Abstract
MicroRNAs are gene expression regulators, associated with several human pathologies, including the ones caused by virus infections. Although their role in infection diseases is not completely known, they can exert double functions in the infected cell, by mediating the virus infection and/or regulating the immunity-related gene targets through complex networks of virus-host cell interactions. In this systematic review, the Pubmed, EMBASE, Scopus, Lilacs, Scielo, and EBSCO databases were searched for research articles published until October 22nd, 2020 that focused on describing the role, function, and/or association of miRNAs in SARS-CoV-2 human infection and COVID-19. Following the PRISMA 2009 protocol, 29 original research articles were selected. Most of the studies reported miRNA data based on the genome sequencing of SARS-CoV-2 isolates and computational prediction analysis. The latter predicted, by at least one independent study, 1266 host miRNAs to target the viral genome. Thirteen miRNAs were identified by four independent studies to target SARS-CoV-2 specific genes, suggested to act by interfering with their cleavage and/or translation process. The studies selected also reported on viral and host miRNAs that targeted host genes, on the expression levels of miRNAs in biological specimens of COVID-19 patients, and on the impact of viral genome mutations on miRNA function. Also, miRNAs that regulate the expression levels of the ACE2 and TMPRSS2 proteins, which are critical for the virus entrance in the host cells, were reported. In conclusion, despite the limited number of studies identified, based on the search terms and eligibility criteria applied, this systematic review provides evidence on the impact of miRNAs on SARS-CoV-2 infection and COVID-19. Although most of the reported viral/host miRNAs interactions were based on in silico prediction analysis, they demonstrate the relevance of the viral/host miRNA interaction for viral activity and host responses. In addition, the identified studies highlight the potential use of miRNAs as therapeutic targets against COVID-19, and other viral human diseases (This review was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database (#CRD42020199290).
1. Introduction
Coronaviruses (CoVs) are single-stranded RNA pathogens that cause health issues in humans and animals, including enteric, respiratory, hepatic, and central nervous diseases (Demirci and Adan, 2020). Six different types of CoV are known to be capable of infecting human cells, HCoV-229E, HKU-NL63, HCoVOC43, HCoV-HKU1, SARS-CoV, and MERS-CoV (Woo et al., 2005). Structurally, all coronaviruses present similarities in their genome organization and are composed of an open reading frame (ORF) 1a/b at the 5′ end that encodes 16 nonstructural proteins (NSP1–NSP16), and an ORF at the 3′ end that encodes four structural proteins (the envelope (E), membrane (M), nucleocapsid (N) and spike (S) proteins). The S protein is responsible for the entry of the SARS-CoV and SARS-CoV-2 virus into the host cell, from its interaction with the host extracellular membrane proteins ACE2 (angiotensin-converting enzyme 2) and TMPRSS2 (transmembrane serine protease 2) (Su et al., 2016; Hoffmann et al., 2020).
The novel coronavirus disease 2019 (COVID-19), caused by the new coronavirus Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), has rapidly turned into a global pandemic since the first case was notified in Wuhan, China, in late December 2019. By the beginning of December (2020), according to The World Health Organization (WHO) official reports, there were 66,243,918 confirmed infected cases, and 1,528,984 COVID-19 related deaths worldwide (WHO, 2020). Considering this alarming global health scenario, an extraordinary number of clinical and research efforts have been put towards the identification of the biological and molecular mechanisms that underline the SARS-CoV-2 infection and its pathogenicity in the human host cells. An urgent goal is to discover biomarkers that can be potentially used as predictors and therapeutic targets for the disease.
MicroRNAs (miRNAs) have emerged as one of these biomarkers that can, among several functions, regulate the immunity-related gene targets through complex networks of virus-host cell interactions (Rad and McLellan, 2020). MiRNAs are conserved endogenous small non-coding RNA molecules that bind to mRNA targets, by base complementarity. These molecules play a role in the regulation of gene expression of various organisms, by suppressing the translation process or by causing the degradation of the target mRNAs (Kincaid and Sullivan, 2012; Canatan and Sanctis, 2020). MiRNA expression dysregulation is associated with the development of pathological processes and chronic diseases, including viral infections (Bernier and Sagan, 2018; Girardi et al., 2018).
