Abstract
Primary aldosteronism is a frequent form of endocrine hypertension caused by aldosterone overproduction from the adrenal cortex. Regulation of aldosterone biosynthesis has been studied in rodents despite differences in adrenal physiology with humans. We therefore investigated pig adrenal steroidogenesis, morphology, and transcriptome profiles of the zona glomerulosa (zG) and zona fasciculata (zF) in response to activation of the renin-angiotensin-aldosterone system by dietary sodium restriction. Six-week-old pigs were fed a low or high sodium diet for 14 days (3 pigs per group, 0.4g sodium/kg feed versus 6.8g sodium/kg). Plasma aldosterone concentrations displayed a 43-fold increase (p=0.011) after 14-days of sodium restriction (day 14 versus day 0). Low dietary sodium caused a 2-fold increase in thickness of the zG (p<0.001) and an almost 3-fold upregulation of CYP11B (cytochrome P450 11B1) (p<0.05) compared with high dietary sodium. Strong immunostaining of the KCNJ5 potassium channel, which is frequently mutated in primary aldosteronism, was demonstrated in the zG. mRNA-seq transcriptome analysis identified significantly altered expression of genes modulated by the renin-angiotensin-aldosterone system in the zG (n= 1,172) and zF (n= 280). These genes included many with a known role in the regulation of aldosterone synthesis and adrenal function. The most highly enriched biological pathways in the zG were related to cholesterol biosynthesis, steroid metabolism, cell cycle and potassium channels. This study provides mechanistic insights into the physiology and pathophysiology of aldosterone production in a species closely related to humans and shows the suitability of pigs as a translational animal model for human adrenal steroidogenesis.
Keywords: aldosterone, cortisol, hyperaldosteronism, adrenal cortex, sodium restriction, steroidogenesis, hypertension
Graphical Abstract
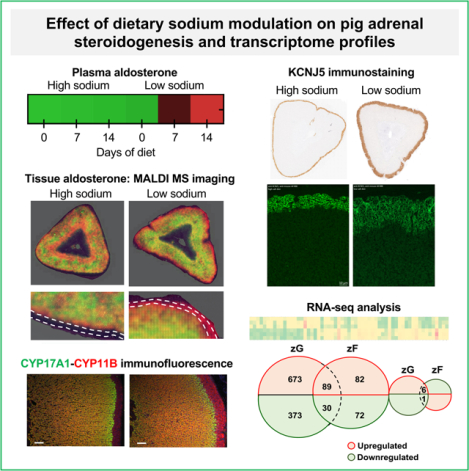
Summary
The pig is an appropriate model to study the regulation of aldosterone secretion and will be useful to discover and assess novel pharmacological targets of aldosterone excess.
Introduction
The mineralocorticoid hormone aldosterone is synthesized in zona glomerulosa (zG) cells of the adrenal cortex and stimulates sodium reabsorption in epithelial cells of the kidney distal tubule and colon for the maintenance of blood volume and blood pressure. The main physiological regulators of aldosterone production are the renin-angiotensin-aldosterone system (RAAS) and circulating potassium; although other factors are likely also involved.1–3 Sodium depletion activates the RAAS and causes expansion of the zG layer and an increase in aldosterone secretion.4,5 Manipulation of dietary sodium in rats has been used as an approach to study the effects of RAAS activation on zG gene expression to identify genes that function in the regulation of aldosterone production.5–7
Humans and commonly used experimental surrogates display distinct differences in adrenal physiology. For example, the Cyp17a1 gene (encoding 17α-hydroxylase and 17,20-lyase) is not expressed in the adrenal glands of laboratory rats and mice resulting in the production of corticosterone as the major glucocorticoid, instead of cortisol as in humans. Another major difference involves the absence of the zona reticularis (zR) and adrenal androgen synthesis in these rodents.8 Furthermore, potassium channels which function in the maintenance of zG cell membrane potential show different gene expression profiles and patterns of immunostaining in rat and human adrenals.9,10 Notably, the rat adrenal does not express the inwardly rectifying potassium channel KCNJ5 which is frequently mutated in primary aldosteronism.9 Regulation of KCNJ5 gene expression and channel activity modulate the zG membrane depolarization that normally initiates aldosterone production in humans.11 KCNJ5 mutations allow uncontrolled zG membrane depolarization and cause aldosterone excess.
The adrenal cortex is divided into three morphologically distinct layers (zG, zF, and zR). In the human adrenal, restricted expression of CYP11B2 (encoding aldosterone synthase) in the zG and CYP11B1 (encoding 11β-hydroxylase) in the zF and zR sustains the functional zonation of aldosterone biosynthesis in the zG and cortisol in the zF. These two CYP11B enzymes, with their specific zonal distributions, are present in multiple species including mice, rats, hamsters, and guinea pigs.8 In contrast, others, such as pigs, cattle, sheep and dogs, express a single CYP11B enzyme that performs the final steps of both aldosterone and cortisol biosynthesis12 and, by way of an unknown mechanism, their biosynthetic zonal specificity is maintained.13,14
Large animals such as pigs are useful to model complex human diseases due to their comparable anatomy and physiology to humans.15–17 Such models provide an opportunity to screen for disease biomarkers and test novel therapeutic strategies.18,19 In this study, we evaluated the role of dietary sodium manipulation on adrenal morphology, steroidogenesis and transcriptome profiles in 6-week-old male pigs. Our objective was to identify the transcriptional response of the adrenal to RAAS activation and determine the suitability of the pig as a translational animal model for human adrenal steroidogenesis.
Methods
An expanded online methods section is available in the online supplementary file.
The authors declare that all supporting data are available within the article and its online supplementary files. mRNA-seq data are publicly available and can be accessed at https://github.com/MedIVLMUMunich/PigAdrenalRNAseq
Animal handling
The study used 6-week-old male German Landrace DanBred pigs on a controlled 14-day diet of 0.04% sodium (n= 3; low sodium group) or 0.7% sodium (n= 3; high sodium group, around 5-times higher than in standard pig feed) of equivalent metabolic energy with free access to water (Table S1).
Immunohistochemistry and immunofluorescence
Primary antibodies for immunohistochemistry and immunofluorescence are shown in Table S2. A pig polyclonal CYP11B antibody was generated using a synthetic peptide (acetyl-95EDVERLQKVEGLHPQR110C) (Figure S1) which was validated for use in Western blotting and immunohistochemistry and immunofluorescence (Figure S2). CYP17A and KCNJ5 monoclonal antibodies were produced as described previously.20,21
Steroid measurements and renin assays
Liquid chromatography tandem mass spectrometry (LC-MS/MS) for adrenal steroid measurements was performed according to Peitzsch et al.22 Renin activity was determined by LC-MS/MS quantification (Attoquant Diagnostics, Vienna, Austria) as reported elsewhere.23
Matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI)
In situ metabolic imaging analysis of fresh frozen adrenal sections was performed with on-tissue derivatization using Girardś T reagent according to Suguira et al.24 and MALDI-MSI analysis was performed as reported by Sun et al.25
Transcriptome profiling by mRNA sequencing
mRNA-seq transcriptome profiling was done by Eurofins Genomics (Ebersberg, Germany).
