Abstract
γ-Glutamyl valine (γ-EV), commonly found in edible beans, was shown to reduce gastrointestinal inflammation via activation of calcium-sensing receptors (CaSRs). The present study aimed to evaluate the efficacy of γ-EV in modulating the tumor necrosis factor-α-induced inflammatory responses in endothelial cells (ECs) via CaSR-mediated pathways. Human aortic ECs (HAoECs) were pretreated (2 h) with γ-EV (0.01, 0.1, and 1 mM). 1 mM pretreatment of γ-EV significantly reduced the upregulation of inflammatory adhesion molecules, VCAM-1 and E-selectin, by 44.56 and 57.41%, respectively. The production of cytokines IL-8 and IL-6 was significantly reduced by 40 and 51%, respectively, with 1 mM pretreatment of γ-EV. Similarly, there was a significant reduction in chemokine MCP-1 from a positive control of 9.70 ± 0.52 to 6.6 ± 0.43 ng/mL, after γ-EV treatment. The anti-inflammatory effect of γ-EV was attenuated by the treatment of the CaSR-specific inhibitor, NPS-2143, suggesting the involvement of CaSR-mediated pathways. Further studies identified the critical role of key modulators, such as β-arrestin2 and cyclic adenosine monophosphate response element-binding protein, in mediating the CaSR-dependent anti-inflammatory effect of γ-EV. Finally, the transport efficiency of γ-EV was evaluated through a monolayer of intestinal epithelial cells (Caco-2), and the apparent permeability (Papp) of the peptide was found to be 1.56 × 10−6 cm/s.
Keywords: anti-inflammation, calcium-sensing receptor, γ-glutamyl peptides, vascular inflammation, endothelial cells
INTRODUCTION
Inflammation of the vascular wall is a major contributor of pathophysiological conditions such as atherosclerosis, hypertension, and the progression of cardiovascular diseases (CVDs).1 Vascular inflammation is promoted by endothelial dysfunction through the production of adhesion molecules, vasoconstrictors, and growth factors including endothelin-I and angiotensin-II.2 Patients with hypertension have demonstrated increased plasma levels of cytokines such as interleukin-6 (IL-6), IL-8, tumor necrosis factor-α (TNF-α), and adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intracellular cell adhesion molecule-1 (ICAM-1), and E-selectin.3 Inflammation and extracellular matrix deposition majorly contribute to vascular remodeling and subsequent vasoconstriction.1 Hypertension or high blood pressure also induces proinflammatory responses and accelerates the advancement of atherosclerosis.4 Therefore, inflammation in blood vessels has a central role in the progression of CVDs.5
CVDs are the major cause of death in the United States, with about 610,000 people dying every year, that is, one in every four deaths.6 The progression of CVDs are also linked to various facets of lifestyle, the most significant one being diet.7 Although diet and exercise have been considered as the two main strategies in reducing CVDs, most of the time, it is either not sustainable or it is hard to incorporate such lifestyle changes. Therefore, to cope with an everincreasing prevalence of CVDs, various upstream preventive methods must be explored to see how the diet or food-derived bioactive compounds can modulate the cardiovascular health.7
Over the past few years, food protein-derived peptides have gained a lot of interest because of their unique bioactive capabilities to exhibit health-promoting activities, including anti-inflammatory activity.8–10 One class of such bioactive peptides is the γ-glutamyl peptides. γ-Glutamyl peptides are found to be naturally occurring in foods such as legumes (soybeans, common beans, and black grams), onion, garlic, and fermented products such as cheese, sourdough, and yeast extract.11 γ-Glutamyl peptides are known as the major contributors of the kokumi flavor. Till now, more than 100 γ-glutamyl peptides, which are kokumi-active, have been shown to activate the calcium-sensing receptor (CaSR) via allosteric modification.12 Thus, the CaSR has been considered as an important receptor that can modulate physiological functions in mammals, given that it is ubiquitously expressed in many mammalian tissues such as the brain, heart, intestines, liver, kidney, and blood vessels.13 The γ-glutamyl dipeptide, γ-glutamyl valine (γ-EV), has been reported earlier for its anti-inflammatory activities, in a dextran sulfate sodium-induced porcine14 and mouse model of colitis.15 γ-EV has also shown to reduce inflammation in the mouse model of lipopolysacchar-ide-induced sepsis.16 Along with its potential anti-inflammatory activities in gastrointestinal cells, γ-EV has also shown anti-inflammatory effects on TNF-α-induced inflammation in mouse adipocytes.14 One similarity highlighted among all the mentioned anti-inflammatory studies was the involvement of the CaSR in regulating the bioactivity of γ-EV. In a study by Zhang et al, it was demonstrated that γ-EV could modulate the cross-talk between TAK1 and β-arrestin2 signaling pathways via allosteric activation of the CaSR, suggesting the ability of γ-EV to significantly affect anti-inflammatory responses via CaSR activation.15 Thus, considering the multiphasic role of the CaSR, especially on gut health, it would be natural to consider the CaSR as a molecular target for improving gut health.17 Beside all the known potential anti-inflammatory effects of γ-EV for gastrointestinal inflammation, very little information is known on the ability of γ-EV to modulate inflammation in vascular endothelial cells (ECs). In ECs, the CaSR regulates blood pressure via activation of intermediate conductance Ca2+-sensitive potassium channels and subsequent K+-induced hyperpolarization.18,19 However, the involvement of the CaSR in modulating vascular inflammation is not completely elucidated yet.
Therefore, considering the potential anti-inflammatory effects of γ-EV on various cell types, the main objectives of the present study were to delineate the role of γ-EV in modulating vascular inflammation via endothelial CaSR-mediated pathways, identify the key signaling regulators in mediating γ-EV-induced CaSR-dependent anti-inflammatory responses, and finally evaluate the transport efficacy of γ-EV through the intestinal epithelial cells.
MATERIALS AND METHODS
Chemicals.
The γ-EV dipeptide was chemically synthesized (purity >98%) by GenScript USA Inc. TNF-α purchased from Abcam, USA (ab9642), was used to induce inflammation in human aortic ECs (HAoECs). The primary antibodies used for western blot and immunofluorescence experiments were as follows: anti-VCAM-1 (Abcam, ab134047, Lot # GR257919-53), anti-ICAM-1 (Santa Cruz, sc-8439, Lot #L1517), anti-E-selectin (Santa Cruz, sc-137054, Lot #G2718), anti-β-actin (Abcam, ab6276, Lot #GR3270852-5), anti-VE-cadherin (Santa Cruz, sc-9989, Lot #K0819), anti-VE cadherin (Invitrogen, #PA5-17401, Lot #VF3007247), anti-ZO-1 (zona occludens-1, Lot #UC280753) (Invitrogen, 61-7300, Lot # UC280753), anti-CaSR (LS Biosciences, LS-C417520, Lot #144764, total endogenous protein), anti-CaSR (Invitrogen, MA1-934, Lot #UE28760, specificity toward the membranal Venus Fly Trap region), anti-β-arrestin2 (Santa Cruz, sc-13140, Lot #L0318), anti-NF-kappaB p65 (Cell Signaling, #6956S, Lot #9), anti-P-NF-kappaB p65 (S536) (Cell Signaling, #3033S, Lot #16), anti-phospho-NFkB p65 (Thr254) (Invitrogen, #PA5-104960, Lot #VF3007485), anti-IkB-alpha (Cell Signaling, #4814S, Lot #17), anti-cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB; Cell Signaling, #9104S, Lot #4), and anti-phospho-CREB (Ser133) (Cell Signaling, #9198S, Lot #18). The secondary antibodies used for western blot were IRDye 800CW goat anti-rabbit IgG (#925-32211, Lot #C80718-11) and IRDye 680RD goat anti-mouse IgG (#925-68070, Lot #C80619-01) from LI-COR Biosciences, while for immunofluorescence were Alexa Fluor 488 (ab150113, Lot #GR3235892-2 and ab150077, Lot #GR3225145-2) and Alexa Fluor 594 (ab150116, Lot #GR3248932-1, and ab150080, Lot #GR3232361-2).
Cell Culture.
HAoECs (PromoCell, Germany, C-12271) were grown in the EC growth medium (C-22010) supplemented with 5% fetal bovine serum (FBS; Gibco, 10437028) at 37 °C and 5% CO2 in a humidified environment; at ~80% confluency, the cells were subcultured using DetachKit (PromoCell, C-41210) consisting of HEPES-buffered salt solution, trypsin/EDTA, and trypsin neutralization solution. HAoECs within the passages 1–5 were used in this study.
