Abstract
The signal enhancement provided by the hyperpolarization of nuclear spins of metabolites is a promising technique for diagnostic magnetic resonance imaging (MRI). To date, most 13C-contrast agents are hyperpolarized utilizing a complex or cost-intensive polarizer. Recently, the in situ parahydrogen-induced 13C hyperpolarization was demonstrated. Hydrogenation, spin order transfer (SOT) by a pulsed NMR sequence, in vivo administration, and detection was achieved within the magnet bore of a 7 Tesla MRI system. So far, the hyperpolarization of the xenobiotic molecule 1-13C-hydroxyethylpropionate (HEP) and the biomolecule 1-13C-succinate (SUC) through the PH-INEPT+ sequence and a SOT scheme proposed by Goldman et al., respectively, was shown. Here, we investigate further the hyperpolarization of SUC at 7 Tesla and study the performance of two additional SOT sequences. Moreover, we present first results of the hyperpolarization at high magnetic field of 1-13C-phospholactate (PLAC), a derivate to obtain the metabolite lactate, employing the PH-INEPT+ sequence. For SUC and PLAC, 13C polarizations of about 1–2 % were achieved within seconds and with minimal equipment. Effects that potentially may explain loss of 13C polarization have been identified, i.e. low hydrogenation yield, fast T1/T2 relaxation and the rarely considered 13C isotope labeling effect.
Introduction
Magnetic Resonance Imaging (MRI) is a widely used clinical imaging modality due to its excellent soft tissue contrast and non-ionizing radiation. In clinical routine, however, the available signal-to-noise ratio (SNR) is limiting its application to imaging and probing the properties of the abundant protons (1H) of water and lipids. While this is sufficient for important and essential diagnostics, many other applications such as sodium imaging1 or metabolic imaging are not feasible in clinical routine. At room temperature and with currently available magnets, only a few out of a million nuclear spins effectively contribute to the Magnetic Resonance (MR) signal according to the Boltzmann distribution. For 1H, the low polarization is compensated by a high concentration mostly in water and lipids. Hence, sufficient SNR is obtained and 1H MRI is feasible. For metabolites or X-nuclei, i.e. a nucleus other than proton, such as 23Na, 13C or 15N, the situation is different as their concentration, polarization, or natural abundance is much lower. Hence, in many cases, the MR signals of metabolites and X-nuclei are well below the detection limit and signal averaging is required.1–4
The hyperpolarization, i.e. the creation of a non-equilibrium overpopulation of the Zeeman eigenstates, is a method to boost the polarization of MR active nuclei (and thus its signal) by many orders of magnitude.5 For instance, hyperpolarized pyruvate, lactate, and fumarate, have been successfully employed to visualize metabolic processes. Cancer was detected and response to treatment was measured in vivo by Magnetic Resonance Spectroscopy Imaging without background signal.6–9 Because of the short lifetime of 13C hyperpolarization the sensitivity-enhanced detection of metabolic conversion is limited to a time-window of rarely more than two minutes.7
The most established and commercially available hyperpolarization technique is dissolution Dynamic Nuclear Polarization (d-DNP)10,11. However, the method is time-consuming and expensive (106 € or more). Typically, achieving a 13C polarization of P > 10 % requires > 30 min per sample.
Another hyperpolarization method is based on the spin order of parahydrogen, i.e. dihydrogen (H2) enriched in its nuclear singlet state (PHIP).12–14 Parahydrogen (pH2) has a combined spin of zero, i.e. it is MR invisible. However, its perfectly ordered spin state can be used to achieve X-nuclei hyperpolarization in several ways. By adding pH2 to an unsaturated molecule (hydrogenation), the spin order becomes available in the product molecules’ spin system. Under PASADENA13 conditions, the 1H spin order can be transferred to 13C through evolution under J-coupling and spin manipulations by spin order transfer (SOT) pulse sequences.15–24 These sequences consist of periods of free evolution and radiofrequency (RF) pulses applied to 1H and 13C.
The pH2 based hyperpolarization procedure is typically executed in an external device (the “polarizer”) operating at low magnetic fields in the range of a few milliTesla.25–33 At these fields, the 13C hyperpolarization of lactate derivatives and (diethyl-) succinate as high as 15 % and 28 %, respectively, have been demonstrated.31,32,34–37 The hyperpolarization of lactate is promising, because high lactate concentrations are sustained well in living organisms and lactate may be used for the investigation of brain and energy metabolism.9,38–40 Please note that purification of the solution from the catalyst and reaction side product is required prior rigorous in vivo application.
For metabolic imaging of molecules hyperpolarized by low-field PHIP or d-DNP, the samples are typically transferred to a high field detection site. During this transfer, T1-relaxation reduces the precious signal enhancement.
Recently, the hyperpolarization of molecules at high field, in an NMR spectrometer or directly in an MRI, has made great progress. 13C polarizations of about 60 % and 9 % for esters of the metabolites acetate and pyruvate, respectively, have been achieved in an NMR spectrometer at 7 T.22,41 Moreover, synthesis amid the magnet bore allows a dramatically enhanced nuclear alignment (SAMBADENA) and we demonstrated that high field methods can be employed to conduct a variant of PASADENA in an MRI system42–44. Dispensing with the need of a transfer, we administrated and imaged an aqueous hyperpolarized solution within no more than 15 s after hyperpolarization.
So far, the SAMBADENA hyperpolarization of the xenobiotic 13C agent 1-13C,2,3,3-2H3-hydroxyethylpropionate (HEP) and the biomolecule 1-13C,2,3-2H2-succinate (SUC) was shown. The different molecular structure of the two substrates required the application of two different SOT sequences for efficient polarization transfer. For hyperpolarization of HEP, the SOT sequence PH-INEPT+23 was used, whereas for hyperpolarization of SUC, the sequence proposed by Goldman et al17. was employed. In both cases, 1H and 13C RF pulses were played out for spin order transfer. Calculations in simplified models predicted a 13C polarization of 49 % and 99 % for HEP and SUC, respectively.42,44,45 Experimentally, reduced 13C polarizations of about 20 % for HEP and 10 % for SUC were found.42,44 The low SUC polarization was attributed to singlet-triplet mixing during hydrogenation that led to a 10-fold reduction of available singlet spin order.44
Here, we present initial experiences of the SAMBADENA hyperpolarization of 1-13C-2H2-phospholactate (PLAC) at 7 T in an MRI. Furthermore, we investigate the hyperpolarization of SUC with two other SOT sequences, denoted as ECHO23,46 and ADAPT19. For both SUC and PLAC, we investigated the hydrogenation and SOT efficiency by varying hydrogenation durations and SOT sequence parameters. Moreover, the influence of pH buffer solutions on the PLAC polarization level was investigated. Quantum mechanical simulations were performed to understand experimental findings better.
