Abstract
Background:
Physical activity (PA) may slow the development of dementia by reducing the accumulation of amyloid.
Objective:
We tested the hypothesis that higher levels of leisure-time PA in mid- or late-life were associated with lower brain amyloid burden in late-life among 326 non-demented participants from the Atherosclerosis Risk in Communities Study of brain florbetapir positron emission tomography (ARIC-PET) ancillary.
Methods:
Self-reported PA was quantified using a past-year recall, interviewer-administered questionnaire in mid-life (1987–1989, aged 45–64 years) and late-life (2011–2013, aged 67–89 years). Continuous PA estimates were classified as 1) any leisure-time PA participation (yes/no); 2) meeting the 2018 United States’ PA guidelines (yes/no); and 3) per 1 standard deviation (SD) higher metabolic equivalent of task (MET) minutes per week (MET·min·wk−1). A brain magnetic resonance imaging scan with Florbetapir PET was performed in late-life. Adjusted odds ratios (OR) of elevated amyloid burden, defined as a global cortical standardized uptake value ratio (>1.2), compared to no elevated amyloid burden were estimated according to PA measures.
Results:
Among the 326 participants (mean age: 76 years, 42% male, 41% Black), 52% had elevated brain amyloid burden. Mid-life leisure-time PA did not show a statistically significant lower odds of elevated late-life amyloid burden (OR=0.71, 95% CI: 0.43–1.18). A 1 SD (970 MET. min. wk−1) higher PA level in mid-life was also not significantly associated with elevated amyloid burden (OR=0.89, 95% CI: 0.69–1.15). Similar estimates were observed for meeting versus not meeting PA guidelines in both mid- and late-life.
Conclusion:
Self-reported higher mid- and late-life leisure-time PA were not significantly associated with lower amyloid burden. Data show a trend of an association, which is, however, imprecise, suggesting replication in larger studies.
Keywords: Amyloid, cohort study, epidemiology, imaging, PET, physical activity
INTRODUCTION
Reducing the burden of cognitive impairment in the United States aging population is a high priority that may be attainable by intervening on modifiable behaviors such as physical activity. Our prior work in the Atherosclerosis Risk in Communities (ARIC) Study suggests that compared to participants who were physically inactive in mid-life (aged 45–64 years), middle or high levels of leisure-time physical activity were associated with less global and domain-specific cognitive decline and a lower incidence of dementia over 14 years of follow-up [1]. The underlying mechanisms that link physical activity to brain-related outcomes are still unknown but hypothesized to occur through several pathways, including increased neurogenesis [2] and the reduction of vascular and metabolic risk factors, including blood pressure [3], blood glucose levels [4], and systemic inflammation [5].
Mouse models have related physical activity (i.e., wheel running) to lower amyloid levels in the brain [6]. However, the role of exercise on amyloid-β burden in the human brain has not been widely examined. The results to date appear inconsistent, and there have been no reports on physical activity in mid-life at a time when amyloid-β begins to accumulate in the brain [6]. The largest human investigation thus far to examine this association was conducted among 268 elderly French community-dwelling individuals with mild cognitive impairment. In this cross-sectional analysis, self-reported physical activity measured continuously was not associated with brain amyloid burden (Odds Ratio = 1.00, 95% Confidence Interval: 0.99–1.00), based on Florbetapir levels (defined as a standardized uptake volume ratio (SUVR)>1.10) [7]. In another cross-sectional analysis, there was no association between levels of self-reported physical activity and amyloid positron emission tomography (PET) among the total sample of 116 cognitively normal individuals. However, lower levels of amyloid-β were observed in the highest versus lowest tertile of self-reported physical activity, but only among apolipoprotein (APOE) ε4 allele carriers [8]. Other prior studies have observed a significant negative association between physical activity and amyloid burden measured with PET, including a report on 60 cognitively normal older adults showing that participants with elevated amyloid had significantly lower reported exercise [9]. An additional report of 317 middle-aged adults from the Wisconsin Registry for Alzheimer’s Prevention found that self-reported physical activity attenuated the adverse effects of age on amyloid burden [10].
Altogether, the current literature on the association between physical activity and brain amyloid burden is constrained by small sample sizes and cross-sectional assessments which are susceptible to a reverse causation interpretation. A further limitation is the lack of repeated measures of physical activity. Considering the variation in physical activity over the adult life span associated with changes in lifestyle and functional abilities, a one-time measurement of physical activity may not be a reliable or informative evaluation of an individual’s activity exposure. Considering the fact that amyloid deposition occurs decades prior to manifest clinical symptoms, quantifying physical activity levels across life epochs with repeated measures is imperative.
