Abstract
Background
The coronavirus disease 2019 (COVID-19) patients with diabetes mellitus (DM) are at high risk of fatal outcomes. This meta-analysis quantifies the prevalence of mortality among (1) diabetic and (2) non-diabetic, and (3) the prevalence of DM, in hospitalized COVID-19 patients.
Methods
Published studies were retrieved from four electronic databases (PubMed, Embase, Scopus, and medRxiv) and appraised critically utilizing the National Heart, Lung, and Blood Institute’s tool. Meta-analyses were performed using the random-effects model. The measures of heterogeneity were ascertained by I- squared (I2) and Chi-squared (Chi2) tests statistics. Predictors of heterogeneity were quantified using meta-regression models.
Results
Of the reviewed 475 publications, 22 studies (chiefly case series (59.09 %)), sourcing data of 45,775 hospitalized COVID-19 patients, were deemed eligible. The weighted prevalence of mortality in hospitlized COVID-19 patients with DM (20.0 %, 95 % CI: 15.0–26.0; I2, 96.8 %) was 82 % (1.82-time) higher than that in non-DM patients (11.0 %, 95 % CI: 5.0–16.0; I2, 99.3 %). The prevalence of mortality among DM patients was highest in Europe (28.0 %; 95 % CI: 14.0–44.0) followed by the United States (20.0 %, 95 % CI: 11.0–32.0) and Asia (17.0 %, 95 % CI: 8.0–28.0). Sample size and severity of the COVID-19 were associated (p < 0.05) with variability in the prevalence of mortality. The weighted prevalence of DM among hospitalized COVID-19 patients was 20 % (95 % confidence interval [CI]: 15–25, I2, 99.3 %). Overall, the quality of the studies was fair.
Conclusions
Hospitalized COVID-19 patients were appreciably burdened with a high prevalence of DM. DM contributed to the increased risk of mortality among hospitalized COVID-19 patients compared to non-DM patients, particularly among critically ill patients. Registration: PROSPERO (registration no. CRD42020196589).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00779-2.
Keywords: Coronavirus infection; Diabetes mellitus; Diabetes mellitus, type 1; Diabetes mellitus, type 2
Submission statement: This manuscript is solely submitted to the Journal of Diabetes & Metabolic Disorders. We have not submitted this paper in part or full to any other journal.
Introduction
In December 2019, the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) emerged in Wuhan, Hubei Province, China, and the disease it caused is called coronavirus disease-2019 (COVID-19) [1–3]. The COVID-19 spread rapidly across continents, and by March 2020, World Health Organization declared the epidemic as a pandemic [4]. By October 04, 2020, the cumulative total COVID-19 cases and deaths reported worldwide exceeded 34.8 million and 1 million, respectively [5].
Diabetes mellitus (DM) is one of the most frequently reported comorbidities in COVID-19 patients that determine their risk of morbidity and mortality. The prevalence of DM in hospitalized COVID-19 patients in China and the United States (US) was 9.7 and 28.3 %, respectively [6, 7]. An Italian study depicted that the prevalence of DM in severe COVID-19 patients admitted in intensive care units (ICU) was 17 % [8]. Previously also, in the 2009-H1N1 pandemic influenza and the Middle East respiratory syndrome, DM was a crucial determinant of mortality [9, 10]. Existing studies have consistently reported increased mortality among COVID-19 patients with DM [11–15].
Research shows, mortality risk among hospitalized COVID-19 patients with DM has an independent association with clinical and biological predictors including age, micro- and macro-vascular complications of DM, shortness of breath, and decreased platelet count [16]. It’s hypothesized that the worse outcomes of COVID-19 patients with DM are attributable to the angiotensin-converting enzyme-2 receptor-mediated entry of the SARS-CoV-2 virus in the host cell, which damages the insulin-producing pancreatic islet cells [17–19].
Given this mortality risk in the SARS-CoV-2 infected DM patients, in the ongoing COVID-19 pandemic situation, it’s vital to estimate the epidemiological burden of mortality among hospitalized COVID-19 patients to ensure the implementation of evidence-based public health initiatives. Therefore, we systemically reviewed published literature and quantified the overall and subgroup weighted prevalence of mortality among diabetic (primary outcome) and non-diabetic (secondary outcome) and the weighted prevalence of DM (secondary outcome), in hospitalized COVID-19 patients.
Methods
This review was reported according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2009 guidelines [20]. The PRISMA checklist is provided in the supplementary information (Supplementary Table: Table S1). The protocol for this review is available in the PROSPERO (registration no. CRD42020196589) [21].
Inclusion criteria
Published research articles reporting quantified or quantifiable estimates on the prevalence of mortality in diabetic hospitalized COVID-19 patients were deemed eligible to be included in this systematic review, considering the following additional eligibility criteria:
Study design: observational, experimental, or case-series studies.
Setting and population: hospitalized COVID-19 patients with confirmed disease regardless of age or gender.
Ascertainment of COVID-19: reverse transcription-polymerase chain reaction (RT-PCR) testing, computer tomography (CT) imaging, clinical, or all.
Ascertainment of DM: accepted as per the study authors ascertainment.
Geographic origin: global.
Language: English only.
Exclusion criteria
We excluded published articles reporting suspected COVID-19 patients without further confirmatory testing (e.g., RT-PCR, CT imaging) or including COVID-19 patients with gestational DM. Editorials, letters, commentaries, and abstracts with no enough data or without full-text did not comprise the inclusion criteria.
