Abstract
Dysfunction of the lymphatic system is associated with a wide range of disease phenotypes. The restoration of dysfunctional lymphatic vessels has been hypothesized as an innovative method to rescue healthy phenotypes in diseased states including neurological conditions, metabolic syndromes, and cardiovascular disease. Compared to the vascular system, little is known about the molecular regulation that controls lymphatic tube morphogenesis. Using synthetic hyaluronic acid (HA) hydrogels as a chemically and mechanically tunable system to preserve lymphatic endothelial cell (LECs) phenotypes, we demonstrate that low matrix elasticity primes lymphatic cord-like structure (CLS) formation directed by a high concentration of vascular endothelial growth factor-C (VEGF-C). Decreasing the substrate stiffness results in the upregulation of key lymphatic markers, including PROX-1, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), and VEGFR-3. Consequently, higher levels of VEGFR-3 enable stimulation of LECs with VEGF-C which is required to both activate matrix metalloproteinases (MMPs) and to facilitate LEC migration. Both of these steps are critical in establishing CLS formation in vitro. With decreases in substrate elasticity, we observe increased MMP expression and increased cellular elongation, as well as formation of intracellular vacuoles, which can further merge into coalescent vacuoles. RNAi studies demonstrate that MMP-14 is required to enable CLS formation and that LECs sense matrix stiffness through YAP/TAZ mechanosensors leading to the activation of their downstream target genes. Collectively, we show that by tuning both the matrix stiffness and VEGF-C concentration, the signaling pathways of CLS formation can be regulated in a synthetic matrix, resulting in lymphatic networks which will be useful for the study of lymphatic biology and future approaches in tissue regeneration.
Keywords: Hyaluronic Acid, Lymphatic Networks, Matrix stiffness, VEGF-C, Mechanoregulation
Introduction:
Lymphatic vasculature pervades the human body and is responsible for lipid transport, immune cell trafficking, extracellular fluid homeostasis, and inflammatory responses.1,2 Consequently, the lymphatic system plays a crucial role in the progression of a wide spectrum of conditions, including congenital disorders, cancer and side-effects of cancer treatments, cardiovascular disease, diabetes, and parasitic infections.3,4 Despite the significance of the lymphatic system and its consequences in numerous disease states, current treatments are limited to primitive and transient management solutions such as compression garments for lymphedema, or entirely absent for other lymphatic complications. Controlling the formation of new lymphatic vessels is postulated as an innovative therapeutic strategy for rescuing various disease phenotypes including metabolic syndrome, Alzheimer’s, lymphedema, cardiovascular disease, and impaired wound healing.2,5 Yet, little is known about the molecular regulation that controls lymphatic cord-like structure (CLS) formation within a synthetic, in vitro system. Beyond conditions that arise from lymphatic dysfunction, a significant bottleneck for the field of tissue engineering is the vascularization of tissues and in vivo endothelial cell organization to form capillaries.6,7 Promoting blood and lymphatic vascular networks within tissue-engineered constructs have been shown to improve their in vivo functionality.8 However, a perennial challenge associated with this goal of controlling in vitro or in vivo morphogenesis of cellular structures includes the need to accurately replicate the morphology and cellular organization of lymphatic vessels.6 To address this challenge, here we utilize a synthetic matrix as a modular scaffold with tunable properties to control CLS formation.
Significant advances in therapeutic strategies that combine material engineering with biotechnological advances to promote vascular regeneration have occurred in recent decades.9–11 Hydrogels have demonstrated success in in vitro applications for blood vasculature regeneration and provide promise for approaches to generate functional lymphatic capillaries.11,12 Hyaluronic acid-based hydrogels (HA-hydrogels) have particularly shown great promise, either as a stand-alone therapy or as a scaffold to deliver molecules and cells.13,14 Hyaluronan/ hyaluronic acid (HA) is a non-sulfated linear polysaccharide of (1-β-4)d-glucuronic acid and (1-β-3)N-acetyl-d-glucosamine. HA is abundant during embryogenesis,15 where it has a crucial role in regulating angiogenesis, lymphangiogenesis, and organ morphogenesis.16,17 HA is ubiquitous in the native ECM, non-immunogenic, and able to be chemically modified, making HA widely used in tissue engineering and medicine.13,18,19 HA also has an important function in maintaining homeostasis and biomechanical integrity of many organs.20 In the lymphatic system, lymphatic endothelial cells (LECs) uniquely express lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), a CD44 homologue, which is responsible for HA binding. LYVE-1 is expressed by LECs and serves as a unique binding receptor for HA.21 LYVE-1 binding to HA presents the potential to use HA-hydrogels as a modular platform to study lymphatic vascular morphogenesis. By controlling ligand binding sites and mechanical properties, HA-hydrogels are investigated in these studies as a platform that can be engineered to generate robust and functional vascular networks from LECs. Overall, due to its developmental relevance, importance for LECs, and ability to support viable cells, HA-hydrogels are an excellent candidate biomaterial to control CLS formation in a biomimetic environment.
Previous developmental biology studies have revealed that the transcription factor prospero-related homeobox 1 (PROX-1) initiates lymphatic sprouting, and that the growth factor VEGF-C promotes the growth of lymphatic vessels.22 Additionally, PROX-1 has been shown to be inversely regulated with the mechanosensitive proteins YAP and TAZ.23 YAP/TAZ expression have extensively been reported to be influenced by substrate stiffness and provide an avenue of modulation in our HA hydrogel system. Another protein associated with lymphangiogenesis, VEGFR-3, has recently been discovered to be influenced by substrate stiffness,24 which raises the potential to create biomaterials to promote expression of this key protein involved in the VEGF-C/VEGFR-3 signaling axis that is crucial for lymphangiogenesis.
Here, we utilize HA-hydrogels with defined composites and tunable elasticity for in vitro studies of CLS formation. Viscoelasticity measurements demonstrate three distinct and physiologically-relevant substrate stiffness profiles: firm, medium, and soft, which allow us to decouple the effects of matrix elasticity and ligand binding density. We first demonstrate that HA-hydrogels preserve key lymphatic markers, including PROX-1 and LYVE-1. Decreasing matrix stiffness results in upregulation of VEGFR-3, which primes LECs to form CLS in response to VEGF-C stimulation in vitro. RNAi studies demonstrate that MMP-14 is required to enable CLS formation and that LECs sense matrix stiffness through YAP/TAZ mechanosensors. Collectively, we demonstrate that by tuning both the matrix stiffness and VEGF-C concentration, the signaling pathways of CLS formation in vitro can be regulated in a synthetic matrix, resulting in lymphatic networks which will be useful for the study of lymphatic biology and future approaches in lymphatic regeneration.
Materials and Methods:
Human LEC Culture
Human LECs derived from the dermis of two adult donors (PromoCell, Heidelberg, Germany) were expanded and used for experiments between passages 5 and 9. Human LECs were grown in endothelial cell growth medium MV 2 (EGM MV2; PromoCell) and incubated at 37°C with 5% CO2. Human LECs were characterized for the positive expression of CD31, LYVE-1, PROX-1, and Podoplanin throughout the experiments. All cell lines were routinely tested for mycoplasma contamination and were negative throughout this study.
Preparation of HA-hydrogels
Hyaluronic acid hydrogels (HyStem-HP, Advanced BioMatrix, Carlsbad, CA) were prepared by mixing 0.4% (w/v) thiol-modified HA conjugated with heparin with 0.4% (w/v) thiol-modified gelatin in a 1:1 volume ratio with 0.25%, 1% and 2% (w/v) polyethylene glycol diacrylate (PEGDA) crosslinker in a 4:1 volume ratio to obtain firm, medium, and soft substrates, respectively. A range of stiffnesses, consistent with those reported in previous studies were screened in preliminary studies23–25. The conditions of 0.25%, 1%, and 2% PEGDA crosslinker resulted in the greatest differences, and were therefore selected for this study. The hydrogel solution was cast in three milliliter syringes for rheology measurements and the images presented in Figure 1A, 4-well glass bottom dishes (Matsunami) for confocal imaging, 96-well tissue culture plates for gene expression and protein assays, and 16-well chamber slides for transmission electron microscopy. Hydrogels were incubated at 37°C with 5% CO2 for 16–24 hours to achieve full cross-linking before use in experiments. By casting the pre-polymer hydrogel solution directly in the wells that were used for each experiment, uniform surfaces were achieved.
Figure 1. Tunable HA-hydrogels as a supportive matrix to preserve LEC phenotypes.
