Abstract
Background and Purpose:
With advancing age, alterations occur to the immune system, including an increase in inflammation (inflammaging) and a reduced ability to respond to new immune challenges. The role of an aging immune system in patients with ischemic stroke remains unclear, though age is an important determinant of stroke risk and outcome. This study assessed the aging immune system in patients with acute ischemic stroke by differences in leukocyte gene expression in relationship to age.
Methods:
Peripheral blood RNA from two cohorts with acute ischemic stroke was measured by whole genome microarray and genes associated with advancing age were identified (FDR-corrected P < 0.05, partial correlation coefficient > |0.3|). Genes were characterized by pathway analysis and compared to age-associated genes from non-stroke studies (n = 3,973).
Results:
There were 166 genes associated with age in Cohort 1 (derivation cohort, n = 94). Sixty-nine of these age-associated genes were verified in Cohort 2 (validation cohort, n = 79). Identified genes included a decrease in CR2, CD27, CCR7, and NT5E. Genes were associated with altered B cell receptor signaling, lymphocyte proliferation, and leukocyte homeostasis. Forty-three of the 69 age-associated genes in stroke were also associated with age in non-stroke studies.
Conclusion:
A relationship between leukocyte gene expression and age in patients with ischemic stroke was identified. The changes include alterations to the adaptive humoral immune system, which may influence age-related stroke risk and outcome.
Keywords: stroke, aging, immune system, outcome, stroke, ischemic, Cerebrovascular Disease/Stroke, Ischemic Stroke
Graphical Abstract
Introduction
Age is an important determinant of stroke risk and outcome.1 Improved understanding of the biological changes that occur with age may reveal novel treatment opportunities for stroke. The effect of aging on the immune system has been characterized in rodent stroke models and traumatic brain injury2–5 as well as in hypertension, diabetes, and hypercholesterolemia.6–8 This study assessed the aging immune system in patients with acute ischemic stroke by leukocyte gene expression analysis.
A number of changes occur in the immune system with age. Immune cells begin to display diminished functional capacity for antigen presentation, cell trafficking, and responses to cytokine stimulation.9 The system becomes immunosenescent, producing immune responses with reduced specificity and effectiveness to antigenic stimulation.10–12 There is also a rise in inflammatory markers with age (e.g. C-reactive protein, IL-6, and TNF), which is associated with stroke morbidity, pneumonia, and death. 13–15
Age-related changes in immune cells may alter the peripheral response to cerebral ischemia. These are important to understand as they may, in part, explain age-related differences in stroke outcome. We examined leukocyte response to cerebral ischemia in relationship to age using transcriptomic analysis. Differences in leukocyte gene expression and pathways associated with age in patients with stroke were identified. These provide insight to age-associated factors that may contribute to worse post-stroke outcomes and increased risk of infection, and may represent potential targets for reducing the impact of age in stroke.16
Methods
Data Availability Statement:
Data from this study will be made available to qualified investigators upon reasonable inquiry.
Study participants
Patients were recruited from the University of California, Davis and the University of Alberta between January 2009 and December 2015. The study was approved by the institutional review board at each site. All participants provided informed written consent.
Two stroke cohorts were studied. Ischemic stroke was diagnosed by two board-certified stroke neurologists using standardized criteria and required evidence of infarction on brain imaging. Cohort 1 (derivation cohort) consisted of 94 patients with acute ischemic stroke (47 women and 47 men) and Cohort 2 (validation cohort) consisted of 79 patients (40 women and 39 men). Comparisons were made to age-associated genes from two non-stroke aging gene expression sources, described further in Methods: Functional Analysis. Additional details (patient criteria, etc.) are presented in the Supplemental Materials.
Sample and data processing to identify age-associated genes in stroke cohorts
Blood samples were collected in Cohort 1 at a median time of 46 hours after stroke onset (IQR: 25 to 72 hours) and in Cohort 2 at a median time of 35 hours after stroke onset (IQR: 22 to 68 hours) (P = 0.46, Mann-Whitney U test). Samples were processed after thrombolysis as described previously.17 Briefly, total RNA was isolated and measured by Affymetrix GeneChip arrays (Affymetrix, Santa Clara, CA). Data processing was completed using Partek Genomics Suite (v7.0, St. Louis, MO)18 and R software version 3.6.3.19 Raw CEL file data were normalized by Robust Multichip Averaging (RMA), then probe sets were summarized to the gene level and filtered to annotated transcripts. The relationship of gene expression with age was evaluated by analysis of co-variance (ANCOVA) across age tertiles, adjusted for sex and study batch.20 Differentially expressed genes were considered significant with a false discovery rate (FDR)-corrected P < 0.05 and partial correlation coefficient > |0.3| (for details see Supplemental Materials).