Data source
Peer-reviewed publications were retrieved from three main electronic databases (PubMed, Embase, and Scopus). The last date of the search was June 30, 2020. The following strategy was used to search the PubMed database - “SARS-CoV-2” OR “Coronavirus” OR “COVID-19” AND “diabetes” NOT “MERS” NOT “Middle East respiratory syndrome.“ Following MeSH Terms were also used in the search: “coronavirus infection,” “diabetes mellitus,” “diabetes mellitus, type 1,” “diabetes mellitus, type 2.”
The literature search was also extended to a pre-print database (medRxiv). Bibliographies of the eligible publications were hand-searched for eligible studies that might have been missed.
Study selection, data abstraction, and risk of bias (RoB) assessment
After uploading the retrieved citations in the Rayyan systematic reviews software [22], the duplicates were removed. Then two reviewers (SS1 and SS2) independently skimmed the titles and abstracts of the remaining papers to assess their eligibility. A full-text reading ensued when the citations seemed to match fully or partially against the pre-stated eligibility criteria.
From the deemed-eligible publications, independently, two reviewers (SS1 and SS2) abstracted the necessary information and data. The abstracted information covered author name, year of publication, study design, study population, number of COVID-19 hospitalized patients with DM, number of deaths among COVID-19 hospitalized patients by the DM status, COVID-19 and DM diagnostic method, and the severity of the COVID-19. From same abstracted publications, whenever reported, additional data on the prevalence of DM and on the prevalence of mortality among non-DM hospitalized COVID-19 patients was also abstracted. Data abstraction was performed into a pre-defined data-extraction sheet. For each included publication, the quality and risk of bias (RoB) was determined, independently, by SS1 and SS2 using the study design-specific quality assessment tool of the National Heart, Lung, and Blood Institute [23].
For particular study types, we eliminated RoB components not applicable to those study designs. RoB components that were adequately addressed by the studies (i.e., the ‘yes’ responses) were scored as one and otherwise 0 (zero). Then, we determined the study design-wise and the overall percentage of scores. Out of the maximum possible ‘yes’ response scores, we categorized the achieved score by the studies as poor, fair, and good when it was between 0–25, 25–76, and 76–100 %, respectively. Disagreements in opinion among the review authors were resolved by discourse.
Evidence synthesis: meta‐analysis
In the meta-analysis, estimation of the weighted prevalence and its 95 % confidence interval (CI) was performed utilizing the DerSimonian and Laird random-effects model. The stabilization of variances in the prevalence estimates was determined by the exact binomial procedure and Freeman-Tukey double arcsine transformation, respectively [24]. Heterogeneity was estimated by I-squared (I2) (categorized as low, moderate, and high based on its values of 25 %, 50 %, and 75 %, respectively) and Chi-squared (Chi2) test statistics (statistically significant at p < 0.1). The predictive intervals estimated the prevalence of future studies.
Subgroup analysis
Subgroup wise weighted prevalence was determined for the country and continent, COVID-19 (RT-PCR only or multiple methods) and DM (specified or unclear) diagnostic method, DM type (type 1 or type 2), the severity of the COVID-19 infection (critically ill or not critically ill), and the sample size (< 100 versus ≥ 100).
Publication bias and heterogeneity assessment
Publication bias was assessed visually by generating funnel plots depicting prevalence against its standard error and statistically by Egger’s test. Heterogeneity was explored statistically by univariate and multivariate meta-regression (random-effects model). The statistical significance of univariate meta-regression analyses was determined at p < 0.1 and was performed for the following potential predictors – country, continent, study design, COVID-19 diagnosis method, COVID-19 severity, diagnostic method, and type of DM, and sample size. Statistically significant predictors from univariate models were included in the multivariate meta-regression model, and the statistical significance was determined at p < 0.05.
Sensitivity analysis
The overall pooled prevalence of the respective outcomes was re-estimated by dropping a study each time. STATA statistical software (version 16; StataCorp, College Station, Texas, USA) was used for all analyses.
Results
Scope of the review
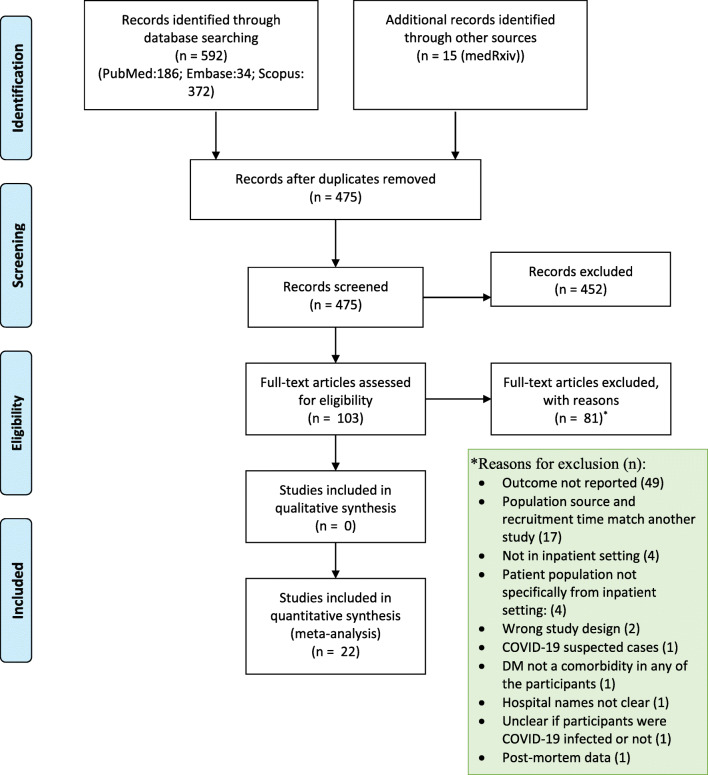
Out of the 607 retrieved citations from the four databases, 22 published articles [16, 25–45] were deemed eligible and included in this review (Fig. 1). The eligible articles reporting on inpatient COVID-19 patients between December 2019 and May 2020 from nine countries (China, India, Iran, Korea, Oman, France, Italy, UK, and the US) dispersed over three continents. Most of these were case series (59.09 %), and the remaining constituted of cross-sectional, case-control, and retrospective cohort studies, attributing to 14 % each.
