Abstract
We present the first description of an antimicrobial stewardship program (ASP) used to successfully manage a multi-antimicrobial drug shortage. Without resorting to formulary restriction, meropenem utilization decreased by 69% and piperacillin-tazobactam by 73%. During the shortage period, hospital mortality decreased (p=0.03), while hospital LOS appeared unchanged.
Keywords: Antimicrobial Stewardship, Antibiotic Stewardship, Antibiotic Shortage
Background:
Despite being common, very little has been published on how healthcare institutions can address antimicrobial shortages.1–4 Antimicrobial stewardship programs (ASPs) have been proposed as ideally suited to deal with antimicrobial shortages.5 To our knowledge there are no publications quantifying how an ASP can impact drug utilization during a shortage. We describe a stewardship program’s response to a critical multi-drug antimicrobial shortage at a large tertiary-care academic hospital, and subsequent effects on antimicrobial use and hospital-level outcomes.
Methods:
Hospital Description and Baseline Antimicrobial Stewardship Activities
Barnes-Jewish Hospital (BJH) is a 1252 bed tertiary care academic medical center located in St. Louis, Missouri. It has had an antimicrobial stewardship program since 1984 which at the time the shortage began, was composed of 2 infectious diseases (ID) trained pharmacists, providing practice guidelines and guidance to other pharmacy staff. Clinical pharmacists tasked with reviewing antimicrobial use as part of their standard duties were embedded with most services, though they did not track their interventions. No pre-existing interventions or use restrictions were in place for most antimicrobials, including for the shortage drugs. Previous shortages were dealt with through messaging alone.
Shortage Timeline and Interventions
Meropenem and Imipenem
On October 5th 2015 procurement failure and low stock of meropenem (MEM) led to a drug shortage alert from the pharmacy leadership. Attempts to purchase imipenem-cilastatin (IPM), an alternative carbapenem, were unsuccessful. Pharmacy and medical leadership formed a task force comprised of two ID physicians, two ID trained clinical pharmacists, an ID pharmacy resident, and an ID fellow. Interventions were executed in a staggered fashion (Supplementary Figure 1), starting with an informational campaign composed of a hospital-wide e-mail announcement, messaging to clinical pharmacy, and guidance on alternatives to MEM. Subsequently, active drug stock tracking was implemented for all anti-pseudomonal antibiotics. Finally, physician-directed prospective auditing of MEM and IPM use was implemented. During the shortage, pharmacy acquired as much additional drug stock as made available from suppliers. Shortage conditions ended on January 1st 2016 after regular supply was re-established.
Piperacillin-tazobactam
On October 20th 2015, lack of purchasable supplies led to an additional shortage declaration for piperacillin-tazobactam (TZP). The shortage task force expanded its scope to include TZP by utilizing an enhanced approach. In addition to aggressive messaging to clinical pharmacy, specialties with high TZP usage including general surgery and emergency medicine were identified. Treatment protocols utilizing TZP were identified and modified to replace TZP with alternative agents, accompanied by corresponding changes to electronic order sets. Prospective auditing of TZP use was also performed through December 28th 2015.
Physician-directed Prospective Auditing of Antimicrobial Use
The electronic medical record was queried to identify all patients actively prescribed the shortage agents. Each chart was then reviewed by a task force member to identify the indication, microbiologic sensitivities, and severity of illness. In cases where a substitution was considered safe and reasonable, the primary team was then called and the change recommended. The final decision on whether to accept or reject this recommendation remained with the primary team. The frequency of review ranged from one to three times a week, adjusted by task force leadership based on shortage severity.