Therefore, we examined the association between leisure-time physical activity and PET-quantified brain amyloid burden in the ARIC cohort by incorporating both late-life and repeated mid-life assessments of physical activity with more than 25 years of follow-up. We also examined whether the associations differed by APOE ε4 carrier allele status, race-study center, and cognitive status. We hypothesized that higher levels of leisure-time physical activity in mid- or late-life were associated with lower brain amyloid burden in late-life.
MATERIALSANDMETHODS
Study population and design
Participants for the ARIC-PET ancillary were recruited from the ongoing ARIC cohort, a community-based prospective study. Enrollment for ARIC began in 1987 with 15,792 participants aged 45–64 years recruited from four U.S. communities (Washington County, Maryland; Forsyth County, North Carolina; selected suburbs of Minneapolis, Minnesota; and Jackson, Mississippi). The baseline ARIC visit (1987–1989) was followed by three triennial visits (visit 2:1990–1992, visit 3:1993–1995, visit 4:1996–1998), and a fifth visit occurring 15 years later in 2011–2013. Details about the cohort have been described [11]. Among those participants who returned for the fifth examination (n = 6,538; 2011–2013), approximately 2,000 were selected to undergo a brain magnetic resonance imaging (MRI) scan, which included both participants with cognitive impairment and an age-stratified sample without impairment [12]. Among participants who received a brain MRI, recruitment for the ARIC-PET ancillary included those participants without dementia, heavy current alcohol use, renal dysfunction (creatinine > 2mg/dL), or prolonged QT-c interval (>450 ms) from three of the ARIC sites (Forsyth County, NC; Jackson, MS; and Washington County, MD). Of those participants recruited in ARIC-PET (n = 346), we excluded participants who were non-Black or non-White (n = 2); had a dementia diagnosis (n = 1); missing APOE ε4 (n = 4); or missing physical activity measurements at visits 1, 3, or 5 (n = 13). Our final analytic sample included 326 adults with measures of amyloid burden and physical activity measured in both mid-life and late-life. ARIC-PET ancillary study protocols and procedures were approved by the Institutional review boards at each participating study center. All participants gave written informed consent.
Exposure: Leisure-time Physical Activity (LTPA)
LTPA was measured at ARIC visits 1, 3, and 5 using the modified Baecke Physical Activity Questionnaire, a standardized interviewer administered questionnaire that utilizes a past-year recall time frame [13]. For the sports and leisure domain, the questionnaire asked, in open-ended form, for up to four of the most common sports or leisure-time types. For each activity type, information related to the duration (hours per week) and frequency (number of weeks per month) were collected. While the Baecke Questionnaire scoring of summary estimates results in index scores for sport, leisure, and work ranging from 1 to 5 (reflecting the highest activity level) [13], several questions from the sports domain were rescored and summarized as metabolic equivalent of task (MET) minutes per week (MET·min·wk−1). This was used because it provides a physiologically meaningful estimate that can be compared to other studies and extrapolated to reflect meeting (or not meeting) public health recommendations for physical activity.
For this, each activity type was assigned a MET value ranging from 1–12 METs based on the 2011 Compendium of Physical Activities [14]. For each activity type reported, MET·min·wk−1 was estimated over the past year by multiplying the frequency, duration, and MET value, and were summed across all activity types reported (up to four) to quantify total volume of LTPA. Participants who reported that they did not participate in any sports or leisure-time activities were assigned a value of 0 MET·min·wk−1. LTPA, in minutes per week (min·wk−1), was also categorized according to the 2018 US Physical Activity Guidelines of at least 150 minutes of aerobic moderate or vigorous intensity per week, with intensity based on reported activities of at least 3 METS [15]. For this study, physical activity was operationalized in mid-life (visit 1) and late-life (visit 5) as: 1) participation in LTPA (yes/no); 2) meeting 2018 physical activity guidelines (yes/no); and 3) per 1 standard deviation (SD; 970 MET·min·wk−1) higher total MET·min·wk−1. We also averaged the total MET·min·wk−1 in mid-life at visits 1 and 3 (1993–1995) to obtain an overall measure of total volume of LTPA across 6 years in mid-life.
The Baecke questionnaire has moderate to good reliability (test-retest reliability ranging from 0.74–0.88) [13]. The questionnaire has also been shown to have moderate validity (Spearman correlation coefficient = 0.54) against energy expenditure measured with doubly-labeled water [16].
