Abstract
Objective
To study the association between sleep duration and the incidence of type 2 diabetes mellitus (T2DM) and to provide a theoretical basis for the prevention of T2DM through a meta-analysis.
Methods
PubMed, Web of Science, Scopus, Embase, Cochrane Library, ProQuest, CNKI, Wanfang, VIP, and SINOMED were searched from their inception until May 2020. All cohort studies on the relationship between sleep duration and T2DM in adults were included. According to the inclusion and exclusion criteria, two authors independently assessed the literature and extracted the data. Metaregression and publication bias were evaluated, and sensitivity and meta-analyses were conducted with RevMan 5.3.
Results
A total of 17 studies were collected, involving 737002 adults. The incidence of T2DM was 4.73% in short sleep duration (SSD) (t ≤ 6 h), 4.39% in normal sleep duration (NSD) (6 h < t < 9 h), and 4.99% in long sleep duration (LSD) (t ≥ 9 h). The meta-analysis demonstrated that SSD increased the risk of T2DM compared with NSD (RR = 1.22, 95% CI: 1.15-1.29, P < 0.001), LSD increased the risk of T2DM compared with NSD (RR = 1.26, 95% CI: 1.15-1.39, P < 0.001), and the risk of T2DM has no significant difference between SSD and LSD (RR = 0.97, 95% CI: 0.89-1.05, P = 0.41). The sensitivity of each study was robust and the publication bias was weak.
Conclusion
SSD or LSD can increase the risk of T2DM.
1. Introduction
Diabetes mellitus (DM) is an epidemic disease in recent years. According to the International Diabetes Federation Diabetes Atlas Ninth Edition published in 2019, it is estimated that 463 million adults (aged between 20 and 79 years) have DM worldwide, and the prevalence has reached 9.3%. This number is expected to reach 578 million (10.2%) by 2030 and 700 million (10.9%) by 2045 [1]. With the aging of the population and the change of lifestyle in China, DM has become an epidemic. The prevalence of DM has soared from 0.67% in 1980 to 10.4% in 2013 [2]. China has 116.4 million people with DM nowadays. In 2019, 0.83 million patients died of DM and complications in China [1].
DM is one of the leading causes of retinopathy, vascular disease, neuropathy, amputation, heart disease, kidney failure, and premature death [3]. There are many risk factors for type 2 diabetes mellitus (T2DM), such as genetic factors and an unhealthy lifestyle. Studies have shown that sleep quality and sleep duration are also risk factors for T2DM [4–6]. However, relevant research conclusions are inconsistent. Some systematic reviews have indicated that short sleep or long sleep are risk factors for T2DM in adult [7–10] and risk factors for gestational diabetes mellitus in pregnant women [11–13]. However, studies for adults have not been updated in time. Therefore, we have conducted a meta-analysis of cohort studies to exhibit the relationship between sleep duration and T2DM in adults to provide the basis for primary prevention of T2DM.
2. Methods
2.1. Literature Search
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines in conducting this systematic review and meta-analysis [14]. We performed a serial literature search for English and non-English papers from inception to May 2020. We have systemically searched the Web of Science Core Collection (Science Citation Index Expanded: 1900–present; Social Sciences Citation Index: 1900–present; Arts & Humanities Citation Index: 1975–present; Conference Proceedings Citation Index—Science: 1996–present; Conference Proceedings Citation Index—Social Science & Humanities: 1996–present; and Emerging Sources Citation Index: 2015–present), PubMed, Scopus, Embase, the Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang, VIP database, and SINOMED from inception to the present. We used the Boolean logic with search terms including “sleep duration,” “sleep amount,” “length of sleep,” “diabetes,” “diabetes mellitus,” “cohort stud∗,” “concurrent stud∗,” and “cohort analy∗.” To search for all terms that begin with a word, enter the word followed by an asterisk. Box 1 provides the detailed search strategy for the Web of Science.
Box 1.

Search strategy in Web of Science.
2.2. Study Eligibility and Selection Criteria
Two authors independently determined study eligibility. Any differences in opinion about eligibility were resolved through another author as a third-party consensus. The inclusion criteria were as follows: (1) cohort studies on the relationship between sleep duration and T2DM in adults, (2) reported sleep duration, (3) diagnosed T2DM, and (4) published English and non-English papers. Studies were not included if (1) they are cross-sectional studies, case-control studies, case reports, and review commentaries; (2) they do not report the incidence of T2DM; (3) they have participants < 10; (4) the subjects were special groups, such as pregnant women and patients after organ transplantation; (5) sleep duration was not reported clearly; (6) they were a duplicate report; and (7) they reported incomplete data and the relevant data were not available.
2.3. Definition of Variables and Outcomes
DM was defined as fasting glucose over 126 mg/dl or 2-hour postprandial glucose (at a glucose tolerance test) over 200 mg/dl or if the subjects were on diabetic drugs or insulin medication. The number of hours of sleep was defined as the average length of their sleep in whole hours at night. The usual sleep duration was self-reported. Taking sleep duration 6 hours < t < 9 hours as a reference, it is categorized as short sleep duration (SSD) (t ≤ 6 hours), normal sleep duration (NSD) (6 hours < t < 9 hours), and long sleep duration (LSD) (t ≥ 9 hours).
2.4. Data Abstraction and Validity Assessment
Data were independently recruited from all included studies on a template adopted from the Cochrane collaboration [15]. For all studies, we extracted the first author, publication year, study design, study location, study period, population, number of T2DM, age, gender (male/female), and data acquisition.
2.5. Assessment of Risk Bias
The risk bias of the included studies was independently assessed by two authors. The cohort study was evaluated by the Newcastle-Ottawa Scale (NOS), which included eight items, categorized into three groups: the selection of study groups, comparability of groups, and ascertainment of outcome [16]. Each study will be evaluated by eight items, and high-quality choices were identified with a star. There are a maximum of one star for each high-quality item within the selection and outcome categories and a maximum of two stars for comparability.
