Abstract
The functional specificity of brain areas is diminished with age and accompanied by the recruitment of additional brain regions in healthy older adults. This process has repeatedly been demonstrated within distinct functional domains, in particular the visual system. However, it is yet unclear, whether this phenomenon in healthy aging, i.e., a reduced activation of task-associated areas and increased activation of additional regions, is also present across different functional systems. In the present functional imaging study, comprising 102 healthy subjects, we therefore assessed two distinct tasks engaging the sensory-motor system and the visual attention system, respectively. We found a significant interaction between age and task in the parietal operculum bilaterally. This area as a part of the sensory-motor system showed an age-related decrease in its BOLD-response to the motor task and an age-related increase of neural activity in response to the visual attention task. The opposite response pattern, i.e., reduced visual attention activation and increased response to the motor task, was observed for regions associated with the visual task: the superior parietal area 7A and the dorsal pre-motor cortex. Importantly, task performance was not correlated with age in either task. This age-by-task interaction indicates that a reduction of functional specificity in the aging brain may be counteracted by the increased recruitment of additional regions not only within, but also across functional domains. Our results thus emphasize the need for comparisons across different functional domains to gain a better understanding of age-related effects on the specificity of functional systems.
Keywords: Aging, Functional specificity, Dedifferentiation, FMRI
Introduction
Physiological processes during healthy aging lead to widespread and apparently massive changes in brain structure and neural activity (for review see Goh 2011). Using functional magnetic resonance imaging, age-related changes in regional activation patterns have been consistently reported in a variety of functional domains including attention (Madden et al. 2002; Cabeza et al. 2004), visual perception (Grady et al. 1994; Levine et al. 2000; Iidaka et al. 2002) and working memory (Rypma and D’Esposito 2000; Grossman et al. 2002). Over the last years, several phenomenological and mechanistical accounts have been proposed for the description of these changes in functional activation (cf. Reuter-Lorenz and Park 2010), e.g., the “posterior–anterior shift with aging” and the “hemispheric asymmetry reduction in older adults (HAROLD, Cabeza 2002). A prominent line of arguments supports the concept of de-differentiation (Grady et al. 1994; Park et al. 2004). This concept postulates that neural representations, which are well segregated in young adults, are considerably less selectively recruited in older subjects (Logan et al. 2002). That is, during a particular task (e.g., face processing) neural activity is reduced within regions associated with task performance in young adults (e.g., the fusiform face-area) and simultaneously increased in regions that are not considered to be task-relevant (e.g., the parahippocampal place area).
Reduction of regional selectivity with healthy aging has already been demonstrated within several functional systems (Grady et al. 1994; Townsend et al. 2006; Carp et al. 2011) and represents a plausible explanation for age-related differences in brain activity. Moreover, Dennis and Cabeza (2011) demonstrated evidence for de-differentiation across tasks within a particular functional domain (learning) by contrasting age-related effects in an implicit to those in an explicit memory task. De-differentiation within a particular functional system thus seems to span multiple tasks, i.e., processing demands. In contrast, very little is known about age-related changes in (regional) specificity across different functional systems. Such “de-differentiation” across functional systems may be a part of the global changes in healthy aging like alterations of local processing or less efficient connectivity (Grady 2008), which entails changes in resource allocation and computational strategies.
In the present study, we address this question by examining age-related changes in the regional specificity of neural activation across functional systems. Therefore, 102 healthy subjects between 21 and 71 years were investigated in a cross-sectional fMRI study. We analyzed two functional systems that show age-related within-domain changes in functional specificity; the visual attention system (Grady et al. 1994) and the motor system (Carp et al. 2011). In detail, we tested for age-related changes in regional specificity across both functional systems in those regions that were significantly associated with one of these. The specific purpose was to identify brain regions that show a decrease of neural activation with age for one task (e.g., visual attention) and an increase of activation in response to the other task (e.g., motor). Given reports of an age-related decline in visual attention (Kramer and Madden 2008) and motor performance (Kaasinen and Rinne 2002; Krampe 2002; Seidler et al. 2010), we deliberately employed tasks in which substantial changes in task performance with age were not expected, i.e., simple letter counting (visual attention) and finger tapping (motor). Also important to our endeavor, these tasks can be expected to feature a limited choice of alternative solution strategies as well as clearly distinguishable neuronal correlates. In particular, we expected to find specific brain areas for each task, in which one task evokes significantly higher activity than the other. Our main focus was then to assess age-related activation shifts across tasks (and hence functional systems) by testing for age-by-task interactions within these task-associated regions. Such cross-domain effects would provide evidence for our hypothesis that an age-related loss of neural specificity is not limited to stimulus-evoked effects (e.g., faces; see Goh et al. 2010) or to “de-differentiation” within a distinct functional system (e.g., visual domain; see Grady et al. 1994), but represents a more general phenomenon.
Materials and methods
Participants
102 subjects participated in the experiment. To provide a balanced age and gender distribution, recruitment was stratified into subgroups [20–30 years: 20 subjects (mean age 25.5 years, 9 females); 30–40 years: 18 subjects (mean age 32.8 years, 8 females); 40–50 years: 22 subjects (mean age 44.9 years, 11 females); 50–60 years: 22 subjects (mean age 55.3, 11 females) and 60–70 years: 20 subjects (mean age 63.9, 11 females)]. All participants had normal or corrected to normal vision and no history of neurological or psychiatric episodes. Participants gave written informed consent to this study, which was approved by the Ethical Committee of the University of Bonn.