Fig. 1.
Prisma flow diagram [20]
A total of 45,775 COVID-19 patients hospitalized in 995 hospitals were reported. Of them, 46.3 % were from the European nations, followed by 31.7 % from the US, while the remaining 20.0 % were from five Asian countries. RT-PCR was used solely in 45.4 % of the COVID-19 patients, whereas for the rest, a combination of lab-based, radiological, and clinical interpretation was used. Although 11,811 COVID-19 patients had DM, the type of it was specified in 14.5 % of these cases. Among the 225 DM patients with available COVID-19 severity information, 28.44 % were critically ill.
Salient features of the reviewed studies are presented in Table 1.
Table 1.
Salient features of the reviewed studies
| Author, year | Country, city | Study time period | Design | Follow up from day of hospitalization | Studied population | COVID-19 diagnosis | Sample size (hospitalized COVID-19 patients) |
DM type (diagnosis criteria) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 patients with DM n (%) |
Death among hospitalized COVID-19 patients with | ||||||||||
| DM n (%) |
No-DM n (%) |
||||||||||
| Alkundi et al. 2020 [25] | UK | 10/03 – 10/05, 2020 | Cross-sectional | NA | COVID-19 patients with a mean age of 70.5 years ±15. | RT-PCR | 232 | T1DM | 11 (4.7) | 6 (54.5) | 49 (33.8) |
| T2DM | 76 (32.8) | 34 (44.7) | |||||||||
| Mixed DM | – | – | |||||||||
| Unclear | – | – | |||||||||
| Cariou et al. 2020 [16] | France | 10/03 – 10/04, 2020 | Case series | 7 days | Hospitalized COVID-19 patients with diabetes of Mean age of 69.8 years + 13 | RT-PCR and/or clinically/radiologically by chest CT | 1317 | T1DM | 39 (3.0) | 2 (5.1) | - |
| T2DM | 1166 (88.5) | 127(10.9) | |||||||||
| Mixed DM | |||||||||||
| Unclear | 112 (8.5) | 11 (9.8) | |||||||||
| Bhandari et al. 2020 [36] | India | 01/03/2020-unlcear | Case series | Unclear (until submission date of the manuscript) | Unclear with a median (range) age 43.5 (2-85) years | RT-PCR | 21 | T1DM | - | - | 19 (0) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 2 (9.5) | 0 (0.0) | |||||||||
| Bode et al. 2020 [39] | US | 01/03 – 06/04, 2020 | Retrospective cohort | In DM patients: 3885 patient days; in non-DM patients: 3793 patient days | Unclear with age of DM and/or uncontrolled hyperglycemia: median (range): 65 (24-95); patients without DM and/or uncontrolled hyperglycemia: Median (range): 61 (18-101) | RT-PCR | 1122 | T1DM | - | - | 64 (6.2) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 88 (7.8) | 13 (14.8) | |||||||||
| Yu et al. 2020 [40] | China | 14/01 – 26/03, 2020 | Case control | NA | Dead and recovered hospitalized COVID-19 positive patients with median (IQR) age 64.0 (51.0–71.0) years | RT-PCR | 1464 | T1DM | - | - | 143 (11.4) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 211 (14.4) | 69 (32.7) | |||||||||
| Docherty et al. 2020 [41] | UK | 06/02 – 03/05, 2020 | Case series | >2 weeks | Inpatient COVID-19 cases with median age (IQR) 72.9 (58.0-82.0) years | RT-PCR and clinical | 20133 | T1DM | - | - | 3696 (24.3) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 4949 (24.6) | 1469 (29.7) | |||||||||
| Ciceri et al. 2020 [42] | Italy | 25/02 – 01/05, 2020 | Case series | >1 month | All adult COVID-19 cases admitted to the emergency department with median (IQR) age 65 (56-75) years | One or more: RT-PCR, clinically, and radiological findings suggesting COVID-19 pneumonia | 410 | T1DM | 8 (2) | 2 (25) | 73 (21.4) |
| T2DM | 61 (14.9) | 20 (32.8) | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | - | - | |||||||||
| Guan et al. 2020 [43] | China |
11/12 – 31/01, 2020 |
Cross-sectional | NA | Unclear with Mean age 48.9+16.3 years | RT-PCR | 1590 | T1DM | - | - | 37 (2.5) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 130 (8.2) | 13 (10) | |||||||||
| Lee et al. 2020 [44] | Korea |
18/02 – 04/03, 2020 |
Case series | >2 weeks | >65 years older hospitalized COVID-19 patients with median (IQR) age 72 (68.0–79.0) years | RT-PCR | 98 | T1DM | - | - | 9 (12.7) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 27 (27.6) | 11 (40.7) | |||||||||
| Zhao et al. 2020 [45] | China |
27/01 – 01/04, 2020 |
Case series | Until 01-Apr-2020 | First 29 severe COVID-19 cases admitted for treatment with median (IQR) age 56.0 (31.5–66.0) years | RT-PCR | 29 | T1DM | - | - | 1 (4.5) |
| T2DM | 7 (24.1) | 0 (0.0) | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | - | - | |||||||||
| Khamis et al. 2020 [26] | Oman |
