Abstract
MR-linac technology enhances the precision of therapeutic radiation by clarifying the tumor-normal tissue interface and provides the potential for adaptive treatment planning. Accurate delineation of tumors on diagnostic magnetic resonance imaging (MRI) frequently requires gadolinium-based contrast agents (GBCAs). Despite generally being considered safe, previous literature suggests that GBCAs are capable of contrast-induced acute kidney injury (AKI). It is unclear if the risk for AKI is enhanced when GBCAs are administered concurrently with ionizing radiotherapy. During irradiation, gadolinium may be liberated from its chelator which may induce AKI. The goal of this work was to determine if radiation combined with GBCAs increased the incidence of AKI. Using a preclinical MRI-guided irradiation system, where MRI acquisitions and radiation delivery are performed in rapid succession, tumor-bearing mice with normal kidney function were injected with GBCA and treated with 2, 8 or 18 Gy irradiation. Renal function was assessed on days three and seven postirradiation to assess for AKI. No clinically relevant changes in blood urea nitrogen and creatinine were observed in any combination of GBCA and radiation dose. From these data, we conclude that GBCA in combination with radiation does not increase the risk for AKI in mice. Additional investigation of multiple doses of GBCA administered concurrently with irradiation is warranted to evaluate the risk of chronic kidney injury.
INTRODUCTION
MR-linac technology is currently in clinical use in several centers worldwide, with many additional centers at various stages of adopting this new radiation delivery system. MR-linac technology provides enhanced image guidance through improved soft-tissue contrast and real-time imaging of both tumor and normal tissues during ionizing radiation delivery, which was not possible with photon-based imaging modalities. This technology may enable enhanced real-time, adaptive radiotherapy (1, 2). However, even with magnetic resonance imaging (MRI), some lesions (e.g., liver tumors) are difficult to visualize without the use of contrast enhancement.
Gadolinium-based contrast agents (GBCAs), e.g., the macrocyclic compound, gadobutrol (known commercially as Gadavist®) are routinely utilized for tumor imaging enhancement. Approximately 25–30% of all patients undergoing MRI use some form of GBCA (3). Initial biodistribution studies of GBCAs indicate they are generally excreted rapidly within 72 h (3, 4). However, there are ongoing concerns about development of acute kidney injury (AKI) and nephrogenic systemic fibrosis (NSF) when GBCAs are combined with radiation treatment (5–7).
Published in vitro data suggest that GBCA exposure is associated with the necrosis and apoptosis of proximal renal tubule cells (8). Additionally, in vivo data in pigs showed a reduction in glomerular filtration rate after injection of GBCAs into the right renal artery, reporting higher nephrotoxicity after GBCA injection compared to iodine contrast media (9). A published 2006 case report demonstrated the potential acute nephrotoxic effects of GBCA administration in humans (10). A 56-year-old woman, originally presenting with a hypertensive crisis, developed AKI after GBCA administration during two consecutive imaging procedures. Subsequent biopsy revealed the presence of acute tubular damage that was attributed to GBCA administration (10). Despite the concern over GBCA-induced AKI, a long-term toxicity study of 22,897 patients who underwent gadoteric acid (Dotarem®)-enhanced MRI demonstrated no increased risk for NSF or AKI (11). However, recently published data suggest that gadolinium may accumulate in tissues throughout the body regardless of renal function. Free Gd3+ is a toxic lanthanide metal that can accumulate in tissues such as kidney, brain and muscle (7). A major concern regarding GBCA administration during radiation therapy is the potential ability for radiation to liberate gadolinium from the agent’s complex. This would thereby release free Gd3+ in the circulation, allowing it to accumulate in the kidney, resulting in contrast-induced AKI.
In the context of MR-linac treatment, patients would receive a dose of radiation within minutes of GBCA administration. In this way, the lesion would be clearly visible pre-treatment thereby allowing clinicians to perform adaptive treatment planning. However, there are currently no data regarding AKI after GBCA administration with concurrent irradiation. In this study, we assessed if GBCAs, administered at standard doses used for imaging procedures, delivered concurrently with radiation-induced acute nephrotoxicity in mice.