Theory and Simulations
13C PASADENA Agents and Spin Order Transfer Sequences
Succinate
As the molecule SUC is expected to form an AA′X spin system to a good approximation,37 spin order transfer sequences tailored to strongly coupled protons are well suited (Figure 1a). AA′X spin systems consist of two chemically equivalent but magnetically inequivalent protons A and A′. The values of the J-couplings were taken from literature as J12 = 7.41 Hz, J13 = −7.15 Hz, and J23 = 5.82 Hz.37 The isotropic chemical shifts of the two 1H nuclei and 13C nucleus were determined by NMR spectroscopy and take the values δ1(1H) ≈ δ2(1H) ≈ 2.75 ppm, and δ3(13C) = 176 ppm. The two protons experience a slightly different effective magnetic field because of isotopic 13C labeling and become chemically inequivalent, i.e. δ1(1H) ≠ δ2(1H). Simulations and experiments indicate that the actual difference of the Larmor frequencies at 7 T is about 4 Hz (Figure 5 and supplementary information). To our knowledge, this effect was not taken into account in simulations of SOT before. Please note that couplings to 2H spins are not considered as the 2H-1H interactions are a factor of γ(1H)/γ(2H) = 6 smaller than the 1H-1H couplings.47 SUC is an intermediate in the tricarboxylic acid cycle and may be used for the diagnosis of brain cancer and potentially other diseases.35,36,48
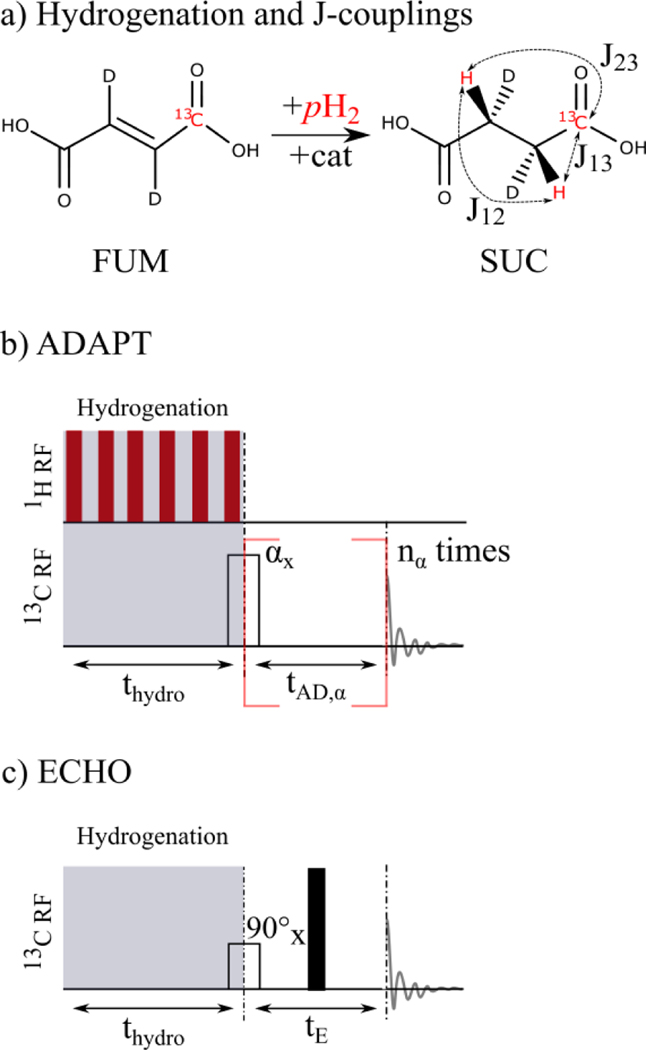
Figure 1: Hydrogenation reaction, J-coupling network, and SOT sequences for succinate and strongly coupled protons.
a) The pH2 protons are added to fumarate (FUM) via a rhodium-based catalyst at 90°C and 20 bar, forming succinate (SUC). The values of the J-couplings read J12 = 7.41 Hz, J13 = −7.15 Hz, and J23 = 5.82 Hz. The SOT sequences ADAPT19 (b) and ECHO23,46 (c) were used to transfer the 1H para order to 13C polarization and consist of RF pulses and free evolution times tAD,α and tE. Note that ADAPT requires heteronuclear decoupling during hydrogenation to preserve singlet state (red bars), whereas ECHO does not. The black bar denotes a 180° spin echo pulse along the x- or y-axis at tE/2.
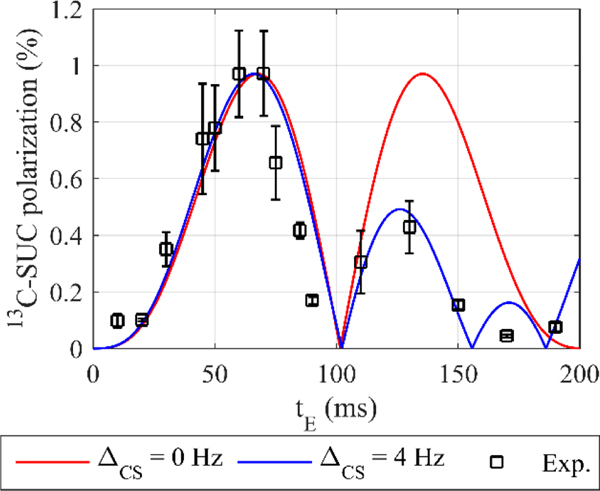
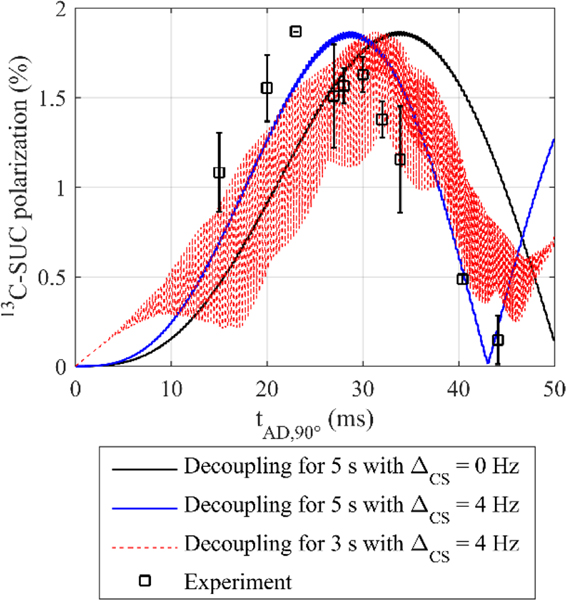
Figure 5: Experimental (squares) and computed (lines) 13C polarization of SUC as a function of tE, the free evolution interval of the ECHO sequence.
The polarization yield of SUC was simulated with and without the effect of the 13C labeling on the 1H chemical shift (red line: ΔCS = 0 Hz, blue line: ΔCS = 4 Hz). The simulated polarization yield was scaled by a constant factor 4.9 to match the maximum of the experimental data (SUC.E2). The simulations matched the experiments better if chemically inequivalent protons were assumed (blue line). All data points were measured three times and the error bars represent the standard deviation.
Here, two SOT sequences, denoted as ADAPT19 and ECHO23,46, tailored to chemically equivalent protons were employed for hyperpolarization of SUC (Figure 1). In contrast to the recently reported hyperpolarization of SUC with Goldman’s sequence,17,44,45 ECHO and ADAPT require 13C RF pulses only and less RF excitations. Hence, ECHO and ADAPT are expected to be less susceptible to erroneous flip angles and off-resonance effects than Goldman’s sequence. In addition, ECHO does not require heteronuclear decoupling during hydrogenation. This is of major importance for MR systems with limited power specifications of the transmit array.
The ADAPT sequence19 converts singlet spin order I1I2 = I1zI2z + I1yI2y + I1xI2x into detectable 13C magnetization (Figure 1b). Therefore, heteronuclear decoupling is essential during hydrogenation to preserve singlet state in the product system. Depending on the flip angle αx ∈ (0°, 180°), the free evolution interval tAD,α and number of ADAPT cycles nα have to be set appropriately. In this contribution, the parameters were chosen as αx = 90° and nα = 2. Please note that according to the original description of the ADAPT sequence19, no 180° spin echo pulses are played out during free evolution. Another sequence, denoted as ECHO here, transfers (I1z-I2z)I3z 1H spin order into 13C polarization and requires neither decoupling during hydrogenation nor 1H RF pulses (Figure 1c). After hydrogenation, a 90° 13C RF pulse followed by free evolution and a 180° spin echo pulse is played out.