Statistical analysis and bioinformatics
IBM SPSS Statistics 26 (IBM Corp., Armonk, New York, USA) and GraphPad PRISM 8.0a (La Jolla, California, USA) were used for statistical analyses. Significant differences were analyzed using a Friedman test for repeated measures, with correction for multiple comparisons, where appropriate, and a Mann-Whitney test for independent measures. P-values less than 0.05 were considered statistically significant. Biological pathway enrichment was analyzed using Reactome (https://reactome.org/PathwayBrowser/#/) and Gene Ontology (http://geneontology.org/) databases.26–28
Results
Low dietary sodium intake and activation of the RAAS
Pigs consumed comparable amounts and ate all daily allocated food. Measurements of urinary sodium excretion levels confirmed the difference in sodium intake between the 2 groups (Figure 1A). At day 14, plasma renin activity increased 7-fold compared with day 0 reaching 116.91 pmol/L/min (9.13 ng/mL/hr ± 3.7) under the low sodium diet. (Figure 1B).
Figure 1. Activation of the pig renin-angiotensin-aldosterone system by dietary sodium restriction.
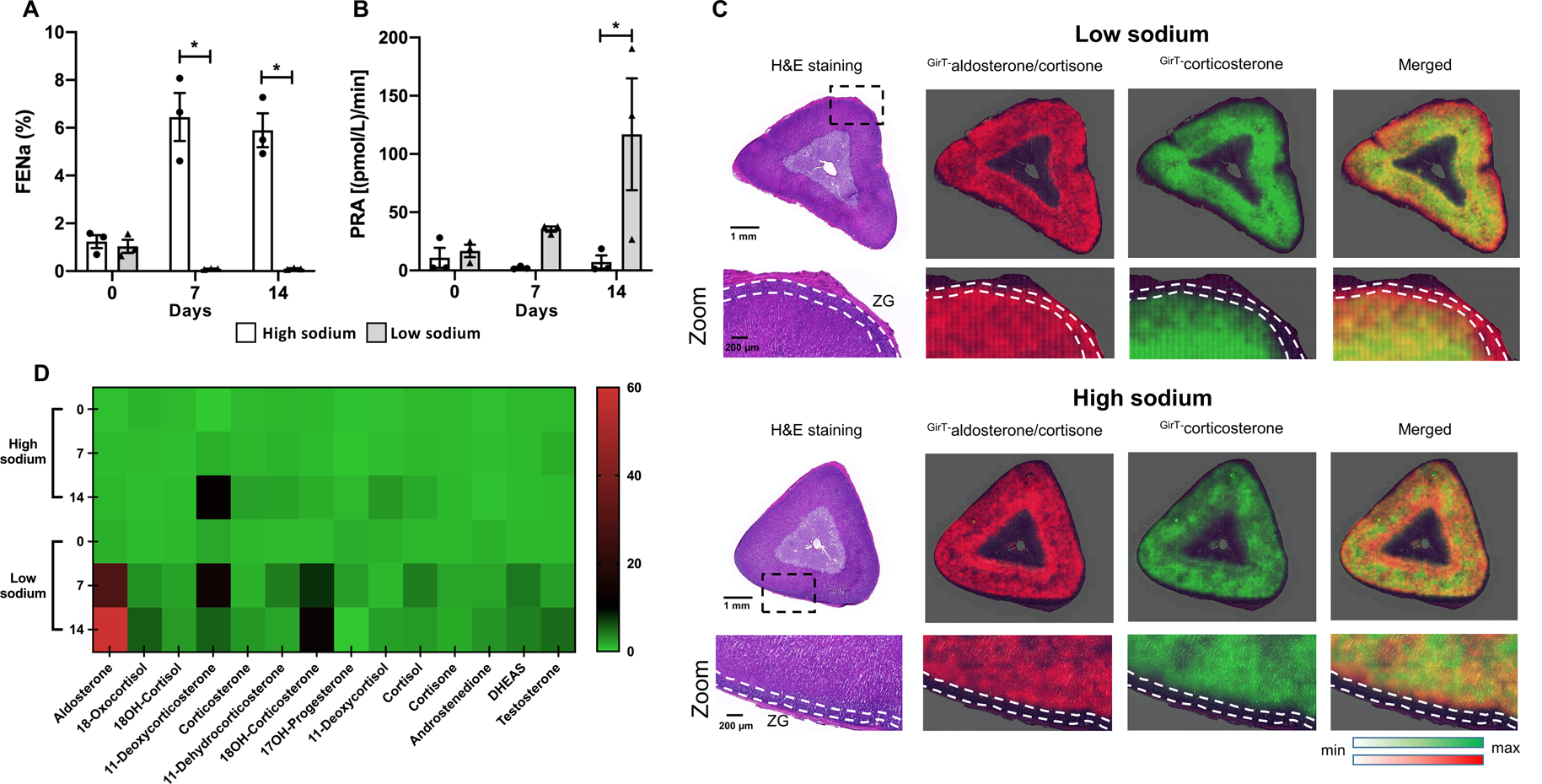
Fractional excretion of sodium measurements confirmed the higher sodium intake at 7 and 14 days of pigs on the high compared with the low sodium diet (Panel A). Plasma renin activity showed a 7-fold increase at day 14 compared with day 0 (Panel B). MALDI-MSI demonstrated the increased aldosterone or cortisone production in the zG of the low sodium group. The absence of CYP17A (required for cortisol synthesis) in the zG indicates that the increased intensity of the aldosterone or cortisone signal in the zG is aldosterone. The zG is delineated with white broken lines (Panel C). Multiple adrenal steroids were measured by LC-MS/MS showing the progressive increase of aldosterone production up to day 14 in pigs under sodium restriction. Data for individual pigs is normalized to the average plasma steroid concentration at day 0 for high and low sodium groups and the intensity scale indicates fold change over baseline (Panel D).
FENa, fractional excretion of sodium; GirT, Girard’s Reagent T; H&E, hematoxylin and eosin; 18OH-cortisol, 18-hydroxycortisol; 18OH-corticosterone, 18-hydroxycorticosterone; 17OH-progesterone, 17-hydroxyprogesterone; PRA, plasma renin activity. Bars represent mean ± SEM (n=3 for each group), a Friedman non-parametric test for repeated measures was used to detect paired differences. Circles, high sodium; triangles, low sodium diet. *p<0.05. Panel C, scale bar = 1mm and 200 μm in zoomed images as shown.
Fourier-transform ion cyclotron resonance MSI of adrenal sections combined with on-tissue derivatization of steroids with Girard T (GirT) reagent facilitated the clear visualization of aldosterone or cortisone (which have an identical m/z ratio) in the zG layer of pigs on the sodium-restricted diet and low detection in the zG of pigs on the high sodium diet (Figure 1C). Because co-expression of CYP17A (which is not expressed in the zG) with CYP11B is required for cortisol and cortisone synthesis, the signal in the zG layer indicates increased synthesis of aldosterone in the zG under dietary sodium restriction.