Functional Analysis
Functional analysis of differentially expressed genes was performed using Ingenuity Pathway Analysis (IPA 8.0, QIAGEN Inc.)21 and by literature review. Age-associated genes in stroke were compared to external sources of genes associated with aging. In the Peters (2015) study, a meta-analysis by Peters et al., 22 the peripheral blood of 7,074 samples from 14 microarray studies was examined for age-associated genes (1,497 genes reported). The Digital Ageing Atlas (DAA) identified age-associated genes from 42 human microarray and RNA sequencing studies (2,577 genes reported).23
Statistical analysis
Unless otherwise noted, P < 0.05 was considered to be statistically significant and statistical analyses and graphs were generated using Partek Genomics Suite (v7.0, St. Louis, MO)18, R software version 3.6.319 and the R package ggpubr (version 0.2.5).24 Patient characteristics were compared over age tertiles and between cohorts using the Chi-squared test for categorical variables and analysis of variance (ANOVA) for continuous variables except where noted; normality was evaluated by the Shapiro–Wilk test. False discovery rate for age-associated genes was adjusted using the Benjamini-Hochberg approach. Hypergeometric overlap of gene lists was performed using Fisher’s exact test (P < 0.001).
Results
Patient characteristics
Two stroke cohorts were studied as summarised in Table 1. In Cohort 1 (derivation cohort, 94 patients), the mean age was 65.9 years (SD 12.5 years, range 37.8 to 89.6 years). Patients were divided into age tertiles. Cohort 1 comprised 50% females, and was of diverse race and ethnicity with 69.1% Caucasian, 11.7% African-American, 5.3% Asian, 3.2% Hispanic, and 10.6% of other races. Demographics were evenly distributed among the age tertiles, except for the lowest tertile which was predominantly Caucasian. Cohort 2 (validation cohort, 79 patients) was similar to Cohort 1 with a mean age of 63.8 years (SD 12.6 years, range 36.4 to 90.8 years), and was 50.6% female. Race and ethnicity were 49.4% Caucasian, 21.5% African American, 6.3% Asian, 15.2% Hispanic, and 7.6% of other races.
Table 1.
Characteristics of Ischemic Stroke Patients Divided by Age Tertiles
| Parameter | Tertile 1 | Tertile 2 | Tertile 3 | P value |
|---|---|---|---|---|
| Cohort 1 (n=94) | n=31 | n=31 | n=32 | NA |
| Age, mean (SD) | 52.2 (6.22) | 66.1 (3.80) | 80.1 (4.34) | NA |
| Sex Female, n (%) | 17 (54.8%) | 14 (45.2%) | 16 (50.0%) | 0.75 |
| Hypertension, n (%) | 19 (61.3%) | 27 (87.1%) | 25 (78.1%) | 0.06 |
| Diabetes Mellitus, n (%) | 10 (32.3%) | 12 (38.7%) | 10 (31.3%) | 0.80 |
| Hyperlipidemia, n (%) | 13 (41.9%) | 18 (58.1%) | 20 (62.5%) | 0.23 |
| Smoking History, n (%) | 15 (48.4%) | 17 (54.8%) | 11 (34.4%) | 0.25 |
| NIHSS admission, median (IQR) | 3 (2-10) | 2 (1-3) | 2.5 (1-5.75) | 0.22* |
| Cohort 2 (n=79) | n=31 | n=26 | n=22 | NA |
| Age, mean (SD) | 51.7 (6.20) | 65.5 (3.84) | 79.9 (4.55) | NA |
| Sex Female, n (%) | 16 (51.6%) | 13 (50.0%) | 11 (50.0%) | 0.99 |
| Hypertension, n (%) | 22 (71.0%) | 18 (69.2%) | 14 (63.6%) | 0.18 |
| Diabetes Mellitus, n (%) | 10 (32.3%) | 8 (30.8%) | 5 (22.7%) | 0.85 |
| Hyperlipidemia, n (%) | 12 (38.7) | 13 (50.0%) | 10 (45.5%) | 0.73 |
| Smoking History, n (%) | 17 (54.8%) | 9 (34.6%) | 10 (45.5%) | 0.31 |
| NIHSS admission, median (IQR) | 3 (2-4.25) | 2 (1-4) | 4.5 (2.5-5.75) | 0.63* |
NA indicates not applicable
evaluated using Kruskal-Wallis test for multiple groups
Age-associated gene expression in patients with stroke
There were 166 genes associated with age (FDR-corrected P < 0.05, partial correlation coefficient > |0.3|) in Cohort 1. Of these, 137 genes (82.5%) decreased with age and 29 genes (17.5%) increased (Table I in the Supplemental Material). The 166 age-associated genes are shown to separate the upper and lower tertiles of patients in a hierarchical clustering (Figure 1A). In Cohort 2, there were 850 genes associated with age (FDR-corrected P < 0.05, partial correlation coefficient > |0.3|), with 588 genes (69.2%) decreasing with age and 262 genes (30.8%) increasing (Figure 1B and Table II in the Supplemental Materials). There were 69 genes associated with age from Cohort 1 that were confirmed in Cohort 2, shown in part in Table 2 and Figure 2A (Figure 2B and Table III in the Supplemental Materials). The probability that this overlap occurred by chance is P = 9.95 x 10−52 (Fisher’s exact test). Among the genes associated with age in Cohort 1 and Cohort 2 were CR2, CD27, CCR7, and NT5E (Figure 2A).
