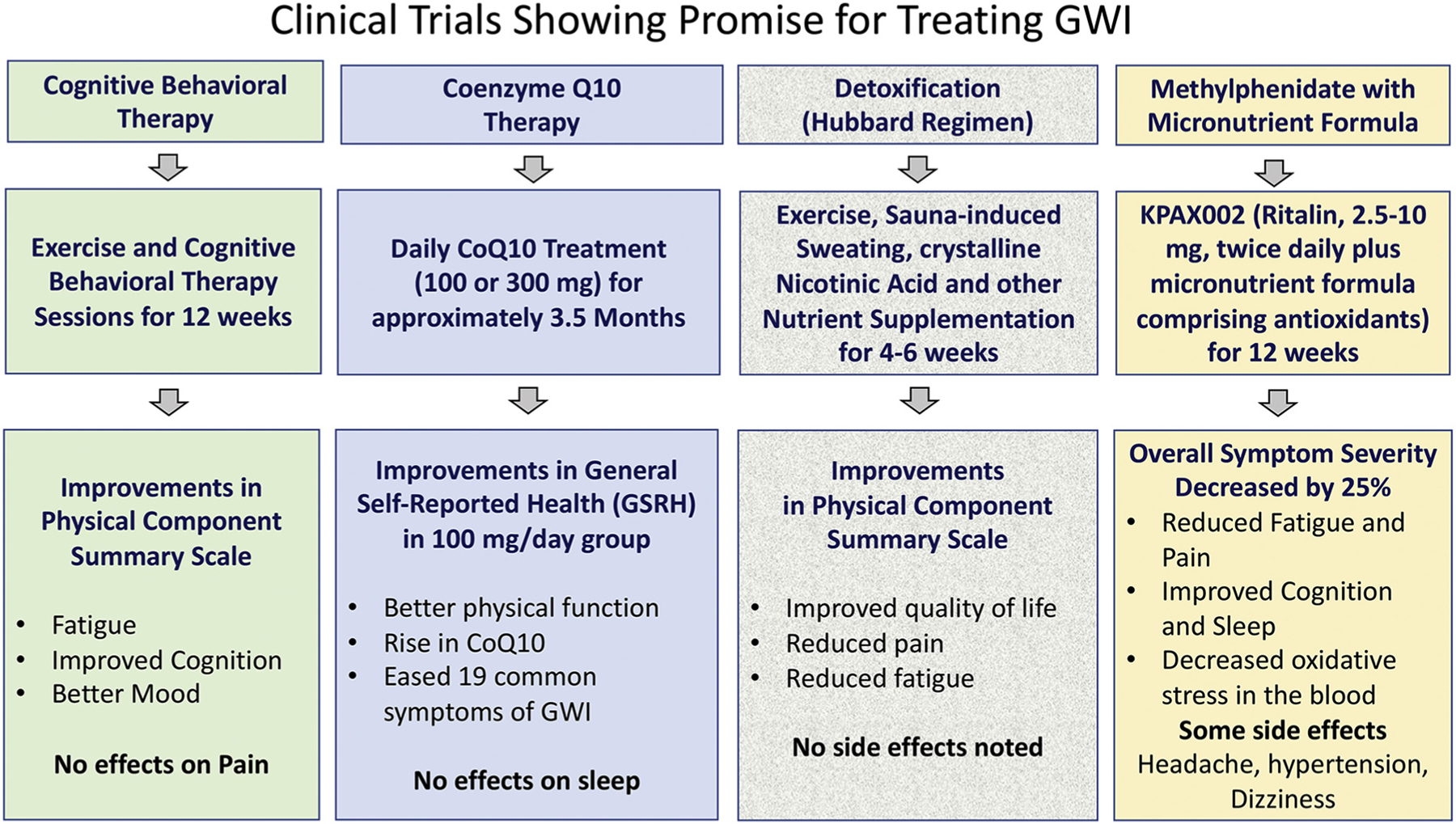
A flowchart depicting some of the clinical trials that showed promise for alleviating the symptoms of Gulf War Illness (GWI). These include cognitive behavioral therapy with exercise, Coenzyme Q10 treatment, Hubbard regimen of detoxification, and combination of methylphenidate and micronutrient formula for improving mitochondrial function. The significant effects are highlighted.
5. Summary and future perspectives
While over 500 million dollars have been expended on GWI research (Chester, Rowneki, Van Doren, & Helmer, 2019), a curative therapy or an approach that significantly improves the quality of life of veterans with GWI is yet to be discovered. However, there have been advances in understanding the pathophysiology of GWI. Numerous studies in animal models have provided significant insights into the pathophysiology underlying cognitive and mood dysfunction in GWI. These comprise incessantly elevated oxidative stress, altered lipid metabolism and peroxisomes, mitochondrial dysfunction, chronic inflammation, inhibitory interneuron loss, altered expression of genes linked to cognitive function, and waned hippocampal neurogenesis in the brain. Several studies have also noted the potential contribution of systemic changes to cognitive and mood impairments in GWI, including increased levels ROS and multiple proinflammatory cytokines in the circulating blood, altered gut microbiome, and activation of the adaptive immune response. However, one of the limitations in most animal studies is the focus on only male subjects to model the GWI. Since GWI is also seen in female service personnel who served in the first GW, future studies will need to utilize both genders to model the illness and develop treatment strategies. Comprehending the sex differences in GWI pathophysiology and treatment efficacy in animal models would improve the health evaluation and treatment of women veterans afflicted with GWI. Such studies’ importance is apparent from a recent report by Sullivan and colleagues showing that women GW veterans continued to report a wide variety of symptoms at a significantly higher excess frequency prevalence than GW-era women (Sullivan et al., 2020).
Studies on veterans with GWI implied that cognitive and mood impairments are linked to multiple changes in the brain. These include chronic activation of the executive control network, impaired functional connectivity between different sensory perception and motor networks, and between different networks in the sensorimotor domain, altered cerebral blood flow, the lack of protective alleles from Class II (HLA) genes, persistent neuroinflammation, and changes in miRNA levels. In addition, altered levels of PL species and proinflammatory cytokines and CRP in the circulating blood have been suggested as factors contributing to long term cognitive and mood impairments in GWI. To further comprehend the pathophysiology of GWI, new research should focus on the collection and maintenance of comprehensive baseline and longitudinal data from blood samples of patients exhibiting varying degrees of GWI symptoms and autopsied brain samples from deceased veterans with GWI for neuropathological analyses.
Clinical trials in GWI patients have tested the effectiveness of several drug and behavioral approaches. Some of the drugs such as doxycycline or Mifipristone have resulted in no functional improvements, whereas other drugs or combination therapies have revealed promise for alleviating multiple symptoms in GWI patients. These include aerobic exercise and CBT, alone or in combination, CoQ10, methylphenidate hydrochloride with a micronutrient formula, and detoxification procedure through Hubbard regimen (Donta et al., 2003; Golomb et al., 2014; Holodniy & Kaiser 2019; Kerr et al., 2019). Nonetheless, larger double-blind, placebo-controlled clinical trials are needed to validate some of the findings observed in these smaller clinical trials. Considering both neuro- and systemic inflammation in chronic GWI, recent therapeutic studies in animal models, have tested the efficacy of systemic administration of several antioxidant and/or antiinflammatory compounds. The results of these studies have suggested several compounds as promising candidates for alleviating cognitive and mood dysfunction in veterans with GWI, including CUR, OEA and MSL (Joshi et al., 2018; Kodali et al., 2018; Shetty et al., 2020). The efficacy of some of these compounds is currently being tested in veterans with GWI in clinical trials. Furthermore, the occurrence of persistent systemic inflammation in GWI brings in more significant question as to whether modulation of systemic inflammation would be efficacious for improving brain function in GWI patients, in addition to relieving other symptoms such as sickness-like behavior and chronic pain. Another issue is the diagnosis of GWI in veteran populations, which remains a challenge for providers. Diagnostic methods that target the inflammatory pathophysiology of GWI would allow for improved identification of the cause of illness and appropriate treatment. Discovery of reliable blood biomarkers and better screening and recruiting of veterans with persistent GWI-like symptoms are needed. For example, a methodology that can discern the extent of pathological changes occurring in the brain by analyzing brain-derived extracellular vesicles in the blood (Madhu et al., 2019) needs to be developed for veterans with GWI.