Blood plasma steroid concentrations measured by LC-MS/MS are shown in Table S3 and are also represented in a heat map (Figure 1D) to illustrate the progressive time-related increase of plasma aldosterone levels in pigs on the sodium-restricted diet only. This corresponded to a 43.2-fold increase in plasma aldosterone concentrations (day 14 versus day 0, p= 0.011) to reach 3.20 nmol/L (1.153 ± 0.583 ng/mL) at day 14 (Table S3). No significant differences in concentrations of steroids other than aldosterone were observed. The plasma 18-hydroxycorticosterone concentration progressively increased on the low sodium diet to 48.66 nmol/L (17.638 ± 11.185 ng/mL) at day 14 compared with 3.92 nmol/L (1.421 ± 0.082 ng/mL) at day 0, but the difference was not significant. The hybrid steroids 18-hydroxycortisol and 18-oxocortisol were identified in all pig blood plasma samples. In the plasma samples measured, both 18-hydroxycortisol and 18-oxocortisol displayed maximum levels in the sodium restricted pigs at day 14 (18-hydroxycortisol, 5.79 nmol/L {2.190 ± 1.178 ng/mL}; 18-oxocortisol, 0.27 nmol/L {0.102 ± 0.046 ng/mL}).
Morphological changes in adrenal cortex in response to dietary sodium restriction
Pig adrenals showed strong immunostaining of VSNL1 and KCNJ5 in the zG layer (Figure 2A and B). VSNL1 immunostaining highlighted an increased thickness of the zG layer from 6.5% ± 0.21 of the total adrenal cortex on the high sodium diet, to 13.8% ± 0.17 (p<0.001) on the low sodium diet. Similar results were observed with measurements from KCNJ5 immunostaining (Figure 2B).
Figure 2. Morphological and functional expansion of the pig adrenal zona glomerulosa by dietary sodium restriction.
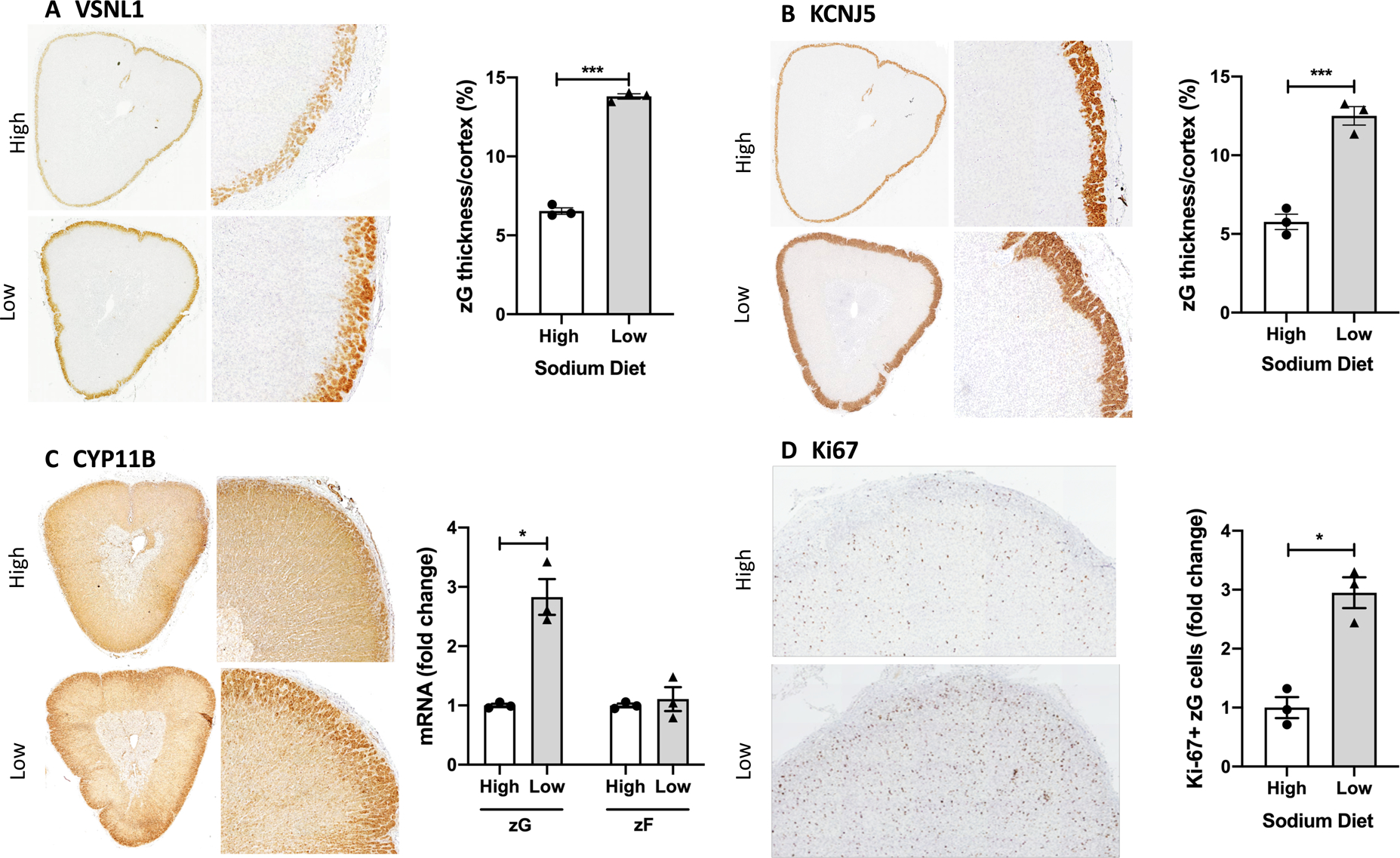
Immunostaining of VSNL1 and KCNJ5 highlighted the increase in the zG in the pigs fed a 14-day low sodium diet. The thickness of the zG layer is shown as a percentage of the total thickness of the adrenal cortex. The average of 6 measurements for each adrenal is shown (Panel A and B). CYP11B immunostaining shows the expansion of the zG layer is complemented by an increase in aldosterone synthase-positive cells in pigs on a low sodium diet (Panel C) and immunostaining for Ki67, a marker of cell proliferation, shows a significant increase in Ki67-positive cells in the zG layer on a low versus high sodium diet (Panel D). Bars represent mean ± SEM (n=3 per group), a Mann-Whitney test was used to detect differences. Circles, high sodium; triangles, low sodium diet. *p<0.05, ***p<0.001.
CYP11B immunohistochemistry showed positive staining throughout the adrenal cortex with more intense immunostaining throughout the thickened zG layer. CYP11B gene expression displayed a 2.8 ± 0.3-fold increase (p=0.045) in the zG under sodium restriction with no change observed in the zF (Figure 2C). Increased zG cell proliferation was demonstrated under sodium restriction with a 2.9 ± 0.26-fold increase (p=0.028) in Ki-67 immunoreactive cells relative to pigs on a high sodium diet (Figure 2D). CYP11B-CYP17A double immunofluorescence delineated the zG layer more clearly, as CYP17A expression and thus immunostaining is restricted to the zF and zR, and confirmed increased intensity of CYP11B immunostaining in the zG but not in the zF under the low versus high sodium diets (Figure 3).
Figure 3. CYP11B-CYP17A double immunofluorescence staining.