24/02 – 24/04, 2020 |
Case series | Until 24-Apr-2020 | RT-PCR confirmed cases admitted to the hospital with mean age 48+16 years | RT-PCR | 63 | T1DM | - | - | 1 (2.3) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 20 (31.7) | 4 (20.0) | |||||||||
| Marcello et al. 2020 [27] | US | 05/03 – 16/04, 2020 | Case series | Until 16-Apr-2020 | RT-PCR confirmed cases with median (IQR) age 61 (49.7-72.9) years | RT-PCR | 6248 | T1DM | - | - | 1145 (27.2) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 2045 (32.7) | 597 (29.2) | |||||||||
| Zhang et al. 2020 [28] | China |
09/01 – 19/02, 2020 |
Case control | NA | Critically ill COVID-19 pneumonia patients with mean age 64.4 + 11.0 years | RT-PCR | 60 | T1DM | - | - | 6 (11.8) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 9 (15.0) | 4 (44.4) | |||||||||
| Nikpouraghdam et al. 2020 [29] | Iran |
19/02 – 15/04, 2020 |
Cross-sectional | NA | Unclear with mean age 55.5+15.15 years | One or more: RT-PCR or clinically by CT-scan | 2964 | T1DM | - | - | 228 (8.0) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 113 (3.8) | 11 (9.7) | |||||||||
| Richardson et al. 2020 [30] | US |
01/03 – 04/04, 2020 |
Case series | Until 04-Apr-2020 | COVID-19 cases admitted to the hospital with median (range) age 63 (0-107) years | RT-PCR | 5700 | T1DM | - | - | 329 (8.5) |
| T2DM | 1808 (31.7) | 224 (12.4) | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | - | - | |||||||||
| Rosenberg et al. 2020 [31] | US |
15/03 – 24/04, 2020 |
Case series | Until 24-Apr-2020 | COVID-19 cases admitted to the hospital with median age 63 years | RT-PCR | 1438 | T1DM | - | - | 158 (16.9) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 504 (35.0) | 134 (26.6) | |||||||||
| Shi et al. 2020 [32] | China |
01/01 – 08/03, 2020 |
Case control | NA | COVID-19 cases with DM who were discharged or admitted to the hospital. Patients with DM: median (IQR) age 64.0 (56.0–72.0) years; Patients without DM median (IQR) age 65.0 (56.0–72.0) years | RT-PCR | 306 | T1DM | - | - | 16 (10.5) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 153 (50.0) | 31 (20.3) | |||||||||
| Wang et al. 2020 [33] | China |
07/02 – 22/02, 2020 |
Case series | Until 22-Feb-2020 | Non-critically ill admitted COVID-19 cases with median (IQR) age 50 (39-58) years | RT-PCR | 1012 | T1DM | - | = | 0 (0.0) |
| T2DM | - | = | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 27 (2.7) | 0 (0.0) | |||||||||
| Cen et al. 2020 [34] | China |
10/02 – 08/03, 2020 |
Case series | 28 days | Mild or moderately ill admitted COVID-19 cases with median (IQR) age 61 (49-68) years | RT-PCR | 1007 | T1DM | - | - | 31 (3.5) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 119 (11.8) | 12 (10.1) | |||||||||
| Zhang et al. 2020 [35] | China | 29-Jan | Retrospective cohort | Until 12-Mar-2020 | All COVID-19 patients admitted with median (IQR) age 64 (56-70) years | RT-PCR | 258 | T1DM | - | - | 8 (4.1) |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 63 (24.4) | 7 (11.1) | |||||||||
| Yan et al. 2020 [37] | China |
10/01 – 24/02, 2020 |
Retrospective cohort | Unclear | Hospital admitted severe COVID-19 cases with median (IQR) age 64 (49-73) years | RT-PCR and chest CT | 193 | T1DM | - | - | 69 (47.6) |
| T2DM | 48 (24.9) | 39 (81.3) | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | - | - | |||||||||
| Jang et al. 2020 [38] | Korea |
19/02 – 15/04, 2020 |
Case series | Until 15-Apr-2020 | >18 years COVID-19 cases admitted via emergency room or outpatient department with mean age 56.9+17.0 years. | RT-PCR | 110 | T1DM | - | - | CD |
| T2DM | - | - | |||||||||
| Mixed DM | - | - | |||||||||
| Unclear | 15* (13.6) | 0 (0.0) | |||||||||
*only for those diabetes mellitus cases with available mortality data
Abbreviations: CD: cannot determine; DM: diabetes mellitus; NA: not applicable; RT-PCR: reverse transcriptase-polymerase chain reaction
Weighted prevalence of DM
The weighted prevalence of DM among hospitalized COVID-19 patients was 20.0 % (95 % CI: 15.0–25.0; I2 = 99.3 %) (Table 2). The joint prevalence of DM in Italy and the UK was similar to that in the US (26 %) but higher than that in five Asian countries (17.0 %). Of the hospitalized COVID-19 patients, 29 %, 24 %, and 18 % were with type 2 DM, type 1DM, or the type of DM was unclear (presumably type 2 DM). The prevalence of DM in critically ill COVID-19 patients was 2.75-time higher than those not critically ill (Table 2).
Table 2.