Study Design
Data on antibiotic utilization were retrospectively obtained for all patients hospitalized at BJH between May 1st and Dec 31st, 2015. Antibiotic data were analyzed individually, as well as categorized into broad spectrum gram-negative antibiotics or anti-methicillin resistant Staphylococcus aureus (MRSA) antibiotics. Broad spectrum antibiotics included cefepime (FEP), IPM, MEM, TZP, and ertapenem. Anti-MRSA antibiotics included daptomycin, ceftaroline, linezolid, and vancomycin. Antibiotic use was captured in days of therapy (DOT), mirroring the definitions used in the CDC’s National Healthcare Safety Network (NHSN) Antimicrobial Use surveillance module.6 Antimicrobial use was calculated and plotted in DOT/1000 patient days (PD).7 The number of audits was tracked by date and plotted against antibiotic utilization figures.
Daily antibiotic utilization for each antibiotic and antibiotic group were plotted, comparing pre- and post-intervention usage. To assess overall impact on antibiotic use, linear regression models with autoregressive integrated moving average (ARIMA) errors were fit to the natural logarithm of daily rates, accounting for the correlation of errors over time. Covariates included calendar day and indicator variables for intervention status for each day. Intervention dates for MEM and TZP were chosen as when shortages were first announced to medical staff (the first intervention). Percent changes in rates were then estimated from the fitted models where the coefficients were assessed to be statistically significantly different from null (i.e. zero).
To assess the impact of interventions on remaining supply we calculated the days of use remaining (DUR), defined as the cumulative amount of stocked drug (in grams) divided by the standard daily pseudomonal dose, the quantity of drug used in 24 hours to treat a pseudomonal infection in an adult with normal renal function. For MEM this was 3 gm (1 gm administered every 8 hours), IPM 2 gm (0.5 gm every 6 hours), FEP 6 gm (2 gm every 8 hours), and TZP 18 gm (4.5 gm every 6 hours).
Change in antibiotic expenditure during the shortage was calculated by comparing antibiotic use during the shortage with use over the prior 5 months. Estimated expenditure per 1000 PD for those periods was calculated by multiplying DOT per 1000 PD with the standard daily pseudomonal dose and the publicly reported average wholesale price (AWP) per gram of drug.8
All-cause mortality for the drug-shortage period (October-December 2015) was compared to the mortality rates for October-December for the preceding three years. Clostridium difficile infection (CDI) rates were obtained as NHSN lab-ID reported cases per 1000 PD. Statistical significance was assessed using Student’s t-test.
Regression models with ARIMA errors adjustment was performed using R version 3.2.4 (R Foundation for Statistical Computing, Vienna, Austria). All other analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with graphs produced in SPSS Statistics version 20 (IBM Corp, NY). P-values < 0.05 were considered statistically significant. This study was approved by the Washington University Institutional Review Board.
Results:
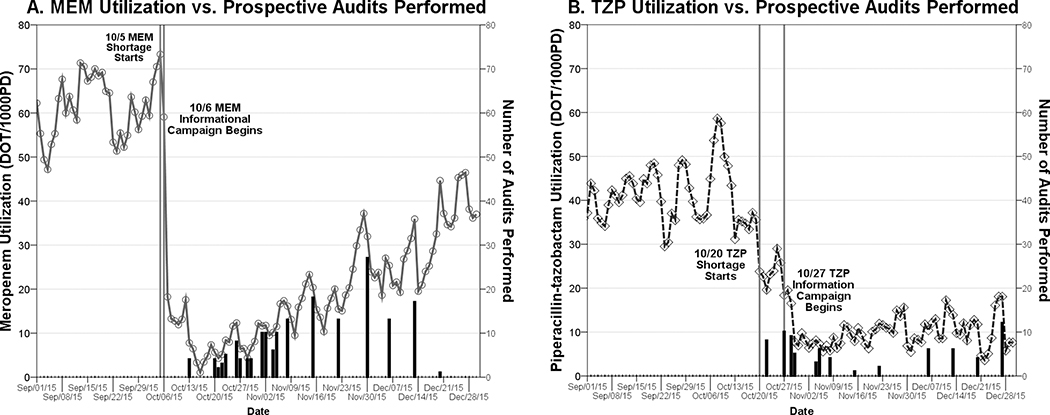
Substantial declines in antimicrobial use were seen for all the shortage drugs during the study period. Decreases in antimicrobial use corresponded with episodes of physician-directed audit and feedback, which totaled 273 audits (172 MEM, 25 IPM, 76 TZP) (Figure 1). Audits were successful in triggering antimicrobial use changes 23% of the time for MEM, 26% for IPM, and 40% for TZP. Shortage intervention periods were associated with statistically significant decreases in antibiotic utilization for MEM, IPM, and TZP, with a compensatory increase in FEP (Table 1). Despite significant changes in MEM, TZP, and FEP utilization, overall broad spectrum, anti-MRSA, and fluoroquinolone agent usage did not change over the course of the interventions.
