Abstract
BACKGROUND:
Following trauma, persistent inflammation, immunosuppression, and catabolism may characterize delayed recovery or failure to recover. Understanding the metabolic response associated with these adverse outcomes may facilitate earlier identification and intervention. We characterized the metabolic profiles of trauma victims who died or developed chronic critical illness (CCI) and hypothesized that differences would be evident within 1-week postinjury.
METHODS:
Venous blood samples from trauma victims with shock who survived at least 7 days were analyzed using mass spectrometry. Subjects who died or developed CCI (intensive care unit length of stay of ≥14 days with persistent organ dysfunction) were compared with subjects who recovered rapidly (intensive care unit length of stay, ≤7 days) and uninjured controls. We used partial least squares discriminant analysis, t tests, linear mixed effects regression, and pathway enrichment analyses to make broad comparisons and identify differences in metabolite concentrations and pathways.
RESULTS:
We identified 27 patients who died or developed CCI and 33 who recovered rapidly. Subjects were predominantly male (65%) with a median age of 53 years and Injury Severity Score of 36. Healthy controls (n = 48) had similar age and sex distributions. Overall, from the 163 metabolites detected in the samples, 56 metabolites and 21 pathways differed between injury outcome groups, and partial least squares discriminant analysis models distinguished injury outcome groups as early as 1-day postinjury. Differences were observed in tryptophan, phenylalanine, and tyrosine metabolism; metabolites associated with oxidative stress via methionine metabolism; inflammatory mediators including kynurenine, arachidonate, and glucuronic acid; and products of the gut microbiome including indole-3-propionate.
CONCLUSIONS:
The metabolic profiles in subjects who ultimately die or develop CCI differ from those who have recovered. In particular, we have identified differences in markers of inflammation, oxidative stress, amino acid metabolism, and alterations in the gut microbiome. Targeted metabolomics has the potential to identify important metabolic changes postinjury to improve early diagnosis and targeted intervention.
LEVEL OF EVIDENCE:
Prognostic/epidemiologic, level III.
Keywords: Metabolomics, chronic critical illness, trauma, PICS
With improvements in trauma systems, early management, and critical care, mortality following severe traumatic injury has declined.1,2 Those who survive the initial physiologic insult, however, are still at risk for late-phase morbidity and mortality associated with chronic critical illness (CCI), with nearly 20% of severe trauma patients developing CCI.3 Although exact definitions vary, CCI is characterized by prolonged intensive care unit (ICU) stays and has been associated with poor functional outcomes, more complications, overall greater mortality, and added cost and resource utilization.3–6 Efforts to characterize trauma patients at risk for CCI have identified potential demographic and clinical characteristics.3 Ongoing catabolism, immunosuppression, and multiple organ failure may contribute to the development of CCI following severe traumatic injury.7 However, our understanding of the many underlying biological changes is incomplete.
The “-omics” fields (e.g., transcriptomics or proteomics) have the potential to identify biologic derangements before phenotypic manifestations of disease are apparent, as well as identify potentially modifiable factors. These fields of study have been applied to the care of injured patients. For example, gene expression profiles have previously demonstrated differences between severely injured patients and healthy controls and may help differentiate patients by outcome; however, these studies often focus primarily on circulating leukocyte subpopulations, and differences in gene expression do not always translate to differences in protein expression.8–11 In addition, identifying genomic differences has not yet translated into effective therapies, that is, personalized medicine. Metabolomics, the study of small molecules, such as amino acids, sugars, lipids, and nucleotides, offers a quantitative and qualitative evaluation of numerous downstream metabolic pathways simultaneously. Studies performed on blood samples provide a comprehensive, cross-sectional view of an organism’s physiologic milieu and has the potential to help bridge the gap between genotypic and phenotypic manifestations of disease. We have previously demonstrated that the metabolic profiles of trauma patients differ from healthy volunteers and change over time, and that metabolites respond differently to enteral and parenteral nutrition;12,13 others have used metabolomics to identify biomarkers associated with early death in combat-injured patients.14
We sought to expand our understanding of the response to injury by characterizing how the metabolomic profiles of trauma victims differ between patients who experienced an adverse outcome (developed CCI or died beyond 7 days) and those who recovered rapidly (discharged alive from ICU within 7 days). The ultimate goal is to determine differences that might be present early enough postinjury whereby their identification could facilitate early intervention. We hypothesized that injured patients who developed CCI or experienced a late death would exhibit different temporal trends in metabolites and pathways involved in inflammation, oxidative stress, and protein metabolism compared with those who recovered rapidly.
PATIENTS AND METHODS
Study Population and Sample Collection
In this cohort study, blunt and penetrating trauma patients 16 years or older admitted to a level I trauma center (Harborview Medical Center, Seattle, WA) between May 2008 and June 2012 with evidence of shock (systolic blood pressure of <90 mm Hg or a base deficit of ≥6 mEq/L) were eligible for inclusion. Patients with an isolated severe neurologic injury (defined as a head, neck, or spine Abbreviated Injury Scale score of 3 or greater) were not eligible. Clinical and demographic data were recorded prospectively, using a standardized case report form.
Venous blood samples were collected from eligible subjects at 12 hours, 1 day, 4 days, and 7 days postadmission. Subjects who were missing samples from one or more time points or who lacked sufficient sample volume for mass spectrometry were excluded. All samples were collected from patients who were hospitalized at least 7 days as patients whose length of stay was less than 7 days (either due to death or discharge) were not at risk for our primary outcome. A threshold of 7 days was selected to differentiate patients who experienced an early death related to their initial injury from those who experienced an adverse outcome related to persistent organ dysfunction and infection. A flow diagram outlining patient inclusion and exclusion criteria can be found in Supplemental Digital Content (Supplementary Fig. 1, http://links.lww.com/TA/B813). A subset of our patient cohort was previously included as the validation cohort in a separate study.15 Samples from healthy uninjured controls were obtained from a commercial source (Solomon Park Research Lab, Burien, WA) and were used primarily to define healthy ranges of metabolites.
Patient and Clinical Outcomes
There are multiple definitions of CCI in the literature.4,5 To be consistent with previous definitions utilized in posttraumatic CCI,3 we have defined CCI by an ICU length of stay of ≥14 days with evidence of organ dysfunction (systolic blood pressure of <90 mm Hg, vasopressor requirement, PaO2 to FiO2 ratio of level of ≤300, or a serum creatinine level of ≥1.9 mg/dL) on day 14 or beyond. We defined an adverse outcome as either death after 7 days or CCI. We compared subjects who experienced this composite adverse outcome to those who recovered rapidly (discharged alive from ICU within 7 days), as well as uninjured control subjects.
Sample Processing and Metabolite Measurements
We collected blood in ethylenediaminetetracetic acid (EDTA) anticoagulant, separated components by centrifugation at 4°C and stored plasma at −80°C. Samples were stored until October 2019, at which time they were further processed and prepared for mass spectrometry. Samples were not thawed and refrozen before being used in this study. The detailed protocol for preparing samples and performing metabolomic analysis is provided in Supplemental Digital Content (Appendix, http://links.lww.com/TA/B811.) Briefly, samples were analyzed using a targeted liquid chromatography and tandem quadrupole mass spectrometry platform described previously,16 and metabolites were identified by comparison with known standards. Metabolite results are reported as relative concentrations. Samples were randomly assigned a run order, and 16 sequential batches of 30 samples were performed. Each batch included three interspersed sets of a blank sample plus two quality-control (QC) samples derived from (1) a pooled subset of patient serum samples and (2) commercially available pooled serum.
Statistical Analysis and Data Presentation
We conducted statistical analyses using R version 3.6.2 (R Core Team, Vienna, Austria). Continuous demographic and clinical data are presented as medians and interquartile ranges and were compared using the Mann-Whitney U test. Categorical data are presented as counts and percentages and were compared using the χ2 test.
