The monkey frontal cortex can flexibly direct covert spatial attention without tapping the circuitry for planning movements.
Abstract
We investigated the spatial representation of covert attention and movement planning in monkeys performing a task that used symbolic cues to decouple the locus of covert attention from the motor target. In the three frontal areas studied, most spatially tuned neurons reflected either where attention was allocated or the planned saccade. Neurons modulated by both covert attention and the motor plan were in the minority. Such dual-purpose neurons were especially rare in premotor and prefrontal cortex but were more common just rostral to the arcuate sulcus. The existence of neurons that indicate where the monkey was attending but not its movement goal runs counter to the idea that the control of spatial attention is entirely reliant on the neuronal circuits underlying motor planning. Rather, the presence of separate neuronal populations for each cognitive process suggests that endogenous attention is under flexible control and can be dissociated from motor intention.
INTRODUCTION
Spatial attention and the planning of movement frequently go hand in hand. For example, the appearance of a stimulus can both capture attention and trigger a saccadic eye movement to its location. This tendency for attention and motor planning to colocalize led to the formulation of the oculomotor readiness hypothesis, which posits that planning an eye movement to a location causes attention to be allocated there and, conversely, that covertly attending to a location facilitates saccades to that place (1, 2). In support of this hypothesis, it has been reported that visual stimulus detection is improved at the endpoint of a planned saccade (3–7) and that saccades in the same direction as an attended location are faster than those away (3, 8–10). Attention has also been reported to influence saccade trajectories, causing them to deviate away from an attended location (8, 11).
Such behavioral cross-talk between spatial attention and motor planning could reflect some shared neuronal substrate. Human neuroimaging studies have shown that some brain areas, including parts of the frontal and parietal cortex, are involved in both covert attention and saccadic planning (12–15). However, it remains unclear whether these two operations are encoded by distinct neuronal populations that occupy the same cortical region and exert some mutual influence on one another or whether they share a common spatial representation at the neuronal level. The popular Premotor Theory of Attention (8) accounts for the interplay between these two processes by positing that selective attention arises solely as a consequence of formulating a motor plan—be it an eye movement or a reach—to some location. Accordingly, this theory holds that spatial attention and motor planning are inextricably yoked and makes the strong prediction that attention is entirely reliant on the neuronal substrates of motor planning. It follows that if a neuron is modulated by covert attention, it must also be involved in motor planning.
Despite the interplay between the two, spatial attention and motor planning can become behaviorally decoupled (3, 16, 17). The obligatory coupling of attention and motor planning is considered pathological, as in the case of utilization behavior, in which patients uncontrollably reach to and manipulate objects that capture their attention (18). The normal decoupling of covert attention and motor planning suggests that, contrary to the Premotor Theory of Attention, these processes may have distinct neuronal underpinnings. To determine whether there are neurons dedicated solely to attention, we recorded from individual neurons in the frontal cortex of monkeys as they performed a task that spatially dissociated covert attention from the saccadic plan. We found that most neurons made contributions to one or the other cognitive process, while a smaller population of neurons contributed to both processes. We found neurons tuned for spatial attention that did not contribute to saccadic planning, suggesting a mechanism by which the frontal cortex can control spatial attention apart from any motor plan.
RESULTS
Behavior
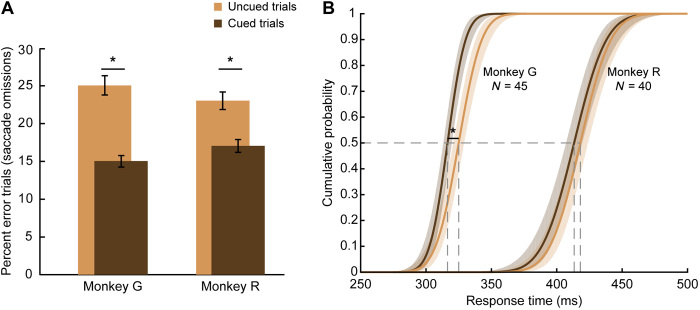
The cognitive processes of motor planning and covert spatial attention were dissociated at the behavioral level using the task depicted in Fig. 1A. Both monkeys performed the task well. Monkey R correctly responded to the “go” signal on 81% of trials, and monkey G did so on 83% of trials. Saccadic responses to the go signal almost always correctly targeted the cued motor location. The small number of erroneous saccades (2% of saccades for monkey G and 3% for monkey R) was disproportionately directed to the attended location (54 and 38% in monkeys G and R, respectively) over the other targets. Much more common were “saccade omission” errors, in which there was no response to the go signal in the allotted time. The absence of a saccade likely indicates that the go signal, a subtle brightening of a peripheral spot, went undetected. Figure 2A shows that such detection failures were significantly more likely for each monkey (paired t test, P < 0.0001) on uncued trials, when the location of the go signal was unpredictable, than on cued trials, when it was symbolically specified (monkey G: 14.8 ± 0.5 versus 25.0 ± 1.1%, 45 sessions, t stat = −9.646; monkey R: 17.3 ± 0.9 versus 23.0 ± 1.4%, 40 sessions, t stat = −5.072; ±SEM). Thus, when provided the opportunity, the monkeys used the cue’s central color to better detect and respond to the brightening of the corresponding peripheral spot. We take this as evidence that the monkeys covertly attended to the peripheral spot indicated by the symbolic attention cue.
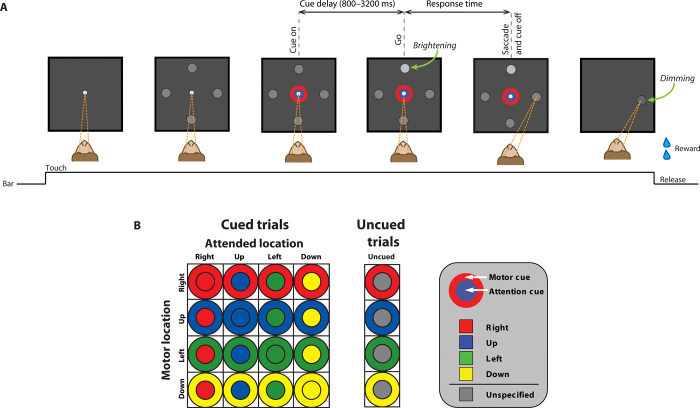
Fig. 1. An example trial of the behavioral task and the bicolored cue stimuli.
(A) The monkey (shown from above) pressed a bar to make a central white fixation spot appear. The monkey had to fixate (convergent dashed lines) this central spot as four peripheral gray spots were presented, followed by a two-part cue stimulus that appeared in and around the fixation point. After a variable cue delay period, a subtle brightening of a peripheral spot (the go signal) indicated when the monkey should direct its gaze to one of the peripheral spots. The cue’s inner color (the circle) indicated which of the four peripheral spots would brighten: the attention cue. The cue’s outer color (the annulus) specified the target of the upcoming saccade: the motor cue. In this example, which is highlighted in (B), the blue circle signifies that the upper spot will brighten (the attended location), and the red annulus specifies a saccade to the rightward spot (the movement target location). (B) The stimuli that served as cues for the different combinations of movement targets and attended locations are represented in a 4 × 4 matrix. On uncued trials (20% of correct trials), the inner circle was gray, and only the annulus was colored. The saccade target was therefore specified, but the attended location was not. The location of the go signal was selected pseudorandomly on these trials.
Fig. 2. Monkeys used symbolic cue to guide spatial attention and improve task performance.
(A) Percent of trials in which each monkey failed to detect the go signal (brightening of a peripheral spot) and complete a saccade within the requisite time (800 ms for monkey R and 600 ms for monkey G). For both monkeys, error rates were significantly lower when the attended location was cued than when it was uncued (paired t test, P < 0.0001). Thus, on cued trials, the monkeys used the cue’s inner color to direct their covert spatial attention and improve detection of the peripheral go signal. Error bars: SEM across sessions. (B) Cumulative probability distributions of RT on correct trials for each monkey. Across monkeys, responses were significantly faster on cued trials than on uncued trials (P < 0.0005). Asterisks mark significant differences. Data are based on 45 sessions from monkey G and 40 sessions from monkey R.