2.6. Statistical Analysis
The meta-analyses were conducted using Review Manager software, version 5.3 (https://community.cochrane.org/help/tools-and-software/revman-5). Dichotomous outcomes eligible in each study are reported as a risk ratio (RR) with an estimated 95% confidence interval (CI). Continuous outcomes are shown as the weighted mean difference (WMD) with the 95% CI, which were calculated from the mean, standard deviation (SD), P value, and sample size in each study. Heterogeneity was assessed using Higgins I2, which evaluates the percentage of total variation across studies that were due to heterogeneity rather than by chance. Thus, if I2 > 50%, which was considered to reflect substantial heterogeneity, a random effect model was used. If I2 ≤ 50%, which was considered to reflect no heterogeneity, a fixed effect model was employed. The chi-square tests were also used to evaluate the heterogeneity: P < 0.1 indicates heterogeneity, while P > 0.1 indicates no heterogeneity. Based on clinical knowledge, the study location and study period were considered to be responsible for heterogeneity, and so, these parameters were set as covariates in the meta-regression. Funnel plots judged the publication biases, and a P < 0.05 was considered statistically significant [17].
2.7. IRB Approval
This meta-analysis study was approved by the institutional review board of the Department of Hepatobiliary and Pancreas Surgery, The First Affiliated Hospital, Xi'an Jiaotong University.
3. Results
3.1. Eligible Studies
A total of 1540 studies were identified, and 325 duplicated articles were excluded. One thousand one hundred and twenty-one studies were excluded after screening the title and abstract. After full-text screening, an additional 77 studies were excluded due to the following criteria: review article (n = 33), reported incomplete data (n = 23), commentary (n = 12), case report (n = 5), and duplicate report (n = 4). Finally, seventeen cohort studies were included in the present meta-analysis [18–34]. Among them, fifteen were in English [18–25, 27, 28, 30–34] and two were in Chinese [26, 29]. The flow chart of the study selection is summarized in Figure 1.
Figure 1.
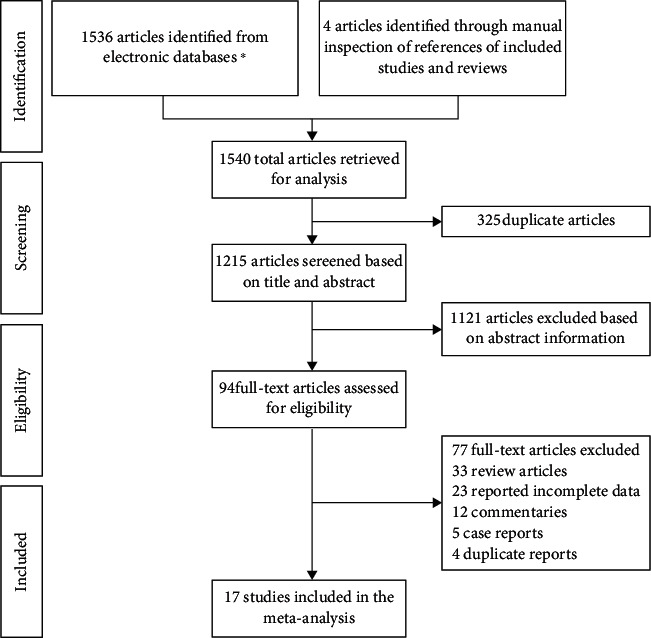
Summary of the literature identification and selection process. ∗ signifies Web of Science (442), PubMed (209), Scopus (490), Embase (117), Cochrane (71), ProQuest (34), CNKI (80), WanFang (61), VIP (10), and SinoMed (22).
3.2. Study Characteristics
A total of 17 studies were selected for inclusion in this meta-analysis [18–34], with 737002 people. Among them were five each from China [21, 23, 25, 26, 29], and the United States [19, 28, 30, 31, 34], and one study each from Norway [18], South Korea [20], Britain [22], Australia [24], Japan [27], Finland [32], and Sweden [33]. Four studies' research subjects, those of Lou et al. [ 25]., Gao et al. [26], Li et al. [29], and Mallon et al. [33] were analyzed independently by gender. The research object of Ayas et al. is male [34]. A summary of the included studies is presented in Table 1.
Table 1.
Characteristics of included studies.
| Study | Study design | Study location | Study period | Total | T2DM | Age | Gender (male/female) | Data acquisition |
|---|---|---|---|---|---|---|---|---|
| Asante et al. (2020) [18] | Prospective cohort study | Nord-Trøndelag, Norway | 2006–2008 | 17058 | 362 | 20–55 | NR | Questionnaires, laboratory measurements |
| Maskarinec et al. (2018) [19] | Prospective cohort study | Hawaii and California, USA | 1993–1996 | 151691 | 8487 | 45–75 | 69097/82594 | Self-reported |
| Kim et al. (2017) [20] | Cohort study | Seoul and Suwon, South Korea | 2011 | 17983 | 664 | 41 ± 5.9 | 11995/5988 | Questionnaires |
| Han et al. (2016) [21] | Cohort study | Hubei, China | 2008–2013 | 16399 | 1123 | 62.5 | 7083/9316 | Face-to-face interviews |
| Leng et al. (2016) [22] | Prospective cohort study | Britain | 1998–2000 | 13465 | 285 | 40–74 | 5887/7578 | Questionnaires |
| Song et al. (2016) [23] | Prospective cohort study | Hebei, China | 2006.6–2015 | 56588 | 4899 | 49 | 43494/13094 | Face-to-face interviews |
| Ding et al. (2015) [24] | Cohort study | New South Wales, Australia | 2006–2010 | 54997 | 888 | ≥45 | 25038/29959 | Self-reported |
| Lou et al. (2015) [25] | Prospective cohort study | Xuzhou, China | 2008–2013 | 11842 | 367 | ≥18 | 5375/6467 | Interviews, laboratory measurements |
| Gao et al. (2014) [26] | Prospective cohort study | Hebei, China | 2006–2011 | 60715 | 3084 | 49.14 ± 11.96 | 47048/13667 | Questionnaires |
| Heianza et al. (2014) [27] | Prospective cohort study | Niigata, Japan | 1999.4–2004.3 | 38987 | 2085 | 18–83 | NR | Questionnaires |
| Boyko et al. (2013) [28] | Cohort study | USA | 2001–2007 | 47093 | 871 | 34.9 ± 9.0 | 35037/12056 | Self-reported, questionnaires |
| Li et al. (2011) [29] | Cohort study | Jiangsu, China | 2004–2007 | 3031 | 76 | 52.28 ± 12.31 | 1381/1647 | Questionnaires |
| Xu et al. (2010) [30] | Prospective cohort study | USA | 2000–2006 | 174542 | 10143 | 50–71 | 99060/75284 | Questionnaires |
| Beihl et al. (2009) [31] | Cohort study | USA | 1992.10–1999.7 | 900 | 146 | 40–69 | 390/510 | Interviews |
| Tuomilehto et al. (2009) [32] | Prospective cohort study | Finland | 2004.10–2005.1 | 515 | 182 | 40–64 | 170/345 | Interviews, laboratory measurements |
| Mallon et al. (2005) [33] | Cohort study | Sweden | 1983–1995 | 1170 | 88 | 45–65 | 550/620 | Questionnaire |
| Ayas et al. (2003) [34] | Cohort study | USA | 1986–1996 | 70026 | 1969 | 30–55 | 0/70026 | Interviews |
NR: not reported.
