Abstract
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder with significant morbidity and mortality. Despite its prevalence, COPD is underdiagnosed, and many patients do not receive a diagnosis until the disease is clinically advanced. Recent basic science and clinical research have focused on the early physiologic and pathobiologic changes in COPD with the hopes of improving diagnosis, providing targets for disease-modifying therapy, and identifying patients most likely to benefit from early intervention. Available treatments for COPD have grown substantially in the past 20 years with the introduction of new oral and inhaled medications as well as novel surgical and bronchoscopic procedures. This article summarizes some of the recent advances in our understanding of disease pathogenesis and treatment paradigms.
Keywords: COPD, chronic bronchitis, emphysema, lung function
Introduction
Emphysema has been recognized as a pathologic entity since the seventeenth century, but the modern definition for chronic obstructive pulmonary disease (COPD) was not introduced until the middle of the twentieth century (1). Over the ensuing decades, the clinical heterogeneity and biologic complexity of COPD have become increasingly appreciated. COPD is the second most common respiratory disease in the world with an estimated 174 million people affected in 2015, an increase of 44.2% compared to 1990 (2). While asthma is more prevalent, COPD is associated with higher morbidity and mortality. A total of 3.2 million people died worldwide from COPD in 2015, an increase of 11.6% compared to 1990 (2). In the United States, COPD is currently the fourth leading cause of death (3). Due to the high prevalence and progressive nature of the disease, there has been increasing interest in the pathophysiology and diagnosis of early COPD, with the hope that better understanding and diagnosis of early disease will improve long-term outcomes.
Pathophysiology
Up until recently, our understanding of disease progression came from the work of Charles Fletcher and Richard Peto, who measured the forced expiratory volume in one second (FEV1) of 800 West London men aged 30–59 years every 6 months for 8 years starting in 1961 (4). They noted a continuous, slow decline in FEV1 that seemed to accelerate with aging. Nonsmokers lost FEV1 slowly over time and almost never developed airflow obstruction. Smokers were either “susceptible” or “nonsusceptible”: Nonsusceptible smokers had an FEV1 decline similar to that of nonsmokers, whereas susceptible smokers had more rapid FEV1 decline, which progressed to airflow obstruction and eventually disability and death. Hence, the dominant paradigm was that exposure to particulate matter—typically in the form of cigarette smoke—led to acceleration of typical age-related lung function decline among those susceptible to its effects.
This assumption was essentially unchallenged until contemporary cohort studies offered new perspectives on lifetime lung function trajectories. Lange et al. (5) used data from three observational cohorts to identify two different trajectories that can lead to COPD. Some subjects reached normal lung function in early adulthood followed by a rapid decline in FEV1, while others never reached normal lung function and developed COPD despite normal age-related decline in FEV1 during adulthood (Figure 1) (5). Furthermore, multiple birth-cohort studies have identified lung function trajectories from birth or childhood to early adulthood that may reflect the influence of potentially modifiable factors such as preterm birth, smoke exposure, recurrent pulmonary infections, and persistent asthma during childhood, which could be the focus of interventions to maximize lung growth and reduce the risk of COPD in older age (6–9). This new understanding of lung function trajectories has motivated researchers to study the early pathophysiologic changes in COPD with the ultimate goal of identifying patients with early disease who may derive greater benefit from intervention.
Figure 1.
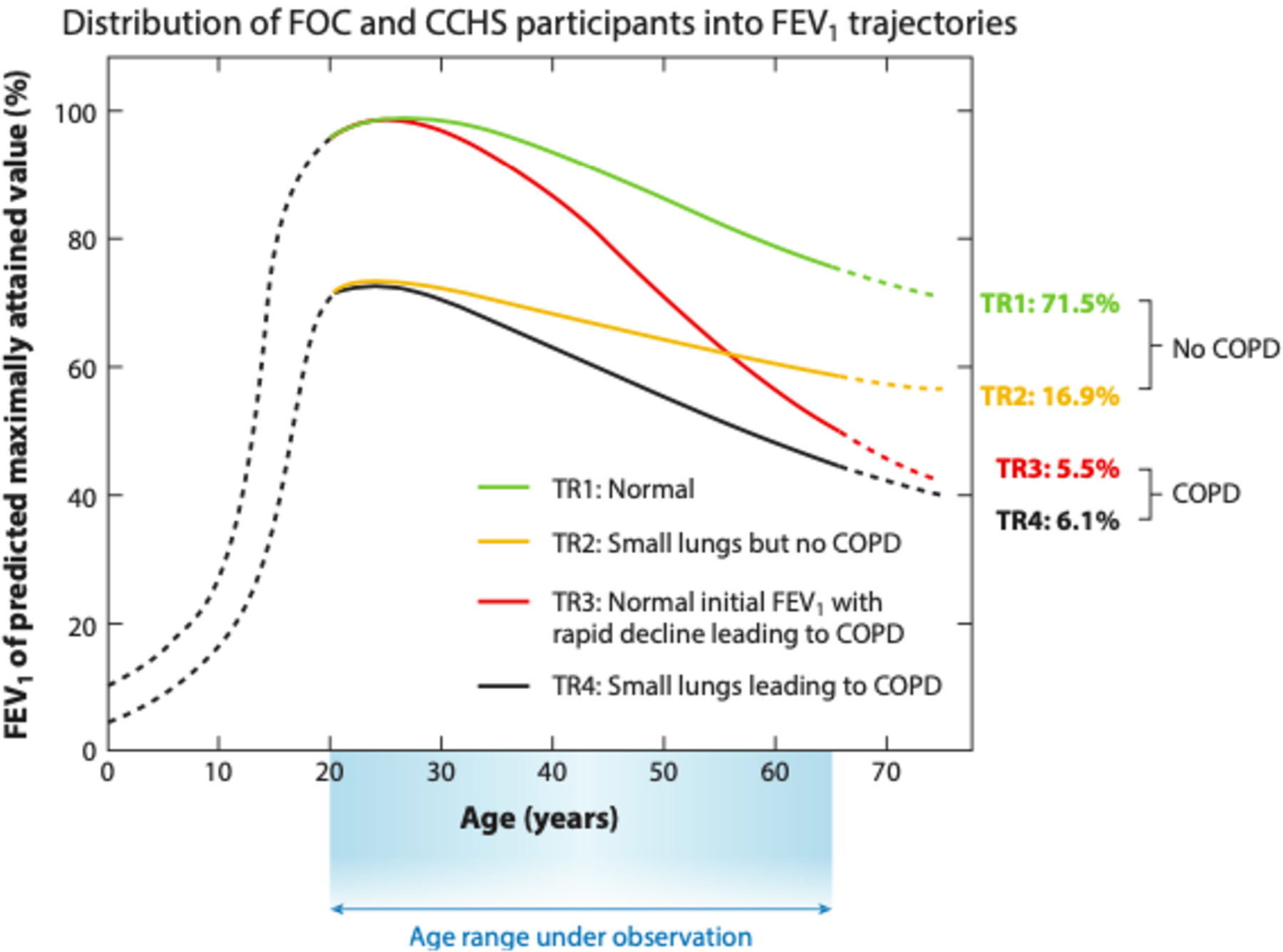
Distribution of participants in the Framingham Offspring Cohort (FOC) and the Copenhagen City Heart Study (CCHS) grouped into four lung function trajectories (TR1 to TR4) according to baseline FEV1 (below or above 80% predicted value) and presence or absence of GOLD grade ≥ 2 COPD at the final examination. Solid lines represent the natural history of FEV1 for the age range of the study while dotted lines represent hypothetical trajectories. Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Obstructive Lung Disease. Copyright © 2015 Massachusetts Medical Society. Figure adapted with permission from Massachusetts Medical Society (5).