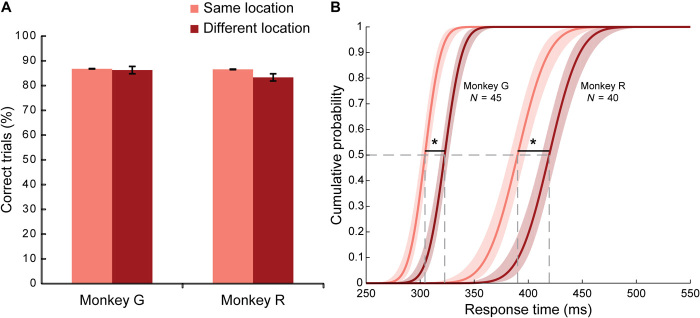
We compared error rates on cued trials for which the attended location and motor location were the same (i.e., the major diagonal of the 4 × 4 matrix shown in Fig. 1B) and for which they differed (i.e., off the major diagonal) and found that they were nearly identical (Mann-Whitney test, monkey R, P = 0.52, U = 18; monkey G, P = 0.86, U = 22; Fig. 3A). Thus, detection of the go signal at the attended location was unaffected by whether the upcoming saccade was to this location or a distinct location. This indicates that the monkeys were capable of dissociating attention and motor planning with no cost to their detection accuracy.
Fig. 3. RTs were faster when the attended and motor locations were the same.
On a quarter of cued trials, the inner and outer parts of the cue stimulus had the same color (major diagonal of the 4 × 4 matrix shown in Fig. 1B). On these “same-location” trials, covert attention was allocated to the same location as the upcoming saccade. On the remaining “different-location” trials, the attended location and motor location were distinct (off-diagonal of the 4 × 4 matrix). (A) Percent correct performance did not differ between same-location and different-location trials for either monkey. Accuracy reflects saccade omission errors only. (B) Cumulative probability distributions of RTs on correctly performed cued trials for each monkey. Responses were significantly faster when the locus of attention and the motor plan were the same than when they differed (P < 0.0005). Asterisks mark significant differences. For other details, see Fig. 2.
Figures 2B and 3B show the cumulative distribution of response times (RTs) on correct trials. RTs were measured from the go signal until the monkey initiated its saccade to the target. A three-way analysis of variance (ANOVA) on the average RTs for each session revealed significant main effects (P < 0.0005) for the factors of monkey, cueing, and whether the attention and motor locations were the same or different. Monkey G was significantly faster than monkey R (F1,332 = 1675.87, 318 versus 409 ms). There was no significant interaction between the monkey and the other factors. As shown in Fig. 2B, RTs on cued trials were slightly smaller than on uncued trials (F1,332 = 12.68, monkey G: 314 versus 322 ms; monkey R: 405 versus 413 ms). RTs were also smaller when the attended location and the motor location were the same than when they differed (F1,332 = 71.06, monkey G: 310 versus 325 ms; monkey R: 398 versus 420 ms). This RT difference was greater on cued trials (shown in Fig. 3B) than on uncued trials (significant interaction of cueing × same versus different location, F1,332 = 5.05, P = 0.025). It is known that, at the time of saccade execution, there is an obligatory shift of attention to the saccade target (3, 19). There is no need for such a shift of attention when attention is already directed to the motor target. We subtracted RTs on cued trials, for which attention and motor planning were colocalized, from those for which they were separated to determine that redirecting attention to the motor target took 19 ms for monkey G (323 versus 304 ms) and 29 ms for monkey R (419 versus 390 ms). This RT difference did not vary systematically with the duration of the cue delay.
The degree to which the peripheral spot brightened (the go signal) varied from trial to trial. Trials with the subtlest brightness change had the highest saccade omission error rates. Error rates decreased monotonically with each greater level of brightness change (fig. S1) and, as previously indicated, were reduced on cued trials. A two-way ANOVA on error rates across all 85 sessions showed significant (P < 0.0001) main effects of degree of brightening (F3,672 = 303.82) and of cueing (F1,672 = 94.67). Thus, detection of the peripheral brightening was improved both by increasing the exogenous salience of the go signal and by instructing the monkey where to direct endogenous attention. There was a significant interaction between these factors (P < 0.002) such that the benefit of top-down attention was more pronounced for subtler bottom-up signals (F3,672 = 5.08).
Correct trial RTs were similarly dependent on both bottom-up and top-down factors. RTs decreased monotonically from the subtlest to the most conspicuous brightness change (monkey G: 337 to 297 ms; monkey R: 429 to 399 ms). As indicated above, RTs were also faster on cued trials. Each monkey’s RTs were assessed with a two-way ANOVA, and both exhibited significant main effects of degree of brightening (monkey G: F3,352 = 71.87, P < 0.0001; monkey R: F3,304 = 23.72, P < 0.0001) and of cueing (monkey G: F1,352 = 21.54, P < 0.0001; monkey R: F1,304 = 4.87, P < 0.05). Thus, task performance, whether measured in terms of detecting the go signal or how rapidly it triggered a response, improved with the salience of the go signal and with prior knowledge of where it would occur.
We tested whether performance faltered during trials with a longer cue delay and found that, to the contrary, saccade omission errors were less likely on longer cue delay trials (fig. S2). The low error rate for the longest cue delay (3200 ms) may indicate that the monkeys were, at least in part, timing their saccades on these trials rather than strictly relying on detection of the go signal. This conclusion is supported by the decrease in RT on these trials relative to the other cue delays (monkey G: 41 ms; monkey R: 7 ms). Despite this alternate strategy for the longest cue delay trials, error rates for each monkey were smaller on cued trials than on uncued trials for each cue delay (fig. S2). Although the behavioral benefit of cueing was smallest for the 3200-ms delay, it was nonetheless significant (6.5 ± 0.9 versus 4.8 ± 0.5%, paired t test, P < 0.05, 85 sessions, t stat = 2.30, ±SEM). Thus, even on the longest cue delay trials, the monkeys’ use of covert attention significantly improved detection of the go signal.
Single-cell analysis
We recorded from 502 neurons in the frontal cortex of two rhesus monkeys: 355 from monkey G and 147 from monkey R. Single-trial firing rates preceding the go signal were assessed by two-way ANOVA to identify motor planning cells, attention cells, “both” cells, and untuned cells (see Materials and Methods).
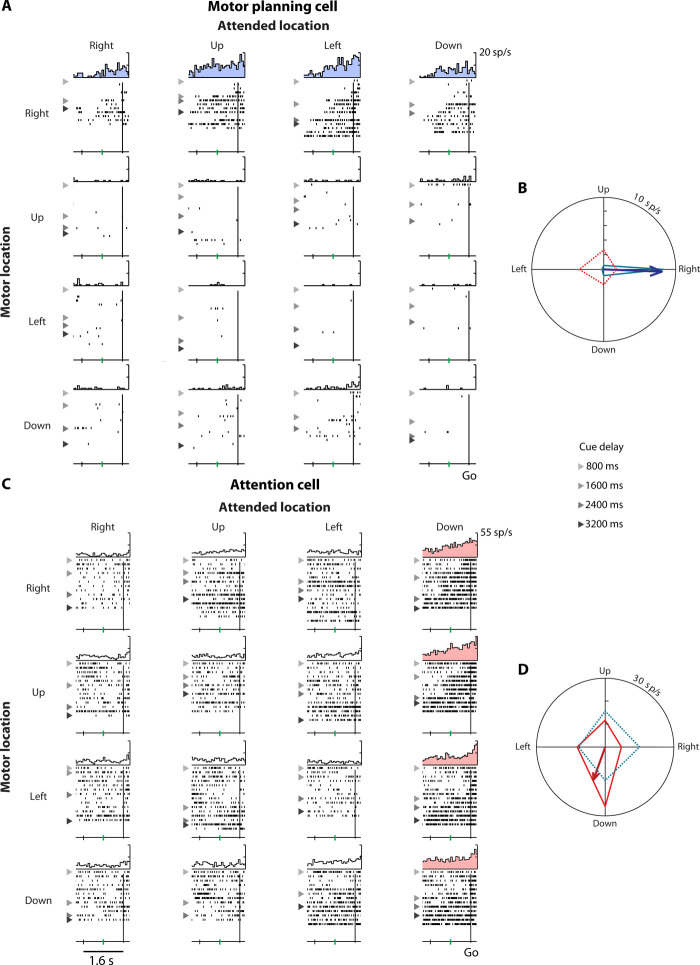
Figure 4 (A and B) shows an example of a motor planning cell. During the cue delay period, this neuron signaled the monkey’s planned saccade direction by firing appreciably more before saccades to the right. Correspondingly, the neuron’s motor planning preferred direction (PD) points to the right (Fig. 4B). This dorsal prefrontal neuron’s activity did not depend on where the monkey had to attend. Conversely, Fig. 4 (C and D) shows an attention cell that fired substantially more when the monkey covertly attended the bottom gray spot. Accordingly, this cell’s attention PD points downward (Fig. 4D). This ventral prefrontal cell’s activity was not dependent on the upcoming saccade. Such purely attention-tuned cells are not consistent with the Premotor Theory of Attention. If attention was truly a consequence of formulating a motor plan, then any cell tuned for the attended location would necessarily also exhibit tuning for the motor location. That is, all attention-tuned neurons would be both cells. The existence of pure attention cells like that shown in Fig. 4 (C and D) demonstrates that spatial attention is its own executive function that can be divorced from motor planning at the single-cell level.