Outcome: Elevated amyloid burden
Brain MRIs were obtained from a 3T MRI scan in late-life at visit 5/ARIC-Neurocognitive Study (NCS) (2011–2013) [12]. Florbetapir PET scans were performed within 1 year of the brain MRI scan with magnetization-prepared rapid gradient echo (MPRAGE) used for coregistration of the PET images. Isotopes were injected 50–70min before a 20 min uptake scan. Each image was reviewed for incidental findings, image quality, and quantified for SUVRs. SUVRs were obtained for each of the 34 total regions of interest, but the primary analysis used the global cortical measure of amyloid-β, calculated as the weighted average of the following regions: orbitofrontal, prefrontal, and superior frontal cortices, lateral temporal, parietal, and occipital lobes, precuneus, and anterior and posterior cingulates. The primary outcome was global amyloid SUVR dichotomized at the analytic sample median (SUVR > 1.2) to indicate elevated brain amyloid burden. The value of 1.2 was chosen due to the highly skewed distribution of the data and is in line with prior ARIC-PET studies [17–19]. Note that this value does not correspond to others in the literature on elevated amyloid levels by PET [20]. In sensitivity analyses, we also examined global amyloid SUVR continuously and using an alternate cutpoint >1.3 (n = 103 (31.6%)) to more accurately reflect elevated amyloid burden in a general population.
Covariates
Covariates include age, sex, education (less than high school, high school or equivalent, and greater than high school), race-ARIC field center (White adults from Minneapolis, Washington County, or Forsyth County or Black adults from Forsyth County and Jackson) to reflect the race-geographic distribution of the ARIC cohort, and APOE ε4 genotype (0 or ≥1 allele). All covariates, except age which references the time of the physical activity exposure assessment, were assessed at the mid-life baseline ARIC visit 1 (1987–1989). Additional analyses considered adjustment for intermediate cardiovascular and lifestyle risk factors measured at the time of the ARIC-PET ancillary study (2011–2013): type 2 diabetes mellitus (defined as fasting glucose ≥126 mg/dL or ≥200 mg/dL non-fasting glucose, self-reported history of physician-diagnosed diabetes, or use of diabetes mellitus medication); hypertension (defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of blood pressure-lowering medication); and body mass index (BMI, calculated as measured weight in kilograms divided by height in meters squared) [21].
Statistical analysis
Descriptive analysis used chi-square and ANOVA tests to examine differences in baseline sociodemographic and disease characteristics among participants who did and did not participate in LTPA in mid-life (visit 1). Multivariable logistic regression was used to estimate the cross-sectional associations of LTPA operationalized in mid-life (visit 1) and late-life (visit 5) as: 1) participation in LTPA (yes/no); 2) meeting 2018 physical activity guidelines (yes/no); and 3) per 1 standard deviation (SD) higher total MET·min·wk−1, with elevated amyloid burden in late-life. Multivariable linear regression was used to estimate the cross-sectional associations of LTPA measures with continuous global amyloid SUVR in late-life. We also examined the associations using LTPA as an average across visits 1 and 3 in mid-life. Sensitivity analyses explored elevated amyloid burden at an SUVR cutpoint >1.3. Models were adjusted for age at time of LTPA assessment, sex, education, race-ARIC field center interaction, and APOE ε4. Additional models further adjusted for visit 5 measures of BMI, hypertension, and diabetes as confounders. We also explored for effect modification by race-study center, APOE ε4 carrier allele status, and cognitive status (normal versus mild cognitive impairment) with inclusion of interaction terms for the exposure and proposed modifier in the model. Stata version 15.0 was used for all analyses (Stata-Corp LLC, College Station, TX, USA).
RESULTS
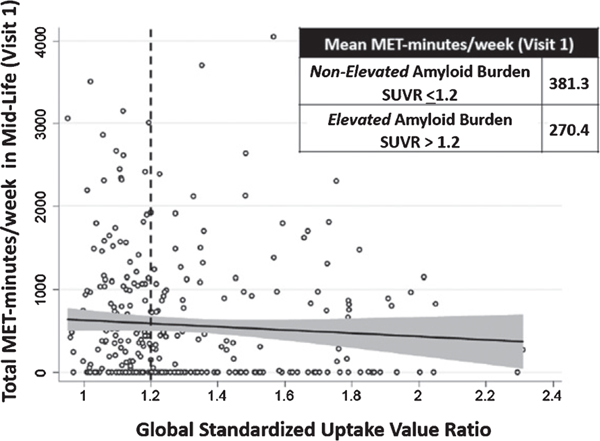
Sociodemographic and clinical characteristics of the study population (n = 326) are provided in Table 1 overall and by reported participation (yes/no) in LTPA in mid-life (visit 1, 1987–1989). Participants who did not participate in LTPA were more often Black and female and had lower educational attainment. Participants not reporting LTPA also had a worse cardiometabolic risk factor profile, including, a slightly higher prevalence of hypertension and smoking and a somewhat higher BMI. Our data suggest a medium/moderate correlation (0.33–0.45) of physical activity (in MET·min·wk−1) across ARIC visits from mid- to late-life [22]. The frequency of elevated amyloid burden (global SUVR > 1.2) was also higher in participants who did not participate in LTPA. Figure 1 shows the negative linear relationship between total volume of LTPA (MET·min·wk−1) in mid-life and global SUVR in late-life. At the median SUVR cutoff of 1.2, participants with non-elevated amyloid burden (SUVR ≤1.2) in late-life had on average higher total LTPA in mid-life (381.3 MET·min·wk−1) compared to participants with elevated amyloid burden (SUVR > 1.2) in late-life (270.4 MET·min·wk−1).