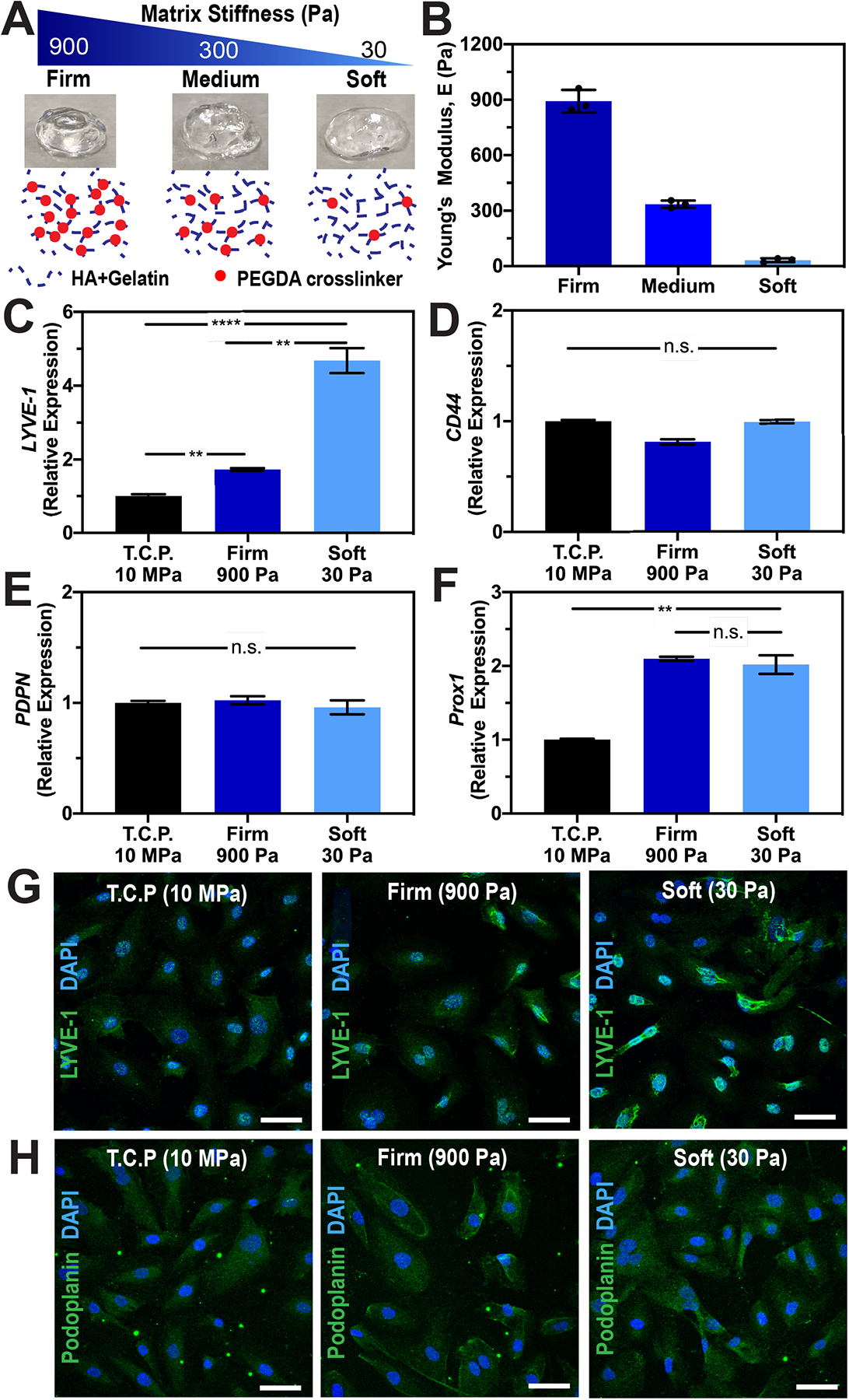
(A) Matrix elasticity of HA-hydrogels can be tuned with varying crosslinking density (red dots) without altering the polymer backbones (blue dotted lines). B) Rheological measurements of HA:gelatin in a 1:1 volume ratio with 2%, 1%, and 0.25% (w/v) of PEGDA crosslinker show three distinct profiles of hydrogel mechanics: firm, medium, and soft, respectively. Values shown are means ± S.D. of three independent hydrogel constructs. Please see Fig. S1 for storage (G’) and loss (G”) modulus data. Real-time qRT-PCR data for key lymphatic markers (C) LYVE-1, (D) CD44, (E) PDPN, and (F) PROX-1 expressed by LECs after being cultured on tissue culture plastic (E~10MPa), firm (E~900Pa), or soft (E~32Pa) HA-hydrogels. Three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings. ANOVA followed by Tukey post hoc analysis was performed to analyze differences between substrate stiffness. Significance levels were set at: n.s. P>0.05, * P<0.05, ** P<0.01 and *** P<0.001. Representative immunofluorescent images of LECs stained for (G) LYVE-1 and (H) Podoplanin after being cultured on tissue culture plastic (E~10MPa), firm (E~900Pa), or soft (E~32Pa) HA-hydrogels. Scale bars are 50 μm.
Hydrogel Mechanical Characterization
A rheometer with parallel plate geometry (8mm in diameter; TA Instruments, New Castle, DE) was utilized for oscillatory shear measurements of the storage modulus (G’) as previously described.25,26 Briefly, oscillatory time sweeps at 1Hz frequency and a constant strain of 5% were performed for 1 minute on three samples (n=3) for firm, medium, and soft hydrogels to characterize the storage modulus of the hydrogels as a function of PEGDA concentration.27,28 These strain and frequency parameters were selected in order to measure G’ in the linear viscoelastic regime.29 Rheological measurements were performed at room temperature and ambient air conditions, as dehydration of the hydrogels was negligible during the short testing duration. The Young’s modulus (matrix stiffness) was calculated by E = 2G′ (1 + v). HA-hydrogels can be assumed to be incompressible,29 such that their Poisson’s ratio (v) approaches 0.5 and the relationship becomes E = 3G′.
Lymphangiogenesis assay
Human LECs were seeded on firm, medium, and soft hydrogels at a density of 100,000 cells/cm2, which was consistent to our previous studies,25,27 and cultured for 12 hours in EGM MV2 media supplemented with either 0.5 or 50ng/mL recombinant human VEGF-C (R&D Systems). Images were acquired from the middle of each well (n=10 per condition) at 4x using an inverted light microscope (ECHO Revolve, San Diego, CA). After an initial screening at time intervals of 3, 6, 9, 12, and 15 hours, the 12 hours timepoint was determined to be the ideal endpoint for imaging as it not only allowed for differences between conditions to develop, but also allowed for images to be captured before some tube contraction occurred.25,26
Quantification of Lymphatic Cord-Like Structures (CLS)
One image field per construct from ten distinct constructs (n=10), captured during the lymphangiogenesis assay, was analyzed using Kinetic Analysis of Vasculogenesis (KAV), a custom plug-in for FIJI.30 Ten parameters per image were quantified, and the tubes/node ratio and network area were selected to compare the degree of CLS formation on each substrate. For each hydrogel condition, at least three independent experiments were performed with three technical replicates.
LEC gene expression
To analyze the effect of HA-hydrogels on lymphatic phenotypes, LECs were seeded on firm and soft hydrogels, as well as tissue culture plastic, and cultured for 48 hours in EGM MV2 media. Similarly, to analyze for gene expression during lymphatic tube morphogenesis, LECs were cultured on firm, medium, and soft hydrogels for 48 hours in EGM MV2 supplemented with 0.5ng/mL or 50ng/mL VEGF-C. The 48 hours timepoint was selected to ensure that the signaling cascade in response to VEGF-C and mechanical stimulation was captured. Each biological replicate was created by pooling RNA from three individual wells to collect a sufficient amount of RNA. At least three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings as previously described.27 RNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher) according to the manufacturer’s protocol. cDNA was then used with the TaqMan Universal PCR Master Mix and Gene Expression Assays for LYVE-1, PROX-1, CD44, Podoplanin, MMP-1, MMP-2, MMP-9, MMP-14, Flt4, YAP, TAZ, MYC, CTGF and GAPDH (Supplementary Table 1). Each sample was prepared in triplicate and the relative expression was normalized to GAPDH and analyzed using the ΔΔCt method.
Immunofluorescence
To analyze lymphatic protein expression, hLECs were seeded on firm and soft hydrogels, as well as tissue culture plastic, and cultured for 72 hours in EGM MV2 media. Samples were fixed with 3.7% formaldehyde, blocked with 1% BSA, permeabilized with 0.1% Triton-X, and stained for LYVE-1 and Podoplanin (Supplementary Table 2). To visualize lymphatic tube formation, hLECs cultured on hydrogels were fixed after 12 hours and samples were incubated with their respective primary antibodies; Phalloidin, YAP, and TAZ. Samples were rinsed and then counterstained with DAPI. Phalloidin stained samples were imaged with a Lionheart Gen5 microscope (BioTek Instruments) and the z-series imaging modality. YAP and TAZ stained samples were imaged at 40x as a z-stack (Nikon A1R-MP Confocal microscope).
Fluorescent Intensity Quantification
Confocal images of samples stained for YAP and TAZ were quantified for total fluorescent intensity using ImageJ (NIH). One field of view per sample was captured and a z-projection was created. Single cells in the field of view were quantified as individual samples. The fluorescent intensity in the nuclei and cytoplasm were gated and measured, and the background was then subtracted to give the Corrected Total Cell Fluorescence (CTCF).
ELISA for VEGFR-3 Protein Quantification
Human LECs were seeded on firm, medium, and soft hydrogels and cultured in EGM MV2 media for 72 hours. A lysis buffer was used to isolate cells from the hydrogels and cell lysates were analyzed using a Human VEGFR-3/Flt4 ELISA (R&D Systems, DY349B-05) kit according to the manufacturer’s protocol.