Fig. 4. Example motor planning cell and attention cell.
Single-unit activity on correct cued trials was arranged by motor location (rows) and attended location (columns) in a 4 × 4 matrix (see Fig. 1B). PETHs and raster plots show spiking activity, aligned to the go signal. Rasters are sorted by the cue delay, with triangles separating trials with progressively longer durations. (A) A motor planning cell (main effect of motor location only, P < 0.001) that preferentially responded to the right motor location (blue histograms). (B) The blue polar tuning curve depicts this cell’s mean firing rate for each motor location (row-wise average of the 4 × 4 matrix). The vector sum of these rates (blue arrow) is the cell’s motor planning PD. Firing rates were computed in the 800 ms before the go signal (from the green tick mark to the vertical line). The red tuning curve shows the cell’s activity for each attended location (column-wise average of the 4 × 4 matrix). The dashed line and absence of a red arrow signify that activity was not significantly modulated by the attended location. (C) An attention cell (main effect of attended location only, P < 0.001) that responded more when the monkey attended the down location (pink histograms). (D) The red polar tuning curve shows this cell’s mean firing rate for each attended location. The vector sum of these rates (red arrow) is the cell’s attention PD. Activity was not significantly modulated by the motor location (dashed blue line), so the motor planning PD is not shown. These example neurons from monkey G’s PF had long latencies. Consequently, the response to cue onset just preceded the go signal on trials with an 800-ms cue delay (lightest triangles).
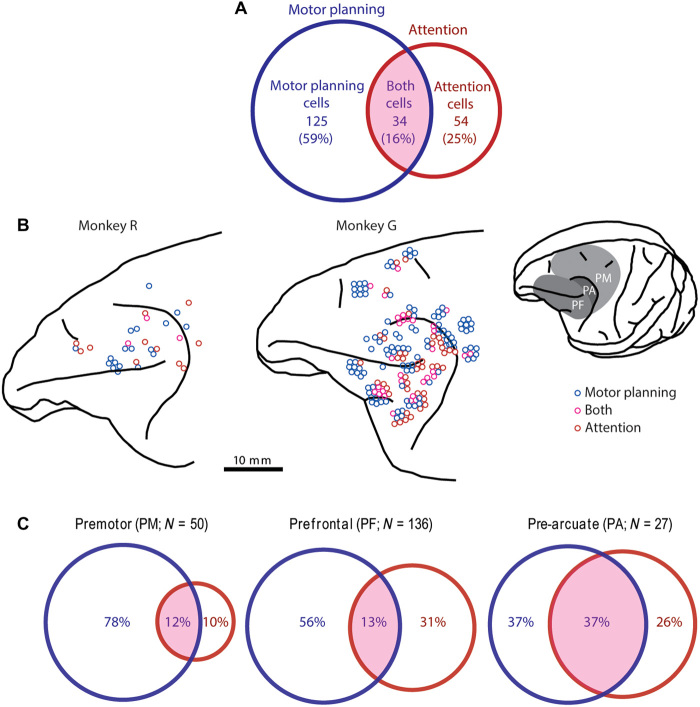
Figure 5A shows the proportion of the three cell types for all 213 neurons that exhibited at least one main effect in the two-way ANOVA. Both cells were the least common type overall (34 of 213, 16%). This was the case for both monkey G (17%) and monkey R (9%). The remaining 84% of cells were like the example neurons shown in Fig. 4 in that they encoded either the motor location or the attended location but not both. Motor planning cells were the most numerous (125 of 213, 59%; monkey G: 59%; monkey R: 56%), whereas attention cells accounted for a quarter of the tuned neurons (54 of 213, 25%; monkey G: 24%; monkey R: 34%). Of those neurons tuned for the attended location (red circle in Fig. 5A), the majority (61%) did not significantly contribute to saccadic planning. This frequent decoupling of attention from motor planning at the single-cell level is not consistent with the Premotor Theory of Attention.
Fig. 5. Classification and distribution of tuned neurons.
(A) Venn diagram showing the number of tuned neurons (N = 213) significantly (P < 0.05; two-way ANOVA, main effects) modulated by the motor location (blue circle) and the attended location (red circle). The overlapping area, labeled “both cells,” represents cells that encode both attention and the motor plan. (B) Lateral views of the left frontal cortex of each monkey. Circles show the point of electrode insertion, jittered for clarity, in which motor planning cells (blue), attention cells (red), and both cells (magenta) were recorded. Inset shows the surface view of monkey G’s left hemisphere with delineation of the PM, PF, and the cortex just anterior to the arcuate sulcus (PA). Cells were localized to an area by histological analysis, which was not necessarily the same as the depicted point of insertion. (C) Distribution of cell types by brain area. Venn diagrams show the tuning of neurons in the PM, PF, and PA. Both cells were uncommon in the PM and PF (<13%) but accounted for 37% of the tuned neurons in the PA, where attention tuning was most common.
Although uncommon (16%), both cells could conceivably provide a mechanism for the known behavioral interplay between attention and motor planning. For example, if both cells had similar (or opposing) spatial preferences for the attended and motor locations, this would accord well with the findings that spatial attention attracts (or repulses) saccade trajectories. We therefore examined whether the spatial preferences of both cells for the attended and motor locations were similar (or at least consistent) and found that they were not. Figure S3 shows the distribution of the angle (ΔΦ) between the attention PD and motor planning PD for all 34 both cells. The distribution does not significantly differ from a uniform one, indicating the lack of a consistent relationship between motor and attention directional preferences. This variety of spatial arrangements between motor and attention fields does not lend itself to a straightforward account of the behavioral interplay between attention and motor planning but does suggest a mechanism for the flexible control of attention with respect to motor plans.
Tuning in different frontal regions
Figure 5B shows the distribution of tuned neurons within the left frontal cortex of each monkey. The recording area was divided into three regions (see inset and Material and Methods): the premotor cortex (PM; N = 296 cells), prefrontal cortex (PF; N = 123 cells), and pre-arcuate cortex (PA; N = 83 cells). The proportion of tuned versus untuned cells did not vary across these areas (χ2 = 4.99, df = 2, P > 0.05, N = 502; 41, 46, and 33% of cell were tuned in the PM, PF, and PA, respectively). However, the proportion of the three tuned cell types did vary across areas (χ2 = 20.45, df = 4, P < 0.0005, N = 213). As shown in Fig. 5C, the majority of tuned cells in each area exhibited tuning for the motor plan (see table S1 for proportions with respect to all cells in an area). The proportion of motor-tuned cells in each area did not vary significantly from their proportion (75%) among all tuned cells (χ2 = 2.14, df = 2, P > 0.2, N = 159). This was also the case for just the motor planning cells (χ2 = 5.52, df = 2, P > 0.05, N = 125).
The frequency of attention tuning did, however, differ significantly by area (χ2 = 7.84, df = 2, P < 0.02, N = 88), being the least common in the PM (22%), twice as common in the PF, and nearly three times as common in the PA (63%). This difference was separately significant for attention cells (χ2 = 6.294, df = 2, P < 0.05, N = 54), which were more than twice as common in the PF and PA than in the PM, and for both cells (χ2 = 8.64, df = 2, P < 0.02, N = 34), which were about three times more common in the PA than in either PM or PF. Attention cells greatly outnumbered both cells in the PF, whereas their counts were similar in the PM and PA. In all three areas, attention cells comprised a substantial fraction of the attention tuning (PF, 70%; PM, 45%; PA, 41%). The implication of the Premotor Theory of Attention, namely that, at the single-cell level, attention tuning would always—or even predominately—be accompanied by tuning for the motor plan, was not borne out in any of these areas.
Analysis of different trial types
We assessed whether neuronal activity differed for those task conditions in which the motor and attended locations were the same (i.e., the major diagonal of the 4 × 4 matrix shown in Figs. 1B and 4, A and C) and those in which they differed. We computed a diagonal PD using same-location trials and compared it with the motor planning and attention PDs, recomputed using only different-location trials. Spatial tuning on same-location trials closely agreed with that on different-location trials. The (smaller) angle between the diagonal PD and the motor planning PD was nonuniformly distributed (χ2 = 228.37, df = 7, P < 0.0001) and was <45° for the majority of cells tuned for the motor plan (70%). Likewise, the angle between the diagonal PD and the attention PD was nonuniformly distributed (χ2 = 51.23, df = 7, P < 0.0001) and was <45° for nearly half of the attention-tuned cells (47%). For both cells, the diagonal PD agreed with the cells’ motor planning PD more often than its attention PD. In all cases, PD angle differences of <22.5° were the most common, indicating that tuning on same- and different-location trials was comparable for most neurons.