Caco-2 (ATCC HTB-37) cells were grown in Eagle’s minimum essential medium (EMEM; ATCC 30-2003) supplemented with 20% FBS and 1% penicillin—streptomycin (Gibco) at 37 °C and 5% CO2 in a humidified environment. Cells were grown for 6 days until they were confluent (80%) and then subcultured and used for further experiments. The cells used for absorption study were then grown for 21 days until they differentiated, and the cell medium was changed every other day. Caco-2 cells within the passages 22—25 were used in this study.
Cell Viability Study.
HAoECs and Caco-2 cells were seeded in clear bottom 96-well plates (VWR, #29444-008) at a cell density of 4 × 104 cells/well. HAoECs were grown up to 90% confluency, while Caco-2 was grown for 15 days to ensure differentiation, with media being changed every other day. The cell viability assay was performed using an MTT cell proliferation assay kit (Abcam, ab211091) using the manufacturer’s protocol. In the case of HAoECs, the highest dose of γ-EV (1 mM) was tested for cell toxicity for different time periods (2, 8, and 24 h), whereas for Caco-2, a cell toxicity of 1 mM γ-EV was tested for 2, 4, and 6 h. After their respective cell treatments, the media were discarded and 50 μL of the MTT reagent and 50 μL of serum-free media were added into each well, along with a background control (no cells). The plates were incubated for 3 h at 37 °C, after which the MTT-supplemented media was removed and 150 μL of the MTT solvent was added into each well. The plates were kept in the dark and placed in an orbital shaker for 15 min. The absorbance of the plate was read out at 590 nm. The cell viability % was calculated with the following formula, after subtracting the background control from each well: % cell viability = (sample/control) × 100, where sample is the γ-EV-treated well and control is the negative control, without γ-EV.
Immunofluorescence.
HAoECs were seeded onto sterile eight-well chamber slides (Sigma, C7182) at a concentration of 3 × 104 cells/well. Once the cells were confluent (~80%) in 3—4 days, the culture medium was removed, and the monolayers were washed with warm Dulbecco’s phosphate-buffered saline (DPBS). The cells were then fixed with 4% paraformaldehyde (Sigma, 158127) for 20 min at room temperature followed by permeabilization using 0.1% saponin (Sigma, 47036) for 5 min. The cells were then blocked using 1% bovine serum albumin in DPBS for 1 h at room temperature. After blocking, the cells were washed again with DPBS and incubated with the suitable primary antibody dilution overnight at 4 °C in a humidified chamber. The following day, cells were washed with DPBS, and hereafter, the chamber slides were kept in the dark. The cells were then incubated with suitable fluorescence-tagged secondary antibodies (1:500 dilution) at room temperature for 1—2 h. Following incubation, cells were washed with DPBS and stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Sigma, #10236276001) at 1:10,000 dilution for 5 min. Cells were washed with DPBS, and the chamber was removed from the slide. The slide was then mounted with a clean glass coverslip using a fluoromount mounting medium (Sigma, F4680). Immunofluorescence was detected using a Nikon A1R-Ti2 confocal system.
In Vitro Inflammation Studies.
For the in vitro inflammation studies, HAoECs were seeded into 24-well TPP tissue culture plates (MidSci, MO, USA) with a seeding density of 5 × 104 cells/well. The cells were grown for 3—5 days until they reached 90% confluency. Confluent cells were washed with HEPES-buffered saline solution (PromoCell, C-40000) and changed to Q-media or low-serum medium (1% FBS) for 1 h. Cells were then pretreated with γ-EV for 2 h at different concentrations (0.01, 0.1, and 1 mM), followed by treatment with TNF-α (5 ng/mL) for another 6 h. Cell medium supernatants were collected for enzyme-linked immunosorbent assays (ELISAs) and for western blots, and cells were harvested with 2× hot Laemmli buffer followed by the addition of dithiothreitol (DTT) and boiled for 3—4 min. To confirm the role of the CaSR, NPS-2143 (1 μM) (Sigma, #SML0362) was used to block the CaSR activation for 1 h before the γ-EV pretreatment.
Western Immunoblot Analysis.
Cell lysates collected in 2× Laemmli buffer were boiled for 5 min, and the proteins were separated in a 4–20% gradient gel (Biorad, #4561096) by sodium dodecyl sulfate—polyacrylamide gel electrophoresis (SDS-PAGE) followed by transfer onto a nitrocellulose membrane (Bio-Rad). The membranes were blocked with Odyssey blocking buffer in TBS (Li-Cor, #927-50000) for 1 h followed by overnight incubation at 4 °C with primary antibodies at their recommended dilutions in Odyssey blocking buffer in Tris-buffered saline with 1% Tween (TBST). The following day, the membranes were washed thrice with TBST and incubated with the secondary antibodies IRDye 800CW goat anti-rabbit IgG and/or IRDye 680RD goat anti-mouse IgG, depending on the host of the primary antibody used, at a concentration of 1:10,000 at room temperature for 1 h. The membranes were washed again after incubation, and the proteins were detected under fluorescent green and red channels in an Odyssey CLx imaging system (Li-Cor Biosciences). The protein expression was quantified using Image Studio software from Li-Cor, and the expression was normalized using β-actin as the loading control.
For the confirmation of CaSR expression in HAoECs, the membrane proteins were extracted and separated from the cytosolic proteins through a Mem-PER Plus membrane protein extraction kit (Thermo Scientific, 89842) using the manufacturer’s manual. The extracted membrane and cytosolic proteins were then detected for CaSR expression through western immunoblotting, as described above.
Enzyme-Linked Immunosorbent Assay.
Cell-free supernatant media from the inflammation studies were used to quantify the levels of monocyte chemoattractant protein-1 (MCP-1), IL-6, and IL-8 through ELISA. ELISAs were performed using specific kits for human CCL-2/MCP-1 (Thermo Scientific, #88-7399-88), IL-6 (#88-7066-880), and IL-8 (#88-8086-88) by following the manufacturer’s guidelines. 96-well high-affinity protein-binding plates (Corning 9018) were coated with 100 μL of the capture antibody (1:250, v/v) diluted in 1× phosphate-buffered saline (PBS) and incubated overnight at 4 °C. Plates were then washed five times with wash buffer (1× PBS, 0.05% Tween) and blocked with 200 μL of 1× ELISA diluent for 1 h at room temperature on an orbital shaker. Plates were again washed five times, followed by the addition of 100 μL of standards and samples and overnight incubation at 4 °C. The following day, plates were washed five times and incubated with 100 μL of the detection antibody (1:250) diluted in 1× ELISA diluent for 1 h at room temperature. After washing, plates were incubated with 100 μL of the horse-radish peroxidase enzyme (1:250, v/v) diluted in 1× diluent for 30 min at room temperature. Finally, after a final wash, plates were incubated with 100 μL of tetramethylbenzidine substrate solution in the dark for 15 min at room temperature. The reaction was stopped with the addition of 50 μL of 1 M H2SO4, and the absorbance was measured at 450 nm. The concentrations of the cytokines and chemokine were quantified from the standard curves.
Peptide Transport Study.
Caco-2 cells were seeded onto 12-well inserts consisting of the 0.4 μm transparent permeable polyester membrane (Corning, Fisher Scientific) at a concentration of 1.5 × 105 cells/insert. The apical side contained 0.5 mL of growth medium, while the basolateral side had 1.5 mL of the medium. The transepithelial electrical resistance (TEER) was also measured every other day for 21 days using EVOM2 paired with STX2 chopstick electrodes (World Precision Instruments). Caco-2 cells having a TEER measurement of 400 Ωcm2 or more were selected for the experiments. On the 21st day, the cells were washed with warm Hanks’ balanced salt solution (HBSS), and then, the apical media of the cells were changed to HBSS (pH 6.5, pH adjusted with MES), while the basolateral media were changed to HBSS (pH 7.4, adjusted with 25 mM HEPES). The cells were incubated at 37 °C and 5% CO2 for 15 min, and then, the TEER values were measured again. HBSS (0.5 mL; pH 6.5) containing 1 mM γ-EV was put into each apical compartment, while the basolateral compartment still contained 1.5 mL of HBSS (pH 7.4). The plates were immediately incubated in an orbital shaker at 37 °C for 2 h. Different wells were allotted for different time periods; therefore, at 0, 1, and 2 h, entire apical and basolateral solutions were collected and analyzed. γ-EV absorbed over time was analyzed by liquid chromatography coupled with tandem mass spectrometry (LC—MS/MS) because of its greater sensitivity. The apparent permeability coefficient (Papp) of the peptide across the Caco-2 monolayer was also calculated with the following formula
where dQ/dt is the transport rate (μmol/L·s), defined as the slope obtained from linear regression of the transport amount, C0 is the initial concentration of the peptide on the apical side (μmol/L), and A is the surface area of the inserts.