3.3. Study Quality
The study quality for all 17 independent studies is shown in Table 2.
Table 2.
Quality of included studies.
| Study | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | |
| Asante et al. (2020) [18] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Maskarinec et al. (2018) [19] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Kim et al. (2017) [20] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Han et al. (2016) [21] | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Leng et al. (2016) [22] | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Song et al. (2016) [23] | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Ding et al. (2015) [24] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Lou et al. (2015) [25] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Gao et al. (2014) [26] | Y | Y | Y | Y | N | N | Y | Y | N |
| Heianza et al. (2014) [27] | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Boyko et al. (2013) [28] | N | Y | Y | Y | Y | Y | Y | Y | N |
| Li et al. (2011) [29] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Xu et al. (2010) [30] | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Beihl et al. (2009) [31] | Y | Y | Y | Y | Y | Y | Y | Y | N |
| Tuomilehto et al. (2009) [32] | N | Y | Y | Y | Y | Y | Y | Y | N |
| Mallon et al. (2005) [33] | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Ayas et al. (2003) [34] | Y | Y | Y | Y | Y | Y | Y | Y | N |
Y: yes; N: no.
4. Meta-Analysis Results
4.1. A Meta-Analysis of the Relationship between SSD and T2DM
We have analyzed a total of 17 studies that involved 712818 participants [18–34]. The incidence of T2DM was 4.73% (10380/219678) in SSD (t ≤ 6 h) and 4.39% (21652/493140) in NSD (6 h < t < 9 h). There was high heterogeneity among the studies, and the random effect model was used. Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD (RR = 1.22, 95% CI: 1.15-1.29, P < 0.001) (Figure 2).
Figure 2.
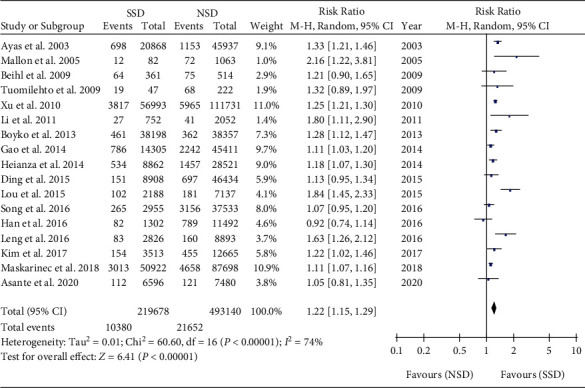
Pooled results for the association between SSD and T2DM.
Subgroup analyses were performed among male [25, 26, 29, 33], female [25, 26, 29, 33, 34], and mixed male and female [18–24, 27, 28, 30–32]. Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in the male subgroup (RR = 1.79, 95% CI: 1.08-2.95, P = 0.02). Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in female subgroups (RR = 1.34, 95% CI: 1.23-1.46, P < 0.001). Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in the mixed male and female subgroup (RR = 1.17, 95% CI: 1.10-1.25, P < 0.001) (Figure 3).
Figure 3.
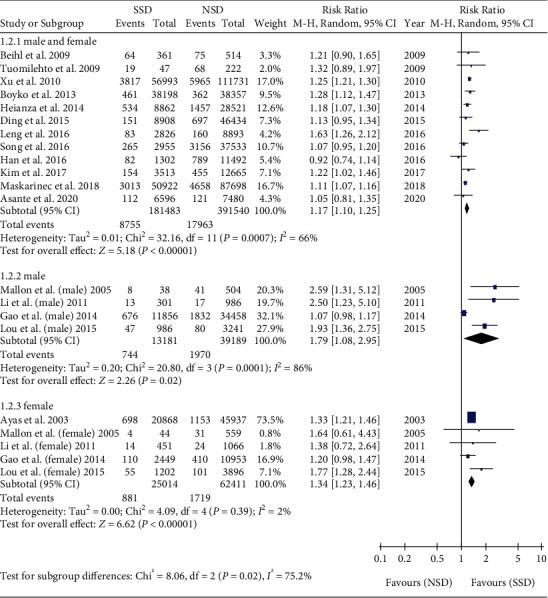
Gender subgroup analysis of the association between SSD and T2DM.
Subgroup analyses were performed among the Asia [20, 21, 23, 25–27, 29], America [19, 28, 30, 31, 34], Europe [18, 22, 32, 33], and Oceania subgroups [24]. Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in the Asia subgroup (RR = 1.20, 95% CI: 1.07-1.35, P = 0.003). Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in the America subgroups (RR = 1.23, 95% CI: 1.14-1.33, P < 0.001). Meta-analysis showed that the incidence of T2DM in SSD was higher than that in NSD in the Europe subgroup (RR = 1.42, 95% CI: 1.06-1.89, P = 0.02) (Figure 4).
Figure 4.
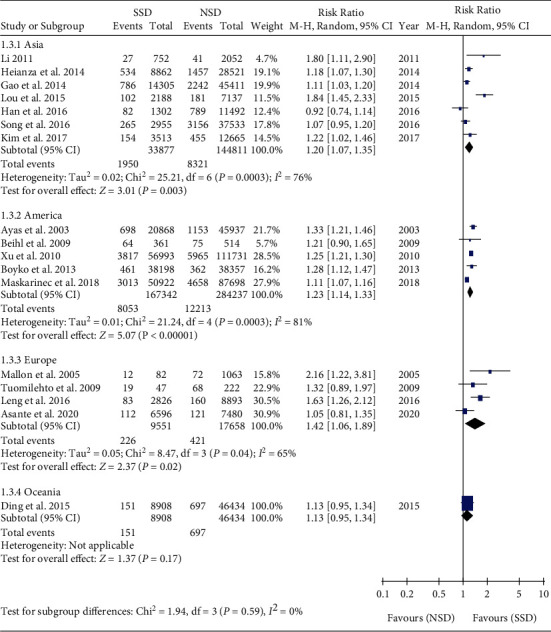
Regional subgroup analysis of the association between SSD and T2DM.