On uncued trials, the attended location was not specified. As expected, cue delay activity on these trials was not tuned for the randomly selected go signal location. However, the motor target was still specified on uncued trials. Cells tuned for the motor plan on cued trials continued to represent this motor plan on uncued trials. We computed an independent motor planning PD using just uncued trials and compared it with the standard motor planning PD, computed from cued trials. The distribution of the smaller angle between these two motor planning PDs was significantly nonuniform for motor planning cells and both cells (χ2 = 129.55 and 71.88, respectively; df = 7; P < 0.0001). The angle was <45° for the majority of these cells (64 and 76%, respectively), was most commonly <22.5°, and was rarely >90° (16 and 0%). Thus, tuning for the motor location on uncued trials was similar to that on cued trials at the single-cell level and across the population. This finding suggests that, although the monkeys were less likely to detect the go signal without the benefit of the attention cue, they continued to plan and execute the saccade specified by the motor cue.
To control for the possibility that the motor planning and attention tuning reported here actually reflected visual selectivity for the cue stimulus, we tested a selection of cells on a fixation task that presented the same central cue stimuli (see Materials and Methods). Of the 59 cells recorded in this task, 8 exhibited a main effect of annulus color (ANOVA, P < 0.05), and only 1 of these cells had the same annulus color preference in the main task and fixation task. Four cells exhibited a main effect of the circle color, and none of these exhibited the same circle color preference in the two tasks. Thus, little, if any, of the spatial tuning observed in the main task can be attributed to selectivity for the color or other visual aspects of the central cue.
Population analysis
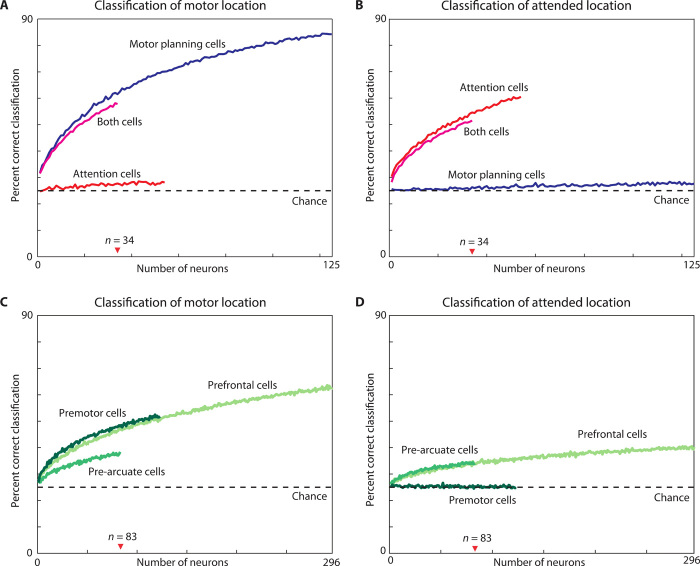
We performed a neuron-dropping classification analysis (see Materials and Methods) to assess the single-trial fidelity of representations of motor plan and covert attention for different neuronal ensembles. Figure 6 shows how frequently the motor (Fig. 6, A and C) and attended locations (Fig. 6, B and D) were correctly classified from single-trial firing rates in neuronal ensembles of different size.
Fig. 6. Decoding the motor and attended locations in different cell types.
The neuron-dropping curves show the percentage of correct single-trial classifications of the motor target location (A and C) and the attended location (B and D) as a function of the number of neurons contributing to the classification. Classification was based on single-trial firing rates computed over the 800 ms before the go signal. (A and B) Classification by motor planning (blue), attention (red), and both cells (magenta) was above chance (dashed line at 25% correct) for even a single neuron tuned to the relevant location and increased as the number of neurons increased. In contrast, even large ensembles of neurons tuned for one location, decoded the other location at close to chance level (e.g., decoding of attended location by motor planning cells). (C and D) Classification performance of ensembles of tuned and untuned cells in the three brain areas. The performance of different cell types should be compared for a given number of neurons. Note that the x axis differs in the top and bottom. SDs for each curve were calculated using ensembles of 34 cells (top) or 83 cells (bottom), corresponding to the smallest cell group in each panel, but were too small (<0.5%) to be plotted. As a result of this low variance, all pairwise differences at N = 34 and N = 83 were significant (P < 0.0001, t test, N = 17 repeats).
This read-out analysis has the advantage that it works equally well for large and small increases or decreases in firing rate, preferred and nonpreferred locations, tuned and untuned cells, and cells with and without a significant interaction effect (N = 43 and 170, respectively). The top row shows decoding for ensembles of each type of tuned neuron. Figure 6A shows that classification of the motor target was above chance level for even a single motor planning cell (blue curve) or both cell (magenta curves) and improved for larger ensembles. Likewise, Fig. 6B shows progressively better decoding of where the monkey was attending for larger and larger ensembles of attention cells (red curve) or both cells (magenta curves). In contrast, decoding of the motor location from attention cells or the attention location from motor planning cells remained near chance level even for large ensembles. These neurons were thus specialized for their preferred spatial variable and carried little or no information about the other one.
For a given ensemble size, decoding of the motor location by motor planning or both cells was more accurate than decoding of the attended location by attention or both cells (Fig. 6, A versus B). These differences were all significant for an ensemble size of 34 cells (t test, P < 0.0001). Classification accuracy was slightly greater for motor planning cells and attention cells than for both cells, for their respective locations (t test, P < 0.0001, 34-cell ensemble). Thus, not only were both cells less common than the other cell types, but they were also less reliable decoders of the planned movement and the allocation of covert attention.
The bottom panels of Fig. 6 show decoding from ensembles of tuned and untuned cells in each brain area. Decoding of the motor target (Fig. 6C) was above chance for each area. PM and PF had more accurate classification than PA for ensembles of a given size. Classification of the attended location (Fig. 6D) was nearly identical for ensembles of PA and PF cells but was at chance level even for the largest PM ensemble. In each area, ensembles of a given size classified the motor location more accurately than the attended location. In general, the greater the proportion of significant motor- and attention-tuned cells, the more accurate the classification is of that location (table S1).
Population dynamics
The time course of population-level tuning for motor planning and spatial attention is shown in fig. S4. The four locations were ranked for each cell by activity during the last 800 ms of the cue delay, as this was the briefest cue delay duration. Tuning was evident earlier than this (e.g., from −1600 to −800 ms) because of trials with longer cue delays. Figure S5 shows the population time courses for just those trials with the longest cue delay. Tuning for the motor plan (fig. S5A) and for attention (fig. S5B) arose after cue onset and remained relatively constant throughout the 3200-ms cue delay. Figures S4 and S5 show that tuning persisted or increased following the go signal until around the time of the saccade. This sustained tuning indicates that cells that preferred a particular motor plan during the cue delay period (fig. S4, A and B) had the same preference, on average, for the execution of that eye movement. Likewise, cells with attention tuning during the cue delay period (fig. S4, C and D) continued to reflect where the go signal had occurred until around the time the monkey shifted its gaze (and thus its overt attention) to the motor target. The tuning of attention cells after the go signal did not differ between trials with different degrees of brightening, suggesting that this tuning continued to reflect endogenous rather than exogenous attention.
The degree of tuning was similar in all the subpopulations considered. We used the magnitude of the motor planning PD and/or attention PD to quantify each neuron’s tuning strength during the 800 ms before the go signal. Tuning for the motor plan was, on average, no stronger than attention tuning. This was the case whether we compared all motor-tuned cells versus all attention-tuned cells, with both cells included in each group (8.1 ± 0.5 versus 7.6 ± 0.7 sp/s, t test, P > 0.55, t stat = 0.60, df = 245, ±SEM), motor planning cells versus attention cells (8.3 ± 0.5 versus 7.9 ± 1.0 sp/s, t test, P > 0.74, t stat = 0.33, df = 177; fig. S4, A versus C), or the motor and attention PDs of both cells (7.2 ± 0.5 versus 6.9 ± 0.5 sp/s, paired t test, P > 0.28, t stat = 1.09, df = 33; fig. S4, B versus D). There was also no significant difference in the degree of tuning for the motor plan between motor planning cells and both cells (P > 0.38, t stat = 0.88, df = 157; fig. S4, A versus B) or the degree of attention tuning between attention cells and both cells (P > 0.49, t stat = 0.69, df = 86; fig. S4, C versus D). Hence, nothing about the tuning strength of both cells would suggest that they exert a disproportionate influence on behavior.
