Abstract
Background:
Given the persistent stigma and discrimination against HIV worldwide, preventive HIV vaccine trials face unique challenges. Negative social impacts (NSIs) - problems that HIV vaccine trial participants face in many different spheres of their lives related to trial participation – have received a great deal of attention. Beneficial social impacts (BSIs) - perceived benefits experienced by a participant and resulting from their trial participation - are a critical component of participants’ experiences, yet they have received little attention.
Setting:
All HVTN trial participants for whom social impact data were available - 8,347 participants in 13 countries who enrolled in 48 phase 1, 2a and 2b trials
Methods:
A cross protocol analysis to assess self-reported BSIs and NSIs related to participating in a preventive HIV vaccine trial. Data were obtained from 48 completed HVTN vaccine trials from December 2000 to September 2017
Results:
Overall 6572 participants (81%) reported at least one BSI and 686 participants (8%) reported 819 NSI events. Altruism/feeling good helping others was the BSI most often endorsed by study participants (43%) followed by receiving risk reduction counseling (30%). The majority of NSI events (81%) were reported by US/Swiss participants and most (79%) trial related NSIs were negative reactions from friends, family, and partners. Of the NSIs reported, 7% were considered to have a major impact on the participant’s quality of life.
Conclusion:
Our results underscore the relatively common experiences of BSIs among preventive HIV vaccine trial participants and mirror the results of other studies that find infrequent reports of NSIs.
Keywords: social impact, research participation, HIV vaccine, clinical trials
Preventive HIV vaccine trials are faced with unique challenges given the persistent stigma and discrimination against people living with HIV worldwide.1,2 The problems that HIV vaccine participants face in multiple different spheres of their lives related to trial participation are referred to by different terms including social impacts, adverse social events and trial related discrimination.3–6 Beneficial social impacts (BSIs) include any perceived benefit experienced by a participant as a result of their participation in a trial and attributable to their trial participation. Negative social impacts (NSIs) include impact on personal relationships, stigma or discrimination resulting from disclosure of vaccine trial participation, and in rare instances challenges related to vaccine induced seropositivity (VISP).7,8 VISP is a particular concern for HIV vaccine recipients. Participants who are in the vaccine treatment arm, as opposed to getting a placebo, may develop HIV antibodies detectable on standard ELISA screening tests, although they are HIV uninfected.9 Distinguishing VISP from HIV infection may require nucleic acid-based tests (RNA PCR, DNA PCR) that require additional laboratory resources, and time, at greater cost.10 HIV antibodies are expected to be induced in the majority of participants receiving experimental vaccines currently in the clinical trials pipeline. A greater understanding of participant experiences of negative and beneficial social impacts is required for a number of reasons. First, these data inform processes and procedures that promote positive participant experiences across the Network. Second, knowledge of social impacts assists in identifying strategies to mitigate negative impacts. Finally, these data ensure adequate trial participation that supports the successful development of a preventive HIV vaccine.
Preventing and addressing NSIs is a priority and the HIV Vaccine Trials Network (HVTN) adheres to a NIAID-developed model to prevent and resolve social impacts related to study participation.7 NSIs have been documented in different phases of HIV vaccine trials, in different geographical regions and are not limited to populations with increased vulnerability to HIV.6,11,12 NSIs may also persist or occur even when active participation has ended.13 Although NSIs are rarely life-altering when they occur,6,14,15 the way they are dealt with is important. One study found that participants’ willingness to participate in HIV vaccine trials was influenced by what they heard about trial participation.16 Across most studies assessing expressed willingness to participate in a hypothetical HIV vaccine trial, the potential for NSIs was consistently ranked high as a deterrent to participation.2,17,18 These concerns have the potential to override altruistic motives, although there is limited evidence of the effect they may have on actual enrollment in HIV vaccine trials.
Although BSIs associated with HIV vaccine trial participation are a critical component of participants’ experiences, they have received little attention. Due to the rigorous consent process and counselling required for participation, increase in knowledge is a benefit that often results from trial participation.19 Trial participants also report feeling good about playing an important role in the search for an effective vaccine, and view their participation as a means of giving back to society.19,20 In addition, participants who may know someone living with HIV, or have lost loved ones to HIV, may view participation as a way to honor their loved ones.21,22 Some participants have also enjoyed positive recognition for their role, increasing their feeling of social importance.7
While there is hope for an efficacious HIV vaccine, the length of time needed to make it a reality is unknown, and it is possible that HIV vaccine trials will continue for many years. To achieve this goal, it is imperative that we continuously characterize the experiences reported by HIV vaccine trial participants, implement mechanisms to amplify the beneficial and reduce the negative impacts, and ensure that negative impacts are handled in the best way possible by trial staff. The published data on NSIs and BSIs of trial participation are limited, especially for sub-Saharan Africa where there are fewer phase 1 and 2 trials, resulting in fewer opportunities to collect data in a region disproportionately impacted by HIV.12,13,20,26 Furthermore some of the studies conducted have been limited by small sample size, inclusion of volunteers at lower risk of HIV only and choice of study design (e.g., retrospective in nature, anonymous convenience sampling).6,11–14
Our analysis aims to broaden understanding of social impacts (BSIs and NSIs) associated with preventive HIV vaccine trials and serves as a resource to other researchers and stakeholders. Our analysis eliminates some of the restrictions ascribed to other social impact studies by including all HVTN trial participants for whom social impact data were available - over 8,000 participants in 13 countries who enrolled in 50 phase 1, 2a and 2b trials. We explore all BSIs and NSIs, describe the most common ones, highlight differences in the reporting of these events by region and study phase, and provide recommendations for future HIV vaccine trials based on our findings.
METHODS:
Study design
A cross protocol analysis was employed to assess self-reported BSIs and NSIs related to participating in a preventive HIV vaccine trial. Data were obtained from 48 completed vaccine trials conducted by the HVTN from December 2000 to September 2017, and included 43 phase 1, 3 phase 2a, and 2 phase 2b trials sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), or for which NIAID provided full regulatory and operational support. Participants were enrolled from 43 clinical research sites in Botswana, Brazil, Haiti, Jamaica, Peru, South Africa, Switzerland, Thailand, Trinidad and Tobago, and the United States. The sites were predominantly located in large and medium-sized cities with accessible means of transportation.
Study participants
Eligibility varied by protocol, but in general participants were aged 18 to 50 years (6 were aged 51–58) and deemed to be in overall good health as determined by physical exam, laboratory tests, and medical history. Participants in phase 1 trials were at low risk of acquiring HIV infection; those in phase 2b trials were at higher risk; and participants in phase 2a trials were a mix of low or higher risk depending on the trial.