The earliest detectable histologic change following cigarette smoke exposure is epigenetic reprogramming of basal epithelial cells, which are essential for effective pulmonary host defense and epithelial remodeling following lung injury (10, 11). Due to epigenetic reprogramming of these cells, distal airways exhibit squamous metaplasia, ciliary dysfunction, basal and goblet cell hyperplasia, and mucus hypersecretion, thereby creating a local inflammatory milieu prone to damage and infection (Figure 2a,b) (12). In fact, a gene expression analysis of respiratory epithelial samples collected from healthy nonsmokers, healthy smokers, and smokers with COPD demonstrated reprogramming of the distal airways to more closely resemble proximal airways in smokers, especially those with COPD (13). Interestingly, this distal-to-proximal repatterning may be mediated by epidermal growth factor signaling in basal cells of small airways, which could represent a new target for therapy (13).
Figure 2.
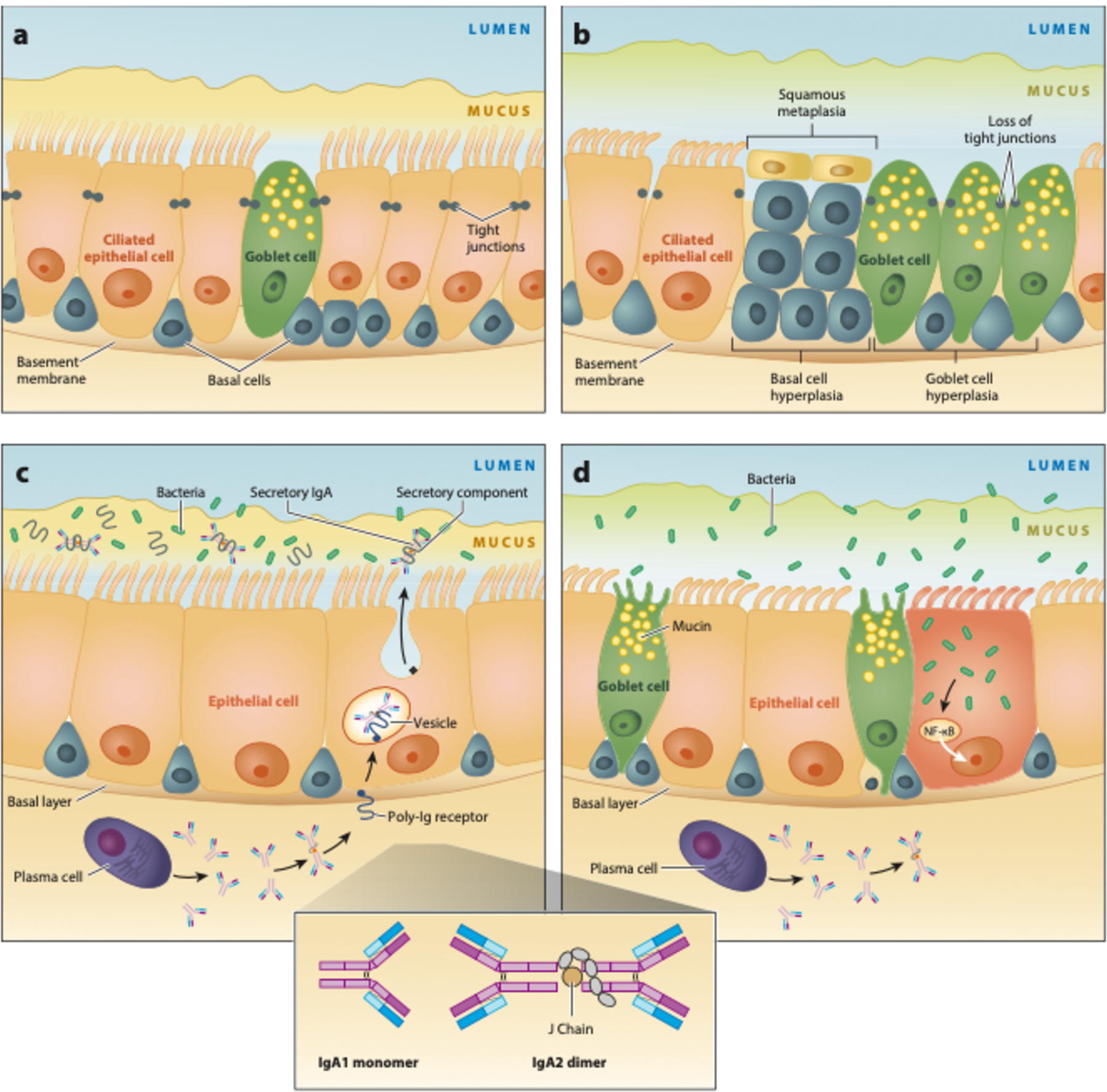
Small airway damage induced by epigenetic changes in chronic obstructive pulmonary disease (COPD). (a) Normal distal respiratory epithelium. Its self-renewing basal cells differentiate into ciliated and mucus-producing goblet cells, which are joined by tight junctions forming an impermeable barrier. Mucus is separated from the epithelial surface by an aqueous periciliary layer. (b) Smoking-related abnormalities: basal and goblet cell hyperplasia, squamous metaplasia, loss of ciliated cells, decrease in the periciliary layer with ciliary damage and crowding, and junctional barrier loss. (c) In normal small airways, dimeric IgA (structure shown in inset) is transcytosed by the polymeric Ig receptor (pIgR) into the mucosal lumen. Liberation of secretory IgA after pIgR cleavage at the luminal surface prevents bacterial invasion. (d) Expression of pIgR is reduced by smoking, which leads to a localized secretory IgA deficiency in small airways and allows bacteria to invade and induce sustained airway inflammation. Abbreviations: Ig, immunoglobulin; NF-κB, nuclear factor-κB. (Figure adapted with permission from Reference 16. Copyright © 2020 American Thoracic Society. All rights reserved. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society. Readers are encouraged to read the entire article for the correct context at https://doi.org/10.1164/rccm.201710-2028PP. The authors, editors, and The American Thoracic Society are not responsible for errors or omissions in adaptations.)