Phospholactate
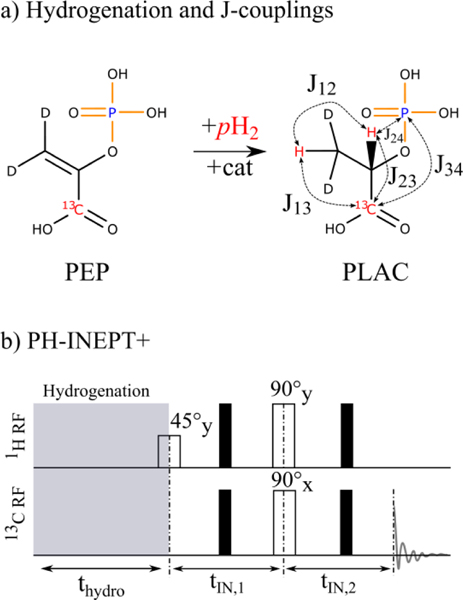
The molecule PLAC forms an ABDX 4-spin system at 7 T with two chemically inequivalent 1H spins A and B, one 31P spin D, and one 13C spin X. The chemical shift difference of the two protons, 225 Hz at 7 T, is much larger than the mutual J-coupling of 6.90 Hz (Figure 2). The values of the chemical shifts δi in ppm and J-couplings Jij in Hz were taken from literature31 and read δ1(1H) = 1.29, δ2(2H) = 4.33, δ3(13C) = 182.1, δ4(31P) = 4.53, J12 = 6.90, J13 = 4.07, J23 = 3.80, J34 = 6.52, and J24 = 8.60. The sequence PH-INEPT+23 was employed for hyperpolarization as it was designed for systems containing weakly coupled protons. Originally, it was assumed that the singlet spin order I1I2 of pH2 is converted into I1zI2z spin order by averaging of the transversal I1yI2y-and I1xI2x-components during hydrogenation at high field.23 1H and 13C 180° spin echo pulses are played out in between the other pulses to compensate for different precession frequencies.
Figure 2: Hydrogenation reaction, J-coupling network, and PH-INEPT+ sequence used for PLAC.
By addition of pH2, phosphoenolpyruvate (PEP) is converted to PLAC (a). By means of the PH-INEPT+ pulse sequence23, spin order is transferred to 13C (b). In a simplified model, PLAC is a 4-spin system composed of two weakly coupled protons, one 13 C nucleus, and one 31P nucleus (blue) with the corresponding J-couplings J12 = 6.90 Hz, J13 = 4.07 Hz, J23 = 3.80 Hz, J34 = 6.52 Hz, and J24 = 8.60 Hz.31 The sequence consists of two free evolution intervals and 1H/13 C RF pulses. Black bars denote 180° spin echo pulses.
PLAC was previously hyperpolarized in low magnetic fields and a maximum polarization of up to 15 % was achieved inside a low-field polarizer.28,31,32,34,49 It was reported that the phosphate group in PLAC (Figure 2a, orange) is removed by blood dephosphorylation quickly after in vivo administration such that the metabolite 1-13C-lactate is obtained.49 Please note, that for now, the PHIP-SAH strategy proposed by Reineri et al. is the only method by which pH2 hyperpolarized 1-13C-lactate has been obtained.50,51
Spin Hamiltonian, Density Matrix, and Strategy for SOT Computations
Spin Hamiltonian
The spin system was driven by the Zeeman interaction considering the isotropic chemical shift (δi) and J-couplings (Jij). The Hamiltonian of PLAC in the laboratory frame of reference reads (ħ = 1)
| (Equation 1) |
where the summation runs over all spins and all coupling pairs with IiIj = IixIjx + IiyIjy + IizIjz. Here, γi denotes the gyromagnetic ratios of 1H, 13C, and 31P (γ1 = γ2 = γH, γ3 = γC, and γ4 = γP).
The Hamiltonian of SUC is of the form
| (Equation 2) |
The factor ΔCS was introduced to account for a difference of the 1H Larmor frequencies induced by the 13C labeling and consequential symmetry breaking.52
Density Matrix of PLAC and SUC
The density matrices representing the spin system after hydrogenation were obtained by numerical simulations. As the reaction takes place over a finite period of time thydr, each hydrogenated molecule experiences a different evolution time t. This condition is approximated by calculating the time-averaged density operator
| (Equation 3) |
where p(t) is a weighting function that reflects the amount of hydrogenated molecules at different points in time. Q is an operator that either describes free evolution (ECHO and PH-INEPT+, Figures 1 and 2) or heteronuclear MLEV16 decoupling53 (ADAPT, Figure 1b). The free evolution operator is given by the matrix exponential of –it. The exponential was computed numerically employing the MatLab function “expm”. Ideal RF pulses (on-resonant and instantaneous rotations) are approximated by the exponential of −iαIjk where α denotes the flip angle and Ijk represents the spin operator of spin j in k-direction (k = x,y, or z). MLEV16 decoupling pulses of finite duration tMLEV were approximated by a single rotation with subsequent time evolution tMLEV. The pulse duration of a single 90° excitation within the MLEV16 pulse train was chosen as tMLEV(90°) ≈ 1 ms in order to simulate the experimental conditions (Table 1).
Table 1: Performed experiments with SUC at 7 T and pH 2.9 using buffer B.1.
The hyperpolarization was investigated in three experimental series: In SUC.E1, ECHO was applied and the polarization was measured as a function of the hydrogenation duration by variation of tdelay with a fixed free evolution interval. In SUC.E2, the free evolution interval tE was varied while tdelay was fixed. To investigate the polarization scheme of the ADAPT sequence, the free evolution interval tAD,90° was varied with fixed hydrogenation duration thydr = 5 s (SUC.E3).
| SOT | Timings / Parameters | |
|---|---|---|
| SUC.E1 | ECHO |
tpH2 = 2 s, Variation of tdelay tE = 70.0 ms Setup 1, 90° C |
| SUC.E2 | ECHO |
tpH2 = 2 s, tdelay = 3 s Variation of tE Setup 1, 90° C |
| SUC.E3 | ADAPT |
tpH2 = 2 s, tbypass = 3 s αx = 90°, nα = 2 Variation of tAD,90° Setup 2, 90° C tMLEV(90°) = 1 ms |
When simulating the hydrogenation process of PLAC numerically, the sudden addition of pH2 was assumed, resulting in a density matrix of the form
| (Equation 4) |
The first factor represents singlet state of the two protons, i.e.
| (Equation 5) |
in Zeeman basis. The density matrices of the target nucleus and 31P spin were assumed to be a normalized unity matrix, i.e. non-polarized, neglecting the low thermal 13C and 31P polarization.
For SUC, two different initial density matrices were considered. In an idealized model the density matrix of a single SUC-molecule was given by
| (Equation 6) |
However, in previous experiments conducted at 7 T using the same hydrogenation catalyst as used in this work, singlet-triplet mixing during hydrogenation led to a significant decrease of singlet spin order.44 Analysis of 1H PASADENA spectra revealed that the amount of singlet and triplet spin order was about 55 % and 45 %, respectively. Hence, the density matrix ρ0 of a single SUC-molecule directly after hydrogenation with pH2 was further assumed to be of the form
| (Equation 7) |
Please note that analytical equations of the time-averaged density matrix were derived for AB and AA’X spin systems by Hübler et al. and Natterer et al.54,55 In the latter paper, however, a potential 13C isotope effect was neglected.
Computations of SOT Sequences
To simulate the effect of free evolution and RF pulses on 1H and 13C, rotation and time evolution operators were applied to the time-averaged density matrix For different initial states of the spin system (Equations 4–7) and different values of ΔCS (Equation 2), the 13C polarization yield was calculated as a function of the free evolution intervals of the SOT sequences (0.05 ms time increments). Moreover, the influence of the 31P spin in PLAC was investigated as computations were conducted excluding or including the 31P spin. The transversal polarization was determined by taking the trace over the matrix product of the final density matrix (after application of the SOT sequence) and the polarization operator 2(I3x+iI3y). Relaxation effects, for instance due to dipole-dipole interaction or chemical exchange in hydroxyl groups, were neglected in all simulations.
Two-Site Kinetic Model of the Hydrogenation Reaction
To estimate the reaction kinetics and relaxation of 1H spin order during hydrogenation experimentally, the 13C polarization was measured as a function of the total hydrogenation duration thydr. A model was developed to fit the data (Equation 8), consisting of an asymptotical increase of the hydrogenated molecules (∝ 1 − exp[−thydr/tcat]), and a mono-exponential decay of the polarization due to relaxation (∝ exp[−thydr/tspin].42,56
| (Equation 8) |
The coefficients Pmax and t0 represent fit parameters that allow the maximum polarization to be adjusted and to consider an activation delay between application of pH2 pressure and the onset of the hydrogenation reaction, respectively. The time constants for hydrogenation and relaxation of the initial 1H spin order are denoted by tcat and tspin, respectively.