Figure S6 shows an analysis to determine whether tuned neurons had a common spatial preference for saccade planning and covert attention. The response associated with planning a movement to a location was ranked according to the neuronal preference for that location as an attended location (and vice versa) and then averaged by rank across cells. This eliminated the population tuning of motor planning and attention cells (fig. S6, A and C) and greatly diminished the tuning of both cells (fig. S6, B and D), indicating that the spatial preferences of these cells for motor planning and attention were entirely or largely independent, respectively. This provides further evidence that there is not a common spatial representation in the frontal cortex that can be accessed interchangeably for purposes of motor planning and covert attention.
We assessed the latency with which motor and attention tuning developed among tuned neurons in each brain area following the presentation of the cue stimulus (table S1). Both kinds of tuning developed fastest in the PA and ≥120 ms later in the PF. In both areas, tuning for attention preceded that for the motor plan by ~100 ms. Tuning for the motor plan developed in the PM at around the same time as in the PF. The 11 attention-tuned cells in the PM were too weakly tuned to assess the latency of their attention tuning.
DISCUSSION
Independent control of attention and motor planning
We found that neurons signaling the focus of covert attention are largely distinct from those that signal an upcoming movement. This neuronal division of labor suggests that attention and motor plans can be flexibly controlled. Our findings accord poorly with the idea of obligatory coupling between these processes (1, 2) and specifically with the Premotor Theory of Attention (8, 11), which contends that the allocation of covert attention requires and is functionally equivalent to generating a motor plan that is not then executed. If this were the case, all cells tuned for where attention is directed would also have to be involved in motor planning. This prediction was not confirmed. We found neurons that signaled where the monkey was attending but not the target of the movement plan. Such attention cells comprised a quarter of all tuned frontal neurons and the majority of attention-tuned cells.
There was also no evidence for the converse form of obligatory coupling, namely that planning a movement requires and is a manifestation of a covert shift of attention. If this were the case, all cells that encoded the target of an upcoming movement would also have to play a role in directing attention. In actuality, motor planning cells, which signaled the upcoming saccade goal but were unmodulated by attention, were the most numerous cell type in each brain area. Thus, attention processing and motor planning were largely relegated to separate neurons (Fig. 5A) that, even in aggregate, conveyed almost no information about the other cognitive process (Fig. 6, A and B).
The existence of separate cells subserving endogenous (voluntary) attention and motor planning is sufficient to rule out a strong formulation of the Premotor Theory of Attention or its converse. Of course, this conclusion does not mean that these spatial variables never correspond. They often do. However, our results suggest that the coupling of endogenously attended locations and movement goals is elective, not obligatory. Whether the neuronal and behavioral separability we report also applies in the case of exogenous (bottom-up) attention remains to be investigated.
Both cells, with their significant tuning for attention and motor planning, would seem well suited to mediate the coupling between these processes. However, both cells were uncommon in the frontal cortex (Fig. 5A), occurring less frequently than if tuning for attention and the motor plan combined randomly. They were especially rare in the PF and PM (13% of tuned cells) but comprised 37% of the tuned cells and half of all the motor-tuned cells in the PA, which included the frontal eye field (FEF) (Fig. 5C). If both cells accounted for the behavioral interplay between attention and saccadic planning, one might expect their spatial preferences for these processes to be the same—or at least predictably related. Instead, the angle between the preferred attention and motor directions was uniformly distributed (fig. S3), indicating that these processes lacked a consistent spatial relationship across the population.
Functional neuroimaging studies have shown that attended locations and movement goals are represented by largely overlapping regions, including portions of the frontal cortex (12–15). While these overlapping functional activations were taken as support for the Premotor Theory of Attention, they could arise from either neurons that contributed to both the covert and overt attention tasks or distinct neurons interspersed within the same region that contributed to one task or the other (15). Consistent with these studies, we found representations of both processes in the PF and PA. We determined that, at the single-cell scale, some cells in each area contributed to both processes but that the majority contributed specifically to either attention or movement goals. Other areas may read out these intermingled signals to support functions such as the allocation of attentional resources, the maintenance of spatial memories, and motor programming (12, 20).
It could be argued that our finding arose as a consequence of the training on and requirements of the dual four-way conditional task. Similar to many laboratory tasks, our task has an element of artificiality. It does, however, draw on capacities that occur in the natural habitat of monkeys (21). For example, stimuli such as fruit peels can signal the presence of nearby food, which a monkey might look and reach toward while simultaneously covertly attending elsewhere to assess the presence of additional resources and/or threats.
Behavioral validation
Our behavioral results show two performance advantages that are mainstays of the spatial attention literature: improved detection of a subtle stimulus at a covertly attended location and faster movements to covertly attended locations. First, both monkeys were significantly more likely to detect and respond to the go signal on cued trials than uncued trial (Fig. 2 and figs. S1 and S2), demonstrating that the monkeys used the color cue to attend to the appropriate peripheral location during the cue delay period. Second, RTs were faster when the attended and motor locations agreed (Fig. 3B), thus obviating the need to shift attention before executing the saccadic response. Our behavioral results are thus consistent with some of the observations that motivated the oculomotor readiness hypothesis and the Premotor Theory of Attention, but our finding of independent attention and motor coding at the single-cell level points to separate neural mechanisms.
We also know that the monkeys were planning their saccade during the cue delay period. They performed the task well above chance levels and virtually never made erroneous saccadic eye movements. Furthermore, their performance accuracy did not depend on whether the attended and motor locations were distinct or the same (Fig. 3A), suggesting that attention and motor planning operated independently and in parallel in our task, as intended. Last, three quarters of the tuned cells had a sustained representation of the motor location throughout the cue delay period. We take this as evidence of an ongoing motor plan. This tuning cannot credibly be interpreted as attention toward the motor target because there is no behavioral incentive to attend there and because attention is already divided between the fixation point and the attended location (22).
Population coding
Population analysis (Fig. 6, A and B) showed that attention cells accurately decoded where the monkey was attending but failed to signal where the monkey would saccade. The reverse was true of motor planning cells. This dissociation was more extreme than in our earlier work, where we found that PF neurons significantly tuned for attention carried at least some information about spatial working memory and vice versa (23, 24). These findings indicate that the attention and movement goals are encoded more distinctly than attention and spatial memory.
Ensembles of both cells (Fig. 6, A and B), PF cells, or PA cells (Fig. 6, C and D) carried information about the motor plan and the focus of attention. In contrast, ensembles of PM cells decoded the motor plan well but were at chance for decoding the attended location. This presumably reflects that attention tuning (table S1), and attention cells in particular (Fig. 5C), was rare in the PM. Conversely, the lower accuracy of motor location decoding in the PA presumably reflects this area’s smaller proportion of motor-tuned cell and motor planning cells in particular. The latencies reported in table S1 suggest that tuning for attention and the motor plan may have arisen first in the PA and then been transmitted to the other areas, where at least the motor plan was more widely (table S1) and robustly encoded (Fig. 6C).
Previous attempts to dissociate attention and motor plans
The spatial tuning of PF, FEF, and PM neurons has been variously interpreted in terms of sensory processing, attention, memory, and motor planning (23–30). Distinguishing between these possibilities is challenging because exogenous attention is invariably drawn to sensory cues and the movement targets they instruct. Several neurophysiological experiments have dissociated some—but not all—of these sources of spatial tuning. For example, Wise and colleagues (26–28) have used experimental paradigms that permitted cells encoding motor plans and responses to be dissociated from those encoding the position of an initial visual cue that was important for determining when or where to move. Because the cue both attracted attention and had to be held in spatial memory, cells encoding the cue’s initial location might have reflected either of these processes. Subsequently, Wise and colleagues (30) used a task with no spatial memory requirement to differentiate between PM neurons that encoded the goal of a planned reaching movement and those that tracked spatial attention. However, this study did not dissociate overt (gaze angle) and covert attention, so either of these might account for the observed attention tuning.