Overall and subgroup wise prevalence of diabetes mellitus in hospitalized COVID-19 patients
| Subgroup | Category | Number of Studies | Number of admitted COVID-19 patients | Number of DM patients | Mean prevalence of DM | 95 % prediction interval | Heterogeneity measures | ||
|---|---|---|---|---|---|---|---|---|---|
| % | 95 % CI | I2 (%) | Q (p-value) | ||||||
| Continent | Asia | 14 | 9,175 | 944 | 17.0 | 11.0–24.0 | 0.0–48.0 | 98.13 | < 0.01 |
| Europe | 3 | 20,775 | 5105 | 26.0 | 18.0–34.0 | - | - | - | |
| North America | 4 | 14,508 | 4445 | 26.0 | 17.0–35.0 | 0.0–75.0 | 99.31 | < 0.01 | |
| Country | China | 9 | 5,919 | 767 | 18.0 | 10.0–26.0 | 0.0–54.0 | 98.26 | < 0.01 |
| India | 1 | 21 | 2 | 10.0 | 1.0–30.00 | Inestimable | - | - | |
| Iran | 1 | 2,964 | 113 | 4.0 | 3.0–5.0 | Inestimable | - | - | |
| Italy | 1 | 410 | 69 | 17.0 | 13.0–21.0 | Inestimable | - | - | |
| Korea | 2 | 208 | 42* | 20.0 | 15.0–25.0 | Inestimable | - | - | |
| Oman | 1 | 63 | 20 | 32.0 | 21.0–45.0 | Inestimable | - | - | |
| UK | 2 | 20,365 | 5036 | 25.0 | 24.0–25.0 | Inestimable | - | - | |
| US | 4 | 14,508 | 4445 | 26.0 | 17.0–35.0 | 0.0–75.0 | 99.31 | < 0.01 | |
| COVID-19 diagnosis | RT-PCR | 17 | 20,758 | 5315 | 21.0 | 15.0–28.0 | 1.0–55.0 | 99.13 | < 0.01 |
| Multiple modes | 4 | 23,700 | 5179 | 16.0 | 4.0–34.0 | 0.0–98.0 | 99.72 | < 0.01 | |
| DM diagnosis | Method specified | 4 | 1,918 | 391 | 28.0 | 9.0–53.0 | 0.0–100 | 99.02 | < 0.01 |
| Unclear | 17 | 42,540 | 10,103 | 18.0 | 13.0–24.0 | 1.0–48.0 | 99.40 | < 0.01 | |
| DM types | Mixed T1DM patients and T2DM | 2 | 642 | 156 | 24.0 | 20.0–27.0 | Inestimable | - | - |
| Type 2 | 3 | 5,922 | 1863 | 29.0 | 23.0–34.0 | Inestimable | - | - | |
| Unclear | 16 | 37,894 | 8475 | 18.0 | 12.0–25.0 | 0.0–51.0 | 99.44 | < 0.01 | |
| COVID-19 severity | Critically ill | 3 | 282 | 64 | 22.0 | 16.0–28.0 | Inestimable | - | - |
| Not Critically ill | 3 | 2,129 | 161 | 8.0 | 2.0–18.0 | Inestimable | - | - | |
| Unclear | 15 | 42,047 | 10,269 | 22.0 | 17.0–29.0 | 3.0–53.0 | 99.42 | < 0.01 | |
| Sample size | < 100 | 12 | 3,608 | 462 | 18.0 | 11.0–27.0 | 0.0–56.0 | 96.80 | < 0.01 |
| ≥ 100 | 9 | 40,850 | 10,032 | 22.0 | 15.0–30.0 | 2.0–55.0 | 99.65 | < 0.01 | |
| Overall | NA | 21 | 44,458 | 10,494 | 20.0 | 15.0–25.0 | 0.02–0.50 | 99.33 | < 0.01 |
*only diabetes patients for whom mortality data was available were included
Weighted prevalence of mortality in diabetic and non‐diabetic patients
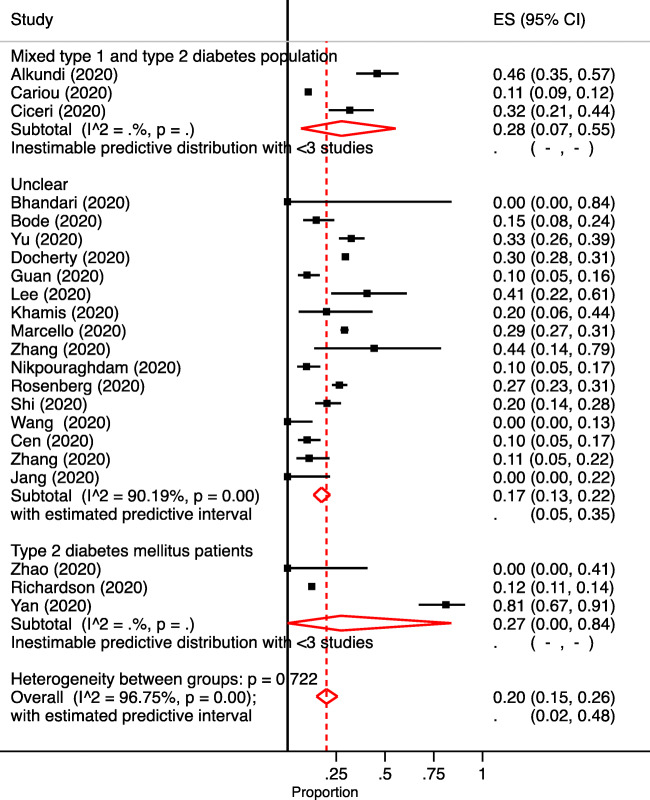
The weighted prevalence of mortality was 1.82-time higher in DM (20.0 %, 95 % CI: 15.0–26.0; I2, 96.8 %) (Fig. 2) than non-DM (11.0 %, 95 % CI: 6.0–16.0; I2, 99.32 %) hospitalized COVID-19 patients (Table 3). The prevalence of mortality in COVID-19 patients with DM in France, Italy, and UK was higher (28 %; 95 % CI: 14.0–44.0) than the US (20.0 %, 95 % CI: 11.0–32.0) and the Asian countries (17.0 %, 95 % CI: 8.0–28.0) (Table 3).