LC—MS Analysis Using MRM.
The samples were diluted using MilliQ water. For LC separation, an Eclipse-Plus C18 RRHD (1.8 μm, 50 × 2.1 mm, Agilent) was used at a flow rate of 0.5 mL/min. The gradients of the mobile phases A (0.1% formic acid in MilliQ water) and B (0.1% formic acid in acetonitrile) were 2% B for 2 min, to 80% B in 2 min, hold at 80% B for 2 min, and then back to 2% B in 0.2 min. The LC system was interfaced with a Sciex QTRAP 6500+ mass spectrometer equipped with a TurboIonSpray (TIS) electrospray ion source. The sample acquisition control and data analysis were performed using Analyst software (version 1.6.3). The SCIEX QTRAP 6500+ mass spectrometer was tuned and calibrated according to the manufacturer’s recommendations. The target peptide was detected using multiple reaction monitoring (MRM) transitions that were previously optimized using standards Q1 to Q3, 247.1 m/z to 118.1 m/z (transition 1) and 247.1 m/z to 84.0 m/z (transition 2). Both transitions had the same optimized declustering potential (DP) at 50, entrance potential (EP) at 11, and collision cell exit potential (CXP) at 13.5. The optimal collision energies for transitions 1 and 2 were 16 and 34, respectively. The instrument was set up to acquire using electrospray ionization (ESI) in the positive ion mode. The ESI operation parameters were as follows: source temperature (Tem), 500 °C; ion spray voltage (IS), 5500 V; ion source gas 1 (GS1), gas 2 (GS2), and curtain gas (CUR), 50, 50, and 25, respectively; and collision gas (CAD), high. For quantification, an external standard curve was prepared with water using a dilution series containing different concentrations of the peptide and run in triplicate alongside the samples (in HBSS buffer). The linear range of the standard curve ranged from 0.0032 to 10 μM with a linear regression slope R2 of 1 and a CV of <4% for all the dilution points.
Statistical Analysis.
Data sets were first confirmed to be normally distributed via the D’Agostino & Pearson test. Statistical analysis was then performed between multiple groups using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using the software GraphPad Prism (version 8.0.1). Data were presented as ± standard error of the mean (SEM), and statistical significance was considered at p < 0.05.
RESULTS
CaSR Expression in HAoECs.
To confirm the presence of the CaSR in HAoECs, immunostaining was performed with the anti-CaSR antibody, along with anti-VE-cadherin to mark the membrane region. As shown in Figure 1A(iv), the anti-CaSR antibody (LS-C417520) detects endogenous levels of total CaSR protein, and it was found to be mainly localized in the cytoplasmic region, despite the common notion of the CaSR being a membrane-bound protein. To confirm the cellular localization of the CaSR, western immunoblotting was performed on proteins isolated simultaneously from the cytoplasm and the membrane of HAoECs, through a membrane protein extraction kit. As shown in Figure 1B, a single dark band was visualized at ~130 kD only in the cytoplasmic protein fraction, which corresponded to immature CaSR protein.20 However, in the membrane fraction, instead of getting a band at the same position, a much lower molecular weight band was observed at ~40 kD. The cytoplasmic and membrane fractions were distinguished via specific markers, such as VE-cadherin (for the membrane) and β-actin (for cytoplasm), to ensure the separation was clear. The immunoblot result was in accordance with the immunostaining of the CaSR in HAoECs with the anti-CaSR antibody (LS-C417520), which confirmed that the CaSR is majorly localized in the cytoplasm. Figure S1 provides the sequential blotting pattern used for obtaining the final Figure 1B.
Figure 1.
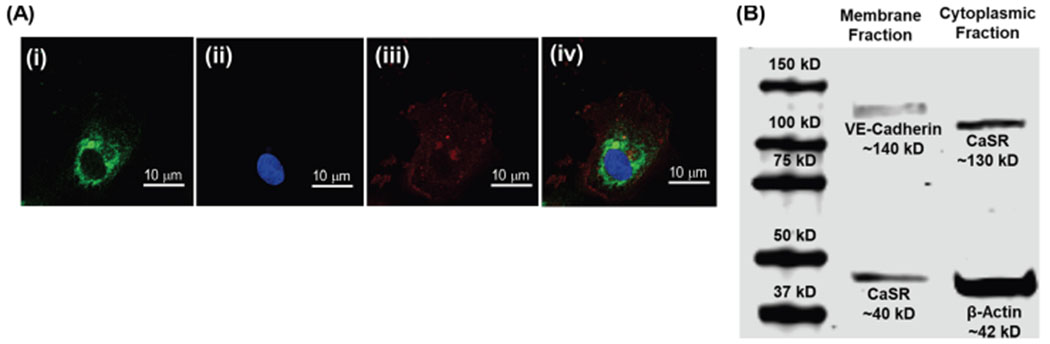
Confirmation of CaSR expression in HAoECs through immunostaining and western immunoblotting. [A (i)] Immunostaining of the HAoEC showing the expression and localization of the CaSR protein with the anti-CaSR antibody (LS-C417520, green), (ii) DAPI staining of the nucleus, (iii) immunostaining of HAoEC membrane protein, VE-cadherin (red), and (iv) merged image of the cell confirming cytosolic localization of the CaSR protein. The anti-CaSR antibody (LS-C417520) targets endogenous CaSR. (B) Western immunoblot showing CaSR protein expression in the cytoplasmic protein fraction of HAoECs at ~130 kD but a much smaller molecular weight protein (~40 kD) in the membranal fraction of HAoECs. The purity of the membrane and cytoplasmic fractions were confirmed by the presence of VE-cadherin (in the membranal fraction) and β-actin (in the cytoplasmic fraction).
To further confirm the localization, a second anti-CaSR antibody (MA1-934), which detects the sequence (214) ADDDYGRPGIEKFREEAEERDI (235), present in the membrane-bound Venus flytrap (VFT) region of the CaSR,21 was used for immunoblotting (Figure S2). Similar to the previous expression pattern, a single band at ~37 kD was observed only in the membranal fraction, but not the cytosolic fraction. The absence of the CaSR in the cytoplasmic fraction confirms its membrane specificity and thus suggests that the CaSR might undergo some post-translational modifications during the translocation of the protein from the cytosol to the membrane of the HAoEC. Therefore, that particular VFT region might be more accessible in the membrane protein fraction as compared to the cytosolic fraction in western immunoblotting. This is consistent with the previous literature of CaSR expression in HAoECs,22 wherein CaSR’s mature cell surface form was owing to the carbohydrate content which is about ~35—40 kD/receptor.20
Anti-Inflammatory Activity of γ-EV.
Modulation of the inflammatory responses induced by TNF-α (5 ng/mL) was examined on γ-EV pretreated HAoECs. As shown in Figure 2, the highest dose of γ-EV pretreatment, that is, 1 mM, could significantly (p < 0.001) reduce the inflammatory response with regard to expression levels of the cellular biomarkers, VCAM-1 and E-selectin, when compared to the positive control. It was observed that the expression of VCAM-1 and E-selectin was reduced by 44.56 and 57.41%, respectively. In the case of VCAM-1, a γ-EV pretreatment dose of 0.1 mM was also shown to have a reducing inflammatory effect with p < 0.05. For the biomarker ICAM-1, a reducing trend was observed, although it was not statistically significant. This may be due to a different regulatory mechanism of ICAM-1 (which may regulate post-translational modification), and such similar observations have also been reported in the literature.23,24
Figure 2.