Although the mechanisms of the interaction among viruses and cellular miRNAs are not completely known, they can directly interfere with the viral replication inside the host cells. Some of the proposed mechanisms include the inhibition of the viral genome translation and the prevention of viral replication by triggering the deregulated expression of several endogenous miRNAs to induce an anti-viral reaction (Scheel et al., 2016; Trobaugh and Klimstra, 2017). This anti-viral response by the miRNAs may involve the regulation of their mRNA targets that are components of signaling pathways that participate in the cellular response triggered by the viral infection, such as WNT, INF, PIK3/AKT, MAPK, and NOTCH (Barbu et al., 2020). On the other hand, other mechanisms, including the “evasion” of cellular miRNAs, and defects in the miRNA biogenesis, can favor the viral replication and the survival of the virus in the host cells (Bernier and Sagan, 2018).
This systematic review focused on describing the roles, functions, and/or associations of miRNAs in mediating the SARS-CoV-2 infection in human cells. The main objective of most of the studies included was to identify biomarkers that can be associated with the distinct forms of the disease and be potentially used for the prevention, diagnosis, and treatment of COVID-19.
2. Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). The review protocol was properly registered at the International Prospective Register of Systematic Reviews (PROSPERO) database under the identifier CRD42020199290.
2.1. Data sources and search strategy
The databases Pubmed, Scielo, Lilacs, EMBASE, Scopus, and EBSCO were searched using the terms “microRNA” and synonyms (microRNA OR miR OR miRNA OR small non-coding RNA OR small ncRNA) in Title/Abstract and “SARS-CoV-2” and synonyms (COVID19 OR SARS-COV2 OR SARS-CoV-2 OR coronavirus disease 2019 OR coronavirus disease-19 OR 2019-nCoV) in Title/Abstract. The end of the search date was October 22nd, 2020. All the searches were independently carried out by two researchers and conducted similarly for all the databases. Duplicate articles were excluded, and the remaining articles were screened based on the title and abstract, followed by the assessment of the full text for relevance and eligibility.
2.2. Study selection and eligibility criteria
The studies were evaluated independently by two researchers and selected according to the following inclusion and exclusion criteria. The inclusion criteria were: (1) articles reporting on human cells infected by SARS-CoV-2 and on the potential role of miRNAs on several cellular mechanisms associated with the viral activity and infection, including, but not limited to, viral-host interaction and host responses; (2) peer-reviewed articles written in English. The exclusion criteria were: (1) articles on SARS-CoV-2 that did not report on the role, function, association, and/or involvement of miRNAs; (2) articles on miRNA analysis performed in animals; (3) non-original articles (reviews), editorials, letters from editors, book chapters, unpublished or non-peer-reviewed studies; (4) articles which full text was not available.
2.3. Data extraction
After the selection and eligibility assessment of the studies, two reviewers extracted the following information independently: name of the first author, year of publication, country of study, methodology (miRNA related and other relevant methods), sample source (biological material (patients' samples and/or cell lines) and online databases), miRNAs analyzed, main results, and conclusions.
2.4. Quality and Bias evaluation
The quality of the studies and the risk of bias was assessed using the Quality in Prognosis Studies (QUIPS) tool, which evaluates studies in the following domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting (Hayden et al., 2006). The criteria were evaluated for quality according to the following classification: high quality (+): with little or no risk of bias; acceptable (+/−): moderate risk of bias; low quality (−): with a high risk of bias, and unsure (?). Based on this classification, the articles received a general evaluation as low, moderate, or high risk of bias.