Transmission Electron Microscopy
Human LECs cultured on hydrogels for 6 and 12 hours were prepared for TEM samples as previously described.25 Briefly, cells were fixed with 3.0% formaldehyde, 1.5% glutaraldehyde in 0.1 M Na cacodylate, 5mM Ca2+ and 2.5% sucrose at room temperature for 1 hr and washed three times in 0.1 M cacodylate/2.5% sucrose pH 7.4 for 15 minutes each. The cells were post-fixed with Palade’s OsO4 on ice for 1 hour, rinsed with Kellenberger’s uranyl acetate and then processed conventionally through Epon embedding on a 16-well Lab-Tek chamber slide (NUNC). Serial sections were cut, mounted onto copper grids, and viewed using a Phillips EM 410 transmission electron microscope (FEI, Hillsboro, OR). Images were captured with a FEI Eagle 2k camera.
RNAi Transfection
Human LECs were transfected with siGENOME SMARTpool human MMP-14 or human PROX-1 (Dharmacon, Lafayette, CO) using the manufacturer’s protocol. Human LECs were cultured to 90% confluency in 6-well plates with EGM MV2 media (PromoCell) and no additional VEGF-C supplementation. The RNAi transfection solution was prepared by mixing DharmaFECT2 RNAi transfection reagent (Dharmacon) with serum-free and antibiotic-free EGM MV2 media. To transfect the cells, EGM MV2 media was removed and replaced with 1.6mL of antibiotic-free EGM MV2 and 400μL transfection solution in each well to achieve a final RNAi concentration of 50nM. Transfected cells were incubated at 37°C and 5% CO2. After 72 hours, total RNA was isolated and real-time qRT-PCR was performed, as described in the previous sub-section, to confirm the knock-down of MMP-14 or PROX-1 expression.
Statistical Analysis
Statistical analysis of KAV parameters was performed with GraphPad Prism (GraphPad Software Inc., La Jolla, CA). For each hydrogel condition, at least three independent experiments were performed with three technical replicates. Statistical comparisons were made using Student’s t test for paired data, analysis of variance (ANOVA) for multiple comparisons, and with Tukey post hoc analysis for parametric data. Specifically, ANOVA followed by Tukey post hoc analysis was performed to analyze differences between substrate stiffness with the same VEGF-C concentrations, and Student’s t test was used to analyze differences between low and high VEGF-C concentrations with the same substrate stiffness. Significance levels were set at the following: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Results:
Hyaluronic Acid (HA)-Hydrogels preserve LEC phenotypes
Since LECs uniquely express LYVE-1 to bind to HA and activate intracellular signaling to promote lymphangiogenesis,21,31 we postulate that tunable HA-hydrogels can serve as a supportive matrix for lymphatic tube formation in vitro. Toward this goal, our initial investigations focused on studying the effect of HA-hydrogels and their mechanical influence on LEC phenotypes. Employing a similar method to our previous studies, where we have extensively characterized HA-hydrogels to control vascular tube morphogenesis,25–28 we generated HA-hydrogels with varied crosslinker concentrations to mimic a wide range of physiologically-relevant matrix stiffnesses, while preserving uniform presentation of cell adhesion molecules (Figure 1A). It is important to note that one advantage of synthetic HA hydrogels is that the chemistry of the polymeric networks is easily controlled via reaction conditions and is uniform between various batches,13,32 which is difficult to impossible to achieve with naturally derived matrices, such as collagen and Matrigel.33,34 Moreover, while enzymatic crosslinking of such natural gels as collagen and Matrigel allows studies of increased stiffness, it also results in increased protein concentration and ligand density, which make it difficult to decouple the effects of matrix stiffness from other matrix properties.26,35
For the present study, we utilized thiol-modified HA-gelatin hydrogels to study lymphatic cellular response to a wide range of tunable mechanical stimuli.25,26,29 By varying PEGDA crosslinker concentrations (from 0.25 to 4%), while preserving the HA:gelatin ratio, hydrogels with a wide range of Young’s Moduli (matrix stiffness) were established (Supplementary Figure 1). After our initial screening, we selected three crosslinker conditions that created hydrogels with distinct Young’s moduli (E, matrix stiffnesses): firm (890 ± 61 Pa), medium (335 ± 20 Pa), and soft (32 ± 10 Pa) for our subsequent studies (Figure 1B). Next, we investigated the effect of HA-hydrogel matrix stiffness on key lymphatic markers that are uniquely expressed by LECs. Real-time qRT-PCR data revealed that LECs cultured on HA-hydrogels showed increased expression of PROX-1 and LYVE-1 compared to LECs cultured on plastic tissue culture plates, while expression of CD44 and PDPN were relatively constant across different conditions (Figure 1C–F). Interestingly, the expression level of LYVE-1 and PROX-1 increased with decreasing matrix stiffness (Figure 1C and F). The soft matrix demonstrated the highest PROX-1 (two-fold increase) and LYVE-1 (five-fold increase) expression, which is quite significant given that LECs are notoriously known to lose LYVE-1 expression during in vitro culture,36 and therefore need to be cultured on fibronectin coated plate to maintain their lymphatic phenotypes.37 While PROX-1 expression was elevated but constant on different hydrogel stiffnesses compared to tissue culture plates, LYVE-1 expression was influenced by substrate stiffness and was greater on the soft hydrogels compared to the firm hydrogels. These observations regarding LYVE-1 expression were also qualitatively confirmed using immunofluorescent imaging (Figure 1G). Although LYVE-1 is a CD44 homolog capable of HA binding,21 we observed that decreasing the matrix stiffness of HA hydrogels effected LYVE-1 but not CD44. PDPN expression, a membrane marker of LECs, was also not altered by changes in matrix stiffness (Figure 1E and H). Collectively, these results underscore the enhanced ability of HA-hydrogels to preserve LEC phenotypes which is an important enabling step towards utilizing tunable HA-hydrogels to control lymphatic tube formation in tissue engineering approaches.
Effect of VEGF-C and matrix stiffness on CLS formation
High concentrations of VEGF-C (i.e., 50 ng/ml) were previously demonstrated to induce differentiation into LECs,38 as well as lymphangiogenesis in both in vitro and in vivo models.39,40 Therefore, to study CLS formation in a controllable in vitro system, we seeded human LECs on HA hydrogel substrates and cultured them for 12 hrs in media supplemented with either 0.5 ng/mL (low) or 50 ng/mL (high) VEGF-C. We observed minimal branching of LECs seeded on the firm hydrogels supplemented with low VEGF-C (data not shown) or even with high VEGF-C (Figure 2A and Supplementary Figure 2). Conversely, when LECs were seeded on soft hydrogels with only 0.5 ng/mL VEGF-C, the mechanical environment allowed some cellular branching to occur (Supplementary Figure 3). Moreover, CLS formation on soft hydrogels was further enhanced with a higher VEGF-C supplementation, leading to greater network areas and a higher extent of branching (Figure 2A), demonstrating that given the right mechanical environment, VEGF-C can amplify CLS formation in vitro.
Figure 2. VEGF-C and matrix stiffness regulate lymphatic cord-like structures formation.