Motor preparation and covert attention have been disentangled using the antisaccade task, in which a peripheral visual cue instructs a saccade to either its location or the opposite location. Covert attention is always initially directed toward the cue, whose identity determines whether the motor target and attention coincide. Using this task, Schall and colleagues (31) demonstrated that some FEF neurons reflected the monkey’s upcoming saccade, while others reflected where the monkey was attending. Using electrically evoked eye movements as a readout, they determined that the monkey’s ongoing motor plan was always directed toward the antisaccade target on such trial (32). This allowed them to rule out the possibility that attention-tuned neurons actually reflected a transient motor plan toward the cue.
Further evidence that attention and saccade planning have distinct neuronal substrates comes from the finding that visually responsive FEF neurons are modulated by covert attention, but movement-related cells are not (33, 34). Tremblay and colleagues (25) reached a similar conclusion using a peripherally cued go/no-go task. They found that most PA neurons that reliably decoded where the monkey was covertly attending also responded to the preceding spatial cue, while few responded during saccade execution. Furthermore, population decoders trained on activity during the sustained attention period were unable to decode the direction of the subsequent saccade and vice versa, indicating that these two epochs lacked a common spatial representation.
Our task design overcame many of the limitations of previous experiments by cueing where to attend and where to saccade using conditional visuospatial (i.e., color-location) associations. This allowed us to use spatially identical cues, presented centrally, to simultaneously and independently specify both locations from among four possibilities. Because the colored cue was presented centrally throughout the cue delay period, there was no demand on spatial working memory nor were there peripheral cues or distractors that could exogenously capture attention or otherwise account for any observed spatial tuning. By requiring central fixation, we dissociated eye position (overt attention) from covert attention. Because we used a conditional (nonspatial) cue, our study specifically addresses the endogenous allocation of covert spatial attention. The cues were 100% reliable and never countermanded midtrial so there was no ambiguity that might foster distributed attention (except on uncued trials), alternate motor plans, or dynamic changes. Thus, the task provided a single sustained time period for examining the neuronal encoding of endogenous covert spatial attention and motor planning.
Prefrontal and pre-arcuate cortex
Here, we use the terms motor planning cell and tuning for the motor plan as a convenience. However, the present study cannot distinguish whether these motor signals reflect the metrics of the planned movement or a spatial goal. In light of previous studies that contrasted goal and movement coding in the PF (35), it seems most likely that PF tuning for the motor location reflects a goal signal rather than the metrics of movement. Our findings therefore agree with the general idea that the PF contributes to goal generation (21, 29).
Our findings also highlight the importance of the PF and PA in top-down spatial attention. Neuronal activity in these areas has been shown to reflect both top-down attention (23, 24, 34, 36) and attentional filtering (25, 33).
PF and FEF are thought to account for the attentional modulation of posterior visual areas. In V4, visual responses and selectivity are enhanced when attention is directed at a cell’s receptive field (34). Lesions of PF and FEF reduce this attentional modulation and synchrony in the deprived V4 (36).
Subthreshold stimulation of FEF likewise enhanced the visual response of V4 cells with receptive fields that coincided with the endpoint of saccades evoked with higher currents (37, 38). Behaviorally, subthreshold FEF stimulation selectively improved target detection at the endpoint of evoked saccades (39). Stimulation of the superior colliculus likewise produced behavioral benefits in attention-demanding visual tasks (40). Stimulation of these oculomotor structures not only presumably generates a saccade plan but also appears to direct covert attention to the saccade target. Such local stimulation may activate a group of neurons that each contribute to both saccade planning and covert attention, with a common spatial preference. An alternative scenario, suggested by others (41) and supported by our findings, is that stimulation activates a mixture of neurons with similar spatial preferences, some of which contribute to motor planning and others to guiding attention.
The present results show a predominance of motor over attention signals in the PF. Together with our previous results (23, 24), which revealed a prevalence of attention over memory signals, it seems likely that the PF encodes all three spatial variables. A comparison of these results, although necessarily indirect, suggests that spatial-goal signals are more prevalent in the PF than are spatial-attention signals, which, in turn, are more prevalent than spatial-memory signals.
Premotor cortex
We found the PM to be predominantly involved in motor planning. Consistent with this finding, previous reports have generally interpreted delay period activity in the PM in terms of motor planning and visuomotor transforms (42, 43). However, our findings and previous reports indicate that a minority of PM neurons appear to play a role in attentional functions (44).
Summary
In summary, we observed largely separate neural representations of attention and motor planning. This demonstrates that motor planning cannot fully account for the allocation of attentional resources, thus ruling out the obligatory yoking of attention to motor planning. Attention is not a by-product of motor plans but rather a distinct resource with dedicated neural circuits.
MATERIALS AND METHODS
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved in advance by the Animal Care and Use Committee of the National Institute of Mental Health.
Behavioral task
We trained two male rhesus monkeys (Macaca mulatta), monkey R (9.0 kg) and monkey G (7.5 kg), to perform the task illustrated in Fig. 1. Each monkey sat in a primate chair facing a video screen 57 cm away, with its head fixed by a surgically implanted head post.
The experimental design dissociated the locus of covert spatial attention from the target of a future saccadic eye movement. To accomplish this objective, we used a dual conditional visuospatial task. One four-way conditional association provided the spatial goal for a saccade; another four-way conditional association guided the allocation of covert attention.
The monkey began each trial by touching a metal bar, which led to the appearance of a small white circle (0.15° radius), the fixation point, at the center of the video screen (Fig. 1). If the monkey continued to fixate this spot [within a square window of 3.3° for a variable period (200 to 1000 ms)], four circular gray spots appeared (0.35° radius): left, right, up, and down from the fixation point at an eccentricity of 4.0°. After an additional fixation period of 200 to 1000 ms, a bicolored cue stimulus appeared at the fixation point. The color of the cue’s outer component, a 0.25°-wide annulus, informed the monkey about which of the four gray spots to fixate after a forthcoming go signal. For convenience, we call this spot the motor location, and we call the instructing stimulus the motor cue. The color of the cue’s inner component, a 0.6° radius circle, indicated which of the four gray spots would subsequently brighten as the go signal. We call this spot the attended location, and we call the central instructing stimulus the attention cue. By design, the monkey should direct covert attention to the attended location to facilitate detection of the brightening event, which triggered a saccade to the motor location. The degree of brightening was selected at random among four levels that were calibrated such that each monkey failed to respond to the smallest degree of brightening on ~30% of trials and responded reliably to the largest degree of brightening.
We refer to the variable and randomly selected interval (800, 1600, 2400, or 3200 ms) between the appearance of the cue stimulus and the go signal as the cue delay period. During this interval, the monkey should direct covert attention to the spot at the attended location while simultaneously planning a saccade to the spot at the (usually distinct) motor location. Following the go signal, the monkey had to complete a saccade to the motor location within 800 ms (for monkey R) or 600 ms (for monkey G). If it did so, then all other stimuli disappeared from the screen, and the monkey had to fixate the motor location (within a square window of 4.0°) for an additional 800 to 1600 ms until it dimmed. At that point, the monkey could release the bar to receive a juice reward. If the monkey broke fixation before the go signal, failed to make a saccade within the requisite time after the go signal, shifted fixation anywhere other than to the motor location, failed to fixate the spot at the motor location for the required interval, or released the bar before this spot dimmed, the trial ended without reward delivery, and the normal interval between trials (600 to 1800 ms) was extended by 1600 ms.
The same conditional mapping between the four cue stimulus colors and the four peripheral spots was used for the motor and attention cues. As illustrated in Fig. 1B, red was associated with the gray spot to the right of the fixation point, blue with up, green with left, and yellow with down. This arbitrary mapping was overlearned by trial and error before the neuronal recordings. On 20% of the trials, the colored attention cue was replaced with a gray cue of the same dimensions. On these uncued trials—i.e., uncued with respect to covert attention—the go signal was the brightening of a pseudorandomly selected peripheral spot. Comparing the performance on cued and uncued trials allowed us to assess whether the monkeys made use of the attention cue on the remaining 80% of trials.
A subset of the neurons was also recorded during a simple fixation task. The objective of this task was to determine whether neurons were selective for the colored cue stimuli. This task had the same timing as the main task, but no peripheral spots appeared on the screen. The monkey had to touch the bar and maintain central fixation during cue stimulus presentation. When both parts of the cue stimulus dimmed, which always occurred simultaneously, the monkey could release the bar to obtain a reward.
Surgery and neuronal recording
A metal head post was surgically implanted under anesthesia before training began. After each monkey had learned the task, we surgically implanted a 27 × 36 mm recording chamber over the left frontal cortex, with its long side oriented in an anterior-posterior (AP) plane and its short side in a medial-lateral (ML) plane. A craniotomy was centered at AP +29.5, ML +12 in monkey R and AP +22, ML +15 in monkey G.