Acknowledgments
The authors are supported by the Department of Defense grants (W81XWH-14-1-0572, W81XWH-16-1-0480, W81XWH-17-1-0447 to A.K.S.), and an R01 grant from the National Institutes of Health - National Institute of Neurological Disorders and Stroke (NIH-NINDS R01NS106907-01 to A.K.S.). Brandon Dickey is supported by the Texas A&M College of Medicine.
Abbreviations:
- AA
Arachidonic acid
- ACh
Acetylcholine
- AChE
Acetylcholinesterase
- ADEVs
Astrocyte-derived extracellular vesicles
- BBB
Blood-brain barrier
- BDL
Bile duct ligation
- BDNF
brain-derived neurotrophic factor
- BOLD
Blood oxygenation level-dependent
- [Ca2+]i
Calcium intracellular
- CaMKII
calcium-calmodulin kinase II
- CBF
cerebral blood flow
- CBT
Cognitive behavioral therapy
- CFS
Chronic fatigue syndrome
- CGJ
Concord grape juice
- CMI
Chronic multisymptomatic illness
- CoQ10
Co-enzyme Q10
- CORT
Cortisol/Corticosterone
- CPF
Chlorpyrifos
- CPG
C-phosphate-G
- CRP
C-reactive protein
- CUR
Curcumin
- DEET
N, N-Diethyl-meta-toluamide
- DFP
Diisopropyl fluorophosphate
- DHA
Docosahexaenoic acid
- DMP
Differentially methylated promoters
- ePC
Ether phosphatidylcholine
- ePE
Ether phosphatidylethanolamine
- eLPE
Ether lysophosphatidylethanolamine
- ETC
Electron transport chain
- FABP3
fatty acid-binding protein 3
- FGF-β
Fibroblast growth factor-beta
- fMRI
Functional magnetic resonance imaging
- FST
Forced swim test
- GFAP
Glial Fibrillary Acidic Protein
- GSRH
General self-rated health
- GW
Gulf war
- GWI
Gulf war Illness
- GWIR
Gulf War Illness-related
- HLA
human leukocyte antigen
- HMGB1
High mobility group box-1
- IL
Interleukin
- IP10
interferon gamma-induced protein 10
- KPAX002
a combination of methylphenidate hydrochloride plus a micronutrient formula designed to support mitochondrial function
- LIF
Leukemia inhibitor factor
- LPC
Lysophosphatidylcholine
- LPE
Lysophosphatidylethanolamine
- lyso-PAF
Lyso-platelet activating factors
- MBP
myelin basic protein
- MCP-1
Monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MEG
magnetoencephalography
- MIP-1α
macrophage inflammatory protein 1 alpha
- MSL
Monosodium luminol
- mtDNA
mitochondrial DNA
- MUFA
Monounsaturated fatty acids
- NDEVs
Neuron-derived extracellular vesicles
- NLRP3
NLR Family Pyrin Domain Containing 3
- NPY
Neuropeptide Y
- NR
nicotinamide riboside
- NRF2
Nuclear factor erythroid 2-related factor 2
- NSC
Neural stem cells
- OEA
Oleoylethanolamide
- OP
Organophosphate
- PB
Pyridostigmine bromide
- PC
Phosphatidylcholine
- PCr-R
Phosphocreatine-recovery time constant
- PE
Phosphatidylethanolamine
- PER
Permethrin
- PET
Positron emission tomography
- pgf2α
prostaglandin F2 alpha
- PI
Phosphatidylinositol
- PL
Phospholipid
- PUFA
Polyunsaturated fatty acids
- PV
Parvalbumin
- ROS
Reactive oxygen species
- SCF
stem cell factor
- SFA
Saturated fatty acids
- Sirt
sirtuin
- SM
sphingomyelin
- SNI
synchronous neural interactions
- SOD
Superoxide dismutase
- START
Stress test activated reversible tachycardia
- STOPP
Stress test originated phantom perception
- TLR4
Toll-like Receptor 4
- TNFα
Tumor necrosis factor-alpha
- TSPO
translocator protein
- VEGF
Vascular endothelial growth factor
- 3-PBA
3-phenoxybenzoic acid
- 4-HNE
4-hydroxynonenal
Footnotes
Declaration of competing interest
The authors declared no conflicts of interest.
Publisher's Disclaimer: Department of Defense, and United States Government Disclaimer
Publisher's Disclaimer: The contents of this article suggest the views of authors and do not represent the official policy or position of the Department of Defense or the United States Government.
References
- Abdel-Rahman A, Abou-Donia S, El-Masry E, Shetty A, & Abou-Donia M (2004). Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. Journal of Toxicology and Environmental Health. Part A 67, 163–192. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A, Shetty AK, & Abou-Donia MB (2001). Subchronic dermal application of N,N-diethyl m-toluamide (DEET) and permethrin to adult rats, alone or in combination, causes diffuse neuronal cell death and cytoskeletal abnormalities in the cerebral cortex and the hippocampus, and Purkinje neuron loss in the cerebellum. Experimental Neurology 172, 153–171. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman A, Shetty AK, & Abou-Donia MB (2002). Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiology of Disease 10, 306–326. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, et al. (2011). Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromolecular Medicine 13, 275–288. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Bishop A, Reed JM, Crynen G, Phillips J, et al. (2012). Lipidomic profiling of phosphocholine-containing brain lipids in mice with sensorimotor deficits and anxiety-like features after exposure to Gulf War agents. Neuromolecular Medicine 14, 349–361. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Joshi U, Crynen G, Reed J, Mouzon B, et al. (2016). Translational potential of long-term decreases in mitochondrial lipids in a mouse model of Gulf War Illness. Toxicology 372, 22–33. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Evans JE, Montague H, Reed JM, Moser A, Crynen G, et al. (2013). Chronic elevation of phosphocholine containing lipids in mice exposed to Gulf War agents pyridostigmine bromide and permethrin. Neurotoxicology and Teratology 40, 74–84. [DOI] [PubMed] [Google Scholar]
- Abou-Donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, et al. (2017). Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicology and Teratology 61, 36–46. [DOI] [PubMed] [Google Scholar]
- Abraham E, Arcaroli J, Carmody A, Wang H, & Tracey KJ (2000). HMG-1 as a mediator of acute lung inflammation. Journal of Immunology 165, 2950–2954. [DOI] [PubMed] [Google Scholar]
- Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, et al. (2017). Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One 12, e0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelh Z, Albrecht DS, Bergan C, Akeju O, Clauw DJ, Conboy L, et al. (2020). Invivo imaging of neuroinflammation in veterans with Gulf War illness. Brain, Behavior, and Immunity 87, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrioli A, Alonso-Nanclares L, Arellano JI, & DeFelipe J (2007). Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience 149, 131–143. [DOI] [PubMed] [Google Scholar]
- Angoa-Perez M, Zagorac B, Francescutti DM, Winters AD, Greenberg JM, Ahmad MM, et al. (2020). Effects of a high fat diet on gut microbiome dysbiosis in a mouse model of Gulf War Illness. Scientific Reports 10, 9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton M, Alen F, Gomez de Heras R, Serrano A, Pavon FJ, Leza JC, et al. (2017). Oleoylethanolamide prevents neuroimmune HMGB1/TLR4/NF-kB danger signaling in rat frontal cortex and depressive-like behavior induced by ethanol binge administration. Addiction Biology 22, 724–741. [DOI] [PubMed] [Google Scholar]
- Arranz AM, & De Strooper B (2019). The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurology 18, 406–414. [DOI] [PubMed] [Google Scholar]
- Atochina O, Da’dara AA, Walker M, & Harn DA (2008). The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology 125, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk JN, El-Amin S, Corey R, Rayhan R, & Timbol C (2013). Carnosine treatment for gulf war illness: A randomized controlled trial. Global Journal of Health Science 5, 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk JN, & Shivapurkar N (2017). Exercise – Induced changes in cerebrospinal fluid miRNAs in Gulf War Illness, Chronic Fatigue Syndrome and sedentary control subjects. Scientific Reports 7, 15338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier L, Diserbo M, Lamproglou I, Amourette C, Peinnequin A, & Fauquette W (2009). Repeated stress in combination with pyridostigmine Part II: Changes in cerebral gene expression. Behavioural Brain Research 197, 292–300. [DOI] [PubMed] [Google Scholar]
- Barker GR, & Warburton EC (2011). When is the hippocampus involved in recognition memory? The Journal of Neuroscience 31, 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani TB, Turnes JM, Moura ELR, Bonato JM, Coppola-Segovia V, Zanata SM, et al. (2017). Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behavioural Brain Research 335, 41–54. [DOI] [PubMed] [Google Scholar]