3. Results
3.1. Search results
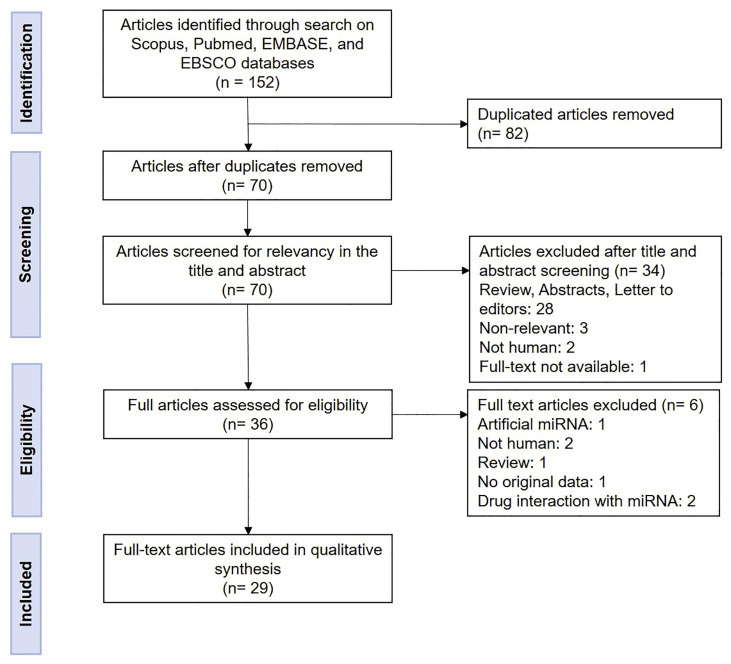
The search for articles in the six selected databases following the described search strategy resulted in 152 articles. After removing duplicates, 70 articles remained and were screened for relevance and compliance based on the inclusion and exclusion criteria. This analysis resulted in 29 full-text articles which were included for qualitative synthesis (Fig. 1 ).
Fig. 1.
Flow diagram of the identification and selection of the studies in this review.
3.2. Risk of bias and quality of the studies
The risk of bias was determined for all the studies using the six categories of the QUIPS tool, as shown in Table S1. The overall assessment of the six categories resulted in 24 studies with a low risk of bias and five with a moderate risk of bias. Following this evaluation, no study was removed from further analysis.
3.3. Studies main characteristics
The selected 29 original articles of the performed systematic review were published between April 14th and October 2nd 2020, and were obtained from 17 countries: India (n = 7), Russia and United States (n = 3), China and Iran (n = 2), Australia, Bangladesh, Canada, France, Germany, Italy, Jordan, New Zealand, Pakistan, Turkey, United Arab Emirates, United Kingdom (n = 1 each).
Fourteen studies (studies #1 to #13, and #22) aimed to comprehensively analyze the SARS-CoV-2 genome and to screen for the potential human miRNAs that targeted the viral genome. Five studies (#12 to #16) focused on the identification of miRNA-like sequences presented on SARS-CoV-2 genome and/or viral miRNAs. Demirci et al., (2020, #13) reported both human and viral miRNAs, while Sardar et al., (2020, #11) reported human miRNAs and anti-viral miRNAs (VIRmiRNA database). Only two studies (#10 and #17) reported on the miRNA expression analysis in COVID-19 patients when compared to non-COVID-19 samples (controls). Three studies (#18 to #20) focused on HCOV-host interactome, considering human miRNAs that targeted human genes, with two of them integrating miRNA data with the expression levels of predicted target genes. Two studies (#21 and #22) analyzed the impact of mutations on the SARS-CoV-2 genome on the affinity of human miRNAs to bind to their target sequence. The remaining seven studies (#23 to #29) focused on the evaluation of ACE2 and TMPRSS2 expression, their activities, and potential interaction with human miRNAs (Table 1 ).
Table 1.
Main characteristics of the 29 articles included in this systematic review.