Neuropsychological and behavioral testing
All volunteers underwent neuropsychological and behavioral testing. They were right-handed as assessed by the Edinburgh Handedness Inventory (median: 92, IQR: 28.9; Oldfield 1971). Each participant was tested on the Mini-Mental State Examination (MMSE, Folstein et al. 1975) to exclude potentially sub-clinical cognitive impairment. The Trail Making Test A and B (Reitan 1955), and the Digit Symbol Substitution Test (DSST) of the German Version of the Wechsler Adult Intelligence Scale-III (Wechsler 1997; Aster et al. 2006) were administered to assess processing speed and task switching. For assessing motor speed, motor control, and dexterity, the finger tapping test (FTT; Halstead 1947; Behrwind et al. 2011) and the pointing task (PT; similar to the CAPSIT Parkinson’s disease test battery; Defer et al. 1999) were performed.
fMRI paradigm
As we were interested in age-related changes in regional activation patterns, differential performance across the assessed age-range may represent an important confound. Hence, the experimental paradigm consisted of two simple tasks for which age-related changes in performance were not expected a priori: a visual attention task (COUNT) and a motor task (TAP). The former (COUNT) consisted of a letter counting task in which a random series of the letters ‘E’ and ‘F’ were visually presented on a screen. The black colored letters appeared on a white background for 400 ms with an stimulus-onset-asynchrony (SOA) of 400 ms. Subjects were asked to identify the number of times they saw a target letter (‘E’) and report the number of target letters after each block. This verbal answer was recorded as a measure of successful task-completion, but for technical limitations initiation time to answer could not be recorded. Given the sustained nature of our task, this reaction time would not have been representative of task performance anyways.
The motor task consisted of a bimanual repetitive finger tapping task, requiring the subjects to press response-buttons alternatively with their left and right index finger, respectively. To minimize inter-individual performance differences, participants practiced this task prior to scanning, attempting to match a tapping speed frequency of 5 Hz. During training, subjects were given feedback if they were tapping too slow (<4.6 Hz) or too fast (>5.4 Hz) and were instructed to speed up or slow down, respectively. After maintaining the requested tapping frequency seven times in a row, tapping speed was considered stable and subjects were moved to the scanner. To match the visual input between both tasks, the letter ‘X’ was presented in the same manner as the letter-sequence in the COUNT task (stimulus duration 400 ms, SOA 400 ms). Subjects were instructed to look on the screen but to disregard the letter ‘X’. As the finger tapping was no speeded reaction task but rather asked the subjects to perform internally triggered movements (cf. Hoffstaedter et al. 2013), this task likewise yielded no response times that could be analyzed in a meaningful fashion. Performance indicator for this task was determined by the response variability, i.e., the variance for the inter-response interval (IRI, Apitz et al. 2010) between the finger movements (left/right), reflecting motor coordination.
The study itself was set up as a block-design with either the TAP or the COUNT task being presented in each individual block for 24 s. Blocks of either task were repeated five times in a randomized sequence with breaks of 8 s between blocks served as implicit resting baseline. The paradigm was presented via a mirror installed on the head-coil through which the subjects followed the presentation of the paradigm on a TFT screen behind the scanner. To minimize head movements every subject was stabilized with pads within the head-coil.
fMRI data acquisition and pre-processing
Images were acquired on a Siemens Tim-Trio 3T whole-body scanner (Erlangen, Germany), using blood oxygenation level dependent (BOLD) contrast (2D-echo-planar imaging (EPI) pulse sequence, repetition time (TR) = 2,200 ms, echo time (TE) = 30 ms, in-plane resolution = 3.1 × 3.1 mm, 36 axial slices, 3.1 mm thickness) covering the whole brain. Image acquisition was preceded by three dummy scans to allow for longitudinal equilibrium that were discarded prior to further processing with SPM8 (www.fil.ion.ucl.ac.uk/spm). In the preprocessing, the EPI images were first corrected for head movement by affine registration using a two-pass procedure, by which images were initially realigned to the first image and subsequently to the mean of the realigned images. After realignment, the mean EPI image for each subject was co-registered to the Montreal Neurological Institute (MNI) gray matter template. For normalization the mean EPI images were segmented into gray matter, white matter and cerebral spinal fluid using the “unified segmentation” approach (Ashburner and Friston 2005). The resulting parameters of a discrete cosine transform, which define the deformation field necessary to move subject data into MNI space, were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes which thereby were transformed into the MNI single subject space and resampled at 1.5 mm isotropic voxel size. The normalized images were spatially smoothed using an 8-mm full width at half maximum (FWHM) Gaussian kernel to meet the statistical requirements for statistical inference by Gaussian random field theory and to compensate for residual macroanatomical variations across subjects.
fMRI image analysis
The imaging data were analyzed using a General Linear Model as implemented in SPM8. Each experimental condition (TAP, COUNT) was separately modeled as a block-vector input function using the stimulus onset and the time of the respective block. The verbal answer of the COUNT condition and the instruction preceding each task-block were modeled separately to reduce confounding variance within the implicit baseline (breaks between blocks). Each input function was convolved with a canonical hemodynamic response function and its first-order temporal derivative to yield the final regressors. To improve analysis specificity the movement parameters (x-translation, y-translation, z-translation, pitch, roll, and yaw) as estimated during image realignment were also included as confound regressors of no interest. Low-frequency signal drifts were filtered using a cut-off period of 128 s. Parameter estimates were subsequently calculated for each voxel using weighted least squares to provide maximum likelihood estimators based on the temporal autocorrelation of the data (Kiebel et al. 2003). For each subject, simple main effects for each of the two experimental conditions were computed by applying appropriate baseline contrasts. These individual first-level contrasts were then fed to a random-effects group-analysis using an ANOVA (condition factor: TAP or COUNT, blocking factor subject) with age x condition effects entered as a covariate. Thus, the variance explained by age is estimated for each factor separately. The statistical design allowed, therefore, testing the effects of condition (mean across all subjects) and the effects of age on each condition separately. In a subsequent analysis, we also assessed potential gender differences and in particular possible task × gender interactions (indicating different cognitive strategies or neuronal correlates thereof between males and females) again using an AV-OVA design with the same general setup but modeling male and female subjects as separate groups. In the modeling of variance components, violations of sphericity were allowed by modeling non-independence across images from the same subject and allowing unequal variances between conditions and subjects using the standard implementation in SPM8.