4.2. Meta-Analysis of the Relationship between LSD and T2DM
We have analyzed a total of 17 studies that involved 538593 participants [18–34]. The incidence of T2DM was 4.99% (2270/45453) in LSD (t ≥ 9 h) and 4.39% (21652/493140) in NSD (6 h < t < 9 h). There was high heterogeneity among the studies, and the random effect model was used. Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD (RR = 1.26, 95% CI: 1.15-1.39, P < 0.001) (Figure 5).
Figure 5.
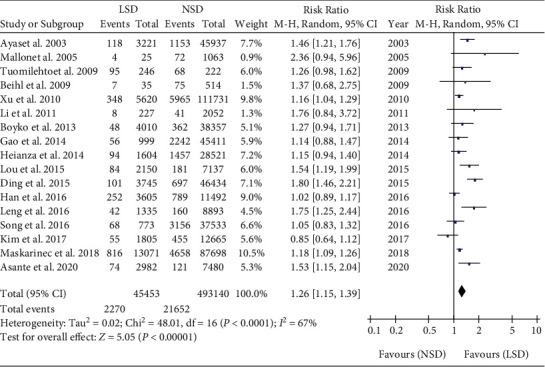
Pooled results for the association between LSD and T2DM.
Subgroup analyses were performed among male [25, 26, 29, 33], female [25, 26, 29, 33, 34], and mixed male and female [18–24, 27, 28, 30–32]. Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in the male subgroup (RR = 1.55, 95% CI: 1.08-2.21, P = 0.02). Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in female subgroups (RR = 1.41, 95% CI: 1.20-1.64, P < 0.001). Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in the mixed male and female subgroup (RR = 1.22, 95% CI: 1.10-1.35, P = 0.0001) (Figure 6).
Figure 6.
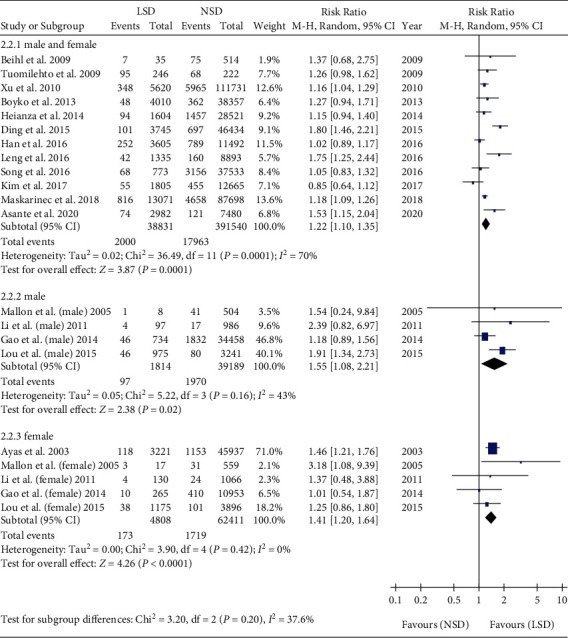
Gender subgroup analysis of the association between LSD and T2DM.
Subgroup analyses were performed among the Asia [20, 21, 23, 25–27, 29], America [19, 28, 30, 31, 34], Europe [18, 22, 32, 33], and Oceania subgroups [24]. Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in the Asia subgroup (RR = 1.12, 95% CI: 1.01-1.25, P = 0.03). Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in the America subgroups (RR = 1.27, 95% CI: 1.12-1.44, P = 0.0003). Meta-analysis showed that the incidence of T2DM in LSD was higher than that in NSD in the Europe subgroup (RR = 1.49, 95% CI: 1.24-1.79, P < 0.001) (Figure 7).
Figure 7.
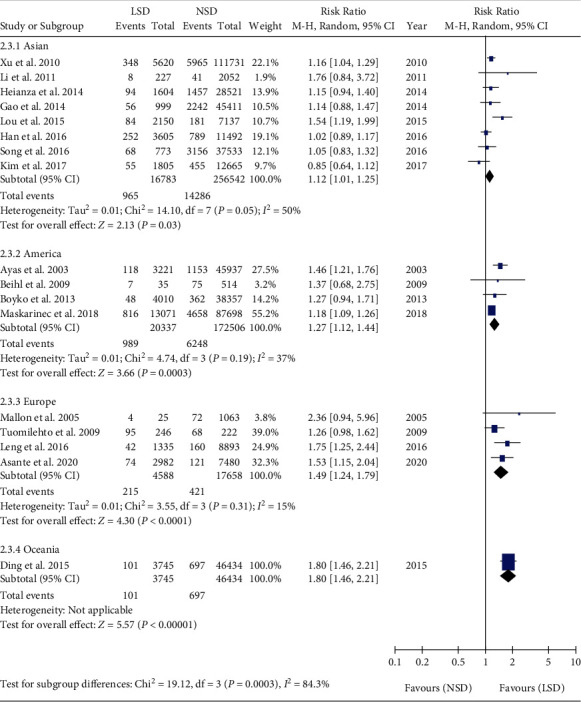
Regional subgroup analysis of the association between LSD and T2DM.
4.3. Meta-Analysis of SSD versus LSD for T2DM
There was high heterogeneity among the studies, and the random effect model was used. Meta-analysis showed that the incidence of T2DM was not significantly different between SSD and LSD (RR = 0.97, 95% CI: 0.89-1.05, P = 0.41) (Figure 8).
Figure 8.
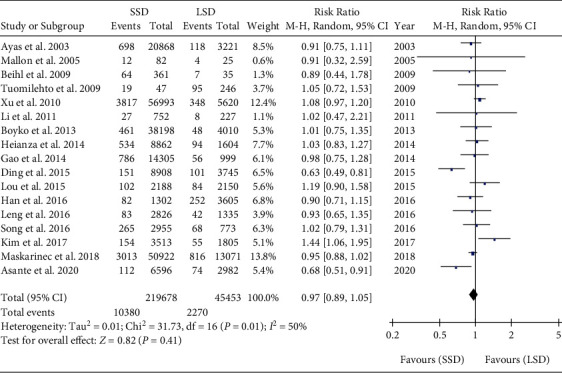
Pooled results of SSD versus LSD for T2DM.