Epithelial reprogramming induced by chronic cigarette smoke exposure also changes the volume as well as water and mucin contents of the airway surface liquid. The normal structure and function of this physiologic interface prevent airway obstruction, inflammation, and infection through effective mucus clearance (14). Mucins, which are high-molecular-weight carbohydrate polymers produced by goblet cells, have been found in higher concentrations in sputum samples of ever-smokers with COPD compared to healthy controls and have been associated with the clinical phenotype of chronic bronchitis (15). Therefore, airway mucin concentration may both serve as a diagnostic biomarker and identify a potential therapeutic target.
In the healthy lung, the small airway cells secrete dimeric immunoglobulin (Ig) A into the mucosal lumen from the polymeric Ig receptor (pIgR) (16). Cleavage of pIgR at the luminal surface liberates secretory IgA (SIgA), still bound to a portion of pIgR called the secretory component. SIgA helps to prevent bacterial invasion of respiratory epithelium. Smoking reduces pIgR expression, leading to localized SIgA deficiency in small airways (Figure 2c,d). In the absence of SIgA, bacteria can invade respiratory epithelial cells. The resulting nuclear factor-κB activation initiates and sustains airway inflammation. Differences in lung microbial community structures may also help explain why not all smokers develop COPD, but which specific microbes drive disease progression and when they do so are important unanswered questions.
These findings suggest that bacterial invasion may be a trigger leading to airway remodeling, which may be a key early pathologic lesion preceding the development of emphysema. While it was originally thought that the small airways contributed very little to the total resistance within the lungs (17), further work in the late 1960s and early 1970s demonstrated that the small airways are, in fact, the major site of increased airway resistance among those with COPD and that these changes may occur in the absence of other morphologic lung disease (18). Further, histologic and computed tomography (CT) data suggest that distal airway narrowing and ultimately dropout are present in mild disease, even before overt emphysema develops (19).
Diagnosis
COPD remains both underdiagnosed and misdiagnosed (20). An analysis of the National Health and Nutrition Examination Surveys (NHANES) demonstrated that >70% of participants with chronic airway obstruction on spirometry did not have a formal diagnosis of COPD (20). In another analysis of five health plans, only 32% of patients with a new presumed diagnosis of COPD had undergone spirometry to confirm their diagnosis (21). In 2016, the US Preventive Services Task Force upheld a prior recommendation against screening asymptomatic adults with spirometry due to lack of evidence that screening improves long-term clinical outcomes (22). One caveat is that some patients with COPD may underreport their symptoms because they avoid symptom-inducing activities or attribute them to physical deconditioning or older age. Hence, as opposed to population-based screening with spirometry, a variety of COPD case-finding approaches have been proposed (23). One of them combines a five-item questionnaire called CAPTURE (COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk) with peak expiratory flow (24). This pragmatic and low-cost approach, which is currently being evaluated in a primary care population (NCT03583099, NCT03653611, NCT03581227), was both sensitive and specific for identifying patients at risk for COPD who could then be further evaluated with spirometry.
The definition of COPD requires postbronchodilator spirometry demonstrating fixed airflow obstruction defined as a FEV1/forced vital capacity (FVC) ratio <0.70, although there is some debate as to whether this is the best way to define obstruction. Using a fixed FEV1/FVC cutoff to define airflow obstruction has been recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (25). The American Thoracic Society and European Respiratory Society recommend using the lower limit of normal (LLN) for FEV1 /FVC, which is derived from age, race, sex, and height based on population-level data (26). While the fixed cutoff is easier to apply, it may result in more false negatives among younger patients who may benefit from early intervention, and more false positives among older patients who may be unnecessarily treated. A prospective cohort study of 95,288 participants in the Copenhagen General Population Study showed that individuals with FEV1/FVC < LLN but ≥0.70 had a median age of 45 years and were at an increased risk of pneumonia, heart failure and all-cause mortality compared to those without airflow obstruction by either definition over a median follow-up of 6 years (27). In a combined analysis of four US population-based cohorts (mean age 62.8 years) investigating different fixed FEV1/FVC thresholds as well as the LLN, Bhatt et al. (28) found that a FEV1/FVC cutoff of 0.71 was best at discriminating COPD-related hospitalizations and mortality, lending support to use of the 0.70 fixed threshold for the identification of individuals at risk for clinically significant COPD. Based on these studies, the optimal FEV1/FVC cutoff (fixed versus LLN) to define airflow obstruction in clinical practice remains a topic of debate but is clearly influenced by age. Additional research is needed to further inform this area of investigation.
The 2001 GOLD report designated patients with FEV1/FVC > 0.70 but with chronic respiratory symptoms (chronic cough, sputum production, and mucus hypersecretion) as GOLD 0 because they were felt to be at risk for future development of airflow obstruction (29). GOLD 0 remained a controversial classification and was eventually removed from following reports as these patients do not represent the majority of those who develop obstruction (30, 31). Despite the removal of GOLD 0, smokers without airflow obstruction but with chronic respiratory symptoms were found to experience significant respiratory morbidity (32, 33). A subset of these smokers with FEV1/FVC ≥ 0.7 but FEV1 <80% predicted, referred to as preserved ratio impaired spirometry (PRISm), has become a focus of research in a number of observational cohorts. A recent longitudinal analysis of participants in the Rotterdam Study showed that a third of participants with PRISm transitioned to COPD over 4.5 years of follow-up and that the presence of PRISm and COPD GOLD 2–4 were both significant predictors of all-cause mortality (34). Further research is needed to fully understand the demographic, clinical, and genetic factors associated with the development of COPD among individuals with PRISm and whether specific therapeutic strategies can delay or prevent COPD in this group.
There has been increasing appreciation of the importance of respiratory symptoms, respiratory exacerbations, and abnormal chest CT findings to better characterize and understand smoking-related lung disease in the absence of airflow obstruction. Among ever-smokers with FEV1 /FVC > 0.70 in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), those with a high symptom burden [defined as a COPD Assessment Test (CAT) score ≥10] were more likely to experience respiratory exacerbations, have lower exercise tolerance, and show evidence of airway thickening on chest CT when compared to those with a low symptom burden (CAT < 10) (35). Interestingly, 42% of the symptomatic group was already on inhaled bronchodilators and 23% on inhaled corticosteroids despite no indication for such prescriptions given the lack of airflow obstruction. A National Institutes of Health–sponsored study evaluating the efficacy of inhaled bronchodilators in a similar population is under way (NCT02867761).