Table 1.
Participant characteristics overall and by participation in leisure-time physical activity in mid-life (Visit 1, 1987–1989), n = 326
| Participant characteristics | Participation in physical activity |
||
|---|---|---|---|
| Total (n = 326) | No (n = 126) | Yes (n = 200) | |
| Age, y, mean (SD) | 52.3 (5.2) | 52.1 (4.9) | 52.4 (5.4) |
| Female sex*, n (%) | 187 (57.4) | 84 (66.7) | 103 (51.5) |
| Black race*, n (%) | 135 (41.4) | 68 (54.0) | 67 (33.5) |
| APOE ε4 | |||
| 0 allele, n (%) | 224 (68.7) | 84 (66.7) | 140 (70.0) |
| 1 allele, n (%) | 94 (28.8) | 39 (31.0) | 55 (27.5) |
| 2 allele, n (%) | 8 (2.5) | 3 (2.4) | 5 (2.5) |
| <High school education*, n (%) | 52 (16.0) | 26 (20.6) | 26 (13.0) |
| Current smoker, n (%) | 57 (17.5) | 24 (19.0) | 33 (16.5) |
| Hypertension*, n (%) | 95 (29.3) | 41 (32.8) | 54 (27.1) |
| Diabetes*, n (%) | 18 (5.5) | 8 (6.4) | 10 (5.0) |
| Body Mass Index*, kg/m2, mean (SD) | 29.0 (5.4) | 30.1 (5.9) | 28.3 (4.9) |
| Late-life Cognitive Status | |||
| Normal, n (%) | 239 (73.3) | 92 (73.0) | 147 (73.5) |
| Mild cognitive impairment, n (%) | 87 (26.7) | 34 (27.0) | 53 (26.5) |
| Mid-life Meeting PA guidelines*, n (%) | 101 (31.0) | 0 (0.0) | 101 (50.5) |
| Mid-life MET. min. wk−1*, mean (SD) | 572.4 (750.9) | 0 (0.0) | 933.0 (763.4) |
| Late-life Participation in PA*, n (%) | 224 (68.7) | 69 (54.8) | 155 (77.5) |
| Late-life Meeting PA guidelines*, n (%) | 146 (44.8) | 38 (30.2) | 108 (54.0) |
| Late-life MET. min. wk−1*, mean (SD) | 667.0 (738.4) | 454.2 (623.8) | 801.1 (774.1) |
| Late-life Global SUVR, mean (SD) | 1.30 (0.26) | 1.32 (0.26) | 1.28 (0.26) |
| Late-life Elevated amyloid burden (SUVR>1.2)*, n (%) | 169 (51.8) | 75 (59.5) | 94 (47.0) |
p<0.05 for differences between participation in physical activity (yes/no). Characteristics are measured at mid-life (visit 1, 1987–1989) unless specified. SD, standard deviation; SUVR, standardized uptake value ratio; PA, physical activity. Mid-life, Visit 1 (1987–1989); Late-Life, Visit 5 (2011–2013).
Fig. 1.
Scatterplot of mid-life (visit 1) total leisure-time physical activity in MET·min·wk−1 and global standardized uptake value ratio measured in late-life (visit 5). SUVR, standardized uptake value ratio.