MATERIALS AND METHODS
Mouse Xenografts
Three-month-old female nude athymic mice (Nu/J) (Jackson Laboratory, Bar Harbor, ME) were subcutaneously injected with 2 × 106 glioblastoma cells (U251) into their left flank. Mice were housed in a barrier facility at the University of Iowa (Iowa City, IA) for 10–14 days to allow tumor formation. All experiments were approved and performed in ethical compliance with the University of Iowa institutional Animal Care and Use Committee (IACUC) protocols (ACURF no. 7021022).
Magnetic Resonance Imaging
Gadobutrol was intraperitoneally injected (4 mmol kg−1) immediately prior to MRI (12). T1-weighted anatomical images were collected on a GE 7T small animal scanner (GE Healthcare, Pittsburgh, PA). To generate T1-weighted images, a fat-saturated, spin-echo sequence was obtained (TE=9.3 ms, TR=900 ms, FOV=3 × 2.25 cm, slice thickness/gap = 0.4/0.1 mm, matrix = 256 × 192 resolution). Images were analyzed using the 3D Slicer software.
Irradiations
Animals (n = 8 per group) were irradiated using a small animal radiation research platform (SARRP) at the University of Iowa Radiation and Free Radical Research Core with a 220-kVp radiation beam (HVL = 0.625 mm copper). Animals were aligned with tumor-bearing flank at isocenter with a 35-cm source-to-axis distance (SAD) verified with the on-board laser system. Alignments were validated using a whole field, electronic portal imaging device (EPID) with a 5-mm aluminum metal marker placed externally at the site of the tumor (Fig. 1A). The marker was removed prior to irradiation. Radiation dose of 2, 8 or 18 Gy, at a depth of 1.5 mm, was delivered using a 10 × 10 mm2 collimator. Delivered radiation doses were validated on a representative mouse in each cohort by the University of Wisconsin Medical Radiation Research Center (Madison, WI) using thermoluminescence dosimetry. Doses were validated within 8% based on a calibration curve. Mice that did not exhibit tumor gadolinium uptake prior to irradiation were excluded.