There is mounting evidence that changes on CT can identify patients with airflow obstruction among current and former smokers (36). There is also evidence that a large number of ever-smokers with FEV1/FVC ≥ 0.70 have radiologic evidence of lung disease (significant emphysema or airway wall thickening), as well as more dyspnea, lower exercise tolerance, and worse respiratory health scores when compared to never-smokers (37). Taken together, these results suggest that current guidelines that require airflow obstruction for the diagnosis of COPD may not adequately describe the spectrum of symptomatic smoking-related lung disease. Future guidelines may need to incorporate a broader definition, including the presence of changes on chest CT, to help guide clinicians.
A promising method using CT to identify those at greatest risk for disease progression comes from an analytic technique designed to identify small airway abnormalities. Parametric response mapping (PRM) is an image processing technique that uses dynamic image registration of paired inspiratory and expiratory CTs to classify areas of the lung as normal, emphysematous, or nonemphysematous air trapping [referred to as functional small airways disease (fSAD)] (38). In ever-smokers with no or mild-to-moderate airflow obstruction, PRM fSAD was more strongly associated than PRM emphysema with FEV1 decline over 5 years of follow-up (39). Importantly, the PRM metrics have been validated with human lung tissue removed from patients with advanced COPD at the time of lung transplantation to show that PRM fSAD correlates with loss of terminal bronchioles as well as narrowing, thickening, and obstruction of the surviving terminal bronchioles (40). These results support the hypothesis that the loss of small airways occurs prior to detectable emphysema or a significant decline in lung function and that these changes can be identified using clinical chest CTs with appropriate image processing techniques. As such, PRM fSAD may be a good biomarker to study and identify early COPD.
Serum biomarkers are another area of ongoing research in order to find novel biomarkers that are modifiable, have an independent association with hard clinical outcomes, and result in significant clinical changes (41). The inflammatory milieu seen in the pathogenesis of COPD has been a focus of research with many different inflammatory markers identified as potential targets (42). C-reactive protein (CRP) has been identified as a strong predictor of COPD hospitalizations and mortality independent of lung function and has been shown to help guide antibiotic use in outpatient COPD exacerbations (43, 44). High fibrinogen levels are similarly associated with an increased risk of severe COPD exacerbations resulting in hospitalization and mortality (45). Blood eosinophils have a relationship to exacerbations as well as response to inhaled corticosteroid (ICS) (46, 47). sRAGE (soluble receptor for advanced glycation end product) is another promising biomarker which seems to play an important mechanistic role in the pathogenesis of emphysema (48). Current research is ongoing with multiple, large, prospective cohort studies hoping to identify clinically relevant markers.
When thinking about the concept of early or pre-COPD, one must consider the importance of age. So-called mild disease in an 80-year-old smoker might present different risk than the same amount of disease in a 40-year-old smoker. Recognizing this difference, a definition for early COPD that incorporates age has been proposed (16). It requires that patients be <50 years old and have a smoking history ≥10 pack-years. They also need one or more of the following: (a) evidence of airflow obstruction, which is defined as FEV1/FVC < LLN in order to capture more patients than the more strict FEV1/FVC <0.70; (b) CT findings compatible with COPD (visual emphysema, air trapping, or bronchial thickening); or (c) rapid FEV1 decline (≥60 ml/yr) (16). A recent analysis of the Copenhagen General Population Study found the prevalence of early COPD (FEV1 /FVC < LLN, age <50 years with ≥10 pack-year smoking history) to be 15% (49). Subjects with early COPD had more respiratory symptoms, more respiratory-related hospitalizations, and higher all-cause mortality in the 14.4 years of follow-up than did those without COPD.
These data highlight the importance of considering multiple facets of lung disease, including symptoms, spirometry, and CT. In an attempt to incorporate this ongoing research into the definition of COPD, the Genetic Epidemiology of COPD (COPDGene) investigators have recently proposed new diagnostic criteria using a combination of exposure history, spirometry, symptoms, and CT imaging to classify patients as No COPD, Possible COPD, Probable COPD, and Definite COPD, with increasing numbers of features present leading to increased confidence in the diagnosis (50). Greater confidence in the diagnosis was associated with higher odds ratios for decline in FEV1 > 350 ml over 5 years as well as higher hazard ratios for all-cause mortality. It is un-clear how pragmatic this definition would be in primary care, rural, or underserved settings where resources such as spirometry or chest CT may not be consistently available. As COPDGene participants had a significantly higher smoking exposure and prevalence of airflow obstruction than the general population, future population-based studies are needed to further assess the broader impact of this proposed definition. Additionally, it may be that certain aspects of symptoms or specific CT abnormalities have more predictive value than others, which is not accounted for in this construct.
Treatment
Pharmacotherapy
Based on GOLD recommendations, the initial treatment for most symptomatic patients with COPD typically includes a bronchodilator, such as a long-acting muscarinic antagonist (LAMA). Understanding Potential Long-Term Impacts on Function with Tiotropium (UPLIFT) was a double-blind trial comparing tiotropium to placebo in nearly 6,000 participants with COPD and a ≥10-pack-year smoking history (51). While tiotropium did not slow FEV1 decline, it did result in improved health-related quality of life and fewer COPD exacerbations after 4 years of follow-up (51). Of note, the majority of participants enrolled in this trial had moderate-to-severe COPD. A separate, smaller study of tiotropium versus placebo in participants with mild or moderate COPD (n = 841) found that those treated with tiotropium had a significantly higher postbronchodilator FEV1 at 24 months, reduced annual FEV1 decline [between-group difference: 157 ml (95% CI 123–192 ml, p <0.001)] and a lower exacerbation rate (52). While tiotropium did not improve FEV1 decline among those with severe disease, it may have a lung function benefit in early or mild disease. Regardless of disease severity, the utility of the LAMA class of inhalers to improve symptoms, increase exercise capacity, and reduce exacerbation frequency is well established in patients with COPD, supporting the use of LAMAs as first-line therapy in patients with symptomatic COPD (25). For particularly symptomatic patients (e.g., CAT ≥ 20), dual bronchodilator therapy with a LAMA and long-acting β2 agonist (LABA) has been demonstrated to provide more symptomatic relief than a single bronchodilator alone (53).
COPD exacerbations are characterized by acute worsening of shortness of breath, cough, or mucus production beyond baseline day-to-day variation. They are detrimental events in the natural history of the disease as they accelerate lung function decline, increase mortality, decrease health status, and increase cardiac events (54). Prevention of exacerbations is essential in the management of COPD, as demonstrated by the GOLD ABCD assessment tool, which incorporates exacerbation history as well as symptom burden to help guide pharmacologic management (25).