Experiments
Enrichment of Parahydrogen
The fraction of pH2 was enriched to ≈ 90 % using a custom-built pH2 converter at a temperature of 21 K as described previously.42–44,57 The pH2-enriched gas was stored in 2.7 L steel or 0.5 L aluminum reservoirs at a pressure of 50 bar and was used within 10 hours.
Sample Preparation
1-13C,2,3-2H2-succinate was formed by hydrogenation of 1-13C,2,3-2H2-fumarate (FUM, 118.08 g/mol molecular weight, CAS: 24461–32-3, Cambridge Isotope Laboratories, deionized H2O as solvent).
By hydrogenation of the precursor 1-13C-2H2-phosphoenolpyruvate (PEP, 170.0 g/mol, in-house synthesis as described in literature32, D2O as solvent) PLAC is obtained.
For hyperpolarization experiments, reaction solutions were prepared with different concentrations of substrate (cFUM = 5 mM, cPEP = 10 mM) and catalyst (ccat ≈ 2 mM) using a Schlenk line and degassing as described previously42–44. The catalyst was obtained by mixing 1,4-bis-[(phenyl-3-propane sulfonate) phosphine] butane disodium salt (≈ 2.2 mM, Q36333, 562.5 g/mol, Sigma Aldrich) with bis(norbornadiene)rhodium (I) tetrafluoroborate (≈ 2.0 mM, CAS: 36620–11-8, StremChemicals).
Previous reports of pH2-induced polarization of SUC and PLAC suggested adjusting the pH value to either low (3–4) or high (10–11) pH for well-resolved J-couplings, to reduce relaxation due to chemical exchange, and to achieve a high hydrogenation yield.32,37 Hence, different substances B.1-B.3 were used to buffer the reaction solution to a pH of 3 for SUC and about 10 for PLAC.
B.1: A pH buffer based on potassium dihydrogen phosphate (KH2PO4, CAS: 7778–77-0) and phosphoric acid (H3PO4, CAS: 7664–38-2) for experiments with SUC was prepared (cKH2PO4 = 40 mM, cH3PO4 = 3 mM). A pH value of 2.9 was measured at room temperature and before hydrogenation (HI 83141, Hanna Instruments).
B.2: For hyperpolarization of PLAC, sodium phosphate in a powdery form was added to the precursor solution until a pH of 10.6 was measured (Na3PO4, CAS: 7601–54-9, Sigma Aldrich). The final Na3PO4 concentration was ≈ 22 mM.
B.3: A commercially available stock buffer solution based on boric acid, potassium chloride, and sodium hydroxide with a pH value of 10.0 at 20°C was used for experiments with PLAC (material number 9438, Merck).
Experimental Setup, Workflow, and Performed Experiments
As the experimental setup, workflow, and hydrogenation reactor were described in detail in several previous reports42–44, only a brief overview is provided here. The experimental setup consists of a 7 T preclinical MRI system (Biospec 70/20, Bruker) and a linearly polarized 1H-13C volume coil (Rapid Biomed). The hyperpolarization experiments were conducted with the help of two or three electromagnetic valves V (type 124, Bürkert) that were controlled with a custom written software (MatLab, National Instruments, Figure 3). To start the experiment, gaseous pH2 at a pressure of 20 bar was injected into a reactor filled with substrate solution through an inlet at the bottom of the reactor. After the experiment and data acquisition, pressure was released through an outlet at the top. Prior to experiments, the reactor was heated in a 60° C or 90° C water bath.
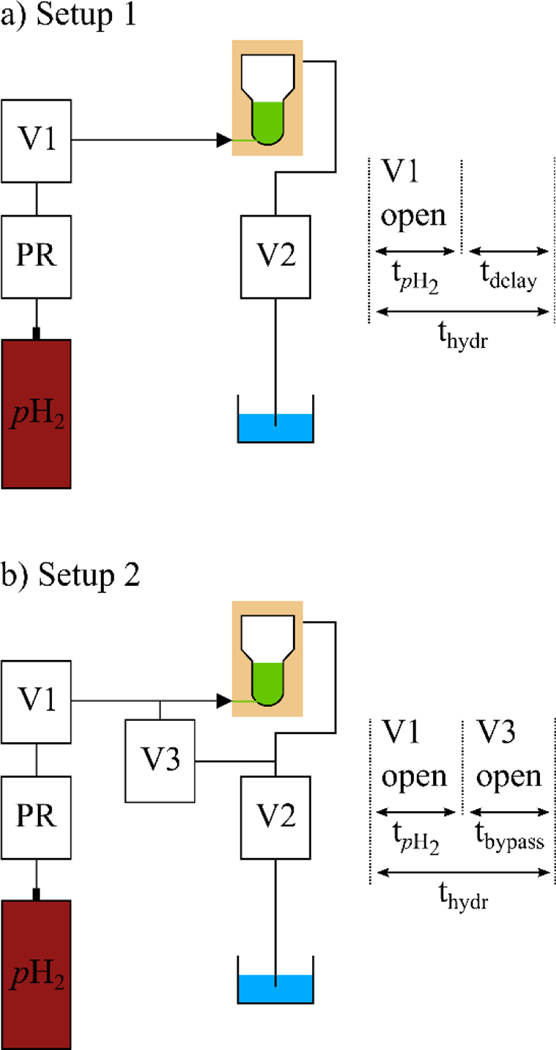
Figure 3: Experimental setups and timetables showing the actuation of the magnetic valves.
Setup 1: The pH2 reservoir was connected to the inlet of the reaction chamber via a pressure regulator (PR), valve V1, and a non-metallic check valve. V1 was opened for a period of tpH2 to initialize the chemical reaction. The SOT sequence was executed after an additional delay (tdelay), resulting in a total hydrogenation time thydr. The outlet valve V2 was closed during the entire hydrogenation and hyperpolarization process. After signal detection, V2 was opened and the solution was flushed out from the reactor and caught in a water bath (blue). Setup 2 was identical to setup 1 but an additional valve (V3) was installed to bypass the reactor for equilibration of the pressure between in- and outlet. This valve was opened after tpH2 for a period of tbypass to stop the pH2 injection and to prevent the formation of gas bubbles. When heteronuclear decoupling was required, decoupling was played out only during tbypass.
The MRI system was adjusted by placing the reactor filled with 1 mL of deionized H2O in the isocenter of the MRI. The RF coil was tuned and matched manually. The field homogeneity was improved by an automatic first order shimming routine provided by the manufacturer. For experiments with SUC, the 1H center frequency was set to the resonance frequency of the SUC-protons at ≈ 2.8 ppm. For hyperpolarization of PLAC, the 1H frequency was centered between the two resonances of the pH2-nascent protons at 4.33 and 1.29 ppm, i.e. at ≈ 2.8 ppm. The durations of the 1H and 13C SOT RF pulses were chosen as 0.5 ms in all experiments. When heteronuclear decoupling during hydrogenation was required, the 1H MLEV16 decoupling scheme was applied. The pulse length of a single 90° 1H RF pulse of the MLEV16 sequence was set to 1 ms.
After hyperpolarization experiments, the 13C polarizations were quantified by comparing the hyperpolarized 13C signals with a thermally polarized acetone reference sample (4 mL, 13.5 M, 1.1 % 13C, 50 averages, 90° excitation, numerical integration with TopSpin, Bruker) assuming a full conversion to SUC and PLAC.
For SUC and ECHO (Figure 1), the hydrogenation reaction was initialized by injecting pressurized pH2 gas into the reaction chamber by opening the pH2 valve (V1) for time duration tpH2 = 2 s (Figure 3, setup 1). After an additional delay of tdelay, i.e. a total hydrogenation duration thydr = tpH2 + tdelay, spin order was transferred.
For SUC/ADAPT and PLAC/PH-INEPT+ (Figures 1 and 2), another setup and workflow was used. This was of particular importance when employing the ADAPT sequence: As previously reported,44 heteronuclear decoupling is strongly disturbed during pH2 injection. Distortions of the field homogeneity because of susceptibility gradients induced by the gas bubbles in aqueous solution during tpH2 lead to erroneous RF excitations. Therefore, 1H MLEV16 decoupling pulses were only applied during tbypass using setup 2. Thus, the total hydrogenation duration was thydr = tpH2 + tbypass.