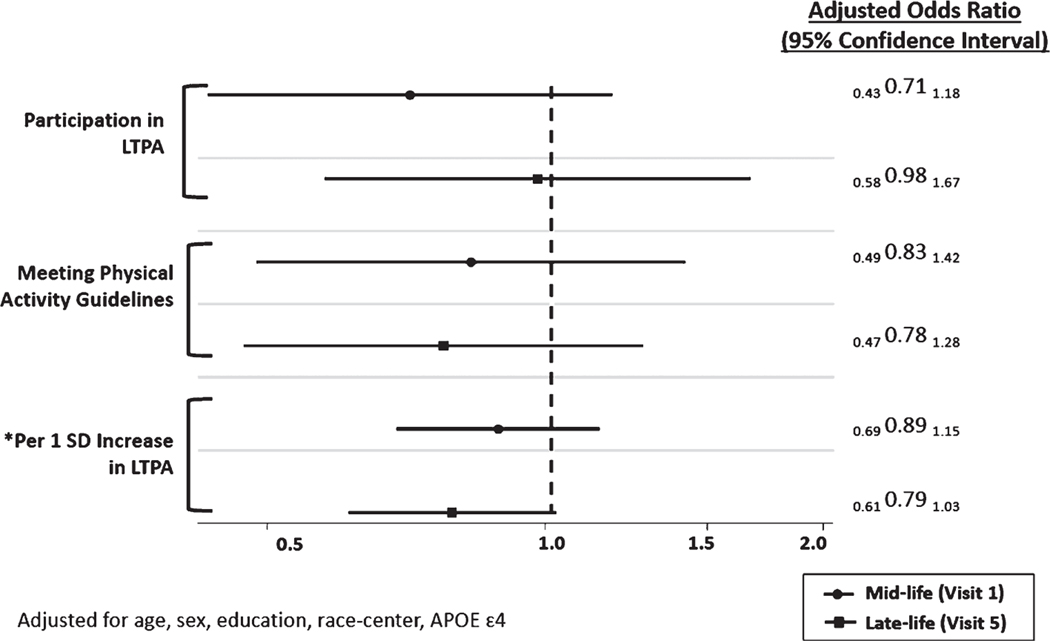
The prevalence of SUVR > 1.2 in late-life in the analytic sample was 52% (n = 169; Table 1). Participation in LTPA in mid-life was not significantly associated with a lower prevalence of elevated amyloid burden (Odds ratio [OR]=0.71 (95% confidence interval [CI]: 0.43, 1.18)) compared to non-participation in LTPA in mid-life (Fig. 2). The OR of elevated amyloid burden for those who met the 2018 U.S. Physical Activity Guidelines in midlife was 0.83 (95% CI: 0.49, 1.42) compared to those who did not meet the guidelines in mid-life. A per standard deviation (SD) higher total volume of LTPA (970 MET·min·wk−1) in mid-life was also not significantly associated with an elevated amyloid burden OR=0.89 (95% CI: 0.69, 1.15) in late-life, although each point estimate favored an inverse association between physical activity and amyloid burden. No significant associations were observed for all measures of LTPA in mid- and late-life with global amyloid SUVR analyzed continuously (Table 2) or when using a more conservative global amyloid SUVR cut point >1.3. Specifically, the OR of elevated amyloid burden (based on an SUVR cut point >1.3) for participation in LTPA in mid-life compared to non-participation in LTPA in mid-life was 0.77 (95% CI: 0.44, 1.33).
Fig.2.
Adjusted odds ratio (OR) and 95% confidence interval of elevated brain amyloid burden (standardized uptake value ratio (SUVR) > 1.2) by measures of leisure-time physical activity (LTPA) in mid- and late-life. Models for mid-life and late-life leisure-time physical activity were run separately. *1 standard deviation (SD) = 970 MET·min·wk−1.
Table 2.
Adjusted association of measures of leisure-time physical activity in mid- and late-life with continuous global amyloid SUVR in late-life, n = 326
| see | Beta (95% Confidence Interval) | p |
|---|---|---|
| MID-LIFE PHYSICAL ACTIVITY | ||
| Participation in Physical Activity (yes versus no) | –0.02 (–0.08, 0.03) | 0.406 |
| Meeting physical activity guidelines (yes versus no) | –0.04 (–0.10, 0.02) | 0.187 |
| Per 1 SD Higher Physical Activity | –0.01 (–0.04, 0.01) | 0.297 |
| LATE-LIFE PHYSICAL ACTIVITY | ||
| Participation in Physical Activity (yes versus no) | 0.03 (–0.03, 0.09) | 0.316 |
| Meeting physical activity guidelines (yes versus no) | 0.03 (–0.03, 0.08) | 0.285 |
| Per 1 SD Higher Physical Activity | 0.01 (–0.02, 0.03) | 0.720 |
Models adjusted for age, sex, education, race-center, APOE ε4.
p < 0.05. SUVR, standardized uptake value ratio; SD, standard deviation.