Figure 1. Hierarchical cluster plot of genes associated with age in patients with ischemic stroke in (A) Cohort 1 and (B) Cohort 2.
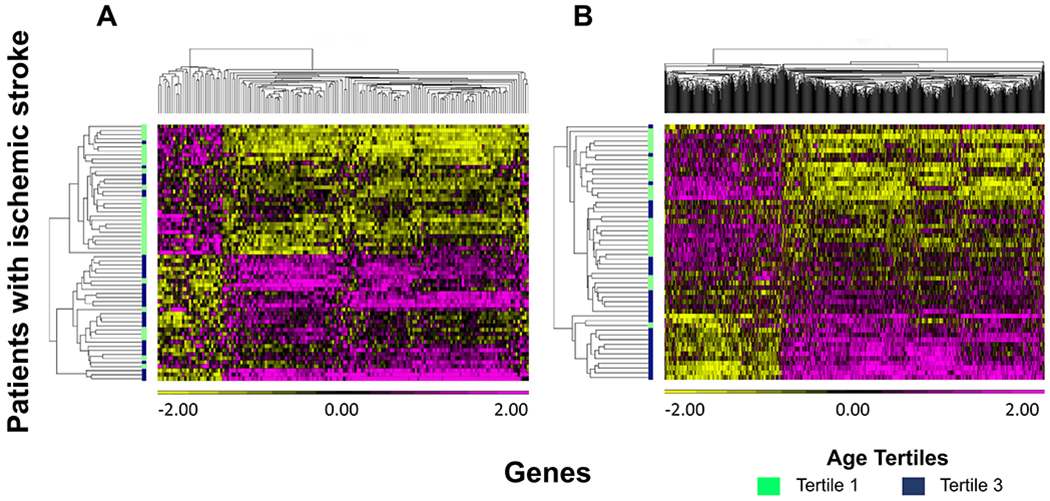
Colour scale below genes on x-axis shows the range in normalized gene expression changes (z-score). Increased gene expression is shown in pink and decreased expression in yellow. Patients are on the y-axis with the lowest age tertile in green (36.4 to 60.8 years) and the highest age tertile in navy blue (73.4 to 90.8 years).
Table 2.
Genes Associated with Age Expressed in Cohort 1 and Confirmed in Cohort 2 in Patients with Ischemic Stroke
| Gene Symbol | Gene Name |
P value (FDR-adjusted) |
r (partial correlation coefficient) | Present In* | |||
|---|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | Peters (2015) Study22 | Digital Ageing Atlas23 | ||
| BANK1 | B cell scaffold protein with ankyrin repeats 1 | 6.27 x 10−6 | 5.67 x 10−6 | −0.46 | −0.53 | 1 | 0 |
| BCL11A | BAF chromatin remodeling complex subunit BCL11A | 2.76 x 10−4 | 4.41 x 10−4 | −0.38 | −0.42 | 1 | 1 |
| BLNK | B cell linker | 1.23 x 10−5 | 5.61 x 10−6 | −0.45 | −0.53 | 0 | 0 |
| BTLA | B and T lymphocyte associated | 3.66 x 10−6 | 1.80 x 10−3 | −0.47 | −0.38 | 1 | 0 |
| CAMK4 | calcium/calmodulin dependent protein kinase IV | 2.45 x 10−4 | 3.26 x 10−4 | −0.38 | −0.43 | 1 | 1 |
| CCR6 | C-C motif chemokine receptor 6 | 2.04 x 10−7 | 2.27 x 10−6 | −0.52 | −0.55 | 1 | 0 |
| CCR7 | C-C motif chemokine receptor 7 | 1.62 x 10−4 | 1.82 x 10−4 | −0.39 | −0.45 | 1 | 0 |
| CD27 | CD27 molecule | 1.87 x 10−4 | 1.33 x 10−3 | −0.39 | −0.39 | 1 | 0 |
| CD79B | CD79b molecule | 3.29 x 10−5 | 2.31 x 10−5 | −0.43 | −0.50 | 1 | 1 |
| CR2 | complement C3d receptor 2 | 2.75 x 10−6 | 3.22 x 10−6 | −0.47 | −0.54 | 1 | 0 |
| CXCR5 | C-X-C motif chemokine receptor 5 | 9.97 x 10−5 | 4.62 x 10−7 | −0.40 | −0.57 | 1 | 0 |
| FCER2 | Fc fragment of IgE receptor II | 1.84 x 10−4 | 2.92 x 10−4 | −0.39 | −0.43 | 0 | 0 |