Metabolites with more than 20% missing were removed, and the remaining missing values were imputed with the k-nearest neighbors. Variation due to batch and run order was addressed using the commercially available QC samples and QC sample–based robust locally estimated scatterplot smoothing signal correction.17 Metabolite measurements were then log2 transformed. Metabolite measurements were analyzed using MetaboAnalyst 4.0 (Xia Lab, McGill University, Montreal, Quebec, Canada), which was used to generate heat maps, and perform partial least squares discriminant analyses (PLS-DAs) and pathway enrichment and topology analyses, in addition to Student’s t test.18
Partial least squares discriminant analysis was used to describe global differences in metabolomic profiles and was performed on the QC sample–based robust locally estimated scatterplot smoothing signal correction–adjusted and log2-transformed metabolite measurements. Partial least squares discriminant analysis is a supervised method of maximizing the explained variance between defined groups and is commonly used in the field of metabolomics.19,20 Trauma patients overall were compared with uninjured controls, and injured patients from each outcome group were compared with each other at each time point. Models were evaluated using cross validation and permutation tests.21 Pathway enrichment and topology analysis were then used to identify biological pathways that differed between outcome groups.
Individual metabolites which distinguished injury outcome groups were identified using three methods: Student’s t test, variable importance in projection (VIP) score from PLS-DA, and linear mixed effects modeling. Metabolites that made the greatest contribution to distinguishing injury outcome groups at each time point were identified using the VIP scores. Metabolites with a VIP score of >2 were reported; for reference, a VIP score of >1 is generally considered to be influential.22 Linear mixed effects modeling was used to identify whether the change in individual metabolite measurements differed over time between outcome groups. Two models, with and without an interaction term for patient outcome, were compared using analysis of variance to determine whether the rate of change differed significantly between outcome groups.23
Benjamini-Hochberg adjusted p values were calculated, and statistical significance was defined as an adjusted p < 0.01 for both t tests and analysis of variance. Pathways were considered important if they met this significance threshold and demonstrated a pathway impact value of >0.1 using MetaboAnalyst.24,25
RESULTS
Of the 120 injured subjects whose outcome was known and who had samples from all four time points, 27 experienced an adverse outcome: 22 developed CCI, 1 developed CCI then died, and 4 died without meeting criteria for CCI. Thirty-three met the criteria for rapid recovery. The remaining 60 patients survived without meeting criteria for rapid recovery or CCI and were excluded from analysis. We also obtained samples from 48 healthy uninjured controls. Table 1 provides the demographic and clinical characteristics of the trauma cohort, as well as the uninjured controls. Briefly, the median age of trauma victims was 43 years (interquartile range, 25–57 years). The majority were male and severely injured. Uninjured controls had similar age, sex, and body mass index (BMI) distributions as the injured patients.
TABLE 1.
Demographic and Clinical Characteristics of Injured Patients, Stratified by Injury Outcome Group, and Uninjured Controls
| Injury Outcome Group |
p | Standardized Mean Difference | Uninjured Controls (n = 48) | ||
|---|---|---|---|---|---|
| Rapid Recovery (n = 33) | CCI or Death (n = 27) | ||||
| Age, median (IQR), y | 41 (25–54) | 57 (39–65) | <0.01 | 0.66 | 55 (43–61) |
| Male sex, n (%) | 22 (66.7) | 17 (63.0) | NS | 0.08 | 31 (64.6) |
| White race, n (%) | 30 (90.9) | 23 (85.2) | NS | 0.17 | |
| BMI | 27 (24–33) | 30 (24–36) | <0.05 | 0.67 | 28 (24–31) |
| Preexisting conditions, n (%) | |||||
| Hypertension | 2 (6.1) | 6 (22.2) | NS | 0.48 | |
| Diabetes | 1 (3.0) | 3 (11.1) | NS | 0.31 | |
| Hyperlipidemia | 6 (6.9) | 3 (3.2) | NS | 0.50 | |
| Blunt mechanism of injury, n (%) | 30 (90.0) | 26 (96.3) | NS | 0.22 | |
| Injury Severity Score, median (IQR) | 34 (27–43) | 43 (34–50) | NS | 0.52 | |
| Admission vital signs and laboratory values | |||||
| Heart rate, BPM | 111 (92–133) | 112 (93–124) | NS | 0.32 | |
| Systolic blood pressure, mm Hg | 122 (106–135) | 115 (85–133) | NS | 0.10 | |
| Lactate, mmol/L | 4.2 (2.6–5.3) | 4.8 (2.8–6.9) | NS | 0.27 | |
| Base deficit, mEq/L | −6.1 (−8.5 to −3.8) | −7.2 (−12.2 to −5.6) | NS | 0.44 | |
| Transfer, n (%) | 11 (33.3) | 7 (25.9) | NS | 0.16 | |
| Acute kidney injury, n (%)** | 2 (6.1) | 7 (25.9) | <0.05 | 0.64 | |
| Day of enteral nutrition initiation, median (IQR) | 2 (2–4) | 2 (2–4) | NS | 0.17 | |
| Enteral nutrition, n (%)† | 33 (100) | 26 (96.4) | NS | 0.27 | |
| ICU-free days, median (IQR)* | 23 (22–25) | 6 (1.5–9.5) | <0.001 | 4.12 | |
| Ventilator-free days, median (IQR)* | 25 (24–27) | 10.0 (6.5–13) | <0.001 | 3.45 | |
| Hospital length of stay, median (IQR), d | 12 (10–14) | 31 (22–38) | <0.001 | 1.47 | |
| Mortality, n (%) | NA | 5 (18.5) | NA | NA | |
| CCI, n (%) | NA | 23 (85.2) | NA | NA | |
Continuous data are shown as medians (IQRs), and categorical data as numbers (%).
Out of 28 days.
Defined as an increase in serum creatinine level of ≥0.3 mg/dL within a 48-hour period in the first 7 days.
Proportion of patients who received enteral or parenteral nutrition during the first 7 days.
BPM, beats per minute; IQR, interquartile range; NS, not statistically significant; NA, column/row combination that is not valid.
Those who experienced an adverse outcome were older than those who recovered rapidly. There were no significant differences in admission vital signs or markers of shock, overall Injury Severity Score, or body site–specific Abbreviated Injury Scale. In addition, no differences in day of initiation or mode of nutrition (enteral versus parenteral) between injury outcome groups were noted. Nutritional support at our facility focused on early initiation of isocaloric enteral nutrition within 48 hours postinjury. Enteral nutrition was preferred over parenteral whenever possible.
A total of 214 metabolites were measured, of which, 57 had greater than 20% missingness and were removed. Six metabolites, that is, caffeine, theophylline, cotinine, trigonelline, thiamine, and mannitol, were removed from the analyses because they were deemed to be primarily or exclusively exogenously derived, because of either patient consumption or iatrogenic administration. Ultimately, 151 metabolites were included in this study (Supplemental Digital Content, Supplementary Table 1, http://links.lww.com/TA/B812)). The mean coefficient of variance for the metabolite measurements was 12% for QC samples and 3% for 30 metabolites when compared to their matching, isotope-labeled internal standards.
Global Differences Between Injury Outcome Groups Apparent by Day 1
The two groups were indistinguishable at 12 hours. Global differences between injury outcome groups were evident by day 1 postinjury (Fig. 1). R2, Q2, and permutation p values are available in Supplemental Digital Content (Supplementary Table 2, http://links.lww.com/TA/B812). The predictive accuracies were overall moderate to high and improved with greater time since injury.
Figure 1.