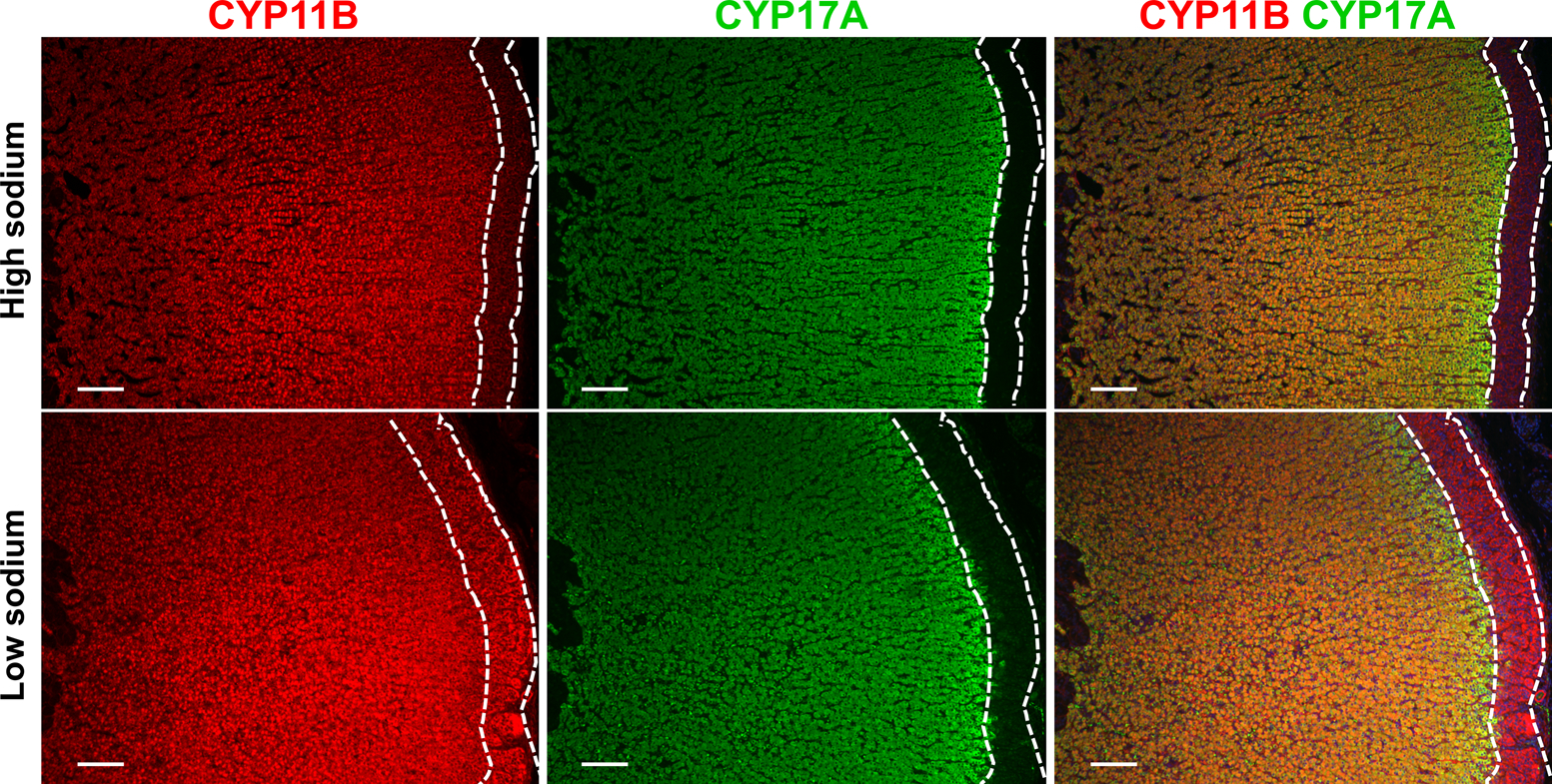
In the adrenal, CYP17A is exclusively expressed in the zF and the zR and CYP11B-CYP17A double immunostaining allows the clear visualization of the zG layer showing the increased thickness and increased CYP11B immunostaining in the zG compared with the zF in pigs on the low compared with the high sodium diet. The zG is delineated with white broken lines. Scale bar= 50 μm
mRNA-seq transcriptome analysis of the zG under a low and high sodium diet
An overview of the mRNA-seq analysis is shown in Figure S3. Comparison of the transcriptomes of the zG and zF demonstrated a higher number of transcripts with significantly altered expression levels in the zG than the zF (Figure S3, Figure 4A–C). These included 1,172 significantly altered annotated genes in the zG of the low versus high sodium groups comprising 768 upregulated and 404 downregulated genes:
Figure 4. Transcriptome alterations in the zona glomerulosa and the zona fasciculata under low versus high sodium diets.
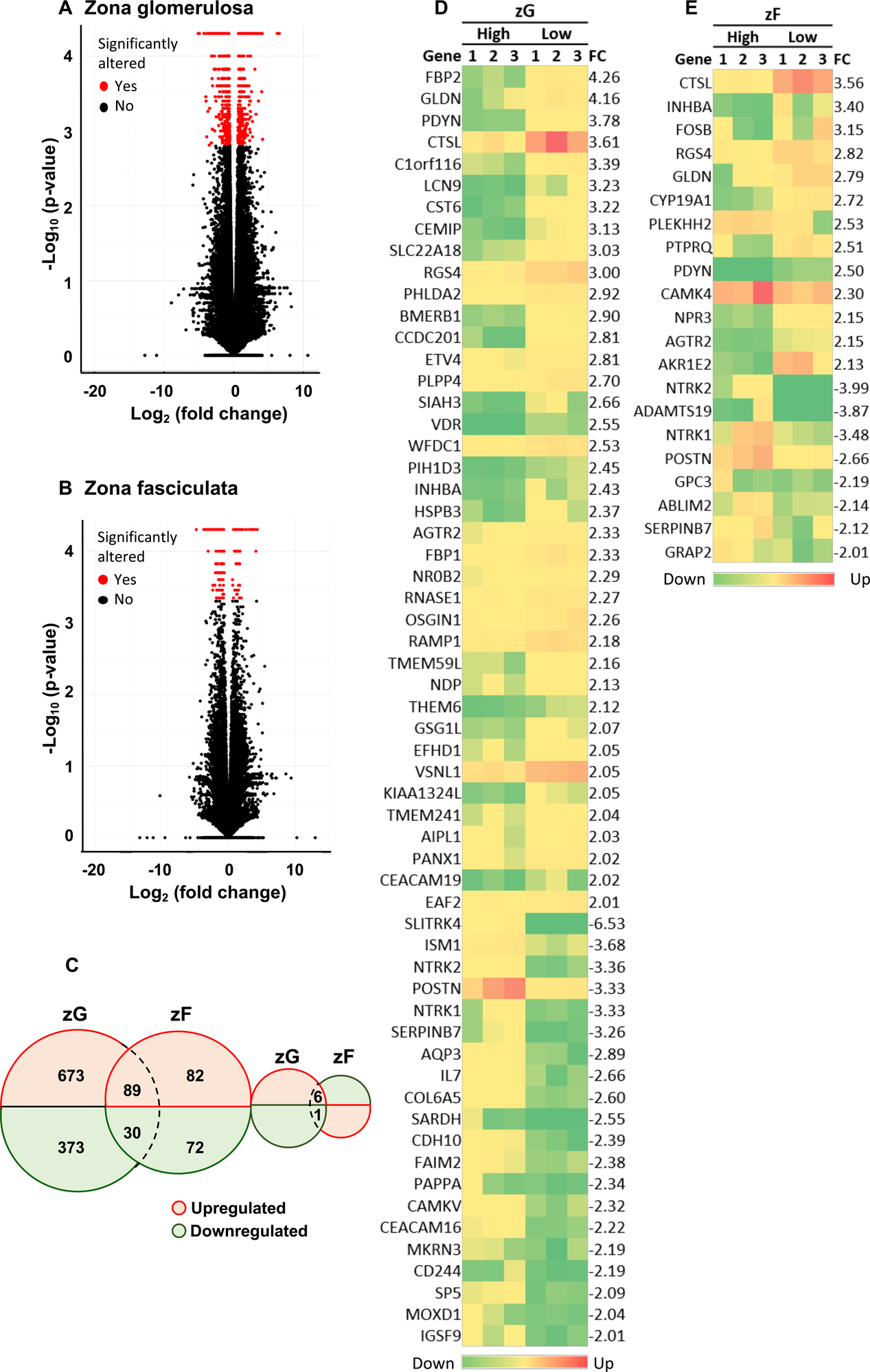
Volcano plots indicate transcripts with significantly altered levels (red dots) by dietary sodium manipulation in the zG (Panel A) and zF (Panel B). The numbers of significantly altered transcripts in the zG and zF (low versus high dietary sodium) are indicated in the Venn diagram (Panel C). There were 6 transcripts upregulated in response to sodium restriction in the zG which were downregulated in the zF and one transcript which was downregulated in the zG but upregulated in the zF (low versus high sodium). The heat maps represent the up- and down-regulated genes (defined as log2-fold change>2) in the zG (Panel D) and zF (Panel E) of each pig (n= 3 in each of the high and low sodium diet groups). The gene names are indicated on the left of each heat map and log2-fold change on the right. FC, log2-fold change; zG, zona glomerulosa; zF, zona fasciculata
https://github.com/MedIVLMUMunich/PigAdrenalRNAseq/raw/master/1)zG_All%20annotated%20DEGs.xlsx. In the zF, 280 significantly altered annotated genes were identified with 172 upregulated and 108 downregulated:
https://github.com/MedIVLMUMunich/PigAdrenalRNAseq/raw/master/2)zF_All%20annotated%20DEGs.xlsx.
Significantly altered transcripts which were common to the zG and the zF under low versus high dietary sodium comprised 89 upregulated and 30 downregulated genes. In addition, 6 transcripts were upregulated in the zG but downregulated in the zF (FHIT, ssc-mir-202, WWOX, GTDC1, ZNF592 and SLC2A9) and 1 transcript was downregulated in the zG and upregulated in the zF (HEATR3) in response to salt restriction (Figure 4C).