| FCRL1 | Fc receptor like 1 | 3.67 x 10−5 | 3.26 x 10−4 | −0.42 | −0.43 | 0 | 0 |
| FCRLA | Fc receptor like A | 7.05 x 10−5 | 3.99 x 10−6 | −0.41 | −0.53 | 1 | 0 |
| IGHM | immunoglobulin heavy constant mu | 5.17 x 10−4 | 3.04 x 10−6 | −0.36 | −0.54 | 0 | 0 |
| MGAT5 | alpha-1,6-mannosylglycoprotein 6-beta-N-acetylgluc-osaminyltransferase | 7.32 x 10−6 | 1.45 x 10−4 | −0.46 | −0.45 | 0 | 0 |
| NT5E | 5′-nucleotidase ecto | 2.44 x 10−8 | 1.46 x 10−6 | −0.55 | −0.55 | 1 | 0 |
| POU2AF1 | POU class 2 associating factor 1 | 3.34 x 10−4 | 2.23 x 10−5 | −0.37 | −0.50 | 1 | 0 |
| RASGRP3 | RAS guanyl releasing protein 3 | 3.83 x 10−6 | 4.85 x 10−4 | −0.47 | −0.42 | 1 | 0 |
| SPIB | Spi-B transcription factor | 1.14 x 10−4 | 3.81 x 10−5 | −0.40 | −0.48 | 1 | 0 |
1 = present, 0 = not present.
Figure 2. Gene boxplots (A) and Venn Diagram (B) of age-associated genes found in Cohort 1 and Cohort 2 (P < 0.05, partial correlation coefficient > |0.3|).
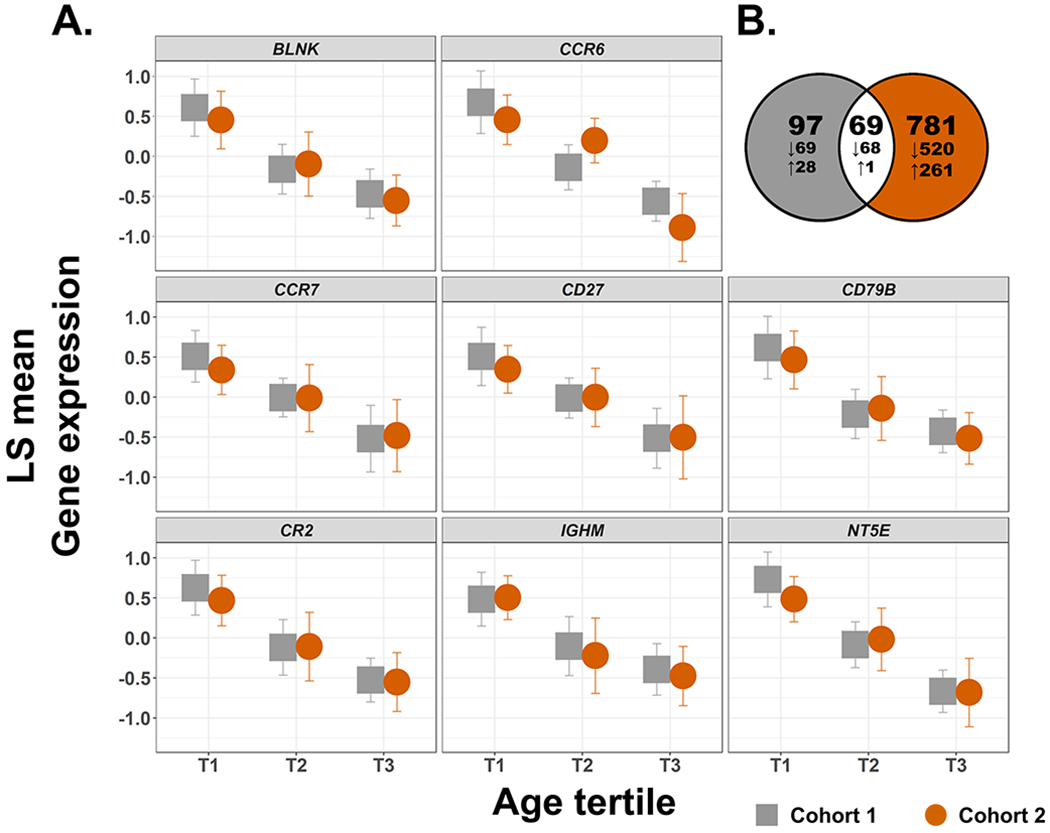
(A) Genes differentially expressed with age in both Cohorts 1 and 2. From left to right, top to bottom; BLNK, CCR6, CCR7, CD27, CD79B, CR2, IGHM, NT5E. Cohort 1 (n = 94) is shown in grey and Cohort 2 (n = 79) is shown in orange. Partial correlation coefficients (r) and P values of significance for Cohort 1 range from −0.55 to −0.36 and 2.44 x 10−8 to 1.62 x 10−4, respectively, and for Cohort 2 from −0.55 to −0.38 and 2.27 x 10−7 to 1.33 x 10−3, respectively (all P values are adjusted by the Benjamini-Hochberg method). Gene expression on the y-axis is displayed as least square (LS) means with 95% confidence intervals. Gene expression was standardized to a mean of zero and standard deviation of one. (B) Venn diagram of age-associated genes found in Cohort 1 (grey) and Cohort 2 (orange). The total number of genes is indicated and associated gene expression direction (increased or decreased) is indicated with arrows.