Multiple clinical trials have been conducted in recent years to assess the efficacy of various inhaler therapies to reduce COPD exacerbation rates. The Effect of Indacaterol–Glycopyrronium versus Fluticasone–Salmeterol on COPD Exacerbations (FLAME) trial showed that a LAMA/LABA combination (indacaterol–glycopyrronium) was more effective than a LABA/ICS combination (salmeterol–fluticasone) at preventing exacerbations (rate ratio 0.89; 95% CI 0.83–0.96; p = 0.003) (55). The Informing the Pathway of COPD Treatment (IMPACT) trial compared the efficacy of the combined triple inhaler therapy of umeclidinium (a LAMA), vilanterol (a LABA), and fluticasone furoate (an ICS) to either umeclidinium–vilanterol or fluticasone furoate–vilanterol at preventing COPD exacerbations in patients with a history of frequent exacerbations and at least moderate obstruction on spirometry (56). The three-inhaler regimen reduced the rate of moderate or severe exacerbations (defined as exacerbations requiring systemic corticosteroids, antibiotics, or hospital admission) compared to either of the dual therapies. In contrast to FLAME, IMPACT showed greater exacerbation reduction with LABA/ICS than with LAMA/LABA. To reconcile these discrepant data, it should be noted that FLAME required a 4-week run-in of tiotropium monotherapy and may have been enriched for patients who were stable without ICS. Currently, for the GOLD D patient population (high symptom burden and increased risk of exacerbations), LAMA, LAMA/LABA, and LABA/ICS are all potential options for initial therapy, with triple therapy reserved for those with persistent exacerbations despite initial therapy (25). Triple therapy also reduced all-cause mortality as compared to the LAMA/LABA combination therapy (57).
There was previously little evidence to help guide clinicians in de-escalation from ICS in patients without frequent exacerbations who may not have as clear a benefit. The Study to Understand the Safety and Efficacy of ICS Withdrawal from Triple Therapy in COPD (SUNSET) enrolled stable COPD patients (FEV1 40–80% predicted) on long-term triple therapy with ≤1 moderate or severe exacerbation in the previous year (58). Participants experienced a significant decline in FEV1 (26 ml) after de-escalation from triple therapy to LAMA/LABA, although no change in exacerbation rate was observed. Notably, patients with high baseline blood eosinophil levels (≥300 cells/μl) had a greater decline in lung function and experienced more exacerbations after withdrawal of ICS (58). A similar association between ICS use, exacerbation rate, and eosinophil count was also seen in a post hoc analysis of the Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD (WISDOM) trial, which found increased exacerbations after ICS withdrawal among participants with higher blood eosinophil counts at screening (59). Further, a secondary analysis of IMPACT modeled eosinophil count as a continuous variable and showed no difference in exacerbation reduction between the LAMA/LABA/ICS and LAMA/LABA arms at eosinophil counts <100 cells/μl but progressively greater treatment effects in the ICS-containing regimens with higher eosinophil levels (47). These findings with regard to eosinophils have been incorporated into the 2019 GOLD guidelines, which integrate serum eosinophil level, dyspnea, and exacerbation history to guide treatment escalation and de-escalation, highlighting one of the ways inhaler management is moving toward a more precision-based approach (25).
Given this association between eosinophilia and COPD exacerbations, trials have evaluated the interleukin-5 monoclonal antibodies mepolizumab and benralizumab, which are currently approved by the US Food and Drug Administration for eosinophilic asthma (61). Results have been mixed, with only one study clearly showing reduced exacerbation rates (62, 63). Further studies to evaluate the efficacy of monoclonal antibodies for the prevention of COPD exacerbations are ongoing (NCT04133909, NCT03930732).
Oral medications have become commonly used among patients who experience frequent exacerbations despite maximal inhaler therapy and in those with eosinophil counts <100 cells/μl who are less likely to benefit from ICS. Azithromycin has been shown to reduce exacerbations when given prophylactically to patients with COPD and an increased risk of exacerbation (64). Subsequent research showed little treatment effect among active smokers, supporting its use in former smokers only (65). Known side effects of azithromycin include QTc prolongation and hearing loss, both of which should be monitored regularly in patients on the medication. Antibiotic resistance is another potential concern. Roflumilast, a phosphodiesterase-4 inhibitor, has been shown to reduce exacerbations and is indicated for patients with recurrent exacerbations, FEV1 < 50% predicted, and the chronic bronchitis phenotype (66).
Loss of muscle mass has been associated with mortality and risk of exacerbation among patients with COPD (67–69). In a recent placebo-controlled trial enrolling sarcopenic COPD patients, bimagrumab, a monoclonal antibody that blocks activin type II receptors, produced an increase in thigh muscle volume and total lean body mass up to week 24 but did not improve physical function (70). It remains unclear whether such an increase in muscle mass could translate into decreased respiratory morbidity and mortality.
Pulmonary Rehabilitation
Pulmonary rehabilitation (PR) is a comprehensive longitudinal intervention that integrates aerobic exercise, muscle strength training, and education programs to improve the physical and psychological well-being of patients with chronic respiratory disease (71). PR has been shown to increase functional capacity, improve health-related quality of life, and reduce hospitalizations (71). Despite its substantial benefits, PR remains underutilized. Likely reasons include lack of awareness among providers, payers, and patients, as well as lack of access (71, 72). A recent large observational cohort study of Medicare beneficiaries discharged after hospitalization for COPD showed that initiation of PR within 90 days of hospital discharge was associated with lower all-cause mortality at 1 year as compared to later initiation or no initiation (73). While less than 2% of the nearly 200,000 patients included in the analysis initiated PR within 90 days of discharge, these findings should encourage increased utilization of and funding for PR (74).
Lung Volume Reduction
Lung volume reduction surgery is one of the few surgical treatment options for patients with COPD. Its underlying premise is that removal of diseased, emphysematous lung allows for re-expansion of adjacent comparatively healthy lung. Lung volume reduction has been shown to confer a mortality benefit among patients with upper-lobe predominant emphysema and low exercise tolerance despite completion of pulmonary rehabilitation (75, 76). Although it is one of the few therapies with a demonstrated survival benefit in COPD, it remains infrequently utilized, likely owing to accreditation restrictions, misconceptions among physicians of the benefits and risks of the procedure, and its stringent eligibility criteria (77, 78).
Endobronchial valves (EBVs) have become a possible alternative for patients who do not qualify for lung volume reduction surgery. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE) and Improving Lung Function in Severe Heterogenous Emphysema with the Spiration Valve System (EMPROVE) were recent multicenter randomized controlled trials evaluating two different types of EBVs compared to optimal medical therapy (79, 80). Inclusion criteria included severe obstruction on spirometry, evidence of significant hyperinflation on lung volume measurements, a heterogenous distribution of emphysema as determined by quantitative CT analysis, and little to no collateral ventilation between the targeted and ipsilateral lobes. Both trials reported an improvement in FEV1 and residual volume in their respective EBV arms. Improvements in respiratory health status as well as six-minute walk distance were also noted at 6 and 12 months. These studies have led to US Food and Drug Administration approval for use of these devices in patients with COPD. There is a high risk of pneumothorax associated with valve placement, although postprocedure pneumothorax did not lead to differences in clinical outcomes at 6 and 12 months. Evaluation of outcomes beyond 12 months is currently ongoing (NCT04302272).