| Study # | First Author | Date of Publication | Country | Aims | References |
|---|---|---|---|---|---|
| 1 | Ahmed SSSJ | June 30, 2020. | India | To dissect the mechanism of SARS-CoV-2 infection in human lung host cells from the initial phase of receptor binding to viral replication machinery, by proteome, transcriptome, text mining and miRNA data analysis. | (Ahmed et al., 2020) |
| 2 | Arisan ED | June 4, 2020. | UK | To identify human miRNAs that present sequence similarities to the SARS-CoV-2 genome and their conservation ratios in SARS-CoV-2 isolates from different geographical regions | (Arisan et al., 2020) |
| 3 | Balmeh N | August 7, 2020. | Iran | To investigate the usage of alternative therapeutic methods against viral infection of SARS-CoV-2 focusing on ACE2, TMPRSS2, GRP78a and AT1R receptors, and comprehensively investigate the SARS-CoV-2 genome inhibitors, including miRNAs. | (Balmeh et al., 2020) |
| 4 | Chen L | April 14, 2020. | China | To comprehensively analyze SARS-CoV-2 whole genome to screen for potential druggable targets for drug development, including miRNAs. | (Chen and Zhong, 2020) |
| 5 | Fulzele S | May 13, 2020. | USA | To understand the pathophysiology of SARS and COVID-19 and identify novel therapeutic targets through in silico miRNA analysis | (Fulzele et al., 2020) |
| 6 | Haddad H | August 14, 2020. | Jordan | To determine predicted human miRNAs that to bind to the ss-RNA of the SARS-CoV-2 whole-genome and specifically to the virus spike glycoprotein gene | (Haddad and Al-Zyoud, 2020) |
| 7 | Srivastava R | September 25, 2020. | USA | To understand the role of dysregulated post-transcriptional regulatory networks (RNA-binding proteins and miRNAs) during a SARS-CoV-2 infection. | (Srivastava et al., 2020) |
| 8 | Mukherjee M | August 11, 2020. | India | To identify viral genomic changes across different isolates focusing on the variations in the 5′ and 3′ untranslated regions and host microRNA binding sites and their consequences in host-mediated regulation of the viral RNA genome. | (Mukherjee and Goswami, 2020) |
| 9 | Nersisyan Sa | September 14, 2020. | Russia | To predict miRNA binding sites within human coronavirus RNAs using bioinformatic tools. | (Nersisyan et al., 2020a) |
| 10 | Chow JT-S | August 26, 2020. | Canada | To identify human miRNAs with the potential to target the SARS-CoV-2 genome and alterations of miRNA expression levels upon infection. | (Chow and Salmena, 2020) |
| 11 | Sardar R | August 4, 2020. | India | To comprehensively analyze the SARS-CoV-2 genomes from different geographical locations and identify the factors involved in host-pathogen interactions, including mutation analysis. To identify host-miRNAs that target the virus genome. | (Sardar et al., 2020) |
| 12 | Khan AAK | July 10, 2020. | Bangladesh | To elucidate the interplay between the SARS-CoV and SARS-CoV-2 viruses' and host's miRNAs, and viral miRNAs and host genome. | (Khan et al., 2020b) |
| 13 | Demirci MDS | June 5, 2020. | Turkey | To search the SARS-CoV-2 genome for miRNA-like sequences and potential host-virus interactions based on human miRNAs actions targeting the SARS-CoV-2 genome. | (Demirci and Adan, 2020) |
| 14 | Demongeot J | August 31, 2020. | France | To describe a potential miRNA-like action by viral RNA, at the level of oxygen transport by hemoglobin and immune response, involving the type I interferon synthesis inhibition. | (Demongeot and Seligmann, 2020) |
| 15 | Saini S | June 2020. | India | To predict mature viral miRNAs and scan for target genes in the human genome. | (Saini et al., 2020) |
| 16 | Sarma A | July 28, 2020. | India | To demonstrate association and sequence similarities shared between miRNAs of SARS-CoV2 and the human host. | (Sarma et al., 2020) |
| 17 | Li C | August 27, 2020. | China | To provide a list of the differential expressed miRNAs in the analysis of peripheral blood from human patients with COVID-19 and healthy controls. | (Li et al., 2020) |