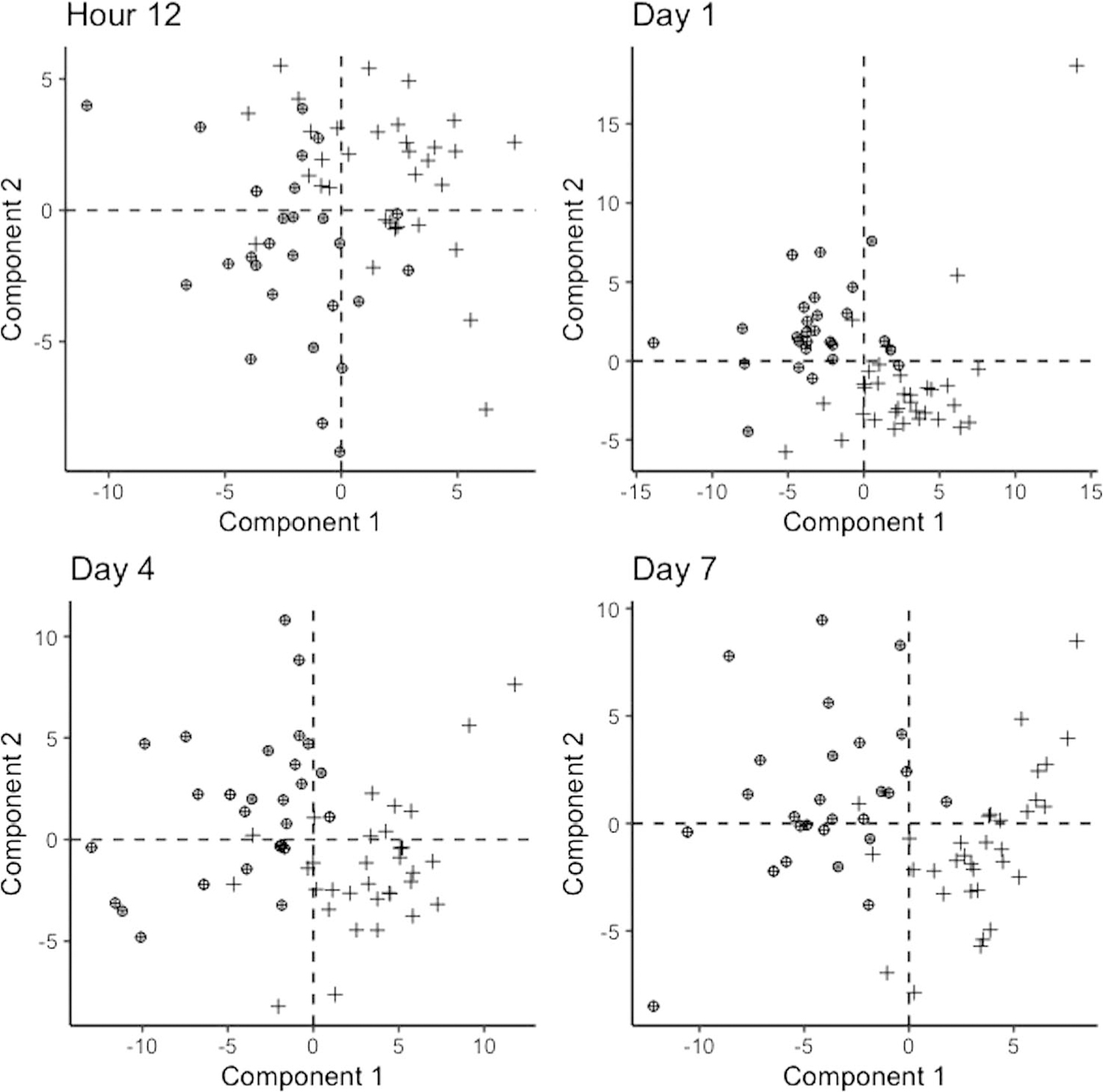
Partial least squares discriminant analysis comparing rapid recovery patients (+) with those who experienced an adverse outcome (⊕) at each time point.
Metabolic Pathways Differ Between Outcome Groups by Day 1
Figure 2 displays the metabolomic pathways that differ between injury outcome groups across time points; Figure 3 is a Venn diagram summary of the overlap between pathways that differed on individual days. Consistent with the global changes noted previously, there were no clear differences in metabolic pathways between the two groups at 12 hours postinjury. However, by 1 day, differences in a number of metabolic pathways emerged. Differences in the key metabolites in the citric acid cycle (TCA) cycle and in glycolysis, gluconeogenesis, and pyruvate metabolism began on day 1 and continued through day 4. Tyrosine, phenylalanine, and tryptophan metabolism; ascorbate and aldarate metabolism; and inositol phosphate metabolism differed on days 1 to 7. Cysteine and methionine metabolism; pentose and glucuronate interconversions; and glycine, serine, and threonine metabolism differed on days 4 and 7.
Figure 2.
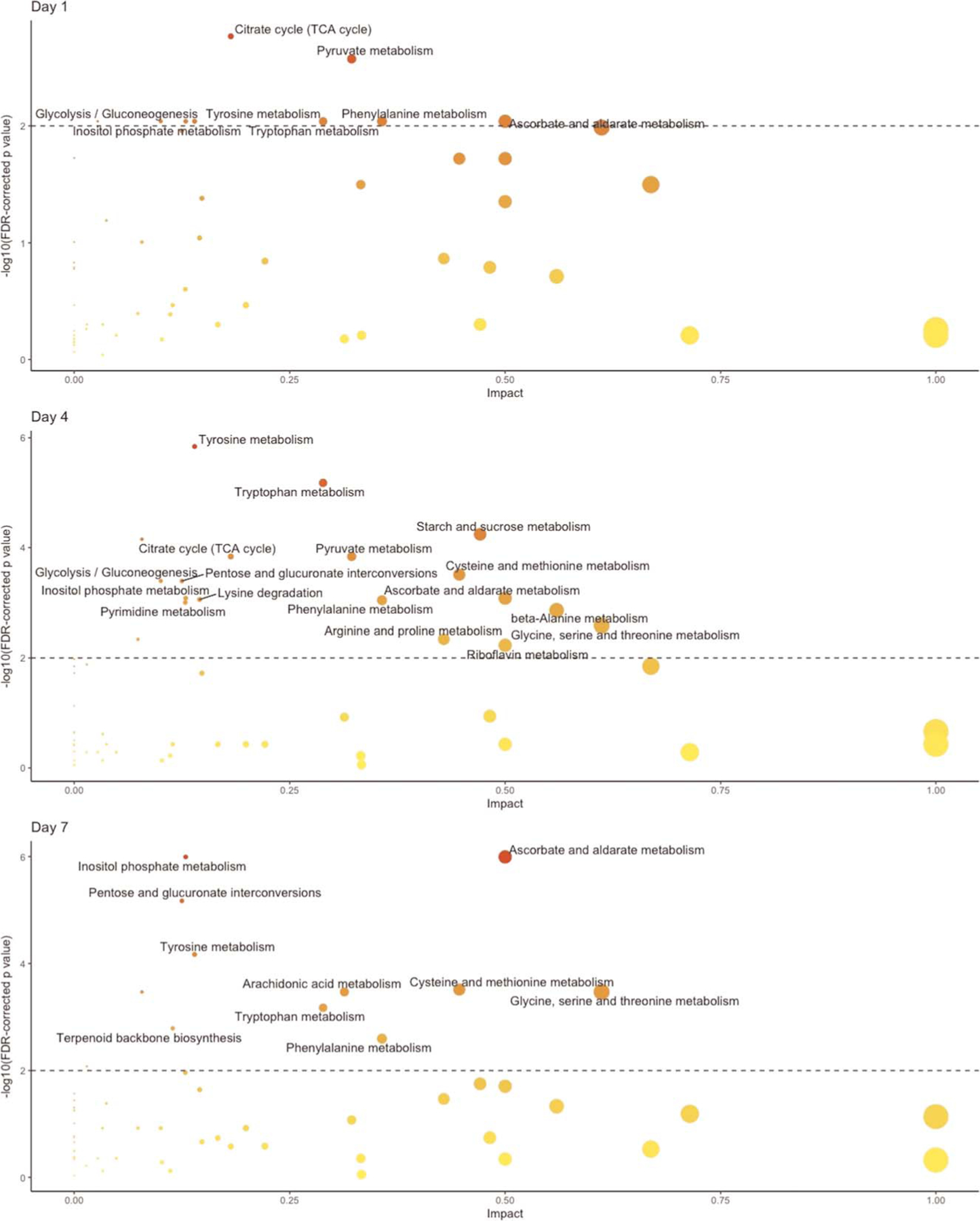
Metabolic pathways that differed significantly between outcome groups (corrected p < 0.01) and that had an impact of >0.1 at each time point. No pathways were significantly different at 12 hours. The dashed horizontal line indicates the level of confidence. Each point indicates a metabolomic pathway, with size corresponding with impact and shade corresponding to corrected p value.