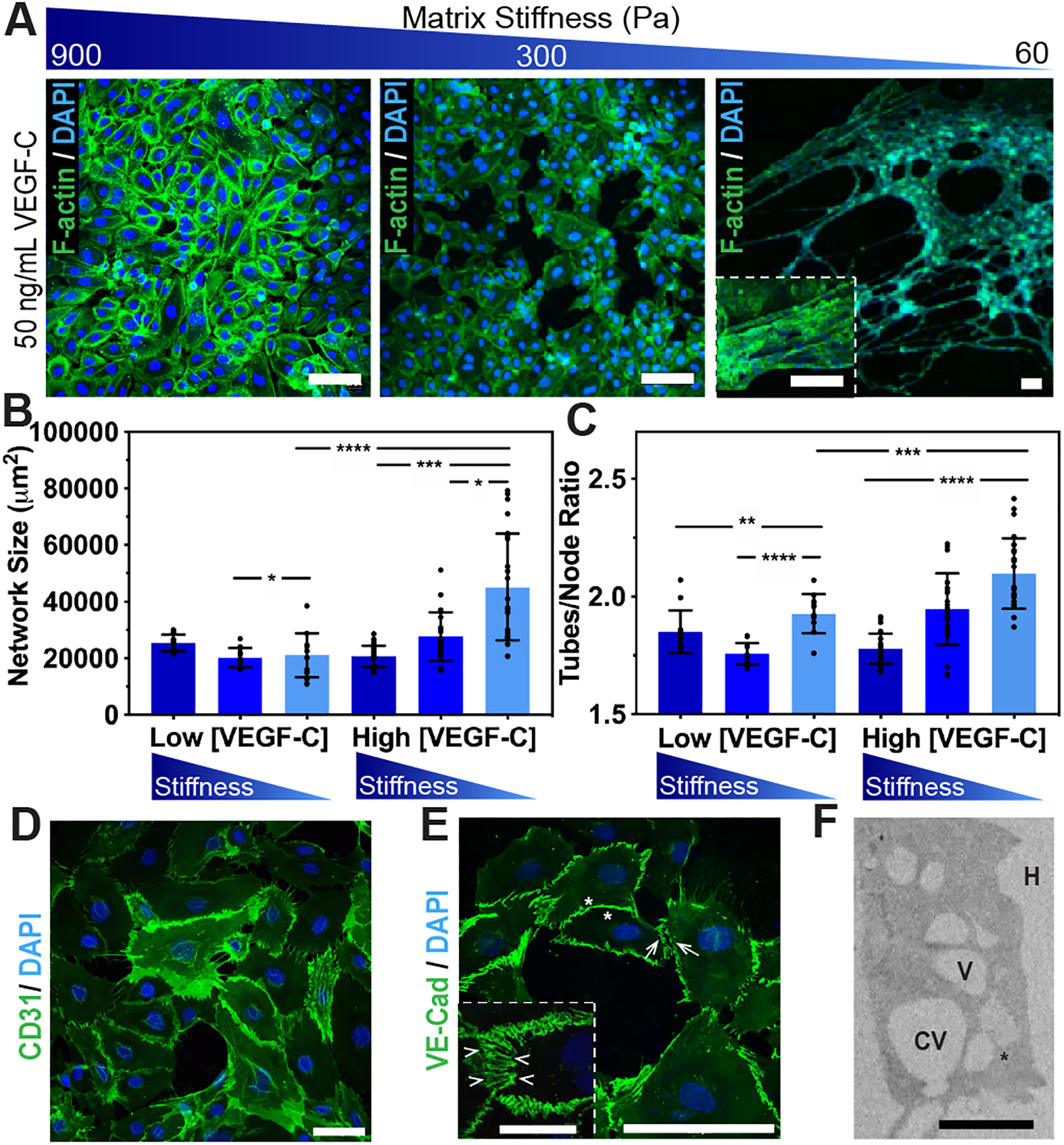
(A) Human LECs were seeded on firm, medium, and soft substrates for 12 hours supplemented with 50 ng/mL VEGF-C and formed CLS, as demonstrated by fluorescence microscopy of F-actin (green) and nuclei (blue). Scale bars are 50 μm. (B) Kinetic analysis of vasculogenesis (KAV) revealed a significant increase in network size and (C) tubes/node ratios as substrate stiffness decreased. Data represents the mean ± S.D. of ten biological replicates performed. Confocal images of CLS formed on soft substrates showing junctional markers (D) CD31 and (E) VE-Cad. Enlarged rendering of confocal image stack indicate cellular junctions (arrowheads) with discontinuous (arrows) and overlapping (asterisks) junctions. (F) TEM analyses of CLS formed after 12 hours showed LECs degrading the hydrogels (H) to generate intracellular vacuoles (V), some of which were observed in the process of merging (asterisk) into coalescent vacuoles (CV). ANOVA followed by Tukey post hoc analysis was performed to analyze differences between substrate stiffness within the same VEGF-C concentrations, and Student’s t test was used to analyze differences between low and high VEGF-C concentrations for the same substrate stiffness. Significance levels were set at: *P<0.5, **P<0.01, ***P<0.001, ****P<0.0001. Scale bars are: (A) 50 μm; (D) 50 μm; (E) 50 μm and 25 μm (inset); and (F) 20 μm.
Next, we utilized the kinetic analysis of vasculogenesis (KAV) Fiji plug-in to quantitate lymphatic tube formation and provide high-throughput lymphangiogenic analysis (Supplementary Figure 4).30,41 Quantification reveals that substrate stiffness influences LEC network assembly and decreasing matrix elasticity results in a significant increase in lymphatic network size – from (20.7 ± 3.8) × 103 μm2 on firm substrates to (27.7 ± 8.6) × 103 μm2 on medium substrates to (44.0 ± 8.4) × 103 μm2 on soft substrates (Figure 2B). Similarly, the tubes/node ratio, or the extent of branching, also increased – from 1.78 ± 0.07 on firm substrates to 1.99 ± 0.13 on medium substrates to 2.08 ± 0.13 on soft substrates (Figure 2C). It should be noted that while the network structures in Figure 2A are slightly less extensive than the networks shown in Supplementary Figures 2 and 3, the differences resulted from the networks being perturbed during the immunostaining process and did not arise during the tube formation process. Phase contrast images like the ones presented in Supplementary Figure 3 were used for quantification with KAV, and immunofluorescence images were used for cytoskeleton visualization.
Very limited CLS formation occurred on firm and medium substrates supplemented with 50 ng/mL VEGF-C, while extensive lymphatic tubes, similar to the tubes formed when LECs were seeded on Matrigel,42 formed on the soft substrates supplemented with 50 ng/mL VEGF-C (Figure 2A–C). Moreover, to study cellular junctions formed on the soft substrates, we stained CLS with CD31 and VE-Cadherin as junctional markers (Figure 2D–E). Using confocal analysis and 3D rendering, we observed lymphatic CLS with discontinuous and overlapping junctions (Figure 2E). To further confirm these observations and investigate the cell-matrix interactions that facilitate the formation of intracellular vacuoles, TEM analysis was performed following our previously published protocols.25,27 After 12 hours of culture, LECs on soft substrates were found elongated and degrading the hydrogels to form intracellular vacuoles (Figure 2F). Some of these intracellular vacuoles were in the process of merging into coalescent vacuoles, which is consistent with the tunneling model of lymphatic vessel formation observed in others in vitro and in vivo models of lymphatic vessel formation.43,44
Matrix stiffness primes LECs for VEGF-C induced lymphatic tube formation in vitro
To examine how matrix elasticity and high concentrations of VEGF-C would co-regulate lymphatic tube formation in vitro, our initial investigations focused on the expression of VEGFR-3, also known as Flt4. Real time qRT-PCR indicates that decreasing matrix stiffness correlated with an increase in VEGFR-3 expression by LECs cultured with either low or high concentrations of VEGF-C (Figure 3A). Interestingly, this trend seemed to be influenced by matrix stiffness and not VEGF-C concentrations. This trend was also confirmed via ELISA to detect the presence of total VEGFR-3 expressed by LECs (Figure 3B and Supplementary Figure 5A). These findings suggest that softer matrices, in particular the soft substrate, primes the LECs to express more VEGFR-3, which enables effective stimulation of LECs with VEGF-C.
Figure 3. Expression of MMP-2 and MMP-14 is dependent on VEGF-C concentration and matrix stiffness.
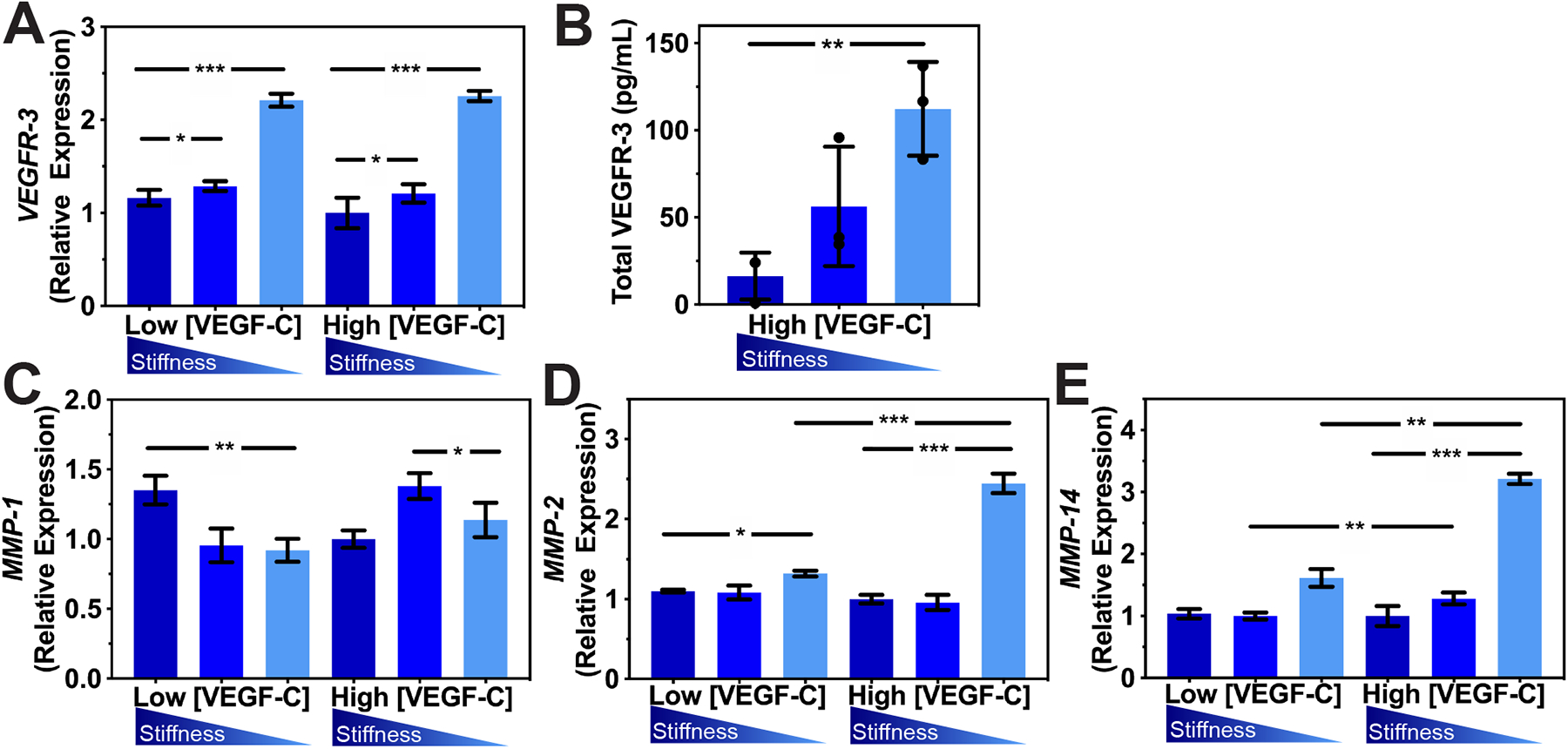
(A) Real-time qRT-PCR data for VEGFR-3 expressed by LECs after being cultured on firm, medium, or soft hydrogels for 48 hrs. Three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings (B) ELISA analysis of soluble total VEGFR-3 secreted by LECs cultured on firm, medium, or soft hydrogels for 72hrs. Real-time qRT-PCR data for MMP-1 (C), MMP-2 (D), and MMP-14 (E). mRNA expression of MMP-1, MMP-2, and MMP-14 increases with decreases in matrix stiffness. Values shown are means ± S.D. from three independent experiments (n=3) performed with three technical replicates. ANOVA followed by Tukey post hoc analysis was performed to analyze differences between substrate stiffness with the same VEGF-C concentrations, and Student’s t test was used to analyze differences between low and high VEGF-C concentrations with the same substrate stiffness. Significance levels were set at: *P<0.5, **P<0.01, ***P<0.001.