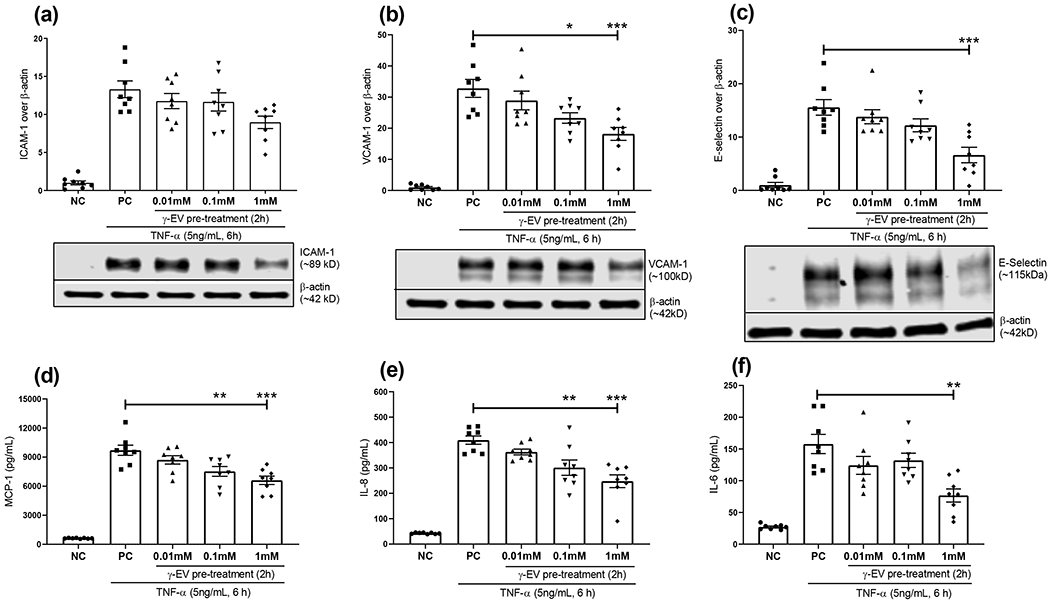
Anti-inflammatory effects of the γ-EV peptide on HAoECs. The inflammatory response induced by TNF-α on HAoECs was evaluated by the expressions of cellular biomarkers, (a) ICAM-1, (b) VCAM-1, and (c) E-selectin, and by the secretion of chemokine, (d) MCP-1 and cytokines, (e) IL-8, and (f) IL-6. The ECs were preincubated with different doses (0.01, 0.1, and 1 mM) of the γ-EV peptide for 2 h, followed by the addition of TNF-α (5 ng/mL) for 6 h. Data presented as the mean ± SEM of at least four independent experiments. Statistically, the data are represented as p < 0.001 for (***), p < 0.01 for (**), and p < 0.05 for (*).
Furthermore, in the case of MCP-1 chemokine production, there was also a significant (p < 0.001) reduction to 6.6 ± 0.43 ng/mL from a positive control of 9.70 ± 0.52 ng/mL (approx. 32%), when HAoECs were pretreated with 1 mM γ-EV. γ-EV (0.1 mM) was also effective in reducing MCP-1 production to 7.52 ± 0.50 ng/mL (~22.5%) from the positive control (p < 0.01). Similarly, the significantly elevated levels of inflammatory cytokines IL-8 and IL-6 in the positive control were reduced by 1 mM γ-EV pretreatment by about 40 and 51%, respectively (p < 0.001). In the case of IL-8, 0.1 mM dose γ-EV was also effective in reducing the inflammatory levels by ~26.5% (p < 0.01).
In summary, the decrease in the production of the major inflammatory mediators (with the exception of ICAM-1) was found to be dose-dependent. At 1 mM, γ-EV pretreatment was found to be significantly effective in reducing the inflammatory responses induced by TNF-α in HAoECs. Henceforth, this dose was chosen for all the subsequent studies with γ-EV. The cell viability assay showed that 1 mM γ-EV was nontoxic to the HAoECs (Figure S3a,b).
Role of the CaSR in the Anti-Inflammatory Activity of γ-EV.
To verify the role of the CaSR in the anti-inflammatory activity of γ-EV in HAoECs, a CaSR-specific antagonist, NPS-2143, was used to block the activation of the CaSR mediated by γ-EV.15,25 The result of this study showed that the preincubation of NPS-2143 reversed the γ-EV-mediated reduction of VCAM-1 and E-selectin biomarkers, and their expression levels became almost similar to the positive control (Figure 3). Similar results were observed in the case of MCP-1, IL-8, and IL-6 secretion, where the γ-EV-mediated reduction of the chemokine and cytokine secretion was reversed, suggesting that the anti-inflammatory effect of γ-EV might be through CaSR-dependent pathways. However, it was also observed that in the case of MCP-1 and IL-8, the reversal was not up to the level of positive control, indicative of the fact that other CaSR-independent pathways might also be involved in this anti-inflammatory effect.
Figure 3.
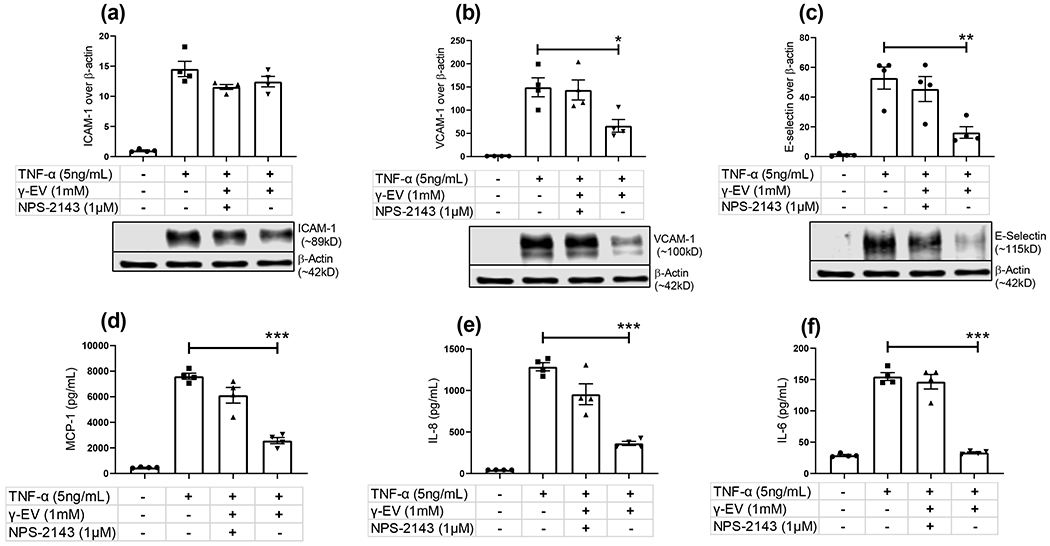
Evaluating the role of CaSR on modulating the anti-inflammatory activity of γ-EV on HAoECs. NPS-2143 (1 μM), a CaSR-specific antagonist, was used to inhibit γ-EV-mediated CaSR activation in HAoECs for 1 h. The cells were then preincubated with the highest dose of γ-EV (1 mM) for 2 h, and the inflammatory responses induced by TNF-α (5 ng/mL) were evaluated by expression levels of (a) ICAM-1, (b) VCAM-1, (c) E-selectin, (d) MCP-1, (e) IL-8, and (f) IL-6. Data presented as the mean ± SEM of four independent experiments. Statistically, the data are represented as p < 0.001 for (***), p < 0.01 for (**), and p < 0.05 for (*).
Investigation of Key Signaling Regulators of γ-EV-Mediated Anti-Inflammatory Response.
The study investigated the role of few key signaling regulators involved in the anti-inflammatory activity of γ-EV, although further validations are required to completely delineate the molecular mechanisms of action. First, to ensure that the TNF-α signaling cascade was activated through the TNF-α receptor (TNFR), degradation of the inhibitor of the B-cell kappa polypeptide gene enhancer, alpha (IkBα) was verified in both positive control and the 1 mM γ-EV treatment with TNF-α. There was no significant difference between the treatment and positive control, suggesting that the effect was downstream of IkBα (Figure 4a). IkBα binds to the nuclear factor kappa-light-chain-enhancer of the activated B cell (NF-kB) transcription factor in the cytoplasm and inhibits it from entering the nucleus and binding to the DNA to activate proinflammatory genes, as was seen in the case of the negative control (Figure 4a). Second, being the master regulator of the inflammatory responses, NF-kB has a crucial role in maintaining the equilibrium in the immune system, and much of that control occurs via NF-kB phosphorylation of its subunits. 26 It was found that phosphorylation at the Ser536 residue of the p65 subunit was significantly higher in the positive control as compared to the negative control. Moreover, this phosphorylation was further enhanced in the presence of γ-EV (Figure 4b). Phosphorylation at the Ser536 residue has been shown to enhance proteasomal degradation of p65 to resolve inflammation as a negative feedback loop mechanism.27,28 Thus, it was suggestive that one of the ways γ-EV promoted its anti-inflammatory effects was through proteasomal degradation of p65, making it unable to activate proinflammatory genes in the nucleus. On the other hand, phosphorylation at the Thr254 residue of p65 has a positive correlation with inflammation.29,30 It was found that 1 mM γ-EV treatment could significantly reduce the phosphorylation of p65 (Thr254) by 54.3% from the positive control (Figure 4c).
Figure 4.