FIG. 1.
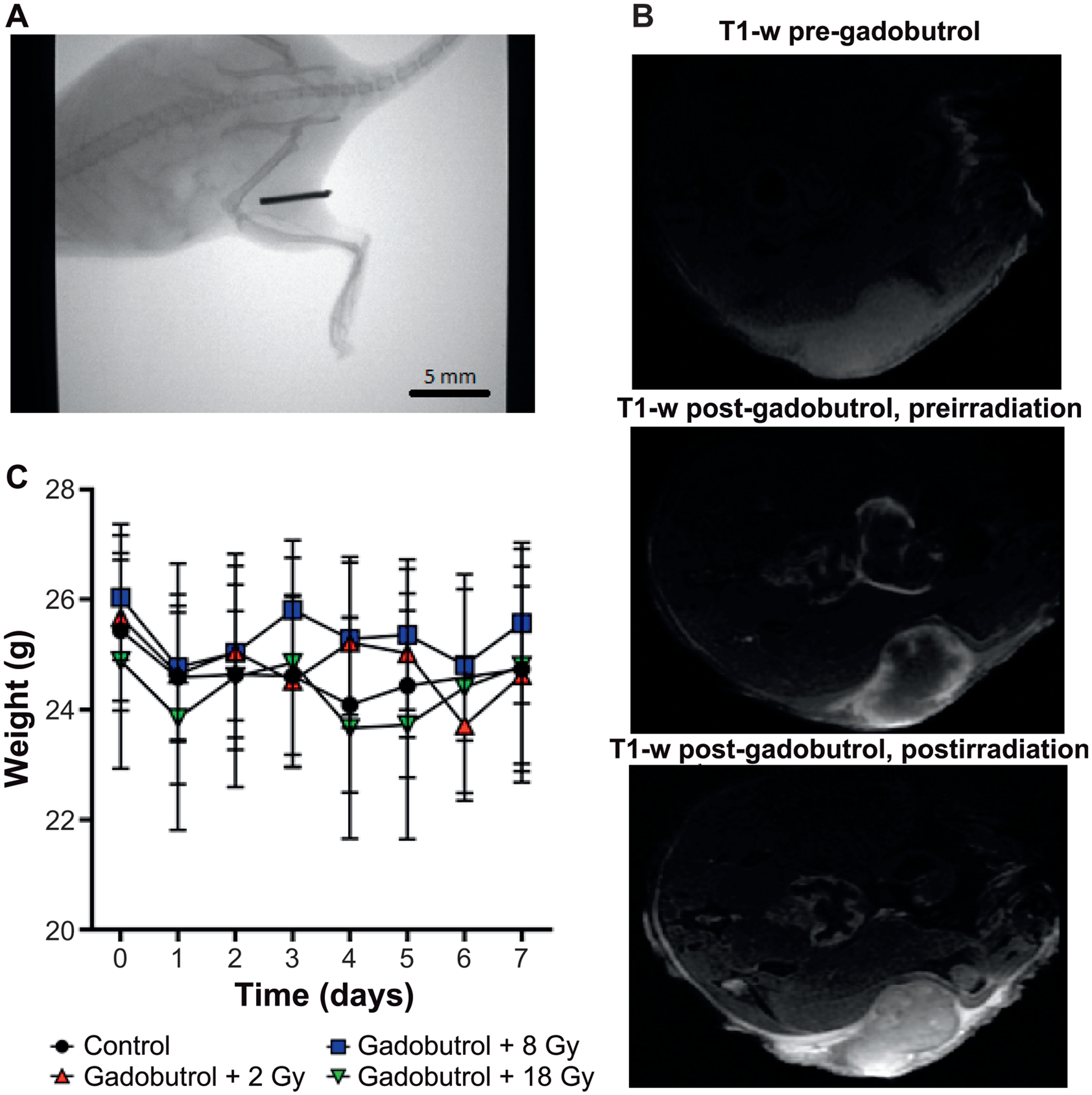
Preclinical contrast enhancing MRI-guided radiotherapy model. Panel A: Representative whole-field portal CT image for tumor localization after animal set up. A 5-mm piece of aluminum wire overlays the flank tumor to confirm that the tumor is aligned at isocenter; the wire is removed prior to treatment. Panel B. Representative T1-weighted (T1-w) images show presence of Gadavist uptake before and after irradiation in a U251 flank tumor. Panel C: Mice (n = 8 per group) were weighed for seven days postirradiation to investigate potential obvious, systemic toxicity.
Blood Collection for Assessment of Nephrotoxicity
Blood samples were collected by tail snip in serum separator tubes at three- and seven-days post-treatment (4). Mice were euthanized on day seven postirradiation, at which point submandibular blood collection was performed. Blood urea nitrogen (BUN) and creatinine analysis were performed by Antecht® Diagnostics (Des Moines, IA) who was blinded to treatment groupings. The elevation of serum BUN/creatinine 72 h post-treatment are considered useful markers of AKI (13, 14). Statistical analysis was performed using GraphPad Prizm software and one-way analysis of variance (ANOVA).
RESULTS
Gadolinium Enhances Murine Tumors on MRI
To confirm the presence of gadolinium during irradiation, we visualized gadolinium tumor uptake using MRI prior to irradiation (Fig. 1B). The mean time to irradiation after administration of gadolinium was 18 ± 3 min. Mice from each treatment group underwent a subsequent T1-weighted MRI after irradiation to confirm continued gadolinium presence with a mean time to MRI data acquisition after irradiation of 28 ± 1 min. Gadolinium contrast enhancement was still evident after irradiation in all assessed mice (Fig. 1B). To assess for systemic toxicity after gadolinium injection and irradiation, mouse weights were tracked for seven days. No significant changes in mouse weights post-treatment were detected (Fig. 1C).