A heat map representation of differentially expressed genes (DEGs) (defined as a log2-fold change ≥2) under low versus high sodium intake in the zG (59 genes) and the zF (21 genes) is shown in Figure 4D–E. The top 20 upregulated and downregulated genes in the zG (low versus high sodium diet) with gene loci, expression levels and respective functions are shown in Table S4 and in the zF in Table S5. Several DEGs were identified with a described role in aldosterone production or adrenal function. These included genes encoding the transcription factors FOSB, FOS, VDR and NR5A1 (also called SF-1), RGS4 (regulator of G protein signaling 4), the calcium-binding proteins VSNL1 (visinin-like 1) and SMOC (secreted modular calcium-binding protein 1), STARD4 (StAR-related lipid transfer protein 4), the cytochrome P450 enzymes CYP27A1, CYP21A2 and CYPB1, and VAV2 (Vav Guanine Nucleotide Exchange Factor 2) (Table).29–43
Table.
Top differentially expressed genes with a described functional role in the adrenal
| Gene | zG_FPKM | Log2_fc LS vs. HS |
P value | zF_FPKM | Log2_fc LS vs. HS |
P value | REF | ||
|---|---|---|---|---|---|---|---|---|---|
| LS | HS | LS | HS | ||||||
| FOSB | n.d. | n.d. | - | - | 8.25 | 0.93 | 3.15 | 0.00005 | 29 |
| RGS4 | 174.01 | 21.70 | 3.00 | 0.00005 | 21.96 | 3.10 | 2.82 | 0.00005 | 6, 30 |
| VDR | 1.63 | 0.28 | 2.55 | 0.00005 | n.d. | n.d. | - | - | 29 |
| AGTR2 | 19.85 | 3.93 | 2.33 | 0.00005 | 1.94 | 0.44 | 2.15 | 0.00005 | 31–33 |
| VSNL1 | 346.14 | 83.42 | 2.05 | 0.00005 | 34.53 | 8.78 | 1.98 | 0.00005 | 34 |
| SMOC2 | 4.31 | 14.45 | −1.75 | 0.00005 | 3.11 | 6.91 | −1.15 | 0.00005 | 30 |
| STARD4 | 16.91 | 8.72 | 0.95 | 0.0001 | 12.66 | 3.76 | 1.75 | 0.00005 | 35 |
| FOS | 55.93 | 28.42 | 0.98 | 0.00005 | 164.14 | 55.98 | 1.55 | 0.00005 | 36 |
| CYP27A1 | 14.10 | 32.12 | −1.19 | 0.00005 | n.d. | n.d. | - | - | 37 |
| NR5A1 | 229.67 | 118.83 | 0.95 | 0.00005 | n.d. | n.d. | - | - | 38 |
| VAV2 | 27.20 | 14.46 | 0.91 | 0.00005 | n.d. | n.d. | - | - | 39 |
| KCNJ5 | 221.11 | 118.62 | 0.90 | 0.00005 | n.d. | n.d. | - | - | 40 |
| CYP21A2 | 5786.23 | 3275.67 | 0.82 | 0.00025 | n.d. | n.d. | - | - | 41 |
| CEBPB | 74.41 | 42.09 | 0.82 | 0.00005 | n.d. | n.d. | - | - | 42 |
| CYP1B1 | 23.04 | 38.24 | −0.73 | 0.00025 | n.d. | n.d. | - | - | 43 |
The genes with the highest level of differential expression with a described functional role in the adrenal are shown. The table indicates the expression levels and log2-fold changes under a low versus high sodium diet in either the zG or zF.