Figure 3.
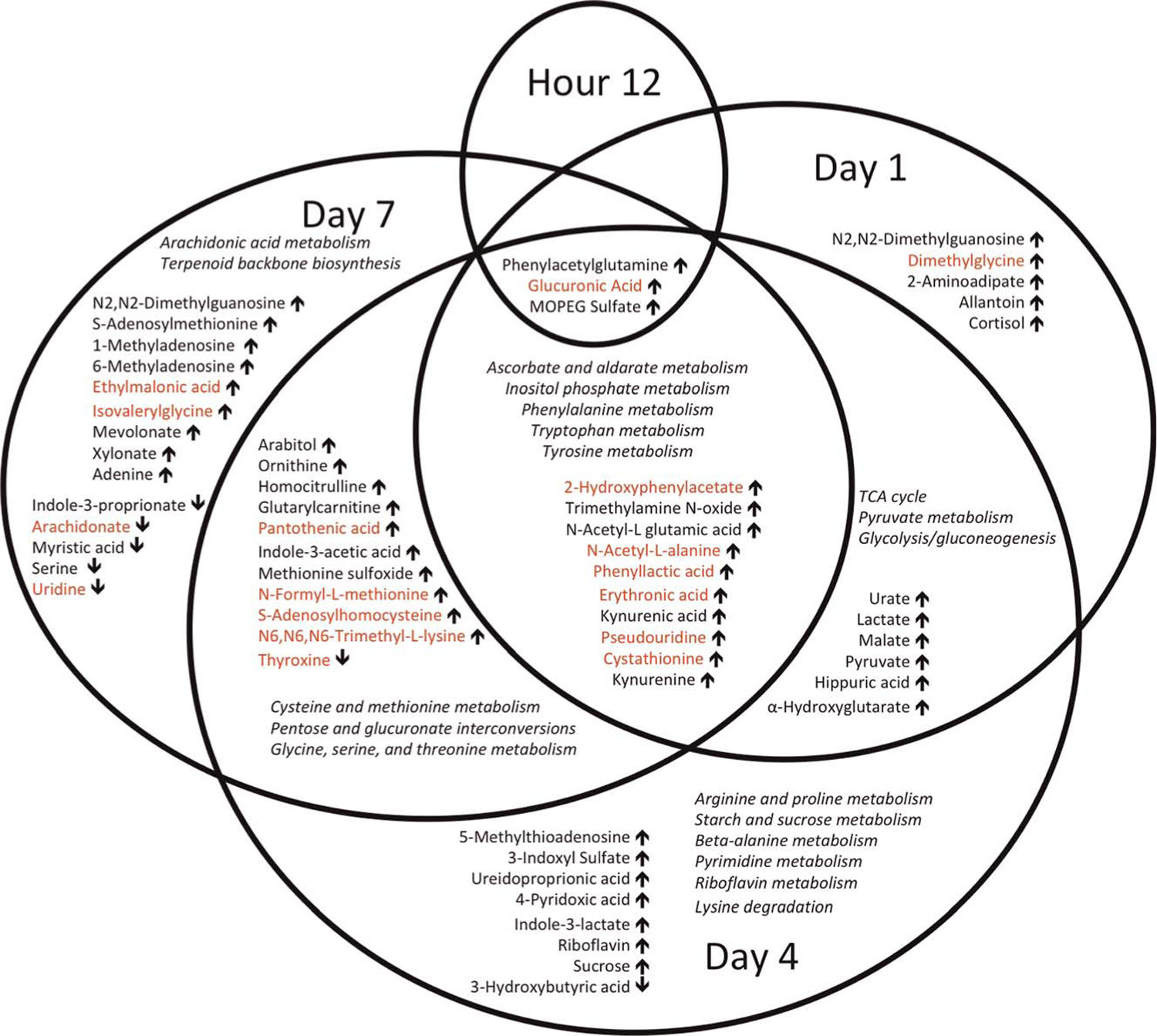
Venn diagram of statistically significantly different metabolites between injury outcome groups one each day, after correcting for multiple comparisons. Arrows indicate the direction of the relative metabolite concentration in the adverse outcome group compared with rapid recovery. Red font indicates metabolites that also differed in their rate of change on linear mixed effects regression between injury outcome groups. Pathways that differ at different time points are also shown in italics.
Identifying Individual Metabolites That Differ Between Injury Outcome Groups
Using t tests to compare patients who experienced an adverse outcome with those with rapid recovery, we identified 3 metabolites that differed by hour 12, 24 by day 1, 39 by day 4, and 38 by day 7 (all after correcting for multiple comparisons). Figure 3 provides a Venn diagram summary of the overlap between metabolites that differed on individual days. All of the metabolites identified at hour 12 and day 1 were increased in the adverse outcome group compared with rapid recovery. By day 4, the majority of metabolites remained higher in the adverse outcome group, with the exception of 7 metabolites: 3-hydroxybutyric acid on day 4; thyroxine on days 4 and 7; and arachidonate, serine, uridine, indole-3-proprionate, and myristic acid on day 7. Supplemental Digital Content (Supplementary Fig. 2, http://links.lww.com/TA/B814) displays heat maps for metabolites that differed significantly at each time point between injury outcome groups.
Two metabolites, phenylacetylglutamine and trimethylamine N-oxide (TMAO), were influential for differentiating patients who rapidly recovered from those who died or developed CCI at all four time points on PLS-DA. The complete list of metabolites with VIP score of >2 for the first two components for each PLS-DA is provided in Supplemental Digital Content (Supplementary Table 3, http://links.lww.com/TA/B812).
Trends in metabolite concentrations over time may provide additional insight into processes associated with recovery and, conversely, those associated with failure to recover. Therefore, we identified metabolites whose rates of change differed between injury outcome groups based on linear mixed effects modeling. These metabolites are highlighted in Figure 3 in red. Two temporal patterns in metabolite concentration dominated: (1) increasing toward control concentrations in those who recovered rapidly while remaining low or decreasing in those who experienced an adverse outcome (e.g., arachidonate, thyroxine) or (2) decreasing toward control concentrations in rapid recovery subjects while remaining high or increasing in those who experienced an adverse outcome (e.g., S-adenosylhomocysteine [SAH], ethylmalonic acid; see Fig. 4 for examples).
Figure 4.