| 18 | Politano G | September 30, 2020. | Italy | To perform systems biology analysis on the HCoV-host interactome, providing reliable miRNA and drug interactions information. | (Politano and Benso, 2020) |
| 19 | Taz TA | August 10, 2020. | Australia | To elucidate the potential relationship between COVID-19 infection and idiopathic pulmonary fibrosis, using a transcriptomic approach, including miRNA interaction analysis. | (Taz et al., 2020) |
| 20 | Vastrad B | September 11, 2020. | India | To understand the mechanisms of SARS-CoV-2 infection and identify potential novel diagnostic or therapeutic targets through bioinformatics analysis including miRNA regulatory networks. | (Vastrad et al., 2020) |
| 21 | Maitra A | June 4, 2020. | India | To provide information on the prevalence of different viral SARS-CoV-2 clades regional differences and potential human miRNA binding sites affected by mutations of the virus by sequencing the SARS-CoV-2 genome. | (Maitra et al., 2020) |
| 22 | Rad AH | July 7, 2020. | New Zealand | To determine the impact of SARS-CoV-2 mutations on the genomic RNA structure and on the ability of the host miRNAs to target the viral genome. | (Rad and McLellan, 2020) |
| 23 | Khan ATA | October 2, 2020. | Pakistan | To provide a computational and bioinformatics-based analysis of ACE2 and its corresponding regulatory miRNAs, elucidating its role in the COVID-19 pathogenesis. | (Khan et al., 2020a) |
| 24 | Lu D | August 30, 2020. | Germany | To identify miRNAs predicted to regulate ACE2 using an in-silico approach. | (Lu et al., 2020) |
| 25 | Zhang H | July 15, 2020. | US | To gain insight into the expression of ACE2 in the human airway epithelia, including miRNA regulatory activity. | (Zhang et al., 2020) |
| 26 | Mukhopadhyay D | September 25, 2020. | United Arab Emirates | To investigate the miRNAs that regulate the expression of ACE2 and TMPRSS2, essential elements for SARS-CoV-2 infection | (Mukhopadhyay and Mussa, 2020) |
| 27 | Nersisyan SA | April 29, 2020. | Russia | To investigate the mechanisms of the interaction between miRNA isoforms and ACE2/TMPRSS2 genes in the colon tissue of COVID-19 patients | (Nersisyan et al., 2020c) |
| 28 | Nersisyan Sb | July 29, 2020. | Russia | To explore the landscape of ACE2 and TMPRSS2 regulation mediated by miRNAs and isomiRs in different human organs using bioinformatic analysis of publicly available paired miRNA/mRNA- sequencing datasets. | (Nersisyan et al., 2020b) |
| 29 | Paniri A | June 1, 2020. | Iran | To perform a comprehensive computational analysis of TMPRSS2 to investigate pathways, expression profiles, epigenetic mechanisms, SNPs of this gene influencing miRNA function. | (Paniri et al., 2020) |
Most of the studies used online databases containing SARS-CoV-2 genome sequences (eg. NCBI and GISAID), gene expression data (e.g., The Cancer Genome Atlas datasets), and protein expression data (e.g., Human Proteome Map). Only four studies conducted their analysis in clinical biological samples (#4, #17, #21, #25) and three (#2, #10, #24) in human cell lines. A summary of the samples and methodologies used for each study are presented in Table S2.
3.4. Potential roles of miRNAs in SARS-CoV-2 infection
As described above, the final 29 studies selected used different methodological approaches to determine the role of miRNAs in the SARS-CoV-2 infection. These studies were classified into seven distinct groups (G) according to their main focus: G1: human miRNAs that target the SARS-CoV-2 genome, G2: viral miRNA or miRNA-like sequences of SARS-CoV-2 genome, G3: expression of human miRNAs in COVID-19 samples, G4: host miRNAs and their mRNAs targets, G5: impact of SARS-CoV-2 mutation on host miRNA function, G6: host miRNAs targeting the ACE2 expression, and G7: host miRNAs targeting the TMPRSS2 expression. A summary of these studies' main results, including the identified miRNAs, their corresponding mRNA targets, and potential roles in mediating SARS-CoV-2 infection are presented in Table 2, Table 3, Table 4 .
Table 2.