Recordings were made in three broad frontal regions, as shown in the inset of Fig. 5B: PM, PF, and PA. PM sites were located in the dorsal PM, primarily in area 6D, both rostral and caudal to the arcuate sulcus. In monkey G, PM recordings encompassed the more medial supplemental eye field (SEF). As SEF and the remainder of PM had similar proportions of tuned cells (54 and 63%, respectively, in monkey G) and nearly identical proportions of the three types of tuned cells, we treated these areas collectively in subsequent analyses. PF included parts of the dorsolateral and ventrolateral PF on either side of the principal sulcus (areas 46d/v, 8, and 45). In the dorsal PF, the proportions of each cell type were nearly identical in both monkeys. Ventral PF, which was only recorded in monkey G, was combined with the dorsal PF as the proportions of monkey G’s tuned cells (50 versus 54%) were similar in these two subregions. Of the tuned cells in these two subregions of monkey G (N = 52 and 61, respectively), the proportions of motor planning cells (62 versus 49%) and attention cells (33 versus 30%) were comparable, whereas both cells (6 versus 21%) were more common in the ventral PF. PA sites were posterior to the principal sulcus and rostral to the genu of the arcuate sulcus, mostly in its rostral bank (areas 8A and 45b), which includes the FEF.
The location of the FEF and SEF was assessed in monkey G with intracortical microstimulation (biphasic pulses with 0.2-ms pulse width, 300-Hz trains of 50- or 100-ms duration). Stimulation reliably elicited contraversive saccades of fixed amplitude and direction at current thresholds of 10 to 80 μA in FEF and 5 to 160 μA in SEF.
Near the conclusion of the experiment, electrolytic marking lesions were made at multiple depths along four widely spaced penetrations where neuronal activity had been detected. Ten days later, the monkeys were deeply anesthetized and perfused with 10% (v/v) formol-saline. Four marking pins were inserted in the brain, along the same angle as the electrode penetrations, at positions that spanned the recording sites (maximum separation of 21 mm). Histological analysis of the frontal lobe was conducted on Nissl-stained coronal sections at 1-mm spacing. Using the marks left by the pins and the electrolytic lesions, we reconstructed the penetrations and the depth at which each neuron was recorded. A macaque brain atlas (45) was used to designate brain areas.
Recordings were made using a multielectrode microdrive, with independently moveable single-contact electrodes arranged in a circle. The initial penetrations in both monkeys were made with a 7-electrode System Eckhorn drive (Thomas Recording GmbH, Giessen, Germany). Later penetrations were made with Alpha Omega’s 8-electrode MultiDrive (Alpharetta, GA). Neighboring electrodes were 1.0 mm apart, and the largest electrode separation (along the diameter) was 2.62 mm. Neuronal activity was discriminated online using a multi-spike discriminator (Alpha Omega Engineering) and then sorted offline using the Offline Sorter (Plexon, Dallas, TX). The analyses were conducted with custom software (MATOFF, A. R. Mitz developer, National Institute of Mental Health, Bethesda, MD) and MATLAB (MathWorks). Eye position was monitored with an infrared oculometer and recorded with an eye tracking system (Arrington Research Inc., Scottsdale, AZ).
Single-neuron analysis
Neuronal activity on correctly executed trials was aligned on the go signal and grouped by task conditions. Activity on cued trials was arranged into a 4 × 4 matrix, in which rows corresponded to the four motor locations and columns to the four attended locations. Mean firing rates were computed for the last 800 ms of the cue delay period, which was the shortest of the four possible delays between cue onset and the go signal. A neuron’s mean firing rate on each trial was assessed using a two-way ANOVA (α = 0.05, df = 15), with motor location and attended location as factors. Each factor had four levels: right, left, up, and down. The significance of the two main effects determined whether a neuron was classified as being tuned for the motor location, the attended location, both locations, or neither. For convenience, we referred to these four classes of neurons as motor planning cells, attention cells, both cells, and untuned cells, respectively. We used an ANOVA so we could assess interaction effects between the motor and attended locations. As the ANOVA entails some assumptions about neuronal firing rates, we also performed separate nonparametric (Kruskal-Wallis) tests for each factor on each cell. Of the 213 cells with a significant main effect in the two-way ANOVA, almost all (203) were significant on one or both of the Kruskal-Wallis tests, and the proportion of the different cell types was largely unchanged. We therefore used the ANOVA classifications in our subsequent analyses.
We calculated a motor planning PD for each motor planning cell. In this sense, a “direction” also corresponds to a location because eccentricity and saccade amplitude did not vary in the present experiment. The neuron’s average firing rate for each motor location (Rightmot, Upmot, Leftmot, and Downmot) was computed using the mean firing rates for all trials in the same row of the 4 × 4 matrix (e.g., Rightmot is the average of all trials in the first row). The motor planning PD was taken as the sum of four vectors—of length Rightmot, Upmot, Leftmot, and Downmot, respectively—originating at the central fixation point and pointing in the four cardinal directions. The motor planning PD’s angle, Φmot, was measured counterclockwise with respect to the positive x axis (rightward)
Likewise, the attention PD and angle (Φatt) were calculated for each attention cell. This vector depended on the average firing rate for each attended location (Rightatt, Upatt, Leftatt, and Downatt) computed across all trials in the same column of the 4 × 4 matrix. For both cells, we computed Φmot, Φatt, and ΔΦ, the smaller of the angles between them.
Population histograms
For each motor planning cell, we computed four peri-event time histograms (PETHs) across trials that had the same motor location. These PETHs were ordered from the most to least preferred based on the rank order of the neuron’s average rate for each motor location during the 800 ms before the go signal (i.e., Rightmot, Upmot, Leftmot, and Downmot). These single-neuron PETHs were then grouped by rank and averaged across neurons from the two monkeys to generate population histograms. We also computed population histograms for attention tuning by computing PETHs for trials that had the same attended location, ranking these based on the neuron’s average rate for each attended location (i.e., Rightatt, Upatt, Leftatt, and Downatt), and pooling the PETH responses by this rank across attention-tuned cells.
To investigate whether a neuron’s motor location preference was predictive of that neuron’s attended location preference (and vice versa), we computed population histograms where the single-neuron PETH responses to the four motor locations were ranked, not by activity when the monkey was planning a saccade to that location, but rather by that neuron’s corresponding preference for the attended locations (i.e., Rightatt, Upatt, Leftatt, and Downatt) before averaging across neurons. Similarly, the single-neuron PETHs for each attended location were ranked using that neuron’s motor location preferences (i.e., Rightmot, Upmot, Leftmot, and Downmot) and then averaged by rank across neurons. If neurons had identical spatial preferences for motor planning and attention, this procedure (fig. S6) would reveal the same degree of tuning as for the procedure described in the preceding paragraph (fig. S4). Alternatively, if spatial tuning for the two processes was completely independent, this analysis should show no residual spatial tuning.
Neuronal population latencies
To assess when motor and attention tuning emerged in different neuronal populations, we compared mean firing rates following onset of the cue stimulus with the preceding 500-ms baseline period. To ensure that the baseline period was not contaminated by an onset response, we computed each neuron’s baseline rate using only those trials for which the four peripheral spots appeared ≥800 ms before the cue. We then determined the mean baseline rate and its SD across neurons. Motor tuning was measured as the difference in firing rate between responses to the cues signaling the most and least preferred motor locations. This difference was assessed in a 50-ms sliding window (with a 1-ms step) and averaged across motor-tuned neurons. The population latency was the first window for which the mean firing rate difference was 3 SD greater than the baseline firing rate. The latency for attention tuning was calculated in an equivalent way.
Neuron-dropping curves
We assessed how well ensembles of significantly tuned neurons could decode the motor and attended locations from the firing rate on a single trial using a leave-one-out classification algorithm (46). The classification procedure followed our earlier study (24). Neuronal ensembles consisted of a given cell type (attention, motor, or both) selected at random from both monkeys. For each ensemble selected, a single trial of the same cued condition type was selected at random from each neuron. The neuron’s average firing rate under this condition was then recomputed without the selected trial. The absolute difference in rate between the selected trial and that neuron’s average firing rate for each condition was ranked from smallest to largest (1 to 16). This rank was summed across neurons in the ensemble, and the trial was classified as belonging to the condition with the lowest rank. The classification of the motor location was correct if the classified condition had the same motor location as the true condition. Similarly, the classification of the attended location was correct if the classified condition had the same attended location as the true condition. Thus, the probability of correct classification by chance was 25%. The accuracy with which an ensemble of a given size decoded either the motor or attended location was determined by repeating the single-trial classification procedure 1000 times for each of the 16 cued conditions. To assess the variability in the percent correct classification, we repeated the calculation 17 times for each curve in Fig. 6, at an ensemble size corresponding to the entirety of the cell category in each panel with the fewest cells.