Contrasts and thresholding
The main effect of each task (TAP, COUNT) was delineated by contrasting the correspondent task regressor with the implicit baseline. Regional preference for a particular task was investigated by contrasting the task of interest against the other (e.g., TAP > COUNT) in conjunction with the main effect of the relevant task (e.g., TAP > baseline). In this context, it should be noted that such preferential activation does not imply task-specificity in the strict sense as, thus, would require to show the absence of activation in the respective other task. Providing evidence for absent effects, however, is not feasible with classical statistics, as a non-significant test does not imply proof of then null-hypotheses (absence of evidence is not evidence of absence). Within the statistical framework of classical inference, significantly stronger activation by, e.g., TAP relative to COUNT, in combination with significant activation for TAP may thus be deemed the best possible evidence that the respective area is preferentially recruited by (in this example) the motor as compared to the visual attention task.
Given the aim of this study as outlined in the introduction, our main focus rested on testing for an age-by-task interaction within regions significantly associated with the respective task. We were thus interested in COUNT-associated regions (significantly higher activated during COUNT than during TAP) where activation decreases with age during COUNT but increases with age during TAP. Key to the investigated interaction-effects is thus the reduction of activation for one task in combination with an increase of activation for the other, i.e., a reduction of the relative differences in BOLD-response that results from activity levels across the two tasks becoming more similar.
To delineate such effects indicating a change in regional specificity across functional systems, we employed global conjunctions across the contrast for task-associated regions (e.g., TAP > baseline and TAP > COUNT) in conjunction with the respective age regressors (in this example, negative weighting of the age regressor for the TAP condition and positive weighting of the age regressor for the COUNT condition). Finally, age-related correlations on neural activation for both experimental tasks were identified by contrasting the task main effect with the correspondent age regressor. This was done for both the positive and negative weighted age regressor. As the present study not focuses on these age-related effects, these results will be reported in the supplementary material.
All different effects and covariate-analyses were thresholded at p < 0.05 (family wise error (FWE)-corrected for multiple comparisons at the voxel cluster level; clusterforming threshold at voxel level: p < 0.001), while the obtained activations were anatomically localized using the cytoarchitectonic maps of the Juelich-Duesseldorf Cytoarchitectonic Atlas (Zilles and Amunts 2010) as implemented in version 1.8 of the SPM Anatomy toolbox (Eickhoff et al. 2005, 2006b, 2007; www.fz-juelich.de/inm/inm-1/spm_anatomy_toolbox).
Test for changes of gray matter probability
The repeatedly demonstrated age-related change of gray matter volume in the human brain (Ge et al. 2002; Sowell et al. 2003; Walhovd et al. 2005; Lehmbeck et al. 2006; Giorgio et al. 2010) provokes the assumption that such structural alterations may confound age-related findings in terms of neural activity. We therefore tested the gray matter probability for each region showing an age-by-task interaction. To this, normalized and segmented T1 images were used in which for every brain voxel a specific probability value for each brain tissue class (gray matter, white matter, and cerebrospinal fluid) is denoted. By correlating the mean gray matter probability values of all voxels within a relevant brain region with age, changes of the gray matter distribution were estimated.
Results
Neuropsychological and behavioral results
Performance for the MMSE was within the clinically normal range (mean: 29.2 ± 1.0, minimum: 27), indicating that no subject suffered from cognitive impairment. Performance for the neuropsychological tests performed outside the scanner was significantly correlated with age in our group of 102 subjects. In particular, we found that performance correlated negatively with age in tests related to attention (TMT-A, TMT-B and DSST; cf. Table 1) and motor control (PT and FTT; cf. Table 1). These results thus confirm that our cohort showed the expected decline in processing-speed and executive functions with increasing healthy age.
Table 1.
Neuropsychological test scores correlated with age
| Test | ρ (rho) | p value |
|---|---|---|
| Trail Making Test A (TMT-A)a | 0.48 | <0.001 |
| Trail Making Test B (TMT-B)a | 0.55 | <0.001 |
| Digit symbol substitution test (DSST)b | −0.47 | <0.001 |
| PT (right)a | 0.23 | 0.022 |
| PT (left)a | 0.19 | 0.049 |
| FTT (right)b | −0.45 | <0.001 |
| FTT (left)b | −0.31 | 0.002 |
Data failed test for normality (Spearman rank correlation)
Data passes test for normality (Pearson product moment correlation)
Analysis of the motor task specific performance indicator yielded no significant correlation of the individual IRI-variance with age, demonstrating stable task performance with increasing age. The performance for the letter counting task was determined by correlating the error rate (deviation from correct number of target letters) with age. Again, no significant correlation with age was found, indicating likewise a comparable performance between younger and older adults. In summary, the significant age-correlations for the more challenging neuropsychological tests administered outside the scanner together with the absent age-effects for the experimental conditions indicates that our subjects indeed show the expected general decline in cognitive-motor functioning, but this did not impact their performance in the deliberately simple experimental tasks.
Imaging results
First, the general activation pattern for each task is described, respectively. Afterward, task-associated regions, i.e., regions showing significantly higher activation during one task compared to the other task are reported. Several task-associated regions showed an age-by-task interaction. These regions are specified finally.