Next, we investigated how stimulation with VEGF-C would induce MMP expression to enable cell migration, which is a crucial step in enabling lymphatic tube formation. Real time qRT-PCR was performed to compare MMP expression in LECs cultured on firm, medium, and soft substrates supplemented with 50 ng/mL (high) VEGF-C versus with 0.5 ng/mL (low) VEGF-C (Supplementary Table 1 and Supplementary Figure 5B). After 48 hrs of incubation in media supplemented with high VEGF-C, LECs showed increased production of MMP-1, −2, and −14. The increase in MMP production became more significant for LECs cultured on the soft substrate (Figure 3C–E). Specifically, LECs cultured on soft substrates with high VEGF-C produced two times the MMP-2 (Figure 3D) and three times the MMP-14 (Figure 3E) produced by LECs cultured in media supplemented with low VEGF-C. The increase in MMP expression that correlates with the decrease in matrix stiffness is very intriguing, especially given the importance of MMPs in regulating lymphatic tube formation.45 In particular, MMP-14 can activate pro-MMP-2 to localize MMPs activity at the direction of cell migration, which is responsible in the formation of lumen compartments.44,46 This result highlights the importance of MMP-1, −2, and −14 during lymphatic tube formation, particularly in the soft substrates. Collectively, these findings suggest that matrix elasticity primes LECs to express VEGFR-3 on the cell surface, which in turns enable effective VEGF-C stimulation and increased expression of MMPs to form lymphatic tube networks in vitro.
YAP/TAZ are mechanosensors of matrix stiffness in LECs
To investigate the effect of matrix elasticity on in vitro lymphatic tube formation, we focused our investigation on the Hippo pathway YAP/TAZ, which are known as sensors and mediators of mechanical cues. YAP/TAZ are highly enriched in blood ECs of growing blood vessels and play crucial roles in angiogenesis by regulating cytoskeletal arrangement, proliferation, and cell motility.47,48 More recently, the roles of YAP/TAZ in lymphatic vessel morphogenesis during development have been elucidated in mice and zebrafish.23,49 Here, we demonstrate that the expression of YAP/TAZ by LECs during CLS formation in HA-hydrogels is regulated by matrix stiffness. Real time qRT-PCR reveals that decreased matrix stiffness results in decreased TAZ expression but a non-significant decrease in YAP expression (Figure 4A–B), which led us to investigate their downstream targets MYC and CTGF, as well as Prox-1 (Figure 4C–E). As matrix stiffness decreases, MYC and CTGF decrease to 0.33-fold and 0.5-fold, respectively. On firm substrates, YAP/TAZ enters the nucleus and binds to the PROX-1 promoter which inhibits transcription of PROX-1 and its targets, such as VEGFR-3 and MMP-14.23,50 With decreasing matrix elasticity, YAP/TAZ are translocated into the cell membrane, leading to their cytoplasmic degradation (Figure 4F–H). Furthermore, decreasing matrix elasticity results in decreased nuclear localization of YAP and increased cytoplasmic localization of TAZ (Figure 4I–J). Subsequently, cytoplasmic degradation of YAP/TAZ enhances the transcription of PROX-1 (Figure 4E), including its targets VEGFR-3 and MMP-14 which are highly expressed on LECs cultured on the soft substrate with high concentrations of VEGF-C. It is important to note that although the soft matrix promotes expression of VEGFR-3, that trend does not occur for PROX-1 in the case of low VEGF-C supplementation (Supplementary Figure 6) and may explain the limited CLS formed on soft matrices with low VEGF-C. Overall, these observations suggest the roles of YAP/TAZ as mechanosensors of matrix stiffness to enable lymphatic tube formation in vitro through transcription of PROX-1, including its targets VEGFR-3 and MMP-14.23,50
Figure 4. Mechanical regulation of lymphatic cord-like structures formation.
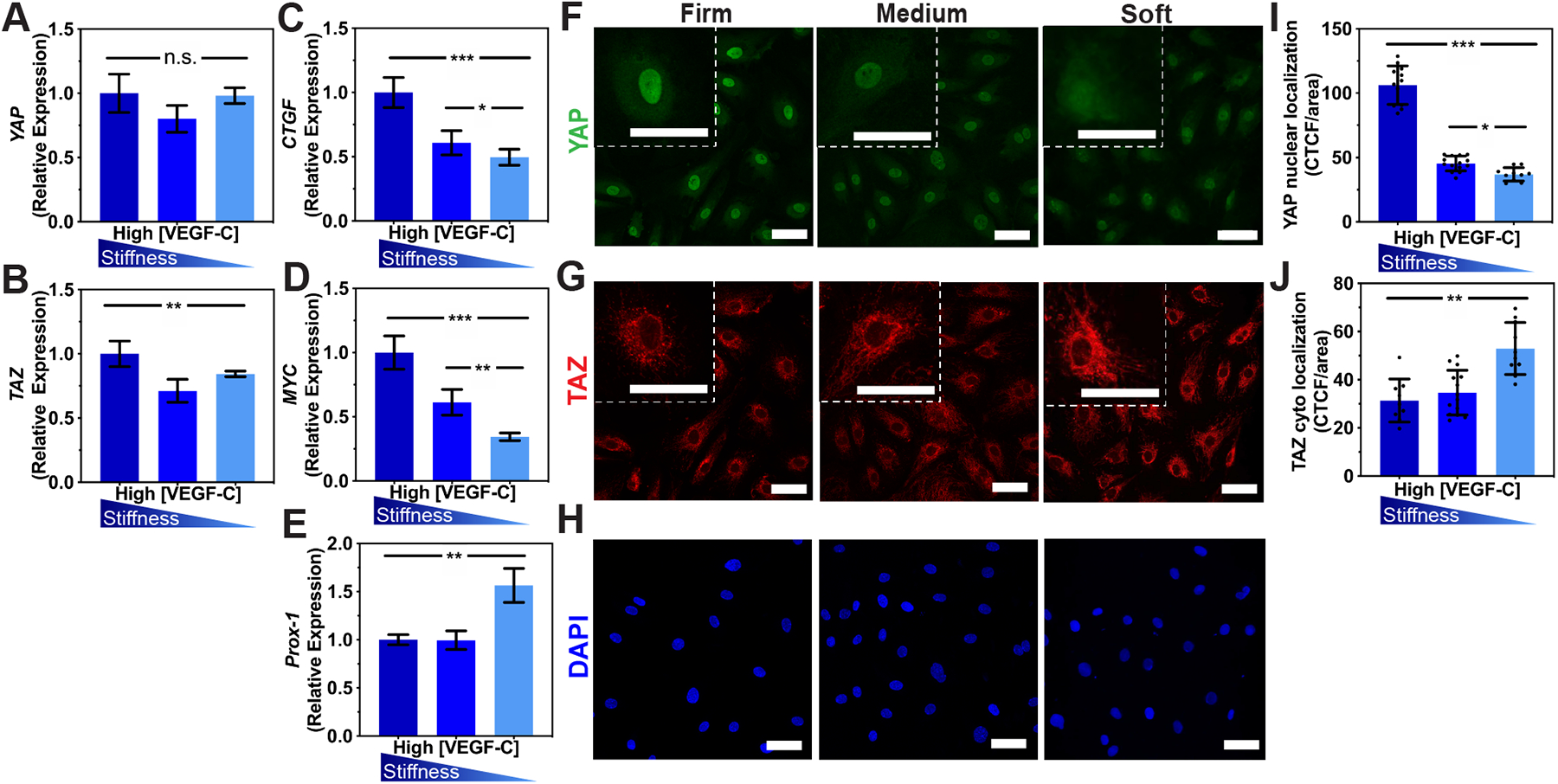
Real-time qRT-PCR data for (A)YAP, (B) TAZ (C), CTGF, (D) MYC, and (E) PROX-1 expressed by LECs after being cultured on firm, medium, or soft hydrogels supplemented with high VEGF-C (50 ng/mL) for 48 hrs. Three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings. Confocal microscopy z-stacks of (F) YAP (green), (G) TAZ (red), and (H) nuclei (blue) indicate the localization of YAP/TAZ in lymphatic networks formed on the firm, medium, and soft hydrogels. Scale bars are 50 μm. Fluorescent intensity quantification demonstrates (I) a decrease in nuclear localization for YAP and (J) and an increase in cytoplasmic localization of TAZ as matrix stiffness decreases. CTCF: Corrected Total Cell Fluorescence. Values shown are means ± S.D. ANOVA followed by Tukey post hoc analysis was performed to analyze differences between substrate stiffness. Significance levels were set at: n.s.P>0.5, *P<0.5, **P<0.01, ***P<0.001.