4.4. Sensitivity Analyses
Sensitivity analyses of the association between SSD, LSD, and T2DM were conducted. The results indicated that the sensitivity of the association between SSD and T2DM was robust after each study was excluded one by one. The RR was 1.22 and 95% CI was 1.15-1.29 (Figure 9). The results indicated that the sensitivity of the association between LSD and T2DM was robust after each study was excluded one by one. The RR was 1.26 and 95% CI was 1.15-1.39 (Figure 10). When the Chinese literature was excluded, the meta-analysis results and heterogeneity did not change significantly. However, according to gender subgroup analysis, the heterogeneity of the male subgroup and female subgroup decreased rapidly. The sensitivity of each study was robust.
Figure 9.
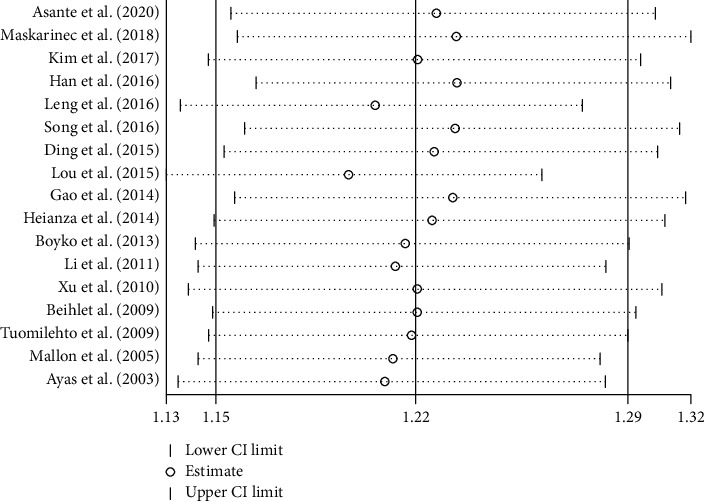
Sensitivity analyses of the association between SSD and T2DM.
Figure 10.
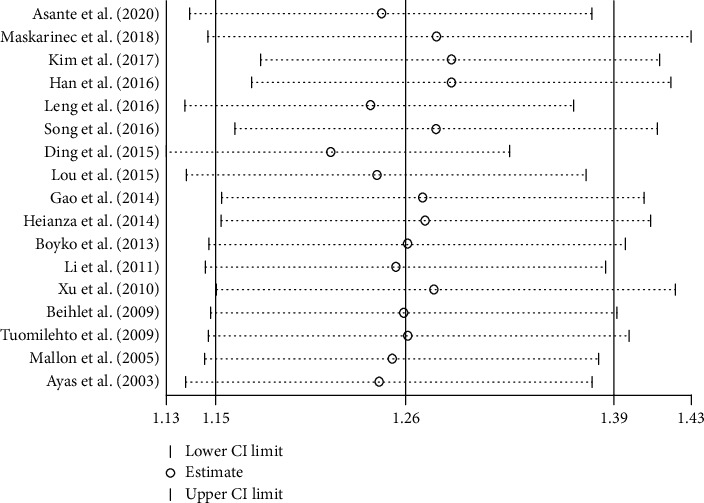
Sensitivity analyses of the association between LSD and T2DM.
4.5. Metaregression for the Sleep Duration and T2DM
Metaregression analyses were performed in the meta-analysis of the relationship between SSD and T2DM. The results indicated that neither the study location (coefficient = 0.021, SE = 0.050, t = 0.42, P = 0.678, 95% CI: −0.085 to 0.127) nor the study period (coefficient = 0.056, SE = 0.064, t = 0.87, P = 0.398, 95% CI: −0.082 to 0.194) was responsible for this heterogeneity. Other factors, such as age, cannot be fully extracted from the text.
Metaregression analyses were performed in the meta-analysis of the relationship between LSD and T2DM. Metaregression indicated that study location may have been responsible for this heterogeneity (coefficient = 0.154, SE = 0.040, t = 3.83, P = 0.002, 95% CI: 0.068 to 0.239). And no significant association was observed in the study period (coefficient = 0.135, SE = 0.075, t = 1.79, P = 0.095, 95% CI: −0.027 to 0.297).
4.6. Publication Bias Analyses
Funnel plots of publication bias for the association between SSD, LSD, and T2DM were assessed. The symmetry found in the funnel plots indicated that the publication bias was weak. (Figures 11 and 12) Most of the studies are at the top of the funnel plot, indicating that the quality of the studies is good.
Figure 11.
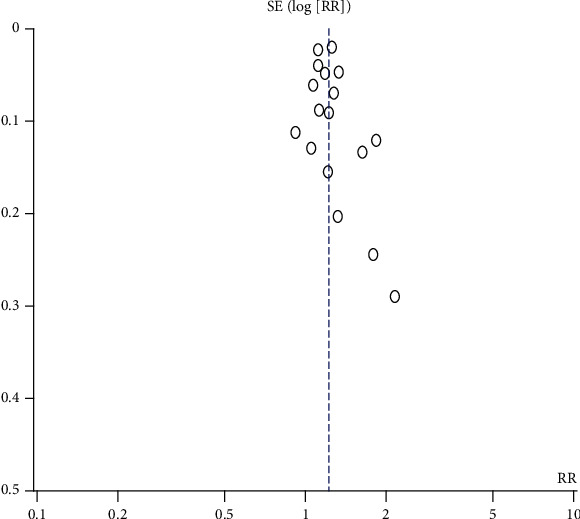
Funnel plots of publication bias for the association between SSD and T2DM.
Figure 12.
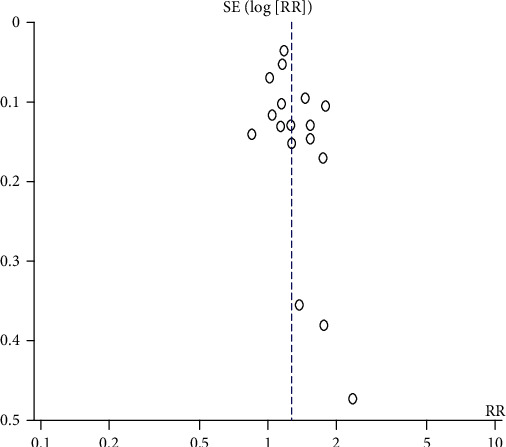
Funnel plots of publication bias for the association between LSD and T2DM.