The full list of annotated DEGs in the zG can be downloaded at: https://github.com/MedIVLMUMunich/PigAdrenalRNAseq/raw/master/1)zG_All%20annotated%20DEGs.xlsx
The full list of annotated DEGs in the zF can be downloaded at: https://github.com/MedIVLMUMunich/PigAdrenalRNAseq/raw/master/2)zF_All%20annotated%20DEGs.xlsx
Fc, fold change; FPKM, fragments per kilobase of transcript, per million mapped reads; n.d., not detected; REF, reference
Enriched biological pathways in the zG in response to dietary sodium restriction
Biological pathway analysis of significantly DEGs in the pig adrenal zG transcriptomes (low versus high sodium diet) identified enriched pathways related to steroid metabolism (p= 5.17e-2; number of DEGs= 31), cholesterol biosynthesis (p= 2.51e-4; DEGs= 12) and regulation of cholesterol biosynthesis by SREBP (sterol regulatory-element binding protein; p= 9.79e-6; DEGs= 12), the cell cycle (p= 9.82e-1; DEGs= 40) and potassium channels (p= 5.18e-1; DEGs= 9) (Table S6). The DEGs in the steroid metabolism pathway included VDR (vitamin D receptor), the top DEG related to this pathway, and STARD4 (StAR related lipid transfer domain containing 4). Seven DEGs were common to the regulation of cholesterol biosynthesis by SREBP and the cholesterol biosynthesis pathways (HMGCS1, HMGCR, FDFT1, PMVK, MVK, SQLE and MVD). DEGs associated with the cell cycle included AGTR2 (angiotensin II receptor type 2) and PRKAR2B (cAMP-dependent protein kinase type II-beta regulatory subunit). KCNJ5 was the DEG in the potassium channel pathway with the highest expression level in the zG (Table S6).
Discussion
We demonstrate the effect of RAAS activation by dietary sodium restriction on adrenal morphology, steroidogenesis and transcriptome profiles in pigs as a translational animal model for humans. In pigs, as described previously in rats,4,5 RAAS activation induced by dietary sodium restriction caused zG expansion, increased zG expression of aldosterone synthase (called CYP11B in pigs and CYP11B2 in rats and humans) at both transcript and protein levels and an increased production of aldosterone from the zG. After 14 days, pig plasma aldosterone concentrations were comparable to those reported in Yanomami Indians (3.20 nmol/L versus 2.38 nmol/L, respectively), a population noted for their low salt consumption44, and 20-fold higher than in a group of 525 adult humans from Europe.45
We show strong immunostaining of KCNJ5 in the zG of the pig adrenal as in humans21 and KCNJ5 transcripts were detected in the zG but not the zF. KCNJ5 is a potassium inwardly rectifying channel that contributes to normal membrane polarization and may contribute to the mechanism of aldosterone synthesis in response to angiotensin II stimulation.11 Germline and somatic KCNJ5 mutations that cause membrane depolarization of the zG cell have been identified as the main known molecular variants causing primary aldosteronism in humans.46 However, there are redundant mechanisms for the maintenance of membrane potential and knocking down the expression of KCNJ5 in human adrenal cortical carcinoma cells did not alter basal aldosterone synthesis nor abrogate its stimulation by angiotensisn II.11 KCNJ5 transcripts were undetectable in laser-captured samples of rat adrenal zG and specific KCNJ5 immunostaining was undetectable indicating a difference between rats and humans in the regulation of zG membrane potential and aldosterone production.9
We used mRNA-seq analysis to gain further insight into transcriptome changes of zG cells associated with increased aldosterone synthesis in the pig as a model for the regulation of human aldosterone production. RNA-seq analysis offers the possibility to accurately quantify expression of genes from very low to high levels and allowed the identification of a large number of genes in the zG transcriptome (768 and 404 up- and down-regulated annotated genes) which are regulated by chronic RAAS activation compared with the rat using microarray analysis (201 and 68 up- and down-regulated genes, low versus high sodium diet).5 In the latter case, the rats were fed a sodium-controlled diet for 3 days, compared with the 14-days in the current study, and the high dietary sodium levels used in the rat study were almost 10-times greater than in the present study, with relatively mild high sodium conditions, which may also account for this difference.
We report several upregulated genes in the zG in response to sodium restriction with a previously reported role in aldosterone production. VDR (encoding the vitamin D receptor) was the top DEG in the zG with an annotated function related to steroid metabolism which was expressed at a low level in the zG and undetected in the zF. VDR gene expression was upregulated by angiotensin II stimulation of a human adrenocortical cell line and VDR overexpression caused an increase in aldosterone secretion under basal and angiotensin II stimulated conditions.29 The VDR gene is upregulated in aldosterone-producing adenomas, a major cause of primary aldosteronism, compared with normal adrenals47 and VDR gene expression is positively correlated with CYP11B2 mRNA levels consistent with a role in pathophysiological aldosterone production.48 Other upregulated genes in the pig zG with a previously reported role in aldosterone secretion include RGS4 (regulator of G protein signaling 4) which is upregulated in the rat adrenal by a low sodium diet and angiotensin II infusion6,30 and VSNL1 encoding the calcium sensor protein VSNL1 which regulates basal and angiotensin II-stimulated CYP11B2 gene expression in human adrenocortical cells in vitro.34
Transcriptome alterations were also identified in the zF transcriptome by stimulation of the RAAS. This is consistent with the report of a more than 2-fold significant increase in cortisol secretion, in addition to an increase in aldosterone secretion, by angiotensin II stimulation of human adrenocortical cells in vitro.49 Furthermore, an analysis of transcription regulatory genes modulated by angiotensin II in cultured human adrenocortical cells identified several genes which significantly activate an 11β-hydroxylase reporter gene, with a relatively much smaller effect on the expression of an aldosterone synthase reporter plasmid.50 These genes included members of the FOS (fos proto-oncogene) gene family, FOS and FOSB, which encode leucine zipper proteins which dimerize with proteins of the JUN family (Jun proto-oncogene), to form the transcription factor complex AP-1 (activator protein-1). FOS and FOSB were highly upregulated in the zF by sodium restriction in the present study. FOSB transcripts were not detected in the zG whereas FOS was expressed in both zones with a greater transcriptional response in the zF.
The cell cycle was one of the most enriched biological pathways in the zG in sodium restricted pigs reflecting the expansion of the zG layer, in agreement with a zG microarray analysis in rats.5 AGTR2 (angiotensin II type 2 receptor) transcription showed the highest response of the DEGs annotated to the cell cycle in the zG. The AGTR2 is highly expressed in the human fetal adrenal,31 strikingly higher than in the adult,32 where it may mediate apoptosis during adrenal gland development.33 The pronounced altered expression of AGTR2 in the zG transcriptome in response to sodium restriction suggests a potential role in the control of adrenal morphology after birth.
The strengths of our study include the use of an animal species closely related to humans. We used a relatively mild high sodium diet to represent a level of salt intake relevant to that of many humans which is in contrast to the highly abnormal experimental conditions of 4–8% NaCl in high salt diets given to rats. Moreover, unlike in rats and mice, pigs display clear, high-level KCNJ5 expression in the zG, and produce the hybrid steroids 18-hydroxycortisol and 18-oxocortisol which display elevated levels in patients with primary aldosteronism carrying KCNJ5 mutations.51,52 This highlights the superiority of the pig over rodents for the study of human adrenal biology and indicates the potential utility of the pig to model aspects of primary aldosteronism.
A limitation of our study is the low number of animals used in each diet group, which may explain why differences in aldosterone precursor steroids were not detected despite the identification of differences in aldosterone production. We also used only male animals to circumvent gender-related effects on steroid production45 and thus we could not address sex-related effects of dietary sodium on aldosterone production.53 An additional limitation of the pig is the expression of a single CYP11B enzyme in the adrenal cortex. Despite this, shared mechanisms of aldosterone physiology with animals that express both CYP11B2 and CYP11B1 in their adrenals are likely because many genes involved in the transcriptional response to sodium restriction were identified common to pigs and rats.