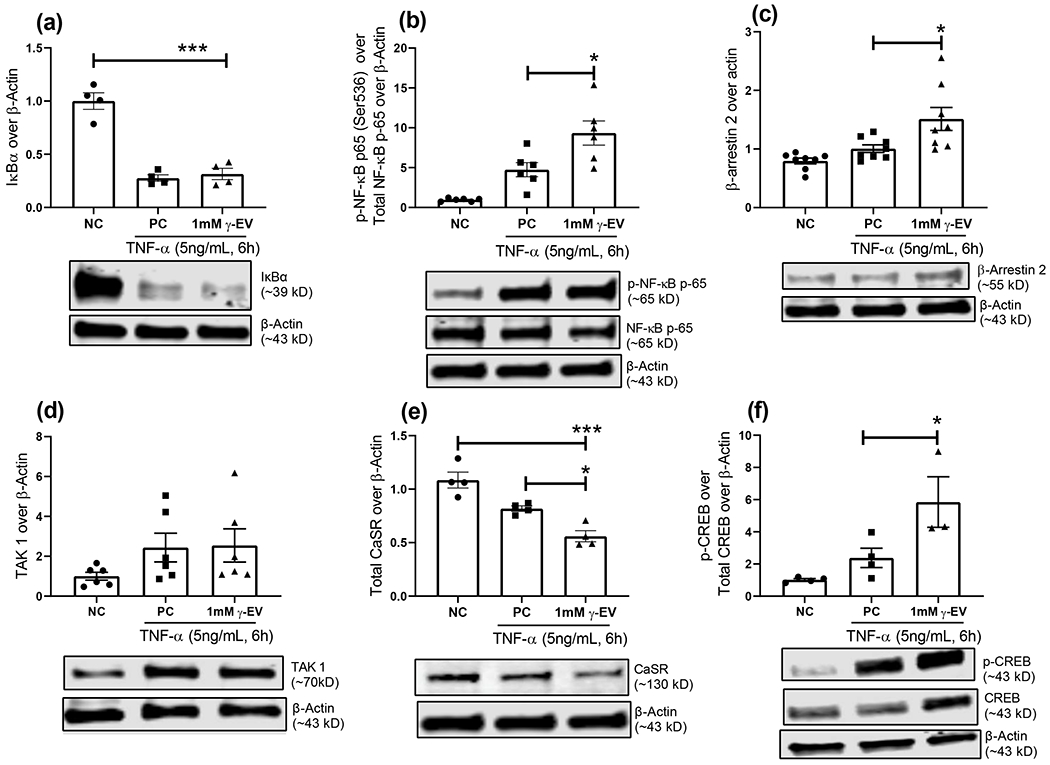
Proposed mechanism of the γ-EV-mediated anti-inflammatory effect. For the inflammatory response generated with TNF-α after 1 mM γ-EV pretreatment, few intracellular proteins were analyzed through western immunoblotting to suggest a possible mechanism for the anti-inflammatory effect based on the literature. The expression levels in the fold changes were measured for (a) IkBα (b) p65 phosphorylation at the Se536 site, (c) p65 phosphorylation at the Thr254 site, (d) β-arrestin2, (e) total CaSR, and (f) CREB phosphorylation. Data presented as the mean ± SEM of at least four independent experiments. Statistically, the data are represented as p < 0.001 for (***), p < 0.01 for (**), and p < 0.05 for (*).
The crosstalk between the TNFR and endothelial CaSR is possibly regulated via two pathways, and both the pathways may involve β-arrestin2 protein, as shown in other cell types.15 The first potential pathway was through inhibition of TGF-β-activated kinase 1 (TAK1) and TAK1 binding protein (TAB1) interaction via β-arrestin2 protein, as shown previously by Zhang et al in gastrointestinal epithelial cells.15 TAK1–TAB1 interaction led to the downstream activation of the NF-kB signaling pathway.15 Our study showed that there was an increase in β-arrestin2 protein in the γ-EV-treated cells (Figure 4d), with no significant change in the level of TAK 1 proteins (data not shown). This suggested that γ-EV treatment led to a higher expression of β-arrestin2 protein so that it could possibly bind to TAB1 more in order to inhibit the TAK1–TAB1 interaction.
The second pathway of the crosstalk involved the desensitization and internalization of the CaSR via β-arrestin2-mediated pathways. In ECs, cAMP was shown to inhibit TNF-α-mediated induction of the E-selectin promoters as TNF-α and cAMP altered the binding of the nuclear factors competitively to the cAMP-responsive element/activating transcription factor (CRE/ATF) region of the E-selectin promoter.31 Because activation of the CaSR led to reduction in cAMP,20,21 it was suggestive that γ-EV treatment led to the reduction of CaSR levels (Figure 4e) by promoting internalization through β-arrestin2.32 This was also verified with the increase in CREB phosphorylation (Figure 4f).
This demonstrates the fact that γ-EV can influence several molecular pathways during an inflammatory response in ECs, some of which has been realized in this study, whereas there might be some other mechanisms which needs further study. A cumulative schematic of the proposed mechanism of action of γ-EV in ECs is illustrated in Figure 5.
Figure 5.
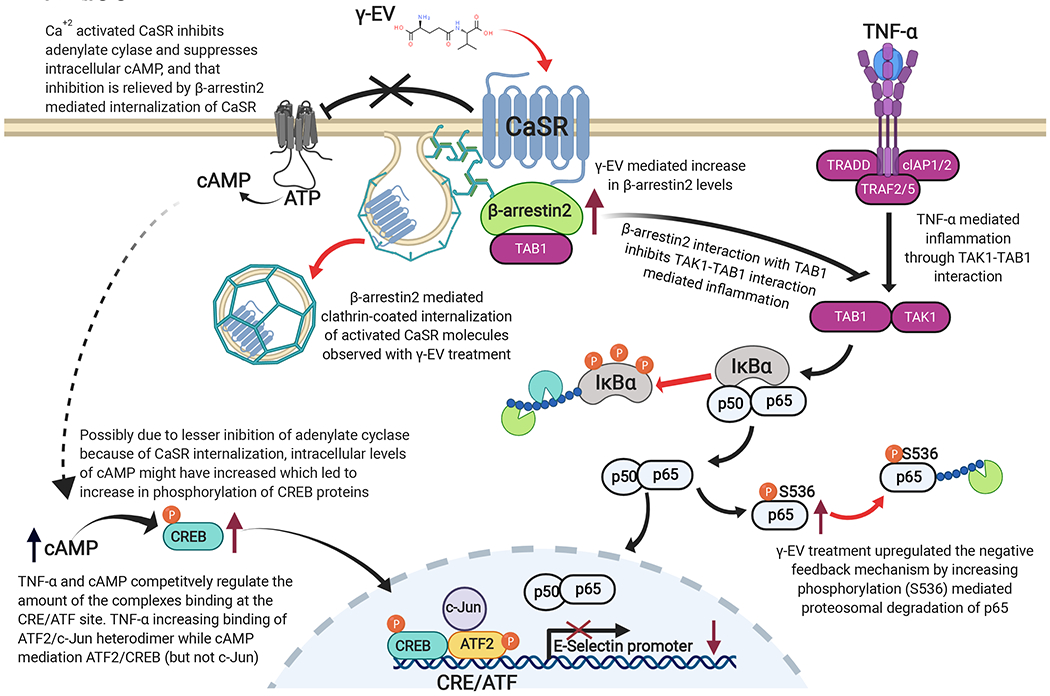
Schematic for a possible mechanism of the γ-EV mediated anti-inflammatory effect in HAoECs. The arrows marked in red correspond to data found in the present study, while the arrows marked in black are the knowledge that exists in the present literature.15,20,23–27 Upon treatment with γ-EV, β-arrestin2 levels increased, and the protein might play a crucial role in the anti-inflammatory response. β-arrestin2 interacts with TAB1 and thus mitigates TAK1-TAB1-mediated inflammatory response. Furthermore, because the Ca2+-activated CaSR is involved in inhibition of adenylate cyclase and thus reduction of intracellular cAMP, it is possible that β-arrestin2 is also relieving the inhibition by mediating internalization of CaSR. The role of cAMP has been shown previously in countering TNF-α-induced inflammation in ECs by competitively regulating factors (such as CREB proteins) which bind to the CRE/ATF site of the E-selectin promoter. Finally, γ-EV has also shown an increase in p65 phosphorylation at the Ser536 residue which is involved in the proteasomal degradation of p65 as a negative feedback mechanism to counter inflammation in cells.
Apical to Basal Transport of γ-EV.