An overall measure of total volume of LTPA in mid-life was estimated by averaging the MET·min·wk−1 at visits 1 and 3. Results were similar to those observed with total volume of LTPA measured at visit 1 only (data not shown). In summary, regardless of how LTPA was operationalized, mid-life LTPA was not significantly associated with amyloid burden in late- life. For all models considering either mid- or late-life LTPA, we did not observe any significant interactions by race, APOE ε4, or cognitive status. Although results were slightly attenuated, the overall inferences were also not affected by additional adjustment for BMI, diabetes, and hypertension (data not shown).
DISCUSSION
In this largest known community-based sample of non-demented older adults with amyloid imaging and repeated measures of LTPA, neither mid- nor late-life LTPA was statistically significantly associated with brain amyloid burden. Our findings fail to support our a priori hypothesis that a mid-life measure of LTPA, free of any question of reverse causality, is significantly associated with brain amyloid burden 25 years later. This result is important because it shows the troublesome independence of amyloid accumulation from the healthy behavior of physical activity.
Our study adds new evidence to the literature relating physical activity to brain amyloid. Although previous findings from human studies have been inconsistent, rodent studies have consistently shown lower brain amyloid burden after intense exercise. Our lack of significant associations is consistent with those of the largest human study to date, based on 268 mild cognitively impaired participants with PET-quantified amyloid-β and self-reported physical activity [7]. This largest study to date raises potential concerns pertaining to its more cognitively impaired participant population, because the cognitive impairment might both affect habitual physical activity levels and reduce the accuracy of reported physical activity. However, the results of studies limited to cognitively normal participants have also been conflicting. In an investigation of 85 late-middle-aged adults from the Wisconsin Registry for Alzheimer’s Prevention, moderate intensity physical activity, ascertained with 1-week actigraphy, was inversely associated with cerebrospinal fluid measured amyloid-β [23]. In contrast, in a study of 139 cognitively normal participants, self-reported physical activity was not associated with cerebrospinal fluid measured amyloid-β in the Dominantly Inherited Alzheimer Network [24]. Using PET-quantified amyloid imaging, the data among cognitively normal participants has also been inconsistent, with some reports showing that higher levels of self-reported physical activity are associated with lower levels of amyloid burden [8–10, 25]. Conversely, data from 182 clinically normal older adults enrolled in the Harvard Aging Brain Study did not show a statistically significant cross-sectional association between accelerometer-measured physical activity and amyloid burden after adjusting for age and sex [26]. Interpretation of these studies, however, is clouded by the fact that they relate amyloid burden with concurrent levels of physical activity, possibly indicating an effect rather than a cause of the amyloid burden.
There are limitations to the present study. The exposure assessment relied on self-report, which typically overestimates vigorous physical activity and underestimates and/or does not quantify light physical activity [27]. This supports the need for studies to also include device-based assessments of physical activity into data collection protocols. The criteria by which participants were selected for the ARIC-PET study may constrain generalizability. Specifically, participants enrolled in PET studies are those willing and able to tolerate the MRI and PET scans, and therefore may be potentially healthier. Lastly, though the results show a consistent, but not statistically significant, protective association of physical activity on amyloid burden, our study’s size potentially limits its power to detect small differences. A post-hoc power analysis conducted in G*Power 3.1 suggests that for an OR = 0.71 for elevated amyloid burden comparing participants who participate versus do not participate in physical activity, a larger sample size (n > 336) is required to detect a significant association at 80% or greater power and an alpha = 0.05. Another limitation of this analysis is that the outcome cut point was based on a median split of global amyloid PET SUVR which almost certainly includes persons with amyloid burdens that are closer to background levels than to levels that are more concerning. Thus, the elevated amyloid group will contain persons who are not destined to develop overt Alzheimer’s disease. This may have made it more difficult to detect an association with physical activity. However, the same inferences were supported with a more conservative SUVR cutpoint >1.3 conducted in sensitivity analyses. Several strengths should be mentioned. First, the well-characterized ARIC cohort provides over 25 years of collected data, allowing us to examine the role of physical activity across different life epochs on brain amyloid in older adulthood. Second, the cross-temporal analysis (mid-life exposure and late-life outcome) results provide much clearer results than those seen at late-life only due to the potential effects of reverse causation. Lastly, this is the largest known sample of non-demented older adults with amyloid imaging and repeat measures of physical activity.
Given our large sample size, we can reasonably conclude that while the point estimates favored an inverse association of LTPA and brain amyloid burden, the hypothesized association was not supported. Although we found that physical activity is not associated with Alzheimer’s disease pathology, it is possible that physical activity is related to cerebrovascular disease, which has important contributions for later life cognitive impairment. It has been documented that physical activity can reduce the risk of diabetes [4], hypertension [3], and obesity [28]. Reductions in these vascular risk factors may reduce the burden of cerebral atherosclerosis and arteriolosclerosis and subsequently reduce ischemic cerebrovascular pathology [29]. In this setting of less cerebrovascular pathology, individuals may be more tolerable to higher burdens of Alzheimer’s disease pathology. Therefore, it is important that future studies quantify the role of physical activity and its changes over time with measures of cerebrovascular disease.