MMP-14 and PROX-1 are required for in vitro lymphatic tube formation
MMP-14, which is also known as MT1-MMP, has been reported to support blood vascular morphogenesis by allowing matrix degradation at the migrating cell front,46 as well as by creating a vascular guidance tunnel to control lumen formation.44 Recent evidence suggests that MMP-14 plays a crucial role in lymphatic formation during development and lymphatic metastasis.24,51,52 We utilized a siRNA suppression approach to investigate the function of MMP-14 in lymphatic tube formation in soft substrates cultured with a high concentration of VEGF-C, where CLS formation was found to be optimized. LECs treated with siRNA targeting MMP-14 showed a significant decrease in MMP-14 expression compared to LECs treated with the Luciferase non-targeting control (Figure 5Ai). In contrast to the Luciferase-treated LECs (control), siRNA suppression of MMP-14 abrogated lymphatic tube formation on the soft substrates, with more rounded cell morphology (Figure 5B). KAV analysis indicates a reduction in network size of CLS formed in the siMMP-14 group compared to the siLuciferase control (Figure 5C).
Figure 5. MMP-14 and Prox-1 are required for lymphatic cord-like structures formation.
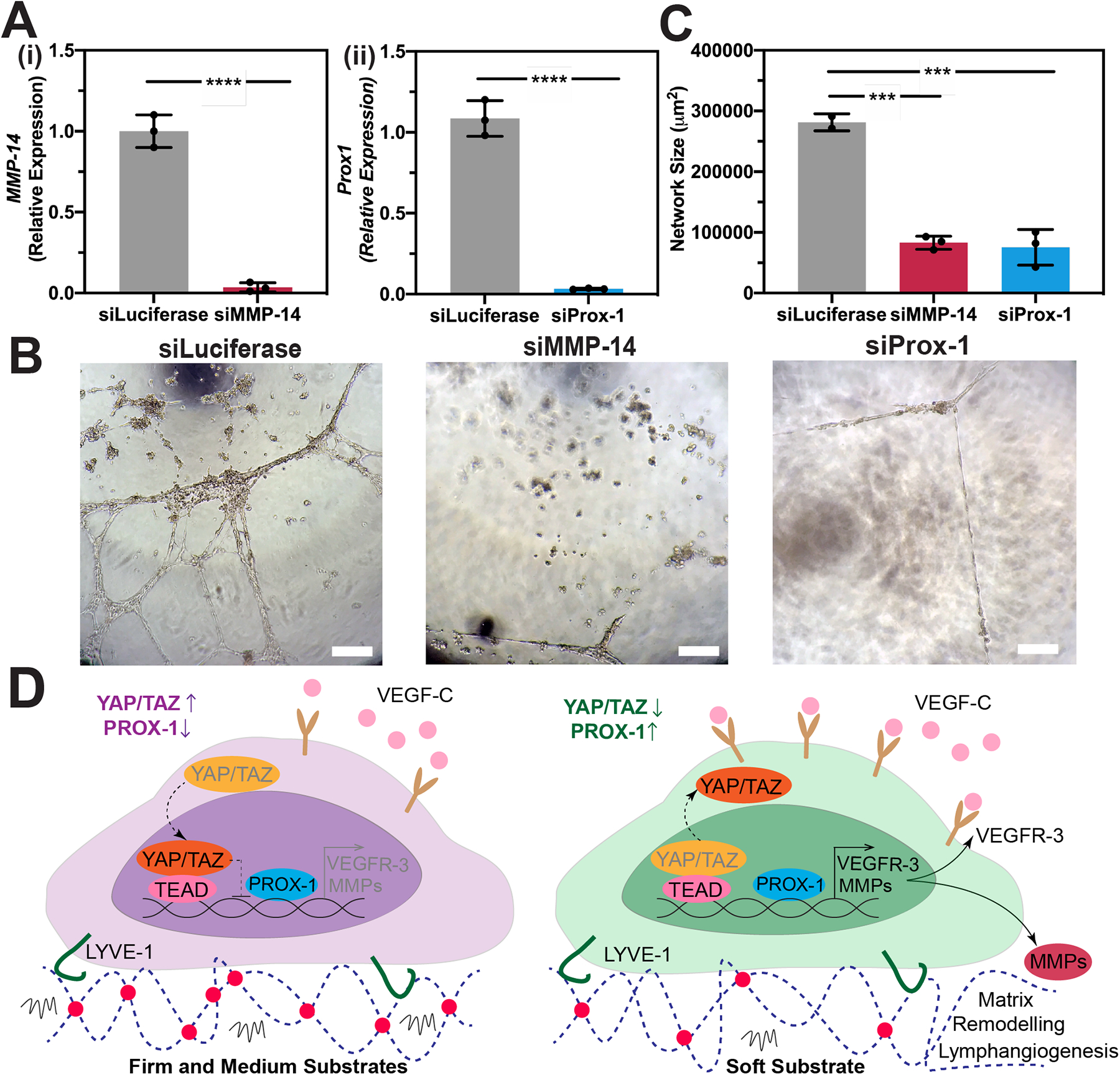
(A) Human LECs transfected with siRNA for (i) MMP-14 or (ii) Prox-1 demonstrated a significant reduction in their expression for MMP-14 or Prox-1, respectively compared to non-targeting control (siLuciferase). Three biological replicates (n=3) were collected per condition and analyzed with real-time qRT-PCR with triplicate readings. Statistical significance was assessed using Paired Student’s t-test. Significance levels were set at ****P<0.001.(B) Human LECs transfected with either siLuciferase, siMMP-14, or siProx-1 were seeded on the soft substrates and supplemented with 50 ng/mL VEGF-C for 12 hrs. Scale bars are 50 μm. (C) Kinetic analysis of vasculogenesis (KAV) revealed a significant reduction in network size in siMMP-14 and siProx-1 treated groups compared to the siLuciferase control. Data represents the mean ± S.D. of three biological replicates performed. Statistical significance was assessed using Paired Student’s t-test to analyze differences between RNAi-treated groups and the Luciferase control. Significance levels were set at ***P<0.01. (D) Schematic diagram depicting the role of matrix elasticity in priming lymphatic tube formation directed by VEGF-C. HA-hydrogels were able to preserve key lymphatic markers, including LYVE-1. When LECs are cultured in firm and medium substrates, YAP/TAZ enter the nucleus and bind to the PROX-1 promoter, inhibiting its transcription, including its targets, such as VEGFR-3 and MMPs. However, decreasing matrix stiffness further primes LECs and enables YAP/TAZ to undergo cytoplasmic degradation, which subsequently enhance transcription of PROX-1, including its targets, such as VEGFR-3 and MMPs. Consequently, high MMP expression and binding of VEGF-C to VEGFR-3 results in matrix remodeling and lymphatic tube formation in vitro.
Our next investigation focused on the role of PROX-1 in regulating lymphatic tube formation in response to matrix elasticity. A previous study reported that YAP/TAZ negatively regulate PROX-1 during lymphatic development.23 We utilized a siRNA suppression approach to examine whether PROX-1 is the connecting link by which LECs respond to substrate stiffness during lymphatic tube formation. LECs treated with siRNA targeting PROX-1 showed a significant decrease in PROX-1 expression compared to LECs treated with the Luciferase non-targeting control (Figure 5Aii). In contrast to the Luciferase-treated LECs (control), siRNA suppression of PROX-1 mitigated lymphatic tube formation on the soft substrates, with more elongated cell morphology (Figure 5B). KAV analysis indicates a reduction in network size of CLS formed in the siProx-1 group compared to the siLuciferase control (Figure 5C). It is important to note that, when siLuciferase, siMMP-14, or siProx-1 treated LECs were cultured on firm and medium substrates, they maintained a cobblestone morphology on monolayer culture with no indication of CLS formation (Supplementary Figure 7). Collectively, these observations suggest that both MMP-14 and PROX-1 are required for matrix stiffness primed lymphatic tube formation induced by VEGF-C.