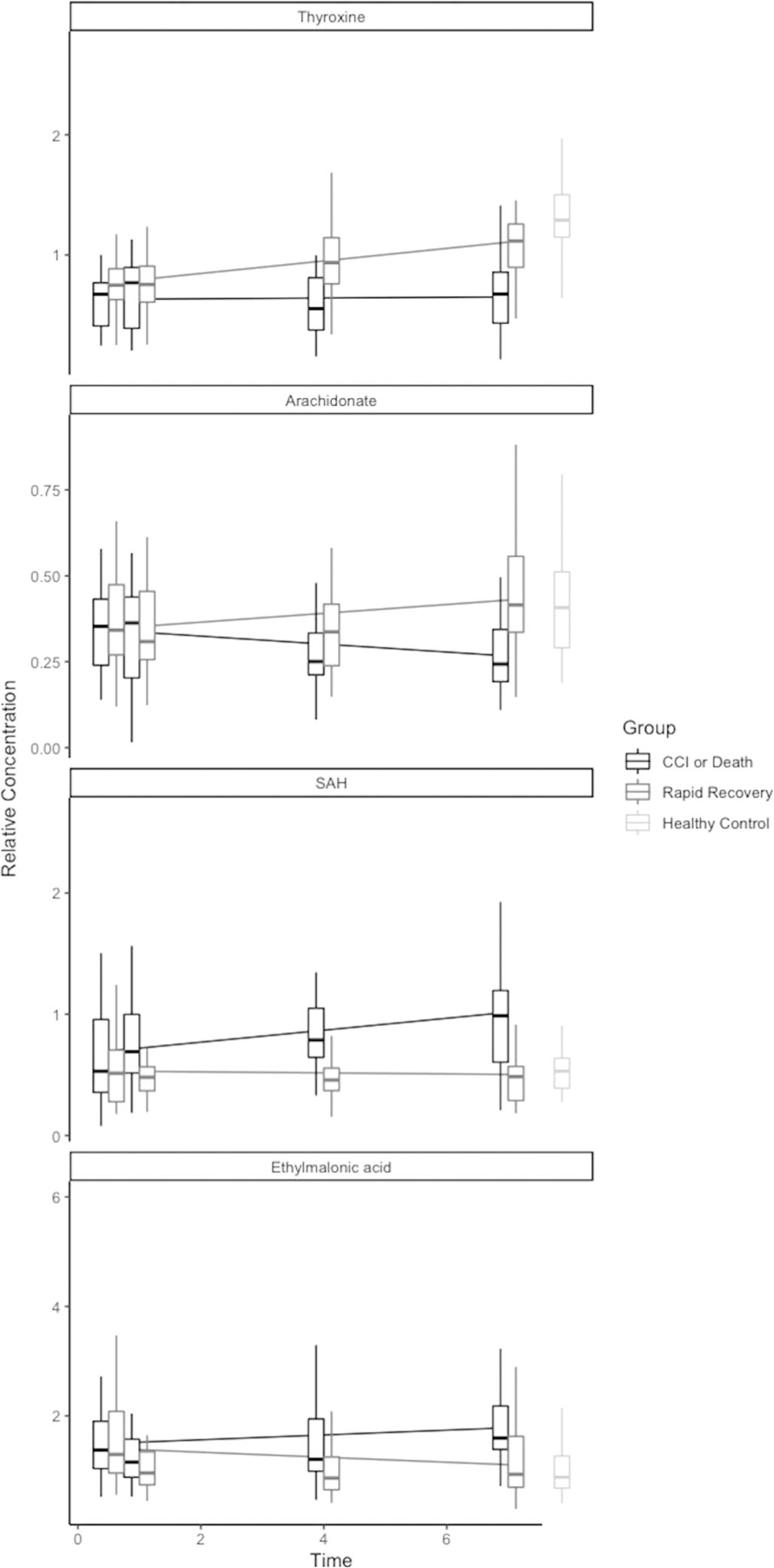
Select metabolites with differential rate of change over time between injured patient outcome groups, compared with uninjured controls. Each line represents Boxplots depict relative metabolite concentrations on the y axis at each time point. The horizonal lines represent the metabolite trend over time, stratified by patient outcome.
DISCUSSION
Unpredictable and unexplained variation in the development of adverse outcomes following traumatic injury is likely related to complex, intertwined metabolic, proteomic, and genomic differences, which transcend injury patterns and severity, age, and underlying comorbidities. In this study, we have demonstrated that the metabolite profiles of traumatically injured patients vary based on clinical outcome. Over the first 7 days following injury, patients who experienced a rapid recovery saw normalization of key metabolites, whereas patients who would go on to experience an adverse outcome, late death or CCI, continued to experience significant derangements. Metabolomics allowed us to evaluate the complex metabolic path ways involved in CCI, identifying multiple distinct metabolites and pathways, representing markers of global metabolic and multiorgan dysfunction, inflammation and immune regulation, oxidative stress, amino acid metabolism, and, potentially, gut microbiome disruption.
Markers of Global Metabolic and Multiorgan Dysfunction
Phenylacetylglutamine and TMAO, two VIPs that were influential at all time points and which were elevated in patients who experienced an adverse outcome compared with rapid recovery as early as 12 hours, are potential markers of renal insufficiency.26,27 N-acetylalanine, upregulated on days 1 to 7, has also been associated with kidney dysfunction.28 These metabolites may signify more severe acute kidney injury as a higher incidence of AKI in the first 7 days was observed in patients who developed CCI or death compared with those who recovered rapidly. Alternatively, preexisting and predisposing conditions (e.g., older age and underlying comorbidities) may put patients at higher risk of CCI or death. Differences in liver function or antimicrobial administration may also explain variation in TMAO, as it is oxidized from TMA (a product of the gut microbiome) by the liver.
Kynurenic acid (increased in days 1–7 in patients experiencing an adverse outcome) is a key product of tryptophan metabolism (also altered on days 1–7) and may contribute to both acute brain dysfunction and increased delirium during critical illness.29 Delirium has long been recognized as a poor prognostic indicator in critically ill patients, with higher mortality and greater length of stay.30 These findings may reflect global metabolic derangements that impact future clinical outcomes though impaired neurologic function.
Thyroid dysfunction has also been described in critical illness. In particular, nonthyroidal illness syndrome, characterized by lower triiodothyronine and thyroxine levels without alterations in TSH, is particularly pronounced in severe and prolonged cases of critical illness and has been associated with worse outcomes in acute trauma.31–33 These findings are mirrored in our study, with thyroxine levels being notably and persistently suppressed by day 4 in adverse outcome patients, whereas rapid recovery patients experience increasing thyroxine levels over time, ultimately approaching levels comparable with healthy controls. Our observations support the prognostic value of thyroid function in critically injured patients, although the clinical implications of these findings are uncertain, as no clear evidence exists to support directed therapy in critically ill patients with nonthyroidal illness syndrome.34
Evidence of Inflammation and Immunomodulation
Several metabolites identified have potential implications in the immune response to injury: glucuronic acid, kynurenine, kynurenic acid, N-formyl-methionine, and arachidonate. Glucuronic acid was elevated at all time points and increased over time in patients who progressed to CCI or died, which may be related to altered phase II metabolism. Glucuronic acid has been noted to induce toll-like receptor 4 signaling in vitro.35 Toll-like receptor 4 recognizes pathogen- and damage-associated molecular pattern molecules, resulting in a pro-inflammatory response, and dysregulated toll-like receptor 4 activation may play a role in disease.36 Kynurenine and kynurenic acid have been shown to alter the expression of interleukin 23 and interleukin 17 in vitro and, as tryptophan degradation products, may have a role in preventing T cell activation and expansion by inducing apoptosis in activated T cells.37 N-formyl-methionine was elevated on days 4 and 7 in patients who experienced an adverse outcome and demonstrated significantly different trends over time between injury outcome groups. N-formyl-methionine is a derivative of methionine found primarily in bacteria and may also be released with tissue damage involving the mitochondria.38 Serving as a signal for infectious burden or tissue damage, formyl peptides such as N-formyl-methionine stimulate the innate immune response by attracting and activating polymorphonuclear leukocytes.38 Arachidonate is a precursor of both pro-inflammatory and anti-inflammatory mediators, including prostaglandins, prostacyclin, thromboxane, leukotrienes, and lipoxins.39 Arachidonate was lower and declined over time in the adverse outcome group, while it increased toward control concentrations in those who recovered rapidly. It is unclear whether the depletion of this metabolite in patients who experienced an adverse outcome is related to decreased production or increased metabolism to downstream pro-inflammatory or anti-inflammatory mediators. Regardless, these findings suggest potential implications in inflammatory cytokine production and immune system regulation.