The long-term impact of mid-life leisure-time physical activity as a modifiable element of lifestyle is of considerable interest, both in clinical settings and from a public health perspective. Pharmaceutical therapies aimed at targeting clinical symptoms of AD and underlying AD pathophysiological processes have not been shown to be effective thus far. Therefore, there is a greater need to understand how lifestyle interventions may not only affect AD symptoms but its pathophysiology as well. Randomized clinical trials of a physical activity lifestyle intervention have not shown clear benefits [30–32], and implementing sustained modification of long-term physical activity patterns has proved to be challenging. This is particularly important in the context of amyloid-β accumulation, which is known to progress for decades prior to the appearance of clinical manifestations. Therefore, observational data with prolonged follow-up, and repeated measures of physical activity that are preferably objectively-measured, are still needed to help elucidate the potentially beneficial long-term role of higher physical activity levels in reducing or slowing the accumulation of brain amyloid in adulthood.
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Neurocognitive data is collected by U012U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. The authors thank the staff and participants of the ARIC study for their important contributions. This study was also supported by grant K24 AG052573 by the National Institute on Aging awarded to Dr. Gottesman. The ARIC-PET study is funded by the National Institute on Aging (R01AG040282). Dr. Palta was supported by grant R00 AG052830 from the National Institute on Aging. Dr. Walker was supported by grant K23 AG064122 from the National Institute on Aging. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institute on Aging; the National Institutes of Health; or the U.S. Department of Health and Human Services. The florbetapir isotope used in the study was provided by Avid Radiopharmaceuticals.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0152r1).
REFERENCES
- [1].Palta P, Sharrett AR, Deal JA, Evenson KR, Gabriel KP, Folsom AR, Gross AL, Windham BG, Knopman D, Mosley TH, Heiss G (2019) Leisure-time physical activity sustained since midlife and preservation of cognitive function: The Atherosclerosis Risk in Communities Study. Alzheimers Dement 15, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu PZ, Nusslock R (2018) Exercise-mediated neurogenesis in the hippocampus via BDNF. Front Neurosci 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Diaz KM, Shimbo D (2013) Physical activity and the prevention of hypertension. Curr Hypertens Rep 15, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF (2016) Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39, 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh-Manoux A, Kivimaki M (2012) Physical activity and inflammatory markers over 10 years: Follow-up in men and women from the Whitehall II cohort study. Circulation 126, 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brown BM, Peiffer J, Rainey-Smith SR (2019) Exploring the relationship between physical activity, beta-amyloid and tau: A narrative review. Ageing Res Rev 50, 9–18. [DOI] [PubMed] [Google Scholar]
- [7].de Souto Barreto P, Andrieu S, Payoux P, Demougeot L, Rolland Y, Vellas B, Multidomain Alzheimer Preventive Trial/Data Sharing Alzheimer Study Group (2015) Physical activity and amyloid-beta brain levels in elderly adults with intact cognition and mild cognitive impairment. J Am Geriatr Soc 63, 1634–1639. [DOI] [PubMed] [Google Scholar]
- [8].Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, Taddei T, Ward VK, Rodrigues MA, Burnham S, Rainey-Smith SR, Villemagne VL, Bush A, Ellis KA, Masters CL, Ames D, Macaulay SL, Szoeke C, Rowe CC, Martins RN (2013) Physical activity and amyloid-beta plasma and brain levels: Results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry 18, 875–881. [DOI] [PubMed] [Google Scholar]
- [9].Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D (2010) Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann Neurol 68, 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Okonkwo OC, Schultz SA, Oh JM, Larson J, Edwards D, Cook D, Koscik R, Gallagher CL, Dowling NM, Carlsson CM, Bendlin BB, LaRue A, Rowley HA, Christian BT, Asthana S, Hermann BP, Johnson SC, Sager MA (2014) Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology 83, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].(1989) The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. The ARIC investigators. Am J Epidemiol 129, 687–702. [PubMed] [Google Scholar]
- [12].Knopman DS, Griswold ME, Lirette ST, Gottesman RF, Kantarci K, Sharrett AR, Jack CR Jr., Graff-Radford J, Schneider AL, Windham BG, Coker LH, Albert MS, Mosley TH Jr., ARIC Neurocognitive Investigators (2015) Vascular imaging abnormalities and cognition: Mediation by cortical volume in nondemented individuals: Atherosclerosis risk in communities-neurocognitive study. Stroke 46, 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36, 936–942. [DOI] [PubMed] [Google Scholar]
- [14].Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr., Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS (2011) 2011 Compendium of Physical Activities: A second update of codes and MET values. Med Sci Sports Exerc 43, 1575–1581. [DOI] [PubMed] [Google Scholar]
- [15].United, 2008. Physical Activity Guidelines Advisory Committee Scientific Report, https://health.gov/paguidelines/2008/pdf/paguide.pdf, Accessed August 22.