Figure 1.
Antimicrobial Use During a Drug Shortage: Impact of Number of Daily Drug Use Audits Performed
○ Meropenem (MEM) ◊ Piperacillin-tazobactam (TZP)
*Bars = # Drug Use Audits Performed That Day
Table 1.
Antimicrobial Use During a Drug Shortage: Impact on Antimicrobial Use by Drug-Targeted Intervention Period
| Percent Change in Utilization (95% CI)a | |||
|---|---|---|---|
| Antibiotic Type | MEM/IPM Intervention Period | TZP Intervention Period | Study Period Excluding Intervention Periods |
| Cefepime | 29.4 (16.1, 44.3) | 6.8 (−3.9, 18.6) | 0.0 (−0.1, 0.0) |
| Meropenem | −69.3 (−78.0, −57.2) | NA | 0.0 (−0.2, 0.1) |
| Piperacillin-tazobactam | NA | −72.8 (−76.1, 69.1) | 0.0 (0.0, 0.1) |
| Broad-spectrum β-lactam agentsb | −2.0 (−8.3, 4.6) | −4.6 (−9.7, 0.8) | 0.0 (0.0, 0.1) |
| Anti-MRSA agentsc | 3.6 (−3.6, 11.3) | −2.2 (−8.6, 4.6) | 0.0 (0.0, 0.1) |
| Fluoroquinolone agents | 15.9 (−1.2, 35.9) | −4.6 (−17.5, 10.4) | 0.0 (−0.1, 0.1) |
Note. CI, confidence interval.
intervals that exclude 0 are statistically significant.
Broad-spectrum β-lactams: Cefepime (FEP), imipenem (IPM), meropenem (MEM), piperacillin-tazobactam (TZP), and ertapenem.
Anti-methacillin-resistant Staphylococcus aureus agents: Daptomycin, ceftaroline, linezolid, vancomycin.
Stocks of MEM and IPM increased rapidly after shortage interventions began (Supplementary figure 2). MEM stores increased from a low of 100 DUR to over 3000 DUR. FEP stock remained stable throughout the shortage, with approximately 1000 DUR available on average. TZP supplies averaged 200 DUR throughout the shortage.
Antibiotic expenditure declined by $3,223 per 1000 PD (Table 2). MEM utilization declined from a pre-shortage average of 65.3 to 20 DOT/1000 PD, while TZP use decreased from 38.8 to 16.5 DOT/1000 PD. FEP use increased from 107.3 to 150.9 DOT/1000 PD during the shortage, and smaller increases were seen for IPM (1.7 to 2.7 DOT/1000 PD) and ertapenem (15 to 19 DOT/1000 PD).
Table 2.