Functional analysis of age-associated genes in patients with stroke
Functional pathways of the genes in Cohort 1 and Cohort 2 associated with age are summarized in Table 3. Canonical pathways identified include B cell receptor signaling and development, T helper cell differentiation, and IL-7 signaling. Disease-related pathways associated with age include activation and proliferation of lymphocytes, leukocyte and cellular homeostasis, B cell proliferation and antibody response.
Table 3.
Functional Pathways Derived from Genes Associated with Age in Patients with Ischemic Stroke
| Pathways | Cohort 1 P value |
Cohort 2 P value |
Genes |
|---|---|---|---|
| Canonical | |||
| B cell Development | 1.86 x 10−6 | 7.41 x 10−3 | CD40, CD79B, IGHM, IL7, IL7R , CD79A, HLA-DOA, HLA-DOB |
| B cell Receptor Signaling | 2.19 x 10−5 | 6.46 x 10−4 | AKT1, AKT3, APBB1IP, BLNK, CAMK4, CD79B, CFL2, CREB1, ETS1, FOXO1, IGHM, MAP3K11, MAP3K4, MAP3K9, MEF2C, MTOR, PAX5, PIK3C2B, TCF3, CD22, CD79A, EBF1 |
| IL-7 Signaling Pathway | 1.66 x 10−3 | 5.89 x 10−4 | AKT1, AKT3, BCL2, CXCR5, FOXO1, IGHM, IL7, IL7R, PAX5, PIK3C2B, STAT1, EBF1 |
| T Helper Cell Differentiation | 5.37 x 10−5 | 9.33 x 10−3 | CD28, CD40, CXCR5, GATA3, IFNGR1, RORC, STAT1, TGFBR2, HLA-DOA, HLA-DOB, IL23R |
| Disease Associated | |||
| Antibody response | 8.97 x 10−12 | 1.88 x 10−10 | FCER2, CR2, CXCR5, BTLA, BLNK, PAX5, RASGRP3, CCR6, FCRLA, SPIB, POU2AF1 |
| Leukocyte Homeostasis | 9.79 x 10−5 | 1.83 x 10−9 | MS4A1, HLA-DOA, CXCR5, CD79B, PAX5, IL23R, SPIB, POU2AF1, BLK, CR2, EBF1, BLNK, BCL11A, RASGRP3, CD79A, CCR6 |
| Proliferation of B lymphocytes | 1.27 x 10−11 | 6.20 x 10−9 | CR2, EBF1, CD79B, CD22, BCL11A, BLNK, CD79A, CCR6, SPIB, POU2AF1 |
| Proliferation of lymphocytes | 2.80 x 10−10 | 2.51 x 10−9 | MS4A1, CXCR5, CD79B, BTLA, CD22, PAX5, POU2AF1, BLK, FCRL1, CR2, EBF1, MGAT5, BLNK, CD79A, BANK1 |
| Quantity of B lymphocytes | 1.94 x 10−11 | 3.11 x 10−9 | FCER2, CR2, CXCR5, BTLA, BLNK, PAX5, RASGRP3, CCR6, FCRLA, SPIB, POU2AF1 |
| Quantity of lymphocytes | 2.02 x 10−11 | 1.93 x 10−9 | MS4A1, HLA-DOA, CXCR5, CD79B, PAX5, IL23R, SPIB, POU2AF1, BLK, CR2, EBF1, BLNK, BCL11A, RASGRP3, CD79A, CCR6 |
Comparison to other studies of age-associated gene expression
Age-associated genes in patients with stroke were also compared to age-associated genes from the Peters (2015) study22 and the Digital Ageing Atlas (DAA).23 The overlap of age-associated genes in the two stroke cohorts and these groups is shown in Figure I in the Supplemental Material. Of the 69 age-associated genes confirmed in both stroke cohorts, 42 genes (60.9%) were present in the Peters (2015) study and 7 genes (10.1%) in the DAA study. Compared to aging genes in the DAA study, Cohort 1 shared 18 genes (10.8%) and Cohort 2 shared 114 genes (13.4%). Compared to the Peters (2015) study, Cohort 1 shared 76 genes (45.8%, P = 1.88 x 10−42) and Cohort 2 shared 207 genes (24.4%, P = 1.10 x 10−56) (Figure I and Table II in the Supplemental Materials).