Targeted Lung Devervation and Bronchial Rheoplasty
Targeted lung denervation (TLD) uses bronchoscopic radiofrequency ablation to disrupt the pulmonary parasympathetic nervous system, which mediates smooth muscle tone, reflex bronchoconstriction, mucus hypersecretion, and airway inflammation (81). While a small randomized sham-controlled double-blind trial of TLD showed a reduction in respiratory adverse events in patients with symptomatic moderate-to-severe COPD (81), a larger randomized trial evaluating TLD’s ability to reduce COPD exacerbations is currently recruiting (NCT03639051). Bronchial rheoplasty is another bronchoscopic procedure, which delivers nonthermal pulsed electric fields to the airways in order to ablate mucus-producing cells in patients with chronic bronchitis, which has been shown to reduce respiratory symptoms as well as goblet cell hyperplasia in small, un-controlled studies (82, 83). Research is ongoing to determine if these results are reproducible in larger randomized controlled trials.
Lung Transplantation
More than 1,000 lung transplants are performed every year for patients with severe COPD (84). It is the most common indication for lung transplant worldwide and the second most common indication in the United States (84). The most recent guidelines from the International Society of Heart and Lung Transplantation acknowledge that timing of referral for transplant in COPD is challenging because the clinical course is typically protracted, and short- and intermediate-term survival in COPD is higher than in other lung diseases (85). Patients with COPD and no contraindications to transplant who have a BODE (body mass index, airflow obstruction, dyspnea severity, exertional capacity) index of 5–6 with progressive disease, FEV1 < 25% predicted, and significant hypoxia or hypercapnia should be referred to a transplant center for evaluation (85).
Oxygen Therapy
Supplemental oxygen for severe hypoxemia was one of the first interventions to show a mortality benefit in COPD. The Nocturnal Oxygen Therapy Trial (NOTT), published in 1980, showed that supplemental oxygen therapy improved mortality in those with severe resting hypoxemia defined as either a partial pressure of oxygen (PaO2) ≤ 55 mmHg or oxygen saturation (SpO2) ≤ 88%, or either PaO2 55–60 mmHg or SpO2 88% in patients with pulmonary hypertension, heart failure, or hematocrit >55% (86). Benefits in less severe hypoxemia were not known until the Long-Term Oxygen Treatment Trial (LOTT), which was published in 2016. LOTT evaluated the use of oxygen in moderate resting hypoxemia (SpO2 89–93%) or exercise-induced desaturations (SpO2 < 90% for ≥10 s and ≥ 80% for ≥5 min) and found supplemental oxygen had no effect on any of the measured outcomes, which included mortality and time to first hospitalization (87).
Chronic Noninvasive Ventilation
Noninvasive ventilation (NIV) has been shown to improve mortality among patients with COPD exacerbations and acute hypercapnic respiratory failure. However, the role of routine outpatient NIV for stable patients with COPD and chronic hypercapnia has yet to be fully characterized (88). One recent trial randomized participants with persistent, compensated hypercapnia (day-time PaCO2 > 53 mm Hg, pH > 7.30) and need for long-term oxygen within 2–4 weeks of an admission for acute hypercapnic respiratory failure to receive home NIV and home oxygen or home oxygen alone (89). While the trial was small (n = 116), participants randomized to NIV had a lower incidence of a composite outcome of readmission or death. A similar study that compared NIV with stable hypercapnia and severe COPD (n = 195) showed improved survival in the NIV group (90). Two other studies have failed to show consistent improvement in outcomes among hypercapnic COPD patients randomized to NIV, although it should be noted that neither resulted in statistically significant reductions in PaCO2 among the NIV arms (91, 92). A recent review of NIV in COPD identified adherence as a significant issue and noted that quality of life and survival seem to improve only when chronically elevated CO2 is effectively reduced (88). This remains an area for future research.
Conclusion
COPD is a common respiratory disease associated with significant clinical heterogeneity and high morbidity and mortality. New treatment options, ranging from inhaled and oral pharmacologic treatments to surgical and bronchoscopic interventional treatments, have opened the door to more personalized management approaches for COPD patients in clinical practice. Continued advances in our understanding of pathobiology, diagnosis, and treatment focused on susceptible patients early in the disease course have the potential to improve long-term clinical outcomes and help identify disease-modifying therapies.
Acknowledgements
M.K.H. is supported by NIH HL138188.
Disclosure Statement
W.W.L. reports nonfinancial support from Pulmonx and personal fees from Konica Minolta. M.K.H. reports personal fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca, Verona, Merck, and Mylan. She also reports research support from Novartis and Sunovion. M.C.F. has nothing to disclose.