A series of different hyperpolarization experiments were performed, where the hydrogenation duration, i.e. tdelay, or free evolution intervals, i.e. tIN,1/2, tE, tAD,α, were varied (Tables 1 and 2). Furthermore, the influence of different pH buffer materials on the PLAC polarization was investigated. The 1H/13C relaxation times and the hydrogenation yield were determined by NMR spectroscopy after hyperpolarization experiments on a 7 T NMR spectrometer (see supplementary information).
Table 2: Performed experiments with PLAC using setup 2 and PH-INEPT+.
First, the hydrogenation conditions, i.e. hydrogenation duration and temperature, were optimized (PLAC.E1 and PLAC.E2). The free evolution intervals tIN,1 and tIN,2 of the sequence were varied in another experiment PLAC.E3 with a fixed hydrogenation duration thydr = 5 s. The influence of the buffer solutions B.2 and B.3 was examined in an additional set of experiments (PLAC.E4).
| Buffer | Timings / Parameters | |
|---|---|---|
| PLAC.E1 | B.2 |
tpH2 = 2 s, Variation of tbypass tIN,1 = 60.0 ms, tIN,2 = 50.0 ms 60° C |
| PLAC.E2 | B.2 |
tpH2 = 2 s, Variation of tbypass tIN,1 = 60.0 ms, tIN,2 = 70.0 ms 90° C |
| PLAC.E3 | B.2 |
tpH2 = 2 s, tbypass = 3 s Variation of tIN,1 and tIN,2 90° C |
| PLAC.E4 | B.2/3, or no buffer |
tpH2 = 2 s, tbypass = 3 s tIN,1 = 87.8 ms, tIN,2 = 70.7 ms 90° C |
Results
Hyperpolarization of Succinate
Optimization of the Hydrogenation Duration
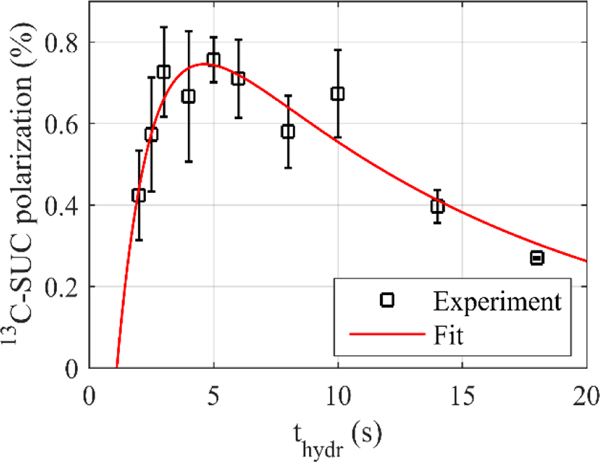
SUC was successfully polarized using both ECHO and ADAPT, however, to a lesser degree compared to the results obtained with Goldman’s sequence.44 When SUC was polarized with different hydrogenation durations thydr (SUC.E1), an optimum was found between 3 s and 6 s (Figure 4). By fitting Equation 8 to the data, the relaxation time of pH2-nascent spin order tspin and the hydrogenation constant tcat were determined as tspin = (13 ± 5) s and tcat = (1.4 ± 0.9) s, respectively. This implies that after 5 s of hydrogenation about (97 ± 6) % of all FUM molecules were hydrogenated. Examination of the hydrogenation kinetics by 1H PASADENA/ALTADENA experiments was not feasible on the MRI system because the 1H resonances were not resolved due to poor field homogeneity and baseline of the MR spectra.
Figure 4: 13C polarization of SUC polarized with ECHO (squares) as a function of the hydrogenation duration thydr and fit (line).
FUM (5 mM) was hydrogenated at 90° C and 20 bar pH2 pressure for different durations (thydr) before ECHO (tE = 70.0 ms) was applied and the polarization was measured (SUC.E1). A function was fitted to the data to extract the hydrogenation and relaxation constant tcat and tspin (Equation 8).
Performance of the ECHO Sequence
Computations assuming pure time-averaged singlet state after hydrogenation and chemically equivalent protons, i.e. 100 % before time-averaging and ΔCS = 0 Hz (Equations 2, 3 and 6), predicted a maximum polarization of P = 48.0 % for tE = 67.7 ms. These is a quite promising result given that the sequence is simple and short. However, when singlet-triplet mixing was taken into account in computations (Equation 7), a 10-fold decrease in polarization was observed (P = 4.8 % for tE = 67.7 ms). Considering the 13C isotope effect with ΔCS = 4 Hz did not lead to significantly different values for tE ≤ 100 ms. However, less polarization was found for tE greater than 100 ms (Figure 5, red and blue lines).
To verify the simulations, experiments with varying evolution intervals tE were conducted. Experimentally, a maximum polarization of P = (0.97 ± 0.15) % was found for tE = 60.0 ms and 70.0 ms after a thydr = 5 s hydrogenation (SUC.E2, Figure 5, squares).
The experimental data was similar to the simulations without isotope effect (ΔCS = 0 Hz), with singlet-triplet mixing, in the range of tE ≤ 110 ms and tE = 190 ms, except for an overall constant scaling factor of 4.94. Significant deviations were observed for tE = 130, 150, and 170 ms (Figure 5, red line and squares). When the isotope effect was taken into account (ΔCS = 4 Hz), good agreement was found for all values of tE (except for tE = 170 ms) and the same overall scaling factor of 4.94 (Figure 5, blue line and squares). An identical value of ΔCS was found by comparing experimental and simulated 1H PASADENA spectra (see supplementary information).
Performance of the ADAPT Sequence
The performance of the ADAPT sequence for hyperpolarizing SUC was investigated in simulations and experiments.
First, a simplified model was assumed, where decoupling was applied for the entire hydrogenation, neglecting reaction intermediates, and relaxation (Equation 6). Here, a polarization of P = 85.3 % was obtained for tAD,90° = 33.63 ms and ΔCS = 0 Hz. By adding singlet-triplet mixing (Equation 7), a significant decrease in 13C polarization to P = 8.5 % was observed (P = 5.7 % with S-T0 mixing and without decoupling).
Next, we investigated the hyperpolarization as a function of tAD,90° (Figure 6). Here, we found that the distribution of the polarization was shifted by ≈ 8 ms to smaller tAD,90° if the isotope effect was considered (ΔCS = 4 Hz and decoupling on). The maximum polarization was ≈ 5 % with S-T0 mixing.
Figure 6: Experimental and simulated (normalized) 13C polarization of SUC using ADAPT.
The polarization yield was simulated assuming decoupling during the entire hydrogenation reaction for chemically equivalent (black) and non-equivalent (blue) protons. Experimentally, decoupling was applied only for 3 s after a 2 s delay (squares). The corresponding simulations yielded an oscillating pattern (red). Experimentally, a maximum polarization of ≈ 1.9 % was observed (squares), while the simulations yielded ≈ 5 %. All measured data points represent the mean of three measurements and the error bars correspond to the standard deviation (except for tAD,90° = 23.0 ms which was measured only once). All simulated polarization yields were scaled by an overall factor to match the experimental data (black, blue, and red lines).
Experimentally, it was necessary to wait for 2 s after the injection of pH2 before the decoupling was tuned on to avoid inhomogeneous B0 and ill - defined pulses because of the bubbles.44 This means that decoupling was switched off and on, resulting in two different density matrices. Using the results from the above experiments (Equation 8 and Figure 4), we estimated that about 75% of FUM was hydrogenated in the first 2 s without decoupling, and 22 % during the following 3 s with decoupling (resulting in 97% total hydrogenation in 5 s). We used these fraction and density matrixes to simulate the expected total polarization as follows:
| (Equation 9) |
These simulations yielded a similar distribution of the polarization as function of tAD,90° as the simplified model, but with an oscillation up to about 10% of the absolute value (Figure 6, red line). The maximum polarization was found to be 5.2 % for ≈ 32 ms.
Experimentally, a similar distribution of the polarization was found. The experiments were most similar to the simulations considering the isotope effect. The maximum polarization was about 1.9 % at tAD,90° = 23.0 ms (SUC.E3, squares).