The increase in TEER of the Caco-2 cells during their growth in the inserts, prior to the experiment, was recorded for 21 days (Figure S4a). The transport efficiency of γ-EV was tested through a monolayer of Caco-2 cells over a period of 2 h. A dose of 1 mM γ-EV was analyzed for the transport study as it showed the best anti-inflammatory activity in vitro. Zonula occludens-1 (ZO-1) immunostaining was performed on the inserts containing Caco-2 cells after the samples were completely retrieved (Figure S4b). It was found that 1 mM dose had a Papp of 1.56 × 10−6 ± 0.7 × 10−6 cm/s (Figure 6a). The TEER values of the monolayers were measured before (513.74 ± 25.50 Ωcm2 for n = 10) and after the experiment (468.38 ± 38.95 Ωcm2 for n = 10), and the TEER values remained unaffected after 2 h of peptide treatment. The cell viability of 1 mM γ-EV was measured on Caco-2, and this dosage was found to be nontoxic to the cells (Figure S3c).
Figure 6.
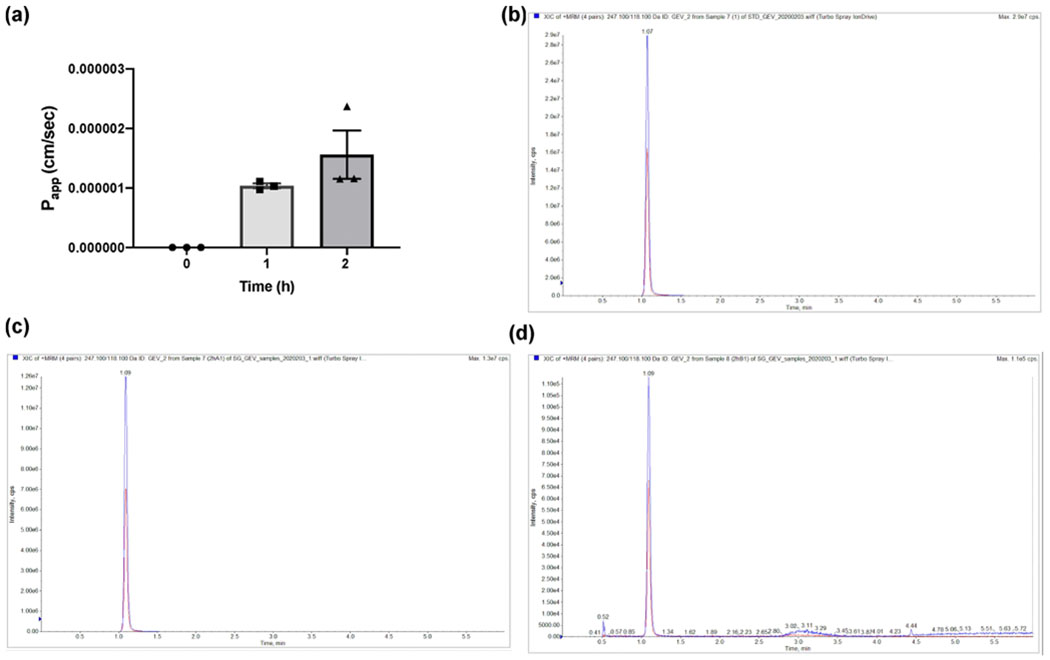
Absorption of 1 mM γ-EV through the Caco-2 monolayer. (a) Apparent permeability coefficient (Papp) of the peptide across the Caco-2 monolayer over 2 h, quantified through LC-MS/MS; (b) MRM of γ-EV in the standard solution at 10 μM; (c) MRM of γ-EV in the apical sample (2 h); (d) MRM of γ-EV in the basal sample (2 h).
The presence of γ-EV in the 2 h basolateral sample was analyzed by LC–MS using MRM to ensure that the peptide was transported intact through the Caco-2 monolayer. The MRM chromatograms shown in Figure 6b–d showed that intact γ-EV could be detected in the 2 h basolateral samples. Figure S5 depicts the calibration curve with the dose range of γ-EV used for the MS/MS analysis.
DISCUSSION
Aberrant expression of the CaSR in noncalcitropic tissues, such as the brain, heart, and vasculature, leads to disease pathogenesis such as cardiovascular diseases, Alzheimer disease, and asthma.18,33 Hence, the CaSR holds the ability to modulate physiological responses in the body. The current study shows that the CaSR is expressed in HAoECs; however, the receptor is seen to be majorly localized in the cytoplasm and expressed lesser in the cell membrane (Figure 1). Such a cytosolic distribution of the CaSR has also been reported in other cell types,34–36 and this may possibly relate to recycling of the membrane receptor, increased rate of biosynthesis of the CaSR, actual intracellular localization, or high rate of post-translational modification.35 Furthermore, in addition to sensing multivalent cations such as Ca2+ and Mg2+, the CaSR can be modulated by L-amino acids which bind to the VFT domain on the extracellular N-terminal of the receptor.37 Molecular modeling of the CaSR VFT domain indicates that the amino acid binding region is relatively unrestricted and hence can bind a large subclass of amino acids, including γ-peptides such as glutathione.38,39 Therefore, after confirming its presence and localization within HAoECs, we analyzed the efficacy of γ-EV to modulate the inflammatory responses in HAoECs via CaSR-mediated pathways. We demonstrated that 1 mM γ-EV exerted the CaSR-mediated anti-inflammatory effect in HAoECs (Figure 2). The anti-inflammatory effect of γ-EV was attenuated by the intervention of the CaSR-specific inhibitor NPS-2143 (Figure 3), suggesting the involvement of the CaSR in modulating the observed effect. However, further studies are required to determine the role of γ-EV in CaSR activation. Anti-inflammatory effects are always a combination of various cellular pathways and crosstalks between cell surface receptors and intracellular signaling proteins. Thus, in the present study, we have made an attempt to identify the critical role of few key signaling molecules in modulating the biological effect of γ-EV.
Endothelial cells, in an unstimulated state, consist of IkB proteins in the cytosol, which inhibit NF-kB dimeric subunits to enter the nucleus. Induction by TNF-α leads to phosphorylation of the IkB proteins, resulting in their ubiquitination and proteasomal degradation,40 as seen in Figure 4a. However, as the inflammation progresses, cells inherently try to resolve the response through NF-kB regulation via the negative feedback loop mechanism. Phosphorylation of the Ser536 residue of the p65 subunit was found to increase the p65 turnover in the cytosol28 and p65 dephosphorylation41 in the nucleus to support resolution of the inflammation. This study demonstrated that γ-EV treatment is possibly promoting this negative feedback mechanism in order to cope up with the inflammation (Figure 4b). Furthermore, it also reduces the phosphorylation of p65 at Thr254, which promotes inflammation (Figure 4c). We also showed that γ-EV increased the levels of β-arrestin2 protein (Figure 4d). β-arrestin2 protein has a crucial role in the anti-inflammatory response as it functions through multiple pathways to reduce the inflammation in a CaSR-dependent manner. First, previous study by Zhang et al. has shown that the CaSR activated with γ-EV recruited β-arrestin2 which subsequently interacted with TAB1 and thereby inhibited the TAK1–TAB1 interaction-mediated inflammation in intestinal epithelial cells.15 Second, β-arrestin2 has also been shown to tightly regulate the activity of the CaSR by mediating its internalization in a Gq/11 and Gi/o independent manner and is modulated by allosteric ligands.32 The internalization of the CaSR might also be a crucial anti-inflammatory mechanism because Ca2+-dependent activation of the CaSR leads to suppression of cAMP, and cAMP, in turn, has been shown to inhibit TNF-α-induced inflammation in ECs.24,42
TNF-α stimulation in ECs activates transcription at the NF-kB site at −94 where NF-kB subunits bind to the E-selectin promoter.43 It has been shown that the CRE/ATF at −153 provides the strongest cytokine responsiveness[36]. This sequence in the E-selectin promoter (TGACATCA) differs from the consensus CRE sequence (TGACGTCA) only by one nucleotide.44 Previous studies have also reported that cAMP is responsible for reducing the TNF-α-induced expression of E-selectin and VCAM-1, but not ICAM-1, in human umbilical vein ECs,24 which is similar to our finding, where we did not observe significant reduction in the ICAM-1 expression levels in HAoECs with 1 mM γ-EV (Figure 2). Although further studies are required, this selective inhibitory activity of cAMP could be either due to its direct role in regulating the transcriptional rate of the E-selectin promoter or through indirect ways by modulating transcription of E-selectin/VCAM-1 inhibitors rather than the E-selectin/VCAM-1 gene itself.24 Furthermore, cAMP also prevents the loss of EC monolayer junctional integrity caused by TNF-α.45 Therefore, cAMP reduces the TNF-induced response of the E-selectin promoter by acting through the CRE/ATF sequences, and the protein factors corresponding to both TNF-α and cAMP compete to bind to the E-selectin CRE/ATF region. TNF-α mediates binding of ATF2/c-jun heterodimers, whereas cAMP mediates binding of ATF2 (without c-Jun) with, possibly, CREB.31 Our study demonstrates an increase in CREB phosphorylation in the presence of γ-EV, indicating a possible role in this effect (Figure 4f). Thus, a combination of all these pathways might have worked toward the anti-inflammatory effect. To summarize, in combination to previous anti-inflammatory studies,15,36,43 our study showed that γ-EV-mediated anti-inflammatory response in ECs could be potentially mediated by internalization of the CaSR, increased β-arrestin2 levels, increased phosphorylation of p65 at the Ser536 residue, and CREB phosphorylation (Figure 5). However, further studies are required to delineate the complete molecular mechanism of the γ-EV-mediated anti-inflammatory effect in ECs.