The local maxima of all reported activations and their anatomic classifications are listed in detail in the supplementary tables S2-S4.
Activation pattern during the motor task (TAP)
During the finger tapping task, a characteristic motor network consisting of the primary motor cortex (area 4a, 4p; Geyer et al. 1996), the supplementary motor area (SMA), the somatosensory cortex (area 1;Geyer et al. 1999), the secondary somatosensory cortex (parietal operculum, OP1-4; Eickhoff et al. 2006a; Eickhoff et al. 2006c), the frontal operculum close to the Pars opercularis (area 44; Amunts et al. 1999), the middle frontal gyrus as well as the thalamus, the basal ganglia and the cerebellum showed significant activation. Moreover, in accordance with the concurrent presentation of continuous visual input (letter ‘X’) we also observed significant activation in extrastriate visual areas (area 18, hOC3v; Amunts et al. 2000; Rottschy et al. 2007) and the inferior temporal gyrus (ventral stream for visual processing).
Activation pattern during the visual attention task (COUNT)
The letter counting task evoked significant activation in the primary visual cortex (area 17; Amunts et al. 2000), the extrastriate visual cortex (area 18, 19, hOC3v, hOC4v; see Rottschy et al. 2007 for hOC4v), the fusiform gyrus (area FG2; Caspers et al. 2012), inferior and superior parietal cortex (area PFm, area hIP3, area 7A; Caspers et al. 2006, 2008; Scheperjans et al. 2008a, 2008b), premotor cortex, pre-SMA, anterior insular cortex, thalamus, basal ganglia and cerebellum (Table S1).
Task-associated regions
During TAP, higher activation compared to COUNT (and significant activation over baseline) was found bilaterally in supplementary motor area (SMA) and adjacent caudal dorsal pre-motor regions, the primary motor cortex (area 4a, 4p), somatosensory cortex (area 3b, 1, 2; Geyer et al. 1999; Grefkes et al. 2001), the frontal (area 44) and parietal operculum (area OP1), the thalamus and the cerebellum. Right-lateralized effects were observed in the middle frontal gyrus and the pallidum (Fig. 1).
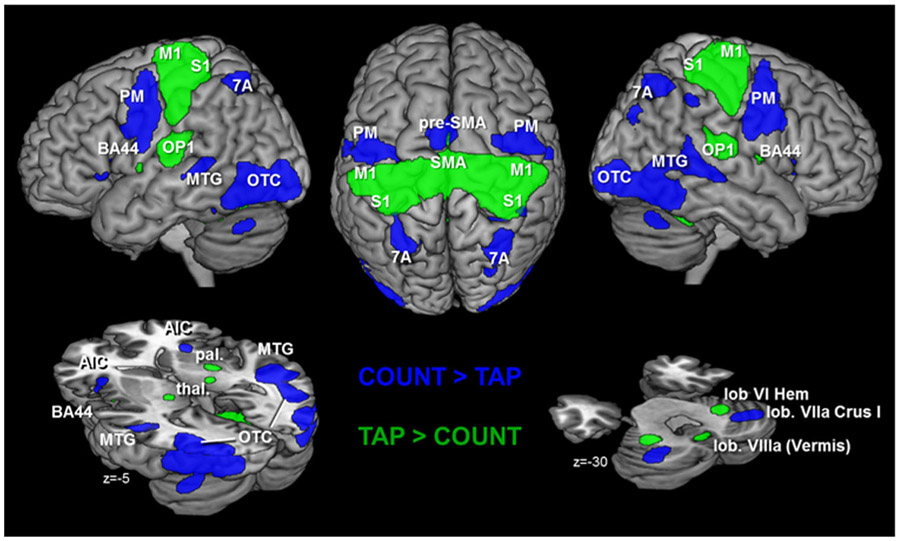
Fig. 1.
Brain activation that is significantly stronger activated to the visual attention task (blue cluster) and to the motor task (green cluster). These activations are independent of age as the covariate (age) was not included in these particular contrasts. M1 primary motor cortex, S1 primary somatosensory cortex, PM pre-motor cortex, OP1 parietal opercular area 1, 7A superior parietal area 7A, SMA supplementary motor area, AIC anterior insular cortex, put. putamen, pal. pallidum, thal. thalamus, MTG middle temporal gyrus, OTC occipital cortex extending into temporal cortex, lob. lobule (cerebellum); all results are FWE corrected on voxel level (p < 0.001)
The COUNT condition evoked significantly higher activity than TAP (and baseline) bilaterally in the extrastriate visual cortex (area hOC3v, hOC4v, hOC5; Malikovic et al. 2007; Rottschy et al. 2007) extending into the middle and superior temporal gyrus (occipito-temporal cortex, cf. Fig. 1), the precentral gyrus and posterior inferior frontal gyrus (pre-motor area 6 extending into area 44), pre-SMA, the intraparietal areas hIP3 and hIP2 (Choi et al. 2006; Scheperjans et al. 2008a; b), superior parietal area 7A, the middle occipital gyrus and the anterior insular (Fig. 1).
Age-related cross-domain effect (age × task interaction)
Subsequently, we tested for regions that were significantly associated with one of the two tasks, i.e., significant in the analyses presented in the last paragraph, and showed a significant age-by-task interaction. More precisely, we tested whether any of the TAP-associated regions (regions significantly more activated during TAP compared to COUNT or baseline) showed an age-related decrease of activation during TAP and an increase of activation during COUNT. Regions were only associated with TAP or COUNT, in the analysis above, if they showed a significant main effect of task, respectively.