Literature Cited
- 1.Fishman AP. 2005. One hundred years of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 171 (9): 941–48 [DOI] [PubMed] [Google Scholar]
- 2.Soriano JB, Abajobir AA, Abate KH, et al. 2017. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med 5(9):691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochanek KD, Murphy SL, Xu J, Arias E. 2017. Mortality in the United States, 2016. NCHS Data Brief 293, National Center for Health Statistics, Hyattsville, MD: [PubMed] [Google Scholar]
- 4.Fletcher C, Peto R. 1977. The natural history of chronic airflow obstruction. Br. Med. J 1 (6077): 1645–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange P, Celli B, Agustí A, et al. 2015. Lung-function trajectories leading to chronic obstructive pulmonary disease. N. Engl. J. Med 373(2):111–22 [DOI] [PubMed] [Google Scholar]
- 6.DenDekker HT, Jaddoe VWV, Reiss IK, et al. 2018. Fetal and infant growth patterns and risk of lower lung function and asthma. Am. J. Respir. Crit. Care Med 197(2):183–92 [DOI] [PubMed] [Google Scholar]
- 7.Belgrave DCM, Granell R, Turner SW, et al. 2018. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med 6(7):526–34 [DOI] [PubMed] [Google Scholar]
- 8.Casas M, DenDekker HT, Kruithof CJ, et al. 2018. The effect of early growth patterns and lung function on the development of childhood asthma: a population based study. Thorax 73(12):1137–45 [DOI] [PubMed] [Google Scholar]
- 9.Bui DS, Lodge CJ, Burgess JA, et al. 2018. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir. Med 6(7):535–44 [DOI] [PubMed] [Google Scholar]
- 10.Heijink IH, Noordhoek JA, Timens W, et al. 2014. Abnormalities in airway epithelial junction formation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 189(11):1439–42 [DOI] [PubMed] [Google Scholar]
- 11.Staudt MR, Buro-Auriemma LJ, Walters MS, et al. 2014. Airway basal stem/progenitor cell shave diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 190(8):955–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgel PR, Bourdin A, Chanez P, et al. 2011. Update on the roles of distal airways in COPD. Eur. Respir. Rev 20(119):7–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Zuo WL, Fukui T, et al. 2017. Smoking-dependent distal-to-proximal repatterning of the adult human small airway epithelium. Am. J. Respir. Crit. Care Med 196(3):340–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boucher RC. 2019. Muco-obstructive lung diseases. N. Engl. J. Med 380 (20): 1941–53 [DOI] [PubMed] [Google Scholar]
- 15.Kesimer M, Ford AA, Ceppe A, et al. 2017. Airway mucin concentration as a marker of chronic bronchitis. N. Engl. J. Med 377(10):911–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez FJ, Han MK, Allinson JP, et al. 2018. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 197(12):1540–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogg JC, Paré PD, Hackett TL. 2017. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol. Rev 97(2):529–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogg JC, Macklem PT, Thurlbeck WM. 1968. Site and nature of airway obstruction in chronic obstructive lung disease. N. Engl. J. Med 278(25):1355–60 [DOI] [PubMed] [Google Scholar]
- 19.McDonough JE, Yuan R, Suzuki M, et al. 2011. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N. Engl. J. Med 365(17):1567–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez CH, Mannino DM, Jaimes FA, et al. 2015.Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann. Am. Thorac. Soc 12(12):1788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han MLK, Min GK, Mardon R, et al. 2007. Spirometry utilization for COPD: How do we measure up? Chest 132(2):403–9 [DOI] [PubMed] [Google Scholar]
- 22.Siu AL, Bibbins-Domingo K, Grossman DC, et al. 2016. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA 315(13):1372–77 [DOI] [PubMed] [Google Scholar]
- 23.Labaki WW, Han MLK. 2018. Improving detection of early chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc 15(Suppl. 4): S243–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FJ, Mannino D, Leidy NK, et al. 2017. A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 195(6):748–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh D, Agusti A, Anzueto A, et al. 2019. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J 53(5):1900164. [DOI] [PubMed] [Google Scholar]
- 26.Pellegrino R, Viegi G, Brusasco V, et al. 2005. Interpretative strategies for lung function tests. Eur. Respir. J 26(5): 948–68 [DOI] [PubMed] [Google Scholar]
- 27.Çolak Y, Afzal S, Nordestgaard BG, et al. 2018. Young and middle-aged adults with airflow limitation according to lower limit of normal but not fixed ratio have high morbidity and poor survival: a population-based prospective cohort study. Eur. Respir. J 51(3):1702681. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt SP, Balte PP, Schwartz JE, et al. 2019. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA 321(24):2438–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauwels RA, Buist AS, Calverley PMA, et al. 2001. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 163(5):1256–76 [DOI] [PubMed] [Google Scholar]
- 30.Rabe KF, Hurd S, Anzueto A, et al. 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med 176(6):532–55 [DOI] [PubMed] [Google Scholar]
- 31.Mannino DM. 2006. GOLD stage 0 COPD: Is it real? Does it matter? Chest 130(2): 309–10 [DOI] [PubMed] [Google Scholar]
- 32.Tan WC, Bourbeau J, Hernandez P, et al. 2014. Exacerbation-like respiratory symptoms in individuals without chronic obstructive pulmonary disease: results from a population-based study. Thorax 69(8):709–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez CH, Kim V,Chen Y,et al. 2014. The clinical impact of non-obstructive chronic bronchitis in current and former smokers. Respir. Med 108(3):491–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijnant SRA, de Roos E, Kavousi M, et al. 2020. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur. Respir. J 55(1):1901217. [DOI] [PubMed] [Google Scholar]
- 35.Woodruff PG, Barr RG, Bleecker E, et al. 2016. Clinical significance of symptoms in smokers with preserved pulmonary function. N. Engl. J. Med 374(19):1811–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mets OM, Buckens CFM, Zanen P, et al. 2011. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA 306(16):1775–81 [DOI] [PubMed] [Google Scholar]
- 37.Regan EA, Lynch DA, Curran-Everett D, et al. 2015. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern. Med 175(9):1539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galbán CJ, Han MK, Boes JL, et al. 2012. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med 18(11):1711–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatt SP, Soler X, Wang X, et al. 2016. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 194(2):178–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasilescu DM, Martinez FJ, Marchetti N, et al. 2019. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 200(5):575–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sin DD, Vestbo J. 2009. Biomarkers in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc 6(6):543–45 [DOI] [PubMed] [Google Scholar]
- 42.Agustí A, Edwards LD, Rennard SI, et al. 2012. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLOS ONE 7(5):e37483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahl M, Vestbo J, Lange P, et al. 2007. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 175(3):250–55 [DOI] [PubMed] [Google Scholar]
- 44.Butler CC, Gillespie D, White P, et al. 2019. C-reactive protein testing to guide antibiotic prescribing for COPD exacerbations. N. Engl. J. Med 381(2):111–20 [DOI] [PubMed] [Google Scholar]
- 45.Mannino D, Tal-Singer R, Lomas D, et al. 2014. Plasma fibrinogen as a biomarker for mortality and hospitalized exacerbations in people with COPD. Chronic Obstr. Pulm. Dis 2(1):23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vedel-Krogh S, Nielsen SF, Lange P, et al. 2016. Blood eosinophils and exacerbations in chronic obstructive pulmonary disease: the Copenhagen General Population Study. Am. J. Respir. Crit. Care Med 193(9):965–74 [DOI] [PubMed] [Google Scholar]
- 47.Pascoe S, Barnes N, Brusselle G, et al. 2019. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir. Med 7(9):745–56 [DOI] [PubMed] [Google Scholar]