Described potential roles for host miRNAs in SARS-CoV-2 infection.
| miRNA | Target | Expression and potential function on SARS-CoV-2 infection | References |
|---|---|---|---|
| miR-1202 | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b. |
| SARS-CoV-2 ORF9a/N | Targets both SARS-CoV-2 and SARS isolates | ||
| SARS-CoV-2 ORF9b | |||
| miR-125a-3p | SARS-CoV-2 gene S | Inhibits the cleavage of the S gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b. |
| Unique to SARS-CoV-2 miRNA compared to SARS | |||
| miR-1307-3p | SARS-CoV-2 3’UTR site | High expression levels in lung tissue | Arisan et al., 2020, Balmeh et al., 2020, Chen and Zhong, 2020, Khan et al., 2020b. |
| This miRNA can also target human gene expression responsible for survival and proliferation (BCL2, PI3K pathway activators), clathrin-dependent endocytosis (AP2, PIP5K), and exocytosis (Actin) associated with virus cell entry and spread. | |||
| miR-138-5p | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Khan et al., 2020b, Sardar et al., 2020. |
| SARS-CoV-2 nsp2 | |||
| SARS-CoV-2 nsp4 | |||
| SARS-CoV-2 3′-to-5’exonuclease | |||
| miR-196a-5p | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Sardar et al., 2020. |
| SARS-CoV-2 S gene | Targets both SARS-CoV-2 and SARS isolates | ||
| miR-197-5p | SARS-CoV-2 ORF1a | Upregulated in patients with cardiovascular disease | Chow and Salmena, 2020, Demirci and Adan, 2020, Khan et al., 2020b, Rad and McLellan, 2020. |
| miR-21-3p | Not reported | Expressed in respiratory epithelial cells in the trachea and lung tissues Targets binding sites of 6 different coronavirus, including SARS-CoV-2 and SARS | Fulzele et al., 2020,Haddad and Al-Zyoud, 2020, Nersisyan et al., 2020a, Srivastava et al., 2020. |
| miR-323a-5p | SARS-CoV-2 ORF1a/b | Inhibits the translation of the ORF1a/b polyprotein gene | Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b, Sardar et al., 2020. |
| SARS-CoV-2 helicase | Targets both SARS-CoV-2 and SARS isolates | ||
| miR-3935 | SARS-CoV-2 ORF1a | Expressed in SARS-CoV-2 target cells | Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b, Rad and McLellan, 2020. |
| miR-4758-5p | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b. |
| Targets both SARS-CoV-2 and SARS isolates | |||
| miR-5047 | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Chow and Salmena, 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b. |
| SARS-CoV-2 ORF8 | Targets both SARS-CoV-2 and SARS isolates | ||
| SARS-CoV-2 M | |||
| miR-506-3p | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene | Ahmed et al., 2020, Demirci and Adan, 2020, Fulzele et al., 2020, Khan et al., 2020b. |
| SARS-CoV-2 N | Targets both SARS-CoV-2 and SARS isolates | ||
| NR3C1, SP3, SMARCC1 | Targets the NR3C1, SP3 and SMARCC1 viral genes that participate in viral replication process (predicted) | ||
| miR-6838-5p | SARS-CoV-2 ORF1a/b | Inhibits the cleavage of the ORF1a/b polyprotein gene (predicted) | Demirci and Adan, 2020, Fulzele et al., 2020, Haddad and Al-Zyoud, 2020, Khan et al., 2020b. |
| Targets both SARS-CoV-2 and SARS isolates | |||
| miR-16-2-3p | Not reported | Upregulation on SARS-CoV-2 infected samples | Chow and Salmena, 2020, Li et al., 2020. |
| miR-1246 | ACE2 | Upregulation associated with Acute Respiratory Distress Syndrome (ARDS) | Khan et al., 2020a, Zhang et al., 2020. |
| Downregulation in the small airway epithelium of smokers compared to non-smokers | |||
| Targets the 3’UTR sequence of ACE2 mRNA | |||
| miR-200c-3p | ACE2 | Induced overexpression resulted in downregulation of ACE2 in human cardiomyocytes. | Lu et al., 2020, Nersisyan et al., 2020b. |