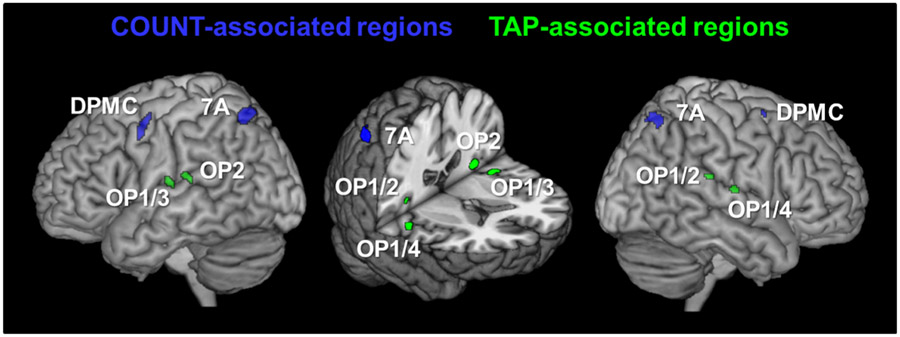
For the identification of COUNT-regions showing such an age-by-task interaction, we tested for COUNT-associated regions (significant main effects of COUNT > TAP and COUNT > baseline) in which activation decreased with age during COUNT, and increased with age during TAP. As depicted in Fig. 2, the superior parietal area 7A and rostral parts of the dorsal premotor cortex (DPMC) bilaterally showed this distinct activation pattern (Fig. 3). In contrast, for TAP-associated regions (defined by featuring significant main effects of TAP > COUNT and TAP > baseline), a pattern of decreased activation with age during TAP and simultaneously increased activation during COUNT would represent the respective age-by-task interaction indicating a shift of functional specificity. This kind of activation pattern was observed in the parietal operculum (parts of areas OP 1, OP 2, OP 3, and OP 4) bilaterally (Figs. 2, 3).
Fig. 2.
Task-associated regions showing an age-related increase of activation in response to their associated task and a decrease in response to the other task, i.e., an age-by-task interaction. Blue cluster COUNT-associated regions showing a decrease of neural activation during COUNT and an increase during TAP; Green cluster TAP-associated regions showing a decrease of neural activation during TAP and an increase during COUNT, DPMC dorsal pre-motor cortex, OP(1–4) parietal opercular areas 1–4, 7A superior parietal area 7A; FWE corrected on voxel level (p < 0.05)
Fig. 3.
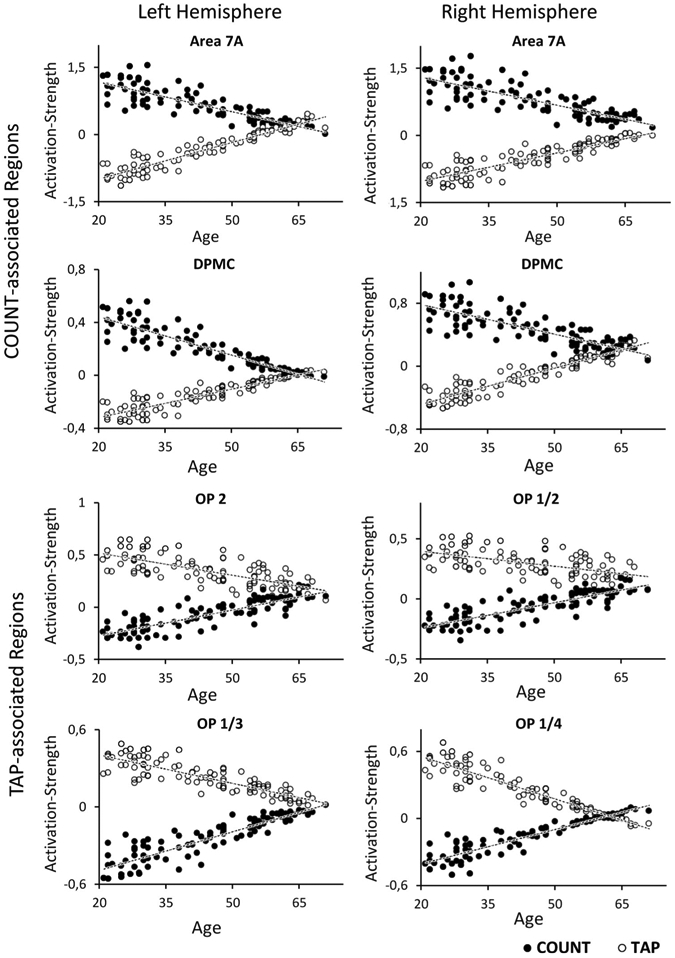
Dependence of brain activation on age in task-associated brain regions for both experimental conditions (TAP and COUNT). The activation strength reflects the beta-value of the voxel with the highest activation (maxima) in the respective cluster for each single subject. Each single dot therefore mirrors the change in BOLD signal by the respective condition in a single subject. COUNT-associated regions are represented by the four upper plots, whereas TAP-associated regions are represented by the four lower plots
In a subsequently conducted categorical analysis using a 2 × 2 factorial design [factor one: age (young/old) factor two: condition (count/tap)], these effects were confirmed, thereby strengthening our findings (cf. supplementary material).
Test for gender-specific effects
The subsequently conducted gender-specific ANOVA yielded no significant interaction between task × gender or age × gender within any of the reported regions.
Test for changes of gray matter probability
The analysis of the gray matter probability for each region showing an age-by-task interaction revealed no significant correlations with age. Hence, confounding results related to structural changes in gray matter can be excluded for these regions.