- Beilharz JE, Kaakoush NO, Maniam J, & Morris MJ (2016). The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain, Behavior, and Immunity 57, 304–313. [DOI] [PubMed] [Google Scholar]
- Belgrad J, Dutta DJ, Bromley-Coolidge S, Kelly KA, Michalovicz LT, Sullivan KA, et al. (2019). Oligodendrocyte involvement in Gulf War Illness. Glia 67, 2107–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Ramirez-Hernandez MH, & Ziegler M (2004). The new life of a centenarian: Signalling functions of NAD(P). Trends in Biochemical Sciences 29, 111–118. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, & Naumann E (2009). Resting cerebral blood flow, attention, and aging. Brain Research 1267, 77–88. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Li C, Stanya KJ, Jacobi D, Dai L, Liu S, et al. (2012). Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nature Medicine 18, 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, Ivanov I, Carpenter DM, Wong EW, Golier JA, Tang CY, et al. (2015). White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: A pilot study. Psychoneuroendocrinology 51, 567–576. [DOI] [PubMed] [Google Scholar]
- Binns JH, Barlow C, Bloom FE, Clauw DJ, Golomb BA, Graves JC, et al. , & (Research Advisory Committee on Gulf War Veterans’ Illnesses)(2008). Gulf War Illness and the health of Gulf War Veterans. Washington, DC: Department of Veterans Affairs. http://www1.va.gov/rac-gwvi/. [Google Scholar]
- Binnewijzend MA, Benedictus MR, Kuijer JP, van der Flier WM, Teunissen CE, Prins ND, et al. (2016). Cerebral perfusion in the predementia stages of Alzheimer’s disease. European Radiology 26, 506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund G, Pivina L, Dadar M, Semenova Y, Rahman MM, Chirumbolo S, et al. (2020). Depleted uranium and Gulf War Illness: Updates and comments on possible mechanisms behind the syndrome. Environmental Research 181, 108927. [DOI] [PubMed] [Google Scholar]
- Blum S, Csurhes P, Reddel S, Spies J, & McCombe P (2014). Killer immunoglobulin-like receptor and their HLA ligands in Guillain-Barre Syndrome. Journal of Neuroimmunology 267, 92–96. [DOI] [PubMed] [Google Scholar]
- Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. (2003). Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. The EMBO Journal 22, 5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick G, Kreitz A, Fuite J, Fletcher MA, Vernon SD, & Klimas N (2011). A pilot study of immune network remodeling under challenge in Gulf War Illness. Brain, Behavior, and Immunity 25, 302–313. [DOI] [PubMed] [Google Scholar]
- Buchholz BM, & Bauer AJ (2010). Membrane TLR signaling mechanisms in the gastrointestinal tract during sepsis. Neurogastroenterology and Motility 22, 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterick TA, Trembley JH, Hocum Stone LL, Muller CJ, Rudquist RR, & Bach RR (2019). Gulf War Illness-associated increases in blood levels of interleukin 6 and C-reactive protein: Biomarker evidence of inflammation. BMC Research Notes 12, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlessi AS, Borba LA, Zugno AI, Quevedo J, & Reus GZ (2019). Gut microbiotabrain axis in depression: The role of neuroinflammation. The European Journal of Neuroscience 00, 1–14. [DOI] [PubMed] [Google Scholar]
- Carpenter JM, Gordon HE, Ludwig HD, Wagner JJ, Harn DA, Norberg T, et al. (2020). Neurochemical and neuroinflammatory perturbations in two Gulf War Illness models: Modulation by the immunotherapeutic LNFPIII. Neurotoxicology 77, 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castinetti F, Conte-Devolx B, & Brue T (2010). Medical treatment of Cushing’s syndrome: Glucocorticoid receptor antagonists and mifepristone. Neuroendocrinology 92(Suppl. 1), 125–130. [DOI] [PubMed] [Google Scholar]
- Chao LL, Abadjian LR, Esparza IL, & Reeb R (2016). Insomnia severity, subjective sleep quality, and risk for obstructive sleep apnea in veterans with Gulf War illness. Military Medicine 181, 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Kriger S, Buckley S, Ng P, & Mueller SG (2014). Effects of low-level sarin and cyclosarin exposure on hippocampal subfields in Gulf War Veterans. Neurotoxicology 44, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Rothlind JC, Cardenas VA, Meyerhoff DJ, & Weiner MW (2010). Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology 31, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. (2018). Cognitive function of patients with treatment-resistant depression after a single low dose of ketamine infusion. Journal of Affective Disorders 241, 1–7. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, et al. (2018). Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-kappaB pathway following experimental traumatic brain injury. Journal of Neuroinflammation 15, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Meyer JN, Hill HZ, Lange G, Condon MR, Klein JC, et al. (2017). Role of mitochondrial DNA damage and dysfunction in veterans with Gulf War Illness. PLoS One 12, e0184832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Pechnick RN, & Wawrowsky K (2016). Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain, Behavior, and Immunity 58, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JE, Rowneki M, Van Doren W, & Helmer DA (2019). Progression of intervention-focused research for Gulf War illness. Military Medical Research 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasserini D, Parnetti L, Andreasson U, Zetterberg H, Giannandrea D, Calabresi P, et al. (2010). CSF levels of heart fatty acid binding protein are altered during early phases of Alzheimer’s disease. Journal of Alzheimer’s Disease 22, 1281–1288. [DOI] [PubMed] [Google Scholar]
- Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, & Georgopoulos AP (2017). Subcortical brain atrophy in Gulf War Illness. Experimental Brain Research 235, 2777–2786. [DOI] [PubMed] [Google Scholar]
- Conboy L, Gerke T, Hsu KY, St John M, Goldstein M, & Schnyer R (2016). The effectiveness of individualized acupuncture protocols in the treatment of Gulf War illness: A pragmatic randomized clinical trial. PLoS One 11, e0149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CM, Briggs RW, Farris EA, Bartlett J, Haley RW, & Odegard TN (2016). Memory and functional brain differences in a national sample of U.S. veterans with Gulf War Illness. Psychiatry Research: Neuroimaging 250, 33–41. [DOI] [PubMed] [Google Scholar]
- Corbel V, Stankiewicz M, Pennetier C, Fournier D, Stojan J, Girard E, et al. (2009). Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biology 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin J (2004). Epidemiology. VA advisers link Gulf War illnesses to neurotoxins. Science 306, 26–27. [DOI] [PubMed] [Google Scholar]
- Davis SM, Collier LA, Leonardo CC, Seifert HA, Ajmo CT Jr., & Pennypacker KR (2017). Leukemia inhibitory factor protects neurons from ischemic damage via upregulation of superoxide dismutase 3. Molecular Neurobiology 54, 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Morales R, Agis-Balboa RC, Esteller M, & Berdasco M (2017). Epigenetic mechanisms during ageing and neurogenesis as novel therapeutic avenues in human brain disorders. Clinical Epigenetics 9, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, & Gage FH (2010). New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature Reviews. Neuroscience 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annual Review of Psychology 66, 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampa C, et al. (2013). Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiology of Disease 52, 229–236. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, & Falzoni S (2017). The P2X7 Receptor in Infection and Inflammation. Immunity 47, 15–31. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. (2010). Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. The Journal of Clinical Psychiatry 71, 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta ST, Clauw DJ, Engel CC Jr., Guarino P, Peduzzi P, Williams DA, et al. (2003). Cognitive behavioral therapy and aerobic exercise for Gulf War veterans’ illnesses: A randomized controlled trial. JAMA 289, 1396–1404. [DOI] [PubMed] [Google Scholar]