Gadolinium and Radiation Show no Evidence of Acute Nephrotoxicity
To investigate the effects of GBCAs and irradiation on kidney function, we measured BUN and creatinine in tumor-bearing mice. Serum levels of BUN and creatinine were obtained three days and seven days after treatment. Three days after treatment, mean serum BUN was 20.3 ± 4.0, 21.3 ± 1.5, 20.9 ± 1.7 and 27.8 ± 2.9 mg dl−1for untreated control, gadobutrol with 2 Gy, gadobutrol with 8 Gy and gadobutrol with 18 Gy irradiated animals, respectively (Fig. 2A). There was a significant increase of BUN 72 h post-treatment compared to healthy controls; however, the values were still within the normal range. Serum creatinine was 0.45 6 0.13, 0.55 6 0.10, 0.47 6 0.1 and 0.61 6 0.1 mg dl−1 for the respective cohorts (Fig. 2B). At day 7, serum BUN remained stable with mean values of 20.3 ± 4.0, 21.3 ± 2.2, 20.6 ± 1.9 and 18.0 ±62.6 mg dl−1 for untreated control, gadobutrol with 2 Gy, gadobutrol with 8 Gy and gadobutrol with 18 Gy irradiated cohorts, respectively (Fig. 2C). Additionally, day 7 serum creatinine followed a similar trend in each cohort and remained stable with mean values of 0.45 ± 0.13, 0.43 ± 0.18, 0.23 ± 0.05, and 0.31 ± 0.06 mg dl−1, respectively (Fig. 2D). No significant BUN changes occurred post-treatment with gadobutrol or 18 Gy irradiation alone (Fig. 2A–D). Seven days post-treatment, there were no significant differences in BUN or serum creatinine values in any cohort. These data suggest that the combination of GBCA with therapeutic levels of radiation does not induce AKI.
FIG. 2.
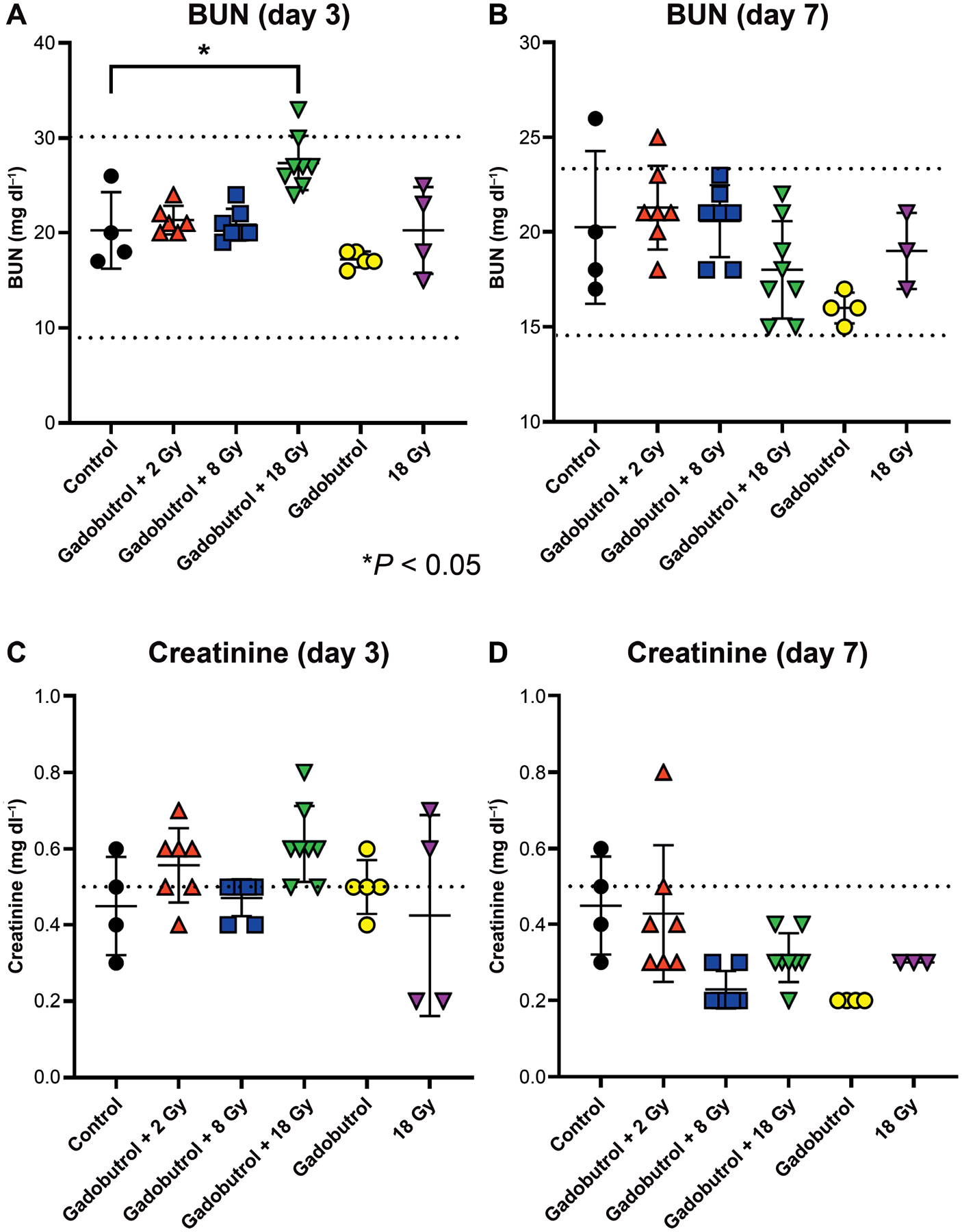
Assessment of nephrotoxicity after Gadavist administration in combination with ionizing radiation. Serum BUN and creatinine levels were measured at three days (panels A and C) and seven days (panels B and D) after: i.p. injection of 4 mmol kg−1 Gadavist and 2, 8 or 18 Gy irradiation; Gadavist alone; or 18 Gy irradiation alone. All groups (n = 8) were compared to a cohort of normal, healthy control animals (n = 4). Dashed lines indicate normal reference levels provided by Antech Diagnostics. *P < 0.05 (one-way ANOVA).
DISCUSSION