Discussion
In this study, we investigated age-dependent changes in regional specificity of fMRI activity across two different functional systems: the motor system (task TAP), and the visual attention system (task COUNT). We first delineated brain regions significantly associated with either the motor or the visual attention task. A clearly differentiated activation pattern was observable for both tasks. Subsequently, we tested for age-by-task interactions within these regions. For the majority of regions within both task-associated networks no interaction was found. However, we identified specific sub-regions within both task-associated, i.e., differentiated, networks, showing such interaction by decreased activation during the associated task and increased activation during the other task, which resulted in reduced differences in evoked activation between both tasks. Such age-related changes of functional specificity were found in the superior parietal area 7A and the dorsal premotor cortex bilaterally within the COUNT-associated regions and the parietal opercular cortex (partly areas OP1, OP2, OP3, and OP4) within the TAP-relevant regions. Notably, the relative increase of activation in the task unrelated area may reflect a reduced de-activation, i.e., less inhibition of the BOLD-response relative to the implicit baseline. The lack of gender-specific differences for the regions showing this age-related interaction argues against potential gender-specific mechanisms and toward a general phenomenon evident in both males and females.
Reduced specificity across functional domains
Activation in motor-associated regions increased during the visual attention task with age but decreases during the motor task, and vice versa, indicating a significant interaction between age and task (functional system). In particular, we found a reduced difference in TAP-COUNT evoked activation strength in elderly subjects within the parietal operculum bilaterally, which was partially attributed to lower activity of these regions during the TAP task (cf. Fig. 3). Moreover, these regions also showed a relative increase of activation below the implicit baseline (less inhibition) during COUNT with age. In analogy, we demonstrated the reverse pattern in two bilateral regions that were functionally associated with the COUNT task, namely area 7A within the superior parietal cortex and the rostral parts of the dorsal premotor cortex (DPMC). The reduced difference in activation strength may reflect reduced functional specificity and, in turn, increased integration between functional systems, which implicates “de-differentiation” across different functional systems. These shifts in functional response patterns may even represent a general pattern of age-related changes in brain activity. In this context, it is important to emphasize that we did not consider any effects within brain regions, which per se showed shared activation between both tasks. All regions reported above showed a clear preference for either the motor or the visual attention task as evident from the significant difference in the main effects. All identified regions actually featured deactivations in the “non-pre-ferred” task in younger subjects (cf. Fig. 3). The obtained effects thus represent changes in recruitment of brain areas that are specifically associated with one of the tasks in younger subjects. To this end, it needs to be stressed that the observed effect do not reflect a “reversal of functional preference”, i.e., there is no region that is associated with TAP in younger and COUNT in older subjects. Rather, activation strength for both tasks (COUNT and TAP) tended to become more similar to each other and less different from the implicit baseline with increasing age for all regions. This pattern thus reflects a loss of neural specificity relative to the implicit resting baseline in one task and a relative de-activation in the other. In this context, it is worth-mentioning that the implicit baseline by no means represents an absolute zero reference without ongoing activity. It is well established that meaningful neural activity is also going on in the human brain during a “resting state” in which subjects are not focused on an external task (Raichle et al. 2001; Buckner et al. 2008; Schilbach et al. 2012). Independently of this relative reference to baseline, the revealed interaction effect may be interpreted as evidence for decreasing functional specificity of task-associated regions with age, in which neural activity becomes more similar to baseline for both tasks.
While the parietal operculum showed a significant preference for the TAP as opposed to the COUNT task and conversely area 7A and the dorsal premotor cortex showed a significantly stronger recruitment in the COUNT as opposed to the TAP task, it should be remembered that these regions are not exclusively recruited by finger tapping or visual attention tasks, respectively. Like any other region in the brain, these areas seem involved in many processes pertaining to motor execution, sensory processing or cognitive functions. The parietal operculum (OP1) as part of the secondary somatosensory cortex is involved in somatosensory integration (Eickhoff et al. 2010) and bimanual processing (Disbrow et al. 2001), but has also been implicated in tactile working memory, stimulus discrimination, and perceptual learning (Romo et al. 2002; Pleger et al. 2003; Burton et al. 2008). Likewise, area 7A is not only part of the dorsal visual stream and involved in visuospatial attention (Hahn et al. 2006; Kelley et al. 2008). It has also been associated with action observation (Buccino et al. 2001; Caspers et al. 2010), motor execution (grasping and sequential finger movements), visuo-motor integration (Battaglia-Mayer and Caminiti 2002; Rizzolatti and Matelli 2003; Pellijeff et al. 2006) and mental simulation (Grezes and Decety 2001). The DPMC, finally, plays a role in several cognitive and motor related processes, e.g., conditional visuo-motor associations (Cieslik et al. 2012), response selection or motor imagery (Grafton et al. 1998; Naito et al. 1999; Toni et al. 1999). All three regions may thus be recruited by different functional systems depending on specific demands of the task at hand. It may hence be argued that these areas may implement processes rather than tasks (Eickhoff and Grefkes 2011), which then with age get differentially recruited to fulfill a given task.
The present data lead to the conclusion that the observed cross-domain effect reflects a functional plasticity throughout the human life-span leading to a less specific recruitment of neuronal processing. While each area maintains its process-specificity, the less specific recruitment of these processes manifests as a loss of specificity at the level of experimental tasks. In this context, a decrease of activation for one task may reflect reduced recruitment of this (in young subjects highly task-associated) process while conversely the same area, i.e., process, gets more recruited (less inhibited) in the context of another task. How the reduced activation of (in young subjects) task-associated regions and relative increase of activation in regions primarily associated with a different task in elderly are causally related, remains to be investigated. It was argued that insufficient activation of originally task-associated regions requires the recruitment of (auxiliary) processes or, conversely, that failure to inhibit competing processes leads to cross-talk and hence reduced task-associated activity (cf. discussion in Goh et al. 2010; Carp et al. 2011). However, the present data indicate that reduced task-specificity is present across functional systems rendering observations of age-related regional hypo-or hyperactivation condition on the actual task at hand.