Data collection
At enrollment and subsequent follow-up study visits, site staff asked participants whether they had experienced any social impacts related to trial participation during screening or follow-up. Participants were instructed to call the clinic staff if an incident occurred between scheduled visits. For each report of a social impact, clinic staff completed a Social Impact Log (SIL) that detailed the incident. At specific time points specified in each protocol, clinic staff also administered a Social Impact Assessment (SIA) questionnaire that asked a series of yes/no questions regarding situations in which perceived NSIs might occur and also asked whether the participant had experienced any BSIs related to trial participation. The specific NSI questions asked about problems in matters of personal relationships, travel, education, medical or dental treatment, health insurance, life insurance, housing, military or other government agency, and any other area not specified. For each affirmative answer, a SIL was completed if the NSI had not been previously reported. These multiple opportunities to report NSI events established confidence that data were collected on events of interest and significance.
Site clinical staff were provided with instructions and an internet-based training module on completing the SIA and SIL. The schedule for administering the SIA varied by protocol, but in general included an early, mid-study and end-of-study assessment. The SIL included: a description of the social impact; onset date; the participant’s rating of the effect of the event on their quality of life (minimal, moderate, or major disturbance); action taken by the participant, clinical site staff, and others to resolve the event; resolution status; whether the situation involved disclosure of HIV vaccine trial participation (and how participation was disclosed); whether HIV testing outside of the study clinic was an issue; and if an HIV test was performed. Study participants self-reported “impact on quality of life” as “no significant impact”, “minimal disturbance”, “moderate disturbance or “major disturbance with significant impact.”
To assess BSIs, the SIA questionnaire asked, “Has participation in the study had a beneficial impact on your life?” Response categories were yes, no and don’t know. In earlier protocols, the type of benefit a participant mentioned was an open-ended response captured in a text field. In later protocols, the form was modified, and staff coded the benefit into check box categories (i.e., personal relationships, feel good helping others, medical care, risk reduction counseling, or other). For the response category of ‘other,’ text describing the benefit was collected. Due to this difference in data collection, a new codebook was developed with expanded categories for the different types of benefits, and text answers to the benefits question were coded using the new codebook.
To develop the codebook, three authors (FS, MPA & LO) independently reviewed all text data and identified data that belonged in one of the existing SIA categories. After independently coding the data, the three authors convened to discuss category assignment. Any discrepancies in assignment were discussed until consensus was reached. For these data, eight new categories were created: (1) increased awareness and knowledge about HIV; (2) increased awareness and knowledge about research; (3) compensation/incentives; (4) connection to site staff; (5) personal satisfaction; (6) advancing science; (7) general increased awareness/knowledge/education/gained information; and (8) facilitating conversation.
For 1,443 (17.7%) of participants, a social benefit was reported, but the text description on the CRF was not entered in the database so the type of benefit could not be determined. These were coded as ‘not specified’ so that they could be included in analyses of any BSI. For 18 (37.5% of studies, 17 phase 1, 1 phase 2a) protocols, all of the benefits were unspecified, and for 6 (12.5% of studies, 6 phase 1, 1 phase 2a) protocols, some percentage of benefits were unspecified.
Statistical Analysis
Assessment of NSIs occurred at all visits and descriptive data are presented on the characteristics of all reported NSIs. Reporting of a BSI was limited to specific study visits specified in protocols where a SIA questionnaire was administered. This introduces problems in comparing BSIs across protocols and time as the SIA schedules varied by protocol and some participants missed their assessment visits. For these reasons, we summarized SIA questionnaire data at a participant level for analyses of BSIs and excluded participants who were never given an assessment. For the analysis, participants who responded ‘yes’ to the benefits question on at least one questionnaire were compared to those who responded, ‘don’t know’ and ‘no.’ Participants could report multiple BSIs and NSIs at each assessment.
To simplify analysis, countries were grouped into regions: three based on geographic locations (Africa, Caribbean, South America) and a fourth by similarity of culture and standard of living (United States and Switzerland combined). Participants from Thailand (N=12 phase 1 participants) were excluded from the analysis because they did not report BSIs or NSIs on their SIAs and SILs.
Differences in the distribution of categorical variables between groups were assessed with Chi-square tests. Multivariable logistic regression was used to assess whether participant characteristics (sex at birth, age at enrollment, race, ethnicity, trial phase, and receipt of an HIV vaccine or control product) were associated with each of the two endpoints, reporting an NSI or BSI. Because of regional differences, models for reporting an NSI and BSI were performed separately for each region. For each region and endpoint, all subset regression using Schwartz’s criteria was used to guide selection of the best model with statistically significant predictors. Confidence intervals and odds ratios (OR) are reported from the best models, with the groups used as references indicated. When more than one variable was a significant predictor, the adjusted OR (aOR) is reported. A 2-sided p-value of < 0.05 is considered statistically significant. There were no corrections for multiple hypothesis testing.
RESULTS:
Participant Characteristics
Among the 8347 participants enrolled, 5685 (68.1%) were assigned male at birth and 2662 (31.9%) were assigned female at birth. As seen in Table 1, the large majority of participants were residents of the United States/Switzerland (78%), White, non-Latinx (55%), Phase 1 participants (47%) and assigned to the vaccine treatment group (69%) with a median age of 27 years (IQR 22, 36).
Table 1.