| Interaction with ACE2 was validated in HEK-293 T cells by luciferase reporter assay | |||
| miR-125a-5p | ACE2 | Expressed in lungs, kidney and esophagus. | Nersisyan et al., 2020c. |
| Repressed transcription of miR-125a-5p as a result of JARID1B action increases ACE2 protein expression levels. | |||
| let-7a-5p | TMPRSS2 | Expression negatively correlated with TMPRSS2 expression | Mukhopadhyay and Mussa, 2020,Nersisyan et al., 2020b. |
| Target the 3’UTR sequence of TMPRSS2 mRNA | |||
| let-7d-5p | TMPRSS2 | Expression negatively correlated with TMPRSS2 expression Target the 3’UTR sequence of TMPRSS2 mRNA |
Mukhopadhyay and Mussa, 2020,Nersisyan et al., 2020b. |
| miR-922 | PI3 | GO molecular function: endopeptidase inhibitor activity, peptidase inhibitor activity, endopeptidase regulator activity | Taz et al., 2020, |
| PDCD1 | This protein was found upregulated in SARS-CoV-2 infected samples. | Vastrad et al., 2020. | |
| KEGG pathways: T cell receptor signaling pathway, Adaptative Immune system | |||
| GO: regulation of immune system process, T cell activation, leukocyte activation, cell activation | |||
| miR-326 | S100A8 | Go biological process: leukocyte aggregation, defense response to fungus, neutrophil chemotaxis, granulocyte chemotaxis, neutrophil migration | Taz et al., 2020. |
| KEGG pathway: IL17 signaling pathway | |||
| C1R | GO: innate immune system, regulation of immune system process, cytokine-mediated signaling pathway, response to biotic stimulus | Vastrad et al., 2020. |
Table 3.
Described potential roles for viral miRNAs or miRNA-like sequences in SARS-CoV-2 infection.
| miRNA | Target | Expression and possible function on SARS-CoV-2 infection | References |
|---|---|---|---|
| 11 predicted miRNA sequences on SARS-CoV-2 genomea | N/A | Immune system (GO:0002520), response to cytokine (GO:0034097), biological adhesion (GO:0065007), regulation of signaling pathways (GO:0009966) | Sarma et al., 2020. |
| SARS-CoV-2-MD241-3p | BMPR2 | Involved in pulmonary vascularity | Saini et al., 2020. |
| SARS-CoV-2-MD3-3p | P53 | Involved in antiviral innate immunity | Saini et al., 2020. |
| 170 predicted miRNA sequences on SARS-CoV-2 genome | N/A | Host immune response: Wnt signaling, MAPK signaling, T cell-mediated immunity, autophagy, FGF receptor binding, TGF-beta signaling | Khan et al., 2020b. |
Viral miRNA or miRNA-like sequences of SARS-CoV-2 reported as human miRNAs due to similarities in RNA sequences.
Table 4.
Described impact of mutations on SARS-CoV-2 genome and TMPRSS2 gene on host miRNAs function.
| miRNAs affected | Target | Mutation/SNP | Results | References |
|---|---|---|---|---|
| miR-3162-3p | SARS-CoV-2 | 28,881-3 Nucleopcasid gene GGG --> AAC | Disruption of target site | Maitra et al., 2020 |
| miR-6826-3p | ||||
| miR-5195-5p | ||||
| miR-24-1-5p | ||||
| miR-3679-3p | ||||
| miR-642b-5p | ||||
| miR-24-2-5p | ||||
| miR-4699-3p | SARS-CoV-2 | 28,881-3 Nucleopcasid gene GGG --> AAC | Creation of target site | Maitra et al., 2020 |
| miR-299-5p | ||||
| miR-12,132 | ||||
| miR-197-5p | SARS-CoV-2 | ORF1a (Nsp3) C3037U | Disruption of target site | Rad and McLellan, 2020 |
| miR-3935-5p | SARS-CoV-2 | ORF1a (Nsp4) A9259G | Disruption of target site | |
| miR-18b-5p | SARS-CoV-2 | ORF1a (Nsp4) G9802U | Disruption of target site | |
| miR-18b-5p | SARS-CoV-2 | ORF1a (Nsp4) C9803U | Disruption of target site | |
| miR-1273d | SARS-CoV-2 | ORF1b (Nsp12) C15293U | Disruption of target site | |
| miR-4661-3p | SARS-CoV-2 | S G25311U | Disruption of target site | |
| miR-338-3p | SARS-CoV-2 | S C24034U | Disruption of target site | |
| miR-338-3p | SARS-CoV-2 | S G24057A | Disruption of target site | |