- 48.Yonchuk JG, Silverman EK, Bowler RP, et al. 2015. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am. J. Respir. Crit. Care Med 192(7):785–92 [DOI] [PubMed] [Google Scholar]
- 49.Çolak Y, Afzal S, Nordestgaard BG, et al. 2020. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease the Copenhagen general population study. Am. J. Respir. Crit. Care Med 201(6):671–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowe KE, Regan EA, Anzueto A, et al. 2019. COPDGene® 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr. Pulm. Dis 6(5):384–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tashkin DP, Celli B, Senn S, et al. 2008. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med 359(15):1543–54 [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Zhong N, Li X, et al. 2017. Tiotropium in early-stage chronic obstructive pulmonary disease. N. Engl. J. Med 377(10):923–35 [DOI] [PubMed] [Google Scholar]
- 53.Martinez FJ, Fabbri LM, Ferguson GT, et al. 2017. Baseline symptom score impact on benefits of glycopyrrolate/formoterol metered dose inhaler in COPD. Chest 152(6):1169–78 [DOI] [PubMed] [Google Scholar]
- 54.Halpin DM, Miravitlles M, Metzdorf N, et al. 2017. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int. J. COPD 12:2891–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wedzicha JA, Banerji D, Chapman KR, et al. 2016. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N. Engl. J. Med 374(23):2222–34 [DOI] [PubMed] [Google Scholar]
- 56.Lipson DA, Barnhart F, Brealey N, et al. 2018. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N. Engl. J. Med 378(18):1671–80 [DOI] [PubMed] [Google Scholar]
- 57.Lipson DA, Crim C, Criner GJ, et al. 2020. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in COPD patients. Am. J. Respir. Crit. Care Med 201(12):1508–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman KR, Hurst JR, Frent SM, et al. 2018. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am. J. Respir. Crit. Care Med 198(3):329–39 [DOI] [PubMed] [Google Scholar]
- 59.Watz H, Tetzlaff K, Wouters EFM, et al. 2016. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir. Med 4(5):390–98 [DOI] [PubMed] [Google Scholar]
- 61.Singh D, Bafadhel M, Brightling CE, et al. 2020. Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 202(5):660–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavord ID, Chanez P, Criner GJ, et al. 2017. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N. Engl. J. Med 377(17):1613–29 [DOI] [PubMed] [Google Scholar]
- 63.Criner GJ, Celli BR, Brightling CE, et al. 2019. Benralizumab for the prevention of COPD exacerbations. N. Engl. J. Med 381(11):1023–34 [DOI] [PubMed] [Google Scholar]
- 64.Albert RK, Connett J, Bailey WC, et al. 2011. Azithromycin for prevention of exacerbations of COPD. N. Engl. J. Med 365(8):689–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han MLK, Tayob N, Murray S, et al. 2014. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am. J. Respir. Crit. Care Med 189(12):1503–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calverley PM, Rabe KF, Goehring UM, et al. 2009. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374(9691):685–94 [DOI] [PubMed] [Google Scholar]
- 67.Swallow EB, Reyes D, Hopkinson NS, et al. 2007. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 62(2):115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marquis K, Debigaré R, Lacasse Y, et al. 2002. Mid thigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 166(6):809–13 [DOI] [PubMed] [Google Scholar]
- 69.Greening NJ, Harvey-Dunstan TC, Chaplin EJ, et al. 2015. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am. J. Respir. Crit. Care Med 192(7):810–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polkey MI, Praestgaard J, Berwick A, et al. 2019. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease: a randomized trial. Am. J. Respir. Crit. Care Med 199(3):313–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rochester CL, Vogiatzis I, Holland AE, et al. 2015. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med 192(11):1373–86 [DOI] [PubMed] [Google Scholar]
- 72.Nishi SPE, Zhang W, Kuo YF, et al. 2016. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2003 to 2012. J. Cardiopulm. Rehabil. Prev 36(5):375–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindenauer PK, Stefan MS, Pekow PS, et al. 2020. Association between initiation of pulmonary rehabilitation after hospitalization for COPD and 1-year survival among medicare beneficiaries. JAMA. 323(18):1813–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rochester CL, Holland AE. 2020. Pulmonary rehabilitation and improved survival for patients with COPD. JAMA. 323(18):1783–85 [DOI] [PubMed] [Google Scholar]
- 75.Fishman A, Martinez F, Naunheim K, et al. 2003. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N. Engl. J. Med 348(21):2059–73 [DOI] [PubMed] [Google Scholar]
- 76.Van Agteren JEM, Carson KV, Tiong LU, et al. 2016. Lung volume reduction surgery for diffuse emphysema. Cochrane Database Syst. Rev 2016(10):CD001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Decker MR, Leverson GE, Jaoude WA, et al. 2014. Lung volume reduction surgery since the National Emphysema Treatment Trial: Study of Society of Thoracic Surgeons Database. J. Thorac. Cardiovasc. Surg 148(6):2651–2658.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Criner GJ, Cordova F, Sternberg AL, et al. 2011. The National Emphysema Treatment Trial (NETT). Part II: Lessons learned about lung volume reduction surgery. Am. J. Respir. Crit. Care Med 184(8):881–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Criner GJ, Sue R, Wright S, et al. 2018. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am. J. Respir. Crit. Care Med 198(9):1151–64 [DOI] [PubMed] [Google Scholar]
- 80.Criner GJ, Delage A, Voelker K, et al. 2019. Improving lung function in severe heterogenous emphysema with the spiration valve system (EMPROVE). A multicenter, open-label randomized controlled clinical trial. Am. J. Respir. Crit. Care Med 200(11):1354–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Slebos DJ, Shah PL, Herth FJF, et al. 2019. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW). A multicenter randomized controlled clinical trial. Am. J. Respir. Crit. Care Med 200(12):1477–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valipour A, Ing A, Williamson JP, et al. 2019. First-in-human results of bronchial rheoplasty: an endobronchial treatment for chronic bronchitis (CB). Am. J. Respir. Crit. Care Med 199:A7037 (Abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valipour A, Ing A, Williamson J, et al. 2018. Late breaking abstract—first-in-human results of bronchial rheoplasty: an endobronchial treatment for chronic bronchitis (CB). Eur. Respir. J 52:OA2162 (Abstr.) [Google Scholar]
- 84.Siddiqui FM, Diamond JM. 2018. Lung transplantation for chronic obstructive pulmonary disease: past, present, and future directions. Curr. Opin. Pulm. Med 24(2):199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weill D, Benden C, Corris PA, et al. 2015. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant 34(1):1–15 [DOI] [PubMed] [Google Scholar]
- 86.Kvale PA, Conway WA, Coates EO. 1980. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease. A clinical trial. Ann. Intern. Med 93(3):391–98 [DOI] [PubMed] [Google Scholar]
- 87.Albert RK, Au DH, Blackford AL, et al. 2016. A randomized trial of long-term oxygen for COPD with moderate desaturation. N. Engl. J. Med 375(17):1617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coleman JM, Wolfe LF, Kalhan R. 2019. Noninvasive ventilation in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc 16(9):1091–98 [DOI] [PubMed] [Google Scholar]
- 89.Murphy PB, Rehal S, Arbane G, et al. 2017. Effect of home noninvasive ventilation with oxygen therapy versus oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA 317(21):2177–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Köhnlein T, Windisch W, Köhler D, et al. 2014. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir. Med 2(9):698–705 [DOI] [PubMed] [Google Scholar]
- 91.Casanova C, Celli BR, Tost L, et al. 2000. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 118(6):1582–90 [DOI] [PubMed] [Google Scholar]
- 92.McEvoy RD, Pierce RJ, Hillman D, et al. 2009. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 64(7):561–66 [DOI] [PubMed] [Google Scholar]