Discussion
Age-associated differences in leukocyte gene expression from patients with acute ischemic stroke were identified, relating to functions of the humoral and adaptive immune system including lymphocyte signaling and immune homeostasis.
A number of genes with B cell-specific expression were associated with advancing age in stroke, including CR2 (CD21), MS4A1 (CD20), and CD79 (Igα/β)25 (Tables 2 and 3, Figure 2A). As part of the humoral immune system, B cells have roles in immune activation and in combating infections.26 The effect of B cells on stroke outcome is an emerging area, with potential beneficial, detrimental, and equivocal roles described.27 B cells have roles in hypertension and diabetes mellitus, which may also contribute to their effect in stroke.6 With age, B cells display impaired antibody production and a shift in subset composition.28 This is consistent with the decrease in CD21 (CR2) we observed, which may suggest a shift in B cells toward a pro-inflammatory CD21low B cell subset. This subset has been linked to age, autoimmune disease, and chronic infection.29 In stroke, a shift toward the CD21low B cell subset with age could increase the risk for post-stroke infection and inflammatory responses to cerebral ischemia.
We also observed a change in IL-7 signaling between older and younger patients (Table 3). This may indicate a shift in T cell functional capacity, which has been reported to occur with age.30–32 Additionally, our data showed a decrease in CD27 and CCR7 with age: markers that, among others, are characteristic of terminally differentiated and exhausted lymphocytes.33 Advancing age is linked to a higher proportion of IL-7Rlow CD8+ T cells, which exhibit the diminished proliferative and cytokine-producing characteristics of terminally differentiated effector memory cells (Table II in the Supplemental Material). Late memory and exhausted B cells have a reduced capacity for antibody generation, which is a necessary component for a robust immune response to pathogens. This, paired with a change in T helper cell differentiation (Table 3), may contribute to increased infection risk in older patients with stroke.34 Additional functional evaluation of immune cell subsets is needed to clarify understanding regarding the role of B cell and T cell populations in this context.
An aged immune system may also increase the risk in developing a potentially harmful pro-inflammatory and autoreactive response following stroke.35 We observed a change in leukocyte homeostasis with age (Table 3). This may affect the ability to maintain peripheral inflammatory balance with age, resulting in more autoreactive pro-inflammatory lymphocytes.36 Genes associated with age in this regard include HLA-DOA, HLA-DOB,37 CD22, CD72,38, 39 RAS-GRP3,40 and NT5E (CD73), which may lead to increased inflammation with age. For example, when CD73 is knocked out in a rodent stroke model infarct volume is increased and associated with increased inflammation.41
Our study findings are bolstered by the confirmation of age-associated genes in a second cohort despite differences in patient characteristics. The overlap of identified age-associated genes in other aging studies also supports the robustness of the findings, as these studies involved a wide selection of subjects across a multitude of phenotypic characteristics. The strength of gene overlap with other aging studies points toward a true “aging signature” being detected, which will be valuable for discerning age-associated impacts in stroke. While important changes in leukocyte gene expression with age were identified, our study has limitations. Sample size remains relatively small, thus further evaluation in larger cohorts is required. Age is complex, and the factors that account for changes in the immune system with age post-stroke warrant further study. The rationale for genes to alter expression with age is also complex and warrants additional investigation. Further evaluation is required to assess the factors that contribute to immune related changes with age in patients with stroke such as antigen exposure, comorbid disease, or epigenetic modifications.42–44 Our gene expression analysis suggests immune cell subsets varied with age in patients with stroke, and therefore further functional analysis will be of interest to delineate specific roles of B and T cell subsets with age in stroke.
Conclusions:
With advancing age there is a change in leukocyte gene expression in patients with ischemic stroke. The observed differences in leukocytes may influence age-related stroke risk, stroke outcome, and risk of infection, thus warrant further study.
Supplementary Material
Figure I. Venn diagram comparing age-associated genes in stroke and other aging studies.
Table I. Differentially expressed genes with age in patients with ischemic stroke from Cohort 1
Table II. Differentially expressed genes with age in patients with ischemic stroke from Cohort 2
Table III. Genes Associated with Age Expressed in Cohort 1 and Confirmed in Cohort 2 in Patients with Ischemic Stroke
Acknowledgements:
Study concept and design, Manuscript Drafting, Data Analysis: GPS, GCJ
Data acquisition: GPS, BS, FRS, GCJ
Data Interpretation: All authors
Study Funding: This study was supported by CIHR, Heart and Stroke Foundation, University of Alberta Hospital Foundation, and NIH.