The Impact of an Antimicrobial Shortage and Subsequent Conservation Efforts on Estimated Hospital Antimicrobial Expenditure
| Drug | Estimated Average AWP Cost Per DOT | Pre-Shortage Utilization (DOT/1000 PD) | Shortage Utilization (DOT/1000 PD) | Change in Estimated Expenditure Per 1000 PD |
|---|---|---|---|---|
| Cefepime | $91.49 | 107.3 | 150.9 | $3,989 |
| Meropenem | $126.21 | 65.3 | 20 | −$5,717 |
| Imipenem-cilastatin | $88.16 | 1.7 | 2.7 | $88 |
| Piperacillin-tazobactam | $91.50 | 38.8 | 16.5 | −$2,041 |
| Ertapenem | $114.63 | 15 | 19 | $458 |
| Estimated total change in expenditure per 1000 PD | −$3,223 | |||
Note. AWP, average wholesale price; DOT, days of therapy; PD, patient day.
The unadjusted hospital mortality rate for the October through December timeframe decreased to 4.09 deaths per 1000 PD for the shortage year 2015, compared to 4.67, 4.78, and 4.60 for the same months in previous years (p-value 0.03). Length of stay did not significantly change for the shortage year timeframe compared to the previous 3 years. CDI rates did not significantly differ between the shortage period compared to the months of 2015 (data not shown; p-value 0.86).
Discussion:
Antimicrobial stewardship programs have many well-established benefits. Our study is the first to characterize another useful function: effective conservation of antibiotics during drug shortages. Messaging and changes in guidelines and decision support were a very effective method at first reducing antibiotic use. However, prospective intervention was required to sustain reduced antibiotic utilization rates. In this study, systematic changes such as the guideline and decision support changes used to intervene on TZP utilization appear to have had a more sustainable effect than messaging alone (as was done for MEM).
Prospective auditing clearly reduced antimicrobial use, if transiently, potentially providing the edge needed to prevent stock depletion. However, auditing was labor-intensive. Six clinicians were required to audit the shortage drugs, likely due to large patient volume at BJH. The system used was also the most rapidly deployable option, not the most efficient.
Unsurprisingly, our study demonstrated that when specific drugs are targeted for conservation, there is a corresponding rise in the use of alternative antibiotics. This response, colloquially referred to as “squeezing the balloon,” is common and expected during both drug shortages and routine antimicrobial stewardship efforts.
Gross hospital outcomes and CDI rates show no discernible immediate deleterious effect of the shortage and conservation efforts; however, long term effects of our emergency stewardship efforts have yet to be seen. This is a well-known limitation to antimicrobial stewardship monitoring, as effects of microbial susceptibility patterns may be subtle, occurring months afterwards.9,10 This supports the need for persistent stewardship programs, where ongoing monitoring can detect delayed effects and allow for corrective response.
Our study has several limitations. The brief shortage duration limits our ability to make more detailed assessment of rare outcomes such as antimicrobial resistant infections or infection-related mortality. Additionally, our interventions were bundled, making it challenging to determine the effect of individual elements. However, initial communications to medical providers appeared highly effective in reducing antibiotic use. Appropriateness of use was not monitored during this study. In shortage conditions the imperative was deemed to be conserving shortage antibiotics for cases where alternatives would be ineffective. Recommendations were often made to take patients off otherwise appropriate antibiotics in favor of effective non-shortage drugs. Lastly, our estimated drug costs were based on average wholesale price, rather than actual expenditure, and did not take into account antibiotic stockpiling, drug price changes related to the shortage, and emergency purchases of alternative antibiotics. For example, emergency supplies of ceftazidime, ceftazidime-avibactam, and ceftolozane-tazobactam were purchased, but a negligible amount of these agents were ever utilized.
In conclusion, antimicrobial stewardship is a successful method to conserve antibiotics during a drug shortage. Our efforts dramatically reduced selected antibiotic utilization, increased reserve stock of critically short antibiotics, saved pharmacy costs, and did not negatively impact overall patient length of stay or mortality. Hospitals facing critical drug shortages should consider utilizing antimicrobial stewardship teams to conserve medications while maintaining a high-standard of patient care.