Participant Characteristics by Region
| Asia (N=12) |
Caribbean (N=116) |
South America (N=255) |
Sub-Saharan Africa (N=1435) |
United States/ Switzerland (N=6529) |
Total (N=8347) |
|
|---|---|---|---|---|---|---|
| Sex at birth | ||||||
| Male | 9 (75.0%) | 63 (54.3%) | 135 (52.9%) | 751 (52.3%) | 4727 (72.4%) | 5685 (68.1%) |
| Female | 3 (25.0%) | 53 (45.7%) | 120 (47.1%) | 684 (47.7%) | 1802 (27.6%) | 2662 (31.9%) |
| Age at enrollment (years) | ||||||
| 18–24 | 1 (8.3%) | 36 (31.0%) | 118 (46.3%) | 894 (62.3%) | 2106 (32.3%) | 3155 (37.8%) |
| 25–34 | 5 (41.7%) | 47 (40.5%) | 77 (30.2%) | 496 (34.6%) | 2311 (35.4%) | 2936 (35.2%) |
| 35–44 | 6 (50.0%) | 26 (22.4%) | 43 (16.9%) | 40 (2.8%) | 1403 (21.5%) | 1518 (18.2%) |
| 45–60 (6 were 51–58) | 0 (0.0%) | 7 (6.0%) | 17 (6.7%) | 5 (0.3%) | 709 (10.9%) | 738 (8.8%) |
| Median (25th, 75th percentile) | 33 (27,40) | 28 (23,36) | 25 (21,34) | 23 (20, 27) | 28 (23, 38) | 27 (22, 36) |
| Min, Max | 24, 42 | 18, 58 | 18, 55 | 18, 55 | 18, 60 | 18, 60 |
| Race/ethnicity | ||||||
| Asian/Hawaiian/ Pacific Islander | 12 (100.0%) | 1 (0.9%) | 0 (0.0%) | 0 (0.0%) | 159 (2.4%) | 172 (2.1%) |
| Black, non-Hispanic/Latino | 0 (0.0%) | 98 (84.5%) | 1 (0.4%) | 1421 (99.0%) | 1039 (15.9%) | 2559 (30.7%) |
| Hispanic/Latino | 0 (0.0%) | 0 (0.0%) | 242 (94.9%) | 0 (0.0%) | 523 (8.0%) | 765 (9.2%) |
| White, non-Hispanic/Latino | 0 (0.0%) | 0 (0.0%) | 10 (3.9%) | 5 (0.3%) | 4542 (69.6%) | 4557 (54.6%) |
| Multiracial | 0 (0.0%) | 17 (14.7%) | 2 (0.8%) | 0 (0.0%) | 195 (3.0%) | 214 (2.6%) |
| Other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 9 (0.6%) | 66 (1.0%) | 75 (0.9%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (0.1%) | 5 (0.1%) |
| Trial phase | ||||||
| Phase 1 | 12 (100.0%) | 80 (69.0%) | 183 (71.8%) | 394 (27.5%) | 3264 (50.0%) | 3933 (47.1%) |
| Phase 2a | 0 (0.0%) | 36 (31.0%) | 72 (28.2%) | 240 (16.7%) | 761 (11.7%) | 1109 (13.3%) |
| Phase 2b Efficacy | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 801 (55.8%) | 2504 (38.4%) | 3305 (39.6%) |
| Treatment group | ||||||
| Vaccine | 10 (83.3%) | 78 (67.2%) | 199 (78.0%) | 845 (58.9%) | 4596 (70.4%) | 5728 (68.6%) |
| Control | 2 (16.7%) | 38 (32.8%) | 56 (22.0%) | 590 (41.1%) | 1933 (29.6%) | 2619 (31.4%) |
Beneficial Social Impacts of Trial Participation
Overall, 6572 participants (81%) reported at least one BSI (Table 2, Figure 1). Altruism/feeling good helping others was the BSI most often endorsed by study participants (43%) followed by receiving risk reduction counseling (30%) and the receipt of medical care or medical care information (including HIV testing) (29%). Statistically significant regional differences were observed in the number of participants reporting a BSI (p<0.0001). South American participants were least likely to report a BSI (57%) while sub-Saharan African participants were most likely to report a BSI (93%). Among participants who reported BSIs, US/Swiss participants were more likely to report altruism/feeling good helping others (50%) as a BSI, while sub-Saharan African participants reported receipt of medical care or medical care information as the most often experienced BSI (65%), followed by receipt of risk-reduction counseling (61%), and South American participants altruism/feeling good helping others (21%) as the most often experienced BSI, followed by reported receipt of medical care or medical care information (20%).There were small but statistically significant differences based on sex assigned at birth observed in the number of participants reporting a BSI across all regions, with females (79%) and males (81%) reporting similar experiences of BSIs (p=0.019). Within sub-Saharan Africa, men (93%) and women (94%) reported similarly high rates of BSIs, but within the United States/ Switzerland, men (80%) were more likely than women (76%) to report a benefit (p=0.0002). Phase 2b efficacy trial participants were more likely to report a BSI (86%) than phase 2a (73%) or phase I (78%) participants (p<0.0001).
Table 2.
Number of Participants Reporting Social Benefits by Type and Participant Characteristics
| Number of participants surveyed1 | Participants reporting a social benefit | Unspecified social benefit3 | Feel good helping others/altruism | Obtained risk-reduction counselling | Obtained medical care or information about medical care, including HIV testing | Improved personal relationships | Received monetary compensation, gifts, food or other incentives | Increased knowledge/awareness of HIV | Increased general knowledge, type not specified | Personal satisfaction/increased self esteem | Increased knowledge of research/vaccines | Contributed to advancing science/ending HIV | Facilitated conversations with others | Connection with site staff | Obtained PrEP information/referral | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | |
| Region | |||||||||||||||||
| Caribbean | 116 | 87, 75.0% | 34, 29.3% | 21, 18.1% | 19, 16.4% | 21, 18.1% | 4, 3.4% | 0, 0.0% | 30, 25.9% | 3, 2.6% | 9, 7.8% | 7, 6.0% | 3, 2.6% | 2, 1.7% | 0, 0.0% | 0, 0.0% | 0, 0.0% |
| South America | 252 | 144, 57.1% | 49, 19.4% | 54, 21.4% | 44, 17.5% | 51, 20.2% | 24, 9.5% | 0, 0.0% | 30, 11.9% | 8, 3.2% | 31, 12.3% | 4, 1.6% | 16, 6.3% | 5, 2.0% | 9, 3.6% | 0, 0.0% | 0, 0.0% |
| sub-Saharan Africa | 1398 | 1307, 93.5% | 242, 17.3% | 291, 20.8% | 855, 61.2% | 905, 64.7% | 263, 18.8% | 7, 0.5% | 431, 30.8% | 20, 1.4% | 9, 0.6% | 115, 8.2% | 13, 0.9% | 30, 2.1% | 11, 0.8% | 0, 0.0% | 2, 0.1% |
| United States/ Switzerland | 6366 | 5034, 79.1% | 1215, 19.1% | 3150, 49.5% | 1496, 23.5% | 1377, 21.6% | 1257, 19.7% | 841, 13.2% | 327, 5.1% | 414, 6.5% | 350, 5.5% | 188, 3.0% | 193, 3.0% | 132, 2.1% | 148, 2.3% | 45, 0.7% | 202, 3.2% |
| Total | 8143 | 6572, 80.7% | 1540, 18.9% | 3516, 43.2% | 2414, 29.6% | 2354, 28.9% | 1548, 19.0% | 848, 10.4% | 818, 10.0% | 445, 5.5% | 399, 4.9% | 314, 3.9% | 225, 2.8% | 169, 2.1% | 168, 2.1% | 45, 0.6% | 204, 2.5% |
| Sex at Birth | |||||||||||||||||
| Female | 2597 | 2057, 79.2% | 699, 26.9% | 773, 29.8% | 517, 19.9% | 629, 24.2% | 282, 10.9% | 181, 7.0% | 288, 11.1% | 57, 2.2% | 92, 3.5% | 95, 3.7% | 49, 1.9% | 33, 1.3% | 22, 0.8% | 0, 0.0% | 63, 2.4% |
| Male | 5546 | 4515, 81.4% | 841, 15.2% | 2743, 49.5% | 1897, 34.2% | 1725, 31.1% | 1266, 22.8% | 667, 12.0% | 530, 9.6% | 388, 7.0% | 307, 5.5% | 219, 3.9% | 176, 3.2% | 136, 2.5% | 146, 2.6% | 45, 0.8% | 141, 2.5% |
| Total | 8143 | 6572, 80.7% | 1540, 18.9% | 3516, 43.2% | 2414, 29.6% | 2354, 28.9% | 1548, 19.0% | 848, 10.4% | 818, 10.0% | 445, 5.5% | 399, 4.9% | 314, 3.9% | 225, 2.8% | 169, 2.1% | 168, 2.1% | 45, 0.6% | 204, 2.5% |
| Trial Phase | |||||||||||||||||
| Phase 1 | 3833 | 3002, 78.3% | 1151, 30.0% | 1404, 36.6% | 575, 15.0% | 661, 17.2% | 539, 14.1% | 345, 9.0% | 176, 4.6% | 104, 2.7% | 162, 4.2% | 79, 2.1% | 72, 1.9% | 32, 0.8% | 31, 0.8% | 0, 0.0% | 93, 2.4% |
| Phase 2a | 1079 | 790, 73.2% | 362, 33.5% | 268, 24.8% | 70, 6.5% | 72, 6.7% | 69, 6.4% | 85, 7.9% | 57, 5.3% | 25, 2.3% | 98, 9.1% | 24, 2.2% | 45, 4.2% | 6, 0.6% | 6, 0.6% | 0, 0.0% | 15, 1.4% |
| Phase 2b/ Efficacy | 3231 | 2780, 86.0% | 27, 0.8% | 1844, 57.1% | 1769, 54.8% | 1621, 50.2% | 940, 29.1% | 418, 12.9% | 585, 18.1% | 316, 9.8% | 139, 4.3% | 211, 6.5% | 108, 3.3% | 131, 4.1% | 131, 4.1% | 45, 1.4% | 96, 3.0% |
| Total | 8143 | 6572, 80.7% | 1540, 18.9% | 3516, 43.2% | 2414, 29.6% | 2354, 28.9% | 1548, 19.0% | 848, 10.4% | 818, 10.0% | 445, 5.5% | 399, 4.9% | 314, 3.9% | 225, 2.8% | 169, 2.1% | 168, 2.1% | 45, 0.6% | 204, 2.5% |
| Region - Sex at Birth | |||||||||||||||||
| sub-Saharan Africa -Female | 667 | 618, 92.7% | 123, 18.4% | 133, 19.9% | 395, 59.2% | 430, 64.5% | 116, 17.4% | 2, 0.3% | 208, 31.2% | 9, 1.3% | 5, 0.7% | 55, 8.2% | 6, 0.9% | 18, 2.7% | 8, 1.2% | 0, 0.0% | 1, 0.1% |
| sub-Saharan Africa - Male | 731 | 689, 94.3% | 119, 16.3% | 158, 21.6% | 460, 62.9% | 475, 65.0% | 147, 20.1% | 5, 0.7% | 223, 30.5% | 11, 1.5% | 4, 0.5% | 60, 8.2% | 7, 1.0% | 12, 1.6% | 3, 0.4% | 0, 0.0% | 1, 0.1% |
| US/Switzerland - Female | 1757 | 1335, 76.0% | 538, 30.6% | 609, 34.7% | 92, 5.2% | 167, 9.5% | 154, 8.8% | 179, 10.2% | 54, 3.1% | 44, 2.5% | 73, 4.2% | 35, 2.0% | 35, 2.0% | 12, 0.7% | 11, 0.6% | 0, 0.0% | 62, 3.5% |
| US/Switzerland - Male | 4609 | 3699, 80.3% | 677, 14.7% | 2541, 55.1% | 1404, 30.5% | 1210, 26.3% | 1103, 23.9% | 662, 14.4% | 273, 5.9% | 370, 8.0% | 277, 6.0% | 153, 3.3% | 158, 3.4% | 120, 2.6% | 137, 3.0% | 45, 1.0% | 140, 3.0% |
| Total | 7764 | 6341, 81.7% | 1457, 18.8% | 3441, 44.3% | 2351, 30.3% | 2282, 29.4% | 1520, 19.6% | 848, 10.9% | 758, 9.8% | 434, 5.6% | 359, 4.6% | 303, 3.9% | 206, 2.7% | 162, 2.1% | 159, 2.0% | 45, 0.6% | 204, 2.6% |
The denominator for the type of social benefit is the number of participants surveyed at least once for the category; a total of 204 enrolled participants missed all assessment visits (Africa=37, Asia=1, Caribbean=0, South America=3, US/Switzerland=163). Percentages may add to more than 100% due to report by a participant of multiple types of events.
The denominator for the total includes the 11 Asian participants surveyed. These participants did not report any social benefits.
Specific type of social benefits not assessed in all protocols.
Figure 1.