Discussion:
LECs express LYVE-1, a specific receptor for HA, and provide a unique advantage for engineered matrices containing HA. We show that matrix stiffness and VEGF-C co-regulate lymphatic tube formation. High levels of VEGF-C are required to initiate CLS formation, as well as activate MMPs to enable LEC migration. Under these conditions, substrate elasticity affects the progression of CLS formation. With decreases in substrate stiffness, we observe increased expression of PROX-1 and activation of VEGFR-3, as well as cytoplasmic degradation of YAP/TAZ and downregulation of YAP/TAZ target genes. Furthermore, MMP-14 is required to enable the movement of LECs on the matrix and YAP/TAZ act as plastic regulators of lymphatic tube formation through PROX-1 transcriptional programming.
A chronic challenge of in vitro culture methods for lymphatic vasculature research is that LECs quickly lose their LEC-specific gene and protein expression.37,53 Previous studies have shown that culturing LECs on fibronectin improved cell adhesion and proliferation, which highlights the critical signaling contribution that the culture substrate provides.37 Here, we show that HA-hydrogels not only protect, but improve LEC-specific markers and provide a more suitable in vitro system. Both LYVE-1 and PROX-1 expression increased in LECs cultured on HA-hydrogels versus plastic tissue culture plates, which highlights a possible strategy for promoting lymphatic-like behavior for more accurate in vitro mechanistic studies. Additionally, the design of this HA-hydrogel system allows mechanical and biochemical signals to be decoupled to probe the effects of their individual contributions. While previous studies have been able to generate preliminary lymphatic vessels in fibrin,8,54,55 collagen,8 and Matrigel,42,56 these hydrogel systems are limited due to both the mechanical and biochemical signaling being altered by any modifications to the system. If these materials are diluted in order to decrease the substrate stiffness, integrin binding sites and other crucial signaling factors are also diluted.25,34 Here, our HA-hydrogel system can mechanically be altered by adjusting only the concentration of the PEGDA crosslinker which allows the substrate composition and ligand density to remain constant for all conditions. By using fully defined components in this HA-hydrogel system, we are also able to study the specific effects of VEGF-C in our system without the noise of additional growth factors that are sometimes included in other in vitro culture systems.
Another important characteristic of this HA-hydrogel system is the ability to generate a range of physiologically relevant substrate stiffnesses. During development, LECs migrate from the cardinal vein which has a Young’s modulus of 3.6 kPa to the surrounding tissue which has a Young’s modulus of only 270 Pa.24 This transition to a significantly lower substrate stiffness highlights the need for softer substrates in order to recapitulate in vivo conditions for lymphangiogenesis. Previous studies have used hydrogels to study lymphatic vessel development, and while those hydrogel systems are advantageous compared to traditional tissue culture plastic culture methods, those systems still have Young’s moduli on the scale of kPa to MPa.24 Here, our modular hydrogel system has been tuned to have Young’s moduli between 30–900 Pa (Figure 1A), representing the range of stiffnesses from the human brain to slightly stiffer than the surrounding tissue measured outside of the cardinal vein.24 It is important to note that while we found that LECs are sensitive to these three substrate stiffness profiles in our HA-hydrogels system, they are still considered in the softer ranges of substrate stiffness profiles, and the sensitivity of substrate stiffness may be unique to the chosen hydrogels system. Nonetheless, these findings highlight the importance of a relatively softer substrate to support lymphatic phenotypes and CLS formation, which is also consistent with previous reports using other hydrogels system.23,24
By decoupling the mechanical and biochemical effects, we show in these studies that the mechanical environment primes LECs for lymphangiogenesis and subsequently, VEGF-C promotes branching. Even with the supplementation of 50 ng/mL VEGF-C, only minimal branching occurs on the firm hydrogels (Figure 2C). Conversely, when LECs were seeded on soft hydrogels with only 0.5 ng/mL VEGF-C, the mechanical environment allowed rudimentary branching to occur (Supplementary Figure 2). The CLS formation on soft hydrogels was further enhanced with a higher VEGF-C supplementation (Figure 2B and 2C), demonstrating that VEGF-C can amplify tube formation, but only if the mechanical environment is suitable. Additionally, our HA-hydrogel model has demonstrated that VEGFR-3 expression is predominantly controlled by the mechanical environment and supplementation of VEGF-C alone cannot induce increased VEGFR-3. This dependency on substrate stiffness also highlights a potential strategy to tune VEGFR-3 expression for specific applications. Collectively, these observations show that while VEGF-C does promote lymphangiogenesis, as extensively reported,39,57 the mechanical environment is also critical for accurate in vitro models.
Beyond tuning VEGFR-3 expression, modifications to the substrate stiffness also modified MMP −1, −2, and −14 expression. MMP-14 is a cell surface activator of MMP-258 and we observe mimetic trends in real time qRT-PCR results for MMP-2 and MMP-14. Although this trend was observed at mRNA level and may not directly translate to protein expression of active MMPs,27,28 our finding was consistent to previous reports. Previous studies have revealed the crucial role of MMP-2 in LEC tube formation, where knocking-down MMP-2 inhibited LEC migration through collagen gels and MMP-2 knockdown in zebrafish caused lymphatic defects.45 MMP-2 degrades gelatin, which is contained in our HA-hydrogel system, allowing for LEC migration and branching, and supports our observed trend of increased MMP-2 expression corresponding with increased LEC branching and tube length. On the other hand, while expression of MMP-1 also increased, the trend was not as significant as MMP-2 and MMP-14. Since MMP-1 degrades collagen, which is not a major component of our hydrogel system, these observations suggest that LECs can adapt to secrete specific MMPs depending on their microenvironment. Additionally, increased VEGFR-3 expression on softer matrices and increased binding with VEGF-C may also contribute to this upregulation of MMP-2 and MMP-14 to promote matrix remodeling and allow for the increased LEC tube formation that is observed. Furthermore, MMP-2 but not MMP-9 has been shown to impact LEC sprouting45 and we observed substantially lower expression levels of MMP-9 compared to MMP-14 and MMP-2 in our screening run (Supplementary Figure 5B). High expression of MMPs is also responsible for cellular elongation and hydrogels degradation to facilitate the formation of intracellular vacuoles and coalescent vacuoles as precursor to open lumen compartments, as observed in other in vitro and in vivo models of lymphatic vessel formation.43,44 These supporting trends in our HA-hydrogel system with previous in vitro and in vivo studies regarding MMPs highlight the ability of our system to accurately recapitulate the native environment that LECs sprout in. Furthermore, this evidence demonstrates that our HA-hydrogel system serves as a novel system that can be utilized for further mechanistic studies in a highly controllable environment.
In addition to VEGFR-3 expression and PROX-1 being modulated by substrate stiffness, we also show that YAP/TAZ are important mechanosensitive proteins and transcription factors that contribute to regulating lymphatic tube formation. YAP/TAZ are influenced by matrix stiffness59 and it was recently revealed that VEGF-C activates the Hippo signaling pathway,23 which includes YAP/TAZ and their target genes, such as MYC and CTGF. PROX-1 expression is required for initial lymphatic specification and budding,22 as well as for continued maintenance of a LEC phenotype,60 and was recently revealed to be negatively regulated by YAP/TAZ.23 Here we show that the highest PROX-1 expression corresponds to samples with the highest degree of tube formation, as expected based on previous findings, and that TAZ expression is inversely related. The trend for YAP remains less clear based on real time qRT-PCR results and shows the need to analyze individual cells and the spatial localization of YAP/TAZ. Upon quantification of YAP/TAZ expression in both the nuclei and cytoplasm, we observe significant degradation of both YAP and TAZ as substrate stiffness decreases, which aligns with PROX-1 upregulation and lymphatic budding. Conversely, when nucleic YAP/TAZ expression is upregulated, PROX-1 is inhibited and lymphatic maintenance occurs, which translates to no tube formation occurring in our HA-hydrogel system here (Figure 5D). It is important to note that there is no clear nuclear TAZ expression and that TAZ may be expressed independently of YAP. These observations seem to agree with previous reports that TAZ expression moves to the cytoplasm in the presence of Prox-1.23 Additionally, when YAP/TAZ target genes, such as MYC and CTGF are downregulated in the presence of softer matrices, expression of PROX-1 targets such as VEGFR-3 and MMP-14 are also upregulated. Complementarily, exposure to soft matrices induces a GATA2-dependent increase in VEGFR-3 as well as LEC migration.24 These mechanosensitive responses by LECs to substrate stiffness highlight the need for more physiologically relevant in vitro models in order to accurately elucidate mechanisms of action, and also highlights the functionality of this HA-hydrogel system for future studies.23
Collectively, we show that by tuning both the matrix stiffness and VEGF-C concentration, the signaling pathways of CLS formation can be regulated in a synthetic matrix. Findings from this simple 2D system will lay an important framework for future work in generating more complex lymphatic networks, which can be used for mechanistic studies and potentially as therapeutics for a range of lymphatic disorders.
Supplementary Material
Acknowledgments
We thank Michael McCaffery (The Johns Hopkins University) for assistance with TEM imaging. We acknowledge support from the University of Notre Dame through “Advancing Our Vision” Initiative in stem cell research, Harper Cancer Research Institute – American Cancer Society Institutional Research Grant (IRG-17-182-04), American Heart Association through Career Development Award (19-CDA-34630012 to D. Hanjaya-Putra), and from the Walther Cancer Foundation (Pre-doctoral Fellowship to L. Alderfer). This publication was made possible, with support from the Indiana Clinical and Translational Science Institute (I-CTSI) funded, in part by Grant Number ULITR00108 from the NIH for Advancing Translational Sciences, Clinical and Translational Science Awards.