The effect of decreased neural specificity across functional systems
Whether the observed less specific recruitment of neuronal processing is beneficial or detrimental for the respective behavioral performance cannot be answered from the current data as both tasks were kept deliberately simple to avoid confounding influences of task performance. Reduced neural specificity (at the level of regional activation in experimental tasks) has repeatedly been linked to age-related impairments of neural processing (Duverne et al. 2009) and performance declines (Li et al. 2001; Li and Sikstrom 2002). The present results deviate somewhat from this view as in spite of clear cross-domains effect resembling what has been termed “de-differentiation” within, e.g., the visual system; older subjects successfully performed both experimental tasks (COUNT and TAP). Given that this performance may be attributed to ceiling-effects in our simple tasks, we would not necessarily conclude a supportive effect of such shifts in recruitment. Nevertheless, we would propose that successful task performance at least argues against a clearly detrimental effect. In line with this view, Cabeza (2002) attributed compensatory effects to another aspect of less specific brain activation in elderly, namely bilateral activations in older adults during tasks that evoke unilateral activation in younger adults (HAROLD, cf. Reuter-Lorenz and Lustig 2005).
Summary
The present results demonstrate a significant age-by-task interaction across different functional domains, mirroring effects of “de-differentiation” previously demonstrated within distinct functional systems (Grady et al. 1994; Carp et al. 2011; Goh 2011). From the obtained behavioral data, we would argue that this less specific task-related recruitment of cortical areas should represent a non-detrimental process. On a more conceptual level, this age effect also prompts considerations on the nature of functional specialization as it suggests that the mapping between regional processes and experimental tasks may undergo age-related changes. Our results thus emphasize that comparing activation patterns across different tasks (from different domains) is necessary to investigate age-related alterations of neural activation (cf. Grady 2012).
Supplementary Material
Acknowledgments
Funding was granted by the Human Brain Project (R01-MH074457-01A1; S.B.E.), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model; S.B.E.), and the Helmholtz Alliance for Mental Health in an Aging Society (HelMA; K.A.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00429-013-0530-x) contains supplementary material, which is available to authorized users.
Contributor Information
Christian Roski, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany.
Svenja Caspers, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany.
Silke Lux, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany.
Felix Hoffstaedter, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany.
René Bergs, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany.
Katrin Amunts, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany; Jülich-Aachen Research Alliance, JARA-BRAIN, 52425 Jülich, Germany; Section Structural–Functional Brain Mapping, Department of Psychiatry, Psychotherapy and Psychosomatics, University Hospital Aachen, RWTH Aachen University, 52074 Aachen, Germany.
Simon B. Eickhoff, Institute of Neuroscience and Medicine (INM-1, INM-2), Research Center Jülich, Leo-Brandt Str. 1, 52425 Jülich, Germany; Institute for Clinical Neuroscience and Medical Psychology, Heinrich-Heine University, 40225 Düsseldorf, Germany
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K (1999) Broca’s region revisited: cytoarchitecture and inter-subject variability. J Comp Neurol 412(2):319–341 [DOI] [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000) Brodmann’s areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage 11(1):66–84 [DOI] [PubMed] [Google Scholar]
- Apitz C, Mackensen-Haen S, Girisch M, Kerst G, Wiegand G, Stuhrmann M, Niethammer K, Behrwind G, Hofbeck M (2010) Neonatal Marfan syndrome: unusually large deletion of exons 24–26 of FBN1 associated with poor prognosis. Klin Padiatr 222(4):261–263 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851 [DOI] [PubMed] [Google Scholar]
- Aster M, Neubauer A, Horn R (2006) Wechsler Intelligenztest für Erwachsene WIE. Deutschsprachige Bearbeitung und Adaptation des WAIS-III von David Wechsler (2.,korrigierte Auflage). Pearson Assessment, Frankfurt [Google Scholar]
- Battaglia-Mayer A, Caminiti R (2002) Optic ataxia as a result of the breakdown of the global tuning fields of parietal neurones. Brain 125(Pt 2):225–237 [DOI] [PubMed] [Google Scholar]
- Behrwind SD, Dafotakis M, Halfter S, Hobusch K, Berthold-Losleben M, Cieslik EC, Eickhoff SB (2011) Executive control in chronic schizophrenia: a perspective from manual stimulus-response compatibility task performance. Behav Brain Res 223(1):24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ (2001) Action observation activates premotor and parietal areas in a somato-topic manner: an fMRI study. Eur J Neurosci 13(2):400–404 [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38 [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, McLaren DG (2008) Cortical network for vibrotactile attention: a fMRI study. Hum Brain Mapp 29(2):207–221. doi: 10.1002/hbm.20384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R (2002) Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17(1):85–100 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L (2004) Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14(4):364–375 [DOI] [PubMed] [Google Scholar]
- Carp J, Park J, Hebrank A, Park DC, Polk TA (2011) Age-related neural dedifferentiation in the motor system. PLoS ONE 6(12): e29411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006) The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage 33(2): 430–448 [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, Amunts K (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212(6):481–495 [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010) ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 50(3):1148–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K (2012) Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain structure & function 212:481–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K (2006) Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495(1):53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Caspers S, Roski C, Kellermann TS, Jakobs O, Langner R, Laird AR, Fox PT, Eickhoff SB (2012) Is there “One” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb Cortex. doi: 10.1093/cercor/bhs256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defer GL, Widner H, Marie RM, Remy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14(4):572–584 [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R (2011) Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging 32 (12):2318 e2317–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disbrow E, Roberts T, Poeppel D, Krubitzer L (2001) Evidence for interhemispheric processing of inputs from the hands in human S2 and PV. J Neurophysiol 85(5):2236–2244 [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD (2009) The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex 19(3):733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C (2011) Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin EEG Neurosci 42(2):107–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25(4):1325–1335 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K (2006a) The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16(2):268–279 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006b) Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32(2):570–582 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K (2006c) The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16(2):254–267 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36(3):511–521 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE (2010) Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30(18):6409–6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198 [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL (2002) Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR. Am J Neuroradiol 23(8):1327–1333 [PMC free article] [PubMed] [Google Scholar]
- Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, Burgel U, Klingberg T, Larsson J, Zilles K, Roland PE (1996) Two different areas within the primary motor cortex of man. Nature 382(6594):805–807 [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K (1999) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10(1):63–83 [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H (2010) Age-related changes in grey and white matter structure throughout adulthood. Neuroimage 51(3): 943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO (2011) Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis 2(1):30–48 [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Suzuki A, Park DC (2010) Reduced neural selectivity increases fMRI adaptation with age during face discrimination. Neuroimage 51(1):336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL (2008) Cognitive neuroscience of aging. Ann N Y Acad Sci 1124:127–144 [DOI] [PubMed] [Google Scholar]
- Grady C (2012) The cognitive neuroscience of ageing. Nat Rev Neurosci 13(7):491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV (1994) Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14(3 Pt 2):1450–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA (1998) Dorsal premotor cortex and conditional movement selection: A PET functional mapping study. J Neurophysiol 79(2):1092–1097 [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001) Human somatosensory area 2: observer-independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14(3):617–631 [DOI] [PubMed] [Google Scholar]
- Grezes J, Decety J (2001) Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp 12(1):1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J (2002) Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage 15(2):302–317 [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA (2006) Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage 32(2):842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead WC (1947) Brain and intelligence: A quantitative study of the frontal lobes. University of Chicago Press, Chicago [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB (2013) The “What” and “When” of self-initiated movements. Cereb Cortex 23(3): 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, Yonekura Y (2002) Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus 12(3):352–362 [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Rinne JO (2002) Functional imaging studies of dopamine system and cognition in normal aging and Parkinson’s disease. Neurosci Biobehav Rev 26(7):785–793 [DOI] [PubMed] [Google Scholar]
- Kelley TA, Serences JT, Giesbrecht B, Yantis S (2008) Cortical mechanisms for shifting and holding visuospatial attention. Cereb Cortex 18(1):114–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Glaser DE, Friston KJ (2003) A heuristic for the degrees of freedom of statistics based on multiple variance parameters. Neuroimage 20(1):591–600 [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ (2008) Attention. In: Craik FIM, Satlhouse TA (eds) The handbook of aging and cognition, 3rd edn. Psychology Press, New York, pp 189–249 [Google Scholar]
- Krampe RT (2002) Aging, expertise and fine motor movement. Neurosci Biobehav Rev 26(7):769–776 [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF (2006) Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. NeuroReport 17(5):467–470 [DOI] [PubMed] [Google Scholar]
- Levine BK, Beason-Held LL, Purpura KP, Aronchick DM, Optican LM, Alexander GE, Horwitz B, Rapoport SI, Schapiro MB (2000) Age-related differences in visual perception: a PET study. Neurobiol Aging 21(4):577–584 [DOI] [PubMed] [Google Scholar]
- Li SC, Sikstrom S (2002) Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neurosci Biobehav Rev 26(7):795–808 [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikstrom S (2001) Aging cognition: from neuromodulation to representation. Trends Cogn Sci 5(11):479–486 [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL (2002) Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 33(5):827–840 [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE (2002) Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol Aging 17(1):24–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Palomero-Gallagher N, Armstrong E, Zilles K (2007) Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex 17(3):562–574 [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19(14):6134–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113 [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR (2004) Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sci USA 101(35):13091–13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellijeff A, Bonilha L, Morgan PS, McKenzie K, Jackson SR (2006) Parietal updating of limb posture: an event-related fMRI study. Neuropsychologia 44(13):2685–2690 [DOI] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M (2003) Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron 40(3):643–653 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. P Natl Acad Sci USA 98(2):676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM (1955) The relation of the trail making test to organic brain damage. J Consult Psychol 19(5):393–394 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C (2005) Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol 15(2): 245–251 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC (2010) Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci 65(4):405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M (2003) Two different streams form the dorsal visual system: anatomy and functions. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale 153(2):146–157 [DOI] [PubMed] [Google Scholar]
- Romo R, Hernandez A, Zainos A, Lemus L, Brody CD (2002) Neuronal correlates of decision-making in secondary somatosensory cortex. Nat Neurosci 5(11):1217–1225 [DOI] [PubMed] [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K (2007) Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp 28(10):1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M (2000) Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci 3(5):509–515 [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008a) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18(9):2141–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008b) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18(4): 846–867 [DOI] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB (2012) Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS ONE 7(2):e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB (2010) Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34(5):721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003) Mapping cortical change across the human life span. Nat Neurosci 6(3):309–315 [DOI] [PubMed] [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE (1999) Signal-, set-and movement-related activity in the human brain: an event-related fMRI study. Cereb Cortex 9(1):35–49 [DOI] [PubMed] [Google Scholar]
- Townsend J, Adamo M, Haist F (2006) Changing channels: an fMRI study of aging and cross-modal attention shifts. Neuroimage 31(4):1682–1692 [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005) Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26 (9):1261–1270; discussion 1275–1268 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997) Wechsler Adult Intelligence Scale - 3rd Edition (WAIS-3). Harcourt Assessment, San Antonio [Google Scholar]
- Zilles K, Amunts K (2010) Centenary of Brodmann’s map-conception and fate. Nat Rev Neurosci 11(2):139–145 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.