- Donta ST, Engel CC Jr., Collins JF, Baseman JB, Dever LL, Taylor T, et al. (2004). Benefits and harms of doxycycline treatment for Gulf War veterans’ illnesses: A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine 141, 85–94. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, & Petrik D (2012). Depression and hippocampal neurogenesis: A road to remission? Science 338, 72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerich T, Zakirova Z, Klimas N, Sullivan K, Shetty AK, Evans JE, et al. (2017). Phospholipid profiling of plasma from GW veterans and rodent models to identify potential biomarkers of Gulf War Illness. PLoS One 12, e0176634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl B, Leuthold AC, Tan HR, Lewis SM, Winskowski AM, Dikel TN, et al. (2010). Post-traumatic stress disorder: A right temporal lobe syndrome? Journal of Neural Engineering 7, 066005. [DOI] [PubMed] [Google Scholar]
- Engdahl BE, James LM, Miller RD, Leuthold AC, Lewis SM, Carpenter AF, et al. (2016). A Magnetoencephalographic (MEG) study of Gulf War Illness (GWI). EBioMedicine 12, 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl BE, James LM, Miller RD, Leuthold AC, Lewis SM, Carpenter AF, et al. (2018). Brain function in Gulf War Illness (GWI) and associated mental health comorbidities. Journal of Neurology & Neuromedicine 3, 24–34. [PMC free article] [PubMed] [Google Scholar]
- Falvo MJ, Lindheimer JB, & Serrador JM (2018). Dynamic cerebral autoregulation is impaired in Veterans with Gulf War Illness: A case-control study. PLoS One 13, e0205393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, et al. (2019). Mitochondrial dysfunction leads to cortical under-connectivity and cognitive impairment. Neuron 102(1127–1142), e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finneran DJ, & Nash KR (2019). Neuroinflammation and fractalkine signaling in Alzheimer’s disease. Journal of Neuroinflammation 16, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M, Do D, & Jack C (2012). Modifiable factors that alter the size of the hippocampus with ageing. Nature Reviews. Neurology 8, 189–202. [DOI] [PubMed] [Google Scholar]
- Fredman G, & Serhan CN (2011). Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. The Biochemical Journal 437, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, et al. (1998). Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA 280, 981–988. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Oka S, Tsuchiya N, Shimada K, Hashimoto A, Tohma S, et al. (2017). The role of common protective alleles HLA-DRB1*13 among systemic autoimmune diseases. Genes and Immunity 18, 1–7. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Oveisi F, & Piomelli D (2003). Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology 28, 1311–1316. [DOI] [PubMed] [Google Scholar]
- Garcia-Faroldi G, Ronnberg E, Orro A, Calounova G, Guss B, Lundequist A, et al. (2013). ADAMTS: Novel proteases expressed by activated mast cells. Biological Chemistry 394, 291–305. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, James LM, Carpenter AF, Engdahl BE, Leuthold AC, & Lewis SM (2017). Gulf War illness (GWI) as a neuroimmune disease. Experimental Brain Research 235, 3217–3225. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, James LM, Mahan MY, Joseph J, Georgopoulos A, & Engdahl BE (2016). Reduced human leukocyte antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine 3, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Karageorgiou E, Leuthold AC, Lewis SM, Lynch JK, Alonso AA, et al. (2007). Synchronous neural interactions assessed by magnetoencephalography: A functional biomarker for brain disorders. Journal of Neural Engineering 4, 349–355. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Tan HR, Lewis SM, Leuthold AC, Winskowski AM, Lynch JK, et al. (2010). The synchronous neural interactions test as a functional neuromarker for post-traumatic stress disorder (PTSD): A robust classification method based on the bootstrap. Journal of Neural Engineering 7, 16011. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, James LM, Mahan MY, Joseph J, Georgopoulos A, & Engdahl BE (2015). Human Leukocyte Antigen (HLA) Protection in Gulf War Illness (GWI). EBioMedicine 3, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschutz A, Heinsen H, Grunblatt E, Wagner AK, Bartl J, Meissner C, et al. (2014). Neuron-specific alterations in signal transduction pathways associated with Alzheimer’s disease. Journal of Alzheimer’s Disease 40, 135–142. [DOI] [PubMed] [Google Scholar]
- Golier JA, Caramanica K, Michaelides AC, Makotkine I, Schmeidler J, Harvey PD, et al. (2016). A randomized, double-blind, placebo-controlled, crossover trial of mifepristone in Gulf War veterans with chronic multisymptom illness. Psychoneuroendocrinology 64, 22–30. [DOI] [PubMed] [Google Scholar]
- Golomb BA, Allison M, Koperski S, Koslik HJ, Devaraj S, & Ritchie JB (2014). Coenzyme Q10 benefits symptoms in Gulf War veterans: Results of a randomized double-blind study. Neural Computation 26, 2594–2651. [DOI] [PubMed] [Google Scholar]
- Golomb BA, Koslik HJ, Christians U, Ritchie J, Wilson P, Elkins N, et al. (2019). Depressed prostaglandins and leukotrienes in veterans with Gulf War illness. Journal of Environmental Science and Health. Part. B 54, 623–639. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Blanco E, Serrano A, Pavon FJ, Parsons LH, Maldonado R, et al. (2014). The systemic administration of oleoylethanolamide exerts neuroprotection of the nigrostriatal system in experimental Parkinsonism. The International Journal of Neuropsychopharmacology 17, 455–468. [DOI] [PubMed] [Google Scholar]
- Gopinath KS, Sakoglu U, Crosson BA, & Haley RW (2019). Exploring brain mechanisms underlying Gulf War Illness with group ICA based analysis of fMRI resting state networks. Neuroscience Letters 701, 136–141. [DOI] [PubMed] [Google Scholar]
- Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, et al. (2009). Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Developmental Biology 325, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw JA, von Haefen C, Poyraz D, Mobius N, Sifringer M, & Spies CD (2015). Chronic alcohol consumption leads to a tissue specific expression of uncoupling protein-2. International Journal of Medical Sciences 12, 995–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, & O’Donnell P (2010). More is less: A disinhibited prefrontal cortex impairs cognitive flexibility. The Journal of Neuroscience 30, 17102–17110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulf War and Health (2017). Treatment for chronic multisymptom illness. Military Medicine 182, 1449–1450. [DOI] [PubMed] [Google Scholar]
- Haley RW, Fleckenstein JL, Marshall WW, McDonald GG, Kramer GL, & Petty F (2000). Effect of basal ganglia injury on central dopamine activity in Gulf War syndrome: Correlation of proton magnetic resonance spectroscopy and plasma homovanillic acid levels. Archives of Neurology 57, 1280–1285. [DOI] [PubMed] [Google Scholar]
- Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, & Fleckenstein JL (2000). Brain abnormalities in Gulf War syndrome: Evaluation with 1H MR spectroscopy. Radiology 215, 807–817. [DOI] [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, Schucany WR, Petty F, et al. (2009). Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Research 171, 207–220. [DOI] [PubMed] [Google Scholar]
- Haley RW, & Tuite JJ (2013). Epidemiologic evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology 40, 178–189. [DOI] [PubMed] [Google Scholar]
- Hart AM, Wilson AD, Montovani C, Smith C, Johnson M, Terenghi G, et al. (2004). Acetyl-l-carnitine: A pathogenesis based treatment for HIV-associated antiretroviral toxic neuropathy. AIDS 18, 1549–1560. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, & Shetty AK (2014). Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Frontiers in Behavioral Neuroscience 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer SD, Rabago DP, Amaza IP, Kille T, Coe CL, Zgierska A, et al. (2015). Effectiveness of nasal irrigation for chronic rhinosinusitis and fatigue in patients with Gulf War illness: Protocol for a randomized controlled trial. Contemporary Clinical Trials 41, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmer DA, Van Doren WW, Litke DR, Tseng CL, Ho L, Osinubi O, et al. (2020). Safety, tolerability and efficacy of dietary supplementation with concord grape juice in Gulf War veterans with Gulf War Illness: A phase I/IIA, randomized, double-blind, placebo-controlled trial. International Journal of Environmental Research and Public Health 17, 3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurology 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen K, Palmfeldt J, Christiansen S, Baiges I, Bak S, Jensen ON, et al. (2012). Candidate hippocampal biomarkers of susceptibility and resilience to stress in a rat model of depression. Molecular & Cellular Proteomics 11(M111), 016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Beck WD, Naughton SX, Poddar I, Adam BL, Yanasak N, et al. (2015). Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology 47, 17–26. [DOI] [PubMed] [Google Scholar]