Hyperpolarization of Phospholactate
SAMBADENA for PLAC was simulated with the SOT sequence PH-INEPT+. When the hydrogenation process was calculated numerically (Equations 3 and 4), the resulting time-averaged 4-spin density matrix was given by pure longitudinal 1H double spin order, i.e. by
| (Equation 10) |
to a good approximation. Based on this density matrix, simulations of the PH-INEPT+ sequence ex- or including the 31P spin predicted a maximum 13C polarization level of P = 41.2 % for tIN,1 = 87.8 ms and tIN,2 = 70.7 ms in both cases.
Optimization of the Hydrogenation Duration
To obtain the optimal hydrogenation duration, we varied thydr in the range of 1 to 30 s. When using buffer B.2 and 90° C or 60° C hydrogenation temperature, a maximum polarization of about 0.7 % or 1.2 %, respectively, was found for thydr = 5 s (PLAC.E1/2). For 90°C reaction temperature, the lifetime of transferred spin order tspin and the hydrogenation constant tcat were determined as (1.3 ± 0.8) s and (17.9 ± 8.8) s, respectively (Equation 8, data not shown). Note that this is quite different to the values obtained for SUC. Fitting the two-site kinetic model to the MRI PLAC data suggested that only (24 ± 10) % of all precursor molecules were hydrogenated after thydr = 5 s at 90° C with a hydrogenation constant of tcat = (17.9 ± 8.8) s. Assuming a (24 ± 10) % hydrogenation yield, an effective 13C polarization of (4.2 ± 0.4) % was reached following Gaussian propagation of errors. This is 10-fold less than predicted by simulations (41.2 %). A 63 % hydrogenation yield was measured by high-resolution NMR spectroscopy of the same samples several hours after initializing the hydrogenation (see supplementary information). This indicates an ongoing hydrogenation after the hyperpolarization experiment and an effective polarization yield of about 1.6 %.
Variation of the Free Evolution Intervals
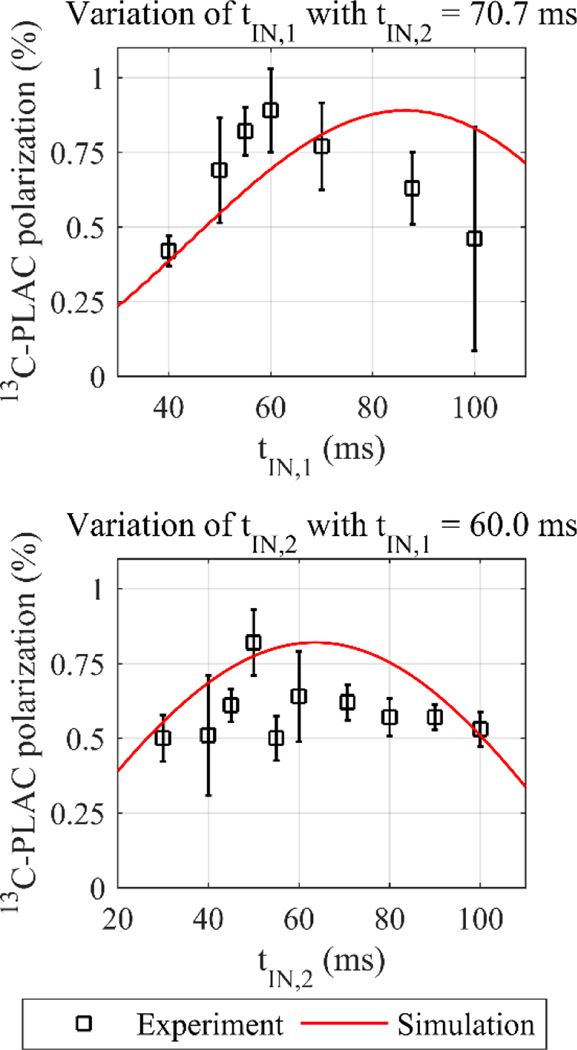
The simulated parameters for PH-INEPT+ yielded a polarization yield of P = (0.63 ± 0.11) % for tIN,1 = 87.8 ms and tIN,2 = 70.7 ms using the sodium phosphate buffer B.2 at 90° C (PLAC.E3, Figure 7). By varying tIN,1 and tIN,2, we found that the experimental polarization increased slightly to P = (0.82 ± 0.11) % when shorter intervals were used, i.e. tIN,1 = 60.0 ms and tIN,2 = 50.0 ms. The measured 13C polarizations were identical for 10 ms ≤ tIN,2 ≤ 110 ms, except for tIN,2 = 50.0 ms. However, the values were identical within two error intervals.
Figure 7: Experimental and theoretical 13C-PLAC polarization as a function of the free evolution intervals of PH-INEPT+.
The intervals were empirically optimized (squares) and compared to simulations (red line). The computations did not suit the experimental outcome after scaling. The mean values and standard deviations are shown.
Please note that the measured polarization yield is about a factor of 65 smaller than predicted by computations. By comparing scaled simulated values and experimental data, mismatches were found for the first and second evolution interval (Figure 7).
Influence of pH Buffer Solution
The hyperpolarization experiments were repeated in differently buffered solutions with tIN,1 = 87.8 ms, tIN,2 = 70.70 ms, and a hydrogenation time of thydr = 5 s at 90° C (PLAC.E4). When adjusting the pH value to 10.6 through the sodium phosphate buffer (B.2), a maximum polarization yield of P = (0.62 ± 0.11) % was found. When a pH buffer based boric acid (B.3, pH 10.0) was used, a decreased polarization of P = (0.17 ± 0.15) % was found. At neutral pH of 7 before hydrogenation when no buffer solution was added to the solution, a mean polarization of P = (0.16 ± 0.05) % was observed.
1H and 13C PLAC relaxation times
The relaxation times of thermally equilibrated PLAC in the B.2 solution at pH 10.6, room temperature, and 7 T were determined as T1 = (6.8 ± 0.4) s for the methyl and T1 = (5.5 ± 0.7) s for methine proton after a hyperpolarization experiment. The T2 relaxation time of the methyl proton at 1.25 ppm was determined as T2 = (3.5 ± 0.2) s. T1 = (31.0 ± 1.0) s and T2 ≈ 2 s were found for the 13C nucleus. See supplementary information for more details.
Discussion
In a previous SAMBADENA related publication, a fundamental hurdle for hyperpolarization of SUC, i.e. singlet-triplet mixing, has been identified.44 Here, the loss of polarization due to the 13C isotope effect, poor hydrogenation, and fast T1/T2 relaxation is discussed for both of the SUC- and PLAC-molecules.
Succinate:
The experimentally observed 13C polarization yield of about 1 % through the ECHO or ADAPT sequence was significantly lower than predicted by simulations considering singlet-triplet mixing, i.e. about 5 % for ECHO and 8 % for ADAPT, respectively (Figures 5 and 6). However, the simulated and measured dependencies on the free evolution intervals were in satisfying agreement when an overall scaling factor was introduced, when chemically inequivalent SUC-protons were hypothesized, and when the experimental workflow was considered. The scaling factor may be explained by ≈ 90 % pH2 enrichment and deviating flip angles within the sample volume. The 13C isotope leads to a break of symmetry within the molecule, resulting in different Larmor frequencies of both protons. The assumed difference of ΔCS = 4 Hz, i.e. a few ppb, is in accordance with previously detected 13C-induced shifts of the 1H Larmor frequency.58,59 Despite optimization of the free evolution intervals and hydrogenation duration, the herein reported polarization yields of 1 % are substantially lower than those previously achieved through Goldman’s sequence, i.e. 10 %.44 However, given the simplicity of ECHO, i.e. 13C RF only and no decoupling, the sequence maybe beneficial for low-budget MR units.
Phospholactate:
The experimental polarization yield of about 1 % is lower than expected from computations where a 13C-PLAC polarization of roughly 41 % was predicted (Figure 7). 1H-31P and 13C-31P J-coupling interaction did not affect the polarization yield in simulations.