In vitro data suggest that GBCA exposure is associated with necrosis and apoptosis in proximal renal tubule cells (8). However, evaluation of over 20,000 patients that received GBCA demonstrated no increased risk for AKI (11). A major concern of GBCA administration during irradiation is the liberation of Gd3+ from its complex. Free Gd3+ may accumulate in the kidney and cause CI-AKI. The current mechanisms for CI-AKI and CI-NSF are still unclear and are an active area of research, but may be related to free Gd3+ being filtered and concentrating in the distal tubules (7). Gd3+ may facilitate mitochondrial reactive oxygen species production leading to distal tubule cell apoptosis via the release of cytochrome c (15). Mapuskar et al. demonstrated that cisplatin-induced AKI and CKD are mediated by mitochondrial disruptions leading to increased reactive oxygen species production (4). Mouse studies have been shown to be increasingly useful tools for evaluating the in vivo development of CI-AKI (16). In preclinical CI-AKI models, there is a rise in serum creatinine 2–3 days after contrast administration, which suggests that the preclinical phenomenon closely mimics the onset of AKI in humans (17). Unfortunately, clinical studies of gadolinium-induced kidney damage have been limited to case reports. Thus, it is difficult to determine how closely preclinical models are able to mimic the pathophysiology of a human.
In this study, we developed a preclinical MRI-guided radiotherapy model to evaluate for AKI when administering gadolinium during irradiation. The combination of gadolinium with irradiation demonstrated no evidence of CI-AKI in a variety of radiation dose cohorts. The combination of 18 Gy with gadobutrol did show an increase in average serum BUN three days post-treatment that was still within the murine normal range (9–30 mg dl−1; Antech Diagnostics). The elevated BUN returned to baseline levels seven days post-treatment. These transient elevations are unlikely clinically relevant and may be the result of transient changes in hydration status. Therefore, despite the large dose of gadobutrol (4 mmol kg−1 compared to 0.1 mmol kg−1 given to humans), administration of gadobutrol followed by irradiation appears to be relatively safe in this context and unlikely to induce acute toxicity.
The mice in these experiments were treatment naïve and were considered to have normal kidney function. Since AKI is more prevalent in patients with reduced renal function, additional investigation of GBCA combined with radiation may be warranted in this setting (4, 5, 18). Additionally, linear compounds are often considered higher risk than macrocyclic for the induction of kidney injury (19). Therefore, the variability in chemical structure between various GBCAs (i.e., macrocyclic vs. linear) may also produce differential, radiation dose-dependent, dissociative properties.