Increased Markers of Oxidative Stress
Critical illness may be characterized by an excess of reactive oxygen species, inducing cellular damage and death, depletion of antioxidants, and accumulation of markers of oxidative stress.40 Several key metabolites and metabolic pathways identified in this study are potential markers of oxidative stress. On days 4 and 7, the methionine metabolism pathway and associated metabolites (cystathionine, S-adenosylmethionine, S-adenosylhomocysteine, and serine) differed between injury outcome groups. In response to oxidative stress, methionine can be oxidized to methionine sulfoxide, a metabolite that was also significantly upregulated on days 4 and 7. Via cystathionine, serine and methionine are converted to glutathione, which acts as a potent antioxidant.41 In addition, we found that ethylmalonic acid was elevated on day 7 and showed differential rate of change over time with rapid recovery patients decreasing over time to become comparable to healthy controls. Ethylmalonic acid has been shown to induce oxidation and superoxide production and reduce glutathione levels in rats.42 These findings suggest a redox imbalance, resulting in depletion of metabolites upstream of key antioxidants, and excess of metabolites produced as byproducts of oxidative stress. Despite these differences between outcome groups, attempts to address oxidative stress in critical illness with antioxidant supplementation have not conclusively demonstrated benefit.43,44 These metabolites may identify at-risk patients early in their clinical course and serve as measures of the adequacy of targeted intervention geared toward minimizing oxidative stress.
Gut Microbiome Disruption
The influence of the gut microbiome on human health is a rapidly progressing line of inquiry, and alterations in the gut microbiome during critical illness and after severe trauma have previously been described.45,46 Indole-3-propionate and TMAO, previously discussed as it relates to kidney insufficiency, are derived from the human gut microbiota.47,48 Lower levels of indole-3-proprionate have been associated with rapid renal decline in patients with chronic kidney disease because of alterations in the intestinal microbiome.47 In this study, indole-3-proprionate was lower, and TMAO was higher in patients who experienced an adverse outcome relative to rapid recovery. As noted before, TMAO is oxidized from TMA, a product of the gut microbiome, by the liver. Alterations in gut microbiome during critical illness are characterized by a loss of taxonomic diversity, and these metabolic byproducts may reflect an imbalance of TMA-producing bacteria over those that produce indole-3-proprionate. Changes in the circulating gut microbiome-derived metabolites may also, at least in part, be due to increases in gut permeability. However, our observations of some metabolites increasing while others are decreasing seem most consistent with a change in the gut microbiome. The etiology of microbiome changes in critical illness is likely multifactorial and includes differences in antibiotic, nutritional support, and proton pump inhibitor administration. Traumatically injured patients who rapidly recover may be experiencing a normalization of their gut microbiome.
Amino Acid Metabolism
Several metabolic pathways related to amino acid metabolism were altered. Our previous study highlighted differences in branched chain amino acid metabolism in trauma patients relative to uninjured controls,12 and isoleucine, leucine, and valine also differed in this study (data not shown). However, no differences in any branched chain amino acid were observed within traumatically injured patients based on outcome. Rather, phenyllactic acid and 2-hydroxyphenylacetate, degradation products of phenylalanine, were increased from day 1 to 7, and phenylalanine has been used as a measure of protein turnover.49 Tryptophan and its metabolites are products of muscle wasting,50 and products of tryptophan metabolism, including kynurenine, kynurenic acid, and indole-3-acetic acid, were also noted to be increased. Together, these findings suggest that muscle catabolism following trauma is more prominent among patients who go onto experience an adverse outcome, highlighting a potential for intervention (i.e., early protein supplementation).
CONCLUSIONS
Failure to normalize or achieve homeostasis relative to healthy controls may be central to experiencing late adverse outcomes following severe traumatic injury. We have demonstrated that it is possible to trend metabolic data over time, which may facilitate early identification of at-risk patients who may benefit from personalized intervention. We identified differences in key metabolites and metabolomics pathways associated with multiorgan dysfunction, changes in inflammation and immune response, greater oxidative stress, and alterations in amino acid metabolism and the gut microbiome. Overlap between these findings and proposed models of persistent inflammation, immunosuppression, and catabolism syndrome following traumatic injury are readily apparent.
This study has a number of limitations. We evaluated serum metabolomics, which may not reflect local environments individual tissues may experience. However, blood and serum samples are a practical medium for the clinical application of this technology and, we posit, more representative of systemic differences than transcriptomic studies, which focus on peripherally circulating leukocytes. The samples used in this study were collected over a decade ago, and metabolite measures may be influenced by storage practices and duration; despite this, we would not expect this phenomenon to differ between patient outcome groups and is unlikely to contribute to the findings presented here. Although age and BMI may influence metabolite concentrations, in a post hoc analysis, neither age nor BMI was associated with any metabolite concentration in this study. Although no statistically significant differences in outcome group by sex or race were noted, unmeasured and unappreciated population differences may also contribute to both metabolite concentrations and predisposition to adverse outcomes following trauma. This study cohort was predominantly white and may not be generalizable to other populations. Finally, one of the biggest challenges to the use of metabolomics is that individual metabolites often have multiple roles, spanning several different pathways such that results can often be interpreted in different lights. For example, it is as yet impossible to know whether alterations in metabolite concentrates are due to differences in synthesis, breakdown, or a combination of the two, which can have important consequences for interpretation. We view this as a potential opportunity for further translational research addressing either substrate or bioproducts of the pathways of interest to change both physiologic and clinical outcomes.
Our observations provide important new insights into the systemic metabolic changes that occur with adverse outcomes following severe traumatic injury. These metabolic changes are apparent early, broadening and persisting to at least 7 days following injury. Differences in markers of organ function, inflammation and immune response, and the gut microbiome, as well as pathways involved in oxidative stress and amino acid metabolism highlight this highly complex and heterogeneous disease process. Ultimately, metabolomic evaluation of injured patients may facilitate identification of high-risk patients, highlight potential therapeutic targets, and improve the delivery of precision medicine.
Supplementary Material
Acknowledgments
This study was supported by NIH T32 Fellowship (5T32GM121290 to D.L.H. and R01 GM078054 to J.C.).
Biographies
DISCUSSION
PAUL E. BANKEY, M.D. (Rochester, New York): Thank you and good afternoon. I would like to thank the organizers for the opportunity to participate in this year’s virtual meeting.
I would also like to thank the authors for a well-written manuscript and congratulate them for their work in the field of metabolomics to improve the overall care of the trauma patient.
The authors have previously reported that plasma metabolic profile, made up of circulating metabolites such as amino acids, sugars, lipids and nucleotides, in trauma patients with shock differs significantly from healthy volunteers the first week after injury and that these metabolites change in response to enteral and parenteral nutrition.
In the big picture these profiles may provide new therapeutic or nutrition targets to improve the care of critically-ill trauma patients.
In the current study the investigators have compared the metabolomics profiles of trauma patients who recovered rapidly – defined as alive, no organ dysfunction and no longer needing the ICU within seven days of admission – to those with chronic critical illness – defined as an ICU length of stay greater than 14 days and evidence of either cardiovascular, respiratory or renal dysfunction after 14 days.
A total of 151 unique metabolites were assayed and plasma samples obtained at 12 hours, one day, four days or seven days after injury in both groups.
Interestingly, and of note, the authors report that as early as one day after injury the circulating metabolite profile was significantly different between the 23 patients that developed chronic critical illness or death versus the 33 patients who had rapid recovery.