Evidence for relaxation of spin order was found by variation of the intervals tIN,1 and tIN,2 as the experimental maximum was observed for shorter tIN,1 intervals than predicted by simulations. Contrary, no prominent shift was observed by variation of the tIN,2 interval. This finding is conclusive as the tIN,2 interval of the PH-INEPT+ sequence serves for the transformation of 13C out-phase in 13C in-phase magnetization where only relaxation of 13C is important.23
Please note that in previous studies, 13C-HEP was polarized to about 20 % through the same SOT sequence at 7 T using a similar setup.42 Comparing the relaxation times and polarization levels of HEP and PLAC leads to the assumption that relaxation may partly explain the tremendous loss of 13C-PLAC polarization. First, the longitudinal relaxation times of PLAC, about 6 s for 1H and 35 s for 13C, are a factor of 2 or 5 lower than for HEP56 (27 s for 1H and 75 s for 13C in aqueous solution with the same catalyst). Second, the polarization transfer was about a factor of 17 more efficient in HEP than in PLAC. This follows by comparison of the simulated and measured HEP42 and PLAC polarization levels, i.e. PHEP,Sim. ≈ 49 %, PHEP,Exp. ≈ 20 %, PPLAC,Sim. ≈ 42 %, and PPLAC,Exp. ≈ 1 %.
Relaxation in PLAC is potentially more pronounced because of dipole-dipole interaction with the additional 31P spin and by the adjacency of the lactate-OH group (Figure 2). This hypothesis is supported by the fact that relaxation times depend on the field strength, temperature, and molecular environment.60 For instance, shorter T1 relaxation times, i.e. 4.3 s for 1H and 16.2 s for 13C, were measured at 9.4 T in earlier studies.34
The observed dependency of the polarization yield on reaction temperature and buffer solution might be caused by changes in the relaxation times and hydrogenation yields.
The experimental polarization yield of 1 % is significantly lower than the 15 % achieved in previous studies conducted at 5.75 mT32 and related to the 1H-13C transfer efficiency, to the hydrogenation reaction, and relaxation times. The achieved signal enhancement of about 1800 at 7 T is likely too low for interesting in vivo applications. Here, faster hydrogenation may prove advantageous to boost 13C polarization yields to make this agent suitable for in vivo studies. Please note, that ions (due to the presence of a pH buffer) may lead to the cancellation of hyperpolarized signals as described in literature.61
Future PHIP Agents and their Application in Biomedical Research
Beside unsaturated double or triple bonds for permanent addition of pH2, additional requirements need to be fulfilled for the generation of PASADENA hyperpolarized 13C agents for biomedical applications. First, chemically inequivalent protons are desirable to prevent polarization loss due to singlet-triplet mixing, challenges with decoupling sequences at high field44, and potential isotope labeling effects. Second, molecules with relatively long 1H and 13C relaxation times are preferable to provide high 13C polarization since the loss of spin coherence during hydrogenation and SOT is less dominant. The influence of relaxation during spin order transfer might be addressed by appropriate choice of the solvents and pH buffer solutions. Third, PASADENA agents with OH-groups close to the pH2-nascnet protons and 13C should be avoided to decrease the influence of spin exchange induced relaxation. When the requirements are (partly) fulfilled, 13C polarization yields of metabolites at high field with pulsed SOT is feasible, e.g. 60 % of ethyl acetate and 9 % of pyruvate, respectively.22,41 SAMBADENA would allow to perform the hyperpolarization directly in the MRI, at low cost, and next to the application target reducing loss by relaxation.
Conclusion and Outlook
In this contribution, the initial experience with the SAMBADENA hyperpolarization of PLAC, a derivate to obtain hyperpolarized 1-13C-lactate, was shown. Moreover, we investigated the hyperpolarization of SUC by means of the ECHO and ADAPT sequences. The low polarizations about 1 % were attributed to a) the 13C isotope effect, b) low T1/T2 relaxation times, and c) low hydrogenation yields. The symmetry of SUC is broken due to 13C labeling (Figure 1a). Consequently, the two SUC-protons precess with different Larmor frequencies (ΔCS = 4 Hz) and the efficiency of spin order transfer is reduced. The value of ΔCS was obtained from simulations and experiments (Figure 5 and supplementary information). In the case of PLAC, low relaxation times of the PLAC-protons and low hydrogenation yields may explain the loss of 13C polarization. These results are of significance for hyperpolarization with SOT sequences at high magnetic fields. Higher hydrogenation yields and precursor molecule concentrations than in recent SAMBADENA experiments are achievable with a more sophisticated reactor design and experimental setup in future work.
Supplementary Material
Acknowledgements
Funding support by the Forschungskommission (University Medical Center Freiburg, SCHM2146/20), the Emmy Noether Program of the DFG (HO 4604/2-1, 4604/2-2), the German Consortium for Translational Cancer Research (DKTK), the Cluster of Excellence “Precision Medicine in Inflammation” (PMI 1267), the RFBR and DFG (HO-4604/3-1, 19-53-12013), the research training group materials4brain (RTG 2154), the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 642773, and the Heinrich-Böll-Stiftung (ABS, P131623) is gratefully acknowledged. E.Y.C thanks the National Science Foundation CHE-1904780, the National Cancer Institute 1R21CA220137, and the National Heart, Lung, and Blood Institute 1 R21 HL154032.
Footnotes
Additional Information
Parts of the methods, results, and discussion were presented in the doctoral thesis of Stephan Berner (Faculty of Mathematics and Physics, University of Freiburg, Germany, 2020, https://freidok.uni-freiburg.de/data/166544).
References
- 1.Platt T. et al. In vivo self-gated 23 Na MRI at 7 T using an oval-shaped body resonator. Magn. Reson. Med (2018) doi: 10.1002/mrm.27103. [DOI] [PubMed] [Google Scholar]
- 2.Nagel AM et al. In Vivo 35Cl MR Imaging in Humans: A Feasibility Study. Radiology 271, 585–595 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Niesporek SC et al. Reproducibility of CMRO2 determination using dynamic 17O MRI. Magn. Reson. Med 79, 2923–2934 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Borowiak R, Groebner J, Haas M, Hennig J & Bock M. Direct cerebral and cardiac 17O-MRI at 3 Tesla: initial results at natural abundance. Magn. Reson. Mater. Phys. Biol. Med 27, 95–99 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Kovtunov KV et al. Hyperpolarized NMR Spectroscopy: d-DNP, PHIP, and SABRE Techniques. Chem. – Asian J 13, 1857–1871 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal R, Vigneron DB & Kurhanewicz J. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur. Urol 72, 1028–1029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller CA et al. Dynamic 2D and 3D mapping of hyperpolarized pyruvate to lactate conversion in vivo with efficient multi-echo balanced steady-state free precession at 3 T. NMR Biomed. 33, e4291 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Laustsen C. et al. Hyperpolarized [1,4– 13 C]fumarate imaging detects microvascular complications and hypoxia mediated cell death in diabetic nephropathy. Sci. Rep 10, 1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JM et al. Hyperpolarized 13C NMR observation of lactate kinetics in skeletal muscle. J. Exp. Biol 218, 3308–3318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardenkjaer-Larsen JH et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A 100, 10158–10163 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardenkjær-Larsen JH et al. Cryogen-free dissolution dynamic nuclear polarization polarizer operating at 3.35 T, 6.70 T, and 10.1 T. Magn. Reson. Med 81, 2184–2194 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Eisenschmid TC et al. Para hydrogen induced polarization in hydrogenation reactions. J. Am. Chem. Soc 109, 8089–8091 (1987). [Google Scholar]
- 13.Bowers CR & Weitekamp DP Parahydrogen and synthesis allow dramatically enhanced nuclear alignment. J. Am. Chem. Soc 109, 5541–5542 (1987). [Google Scholar]
- 14.Bowers CR & Weitekamp DP Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical Reaction and Nuclear Magnetic Resonance. Phys. Rev. Lett 57, 2645 (1986). [DOI] [PubMed] [Google Scholar]