- Hilborne LH, Golomb BA, Marshall GN, Davis LM, Sherbourne CD, Augerson W, et al. (2005). Examining possible causes of Gulf War Illness: RAND policy investigations and reviews of the scientific literature. Santa Monica, CA: RAND Corporation. https://www.rand.org/pubs/research_briefs/RB7544. [Google Scholar]
- Holodniy M, & Kaiser JD (2019). Treatment for Gulf War Illness (GWI) with KPAX002 (methylphenidate hydrochloride + GWI nutrient formula) in subjects meeting the Kansas case definition: A prospective, open-label trial. Journal of Psychiatric Research 118, 14–20. [DOI] [PubMed] [Google Scholar]
- Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. (2018). NAD (+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proceedings of the National Academy of Sciences of the United States of America 115, E1876–E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JQ, Ren FZ, Jiang YY, Xiao C, & Lei XG (2015). Selenoproteins protect against avian nutritional muscular dystrophy by metabolizing peroxides and regulating redox/apoptotic signaling. Free Radical Biology & Medicine 83, 129–138. [DOI] [PubMed] [Google Scholar]
- Huang TT, Leu D, & Zou Y (2015). Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Archives of Biochemistry and Biophysics 576, 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard LR (1990). Clear body clear mind. Los Angeles, CA, USA: Bridge Publications, Inc. [Google Scholar]
- Innamorato NG, Lastres-Becker I, & Cuadrado A (2009). Role of microglial redox balance in modulation of neuroinflammation. Current Opinion in Neurology 22, 308–314. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2001). Gulf War veterans: Treating symptoms and syndromes. Washington, DC: The National Academies Press. 10.17226/10185. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2013). Gulf War and health: Treatment for chronic multisymptom illness. Washington, DC: The National Academies Press. 10.17226/13539. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2014). Chronic multisymptom illness in Gulf War veterans: Case definitions reexamined. Washington, DC: The National Academies Press. 10.17226/18623. [DOI] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, & Langa KM (2010). Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 304, 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Christova P, Engdahl BE, Lewis SM, Carpenter AF, & Georgopoulos AP (2017). Human leukocyte antigen (HLA) and Gulf War Illness (GWI): HLA-DRB1*13: 02 spares subcortical Atrophy in Gulf War veterans. EBioMedicine 26, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Engdahl BE, Leuthold AC, & Georgopoulos AP (2016). Brain correlates of human leukocyte antigen (HLA) protection in Gulf War Illness (GWI). EBioMedicine 13, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LM, Engdahl BE, Leuthold AC, Krueger RF, & Georgopoulos AP (2015). Pathological personality traits modulate neural interactions. Experimental Brain Research 233, 3543–3552. [DOI] [PubMed] [Google Scholar]
- Janulewicz PA, Krengel MH, Maule A, White RF, Cirillo J, Sisson E, et al. (2017). Neuropsychological characteristics of Gulf War illness: A meta-analysis. PLoS One 12, e0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz PA, Seth RK, Carlson JM, Ajama J, Quinn E, Heeren T, et al. (2019). The gut-microbiome in Gulf War veterans: A preliminary report. International Journal of Environmental Research and Public Health 16, 3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey MG, Krengel M, Kibler JL, Zundel C, Klimas NG, Sullivan K, et al. (2019). Neuropsychological findings in Gulf War Illness: A review. Frontiers in Psychology 10, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Scofield VL, Yan M, Qiang W, Liu N, Reid AJ, et al. (2006). Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit). Journal of Virology 80, 4557–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Arribas-Blazquez M, Sanz-Rodriguez A, Concannon C, Olivos-Ore LA, Reschke CR, et al. (2015). microRNA targeting of the P2X7 purinoceptor opposes a contralateral epileptogenic focus in the hippocampus. Scientific Reports 5 17486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi U, Evans JE, Joseph R, Emmerich T, Saltiel N, Lungmus C, et al. (2018). Oleoylethanolamide treatment reduces neurobehavioral deficits and brain pathology in a mouse model of Gulf War Illness. Scientific Reports 8, 12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi U, Evans JE, Pearson A, Saltiel N, Cseresznye A, Darcey T, et al. (2020). Targeting sirtuin activity with nicotinamide riboside reduces neuroinflammation in a GWI mouse model. Neurotoxicology 79, 84–94. [DOI] [PubMed] [Google Scholar]
- Joshi U, Pearson A, Evans JE, Langlois H, Saltiel N, Ojo J, et al. (2019). A permethrin metabolite is associated with adaptive immune responses in Gulf War Illness. Brain, Behavior, and Immunity 81, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MR, & Holton KF (2020). Neurotoxicity in Gulf War Illness and the potential role of glutamate. Neurotoxicology 80, 60–70. [DOI] [PubMed] [Google Scholar]
- Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, & Baum MK (2006). Micronutrient supplementation increases CD4 count in HIV-infected individuals on highly active antiretroviral therapy: A prospective, double-blinded, placebo-controlled trial. Journal of Acquired Immune Deficiency Syndromes 42, 523–528. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, & Kaltschmidt C (2015). NF-KappaB in long-term memory and structural plasticity in the adult mammalian brain. Frontiers in Molecular Neuroscience 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MB (2019). Pharmaceutical approval update. Pharmacy and Therapeutics 44, 251–254. [PMC free article] [PubMed] [Google Scholar]
- Ke X, Ding Y, Xu K, He H, Wang D, Deng X, et al. (2018). The profile of cognitive impairments in chronic ketamine users. Psychiatry Research 266, 124–131. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, & Gage FH (2002). Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Annals of Neurology 52, 135–143. [DOI] [PubMed] [Google Scholar]
- Kerr K, Morse G, Graves D, Zuo F, Lipowicz A, & Carpenter DO (2019). A detoxification intervention for gulf war illness: A pilot randomized controlled trial. International Journal of Environmental Research and Public Health 16, 4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaiboullina SF, DeMeirleir KL, Rawat S, Berk GS, Gaynor-Berk RS, Mijatovic T, et al. (2015). Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis. Cytokine 72, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn KH, Warsaw RH, & Shields MG (1989). Neurobehavioral dysfunction in firemen exposed to polycholorinated biphenyls (PCBs): Possible improvement after detoxification. Archives of Environmental Health 44, 345–350. [DOI] [PubMed] [Google Scholar]
- Kim J, & Wong PK (2012). Targeting p38 mitogen-activated protein kinase signaling restores subventricular zone neural stem cells and corrects neuromotor deficits in Atm knockout mouse. Stem Cells Translational Medicine 1, 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. (2008). Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. The Journal of Biological Chemistry 283, 14497–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Son M, Lee SE, Park IH, Kwak MS, Han M, et al. (2018). High-mobility group box 1-induced complement activation causes sterile inflammation. Frontiers in Immunology 9, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimono D, Bose D, Seth RK, Mondal A, Saha P, Janulewicz P, et al. (2020). Host Akkermansia muciniphila abundance correlates with Gulf War Illness symptom persistence via NLRP3-mediated neuroinflammation and decreased brain-derived neurotrophic factor. Neuroscience Insights 15 2633105520942480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimono D, Sarkar S, Albadrani M, Seth R, Bose D, Mondal A, et al. (2019). Dysbiosis-associated enteric glial cell immune-activation and redox imbalance modulate tight junction protein expression in Gulf War Illness pathology. Frontiers in Physiology 10, 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, & Shetty AK (2018). Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain, Behavior, and Immunity 69, 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, & Monyer H (2010). NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 68, 557–569. [DOI] [PubMed] [Google Scholar]