Acknowledgments
We thank K. Blomstrom for assistance with animal training and data collection, A. Cummins for preparing the histological material, I. Caprara for early stages of the analysis, and M. Leathers for comments on an earlier version of this manuscript. Funding: This research was supported (in part) by the Intramural Research Program of the NIMH and includes the relevant Annual Report number in the following format: Z01MH002875. A.G. was also partially supported by PRIN funding (PRIN 2017, 2017KZNZLN_00). Author contributions: A.M. and S.P.W. designed the study. A.M. collected the data; A.G., R.C., and A.M. analyzed the data. R.C., A.G., S.P.W., and A.M. interpreted the results and wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Access to details of the behavioral and neurophysiological data used in the analysis is available by request. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/14/eabe0716/DC1
REFERENCES AND NOTES
- 1.R. M. Klein, Does oculomotor readiness mediate cognitive control of visual attention?, in Attention and Performance VIII, R. Nickerson, Ed., (Erlbaum, 1980), pp. 259-276. [Google Scholar]
- 2.R. M. Klein, A. Pontefract, Does oculomotor readiness mediate cognitive control of visual attention? Revisited!, in Attention and Performance XV: Conscious Nonconscious Information Processing, C. Umiltà, M. Moscovitch, Eds. (The MIT Press, 1994), pp. 333–350. [Google Scholar]
- 3.Kowler E., Anderson E., Dosher B., Blaser E., The role of attention in the programming of saccades. Vision Res. 35, 1897–1916 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Hoffman J. E., Subramaniam B., The role of visual attention in saccadic eye movements. Percept. Psychophys. 57, 787–795 (1995). [DOI] [PubMed] [Google Scholar]
- 5.Deubel H., Schneider W. X., Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vision Res. 36, 1827–1837 (1996). [DOI] [PubMed] [Google Scholar]
- 6.Baldauf D., Deubel H., Properties of attentional selection during the preparation of sequential saccades. Exp. Brain Res. 184, 411–425 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Harrison W. J., Mattingley J. B., Remington R. W., Eye movement targets are released from visual crowding. J. Neurosci. 33, 2927–2933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzolatti G., Riggio L., Dascola I., Umiltà C., Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25, 31–40 (1987). [DOI] [PubMed] [Google Scholar]
- 9.Hunt A. R., Kingstone A., Covert and overt voluntary attention: Linked or independent? Cogn. Brain Res. 18, 102–105 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Hunt A. R., Kingstone A., Inhibition of return: Dissociating attentional and oculomotor components. J. Exp. Psychol. Hum. Percept. Perform. 29, 1068–1074 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Sheliga B. M., Riggio L., Rizzolatti G., Orienting of attention and eye movements. Exp. Brain Res. 98, 507–522 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Perry R. J., Zeki S., The neurology of saccades and covert shifts in spatial attention: An event-related fMRI study. Brain 123, 2273–2288 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Nobre A. C., Gitelman D. R., Dias E. C., Mesulam M. M., Covert visual spatial orienting and saccades: Overlapping neural systems. Neuroimage 11, 210–216 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Beauchamp M. S., Petit L., Ellmore T. M., Ingeholm J., Haxby J. V., A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage 14, 310–321 (2001). [DOI] [PubMed] [Google Scholar]
- 15.de Haan B., Morgan P. S., Rorden C., Covert orienting of attention and overt eye movements activate identical brain regions. Brain Res. 1204, 102–111 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagnini A., Castet E., Spatiotemporal dynamics of visual attention during saccade preparation: Independence and coupling between attention and movement planning. J. Vis. 7, 8.1–8.16 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Khan A. Z., Blohm G., Pisella L., Munoz D. P., Saccade execution suppresses discrimination at distractor locations rather than enhancing the saccade goal location. Eur. J. Neurosci. 41, 1624–1634 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Lhermitte F., “Utilization behaviour” and its relation to lesions of the frontal lobes. Brain 106, 237–255 (1983). [DOI] [PubMed] [Google Scholar]
- 19.Deubel H., The time course of presaccadic attention shifts. Psychol. Res. 72, 630–640 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Ikkai A., Curtis C. E., Common neural mechanisms supporting spatial working memory, attention and motor intention. Neuropsychologia 49, 1428–1434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R. E. Passingham, S. P. Wise, The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution, and the Origin of Insight (OUP Oxford, 2012). [Google Scholar]
- 22.Müller M. M., Malinowski P., Gruber T., Hillyard S. A., Sustained division of the attentional spotlight. Nature 424, 309–312 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Lebedev M. A., Messinger A., Kralik J. D., Wise S. P., Representation of attended versus remembered locations in prefrontal cortex. PLOS Biol. 2, e365 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messinger A., Lebedev M. A., Kralik J. D., Wise S. P., Multitasking of attention and memory functions in the primate prefrontal cortex. J. Neurosci. 29, 5640–5653 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tremblay S., Pieper F., Sachs A., Martinez-Trujillo J., Attentional filtering of visual information by neuronal ensembles in the primate lateral prefrontal cortex. Neuron 85, 202–215 (2015). [DOI] [PubMed] [Google Scholar]
- 26.di Pellegrino G., Wise S. P., Visuospatial versus visuomotor activity in the premotor and prefrontal cortex of a primate. J. Neurosci. 13, 1227–1243 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boussaoud D., Wise S. P., Primate frontal cortex: Effects of stimulus and movement. Exp. Brain Res. 95, 28–40 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Boussaoud D., Wise S. P., Primate frontal cortex: Neuronal activity following attentional versus intentional cues. Exp. Brain Res. 95, 15–27 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Genovesio A., Tsujimoto S., Wise S. P., Encoding goals but not abstract magnitude in the primate prefrontal cortex. Neuron 74, 656–662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebedev M. A., Wise S. P., Tuning for the orientation of spatial attention in dorsal premotor cortex. Eur. J. Neurosci. 13, 1002–1008 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Sato T. R., Schall J. D., Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 38, 637–648 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Juan C.-H., Shorter-Jacobi S. M., Schall J. D., Dissociation of spatial attention and saccade preparation. Proc. Natl. Acad. Sci. U.S.A. 101, 15541–15544 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson K. G., Biscoe K. L., Sato T. R., Neuronal basis of covert spatial attention in the frontal eye field. J. Neurosci. 25, 9479–9487 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregoriou G. G., Gotts S. J., Zhou H., Desimone R., High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324, 1207–1210 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito N., Mushiake H., Sakamoto K., Itoyama Y., Tanji J., Representation of immediate and final behavioral goals in the monkey prefrontal cortex during an instructed delay period. Cereb. Cortex 15, 1535–1546 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Gregoriou G. G., Rossi A. F., Ungerleider L. G., Desimone R., Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat. Neurosci. 17, 1003–1011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekstrom L. B., Roelfsema P. R., Arsenault J. T., Bonmassar G., Vanduffel W., Bottom-up dependent gating of frontal signals in early visual cortex. Science 321, 414–417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong K. M., Moore T., Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc. Natl. Acad. Sci. U.S.A. 104, 9499–9504 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore T., Fallah M., Microstimulation of the frontal eye field and its effects on covert spatial attention. J. Neurophysiol. 91, 152–162 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Müller J. R., Philiastides M. G., Newsome W. T., Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl. Acad. Sci. U.S.A. 102, 524–529 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awh E., Armstrong K. M., Moore T., Visual and oculomotor selection: Links, causes and implications for spatial attention. Trends Cogn. Sci. 10, 124–130 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Cisek P., Kalaska J. F., Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron 45, 801–814 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Fujii N., Mushiake H., Tanji J., Distribution of eye- and arm-movement-related neuronal activity in the SEF and in the SMA and pre-SMA of monkeys. J. Neurophysiol. 87, 2158–2166 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Vaadia E., Kurata K., Wise S. P., Neuronal activity preceding directional and nondirectional cues in the premotor cortex of rhesus monkeys. Somatosens. Mot. Res. 6, 207–230 (1988). [DOI] [PubMed] [Google Scholar]
- 45.K. Saleem, N. Logothetis, A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates, 2nd Edition (Academic Press, 2012), pp. 1–389. [Google Scholar]
- 46.Foffani G., Moxon K. A., PSTH-based classification of sensory stimuli using ensembles of single neurons. J. Neurosci. Methods 135, 107–120 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/14/eabe0716/DC1