- [16].Hertogh EM, Monninkhof EM, Schouten EG, Peeters PH, Schuit AJ (2008) Validity of the modified Baecke questionnaire: Comparison with energy expenditure according to the doubly labeled water method. Int J Behav Nutr Phys Act 5, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH (2017) Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317, 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gottesman RF, Schneider AL, Zhou Y, Chen X, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH Jr. (2016) The ARIC-PET amyloid imaging study: Brain amyloid differences by age, race, sex, and APOE. Neurology 87, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walker KA, Windham BG, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Selvin E, Jack CR Jr., Gottesman RF (2018) The association of mid-to late-life systemic inflammation with white matter structure in older adults: The Atherosclerosis Risk in Communities Study. Neurobiol Aging 68, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Gunter JL, Senjem ML, Jones DT, Kantarci K, Machulda MM, Mielke MM, Roberts RO, Vemuri P, Reyes DA, Petersen RC (2017) Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 13, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL (1972) Indices of relative weight and obesity. J Chronic Dis 25, 329–343. [DOI] [PubMed] [Google Scholar]
- [22].Cohen J (1988) Statistical power analysis for the behavioral sciences, Lawrence Earlbaum Associates, Hillsdale, NJ. [Google Scholar]
- [23].Law LL, Rol RN, Schultz SA, Dougherty RJ, Edwards DF, Koscik RL, Gallagher CL, Carlsson CM, Bendlin BB, Zetterberg H, Blennow K, Asthana S, Sager MA, Hermann BP, Johnson SC, Cook DB, Okonkwo OC (2018) Moderate intensity physical activity associates with CSF biomarkers in a cohort at risk for Alzheimer’s disease. Alzheimers Dement (Amst) 10, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brown BM, Sohrabi HR, Taddei K, Gardener SL, Rainey-Smith SR, Peiffer JJ, Xiong C, Fagan AM, Benzinger T, Buckles V, Erickson KI, Clarnette R, Shah T, Masters CL, Weiner M, Cairns N, Rossor M, Graff-Radford NR, Salloway S, Voglein J, Laske C, Noble J, Schofield PR, Bateman RJ, Morris JC, Martins RN, Dominantly Inherited Alzheimer N (2017) Habitual exercise levels are associated with cerebral amyloid load in presymptomatic autosomal dominant Alzheimer’s disease. Alzheimers Dement 13, 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC (2012) Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol 69, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rabin JS, Klein H, Kirn DR, Schultz AP, Yang HS, Hampton O, Jiang S, Buckley RF, Viswanathan A, Hedden T, Pruzin J, Yau WW, Guzman-Velez E, Quiroz YT, Properzi M, Marshall GA, Rentz DM, Johnson KA, Sperling RA, Chhatwal JP (2019) Associations of physical activity and beta-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol 76, 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dyrstad SM, Hansen BH, Holme IM, Anderssen SA (2014) Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc 46, 99–106. [DOI] [PubMed] [Google Scholar]
- [28].Jakicic JM, Otto AD (2005) Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr 82, 226S–229S. [DOI] [PubMed] [Google Scholar]
- [29].Knopman DS, Roberts R (2010) Vascular risk factors: Imaging and neuropathologic correlates. J Alzheimers Dis 20, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, Macko R, Marquez DX, Petruzzello SJ, Powell KE, For Physical Activity Guidelines Advisory Committee (2019) Physical activity, cognition, and brain outcomes: A review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc 51, 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, Guralnik J, Hendrie HC, Jennings J, Katula J, Lopez OL, McDermott MM, Pahor M, Reid KF, Rushing J, Verghese J, Rapp S, Williamson JD, LIFE Study Investigators (2015) Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: The LIFE Randomized Trial. JAMA 314, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Richard E, Andrieu S, Solomon A, Mangialasche F, Ahtiluoto S, Moll van Charante EP, Coley N, Fratiglioni L, Neely AS, Vellas B, van Gool WA, Kivipelto M (2012) Methodological challenges in designing dementia prevention trials–the European Dementia Prevention Initiative (EDPI). J Neurol Sci 322, 64–70. [DOI] [PubMed] [Google Scholar]