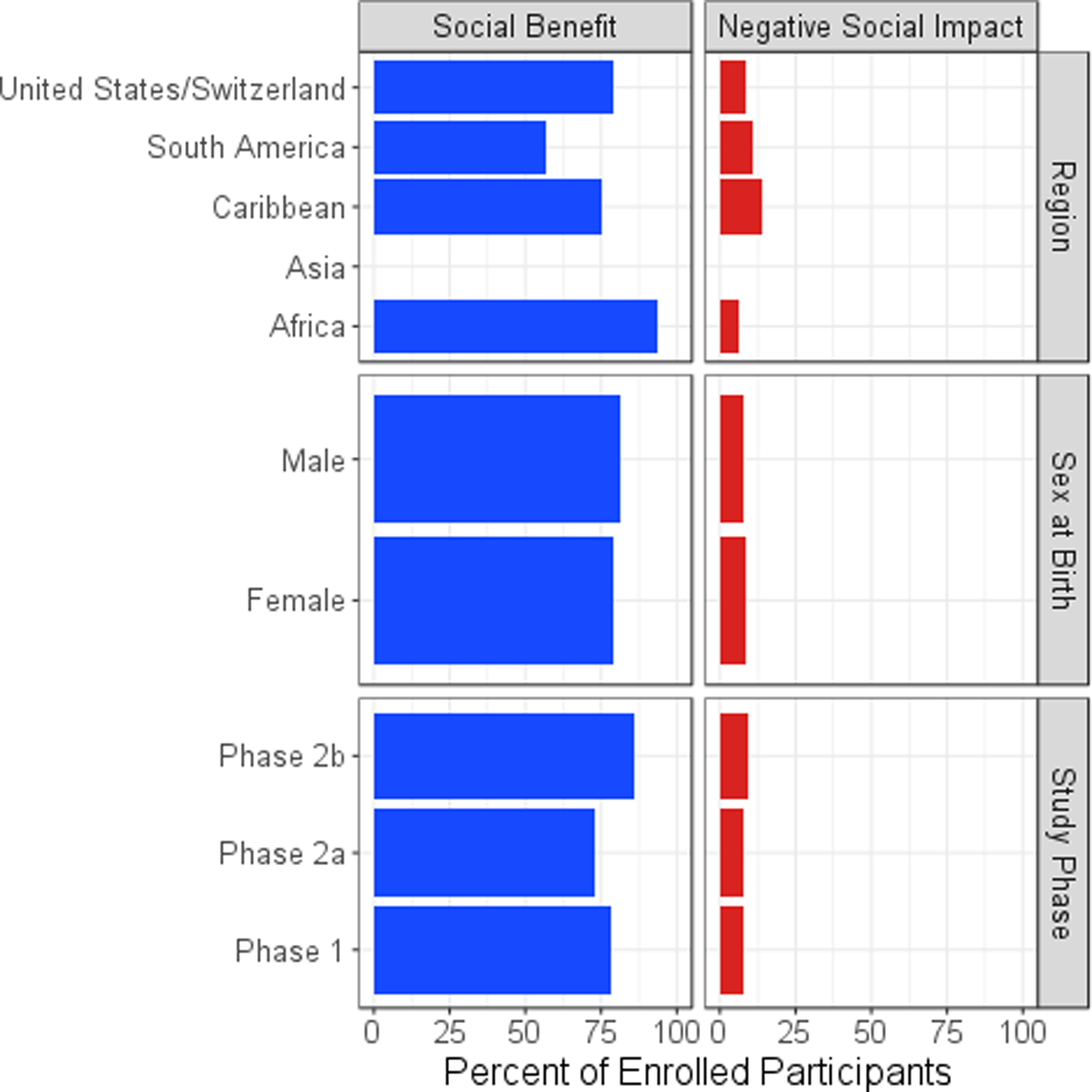
Percentage of Participants Reporting a Social Benefit or a Negative Social Impact by Participant Characteristics
Negative Social Impacts of Trial Participation
Overall, 686 participants (8%) reported 819 NSI events (Table 3, Figure 1). The majority of NSI events (81%) were reported by US/Swiss participants. Negative reactions from friends, family, and partners accounted for most (79%) trial related NSIs. These instances were primarily attributed to disagreements with the participant’s decision to join the study, worry about side effects, and misunderstandings of the participant’s HIV status or risk of infection. Employment related problems were the second most commonly reported NSIs, representing 6% of all reported NSIs. Combined, other NSIs, including problems with life insurance, medical or dental care, health insurance, travel/immigration, education, and military/government agencies were reported by less than 1.4% of participants (Table 4).
Table 3.
Number of Negative Social Impact Events Reported by Type and Participant Characteristics
| Number of events reported | No. participants reporting a negative social impact1 | Personal relationships | Employment | Medical/dental | Life insurance | Military/government agency | Travel/immigration | Education | Health insurance | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | N, % | |
| Region | |||||||||||
| Caribbean | 19 | 16, 13.8% | 15, 78.9% | 2, 10.5% | 0, 0.0% | 1, 5.3% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% |
| South America | 37 | 28, 11% | 27, 73.0% | 5, 13.5% | 2, 5.4% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 2, 5.4% |
| sub-Saharan Africa | 96 | 85, 5.9% | 75, 78.1% | 7, 7.3% | 8, 8.3% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 1, 1.0% | 0, 0.0% | 5, 5.2% |
| US/Switzerland | 667 | 557, 8.5% | 531, 79.6% | 34, 5.1% | 32, 4.8% | 16, 2.4% | 4, 0.6% | 3, 0.4% | 0, 0.0% | 1, 0.1% | 46, 6.9% |
| Total | 819 | 686, 8.2% | 648, 79.1% | 48, 5.9% | 42, 5.1% | 17, 2.1% | 4, 0.5% | 3, 0.4% | 1, 0.1% | 1, 0.1% | 53, 6.5% |
| Sex at Birth | |||||||||||
| Female | 267 | 229, 8.6% | 215, 80.5% | 14, 5.2% | 17, 6.4% | 4, 1.5% | 0, 0.0% | 2, 0.7% | 0, 0.0% | 0, 0.0% | 14, 5.2% |
| Male | 552 | 457, 8.0% | 433, 78.4% | 34, 6.2% | 25, 4.5% | 13, 2.4% | 4, 0.7% | 1, 0.2% | 1, 0.2% | 1, 0.2% | 39, 7.1% |
| Total | 819 | 686, 8.2% | 648, 79.1% | 48, 5.9% | 42, 5.1% | 17, 2.1% | 4, 0.5% | 3, 0.4% | 1, 0.1% | 1, 0.1% | 53, 6.5% |
| Study Phase | |||||||||||
| Phase 1 | 343 | 297, 7.6% | 275, 80.2% | 24, 7.0% | 15, 4.4% | 6, 1.7% | 1, 0.3% | 2, 0.6% | 0, 0.0% | 0, 0.0% | 18, 5.2% |
| Phase 2a | 106 | 89, 8.0% | 81, 76.4% | 8, 7.5% | 6, 5.7% | 1, 0.9% | 1, 0.9% | 0, 0.0% | 1, 0.9% | 0, 0.0% | 8, 7.5% |
| Phase 2b Efficacy | 370 | 300, 9.1% | 292, 78.9% | 16, 4.3% | 21, 5.7% | 10, 2.7% | 2, 0.5% | 1, 0.3% | 0, 0.0% | 1, 0.3% | 27, 7.3% |
| Total | 819 | 686, 8.2% | 648, 79.1% | 48, 5.9% | 42, 5.1% | 17, 2.1% | 4, 0.5% | 3, 0.4% | 1, 0.1% | 1, 0.1% | 53, 6.5% |
| Region - Sex at Birth | |||||||||||
| sub-Saharan Africa -Female | 62 | 55, 8.0% | 51, 82.3% | 2, 3.2% | 6, 9.7% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 3, 4.8% |
| sub Saharan Africa - Male | 34 | 30, 4.0% | 24, 70.6% | 5, 14.7% | 2, 5.9% | 0, 0.0% | 0, 0.0% | 0, 0.0% | 1, 2.9% | 0, 0.0% | 2, 5.9% |
| United States/ Switzerland - Female | 175 | 152, 8.4% | 140, 80.0% | 10, 5.7% | 10, 5.7% | 3, 1.7% | 0, 0.0% | 2, 1.1% | 0, 0.0% | 0, 0.0% | 10, 5.7% |
| United States/Switzerland- Male | 492 | 405, 8.6% | 391, 79.5% | 24, 4.9% | 22, 4.5% | 13, 2.6% | 4, 0.8% | 1, 0.2% | 0, 0.0% | 1, 0.2% | 36, 7.3% |
| Total, US/ Switzerland/ sub-Saharan Africa | 763 | 642, 8.1% | 606, 79.4% | 41, 5.4% | 40, 5.2% | 16, 2.1% | 4, 0.5% | 3, 0.4% | 1, 0.1% | 1, 0.1% | 51, 6.7% |
The denominator for the percentage of participants reporting a negative social impact is the total number of participants in each category. This includes the 12 Asian participants. These participants did not report negative social impacts.