Finally, in order to evaluate the role of this peptide as a functional food or nutraceutical, its transport efficiency through intestinal epithelial cells is very critical, and our study demonstrated that the peptide (γ-EV) can be transported at a Papp of 1.56 × 10−6 ± 0.7 × 10−6 cm/s across the monolayer of intestinal Caco-2 cells at a dose of 1 mM. On comparing the Papp of γ-EV with other known small bioactive peptides, such as Val-Pro-Pro (0.0005 × 10−6 ± 0.001 cm/s),46 Ile-Pro-Pro (0.01 × 10−6 ± 0.009 cm/s),46 Leu-Lys-Pro (0.18 × 10−6 ± 0.01 cm/s),47 it is apparent that the transport efficiency is relatively high. However, there are also other bioactive peptides which have even higher transport efficiencies, such as Leu-Tyr (3.49 × 10−6 cm/s),48 Val-Tyr (6.8 × 10−6 ± 0.7 cm/s),49 and Gly-Sar (38.6 × 10−6 ± 11.4 cm/s).50 One of the main reasons for the differences in the permeability is the sequence and structure of the peptide itself, which in turn affects either their affinity (Km) to PepT1 or their permeability through the plasma membrane.50 Indeed, our study provides novel evidence supporting the efficacy of γ-EV in reducing vascular inflammation and that γ-EV can be transported via intestinal epithelial cells after oral administration, suggesting its future potential use for the prevention and management of CVDs.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by the National Institutes of Health (NIH) P20GM104320 Nebraska Center for the Prevention of Obesity Diseases through Dietary Molecules (NPOD) pilot grant and University of Nebraska Collaboration Initiative, System Science Seed Grant, to KM. The LC—MS/MS data are based upon work done on the 6500+ QTRAP funded by the Nebraska Research Initiative. Figure 5 and the graphical abstract were created with BioRender.com and were exported under a paid subscription.
ABBREVIATIONS
- CaSR
calcium-sensing receptor
- CREB
cAMP response element-binding protein
- CVDs
cardiovascular diseases
- DAPI
4’,6-diamidine-2’-phenylindole dihydrochloride
- Cs
endothelial cells
- FBS
fetal bovine serum
- γ-EV
γ-glutamyl valine
- HAoECs
human aortic endothelial cells
- ICAM-1
intracellular adhesion molecule-1
- TAB1
TAK1 binding proteins
- TAK1
TGF-β-activated kinase 1
- TEER
transepithelial electrical resistance
- TNF-α
tumor necrosis factor-α
- TNFR
tumor necrosis factor-α receptor
- VCAM-1
vascular cell adhesion molecule-1
- VFT
Venus flytrap
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.0c04526.
Sequential immunoblotting of CaSR; confirmation of CaSR expression in HAoECs fractions; cell viability assay of HAoEC and Caco-2; monitoring the growth of Caco-2 cells; and calibration curve with the dose range of γ-EV (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jafc.0c04526
The authors declare no competing financial interest.
Contributor Information
Snigdha Guha, Department of Food Science and Technology, University of Nebraska-Lincoln, Lincoln 68588, Nebraska, United States.
Catherine Paul, Department of Food Science and Technology, University of Nebraska-Lincoln, Lincoln 68588, Nebraska, United States.
Sophie Alvarez, Proteomics and Metabolomics Facility, Nebraska Center for Biotechnology, University of Nebraska-Lincoln, Lincoln 68588, Nebraska, United States.
Yoshinori Mine, Department of Food Science, University of Guelph, Guelph N1G2W1, Ontario, Canada.
Kaustav Majumder, Department of Food Science and Technology, University of Nebraska-Lincoln, Lincoln 68588, Nebraska, United States.
REFERENCES
- (1).Savoia C; Schiffrin EL Inflammation in hypertension. Curr. Opin. Nephrol. Hypertens 2006, 15, 152–8. [DOI] [PubMed] [Google Scholar]
- (2).Ross R Atherosclerosis - An Inflammatory Disease. N. Engl. J. Med 1999, 340, 115–126. [DOI] [PubMed] [Google Scholar]
- (3).Savoia C; Sada L; Zezza L; Pucci L; Lauri FM; Befani A; Alonzo A; Volpe M Vascular Inflammation and Endothelial Dysfunction in Experimental Hypertension. Int. J. Hypertens 2011, 2011, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Libby P; Ridker PM; Maseri A Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [DOI] [PubMed] [Google Scholar]
- (5).Libby P Current Concepts of the Pathogenesis of the Acute Coronary Syndromes. Circulation 2001, 104, 365–372. [DOI] [PubMed] [Google Scholar]
- (6).Heron M Deaths: leading causes for 2017. National Vital Statistics Reportss, 2019, Vol. 68, pp 1–77. [PubMed] [Google Scholar]
- (7).Cam A; de Mejia EG Role of dietary proteins and peptides in cardiovascular disease. Mol. Nutr. Food Res 2012, 56, 53–66. [DOI] [PubMed] [Google Scholar]
- (8).Majumder K; Mine Y; Wu J The potential of food protein-derived anti-inflammatory peptides against various chronic inflammatory diseases. J. Sci. Food Agric 2016, 96, 2303–2311. [DOI] [PubMed] [Google Scholar]
- (9).Santiago-Lopez L; Gonzalez-Cordova AF; Hernandez-Mendoza A; Vallejo-Cordoba B Potential Use of Food Protein-Derived Peptides in the Treatment of Inflammatory Diseases. Protein Pept. Lett 2017, 24, 137–145. [DOI] [PubMed] [Google Scholar]
- (10).Guha S; Majumder K Structural-features of food-derived bioactive peptides with anti-inflammatory activity: A brief review. J. Food Biochem 2018, 43, No. e12531. [DOI] [PubMed] [Google Scholar]
- (11).Yang J; Bai W; Zeng X; Cui C Gamma glutamyl peptides: The food source, enzymatic synthesis, kokumi-active and the potential functional properties – A review. Trends Food Sci. Technol 2019, 91, 339–346. [Google Scholar]
- (12).Amino Y; Nakazawa M; Kaneko M; Miyaki T; Miyamura N; Maruyama Y; Eto Y Structure–CaSR–Activity Relation of Kokumi γ-Glutamyl Peptides. Chem. Pharm. Bull 2016, 64, 1181–1189. [DOI] [PubMed] [Google Scholar]
- (13).Hannan FM; Kallay E; Chang W; Brandi ML; Thakker RV The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol 2019, 15, 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Zhang H; Kodera T; Eto Y; Mine Y γ-Glutamyl valine supplementation-induced mitigation of gut inflammation in a porcine model of colitis. J. Funct. Foods 2016, 24, 558–567. [Google Scholar]
- (15).Zhang H; Kovacs-Nolan J; Kodera T; Eto Y; Mine Y γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta, Mol. Basis Dis 2015, 1852, 792–804. [DOI] [PubMed] [Google Scholar]
- (16).Chee ME; Majumder K; Mine Y Intervention of Dietary Dipeptide Gamma-l-Glutamyl-l-Valine (γ-EV) Ameliorates Inflammatory Response in a Mouse Model of LPS-Induced Sepsis. J. Agric. Food Chem 2017, 65, 5953–5960. [DOI] [PubMed] [Google Scholar]
- (17).Zhang H; Mine Y Is Calcium-Sensing Receptor a New Molecular Target toward Improving Gastrointestinal Health? J. Agric. Food Chem 2018, 66, 3995–3997. [DOI] [PubMed] [Google Scholar]
- (18).Schepelmann M; Yarova PL; Lopez-Fernandez I; Davies TS; Brennan SC; Edwards PJ; Aggarwal A; Graça J; Rietdorf K; Matchkov V; Fenton RA; Chang W; Krssak M; Stewart A; Broadley KJ; Ward DT; Price SA; Edwards DH; Kemp PJ; Riccardi D The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol.: Cell Physiol 2016, 310, C193–C204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bonomini M; Giardinelli A; Morabito C; Silvestre SD; Cesare MD; Pietro ND; Sirolli V; Formoso G; Amoroso L; Mariggio MA; Pandolfi A Calcimimetic r-568 and its enantiomer s-568 increase nitric oxide release in human endothelial cells. PLoS One 2012, 7, No. e30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Brown EM; MacLeod RJ Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev 2001, 81, 239–297. [DOI] [PubMed] [Google Scholar]