Supplementary Material
Supplementary Figure 1. Antimicrobial Use Trends During a Drug Shortage: Antimicrobial Use in Context of Timeline of Antimicrobial Conservation Efforts
□ Cefepime (FEP) ○ Meropenem (MEM) ◊ Piperacillin-tazobactam (TZP) Δ Imipenem (IPM)
Supplementary Figure 2. Antimicrobial Reserve During a Stewardship Managed Drug Shortage
□ Cefepime (FEP) ○ Meropenem (MEM) ◊ Piperacillin-tazobactam (TZP) Δ Imipenem (IPM)
Acknowledgements:
None of the authors have potential conflicts of interest regarding this work. We would like to acknowledge the healthcare informatics team at BJC for their assistance with data collection. Data was collected in part from work supported by the CDC SHEPheRD Grant 2015–010 “Accelerating Hospital Reporting to NHSN’s Antibiotic Use and Resistance Module”.
References:
- 1.Quadri F, Mazer-Amirshahi M, Fox ER, Hawley KL, Pines JM, Zocchi MS, et al. Antibacterial Drug Shortages From 2001 to 2013: Implications for Clinical Practice. Clin Infect Dis 2015;60:1737–1742. [DOI] [PubMed] [Google Scholar]
- 2.Gupta DK, Huang S-M. Drug Shortages in the United States: A Critical Evaluation of Root Causes and the Need for Action. Clin Pharmacol Ther 2013;93:133–135. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn BM. Despite curbing new drug shortages, shortfall of drugs a persistent problem. JAMA 2013;309:532–533. [DOI] [PubMed] [Google Scholar]
- 4.Paskovaty A, Lucarelli CD, Patel P, Ryan M, Seyboth B, Thackray J, et al. Antimicrobial stewardship efforts to manage a pentamidine shortage. Am J Health Syst Pharm 2014;71:2014–2018. [DOI] [PubMed] [Google Scholar]
- 5.Griffith MM, Patel JA, Sutton SH, Bolon MK, Esterly JS, Gross AE, et al. Prospective Approach to Managing Antimicrobial Drug Shortages. Infect Control Hosp Epidemiol 2012;33:745–752. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Antimicrobial Use and Resistance (AUR) Module (updated January 2016) [Internet]. 2016;Available from: http://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf
- 7.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of Adult Antibacterial Drug Use in 130 US Hospitals: Comparison of Defined Daily Dose and Days of Therapy. Clin Infect Dis 2007;44:664–670. [DOI] [PubMed] [Google Scholar]
- 8.RED BOOK Online® [Internet]. RED BOOK Online [cited 2016 May 5];Available from: http://www.micromedexsolutions.com/micromedex2/librarian/CS/780C23/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/ABFF5D/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/redbook.FindRedBook?navitem=topRedBook&isToolPage=true
- 9.Valiquette L, Cossette B, Garant M-P, Diab H, Pepin J. Impact of a Reduction in the Use of High-Risk Antibiotics on the Course of an Epidemic of Clostridium difficile-Associated Disease Caused by the Hypervirulent NAP1/027 Strain. Clin Infect Dis 2007;45:S112–S121. [DOI] [PubMed] [Google Scholar]
- 10.Bantar C, Sartori B, Vesco E, Heft C, Saúl M, Salamone F, et al. A Hospitalwide Intervention Program to Optimize the Quality of Antibiotic Use: Impact on Prescribing Practice, Antibiotic Consumption, Cost Savings, and Bacterial Resistance. Clin Infect Dis 2003;37:180–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Antimicrobial Use Trends During a Drug Shortage: Antimicrobial Use in Context of Timeline of Antimicrobial Conservation Efforts
□ Cefepime (FEP) ○ Meropenem (MEM) ◊ Piperacillin-tazobactam (TZP) Δ Imipenem (IPM)
Supplementary Figure 2. Antimicrobial Reserve During a Stewardship Managed Drug Shortage
□ Cefepime (FEP) ○ Meropenem (MEM) ◊ Piperacillin-tazobactam (TZP) Δ Imipenem (IPM)