Additionally, the global differences in circulating metabolites between the two outcome groups became more significant and predictive at Day 4 and Day 7 after injury.
Digging deeper into the metabolic profiles, there were 24 individual metabolites that differed on Day 1, 39 on Day 4, and 38 on Day 7. However, only two of the metabolites studied were able to differentiate rapid recovery from chronic critical illness at all of the time points.
These two metabolites have been linked with the renal insufficiency, as reported in the preliminary manuscript.
Metabolomics pathway analysis of the altered profiles suggest, not unexpectedly, that inflammation, immune response, oxidative stress, amino acid metabolism, and gut microbiome disruption pathways were altered; however, no one pathway seemed to predominate in the reported results.
The authors conclusion, which I agree with, is that the metabolic profiles after trauma patients that develop chronic critical illness differs from those who rapidly recover and can be differentiated as early as one day after injury.
The authors highlight the potential of metabolomics to identify important metabolic changes after trauma that could improve early diagnosis and targeted interventions. And I look forward to future work by the author towards this ultimate goal.
I do have a few questions that I’d like the author to consider.
First, I do compliment the investigators on the detailed metabolite analysis and quality controls in performing the MS-based measurements. In this study a total of approximately 150 metabolites were measured.
So my question is do you think that this is a large enough or a robust enough dataset to identify key targets or do you believe there may be other key metabolites that are currently not reliably detected with the current methodology and technical approach?
I take the frame of reference from the genomics studies where thousands of genes are expressed and here we have only a couple hundred of these metabolites.
Second, I will quibble with your choice of endpoints of chronic critical illness with a single organ dysfunction as your endpoint. And I think that this might confound your profile comparisons as not being a very specific endpoint.
To clarify my question, the profile of a patient in the ICU at two weeks with an isolated renal failure might be expected to be significantly different from a patient with isolated respiratory failure but in your analysis I would assume, I got the impression that these two profiles would be considered potentially equivalent.
Lastly, my early education in the ICU I was taught that nutrition support was for starvation and that hypermetabolic/ catabolic ICU patients following trauma or with sepsis required so-called metabolic support.
So is there a potential practical takeaway from your data regarding how we assess or provide metabolic support for patients in the ICU currently?
I was thinking of maybe the profiles would provide a better assessment of nitrogen balance, patients that might require a different amino acid formulations, such as more branch chains, give less arachidonic acid, give more glutamine or something else that you may have uncovered in your more detailed familiarity with the data. I ask this because in the end I truly believe “we are what we eat.”
Congratulations, again, on an interesting study and I look forward to your responses to my questions. Thank you.
DARA L. HORN, M.D. (Seattle, Washington): Thank you, Dr. Malhotra and Dr. Bankey for facilitating this discussion today, and I would like to thank the AAST for allowing me to present our work. Those were very insightful questions and I think highlight many important takeaways from in this paper.
Your first question regarding the size or robustness of our data goes back to our use of targeted metabolic analysis versus an untargeted approach, which could have potentially identified several hundred to several thousands of metabolites compared to the approximately 150 metabolites we evaluated. You are correct in thinking that there may be additional metabolites that we are missing by choosing this targeted approach. Untargeted analyses have their own limitations, and may identify unknown or uncharacterized metabolites. Ultimately, I don’t think that our use of targeted analyses negates the findings, and we can move forward, focusing on individual, potentially influential pathways and related metabolites.
Your second question was whether chronic critical illness was too broad of a target endpoint given that we defined it as patients who developed either persistent kidney, pulmonary, or cardiovascular dysfunction at 14 days. That’s an interesting point, as it does create a more heterogeneous population than one might like to analyze in the setting of metabolomics–the underlying disease pathophysiology for someone with persistent renal dysfunction may be very different from someone who has persistent respiratory insufficiency. And I think that’s an interesting insight because some of the metabolites that we identified had been previously tied to acute kidney injury or chronic kidney disease. It may be that if we were to utilize metabolomics for early identification of patients with CCI, perhaps a panel of metabolites would be useful to reflect this heterogeneity.
The third question you asked was whether these data can influence our clinical practice with regard to nutrition. I think that these data—and more broadly, metabolomics–have the potential to influence our practical approach to the nutritional support of patients with chronic critical illness. In particular, we identified metabolites and metabolic pathways which may relate to protein catabolism. Moving forward, we could characterize metabolites over time as they relate to nutritional support, and perhaps whether there are correlates between nitrogen balance, metabolic profiles, and different clinical outcomes. It then be possible to trend the day-to-day differences in nutritional supply and demand. However, there are certainly hurdles to overcome before we see this technology in clinical practice.
DAVID A. SPAIN, M.D. (Stanford, California): Great presentation. Is the metabolomics profile a response to CCI or a contributor to CCI? Is CCI and the metabolomics profile a reflection of severity of illness and/or complications or does this reflect an inherent difference in genomics or transcriptomics of the at-risk population for CCI? Chicken or egg question.
DARA L. HORN, M.D. (Seattle, Washington): Great question. I presented just the day seven findings here; however, we looked at 12-hour, one-day, four-day, and seven-day metabolomic profiles, and evaluated how they changed over time. What we found particularly striking was how similar outcome groups were at the earlier time points, and how groups changed over time and became more divergent. Patients that rapidly recovered became more similar to healthy controls than to patients that would go on to develop an adverse outcome.
Regardless of whether the metabolite differences were a response to or a product of CCI, I think that these data highlight the potential for early identification, and possible intervention. The metabolite differences preceded the diagnosis of CCI– significant global differences were apparent very early (by one day post-injury), which is long before any clinical diagnosis of CCI could reasonably be make or anticipated.
We also saw no differences in injury severity scores between our outcome groups so I cannot attribute the differences in metabolites to one group being more severely injured than the other. It is possible that unmeasured and unappreciated genetic differences may contribute both to differences in predisposition to CCI and metabolite profiles.
ENRIQUE GINZBURG, M.D. (Miami, Florida): Have you implemented Omega-3 supplementation in your ICU and if not why do it and see what effect it has on your metabolomics factors?
DARA L. HORN, M.D. (Seattle, Washington): At the time study samples were collected, Omega-3 fatty acids were not a routine part of supplementation at our institution. There was a protocol for providing Vitamin C and Vitamin E and Selenium supplementations.
I think there is an opportunity to trial a number of interventions to determine their impact—if any—on metabolic profiles, and determine whether this targeted approach can guide therapy (for example, based on the metabolic derangements present, what interventions address those differences, and then trend the effectiveness of the intervention over time).
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
DISCLOSURE
The authors declare no conflicts of interest.
This study was presented at the 79th Annual Meeting of the American Association for the Surgery of Trauma and Clinical Congress of Acute Care Surgery, September 8–18, 2020, virtual meeting.
Contributor Information
Dara L. Horn, Department of Surgery, University of Washington.
Lisa F. Bettcher, Department of Anesthesiology and Pain Medicine, University of Washington.
Sandi L. Navarro, Fred Hutchinson Cancer Research Center.
Vadim Pascua, Department of Anesthesiology and Pain Medicine, University of Washington.
Fausto Carnevale Neto, Department of Anesthesiology and Pain Medicine, University of Washington.
Joseph Cuschieri, Division of Trauma and Critical Care, Department of Surgery, Harborview Medical Center, Seattle, Washington..
Daniel Raftery, Department of Anesthesiology and Pain Medicine, University of Washington; Fred Hutchinson Cancer Research Center, Harborview Medical Center.
Grant E. O’Keefe, Division of Trauma and Critical Care, Department of Surgery, Harborview Medical Center, Seattle, Washington..