- Koslik HJ, Hamilton G, & Golomb BA (2014). Mitochondrial dysfunction in Gulf War illness revealed by 31Phosphorus Magnetic Resonance Spectroscopy: A case-control study. PLoS One 9, e92887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, & Cavanagh J (2012). Depression: An inflammatory illness? Journal of Neurology, Neurosurgery, and Psychiatry 83, 495–502. [DOI] [PubMed] [Google Scholar]
- Kuang X, Yan M, Ajmo JM, Scofield VL, Stoica G, & Wong PK (2012). Activation of AMP-activated protein kinase in cerebella of Atm−/− mice is attributable to accumulation of reactive oxygen species. Biochemical and Biophysical Research Communications 418, 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgrave-Gomez J, Mercado-Gomez O, & Guevara-Guzman R (2015). Epigenetic mechanisms in neurological and neurodegenerative diseases. Frontiers in Cellular Neuroscience 9, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langheim FJ, Leuthold AC, & Georgopoulos AP (2006). Synchronous dynamic brain networks revealed by magnetoencephalography. Proceedings of the National Academy of Sciences of the United States of America 103, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, & Wood ER (2010). Associative recognition and the hippocampus: Differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus 20, 1139–1153. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Innamorato NG, Jaworski T, Rabano A, Kugler S, Van Leuven F, et al. (2014). Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain 137, 78–91. [DOI] [PubMed] [Google Scholar]
- Lee HJ, & Yang SJ (2019). Nicotinamide riboside regulates inflammation and mitochondrial markers in AML12 hepatocytes. Nutrition Research and Practice 13, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Han HW, & Yim SY (2015). Beneficial effects of soy milk and fiber on high cholesterol diet-induced alteration of gut microbiota and inflammatory gene expression in rats. Food & Function 6, 492–500. [DOI] [PubMed] [Google Scholar]
- Li X, Spence JS, Buhner DM, Hart J Jr., Cullum CM, Biggs MM, et al. (2011). Hippocampal dysfunction in Gulf War veterans: Investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology 261, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw MY, You DL, Cheng PT, Kao PF, & Wong AM (1998). Central representation of phantom limb phenomenon in amputees studied with single photon emission computerized tomography. American Journal of Physical Medicine & Rehabilitation 77, 368–375. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Tsai SJ, Kuo PH, Liu YL, Yang AC, Kao CF, et al. (2017). The ADAMTS9 gene is associated with cognitive aging in the elderly in a Taiwanese population. PLoS One 12, e0172440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Aslan S, Li X, Buhner DM, Spence JS, Briggs RW, et al. (2011). Perfusion deficit to cholinergic challenge in veterans with Gulf War Illness. Neurotoxicology 32, 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, & Piomelli D (2005). Regulation of food intake by oleoylethanolamide. Cellular and Molecular Life Sciences 62, 708–716. [DOI] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, & O’Callaghan JP (2017). Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. Journal of Neurochemistry 142, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MD, Smiraglia DJ, & Campbell MJ (2017). The genomic impact of DNA CpG methylation on gene expression; relationships in prostate cancer. Biomolecules 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht VA, Woodruff JL, Maissy ES, Grillo CA, Wilson MA, Fadel JR, et al. (2019). Pyridostigmine bromide and stress interact to impact immune function, cholinergic neurochemistry and behavior in a rat model of Gulf War Illness. Brain, Behavior, and Immunity 80, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, et al. (2019). Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain, Behavior, and Immunity 81, 430–443. [DOI] [PubMed] [Google Scholar]
- Malaeb SN, Davis JM, Pinz IM, Newman JL, Dammann O, & Rios M (2014). Effect of sustained postnatal systemic inflammation on hippocampal volume and function in mice. Pediatric Research 76, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Jung Y, Park BS, Partovi D, & Reddy PH (2005). Time-course of mitochondrial gene expressions in mice brains: Implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. Journal of Neurochemistry 92, 494–504. [DOI] [PubMed] [Google Scholar]
- Mao XW, Green LM, Mekonnen T, Lindsey N, & Gridley DS (2010). Gene expression analysis of oxidative stress and apoptosis in proton-irradiated rat retina. In Vivo 24, 425–430. [PubMed] [Google Scholar]
- Marshall RS (2012). Effects of altered cerebral hemodynamics on cognitive function. Journal of Alzheimer’s Disease 32, 633–642. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Lazar RM, Pile-Spellman J, Young WL, Duong DH, Joshi S, et al. (2001). Recovery of brain function during induced cerebral hypoperfusion. Brain 124, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Mathersul DC, Eising CM, DeSouza DD, Spiegel D, & Bayley PJ (2020). Brain and physiological markers of autonomic function are associated with treatment-related improvements in self-reported autonomic dysfunction in veterans with Gulf War Illness: An exploratory pilot study. Global Advances in Health and Medicine 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy KM, & Sahay A (2017). Targeting adult neurogenesis to optimize hippocampal circuits in aging. Neurotherapeutics 14, 630–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, et al. (2019). Diet and inflammation in cognitive ageing and Alzheimer’s disease. Current Nutrition Reports 8, 53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuade A, & Blurton-Jones M (2019). Microglia in Alzheimer’s disease: Exploring how genetics and phenotype influence risk. Journal of Molecular Biology 431, 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megahed T, Hattiangady B, Shuai B, & Shetty AK (2015). Parvalbumin and neuropeptide Y expressing hippocampal GABA-ergic inhibitory interneuron numbers decline in a model of Gulf War illness. Frontiers in Cellular Neuroscience 8, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, & Ali JA (2004). Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain Research 1009, 189–194. [DOI] [PubMed] [Google Scholar]
- Merali Z, Levac C, & Anisman H (2003). Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biological Psychiatry 54, 552–565. [DOI] [PubMed] [Google Scholar]
- Meuer SC, Hussey RE, Hodgdon JC, Hercend T, Schlossman SF, & Reinherz EL (1982). Surface structures involved in target recognition by human cytotoxic T lymphocytes. Science 218, 471–473. [DOI] [PubMed] [Google Scholar]
- Michalovicz LT, Kelly KA, Sullivan K, & O’Callaghan JP (2020). Acetylcholinesterase inhibitor exposures as an initiating factor in the development of Gulf War Illness, a chronic neuroimmune disorder in deployed veterans. Neuropharmacology 171, 108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovicz LT, Locker AR, Kelly KA, Miller JV, Barnes Z, Fletcher MA, et al. (2019). Corticosterone and pyridostigmine/DEET exposure attenuate peripheral cytokine expression: Supporting a dominant role for neuroinflammation in a mouse model of Gulf War Illness. Neurotoxicology 70, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JV, LeBouf RF, Kelly KA, Michalovicz LT, Ranpara A, Locker AR, et al. (2018). The neuroinflammatory phenotype in a mouse model of Gulf War Illness is unrelated to brain regional levels of acetylcholine as measured by quantitative HILIC-UPLC-MS/MS. Toxicological Sciences 165, 302–313. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Lipschitz DL, Donaldson GW, Kida Y, Williams SL, Landward R, et al. (2017). Investigating clinical benefits of a novel sleep-focused mind-body program on Gulf War Illness symptoms: A randomized controlled trial. Psychosomatic Medicine 79, 706–718. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (2018). Gulf War and health: Volume 11: Generational health effects of serving in the Gulf War. Washington (DC). [PubMed] [Google Scholar]