CONCLUSIONS
Our data suggest that in mice with no previous kidney disease, the addition of GBCAs (gadobutrol) with irradiation does not increase the risk for acute kidney injury based on the analysis of functional markers for AKI. Additional investigation of multiple doses of GBCA administered concurrently with irradiation is warranted to evaluate the risk of chronic kidney injury.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH grant nos. T32 CA078586, P01 CA217797, R01 CA169046, R01 CA182804) and a Gateway for Cancer Research grant (no. G-17-1500). Core facilities were supported in part by the Carver College of Medicine and the Holden Comprehensive Cancer Center (NIH grant no. P30 CA086862). The Radiation and Free Radical Research Core provided invaluable support in the completion of this work. The content is solely the responsibility of the authors and does not represent views of the National Institutes of Health.
REFERENCES
- 1.Winkel D, Bol GH, Kroon PS, van Asselen B, Hackett SS, Werensteijn-Honingh AM, et al. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin Transl Radiat Oncol 2019; 18:54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, Wan L, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med 2019; 8:2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 2009; 30:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mapuskar KA, Wen H, Holanda DG, Rastogi P, Steinbach E, Han R, et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol 2019; 20:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien C-C, Wang H-Y, Wang J-J, Kan W-C, Chien T-W, Lin C-Y, et al. Risk of acute kidney injury after exposure to gadolinium-based contrast in patients with renal impairment. Ren Fail 2011; 33:758–64. [DOI] [PubMed] [Google Scholar]
- 6.Ozkok S, Ozkok A. Contrast-induced acute kidney injury: A review of practical points. World J Nephrol 2017; 6:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 2016; 29:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich MC, Kuhlmann MK, Kohlbacher S, Scheer M, Grgic A, Heckmann MB, et al. Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: In vitro study. Radiology 2007; 242:425–34. [DOI] [PubMed] [Google Scholar]
- 9.Elmstahl B, Nyman U, Leander P, Chai C-M, Golman K, Bjork J, et al. Gadolinium contrast media are more nephrotoxic than iodine media. The importance of osmolality in direct renal artery injections. Eur Radiol 2006; 16:2712–20. [DOI] [PubMed] [Google Scholar]
- 10.Akgun H, Gonlusen G, Cartwright J Joiner, Suki WN, Truong LD. Are gadolinium-based contrast media nephrotoxic?: A renal biopsy study. Arch Pathol Lab Med 2006; 130:1354–7. [DOI] [PubMed] [Google Scholar]
- 11.Young LK, Matthew SZ, Houston JG. Absence of potential gadolinium toxicity symptoms following 22,897 gadoteric acid (Dotarem(R)) examinations, including 3,209 performed on renally insufficient individuals. Eur Radiol 2019; 29:1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howles GP, Bing KF, Qi Y, Rosenzweig SJ, Nightingale KR, Johnson GA. Contrast-enhanced in vivo magnetic resonance microscopy of the mouse brain enabled by noninvasive opening of the blood-brain barrier with ultrasound. Magn Reson Med 2010; 64:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasung ME, Chawla LS, Madero M. Biomarkers of renal function, which and when? Clinica Chimica Acta 2015; 438:350–7. [DOI] [PubMed] [Google Scholar]
- 14.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 2015; 10:147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Yuan L, Yang X, Wang K. La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem Biol Interact 2003; 146:27–37. [DOI] [PubMed] [Google Scholar]
- 16.Skrypnyk NI, Siskind LJ, Faubel S, de Caestecker MP. Bridging translation for acute kidney injury with better preclinical modeling of human disease. Am J Physiol Renal Physiol 2016; 310:F972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol 2008; 51:1419–28. [DOI] [PubMed] [Google Scholar]
- 18.Elmholdt T, Pedersen M, Jorgensen B, Sondergaard K, Jensen J, Ramsing M, et al. Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: a case-control study from Denmark. Br J Dermatol 2011; 165:828–36. [DOI] [PubMed] [Google Scholar]
- 19.Schieda N, Blaichman JI, Costa AF, Glikstein R, Hurrell C, James M, et al. Gadolinium-based contrast agents in kidney disease: A comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis 2018; 5:2054358118778573. [DOI] [PMC free article] [PubMed] [Google Scholar]