Fig. 2.
Forest plot depicting the overall and diabetes mellitus (DM) type-wise prevalence of mortality in hospitalized COVID-19 patients with DM. The diamond is centred on the summary of the prevalence estimate, and the width indicates the corresponding 95 % CI
Table 3.
Overall and subgroup wise prevalence of mortality among hospitalized COVID-19 patients with and without diabetes mellitus
| Hospitalized COVID-19 patients with diabetes mellitus | |||||||||
| Subgroup | Category | Number of studies | Number of DM patients | Number of deaths | Mean prevalence of deaths | 95% prediction interval | Heterogeneity measures | ||
| % | 95% CI | I2 (%) | Q (p-value) | ||||||
| Continent | Asia | 14 | 944 | 201 | 17.0 | 8.0–28.0 | 0.0–66.0 | 92.10 | <0.01 |
| Europe | 4 | 6,422 | 1671 | 28 | 14.0-44.0 | 0.0–96.0 | 98.87 | <0.01 | |
| North America | 4 | 4,445 | 968 | 20.0 | 11.0-32.0 | 0.0–79.0 | 98.35 | <0.01 | |
| Country | China | 9 | 767 | 175 | 20.0 | 8.0–34.0 | 0.0–76.0 | 94.35 | <0.01 |
| France | 1 | 1,317 | 140 | 11.0 | 9.0–12.0 | Inestimable | - | - | |
| India | 1 | 2 | 0 | 0.0 | 0.0–84.0 | Inestimable | - | - | |
| Iran | 1 | 113 | 11 | 10.0 | 5.0–17.0 | Inestimable | - | - | |
| Italy | 1 | 69 | 22 | 32.0 | 21.0–44.0 | Inestimable | - | - | |
| Korea | 2 | 42 | 11 | 21.0 | 9.0–35.0 | Inestimable | - | - | |
| Oman | 1 | 20 | 4 | 20.0 | 6.0–44.0 | Inestimable | - | - | |
| UK | 2 | 5,036 | 1509 | 30.0 | 29.0–31.0 | Inestimable | - | - | |
| US | 4 | 4,445 | 968 | 20.0 | 11.0–32.0 | 0.0–79.0 | 98.35 | <0.01 | |
| COVID-19 diagnosis | RT-PCR | 17 | 5,315 | 1159 | 17.0 | 11.0-23.0 | 0.0-45.0 | 94.34 | <0.01 |
| Multiple modes | 5 | 6,496 | 1681 | 30.0 | 16.0–46.0 | 0.0–89.0 | 98.80 | <0.01 | |
| Diabetes diagnosis | Method specified | 5 | 1,708 | 231 | 19.0 | 10.0–31.0 | 0.0–69.0 | 93.67 | <0.01 |
| Unclear | 17 | 10,103 | 2609 | 21.0 | 15.0-27.0 | 2.0–48.0 | 96.35 | <0.01 | |
| DM type | Mixed T1 and T2 DM | 3 | 1,473 | 202 | 28.0 | 7.0–55.0 | Inestimable | - | - |
| T2DM | 3 | 1,863 | 263 | 27.0 | 0.0–84.0 | Inestimable | - | - | |
| Unclear | 16 | 8,475 | 2375 | 17.0 | 13.0-22.0 | 5.0–35.0 | 90.19 | <0.01 | |
| COVID-19 severity | Critically ill | 3 | 64 | 43 | 40.0 | 0.0–93.0 | Inestimable | - | - |
| Not Critically ill | 3 | 161 | 12 | 3.0 | 0.0–12.0 | Inestimable | - | - | |
| Unclear | 16 | 11,586 | 2785 | 21.0 | 15.0-27.0 | 3.0–47.0 | 97.15 | <0.01 | |
| Sample size | <100 | 12 | 462 | 140 | 21.0 | 8.0-38.0 | 0.0–86.0 | 91.96 | <0.01 |
| >100 | 10 | 11,349 | 2700 | 19.0 | 13.0–25.0 | 2.0–46.0 | 98.21 | <0.01 | |
| Overall | 22 | 11,811 | 2840 | 20.0 | 15.0–26.0 | 2.0–48.0 | 96.75 | <0.01 | |
| Hospitalized COVID-19 patients without diabetes mellitus* | |||||||||
| Subgroup | Category | Number of studies | Number of non-DM patients | Number of deaths | Mean prevalence of deaths | 95% prediction interval | Heterogeneity measures | ||
| % | 95% CI | I2 (%) | Q (p-value) | ||||||
| Continent | Asia | 13 | 8,136 | 549 | 7.0 | 3.0–12.0 | 0.0–0.31 | 97.59 | <0.01 |
| Europe | 3 | 15,670 | 3,818 | 25.0 | 21.0-30.0 | Inestimable | - | - | |
| North America | 4 | 10,063 | 1,696 | 14.0 | 5.0–26.0 | 0.0–0.81 | 99.53 | <0.01 | |
| Country | China | 9 | 5,152 | 311 | 8.0 | 3.0–15.0 | 0.0–0.41 | 98.21 | <0.01 |
| India | 1 | 19 | 0 | 0.0 | 0.0–18.0 | Inestimable | - | - | |
| Iran | 1 | 2,851 | 228 | 8.0 | 7.0–9.0 | Inestimable | - | - | |
| Italy | 1 | 341 | 73 | 21.0 | 17.0–26.0 | Inestimable | - | - | |
| Korea | 1 | 71 | 9 | 13.0 | 6.0–23.0 | Inestimable | - | - | |
| Oman | 1 | 43 | 1 | 2.0 | 0.0–12.0 | Inestimable | - | - | |
| UK | 2 | 15,329 | 3,745 | 24.0 | 24.0–25.0 | Inestimable | - | - | |
| US | 4 | 10,063 | 1,696 | 14.0 | 5.0–26.0 | 0.0–0.81 | 99.53 | <0.01 | |
| COVID-19 diagnosis | RT-PCR | 16 | 15,348 | 1997 | 8.0 | 4.0-14.0 | 0.0–40.0 | 99.05 | <0.01 |
| Multiple modes | 4 | 18,521 | 4,066 | 24.0 | 12.0–38.0 | 0.0–0.91 | 99.45 | <0.01 | |
| COVID-19 severity | Critically ill | 3 | 218 | 76 | 20.0 | 1.0–52.0 | Inestimable | - | - |
| Not Critically ill | 2 | 1,873 | 31 | 1.0 | 1.0–1.0 | Inestimable | - | - | |