The denominator for the type of negative social impact is the number of events reported in each category. Percentages may add to more than 100% due to report by a participant of multiple types of events.
Table 4.
Participants Reporting a Negative Social Impact by Region, Type and by Maximum Impact on Quality of Life1
| Maximum Impact on Quality of Life | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reported a negative social impact | Minimal | Moderate | Major | |||||
| N | % | N | % | N | % | N | % | |
| Any negative social impact | 686 | 8.2 | 503 | 6.0 | 134 | 1.6 | 49 | 0.6 |
| Type of negative social impact | ||||||||
| Personal relationships | 563 | 5.4 | 442 | 4.3 | 96 | 0.9 | 25 | 0.2 |
| Employment | 46 | 0.4 | 24 | 0.2 | 11 | 0.1 | 11 | 0.1 |
| Medical/dental | 41 | 0.4 | 27 | 0.3 | 8 | 0.1 | 6 | 0.1 |
| Life Insurance | 17 | 0.2 | 11 | 0.1 | 5 | <0.1 | 1 | <0.1 |
| Military/government agency | 4 | <0.1 | 2 | <0.1 | 1 | <0.1 | 3 | <0.1 |
| Travel/Immigration | 3 | <0.1 | 1 | <0.1 | 1 | <0.1 | 1 | <0.1 |
| Housing | 2 | <0.1 | 0 | 0.0 | 0 | 0.0 | 2 | <0.1 |
| Education | 1 | <0.1 | 1 | <0.1 | 0 | 0.0 | 0 | 0.0 |
| Health Insurance | 1 | <0.1 | 0 | 0.0 | 1 | <0.1 | 0 | 0.0 |
| Other | 53 | 0.5 | 29 | 0.3 | 21 | 0.2 | 3 | <0.1 |
| Region | ||||||||
| Asia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Caribbean | 16 | 0.2 | 9 | 7.8 | 1 | 0.9 | 6 | 5.2 |
| South America | 28 | 0.3 | 14 | 5.5 | 8 | 3.1 | 6 | 2.4 |
| sub-Saharan Africa | 85 | 1.0 | 56 | 3.9 | 17 | 1.2 | 12 | 0.8 |
| United States/Switzerland | 557 | 6.7 | 424 | 6.5 | 108 | 1.7 | 25 | 0.4 |
The denominator for all percentages is the number of participants, n=8347.
For participants reporting multiple events within a type category, the event with the maximum impact on quality of life is counted. Likewise, for the “Any negative social impact” row, the event with the maximum impact is counted.
For the majority of NSI events, the participant disclosed their trial participation voluntarily (81%). In 2 events (0.5%) an entity (i.e, an employer, the military) required HIV status, which led to disclosure. For other events (7%), participation was disclosed involuntarily by the participant or by others without participant approval, or others saw trial materials or overheard trial related conversations. A total of 154 (19%) events involved obtaining HIV testing, including pressure by a partner, medical or insurance provider, employer, or government entity to have an HIV test performed off-study.
Most of the reported NSIs were considered to have minimal or moderate impact on the participant’s quality of life. Of the 686 participants reporting an NSI event, 503 (73%) considered the impact minimal, 134 (20%) considered it moderate and 49 (7%) reported a major impact (Table 4). Although NSIs related to employment and medical/dental care were rare, these events were more likely to be described by participants as having moderate or major impacts on their quality of life. Although participants in the Caribbean and South America were less likely to report an NSI, the NSIs reported were slightly more likely to have a major impact on quality of life (5.2% and 2.4% endorsed as having a major impact on quality of life in the Caribbean and South America respectively as compared to 0.8% in sub-Saharan Africa and 0.4% in the United States/Switzerland).
Predictors of Beneficial and Negative Social Impact Reporting
Univariate and multipredictor models for the likelihood of an NSI event and the likelihood of reporting any BSI were run separately for the South American, sub-Saharan African, and the United States/Swiss cohorts. In the sub-Saharan African model, women had a greater likelihood of reporting an NSI than men (OR = 2.10, 95% CI: 1.34, 3.36). In the model of BSI, we found that participants in Phase 1 trials (aOR = 19.91, 95% CI: 9.08, 52.6) and Phase 2b trials (aOR = 7.72, 95% CI: 4.78, 12.74) were considerably more likely to report BSIs than Phase 2a trials, and participants who were older were less likely to report a BSI (aOR = 0.94, 95% CI: 0.90, 0.98).
In the US/Swiss NSI Model, white race (aOR = 0.78, 95% CI: 0.60, 1.00), Hispanic/Latinx ethnicity (aOR = 1.50, 95% CI: 1.13, 1.97), enrollment in an efficacy (phase 2b) trial (aOR = 1.49, 95% CI: 1.23, 1.79), and assignment to the vaccine treatment arm (aOR = 1.33, 95% CI: 1.08, 1.64) were associated with a greater likelihood of reporting an NSI. For the model of BSI in the US/Switzerland, higher BSI were reported in efficacy trials than Phase 1/2a trials overall, however there was a significant interaction between race and trial phase. In phase 1 trials, people who identified as black were less likely than white people to experience a benefit (aOR=0.66, 95% CI=0.54, 0.79), whereas people of other races were not significantly different from white people. In efficacy trials, however, people who identified as black were more likely to report a benefit than white people, (aOR=2.60, 95% CI: 1.82, 3.73), and people identifying as other races were also more likely to report a benefit than white people (aOR=1.99, 95% CI: 1.25, 3.18).
In South America, we did not find any significant predictors of reporting an NSI. Participants enrolled in phase 1 trials were more likely to report a BSI than participants in phase 2a trials (OR: 1.76, 95% CI: 1.02–3.06).