- (21).Conigrave AD; Ward DT Calcium-sensing receptor (CaSR): Pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metabol 2013, 27, 315–331. [DOI] [PubMed] [Google Scholar]
- (22).Ziegelstein RC; Xiong Y; He C; Hu Q Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun 2006, 342, 153–163. [DOI] [PubMed] [Google Scholar]
- (23).Majumder K; Chakrabarti S; Morton JS; Panahi S; Kaufman S; Davidge ST; Wu J Egg-derived ACE-inhibitory peptides IQW and LKP reduce blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2015, 13, 50–60. [Google Scholar]
- (24).Pober JS; Slowik MR; De Luca LG; Ritchie AJ Elevated cyclic AMP inhibits endothelial cell synthesis and expression of TNF-induced endothelial leukocyte adhesion molecule-1, and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. J. Immunol 1993, 150, 5114–5123. [PubMed] [Google Scholar]
- (25).Nemeth EF; Delmar EG; Heaton WL; Miller MA; Lambert LD; Conklin RL; Gowen M; Gleason JG; Bhatnagar PK; Fox J Calcilytic Compounds: Potent and Selective Ca2+Receptor Antagonists That Stimulate Secretion of Parathyroid Hormone. J. Pharmacol. Exp. Ther 2001, 299, 323–331. [PubMed] [Google Scholar]
- (26).Christian F; Smith E; Carmody R The Regulation of NF-kB Subunits by Phosphorylation. Cells 2016, 5, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Mattioli I; Sebald A; Bucher C; Charles R-P; Nakano H; Doi T; Kracht M; Schmitz ML Transient and Selective NF-kB p65 Serine 536 Phosphorylation Induced by T Cell Costimulation Is Mediated by IkB Kinase β and Controls the Kinetics of p65 Nuclear Import. J. Immunol 2004, 172, 6336–6344. [DOI] [PubMed] [Google Scholar]
- (28).Lawrence T; Bebien M; Liu GY; Nizet V; Karin M IKKα limits macrophage NF-kB activation and contributes to the resolution of inflammation. Nature 2005, 434, 1138–1143. [DOI] [PubMed] [Google Scholar]
- (29).Xing D; Gong K; Feng W; Nozell SE; Chen YF; Chatham JC; Oparil S O-GlcNAc modification of nfkb p65 inhibits tnf-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLoS One 2011, 6, No. e24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ryo A; Suizu F; Yoshida Y; Perrem K; Liou Y-C; Wulf G; Rottapel R; Yamaoka S; Lu KP Regulation of NF-kB Signaling by Pin1-Dependent Prolyl Isomerization and Ubiquitin-Mediated Proteolysis of p65/RelA. Mol. Cell 2003, 12, 1413–1426. [DOI] [PubMed] [Google Scholar]
- (31).De Luca LG; Johnson DR; Whitley MZ; Collins T; Pober JS cAMP and Tumor Necrosis Factor Competitively Regulate Transcriptional Activation through and Nuclear Factor Binding to the CAMP-responsive Element/Activating Transcription Factor Element of the Endothelial Leukocyte Adhesion Molecule-1 (E-selectin) Promot. J. Biol. Chem 1994, 269, 19193–19196. [PubMed] [Google Scholar]
- (32).Mos I; Jacobsen SE; Foster SR; Bräuner-Osborne H Calcium-Sensing Receptor Internalization Is β-Arrestin-Dependent and Modulated by Allosteric Ligands. Mol. Pharmacol 2019, 96, 463–474. [DOI] [PubMed] [Google Scholar]
- (33).Ruat M; Traiffort E Roles of the calcium sensing receptor in the central nervous system. Best Pract. Res. Clin. Endocrinol. Metabol 2013, 27, 429–442. [DOI] [PubMed] [Google Scholar]
- (34).Chattopadhyay N; Cheng I; Rogers K; Riccardi D; Hall A; Diaz R; Hebert SC; Soybel DI; Brown EM Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am. J. Physiol.: Gastrointest. Liver Physiol 1998, 274, G122–G130. [DOI] [PubMed] [Google Scholar]
- (35).Bruce JIE; Yang X; Ferguson CJ; Elliott AC; Steward MC; Case RM; Riccardi D Molecular and Functional Identification of a Ca2+(Polyvalent Cation)-sensing Receptor in Rat Pancreas. J. Biol. Chem 1999, 274, 20561–20568. [DOI] [PubMed] [Google Scholar]
- (36).Riccardi D; Hall AE; Chattopadhyay N; Xu JZ; Brown EM; Hebert SC Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. Ren. Physiol 1998, 274, F611–F622. [DOI] [PubMed] [Google Scholar]
- (37).Mun H-C; Franks AH; Culverston EL; Krapcho K; Nemeth EF; Conigrave AD The Venus Fly Trap Domain of the Extracellular Ca2+-sensing Receptor Is Required for l-Amino Acid Sensing. J. Biol. Chem 2004, 279, 51739–51744. [DOI] [PubMed] [Google Scholar]
- (38).Wang M; Hampson DR An evaluation of automated in silico ligand docking of amino acid ligands to Family C G-protein coupled receptors. Bioorg. Med. Chem 2006, 14, 2032–2039. [DOI] [PubMed] [Google Scholar]
- (39).Wang M; Yao Y; Kuang D; Hampson DR Activation of Family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem 2006, 281, 8864–8870. [DOI] [PubMed] [Google Scholar]
- (40).Pober JS Endothelial activation: intracellular signaling pathways. Arthritis Res 2002, 4, S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Nelson DE; Ihekwaba AEC; Elliott M; Johnson JR; Gibney CA; Foreman BE; Nelson G; See V; Horton CA; Spiller DG; Edwards SW; McDowell HP; Unitt JF; Sullivan E; Grimley R; Benson N; Broomhead D; Kell DB; White MRH Oscillations in NF- B Signaling Control the Dynamics of Gene Expression. Science 2004, 306, 704–708. [DOI] [PubMed] [Google Scholar]
- (42).Broadhead GK; Mun H.-c.; Avlani VA; Jourdon O; Church WB; Christopoulos A; Delbridge L; Conigrave AD Allosteric Modulation of the Calcium-sensing Receptor by γ-Glutamyl Peptides. J. Biol. Chem 2011, 286, 8786–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Read MA; Whitley MZ; Williams AJ; Collins T NF-kappa B and I kappa B alpha: an inducible regulatory system in endothelial activation. J. Exp. Med 1994, 179, 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Montminy MR; Bilezikjian LM Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987, 328, 175–178. [DOI] [PubMed] [Google Scholar]
- (45).Stolpen AH; Guinan EC; Fiers W; Pober JS Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am. J. Pathol 1986, 123, 16–24. [PMC free article] [PubMed] [Google Scholar]
- (46).Foltz M; Cerstiaens A; van Meensel A; Mols R; van der Pijl PC; Duchateau GSMJE; Augustijns P The angiotensin converting enzyme inhibitory tripeptides Ile-Pro-Pro and Val-Pro-Pro show increasing permeabilities with increasing physiological relevance of absorption models. Peptides 2008, 29, 1312–1320. [DOI] [PubMed] [Google Scholar]
- (47).Xu Q; Fan H; Yu W; Hong H; Wu J Transport Study of Egg-Derived Antihypertensive Peptides (LKP and IQW) Using Caco-2 and HT29 Coculture Monolayers. J. Agric. Food Chem 2017, 65, 7406–7414. [DOI] [PubMed] [Google Scholar]
- (48).Yang Y-J; He H-Y; Wang F-Z; Yu X-R; Yuan J; Wang L-F; Aluko RE; He R Transport of angiotensin converting enzyme and renin dual inhibitory peptides LY, RALP and TF across Caco-2 cell monolayers. J. Funct. Foods 2017, 35, 303–314. [Google Scholar]
- (49).Takeda J; Park H-Y; Kunitake Y; Yoshiura K; Matsui T Theaflavins, dimeric catechins, inhibit peptide transport across Caco-2 cell monolayers via down-regulation of AMP-activated protein kinase-mediated peptide transporter PEPT1. Food Chem. 2013, 138, 2140–2145. [DOI] [PubMed] [Google Scholar]
- (50).Hong S-M; Tanaka M; Koyanagi R; Shen W; Matsui T Structural Design of Oligopeptides for Intestinal Transport Model. J. Agric. Food Chem 2016, 64, 2072–2079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