REFERENCES
- 1.DiMaggio C, Ayoung-Chee P, Shinseki M, Wilson C, Marshall G, Lee DC, Wall S, Maulana S, Leon Pachter H, Frangos S. Traumatic injury in the United States: in-patient epidemiology 2000–2011. Injury. 2016;47(7): 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuschieri J, Johnson JL, Sperry J, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mira JC, Cuschieri J, Ozrazgat-Baslanti T, et al. The epidemiology of chronic critical illness after severe traumatic injury at two level-one trauma centers. Crit Care Med. 2017;45(12):1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn JM, Le T, Angus DC, Cox CE, Hough CL, White DB, Yende S, Carson SS, ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med. 2015;43(2):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meegan A, Rosielle DA. Chronic critical illness in adults. J Palliat Med. 2018;21(1):99–100. [DOI] [PubMed] [Google Scholar]
- 7.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–29; discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laudanski K, Miller-Graziano C, Xiao W, et al. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci. 2006;103(42):15564–15569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren HS, Elson CM, Hayden DL, et al. , Inflammation and Host Response to Injury Large Scale Collaborative Research Program. A genomic score prognostic of outcome in trauma patients. Mol Med. 2009;15(7–8):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb JP, Mindrinos MN, Miller-Graziano C, et al. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci. 2005;102(13):4801–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenca AG, Gentile LF, Lopez MC, et al. , Inflammation and Host Response to Injury Collaborative Research Program. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit Care Med. 2013;41(5):1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent BA, Seaton M, Sood RF, Gu H, Djukovic D, Raftery D, O’keefe GE. Use of metabolomics to trend recovery and therapy after injury in critically ill trauma patients. JAMA Surg. 2016;151(7):e160853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parent BA, Seaton M, Djukovic D, Gu H, Wheelock B, Navarro SL, Raftery D, O’Keefe GE. Parenteral and enteral nutrition in surgical critical care: plasma metabolomics demonstrates divergent effects on nitrogen, fatty-acid, ribonucleotide, and oxidative metabolism. J Trauma Acute Care Surg. 2017;82(4):704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lusczek ER, Muratore SL, Dubick MA, Beilman GJ. Assessment of key plasma metabolites in combat casualties. J Trauma Acute Care Surg. 2017; 82(2):309–316. [DOI] [PubMed] [Google Scholar]
- 15.Cuschieri J, Bulger E, Schaeffer V, et al. , Inflammation and the Host Response to Injury Collaborative Research Program. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34(4):346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagana Gowda GA, Djukovic D, Bettcher LF, Gu H, Raftery D. NMR-guided mass spectrometry for absolute quantitation of human blood metabolites. Anal Chem. 2018;90(3):2001–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn WB, Broadhurst D, Begley P, et al. , Human Serum Metabolome (HUSERMET) Consortium. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011;6(7):1060–1083. [DOI] [PubMed] [Google Scholar]
- 18.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinformatics. 2019;68(1):e86. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh T, Zhang W, Ghosh D, Kechris K. Predictive modeling for metabolomics data. Methods Mol Biol. 2020;2104:313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaraman SP, Anand RJ, Deantonio JH, et al. Metabolomics and precision medicine in trauma: the state of the field. Shock. 2018;50(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szymańska E, Saccenti E, Smilde AK, Westerhuis JA. Double-check: validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics. 2012;8(S1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehmood T, Liland KH, Snipen L, Sæbø S. A review of variable selection methods in partial least squares regression. Chemom Intel Lab Syst. 2012; 118:62–69. Available at: 10.1016/j.chemolab.2012.07.010. [DOI] [Google Scholar]
- 23.Bryan M, Heagerty PJ. Direct regression models for longitudinal rates of change. Stat Med. 2014;33(12):2115–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010;26(18):2342–2344. [DOI] [PubMed] [Google Scholar]
- 25.Navarro SL, Randolph TW, Shireman LM, Raftery D, Mccune JS. Pharmacometabonomic prediction of busulfan clearance in hematopoietic cell transplant recipients. J Proteome Res. 2016;15(8):2802–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmerman L, Egestad B, Jörnvall H, Bergström J. Identification and determination of phenylacetylglutamine, a major nitrogenous metabolite in plasma of uremic patients. Clin Nephrol. 1989;32(3):124–128. [PubMed] [Google Scholar]
- 27.Missailidis C, Hällqvist J, Qureshi AR, Barany P, Heimbürger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekula P, Goek O-N, Quaye L, et al. A metabolome-wide association study of kidney function and disease in the general population. J Am Soc Nephrol. 2016;27:1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voils SA, Shoulders BR, Singh S, Solberg LM, Garrett TJ, Frye RF. Intensive care unit delirium in surgical patients is associated with upregulation in tryptophan metabolism. Pharmacotherapy. 2020;40(6): 500–506. [DOI] [PubMed] [Google Scholar]
- 30.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr., Inouye SK, Bernard GR, Dittus RS. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004; 291(14):1753–1762. [DOI] [PubMed] [Google Scholar]
- 31.Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375–388. [DOI] [PubMed] [Google Scholar]
- 32.Langouche L, Jacobs A, Van den Berghe G. Nonthyroidal illness syndrome across the ages. J Endocr Soc. 2019;3(12):2313–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips RH, Valente WA, Caplan ES, Connor TB, Wiswell JG. Circulating thyroid hormone changes in acute trauma: prognostic implications for clinical outcome. J Trauma. 1984;24(2):116–119. [DOI] [PubMed] [Google Scholar]
- 34.Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am. 2007;36(3):657–672. [DOI] [PubMed] [Google Scholar]
- 35.Lewis SS, Hutchinson MR, Zhang Y, Hund DK, Maier SF, Rice KC, Watkins LR. Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain. Brain Behav Immun. 2013;30:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. 2013;48(1):190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salimi Elizei S, Poormasjedi-Meibod M-S, Wang X, Kheirandish M, Ghahary A. Kynurenic acid downregulates IL-17/1L-23 axis in vitro. Mol Cell Biochem. 2017;431:55–65. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. [DOI] [PubMed] [Google Scholar]
- 40.Bayir H Reactive oxygen species. Crit Care Med. 2005;33(Suppl 12): S498–S501. [DOI] [PubMed] [Google Scholar]
- 41.Martínez Y, Li X, Liu G, Bin P, Yan W, Más D, Valdivié M, Hu CA, Ren W, Yin Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49:2091–2098. [DOI] [PubMed] [Google Scholar]
- 42.Schuck PF, Milanez AP, Felisberto F, Galant LS, Machado JL, Furlanetto CB, Petronilho F, Dal-Pizzol F, Streck EL, Ferreira GC. Brain and muscle redox imbalance elicited by acute ethylmalonic acid administration. PLoS One. 2015;10(5):e0126606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abilés J, de la Cruz AP, Castaño J, et al. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit Care. 2006;10(5):R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedreag OH, Rogobete AF, Sandesc D, et al. Modulation of the redox expression and inflammation response in the critically ill polytrauma patient with thoracic injury. Statistical correlations between antioxidant therapy. Clin Lab. 2016;62(9):1747–1759. [DOI] [PubMed] [Google Scholar]
- 45.Howard BM, Kornblith LZ, Christie SA, et al. Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2(1):e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otani S, Chihade DB, Coopersmith CM. Critical illness and the role of the microbiome. Acute Med Surg. 2019;6(2):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C-Y, Lin C-J, Pan H-C, Lee C-C, Lu S-C, Hsieh Y-T, Huang S-Y, Huang H-Y. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin Nutr. 2019;38:2945–2948. [DOI] [PubMed] [Google Scholar]
- 48.Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of trimethylamine n-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients. 2018;10(10):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews DE. An overview of phenylalanine and tyrosine kinetics in humans. J Nutr. 2007;137(6 Suppl 1):1549S–1575S; discussion 1573S-1575S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ninomiya S, Nakamura N, Nakamura H, et al. Low levels of serum tryptophan underlie skeletal muscle atrophy. Nutrients. 2020;12(4):978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