Perspectives
This study presents a proof of concept for the suitability of the pig as a model of human steroidogenesis and provides a rich source of transcriptome data for studies on aldosterone physiology and pathophysiology and for the identification of potential novel pharmacological targets to treat aldosterone excess.
Supplementary Material
Novelty and Significance.
What is new?
The level of sodium intake significantly alters pig adrenal morphology, steroidogenesis and transcriptome profiles
Under a low sodium diet, the pig adrenal displays zona glomerulosa expansion with increased expression of CYP11B
MALDI-MSI demonstrated that aldosterone production in the zona glomerulosa increased in response to sodium restriction
Plasma aldosterone concentrations and plasma renin activity were increased in pigs fed a low sodium diet for 14-days
The KCNJ5 potassium channel is highly expressed in the pig zona glomerulosa and absent in the zona fasciculata
Use of the pig adrenal as a surrogate to study adrenal steroidogenesis offers advantages over that of the rat or mouse
What is relevant?
Pig adrenal transcriptome profiling by mRNA seq analysis identifies genes responsive to activation of the renin-angiotensin aldosterone system
An abundance of genes in the zona glomerulosa and the zona fasciculata respond to dietary sodium restriction
In some respects, pig adrenal steroidogenesis is more like that of the human than the adrenals of rats and mice.
Acknowledgements
The technical assistance of Christina Blechinger, Diana Jaquin, Petra Rank and Youssra Ahmed-Mohammed is gratefully acknowledged.
Sources of funding
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No [694913] to M Reincke) and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) project number: 314061271-TRR 205 to G Eisenhofer, M Peitzsch, M Reincke, A Walch, and TA Williams and Deutsche Forschungsgemeinschaft SFB 824 C04 to A Walch. CE Gomez-Sanchez is supported by National Heart, Lung and Blood Institute grant R01 HL144847, and the National Institute of General Medical Sciences grant U54 GM115428 and BX004681 from the Department of Veterans Affairs, USA.
Footnotes
Conflicts of interest/Disclosure
None
References
- 1.Spät A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. [DOI] [PubMed] [Google Scholar]
- 2.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wils J, Duparc C, Cailleux AF, Lopez AG, Guiheneuf C, Boutelet I, Boyer HG, Dubessy C, Cherifi S, Cauliez B, Gobet F, Defortescu G, Ménard JF, Louiset E, Lefebvre H. The neuropeptide substance P regulates aldosterone secretion in human adrenals. Nat Commun 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitani F, Suzuki H, Hata JI, Ogishima T, Shimada H, Ishimura Y. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 1994;135:431–438. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto K, Harris RBS, Rainey WE, Seki T. Sodium deficiency regulates rat adrenal zona glomerulosa gene expression. Endocrinology. 2014;155:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero DG, Zhou MY, Yanes LL, Plonczynski MW, Washington TR, Gomez-Sanchez CE, Gomez-Sanchez EP. Regulators of G-protein signaling 4 in adrenal gland: Localization, regulation, and role in aldosterone secretion. J Endocrinol. 2007;194:429–440. [DOI] [PubMed] [Google Scholar]
- 7.Kobuke K, Oki K, Gomez-Sanchez CE, Gomez-Sanchez EP, Ohno H, Itcho K, Yoshii Y, Yoneda M, Hattori N. Calneuron 1 increased Ca2+ in the endoplasmic reticulum and aldosterone production in aldosterone-producing adenoma. Hypertension. 2018;71:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragao-Santiago L, Gomez-Sanchez CE, Mulatero P, Spyroglou A, Reincke M, Williams TA. Mouse models of primary aldosteronism: from physiology to pathophysiology. Endocrinology. 2017;158:4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen AX, Nishimoto K, Nanba K, Rainey WE. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol Cell Endocrinol. 2015;417:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T, He M, Hu C. Regulation of aldosterone production by ion channels: from basal secretion to primary aldosteronism. Biochim Biophys Acta Mol Basis Dis. 2018;1864:871–881. [DOI] [PubMed] [Google Scholar]
- 11.Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. The potassium channel, Kir3.4 participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology. 2012;153:4328–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robic A, Faraut T, Prunier A. Pathways and genes involved in steroid hormone metabolism in male pigs: A review and update. J Steroid Biochem Mol Biol. 2014; 140:44–55. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Sanchez CE, Ferris MW, Foecking MF, Gomez-Sanchez EP. Synthesis of 18-hydroxycortisol and 18-oxocortisol in bovine adrenal slices. J Steroid Biochem. 1989;33:595–598. [DOI] [PubMed] [Google Scholar]
- 14.Boon WC, Coghlan JP, McDougall JG. Late steps of aldosterone biosynthesis: sheep are not rats. Clin Exp Pharmacol Physiol Suppl. 1998;25:S21–S27. [DOI] [PubMed] [Google Scholar]
- 15.Perleberg C, Kind A, Schnieke A. Genetically engineered pigs as models for human disease. Dis Model Mech. 2018;11(1):dmm030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renner S, Blutke A, Clauss S, Deeg CA, Kemter E, Merkus D, Wanke R, Wolf E. Porcine models for studying complications and organ crosstalk in diabetes mellitus. Cell Tissue Res. 2020;380:341–378. [DOI] [PubMed] [Google Scholar]
- 17.Moretti A, Fonteyne L, Giesert F, Hoppmann P, Meier AB, Bozoglu T, Baehr A, Schneider CM, Sinnecker D, Klett K, et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020;26:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renner S, Römisch-Margl W, Prehn C, Krebs S, Adamski J, Göke B, Blum H, Suhre K, Roscher AA, Wolf E. Changing metabolic signatures of amino acids and lipids during the prediabetic period in a pig model with impaired incretin function and reduced β-cell mass. Diabetes. 2012;61:2166–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streckel E, Braun-Reichhart C, Herbach N, Dahlhoff M, Kessler B, Blutke A, Bähr A, Übel N, Eddicks M, Ritzmann M, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide in juvenile transgenic pigs modeling a pre-diabetic condition. J Transl Med. 2015;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida T, Nishimoto K, Fukumura Y, Asahina M, Goto H, Kawano Y, Shimizu F, Tsujimura A, Seki T, Mukai K, et al. Disorganized steroidogenesis in adrenocortical carcinoma, a case study. Endocr Pathol. 2017;28:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Gomez-Sanchez CE, Jaquin D, Aristizabal Prada ET, Meyer LS, Knösel T, Schneider H, Beuschlein F, Reincke M, Williams TA. Primary aldosteronism: KCNJ5 mutations and adrenocortical cell growth. Hypertension. 2019;74:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peitzsch M, Dekkers T, Haase M, Sweep FCGJ, Quack I, Antoch G, Siegert G, Lenders JWM, Deinum J, Willenberg HS, Eisenhofer G. An LC-MS/MS method for steroid profiling during adrenal venous sampling for investigation of primary aldosteronism. J Steroid Biochem Mol Biol. 2015;145:75–84. [DOI] [PubMed] [Google Scholar]