- 15.Kadlecek S, Emami K, Ishii M & Rizi R. Optimal transfer of spin-order between a singlet nuclear pair and a heteronucleus. J. Magn. Reson 205, 9–13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai C, Coffey AM, Shchepin RV, Chekmenev EY & Waddell KW Efficient Transformation of Parahydrogen Spin Order into Heteronuclear Magnetization. J. Phys. Chem. B 117, 1219–1224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman M, Jóhannesson H, Axelsson O & Karlsson M. Design and implementation of 13C hyper polarization from para-hydrogen, for new MRI contrast agents. Comptes Rendus Chim. 9, 357–363 (2006). [Google Scholar]
- 18.Norton VA Efficient generation of hyperpolarized molecules utilizing the scalar order of parahydrogen. (California Institute of Technology, 2010). [Google Scholar]
- 19.Stevanato G. Alternating Delays Achieve Polarization Transfer (ADAPT) to heteronuclei in PHIP experiments. J. Magn. Reson 274, 148–162 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Stevanato G, Eills J, Bengs C & Pileio G. A pulse sequence for singlet to heteronuclear magnetization transfer: S2hM. J. Magn. Reson. San Diego Calif 1997 277, 169–178 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Eills J. et al. Singlet order conversion and parahydrogen-induced hyperpolarization of 13C nuclei in near-equivalent spin systems. J. Magn. Reson 274, 163–172 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Korchak S, Yang S, Mamone S & Glöggler S. Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by utilizing para-Hydrogen. ChemistryOpen 7, 344–348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haake M, Natterer J & Bargon J. Efficient NMR Pulse Sequences to Transfer the Parahydrogen-Induced Polarization to Hetero Nuclei. J. Am. Chem. Soc 118, 8688–8691 (1996). [Google Scholar]
- 24.Kadlecek S. et al. A simple and low-cost device for generating hyperpolarized contrast agents using parahydrogen. NMR Biomed. 24, 933–942 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Borowiak R. et al. A battery-driven, low-field NMR unit for thermally and hyperpolarized samples. Magn. Reson. Mater. Phys. Biol. Med 26, 491–499 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Hövener J-B et al. Quality assurance of PASADENA hyperpolarization for 13C biomolecules. Magma N. Y. N 22, 123–134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hövener J-B et al. PASADENA hyperpolarization of 13C biomolecules: equipment design and installation. Magn. Reson. Mater. Phys. Biol. Med 22, 111–121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coffey AM et al. Open-Source Automated Parahydrogen Hyperpolarizer for Molecular Imaging Using 13C Metabolic Contrast Agents. Anal. Chem 88, 8279–8288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agraz J, Grunfeld A, Cunningham K, Li D & Wagner S. Improved PHIP polarization using a precision, low noise, voltage controlled current source. J. Magn. Reson 235, 77–84 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Agraz J. et al. LabVIEW-based control software for para-hydrogen induced polarization instrumentation. Rev. Sci. Instrum 85, 044705 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Shchepin RV, Coffey AM, Waddell KW & Chekmenev EY PASADENA Hyperpolarized 13C Phospholactate. J. Am. Chem. Soc 134, 3957–3960 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shchepin RV, Coffey AM, Waddell KW & Chekmenev EY Parahydrogen Induced Polarization of 1–13C-Phospholactate-d2 for Biomedical Imaging with >30,000,000-fold NMR Signal Enhancement in Water. Anal. Chem 86, 5601–5605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hövener J-B et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed 57, 11140–11162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salnikov OG et al. Effects of Deuteration of 13C-Enriched Phospholactate on Efficiency of Parahydrogen-Induced Polarization by Magnetic Field Cycling. J. Phys. Chem C 122, 24740–24749 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya P. et al. Towards hyperpolarized 13C-succinate imaging of brain cancer. J. Magn. Reson. San Diego Calif 1997 186, 150–155 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Billingsley KL et al. Hyperpolarized [1,4-(13)C]-diethylsuccinate: a potential DNP substrate for in vivo metabolic imaging. NMR Biomed. 27, 356–362 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chekmenev EY et al. PASADENA Hyperpolarization of Succinic Acid for MRI and NMR Spectroscopy. J. Am. Chem. Soc 130, 4212–4213 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zilberter Y, Zilberter T & Bregestovski P. Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol. Sci 31, 394–401 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Chen AP et al. Feasibility of using hyperpolarized [1–13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magn. Reson. Imaging 26, 721–726 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J. et al. Dynamic 1H imaging of hyperpolarized [1–13C]lactate in vivo using a reverse INEPT experiment. Magn. Reson. Med 79, 741–747 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korchak S, Mamone S & Glöggler S. Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A para-Hydrogen Approach. ChemistryOpen 7, 672–676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt AB et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat. Commun 8, ncomms14535 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt AB et al. In vivo 13C-MRI using SAMBADENA. PLOS ONE 13, e0200141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berner S. et al. SAMBADENA Hyperpolarization of 13C-Succinate in an MRI: Singlet-Triplet Mixing Causes Polarization Loss. ChemistryOpen 8, 728–736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bär S. et al. On the spin order transfer from parahydrogen to another nucleus. J. Magn. Reson 225, 25–35 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Barkemeyer J, Haake M & Bargon J. Hetero-NMR Enhancement via Parahydrogen Labeling. J. Am. Chem. Soc 117, 2927–2928 (1995). [Google Scholar]
- 47.Wasylishen RE & Friedrich JO Deuterium isotope effects on the nitrogen chemical shift and 1J(N,H) in the ammonium ion. J. Chem. Phys 80, 585–587 (1984). [Google Scholar]
- 48.Zacharias NM et al. Towards Real-time Metabolic Profiling of Cancer with Hyperpolarized Succinate. J. Mol. Imaging Dyn 6, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shchepin RV, Pham W & Chekmenev EY Dephosphorylation and biodistribution of 1–13C-phospholactate in vivo. J. Label. Compd. Radiopharm 57, 517–524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reineri F, Boi T & Aime S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun 6, 5858 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Cavallari E, Carrera C, Aime S & Reineri F. 13C MR Hyperpolarization of Lactate by Using ParaHydrogen and Metabolic Transformation in Vitro. Chem. – Eur. J 23, 1200–1204 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Hansen PE Isotope effects in nuclear shielding. Prog. Nucl. Magn. Reson. Spectrosc 20, 207–255 (1988). [Google Scholar]
- 53.Levitt MH, Freeman R & Frenkiel T. Broadband heteronuclear decoupling. J. Magn. Reson 1969 47, 328–330 (1982). [Google Scholar]
- 54.Hübler P, Natterer J & Bargon J. Indirect characterisation of hydrogenation intermediates using PASADENA NMR spectroscopy — evolution of zero-quantum coherence in AB spin systems. Berichte Bunsenges. Für Phys. Chem 102, 364–369 (1998). [Google Scholar]
- 55.Natterer J, Schedletzky O, Barkemeyer J, Bargon J & Glaser SJ Investigating Catalytic Processes with Parahydrogen: Evolution of Zero-Quantum Coherence in AA′X Spin Systems. J. Magn. Reson 133, 92–97 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A Liquid-state nuclear hyperpolarization without a polarizer : synthesis amid the magnet bore allows a dramatically enhanced nuclear alignment. (Albert-Ludwigs-Universität Freiburg, 2020). [Google Scholar]
- 57.Hövener J-B et al. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 26, 124–131 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Tiainen M, Maaheimo H, Soininen P & Laatikainen R. 13C isotope effects on 1H chemical shifts: NMR spectral analysis of 13C-labelled D-glucose and some 13C-labelled amino acids. Magn. Reson. Chem 48, 117–122 (2010). [DOI] [PubMed] [Google Scholar]
- 59.de Graaf RA, Chowdhury GMI & Behar KL Quantification of High-Resolution 1H-[13C] NMR Spectra from Rat Brain Extracts. Anal. Chem 86, 5032–5038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiley: Spin Dynamics: Basics of Nuclear Magnetic Resonance, 2nd Edition - Levitt Malcolm H. http://eu.wiley.com/WileyCDA/WileyTitle/productCd-0470511176.html.
- 61.Bröhl A & Giernoth R. PHIP NMR Spectroscopy in Ionic Liquids: Influence of Salts on the Intensity of Polarization Signals. Anal. Chem 86, 10311–10314 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.