Non-Standard Abbreviations and Acronyms
- DAA
Digital Ageing Atlas
- NIHSS
National Institutes of Health Stroke Scale
- RMA
Robust Multichip Averaging
- FDR
False Discovery Rate
Footnotes
Financial Disclosures: JKT: Bank of Montreal Financial Group and NMHI.
GPS, SF, SZ, DM, BPA, BS, FRS, GCJ: None
Supplemental Materials:
Contributor Information
Gina P Sykes, Division of Neurology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada.
Joseph Kamtchum-Tatuene, Neuroscience and Mental Health Institute, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada.
Sarina Falcione, Department of Medical Microbiology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada.
Sarah Zehnder, Division of Neurology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada.
Danielle Munsterman, Division of Neurology, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada.
Boryana Stamova, Department of Neurology, University of California, Davis, Sacramento.
Bradley P Ander, Department of Neurology, University of California, Davis, Sacramento.
Frank R Sharp, Department of Neurology, University of California, Davis, Sacramento.
Glen Jickling, Division of Neurology, Department of Medicine and Neuroscience and Mental Health Institute, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Canada, and Department of Neurology, University of California, Davis, Sacramento.
References
- 1.Drozdowska BA, Singh S, Quinn TJ. Thinking about the future: A review of prognostic scales used in acute stroke. Front Neurol. 2019;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritzel RM, Lai YJ, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, et al. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 2018;136:89–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hazy A, Bochicchio L, Oliver A, Xie E, Geng S, Brickler T, Xie H, Li L, Allen IC, Theus MH. Divergent age-dependent peripheral immune transcriptomic profile following traumatic brain injury. Sci Rep. 2019;9:8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wesley UV, Bhute VJ, Hatcher JF, Palecek SP, Dempsey RJ. Local and systemic metabolic alterations in brain, plasma, and liver of rats in response to aging and ischemic stroke, as detected by nuclear magnetic resonance (nmr) spectroscopy. Neurochem Int. 2019;127:113–124 [DOI] [PubMed] [Google Scholar]
- 5.Pan M, Wang P, Zheng C, Zhang H, Lin S, Shao B, Zhuge Q, Jin K. Aging systemic milieu impairs outcome after ischemic stroke in rats. Aging Dis. 2017;8:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaraj UM, Poinsatte K, Torres V, Ortega SB, Stowe AM. Heterogeneity of B cell functions in stroke-related risk, prevention, injury, and repair. Neurotherapeutics. 2016;13:729–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv A, Aviv H. Reflections on telomeres, growth, aging, and essential hypertension. Hypertension. 1997;29:1067–1072 [DOI] [PubMed] [Google Scholar]
- 8.Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, Perkins G, Dong H, Kayali AG, Sweet IR, et al. The fractalkine/cx3cr1 system regulates β cell function and insulin secretion. Cell. 2013;153:413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341 [DOI] [PubMed] [Google Scholar]
- 10.Deleidi M, Jäggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: Between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goronzy JJ, Weyand CM. Aging, autoimmunity and arthritis: T-cell senescence and contraction of t-cell repertoire diversity - catalysts of autoimmunity and chronic inflammation. Arthritis Res Ther. 2003;5:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruunsgaard H, Skinhøj P, Qvist J, Pedersen BK. Elderly humans show prolonged in vivo inflammatory activity during pneumococcal infections. J Infect Dis. 1999;180:551–554 [DOI] [PubMed] [Google Scholar]
- 14.Van Den Biggelaar AH, De Craen AJ, Gussekloo J, Huizinga TW, Heijmans BT, Frölich M, Kirkwood TB, Westendorp RG. Inflammation underlying cardiovascular mortality is a late consequence of evolutionary programming. FASEB J. 2004;18:1022–1024 [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: Transition from theory to practice. Circ J. 2010;74:213–220 [DOI] [PubMed] [Google Scholar]
- 16.Prattichizzo F, De Nigris V, La Sala L, Procopio AD, Olivieri F, Ceriello A. “Inflammaging” As a druggable target: A senescence-associated secretory phenotype-centered view of type 2 diabetes. Oxid Med Cell Longev. 2016;2016:1810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dykstra-Aiello C, Jickling GC, Ander BP, Shroff N, Zhan X, Liu D, Hull H, Orantia M, Stamova BS, Sharp FR. Altered expression of long noncoding rnas in blood after ischemic stroke and proximity to putative stroke risk loci. Stroke. 2016;47:2896–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partek Inc. Partek® genomics suite®. 2019 [Google Scholar]
- 19.R Core Team. R: A language and environment for statistical computing - reference index. 2019 [Google Scholar]