- Naughton SX, Hernandez CM, Beck WD, Poddar I, Yanasak N, Lin PC, et al. (2018). Repeated exposures to diisopropylfluorophosphate result in structural disruptions of myelinated axons and persistent impairments of axonal transport in the brains of rats. Toxicology 406–407, 92–103. [DOI] [PubMed] [Google Scholar]
- Navakkode S, Liu C, & Soong TW (2018). Altered function of neuronal L-type calcium channels in ageing and neuroinflammation: Implications in age-related synaptic dysfunction and cognitive decline. Ageing Research Reviews 42, 86–99. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Naviaux JC, Li K, Wang L, Monk JM, Bright AT, et al. (2019). Metabolic features of Gulf War illness. PLoS One 14, e0219531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MB, de Oliveira Junior AN, Goldim M, Mathias K, Fileti ME, da Rosa N, et al. (2018). Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain, Behavior, and Immunity 73, 661–669. [DOI] [PubMed] [Google Scholar]
- Niyazov DM, Kahler SG, & Frye RE (2016). Primary mitochondrial disease and secondary mitochondrial Dysfunction: Importance of distinction for diagnosis and treatment. Molecular Syndromology 7, 122–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, & Lasley SM (2015). Corticosterone primes the neuroinflammatory response to DFP in mice: Potential animal model of Gulf War Illness. Journal of Neurochemistry 133, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Cooper CM, Farris EA, Arduengo J, Bartlett J, & Haley R (2013). Memory impairment exhibited by veterans with Gulf War Illness. Neurocase 19, 316–327. [DOI] [PubMed] [Google Scholar]
- Ogura M, Takarada T, Nakamichi N, Kawagoe H, Sako A, Nakazato R, et al. (2011). Exacerbated vulnerability to oxidative stress in astrocytic C6 glioma cells with stable overexpression of the glutamine transporter slc38a1. Neurochemistry International 58, 504–511. [DOI] [PubMed] [Google Scholar]
- Ojo JO, Abdullah L, Evans J, Reed JM, Montague H, Mullan MJ, et al. (2014). Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34, 109–127. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, & Shetty AK (2011). Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Molecular Psychiatry 16, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, & Shetty AK (2013). Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 38, 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L, Middleton S, Baker K, & Younger J (2015). Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunology 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A, Leoni G, Cervellati F, Canali R, Signorini C, Leoncini S, et al. (2013). Genes related to mitochondrial functions, protein degradation, and chromatin folding are differentially expressed in lymphomonocytes of Rett syndrome patients. Mediators of Inflammation 2013, 137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu AD, Grant S, Frampton G, McMillin M, Kain J, Kodali M, et al. (2018). Gulf war illness-related chemicals increase CD11b/c(+) monocyte infiltration into the liver and aggravate hepatic cholestasis in a rodent model. Scientific Reports 8, 13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KF, & Deshpande LS (2016). Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology 52, 127–133. [DOI] [PubMed] [Google Scholar]
- Phillips KF, & Deshpande LS (2018). Chronic neurological morbidities and elevated hippocampal calcium levels in a DFP-based rat model of Gulf War Illness. Military Medicine 183, 552–555. [DOI] [PubMed] [Google Scholar]
- Phillips KF, Santos E, Blair RE, & Deshpande LS (2019). Targeting intracellular calcium stores alleviates neurological morbidities in a DFP-based rat model of Gulf War Illness. Toxicological Sciences 169, 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter B, Long K, Rull RP, Dursa EK, & Millennium Cohort Study T (2020). Prevalence of chronic multisymptom Illness/Gulf War Illness Over Time among millennium cohort participants, 2001 to 2016. Journal of Occupational and Environmental Medicine 62, 4–10. [DOI] [PubMed] [Google Scholar]
- Rahimifard M, Maqbool F, Moeini-Nodeh S, Niaz K, Abdollahi M, Braidy N, et al. (2017). Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Research Reviews 36, 11–19. [DOI] [PubMed] [Google Scholar]
- Ray DE, & Fry JR (2006). A reassessment of the neurotoxicity of pyrethroid insecticides. Pharmacology & Therapeutics 111, 174–193. [DOI] [PubMed] [Google Scholar]
- Rayhan RU, Raksit MP, Timbol CR, Adewuyi O, Vanmeter JW, & Baraniuk JN (2013). Prefrontal lactate predicts exercise-induced cognitive dysfunction in Gulf War Illness. American Journal of Translational Research 5, 212–223. [PMC free article] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, et al. (2013). Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One 8, e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PV, Lungu G, Kuang X, Stoica G, & Wong PK (2010). Neuroprotective effects of the drug GVT (monosodium luminol) are mediated by the stabilization of Nrf2 in astrocytes. Neurochemistry International 56, 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro ACR, Zhu J, Kronfol MM, Jahr FM, Younis RM, Hawkins E, et al. (2020). Molecular mechanisms for the antidepressant-like effects of a low-dose ketamine treatment in a DFP-based rat model for Gulf War Illness. Neurotoxicology 80, 52–59. [DOI] [PubMed] [Google Scholar]
- Ruggieri S, Orsomando G, Sorci L, & Raffaelli N (2015). Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochimica et Biophysica Acta 1854, 1138–1149. [DOI] [PubMed] [Google Scholar]
- Samarghandian S, Azimi-Nezhad M, Farkhondeh T, & Samini F (2017). Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomedicine & Pharmacotherapy 87, 223–229. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Tetko IV, Tandon P, Silveira DC, Vreugdenhil M, Henzi T, et al. (2004). Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Molecular and Cellular Neurosciences 25, 650–663. [DOI] [PubMed] [Google Scholar]
- Sengupta P (2013). The laboratory rat: Relating its age with human’s. International Journal of Preventive Medicine 4, 624–630. [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yacoubian S, & Yang R (2008). Anti-inflammatory and proresolving lipid mediators. Annual Review of Pathology 3, 279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RK, Kimono D, Alhasson F, Sarkar S, Albadrani M, Lasley SK, et al. (2018). Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War Illness. Toxicology and Applied Pharmacology 350, 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RK, Maqsood R, Mondal A, Bose D, Kimono D, Holland LA, et al. (2019). DNA virome diversity and its association with host bacteria regulate inflammatory phenotype and neuronal immunotoxicity in experimental Gulf War Illness. Viruses 11, 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Attaluri S, Kodali M, Shuai B, Shetty GA, Upadhya D, et al. (2020). Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro- and systemic inflammation in a model of Gulf War Illness. Redox Biology 28, 101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Upadhya D, Bates A, Attaluri S, Shuai B, et al. (2017). Chronic oxidative stress, mitochondrial dysfunction, Nrf2 activation and inflammation in the hippocampus accompany heightened systemic inflammation and oxidative stress in an animal model of Gulf War Illness. Frontiers in Molecular Neuroscience 10, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Zheng Z, Li J, Xiao Z, Qi W, Zhang A, et al. (2015). Curcumin inhibits Abeta-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neuroscience Letters 594, 105–110. [DOI] [PubMed] [Google Scholar]