DISCUSSION:
Our results underscore the relatively common experiences of beneficial social impacts among preventive HIV vaccine trial participants with the large majority of participants experiencing at least one BSI as a result of their participation. Our data mirror the findings of Cleghorn and colleagues27 where 79% of Caribbean Island and South American participants in a HIVNET phase 2 HIV vaccine trial reported benefits from study participation. Among our participants, BSIs were common across regions, with sub-Saharan African participants being most likely to report a BSI. Psychological benefits – such as feeling good helping others and the positive feelings associated with altruistic behaviors – were the BSIs most often endorsed, and participants in the United States and Switzerland were more likely to endorse psychological benefits. Tangible benefits, such as receipt of medical care, medical information, or risk reduction counseling, were the most often endorsed benefits by participants in sub-Saharan Africa and South America. This is likely due to differences in access to resources and medical care across these regions. Our analysis included a small number of Thai participants and although none reported BSIs, other studies have found evidence of social and behavioral benefits to participation.28,29
Individuals assigned male sex at birth reported similarly high rates of BSIs as individuals assigned female sex at birth. In the United States and Switzerland, men were more likely than women to report a benefit. This may be due to the disproportionate impact of HIV on MSM communities in these countries, and the potentially higher likelihood to experience positive feelings when one perceives oneself as contributing to one’s community. This may also explain the greater likelihood of phase 2b efficacy trial participants, who have risk profiles placing them at higher vulnerability for HIV acquisition, to report a BSI than phase 2a and phase 1 participants. It may also be true that by virtue of having a higher risk profile, phase 2b efficacy trial participants obtain more value from risk reduction counseling and medical information and care.
Our findings mirror those of other studies6,10,18,23–25 that find infrequent reports of NSIs experienced by preventive HIV vaccine trial participants. Our study and others6,13,18,22,23 have found that the large majority of NSIs result from negative reactions from friends, family and partners. In our analyses, difficulties in interpersonal relationships accounted for 79% of all trial related NSIs. The large majority (73%) of these NSI events were reported to have a minimal impact on quality of life. Employment and medical dental care NSIs were rare, but when they did occur, were more likely to be experienced by Caribbean and South American participants and were described as having moderate or major impacts on quality of life. It is possible that study participants may hesitate to report NSI events due to concern that such reports could result in loss of tangible study benefits30,31. However, we have seen no evidence of this. Instead, it may be the case that, in certain settings, inadequate housing, limited employment opportunities and risk of violence overshadow potential negative social impacts of trial participation. Concerns may arise that possible loss of funding for future HIV prevention research31 may negatively affect conduct of ongoing studies, not specific to NSI data collection. To ensure NSI data is collected accurately, staff at clinical research sites receive extensive ongoing training on the importance of documenting NSIs. These trainings focus on the development of skills to build rapport and obtain sensitive information.
A number of study limitations should be acknowledged. First, for a large number of participants who reported a BSI, the database entries were missing details regarding the specific BSI experienced. This limits our ability to identify differences in types of BSIs experienced, and certain experiences are likely either not reported or under reported. Second, it is possible reporting of BSIs was affected by social response bias and potential staff influences. There is some evidence of this possible effect in the HVTN 503 BSI data, which included many data points with identical wording. Third, all social impact data were collected by interviewers, which may have negatively impacted reporting of an impact. The use of audio computer-assisted self-interviewing (ACASI), a system that allows for the anonymous collection of social impact data, may lead to more frequent reports of events, but also presents potential risk of inaccurate reporting Fourth, , our analysis does not include data from the Step study, a privately-sponsored phase 2b protocol that included 3000 volunteers at high risk for HIV infection. Finally, our findings are limited to specific geographic areas where preventive HIV vaccine trial participants were enrolled in HVTN trials. As such, they may not reflect the experiences of all types of trial volunteers or the wide range of global settings where clinical trials are conducted.
These data show infrequent reports of NSIs among participants in preventive HIV vaccine clinical trials. NSIs have received a great deal of attention and effective risk mitigation measures are well established.7 BSIs are acknowledged infrequently. These data are being utilized to update our informed consent form to accurately reflect the incidence of NSIs. Our data indicate that, when asked, most participants report experiencing BSIs, and indicate the need for more attention to benefits of participation, particularly in HIV prevention where stigma and discrimination persist.
Acknowledgments:
We thank the many study participants, study site staff, and investigators for their time and effort in successfully conducting the studies with data included in this analysis. The clinical trials reported in this analysis were conducted by the National Institute of Allergy and Infectious Diseases (NIAID) - funded HIV Vaccine Trials Network with the support of NIAID U.S. Public Health Service Grants UM1 AI068614 [LOC: HIV Vaccine Trials Network], UM1 AI068618 [Laboratory Center: HIV Vaccine Trials Network] and UM1 AI068635 [SDMC: HIV Vaccine Trials Network]. This paper was written by author M. Allen in her capacity as an NIH employee, but the views expressed in this paper do not necessarily represent those of the NIH. GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation.
Conflicts of Interest and Source of Funding:
This paper was written by author M. Allen in her capacity as an NIH employee, but the views expressed in this paper do not necessarily represent those of the NIH.
Funding Support:
NIH/NIAID 2UM1AI068614-08
Footnotes
Meetings with Data presentation:
HVTN Regional Meeting, Johannesburg, South Africa, February 2019
HVTN Full Group Meeting, Washington, DC, May 2019
Contributor Information
Michele P. Andrasik, Vaccine and Infectious Disease Division (VIDD), Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Fredericka Albertina Sesay, University of Washington.
Mary Allen, National Institute of Allergy and Infectious Diseases (NIAID).
References
- 1.UNAIDS. Global AIDS Update: Communities At The Centre 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf. Accessed August 10, 2019.