| Unclear | 15 | 31,778 | 5,956 | 11.0 | 7.0-17.0 | 0.0–38.0 | 99.26 | <0.01 | |
| Sample size | <100 | 11 | 3,051 | 280 | 10.0 | 3.0-21.0 | 0.0–61.0 | 98.12 | <0.01 |
| >100 | 9 | 30818 | 5,783 | 11.0 | 6.0–18.0 | 0.0–0.42 | 99.57 | <0.01 | |
| Overall | 20 | 33,869 | 6063 | 11.0 | 6.0-16.0 | 0.0–0.41 | 99.32 | <0.01 | |
Juxtaposed to less severe COVID-19 infection, DM and non-DM patients with severe COVID-19 infection had 37 and 19 % higher prevalence of mortality, respectively, (Table 3). Table S2 presents more weighted estimates on the prevalence of mortality grouped by continents.
The DM patients with COVID-19 who were admitted to ICU had a seven-percentage point higher prevalence of death (26.0 %) compared with those who were not admitted to the ICU (19.0 %) (Table S3). The DM patients who died in the ICU setting were primarily suffering from severe COVID-19 infection (81 %; 95 % CI: 67.0–91.0).
Sources of heterogenity
DM among COVID-19 patients
The univariate meta-regression analyses suggested that COVID-19 severity as the determinant of heterogeneity (Table S4).
Mortality among diabetic COVID-19 patients
An adjusted meta-regression model including sample size and COVID-19 severity as predictors depicted a 452 % (p = 0.014) increased risk of mortality in DM patients with severe COVID-19 infection compared to the reference group (Table S5).
Mortality among non‐diabetic COVID-19 patients
For the prevalence of mortality among non-DM hospitalized COVID-19 patients, nation, continent, and COVID-19 diagnostic methods were the plausible predictors of heterogeneity; however, none was statistically significant in the multivariate models (Table S6).
Publication bias
For all outcomes, the visual inspection of funnel plots (Supplementary Figure: S1-S3) and Egger’s test findings did not suggest any small study effect.
Sensitivity analysis
Upon sensitivity analysis, the overall pooled prevalences of the respective outcomes obtained in each iteration closely resembled the preliminary estimates.
Risk of bias assessment
Overall, the quality of the studies was fair. The average study score was 6.4 out of a maximum possible average score of 9.4. The most common study type, case series, was of good quality (average score 6.5 out of a maximum possible score of 8) (Table S7).
Discussion
Overall, 22 observational studies with a total of 45,775 hospitalized COVID-19 patients were reviewed. The prevalence of DM was appreciably high among hospitalized COVID-19 patients. Compared to inpatient COVID-19 patients with no DM, those with DM had a higher prevalence of death, particularly in those with critically ill SARS-CoV-2 infection.
In comparison to the existing systematic reviews and meta-analysis studies, similar to our study, Zheng et al. (2020) also depicted a high preponderance of diabetes in COVID-19 patients with severe disease compared to non-severe cases [46] ext, Kumar et al. (2020) found that DM patients with COVID-19 have about a twofold increased risk of mortality contrasted to COVID-19 patients without diabetes, [47] supporting our findings. However, the findings of this study were chiefly based on data extracted from case-control studies retrieved from the PubMed database only. Another prevalence meta-analysis study of hospitalized COVID-19 patients reported a DM prevalence of nearly 8 % [48]. In contrast, this estimate was much higher in our study, which might have happened because we included studies that reported mortality information on COVID-19 patients. However, our meta-analysis models included a larger number of studies.
The inclusion of studies irrespective of their study design and geographical origin allowed us to make a comprehensive prevalence estimation of the inpatient deaths in COVID-19 infected DM patients. Additionally, as we did not exclude from meta-analysis the studies with zero numerators, our estimates plausibly did not compromise with the sample size and power. Furthermore, the substantial sample size and the relative geographic diversity of the origin of the study population might ensure better generalizability of our study.