- Sim SE, Lim CS, Kim JI, Seo D, Chun H, Yu NK, et al. (2016). The brain-enriched MicroRNA miR-9–3p regulates synaptic plasticity and memory. The Journal of Neuroscience 36, 8641–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Babic Leko M, Wray S, Harrington CR, Delalle I, Jovanov-Milosevic N, et al. (2017). Monoaminergic neuropathology in Alzheimer’s disease. Progress in Neurobiology 151, 101–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BN, Wang JM, Vogt D, Vickers K, King DW, & King LA (2013). Gulf war illness: Symptomatology among veterans 10 years after deployment. Journal of Occupational and Environmental Medicine 55, 104–110. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, & Cameron HA (2011). Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solleiro-Villavicencio H, & Rivas-Arancibia S (2018). Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4(+)T cells in neurodegenerative diseases. Frontiers in Cellular Neuroscience 12, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector R, & Johanson CE (2007). Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. Journal of Neurochemistry 103, 425–438. [DOI] [PubMed] [Google Scholar]
- Speed HE, Blaiss CA, Kim A, Haws ME, Melvin NR, Jennings M, et al. (2012). Delayed reduction of hippocampal synaptic transmission and spines following exposure to repeated subclinical doses of organophosphorus pesticide in adult mice. Toxicological Sciences 125, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Hamilton T, & Colmers WF (2007). Neuropeptide Y in the dentate gyrus. Progress in Brain Research 163, 285–297. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, & Gallagher M (2013). Hilar interneuron vulnerability distinguishes aged rats with memory impairment. The Journal of Comparative Neurology 521, 3508–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahel PF, Smith WR, Bruchis J, & Rabb CH (2008). Peroxisome proliferator-activated receptors: “Key” regulators of neuroinflammation after traumatic brain injury. PPAR Research 2008, 538141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L (2000). Prevalence and patterns of Gulf War illness in Kansas veterans: Association of symptoms with characteristics of person, place, and time of military service. American Journal of Epidemiology 152, 992–1002. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Krengel M, Heboyan V, Schildroth S, Wilson CC, Iobst S, et al. (2020). Prevalence and patterns of symptoms among female veterans of the 1991 Gulf War Era: 25 years later. Journal of Women’s Health (2002) 29, 819–826. [DOI] [PubMed] [Google Scholar]
- Sullivan N, Phillips LA, Pigeon WR, Quigley KS, Graff F, Litke DR, et al. (2019). Coping with medically unexplained physical symptoms: The role of illness beliefs and behaviors. International Journal of Behavioral Medicine 26, 665–672. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr. (2012). Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacology & Therapeutics 134, 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till A, Lakhani R, Burnett SF, & Subramani S (2012). Pexophagy: The selective degradation of peroxisomes. International Journal of Cell Biology 2012, 512721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, et al. (2014). Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano 8, 76–103. [DOI] [PubMed] [Google Scholar]
- Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, Lyons MJ, et al. (2009). Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. Journal of the International Neuropsychological Society 15, 717–729. [DOI] [PubMed] [Google Scholar]
- Trivedi MS, Abreu MM, Sarria L, Rose N, Ahmed N, Beljanski V, et al. (2019). Alterations in DNA methylation status associated with Gulf War Illness. DNA and Cell Biology 38, 561–571. [DOI] [PubMed] [Google Scholar]
- Tuite JJ, & Haley RW (2013). Meteorological and intelligence evidence of long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology 40, 160–177. [DOI] [PubMed] [Google Scholar]
- Tundup S, Srivastava L, Norberg T, Watford W, & Harn D (2015). A neoglycoconjugate containing the human milk sugar LNFPIII drives anti-inflammatory activation of antigen presenting cells in a CD14 dependent pathway. PLoS One 10, e0137495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugrinova I, & Pasheva E (2017). HMGB1 protein: A therapeutic target inside and outside the cell. Advances in Protein Chemistry and Structural Biology 107, 37–76. [DOI] [PubMed] [Google Scholar]
- Ullah F, Liang A, Rangel A, Gyengesi E, Niedermayer G, & Munch G (2017). High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Archives of Toxicology 91, 1623–1634. [DOI] [PubMed] [Google Scholar]
- Van Riper SM, Alexander AL, Koltyn KF, Stegner AJ, Ellingson LD, Destiche DJ, et al. (2017). Cerebral white matter structure is disrupted in Gulf War Veterans with chronic musculoskeletal pain. Pain 158, 2364–2375. [DOI] [PubMed] [Google Scholar]
- Vasko R, & Goligorsky MS (2013). Dysfunctional lysosomal autophagy leads to peroxisomal oxidative burnout and damage during endotoxin-induced stress. Autophagy 9, 442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, & Sutker PB (1998). Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 12, 125–133. [DOI] [PubMed] [Google Scholar]
- Wada-Isoe K, Imamura K, Kitamaya M, Kowa H, & Nakashima K (2008). Serum heart-fatty acid binding protein levels in patients with Lewy body disease. Journal of the Neurological Sciences 266, 20–24. [DOI] [PubMed] [Google Scholar]
- Wang S, Wan T, Ye M, Qiu Y, Pei L, Jiang R, et al. (2018). Nicotinamide riboside attenuates alcohol induced liver injuries via activation of SirT1/PGC-1alpha/mitochondrial biosynthesis pathway. Redox Biology 17, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, & Brown MW (2013). Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Rayhan RU, Garner R, Provenzano D, Zajur K, Addiego FM, et al. (2020). Exercise alters cerebellar and cortical activity related to working memory in phenotypes of Gulf War Illness. Brain Communications 2 fcz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, et al. (2016). Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Genova H, Dobryakova E, DeLuca J, Chiaravalloti N, Falvo M, et al. (2019). Fatigue in Gulf War Illness is associated with tonically high activation in the executive control network. Neuroimage Clinical 21, 101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Ashbrook DG, Gao J, Starlard-Davenport A, Zhao W, Miller DB, et al. (2020). Genome-wide transcriptome architecture in a mouse model of Gulf War Illness. Brain, Behavior, and Immunity S0889-1591(20), 30604–30608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Song N, Wang R, Jiang H, & Xie J (2016). Preferential Heme Oxygenase-1 activation in striatal astrocytes antagonizes dopaminergic neuron degeneration in MPTP-intoxicated mice. Molecular Neurobiology 53, 5056–5065. [DOI] [PubMed] [Google Scholar]
- Yamanaka D, Kawano T, Nishigaki A, Aoyama B, Tateiwa H, Shigematsu-Locatelli M, et al. (2017). Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. Journal of Anesthesia 31, 25–35. [DOI] [PubMed] [Google Scholar]
- Zaben MJ, & Gray WP (2013). Neuropeptides and hippocampal neurogenesis. Neuropeptides 47, 431–438. [DOI] [PubMed] [Google Scholar]
- Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, et al. (2016). A chronic longitudinal characterization of neurobehavioral and neuropathological cognitive impairment in a mouse model of Gulf War agent exposure. Frontiers in Integrative Neuroscience 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, et al. (2015). Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness. PLoS One 10, e0119579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Avery T, Vakhtin AA, Mathersul DC, Tranvinh E, Wintermark M, et al. (2020). Brainstem atrophy in Gulf War Illness. Neurotoxicology 78, 71–79. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Duan S, Zhou Y, Yu S, Wu J, Wu X, et al. (2015). Sulfiredoxin-1 attenuates oxidative stress via Nrf2/ARE pathway and 2-Cys Prdxs after oxygen-glucose deprivation in astrocytes. Journal of Molecular Neuroscience 55, 941–950. [DOI] [PubMed] [Google Scholar]
- Zhu J, Hawkins E, Phillips K, & Deshpande LS (2020). Assessment of ketamine and its enantiomers in an organophosphate-based rat model for features of Gulf War Illness. International Journal of Environmental Research and Public Health 17, 4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zundel CG, Krengel MH, Heeren T, Yee MK, Grasso CM, Janulewicz Lloyd PA, et al. (2019). Rates of chronic medical conditions in 1991 Gulf War veterans compared to the general population. International Journal of Environmental Research and Public Health 16, 949. [DOI] [PMC free article] [PubMed] [Google Scholar]