5. Discussion
The countries with the largest numbers of adults with DM aged 20–79 years in 2019 are China, India, and America with 116.4 million, 77 million, and 31 million DM patients (aged 20–79 years), respectively, and are anticipated to remain so in 2030 [1]. T2DM is closely related to unhealthy lifestyles [2]. Studies have shown that sleep is closely related to endocrine and metabolism [35]. With the progress of society and the development of the economy, the pace of modern life is getting faster and faster. Sleep discomfort causes many health problems. Studies have found that the average American sleep duration is from 9 hours in 1910 to 6.8 hours in 2005 [36]. Sleep discomfort causes not only endocrine disorders and other pathological states but also affects personal mental health. Therefore, improving sleep is a good measure to improve the quality of life.
In this meta-analysis of the association between SSD and T2DM, the risk of T2DM with SSD was 1.22 times higher than that with NSD, which was consistent with the conclusions of other cohort studies [37–45]. The risk of T2DM in male with SSD was 1.79 times higher than that with NSD. The risk of T2DM in females with SSD was 1.34 times higher than that with NSD. The risk of T2DM in males with SSD was slightly higher than that in females. Subgroup analysis by region showed that there was no significant difference among the Asian, American, and European subgroups. The risk of T2DM in Asia with SSD was 1.79 times higher than that with NSD. The risk of T2DM in America with SSD was 1.34 times higher than that with NSD. The risk of T2DM in Europe with SSD was 1.24 times higher than that with NSD. The mechanism of SSD leading to T2DM is still unclear. The possible reason is that sleep deprivation causes the imbalance of the sympathetic vagus nerve, which leads to the decrease of β cell response ability, the inhibition of insulin secretion, and the further development of insulin resistance and T2DM. Lack of sleep may also cause the release of a large number of inflammatory factors, inhibits the activity of islet receptor L-arginine kinase, and leads to insulin resistance. Some studies found that slow-wave sleep duration decreased insulin sensitivity and increased the risk of T2DM [46–48].
In this meta-analysis of the association between LSD and T2DM, the risk of T2DM with LSD was 1.26 times higher than that with NSD, which was consistent with the conclusions of other cohort studies [37–45]. The risk of T2DM in male with LSD was 1.55 times higher than that with NSD. The risk of T2DM in females with LSD was 1.27 times higher than that with NSD. Subgroup analysis by region showed that there were differences among the Asian, American, and European subgroups. The risk of T2DM in Asia with LSD was 1.12 times higher than that with NSD. The risk of T2DM in America with LSD was 1.27 times higher than that with NSD. The risk of T2DM in Europe with LSD was 1.49 times higher than that with NSD. There are few studies on the relationship between LSD and T2DM. The reason may be that long sleeps are a poor sleep quality actually and they may prolong sleep duration to make up for the impact of poor sleep quality. Sleeping for a long time is harmful to health itself. Excessive sleep duration may be associated with other factors, such as obesity, poor social and economic status, low physical activity, depression, sleep apnea, or other chronic diseases. These factors can produce a confounding effect [46, 47, 49].
The meta-analysis found that LSD or SSD will increase the risk of T2DM. Some studies suggest that LSD or SSD can lead to impaired fasting blood glucose, abnormal HbA1, and insulin resistance [50–56]. There was a U-shaped dose response relationship between sleep duration and risk of T2DM [10, 57]. SSD or LSD increased the risk of T2DM. Therefore, the risk of T2DM can be reduced if the sleep duration of the patients with poor sleep is changed and the sleep duration is maintained between 7 and 8 hours. SSD or LSD is a risk factor for chronic diseases such as DM, coronary heart disease, stroke, and obesity [7, 9, 58, 59]. The sensitivity of each study was robust and the publication bias was weak. While we found that the study location may have been responsible for this heterogeneity in the meta-analysis of the relationship between LSD and T2DM, other factors, such as age, cannot be fully extracted from the text.
5.1. Limitations
Limitations are listed as follows: (1) some studies have not been retrieved. All the included studies are published in Chinese and English, and there may be incomplete literature retrieval, (2) the overall heterogeneity of the study is high, suggesting that there is heterogeneity among the studies. However, after subgroup analyses were performed, the heterogeneity of subgroups decreased significantly, (3) the study explored the association between sleep duration and T2DM but paid less attention to the association between sleep quality and T2DM, which also indicated that we should focus on it in later research, and (4) for special populations such as gestational diabetes, postpartum diabetes, and posttransplant diabetes, there is less attention.
More and more studies have confirmed that an unhealthy lifestyle plays an important role in the pathogenesis of T2DM and glucose control in DM [60]. From the perspective of prevention, appropriate sleep duration can be used as the primary prevention of T2DM [7, 9, 61, 62]. Good sleep should be considered as an important health component in the prevention and treatment of T2DM.
6. Conclusions
The purpose of the current study was to determine the cohort studies of the relationship between sleep duration and T2DM in adults with a systematic review and meta-analysis. This study provides the first systematic assessment of the cohort study of the relationship between sleep duration and T2DM. The findings indicate that SSD or LSD is a risk factor for T2DM. The findings of this study have several important implications for future practice. Further research is required to attend to the association between sleep quality and T2DM.
Acknowledgments
This research was supported by grants from the Ministry of Education Innovation Team Development Program (IRT_16R57).
Contributor Information
Qinling Yang, Email: 13519133867@163.com.
Xuemei Zheng, Email: xazhxm@xjtu.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
This meta-analysis study was approved by the institutional review board of the Department of Hepatobiliary and Pancreas Surgery, The First Affiliated Hospital, Xi'an Jiaotong University. All protocols conformed with the ethical guidelines of the 1975 Helsinki Declaration, and written, informed consent was obtained from all patients.