Despite these strengths, our study has certain limitations. First, due to the incorporation of publications in the English language only, the obtained estimates might biased given not searching for studies published in other languages. However, this is could be unlikely the case, and if so the bias would be very minimal, as due to the nature of the ongoing pandemic and the aim to reach a broader range of readers, the primary publication language was the English. Additionally, we could not account for the mortality of those DM patients who remained hospitalized at the end of the follow-up period of the studies. Third, the obtained weighted prevalence estimates need cautious interpretation since these estimates were based on studies including only hospitalized COVID-19 patients. Finally, the cautious interpretation also should be exercised with regard to the generalizability of the findings as producing estimates from studies reported from a limited number of countries should not be generalized to the whole region, sub-region, or global level.
The chief implication of this study is that it provides an estimate on the burden of mortality in hospitalized COVID-19 patients with and without comorbidity diabetes. These estimates are likely to be useful for health authorities to make better hospital management protocols for such patients like, reviewing the existing management paradigm in a hospital, using efficient triaging, close monitoring, and determining the need for specialist care. Additionally, the substantial mortality burden among ICU admitted COVID-19 infected diabetes patients highlights the urgent need for additional research to ascertain its determinants.
Conclusions
Hospitalized COVID-19 patients with DM were at nearly twice the risk of mortality compared with their non-diabetic counterparts. The risk of mortality frequented when DM patients with SARS-CoV-2 infection required ICU support. It is warranted to review and strengthen the existing management protocols of the hospitals treating COVID-19 patients with diabetes with the implementation of an effective triaging system to ensure prompt and effective care of these patients during the ongoing pandemic.
Supplementary Information
(PDF 525 KB)
Author contribution
SS1 conceptualized and designed the manuscript, analyzed, drafted, and edited all versions of the manuscript. RHA critically reviewed, opined, drafted the ‘introduction’ part, and edited the manuscript. SS2, along with SS1, contributed to data abstraction and quality assessment of the reviewed studies. SS2 also hard edited the manuscript. All authors agree with the final content of the manuscript.
Funding
This study received no specific funding.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Since the study did not require any direct human participation, an ethical approcal was not needed.
Footnotes
Rami H. Al-Rifai shared first author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382:1177–9. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–7. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19–11; 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. Accessed 20 Sept 2020.
- 5.World Health Organization. Coronavirus Disease (COVID-19) Situation Reports, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 6 Oct 2020.
- 6.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–64. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623–8. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 10.Alqahtani FY, Aleanizy FS, Ali El Hadi Mohamed R, et al. Prevalence of comorbidities in cases of Middle East respiratory syndrome coronavirus: a retrospective study. Epidemiol Infect. 2019;147:e35. doi: 10.1017/S0950268818002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onder G, Rezza G, Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 10.1001/jama.2020.4683. [DOI] [PubMed]
- 12.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill patients in the Seattle Region — case series. N Engl J Med. 2020;382:2012–22. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roncon L, Zuin M, Rigatelli G, et al. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–15. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–9. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Z, Xu Y, Bao L, et al. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses. 2019;11. 10.3390/v11010059. [DOI] [PMC free article] [PubMed]
- 19.Yang J-K, Lin S-S, Ji X-J, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–9. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Saha S, Saha S. A systematic review and meta-analysis of observational and experimental studies, and case series to determine the prevalence of mortality among the inpatient novel coronavirus infected diabetes patients. PROSPERO 2020 CRD42020196589. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020196589. Accessed 13 Aug 2020.
- 22.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Study Quality Assessment Tools | National Heart, Lung, and Blood Institute (NHLBI). https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 14 June 2020.
- 24.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Heal. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkundi A, Mahmoud I, Musa A, et al. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: A retrospective single centre study. Diabetes Res Clin Pract. 2020;165:108263. doi: 10.1016/j.diabres.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: An experience from Oman. J Infect Public Health. 2020;13:906–13. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcello RK, Dolle J, Grami S, et al. Characteristics and outcomes of COVID-19 patients in New York City’s Public Hospital System. medRxiv 2020; 2020.05.29.20086645. [DOI] [PMC free article] [PubMed]
- 28.Zhang N, Xu X, Zhou L-Y, et al. Clinical characteristics and chest CT imaging features of critically ill COVID-19 patients. Eur Radiol. 10.1007/s00330-020-06955-x. [DOI] [PMC free article] [PubMed]
- 29.Nikpouraghdam M, Jalali Farahani A, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–91. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26:1063–8. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cen Y, Chen X, Shen Y, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin Microbiol Infect. 2020;26:1242–7. doi: 10.1016/j.cmi.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Cui Y, Shen M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhandari S, Bhargava A, Sharma S, et al. Clinical profile of Covid-19 infected patients admitted in a Tertiary Care Hospital in North India. J Assoc Phys India. 2020;68:13–7. [PubMed] [Google Scholar]
- 37.Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang JG, Hur J, Choi EY, et al. Prognostic factors for severe Coronavirus Disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35:e209. doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–21. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu C, Lei Q, Li W, et al. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. 2020;59:168–75. doi: 10.1016/j.amepre.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan W-J, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JY, Kim HA, Huh K, et al. Risk factors for mortality and respiratory support in elderly patients hospitalized with COVID-19 in Korea. J Korean Med Sci. 2020;35:e223. doi: 10.3346/jkms.2020.35.e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao J, Gao H-Y, Feng Z-Y, et al. A Retrospective Analysis of the Clinical and Epidemiological Characteristics of COVID-19 Patients in Henan Provincial People’s Hospital, Zhengzhou, China. Front Med; 7. 10.3389/fmed.2020.00286. [DOI] [PMC free article] [PubMed]
- 46.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81:e16–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. [DOI] [PMC free article] [PubMed]
- 48.Emami A, Javanmardi F, Pirbonyeh N, et al. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 525 KB)