- 20.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics. 2014;30:523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters MJ, Joehanes R, Pilling LC, Schurmann C, Conneely KN, Powell J, Reinmaa E, Stuphin GL, Zhernakova A, Schram K, et al. The transcriptional landscape of age in human peripheral blood. Nat Commun. 2015;6:8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig T, Smelick C, Tacutu R, Wuttke D, Wood SH, Stanley H, Janssens G, Savitskaya E, Moskalev A, Arking R, et al. The digital ageing atlas: Integrating the diversity of age-related changes into a unified resource. Nucleic Acids Res. 2015;43:D873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kassambara A ggpubr: ‘ggplot2’ Based Publication Ready Plots. Version 0.2.5. 2020
- 25.Online mendelian inheritance in man, omim®. 2020
- 26.Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, Bos NA, Johnsen HE, Orfao A, Perez-Andres M, et al. Circulating human b and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal cd138− and cd138+ plasma cells. Haematologica. 2010;95:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javidi E, Magnus T. Autoimmunity after ischemic stroke and brain injury. Front Immunol. 2019;10:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulati M, Caruso C, Colonna-Romano G. From lymphopoiesis to plasma cells differentiation, the age-related modifications of b cell compartment are influenced by "Inflamm-ageing". Ageing Res Rev. 2017;36:125–136 [DOI] [PubMed] [Google Scholar]
- 29.Charles ED, Brunetti C, Marukian S, Ritola KD, Talal AH, Marks K, Jacobson IM, Rice CM, Dustin LB. Clonal b cells in patients with hepatitis c virus-associated mixed cryoglobulinemia contain an expanded anergic cd21low b-cell subset. Blood. 2011;117:5425–5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HR, Hong MS, Dan JM, Kang I. Altered il-7ralpha expression with aging and the potential implications of il-7 therapy on cd8+ t-cell immune responses. Blood. 2006;107:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ucar D, Márquez EJ, Chung CH, Marches R, Rossi RJ, Uyar A, Wu TC, George J, Stitzel ML, Palucka AK, et al. The chromatin accessibility signature of human immune aging stems from cd8. J Exp Med. 2017;214:3123–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aspinall R, Lapenna A, B-Lynch C, Lang PO. Cellular signalling pathways in immune aging and regeneration. Biochem Soc Trans. 2014;42:651–656 [DOI] [PubMed] [Google Scholar]
- 33.Fülöp T, Larbi A, Pawelec G. Human t cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck-Loebenstein B. Age-related loss of naïve t cells and dysregulation of t-cell/b-cell interactions in human lymph nodes. Immunology. 2005;114:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle KP, Buckwalter MS. Does b lymphocyte-mediated autoimmunity contribute to post-stroke dementia? Brain Behav Immun. 2017;64:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Y, Jensen PE, Chen X. Immunodeficiency and autoimmunity in h2-o-deficient mice. J Immunol. 2013;190:126–137 [DOI] [PubMed] [Google Scholar]
- 38.Dörner T, Shoc A, Smith K. Cd22 and autoimmune disease. International Reviews of Immunology. 2012;31:363–378 [DOI] [PubMed] [Google Scholar]
- 39.Nitschke L The role of cd22 and other inhibitory co-receptors in b-cell activation. Curr Opin Immunol. 2005;17:290–297 [DOI] [PubMed] [Google Scholar]
- 40.Stone JC. Regulation and function of the rasgrp family of ras activators in blood cells. Genes Cancer. 2011;2:320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki K, Uchida K, Nakanishi N, Hattori Y. Cilostazol activates amp-activated protein kinase and restores endothelial function in diabetes. Am J Hypertens. 2008;21:451–457 [DOI] [PubMed] [Google Scholar]
- 42.Krupinski J, Carrera C, Muiño E, Torres N, Al-Baradie R, Cullell N, Fernandez-Cadenas I. Dna methylation in stroke. Update of latest advances. Comput Struct Biotechnol J. 2018;16:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the aric study (atherosclerosis risk in communities). Circ Genom Precis Med. 2018;11:e001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soriano-Tárraga C, Giralt-Steinhauer E, Mola-Caminal M, Vivanco-Hidalgo RM, Ois A, Rodríguez-Campello A, Cuadrado-Godia E, Saylos-Baixeras S, Elosura R, Roquer J, et al. Ischemic stroke patients are biologically older than their chronological age. Aging (Albany NY). 2016;8:2655–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure I. Venn diagram comparing age-associated genes in stroke and other aging studies.
Table I. Differentially expressed genes with age in patients with ischemic stroke from Cohort 1
Table II. Differentially expressed genes with age in patients with ischemic stroke from Cohort 2
Table III. Genes Associated with Age Expressed in Cohort 1 and Confirmed in Cohort 2 in Patients with Ischemic Stroke
Data Availability Statement
Data from this study will be made available to qualified investigators upon reasonable inquiry.