Disclosure
None of the authors have any financial and personal relationships with other people or organizations that could inappropriately influence their work.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
The study design was done by Huapeng Lu, Qinling Yang, Fang Tian, and Yi Lyu. Data collection was done by Huapeng Lu and Qinling Yang. Data analysis was done by Fang Tian and Yi Lyu. The manuscript writing was done by Huapeng Lu and Qinling Yang. Critical revisions were done by Xuemei Zheng, Hairong He, and Xia Xin. Huapeng Lu and Qinling Yang contributed equally to this study.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 9th ed. 2019. 2020, https://diabetesatlas.org/en/ [PubMed]
- 2.Chinese Diabetes Society. Guideline for the prevention and control of type 2 diabetes in China (2017 edition) Chinese Journal of Practical Internal Medicine. 2018;38(4):292–344. doi: 10.3760/cma.j.issn.1674-5809.2018.01.003. [DOI] [Google Scholar]
- 3.Cass A. R., Alonso W. J., Islam J., Weller S. C. Risk of obstructive sleep apnea in patients with type 2 diabetes mellitus. Family Medicine. 2013;45(7):492–500. [PubMed] [Google Scholar]
- 4.Touma C., Pannain S. Does lack of sleep cause diabetes? Cleveland Clinic Journal of Medicine. 2011;78(8):549–558. doi: 10.3949/ccjm.78a.10165. [DOI] [PubMed] [Google Scholar]
- 5.Barone M. T. U., Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Research and Clinical Practice. 2011;91(2):129–137. doi: 10.1016/j.diabres.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Larcher S., Benhamou P. Y., Pépin J. L., Borel A. L. Sleep habits and diabetes. Diabetes & Metabolism. 2015;41(4):263–271. doi: 10.1016/j.diabet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Jike M., Itani O., Watanabe N., Buysse D. J., Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Medicine Reviews. 2018;39:25–36. doi: 10.1016/j.smrv.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y. H., Shi L., Bao Y. P., et al. Association between sleep duration during pregnancy and gestational diabetes mellitus: a meta-analysis. Sleep Medicine. 2018;52:67–74. doi: 10.1016/j.sleep.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Itani O., Jike M., Watanabe N., Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Medicine. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Shan Z., Ma H., Xie M., et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q., Zhang X., Wang Y., et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Medicine Reviews. 2021;58:p. 101436. doi: 10.1016/j.smrv.2021.101436. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X., Zhang R., Cheng L., et al. The effect of sleep impairment on gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sleep Medicine. 2020;74:267–277. doi: 10.1016/j.sleep.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Gao X., Sun H., Zhang Y., Liu L., Wang J., Wang T. Investigating causal relations between sleep-related traits and risk of type 2 diabetes mellitus: a Mendelian randomization study. Frontiers in Genetics. 2020;11:p. 607865. doi: 10.3389/fgene.2020.607865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li T., Higgins J. P., Deeks J. J. Cochrane handbook for systematic reviews of interventions Version 6. John Wiley & Son; 2019. [Google Scholar]
- 16.Wells G. A., Shea B., O’Connell D. A., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Lu H., Hou Y., Chen J., et al. Risk of catheter-related bloodstream infection associated with midline catheters compared with peripherally inserted central catheters: a meta-analysis. Nursing Open. 2020 doi: 10.1002/nop2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asante E. O., Sun Y., Nilsen T. I. L., Åsvold B. O., Sørgjerd E. P., Mai X. M. Hours lying down per day, as a proxy for sedentary behaviour and risk of diabetes in young and middle-aged adults in Norway: an 11-year follow-up of the HUNT study. BMJ Open. 2020;10(3, article e0350103):p. e035010. doi: 10.1136/bmjopen-2019-035010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maskarinec G., Jacobs S., Amshoff Y., et al. Sleep duration and incidence of type 2 diabetes: the multiethnic cohort. Sleep Health. 2018;4(1):27–32. doi: 10.1016/j.sleh.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C. W., Chang Y., Sung E., Ryu S. Sleep duration and progression to diabetes in people with prediabetes defined by HbA1cconcentration. Diabetic Medicine. 2017;34(11):1591–1598. doi: 10.1111/dme.13432. [DOI] [PubMed] [Google Scholar]
- 21.Han X., Liu B., Wang J., et al. Long sleep duration and afternoon napping are associated with higher risk of incident diabetes in middle-aged and older Chinese: the Dongfeng-Tongji cohort study. Annals of Medicine. 2016;48(4):216–223. doi: 10.3109/07853890.2016.1155229. [DOI] [PubMed] [Google Scholar]
- 22.Leng Y., Cappuccio F. P., Surtees P. G., Luben R., Brayne C., Khaw K. T. Daytime napping, sleep duration and increased 8-year risk of type 2 diabetes in a British population. Nutrition, Metabolism, and Cardiovascular Diseases. 2016;26(11):996–1003. doi: 10.1016/j.numecd.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Q., Liu X., Zhou W., Wang X., Wu S. Short-term changes in sleep duration and risk of type 2 diabetes: Kailuan prospective study. Medicine (Baltimore) 2016;95(45, article e5363) doi: 10.1097/MD.0000000000005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding D., Chong S., Jalaludin B., Comino E., Bauman A. E. Risk factors of incident type 2-diabetes mellitus over a 3-year follow-up: results from a large Australian sample. Diabetes Research and Clinical Practice. 2015;108(2):306–315. doi: 10.1016/j.diabres.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Lou P., Zhang P., Zhang L., et al. Effects of sleep duration and sleep quality on prevalence of type 2 diabetes mellitus: a 5-year follow-up study in China. Diabetes Research and Clinical Practice. 2015;109(1):178–184. doi: 10.1016/j.diabres.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Gao M., Li J., Wu Y. Relationship between sleep time and risk of newly onset diabetes. Chinese Journal of Endocrinology and Metabolism. 2014;30(5):393–396. doi: 10.3760/cma.j.issn.1000-6699.2014.05.008. [DOI] [Google Scholar]
- 27.Heianza Y., Kato K., Fujihara K., et al. Role of sleep duration as a risk factor for type 2 diabetes among adults of different ages in Japan: the Niigata wellness study. Diabetic Medicine. 2014;31(11):1363–1367. doi: 10.1111/dme.12555. [DOI] [PubMed] [Google Scholar]
- 28.Boyko E. J., Seelig A. D., Jacobson I. G., et al. Sleep characteristics, mental health, and diabetes risk: a prospective study of U.S. military service members in the Millennium Cohort Study. Diabetes Care. 2013;36(10):3154–3161. doi: 10.2337/DC13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Hong X., Liang Y., Wang Z., Xu F. The relationship between sleeping duration and type 2 diabetes mellitus among adults: a three-year community-based follow-up study in Nanjing. Modern Preventive Medicine. 2011;38(18):3646–3648. [Google Scholar]