- 23.Burrello J, Buffolo F, Domenig O, Tetti M, Pecori A, Monticone S, Poglitsch M, Mulatero P. Renin-angiotensin-aldosterone system triple-A analysis for the screening of primary aldosteronism. Hypertension. 2020;75:163–172. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura Y, Takeo E, Shimma S, Yokota M, Higashi T, Seki T, Mizuno Y, Oya M, Kosaka T, Omura M, Nishikawa T, Suematsu M, Nishimoto K. Aldosterone and 18-oxocortisol coaccumulation in aldosterone-producing lesions. Hypertension. 2018;72:1345–1354. [DOI] [PubMed] [Google Scholar]
- 25.Sun N, Meyer LS, Feuchtinger A, Kunzke T, Knösel T, Reincke M, Walch A, Williams TA. Mass spectrometry imaging establishes 2 distinct metabolic phenotypes of aldosterone-producing cell clusters in primary aldosteronism. Hypertension. 2020;75:634–644. [DOI] [PubMed] [Google Scholar]
- 26.Fabregat A, Sidiropoulos K, Viteri G, Marin-Garcia P, Ping P, Stein L, D’Eustachio P, Hermjakob H. Reactome diagram viewer: data structures and strategies to boost performance. Bioinformatics. 2018;34:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, Sidiropoulos K, Cook J, Gillespie M, Haw R, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidiropoulos K, Viteri G, Sevilla C, Jupe S, Webber M, Orlic-Milacic M, Jassal B, May B, Shamovsky V, Duenas C, et al. Reactome enhanced pathway visualization. Bioinformatics. 2017;33:3461–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero DG, Gomez-Sanchez EP, Gomez-Sanchez CE. Angiotensin II-regulated transcription regulatory genes in adrenal steroidogenesis. Physiol Genomics. 2010;42A:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimoto K, Rigsby CS, Wang T, Mukai K, Gomez-Sanchez CE, Rainey WE, Seki T. Transcriptome analysis reveals differentially expressed transcripts in rat adrenal zona glomerulosa and zona fasciculata. Endocrinology. 2012;153:1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breault L, Lehoux JG, Gallo-Payet N. The angiotensin AT2 receptor is present in the human fetal adrenal gland throughout the second trimester of gestation. J Clin Endocrinol Metab. 1996;81:3914–3922. [DOI] [PubMed] [Google Scholar]
- 32.Xing Y, Nakamura Y, Rainey WE. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol. 2009;300:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamoux E, Breault L, LeHoux J-G, Gallo-Payet N. Involvement of the angiotensin II type 2 receptor in apoptosis during human fetal adrenal gland development. J Clin Endocrinol Metab. 1999;84:4722–4730. [DOI] [PubMed] [Google Scholar]
- 34.Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59:833–839. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Agudo D, Calderon-Dominguez M, Ren S, Marques D, Redford K, Medina-Torres MA, Hylemon P, Gil G, Pandak WM. Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim Biophys Acta - Mol Cell Biol Lipids. 2011;1811:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guang Y, Jary K, Shen-hua Z, Hervonen A. Induction of c-fos-like protein in the rat adrenal cortex by acute stress -immunocytochemical evidence. Mol Cell Endocrinol. 1989;66:163–170. [DOI] [PubMed] [Google Scholar]
- 37.Menzies RI, Zhao X, Mullins LJ, Mullins JJ, Cairns C, Wrobel N, Dunbar DR, Bailey MA, Kenyon CJ. Transcription controls growth, cell kinetics and cholesterol supply to sustain ACTH responses. Endocr Connect. 2017;6:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE. Differential regulation of aldosterone synthase and 11β-hydroxylase transcription by steroidogenic factor-1. J. Mol. Endocrinol 2002;28:125–135. [DOI] [PubMed] [Google Scholar]
- 39.Ruggiero C, Doghman-Bouguerra M, Sbiera S, Sbiera I, Parsons M, Ragazzon B, Morin A, Robidel E, Favier J, Bertherat J, Fassnacht M, Lalli E. Dosage-dependent regulation of VAV2 expression by steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci Signal. 2017;10:eaal2464. [DOI] [PubMed] [Google Scholar]
- 40.Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swierczynska MM, Betz MJ, Colombi M, Dazert E, Jenö P, Moes S, Pfaff C, Glatz K, Reincke M, Beuschlein F, Donath MY, Hall MN. Proteomic Landscape of Aldosterone-Producing Adenoma. Hypertension. 2019;73:469–480. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Demura M, Cheng Y, Zhu A, Karashima S, Yoneda T, Demura Y, Maeda Y, Namiki M, Ono K, Nakamura Y, Sasano H, Akagi T, Yamagishi M, Saijoh K, Takeda Y. Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension. 2014;63:281–288. [DOI] [PubMed] [Google Scholar]
- 43.Zheng W, Jefcoate CR. Steroidogenic factor-1 interacts with cAMP response element-binding protein to mediate cAMP stimulation of CYP1B1 via a far upstream enhancer. Mol Pharmacol. 2005; 67:499–512. [DOI] [PubMed] [Google Scholar]
- 44.Nowaczynski W, Oliver WJ, Neel JV. Serum aldosterone and protein-binding variables in Yanomama Indians: a no-salt culture as compared to partially acculturated Guaymi Indians. Clin Physiol Biochem. 1985;3:289–306. [PubMed] [Google Scholar]
- 45.Eisenhofer G, Peitzsch M, Kaden D, Langton K, Pamporaki C, Masjkur J, Tsatsaronis G, Mangelis A, Williams TA, Reincke M, Lenders JWM, Bornstein SR. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams TA, Monticone S, Mulatero P. KCNJ5 mutations are the most frequent genetic alteration in primary aldosteronism. Hypertension. 2015;65:507–509. [DOI] [PubMed] [Google Scholar]
- 47.Bi C, Li B, Du L, Wang L, Zhang Y, Cheng Z, Zhai A. Vitamin D receptor, an important transcription factor associated with aldosterone-producing adenoma. PLoS One. 2013;8:e82309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X, Yamazaki Y, Tezuka Y, Onodera Y, Ogata H, Omata K, Morimoto R, Nakamura Y, Satoh F, Sasano H. The crosstalk between aldosterone and calcium metabolism in primary aldosteronism: A possible calcium metabolism-associated aberrant “neoplastic” steroidogenesis in adrenals. J Steroid Biochem Mol Biol. 2019;193:105434. [DOI] [PubMed] [Google Scholar]
- 49.Parmar J, Key RE, Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab. 2008;93:4542–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics. 2007;30:26–34. [DOI] [PubMed] [Google Scholar]
- 51.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G, Reincke M. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67:139–145. [DOI] [PubMed] [Google Scholar]
- 52.Tezuka Y, Yamazaki Y, Kitada M, Morimoto R, Kudo M, Seiji K, Takase K, Kawasaki Y, Mitsuzuka K, Ito A, et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019;73:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faulkner JL, Harwood D, Bender L, Shrestha L, Brands MW, Morwitzer MJ, Kennard S, Antonova G, Belin de Chantemèle EJ. Lack of suppression of aldosterone production leads to salt-sensitive hypertension in female but not male Balb/C mice. Hypertension. 2018;72:1397–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