- 2.Newman PA, Duan N, Kakinami L, et al. What can HIV vaccine trials teach us about future HIV vaccine dissemination? Vaccine. 2008;26(20):2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belshe RB, Clements ML, Keefer MC, et al. Interpreting HIV serodiagnostic test results in the 1990s: Social risks of HIV vaccine studies in uninfected volunteers. Ann Intern Med. 1994;121(8):584–589. [DOI] [PubMed] [Google Scholar]
- 4.Grady C The search for an AIDS vaccine: Ethical issues in the development and testing of a preventive HIV vaccine. Indiana University Press; 1995. [Google Scholar]
- 5.Temoshok LR. Behavioral research contributions to planning and conducting HIV vaccine efficacy studies. AIDS Res Hum Retroviruses. 1994;10 Suppl 2:S277–80. [PubMed] [Google Scholar]
- 6.Allen M, Israel H, Rybczyk K, et al. Trial-related discrimination in HIV vaccine clinical trials. AIDS Res Hum Retroviruses. 2001;17(8):667–674. [DOI] [PubMed] [Google Scholar]
- 7.Allen M, Lau C. Social impact of preventive HIV vaccine clinical trial participation: A model of prevention, assessment and intervention. Soc Sci Med. 2008;66(4):945–951. [DOI] [PubMed] [Google Scholar]
- 8.Voronin Y, Zinszner H, Karg C, et al. HIV vaccine-induced sero-reactivity: A challenge for trial participants, researchers, and physicians. Vaccine. 2015;33(10):1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey SE. Unique risks to volunteers in HIV vaccine trials. J Investig Med. 2003;51(Suppl 1):S18–S20. [PubMed] [Google Scholar]
- 10.VISR Working Group of Global HIV Vaccine Enterprise, Voronin Y, Zinszner H, Karg C, et al. HIV Vaccine-Induced Sero-Reactivity: A challenge for trial participants, researchers, and physicians. Vaccine 2015;33(10):1243–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thapinta D, Jenkins RA, Celentano DD, et al. Evaluation of behavioral and social issues among thai HIV vaccine trial volunteers. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(3):308–314. [DOI] [PubMed] [Google Scholar]
- 12.Mutua G, Mutengu L, Mpendo J, et al. Major negative social impacts are rare in phase 1 HIV vaccine trials in Africa. AIDS Res Hum Retroviruses. 2014;30(S1):A190–A191. [Google Scholar]
- 13.Tarimo EA, Munseri P, Aboud S, Bakari M, Mhalu F, Sandstrom E. Experiences of social harm and changes in sexual practices among volunteers who had completed a phase I/II HIV vaccine trial employing HIV-1 DNA priming and HIV-1 MVA boosting in Dar es Salaam, Tanzania. PloS one. 2014;9(3):e90938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheon AR, Wagner L, McElrath MJ, et al. Preventing discrimination against volunteers in prophylactic HIV vaccine trials: Lessons from a phase II trial. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(5):519–526. [DOI] [PubMed] [Google Scholar]
- 15.Pitisuttithum P, Choopanya K, Bussaratid V, et al. Social harms in injecting drug users participating in the first phase III HIV vaccine trial in Thailand. J Med Assoc Thai. 2007;90(11):2442–2448. [PubMed] [Google Scholar]
- 16.Newman PA, Roungprakhon S, Tepjan S, Yim S. Preventive HIV vaccine acceptability and behavioral risk compensation among high-risk men who have sex with men and transgenders in thailand. Vaccine. 2010;28(4):958–964. [DOI] [PubMed] [Google Scholar]
- 17.Newman PA, Roungprakhon S, Tepjan S, Yim S, Walisser R. A social vaccine? social and structural contexts of HIV vaccine acceptability among most-at-risk populations in thailand. Global public health. 2012;7(9):1009–1024. [DOI] [PubMed] [Google Scholar]
- 18.Newman PA, Duan N, Rudy ET, Anton PA. Challenges for HIV vaccine dissemination and clinical trial recruitment: If we build it, will they come? AIDS Patient Care & STDs. 2004;18(12):691–701. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins RA, Thapinta D, Morgan PA, et al. Behavioral and social issues among volunteers in a preventive HIV vaccine trial in Thailand. J Acquir Immune Defic Syndr. 2005;40(5):592–599. [DOI] [PubMed] [Google Scholar]
- 20.Sugarman J, Stalter R, Bokoch K, et al. Positive social impacts related to participation in an HIV prevention trial involving people who inject drugs. IRB. 2015;37(1):17–19. [PMC free article] [PubMed] [Google Scholar]
- 21.Buchbinder SP, Metch B, Holte SE, et al. Determinants of enrollment in a preventive HIV vaccine trial: Hypothetical versus actual willingness and barriers to participation. J Acquired Immune Defic Syndromes. 2004;36(1):604–612. [DOI] [PubMed] [Google Scholar]
- 22.Nyaoke BA, Mutua GN, Sajabi R, et al. Volunteer motivators for participating in HIV vaccine clinical trials in Nairobi, Kenya. PloS one. 2017;12(9):e0183788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belshe RB, Stevens C, Gorse GJ, et al. Safety and immunogenicity of a canarypox-vectored human immunodeficiency virus Type 1 vaccine with or without gp120: a phase 2 study in higher- and lower-risk volunteers. J Infect Dis. 2001;183:1343–1352. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs J, Durham M, McLellan-Lemal E, et al. Negative Social Impacts Among Volunteers in an HIV Vaccine Efficacy Trial. J Acquir Immune Defic Syndr. 2007;46:362–368. [DOI] [PubMed] [Google Scholar]
- 25.Sugarman J, Statler R. Bokoch K, et al. Positive Social Impacts Related to Participation in an HIV Prevention Trial Involving people who inject drugs. Ethics Hum Res. 2015;37(1):17–19. [PMC free article] [PubMed] [Google Scholar]
- 26.Mutua G, Mutengu L, Mpendo J, et al. Major Negative Social Impacts are Rare in Phase I HIV Vaccine Trials in Africa. AIDS Res Hum Retroviruses. 2014;30(S1):A190–A191. [Google Scholar]
- 27.Cleghorn F, Pape JW, Schechter M, et al. Lessons From a Multisite International Trial in the Caribbean and South America of an HIV-1 Canarypox Vaccine (ALVAC-HIV vCP1452) With or Without Boosting With MN rgp120. J Acquir Immune Defic Syndr. 2007;46(2):222–230 [DOI] [PubMed] [Google Scholar]
- 28.Jenkins RA, Thapinta D, Morgan PA, et al. Behavioral and Social Issues Among Volunteers in a Preventive HIV Vaccine Trial in Thailand. J Acquir Immune Defic Syndr. 2005;40(5):592–599. [DOI] [PubMed] [Google Scholar]
- 29.Phanuphak P Teeratakulpixam S, Sarangbin S et al. International Clinical Trials of HIV Vaccines: I. Phase I Trial of an HIV-1 Synthetic Peptide Vaccine in Bangkok, Thailand. Asian Pacific J of Allergy & Immun. 1997;15:41–48. [PubMed] [Google Scholar]
- 30.Newman PA, Rubincam C, Slack C, et al. Towards a Science of Community Stakeholder Engagement in Biomedical HIV Prevention Trials: An Embedded Four-Country Case Study. PLoS One;10(8):e0135937. doi: 10.1371/journal.pone.0135937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Essack Z, Koen J, Slack C, Lindegger G, Newman PA. Civil society perspectives on negative biomedical HIV prevention trial results and implications for future trials. AIDS Care. 2012;24(10):1249–1254. [DOI] [PubMed] [Google Scholar]