Abbreviations
- ANOVA
Analysis of variance
- CLS
Cord-Like Structures
- Ct
Cycle threshold
- E
Young’s modulus, elastic modulus
- EGM MV2
Endothelial Growth Media Microvascular 2
- Flt-4
Fms-related tyrosine kinase 4
- G’
Storage modulus
- G”
Loss modulus
- HA
Hyaluronic Acid
- KAV
Kinetic Analysis of Vasculogenesis
- LEC
Lymphatic Endothelial Cell
- LYVE-1
Lymphatic Vessel Endothelial Hyaluronan Receptor-1
- MMP
Matrix Metalloproteinase
- MT1-MMP
Membrane Type-1 Matrix Metalloproteinase
- PEGDA
Polyethylene glycol diacrylate
- PROX-1
Prospero-related homeobox-1
- RT qRT-PCR
Real time quantitative reverse transcription polymerase chain reaction
- TAZ
PDZ-binding motif
- TEM
Transmission Electron Microscopy
- v
Poisson’s Ratio
- VEGF-C
Vascular Endothelial Growth Factor-C
- VEGFR-3
Vascular Endothelial Growth Factor Receptor-3
- YAP
Yes-associated protein
Footnotes
Conflict of interest statement:
The authors have declared that no conflict of interest exists
References
- 1.Oliver G, Kipnis J, Randolph GJ & Harvey NL The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 182, 270–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrova TV & Koh GY Biological functions of lymphatic vessels. Science (80-. ) 369, eaax4063 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Liao S & Padera TP Lymphatic Function and Immune Regulation in Health and Disease. Lymphat. Res. Biol 11, 136–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y & Oliver G Current views on the function of the lymphatic vasculature in health and disease. Genes Dev 75, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon JB, Raghunathan S & Swartz MA A tissue-engineered model of the intestinal lacteal for evaluating lipid transport by lymphatics. Biotechnol. Bioeng 103, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaupper M, Jeltsch M, Rohringer S, Redl H & Holnthoner W Lymphatic Vessels in Regenerative Medicine and Tissue Engineering. Tissue Eng. Part B Rev 22, 395–407 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Helm C-L, Zisch A & Swartz MA Engineered Blood and Lymphatic Capillaries in 3-D VEGF-Fibrin-Collagen Matrices with Interstitial Flow. Biotechnol. Bioeng 96, (2006). [DOI] [PubMed] [Google Scholar]
- 8.Marino D, Luginbühl J, Scola S, Meuli M & Reichmann E Bioengineering: Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med 6, (2014). [DOI] [PubMed] [Google Scholar]
- 9.Muylaert DEP, Fledderus JO, Bouten CVC, Dankers PYW & Verhaar MC Combining tissue repair and tissue engineering: bioactivating implantable cell-free vascular scaffolds. Heart 100, (2014). [DOI] [PubMed] [Google Scholar]
- 10.Zhang L & Xu Q Stem/progenitor cells in vascular regeneration. Arterioscler. Thromb. Vasc. Biol 34, (2014). [DOI] [PubMed] [Google Scholar]
- 11.Park KM & Gerecht S Harnessing developmental processes for vascular engineering and regeneration. Development 141, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillel AT et al. Photoactivated Composite Biomaterial for Soft Tissue Restoration in Rodents and in Humans. Sci. Transl. Med 3, 93ra67 LP–93ra67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdick JA & Prestwich GD Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater 23, H41–H56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faust HJ et al. A hyaluronic acid binding peptide-polymer system for treating osteoarthritis. Biomaterials 183, 93–101 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Toole BP Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Slevin M, Kumar S & Gaffney J Angiogenic Oligosaccharides of Hyaluronan Induce Multiple Signaling Pathways Affecting Vascular Endothelial Cell Mitogenic and Wound Healing Responses. J. Biol. Chem 277, 41046–41059 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Banerjee SD & Toole BP Hyaluronan-binding protein in endothelial cell morphogenesis. J. Cell Biol 119, 643 LP–652 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Jha A, Harrington D, Farach-Carson M & Jia X Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter 8, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prestwich G Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 155, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf KJ & Kumar S Hyaluronic Acid: Incorporating the Bio into the Material. ACS Biomater. Sci. Eng 5, 3753–3765 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerji S et al. LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-specific Receptor for Hyaluronan. J. Cell Biol 144, 789 LP–801 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wigle JT & Oliver G An essential role for Prox1 in the induction of the LEC phenotype. EMBO J 21, 1505–1513 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyunsoo C et al. YAP and TAZ Negatively Regulate Prox1 During Developmental and Pathologic Lymphangiogenesis. Circ. Res 124, 225–242 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Frye M et al. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat. Commun 9, 1511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanjaya-Putra D et al. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J. Cell. Mol. Med 14, 2436–2447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee D, Hanjaya-Putra D, Bose V, Luong E & Gerecht S Hyaluronic Acid Hydrogels Support Cord-Like Structures from Endothelial Colony-Forming Cells. Tissue Eng. Part A 17, 1351–1361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanjaya-Putra D et al. Controlled activation of morphogenesis to generate a functional human microvasculature in a synthetic matrix. Blood 118, 804 LP–815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanjaya-Putra D et al. Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 33, 6123–6131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderhooft JL, Alcoutlabi M, Magda JJ & Prestwich GD Rheological properties of cross-linked hyaluronan-gelatin hydrogels for tissue engineering. Macromol. Biosci 9, 20–28 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varberg KM et al. Kinetic analyses of vasculogenesis inform mechanistic studies. Am. J. Physiol. - Cell Physiol (2017). doi: 10.1152/ajpcell.00367.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M et al. Low Molecular Weight Hyaluronan Induces Lymphangiogenesis through LYVE-1-Mediated Signaling Pathways. PLoS One 9, e92857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KY & Mooney DJ Hydrogels for Tissue Engineering. Chem. Rev 101, 1869–1880 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Nguyen EH et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng 1, 96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutolf MP & Hubbell JA Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47–55 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Hanjaya-Putra D & Gerecht S Vascular engineering using human embryonic stem cells. Biotechnol. Prog 25, 2–9 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Johnson LA, Prevo R, Clasper S & Jackson DG Inflammation-induced Uptake and Degradation of the Lymphatic Endothelial Hyaluronan Receptor LYVE-1. J. Biol. Chem 282, 33671–33680 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Makinen T et al. Isolated lymphatic endothelial cells transduce growth, surival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J 20, 4762–4773 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kono T et al. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arterioscler. Thromb. Vasc. Biol 26, 2070–2076 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Sweat RS, Sloas DC & Murfee WL VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirculation 21, 532–540 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell KT, Hadley DJ, Kukis DL & Silva EA Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications. PLoS One 12, e0181484–e0181484 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AU - Varberg KM, AU - Winfree S, AU - Dunn KW & AU - Haneline LS Kinetic Analysis of Vasculogenesis Quantifies Dynamics of Vasculogenesis and Angiogenesis In Vitro. JoVE e57044 (2018). doi:doi: 10.3791/57044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kazenwadel J, Secker GA, Betterman KL & Harvey NL In Vitro Assays Using Primary Embryonic Mouse Lymphatic Endothelial Cells Uncover Key Roles for FGFR1 Signalling in Lymphangiogenesis. PLoS One 7, e40497 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Detry B et al. Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol 12, 29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stratman AN et al. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP–dependent proteolysis in 3-dimensional collagen matrices. Blood 114, 237 LP–247 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Detry B et al. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 119, 5048–5056 (2012). [DOI] [PubMed] [Google Scholar]
- 46.W. M, van HV, A. EM & H.A. QP Pericellular Proteases in Angiogenesis and Vasculogenesis. Arterioscler. Thromb. Vasc. Biol 26, 716–728 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Kim J et al. YAP/TAZ regulates sprouting angiogenesis and vascular barrier maturation. J. Clin. Invest 127, 3441–3461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mason DE et al. YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol 218, 1369–1389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimm L et al. Yap1 promotes sprouting and proliferation of lymphatic progenitors downstream of Vegfc in the zebrafish trunk. Elife 8, e42881 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gramolelli S et al. PROX1 is a transcriptional regulator of MMP14. Sci. Rep 8, 9531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yao G et al. MT1-MMP in breast cancer: induction of VEGF-C correlates with metastasis and poor prognosis. Cancer Cell Int 13, 98 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong HLX et al. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun 7, 10824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrance W, Banerji S, Day AJ, Bhattacharjee S & Jackson DG Binding of hyaluronan to the native lymphatic vessel endothelial receptor LYVE-1 is critically dependent on receptor surface clustering and hyaluronan organisation. J. Biol. Chem (2016). doi: 10.1074/jbc.M115.708305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Güç E et al. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 131, 160–175 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Knezevic L et al. Engineering Blood and Lymphatic Microvascular Networks in Fibrin Matrices. Front. Bioeng. Biotechnol 5, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kriehuber E et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med 194, 797–808 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman J, Le TX, Skobe M & Swartz M Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ. Res 96, (2005). [DOI] [PubMed] [Google Scholar]
- 58.Pulyaeva H et al. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis 15, 111–120 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–184 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Johnson NC et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22, 3282–3291 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