- 30.Xu Q., Song Y., Hollenbeck A., Blair A., Schatzkin A., Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2009;33(1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beihl D. A., Liese A. D., Haffner S. M. Sleep Duration as a Risk Factor for Incident Type 2 Diabetes in a Multiethnic Cohort. Annals of Epidemiology. 2009;19(5):351–357. doi: 10.1016/j.annepidem.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Tuomilehto H., Peltonen M., Partinen M., et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: the Finnish Diabetes Prevention Study. Diabetes Care. 2009;32(11):1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallon L., Broman J. E., Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 34.Ayas N. T., White D. P., al-Delaimy W. K., et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 35.Briançon-Marjollet A., Weiszenstein M., Henri M., Thomas A., Godin-Ribuot D., Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetology and Metabolic Syndrome. 2015;7(1):p. 25. doi: 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Egan B. M. Sleep and hypertension: burning the candle at both ends really is hazardous to your health. Hypertension. 2006;47(5):816–817. doi: 10.1161/01.HYP.0000217363.62667.95. [DOI] [PubMed] [Google Scholar]
- 37.Gangwisch J. E., Heymsfield S. B., Boden-Albala B., et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.the HIPOP-OHP Research group, Hayashino Y., Fukuhara S., et al. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the high-risk and population strategy for occupational health promotion (HIPOP-OHP) study. BMC Public Health. 2007;7(1):p. 129. doi: 10.1186/1471-2458-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaggi H. K., Araujo A. B., Mckinlay J. B. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 40.Bjorkelund C., Bondyr-Carlsson D., Lapidus L., et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 41.Li X., Li Y. Sleep patterns, glycolipid metabolism disorders and prospective cohort studies. Sleep Medicine. 2019;64(S1):S225–S226. doi: 10.1016/j.sleep.2019.11.630. [DOI] [Google Scholar]
- 42.Kadotani H., Yamaguchi M., Nagai Y., et al. Poster presentations 1. Sleep and Biological Rhythms. 2011;9(4):254–342. doi: 10.1111/j.1479-8425.2011.00518.x. [DOI] [Google Scholar]
- 43.Holliday E. G., Magee C. A., Kritharides L., Banks E., Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PLoS One. 2013;8(11, article e82305) doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Ruesten A., Weikert C., Fietze I., Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. PLoS One. 2012;7(1, article e30972) doi: 10.1371/journal.pone.0030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kita T., Yoshioka E., Satoh H., et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35(2):313–318. doi: 10.2337/dc11-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L., Wan J., Zhou Y., Deng Q., Sun J. Sleep and type 2 diabetes mellitus. International Journal of Endocrinology and Metabolism. 2019;39(3):182–186. doi: 10.3760/cma.j.issn.1673-4157.2019.03.009. [DOI] [Google Scholar]
- 47.Wu H., Yang L., Yu M., Zhong J., Hu R. Progress in research of association between sleep duration and type 2 diabetes. Chinese Journal of Epidemiology. 2017;38(3):411–416. doi: 10.3760/cma.j.issn.0254-6450.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Pan Z., Peian L., Peipei C., et al. Relationship between sleep duration and risk of type 2 diabetes mellitus. Chinese Journal of Prevention and Control of Chronic Diseases. 2015;23(4):279–281. doi: 10.16386/j.cjpccd.issn.1004-6194.2015.04.021. [DOI] [Google Scholar]
- 49.Gutiérrez-Repiso C., Soriguer F., Rubio-Martín E., et al. Night-time sleep duration and the incidence of obesity and type 2 diabetes. Findings from the prospective Pizarra study. Sleep Medicine. 2014;15(11):1398–1404. doi: 10.1016/j.sleep.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Lee S. W., Ng K. Y., Chin W. K. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Medicine Reviews. 2017;31:91–101. doi: 10.1016/j.smrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Kim C. R., Song Y. M., Shin J. Y., Gim W. Association between sleep duration and impaired fasting glucose in Korean adults: results from the Korean National Health and Nutrition Examination Survey 2011-2012. Korean Journal of Family Medicine. 2016;37(1):51–56. doi: 10.4082/kjfm.2016.37.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upala S., Sanguankeo A., Congrete S., Romphothong K. Sleep duration and insulin resistance in individuals without diabetes mellitus: a systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2015;109(3):e11–e12. doi: 10.1016/j.diabres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Lou P., Chen P., Zhang L., et al. Interaction of sleep quality and sleep duration on impaired fasting glucose: a population-based cross-sectional survey in China. BMJ Open. 2014;4(3):e004436–e004436. doi: 10.1136/bmjopen-2013-004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pyykkönen A. J., Isomaa B., Pesonen A. K., et al. Sleep duration and insulin resistance in individuals without type 2 diabetes: the PPP-Botnia study. Annals of Medicine. 2014;46(5):324–329. doi: 10.3109/07853890.2014.902226. [DOI] [PubMed] [Google Scholar]
- 55.Kim B. K., Kim B. S., An S. Y., et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health and Nutrition Examination Survey 2007-2010. Journal of Korean Medical Science. 2013;28(9):1334–1339. doi: 10.3346/jkms.2013.28.9.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katano S., Nakamura Y., Nakamura A., et al. Association of short sleep duration with impaired glucose tolerance or diabetes mellitus. Journal of Diabetes Investigation. 2011;2(5):366–372. doi: 10.1111/j.2040-1124.2011.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohkuma T., Fujii H., Iwase M., et al. Impact of sleep duration on obesity and the glycemic level in patients with type 2 diabetes: the Fukuoka Diabetes Registry. Diabetes Care. 2013;36(3):611–617. doi: 10.2337/dc12-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akinseye O. A., Ojike N. I., Akinseye L. I., Dhandapany P. S., Pandi-Perumal S. R. Association of sleep duration with stroke in diabetic patients: analysis of the National Health Interview Survey. Journal of Stroke and Cerebrovascular Diseases. 2016;25(3):650–655. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 59.Kubota Y., Iso H., Ikehara S., Tamakoshi A. Relationship between sleep duration and cause-specific mortality in diabetic men and women based on self-reports. Sleep and Biological Rhythms. 2015;13(1):85–93. doi: 10.1111/sbr.12091. [DOI] [Google Scholar]
- 60.Gozashti M. H., Eslami N., Radfar M. H., Pakmanesh H. Sleep pattern, duration and quality in relation with glycemic control in people with type 2 diabetes mellitus. Iranian Journal of Medical Sciences. 2016;41(6):531–538. [PMC free article] [PubMed] [Google Scholar]
- 61.Tang Y., Meng L., Li D., et al. Interaction of sleep quality and sleep duration on glycemic control in patients with type 2 diabetes mellitus. Chinese Medical Journal. 2014;127(20):3543–3547. [PubMed] [Google Scholar]
- 62.Chen H. C., Chou P. Predictors of change in self-reported sleep duration in community-dwelling older adults: the Shih-Pai Sleep Study, Taiwan. Scientific Reports. 2017;7(1):p. 4729. doi: 10.1038/s41598-017-04932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