| miR-6729-5p | TMPRSS2 | rs75036690 miRNA Seed region AGGAGU[G/C] | Disruption of target site | Paniri et al., 2020 |
| miR-433b-3p | TMPRSS2 | rs12473206 miRNA Seed region AGGAGU[G/C] | Creation of target site | |
| miR-548c-3p | TMPRSS2 | rs456142 miRNA target site G/A | Break of target site | |
| miR-127-3p | TMPRSS2 | rs462574 miRNA target sites T/C | Creation of target site | |
| miR-1324 | TMPRSS2 | rs462574 miRNA target sites T/C | Break of target site | |
| miR-5089 | TMPRSS2 | rs456298 miRNA target sites T/A | Creation/enhancement of target site | |
| miR-204-5p | TMPRSS2 | rs12627374 miRNA target sites G/A | Decrease affinity with target site | |
| miR-211-5p | TMPRSS2 | rs12627374 miRNA target sites G/A | Enhance affinity with target site | |
| miR-4685-3p | TMPRSS2 | rs12627374 miRNA target sites G/A | Decrease affinity with target site | |
| miR-4716-5p | TMPRSS2 | rs12627374 miRNA target sites G/A | Creation of target site |
3.4.1. Host miRNAs targeting SARS-CoV-2 genome
Based on the 14 studies (#1 to #13, and #22) on human miRNAs predicted to target the SARS-CoV-2 genome, 1266 human miRNAs were reported. From these, 70.9% (898) were predicted by a single study (#1 to #3, #5, #7, #8, #10 to #13 and #22), 22.5% (285) were predicted by two independent studies (#1 to #3, #5 to #13, and #22), 5.5% (70) were predicted by three (#1 to #3, #5 to #13, and #22) independent studies. The remaining 1.1% (13) miRNAs were predicted by four independent studies (#1 to #7, #8 to #13 and #22). From these analysis, 13 miRNAs were identified as targeting the SARS-CoV-2 genome: miR-1202, miR-125a-3p, miR-1307-3p, miR-138-5p, miR-196a-5p, miR-197-5p, miR-21-3p, miR-323a-5p, miR-3935, miR-4758-5p, miR-5047, miR-506-3p, and miR-6838-5p. In Table 2 it is presented the potential functions of these 13 miRNAs in the SARS-CoV-2 infection, their predicted or experimentally validated targets on the viral genome and, if available, data on their expression levels in the COVID-19 patients' samples analyzed.
It is of note, that one of the studies (#5) provided 68.5% (867) of all the miRNAs predicted to target the SARS-CoV-2 genome, being the main contributor of the miRNAs presented in this systematic review.
Interestingly, using the AVIRmiR module from the VIRmiRNA database (http://crdd.osdd.net/servers/virmirna/index.html), one group reported on 13 experimental antivirals miRNAs (#11), which were not cataloged on the miRbase database. The antiviral activity of these miRNAs was experimentally validated against the HIV or HCV virus and has yet to be confirmed in SARS-CoV-2 infection (Table 2).
3.4.2. Viral miRNAs or miRNA-like sequences of SARS-CoV-2 genome
By using bioinformatics tools, support vector machine, and/or machine learning approach, five (#12 to #16) of the 29 selected studies identified miRNA-like sequences in the SARS-CoV-2 genomes analyzed. Even considering that different technologies were used in each of these studies, their methods were commonly applied to predict potential pre-miRNAs based on DNA sequences and to determine the stability of the pre-miRNA through minimum-free energy calculation of the secondary structure, and the similarity of the candidate mature miRNA-like sequences with known human mature miRNAs.
Two studies (#14 and #16) reported on the comparison of the selected viral miRNA-like sequences with human miRNAs that were present on the miRbase v 22.1: 16 non-overlapped miRNAs were found in these studies. On the other hand, three studies (#12, #13, #15), did not use this miRNA database to compare their candidate viral miRNAs and did not report them according to this database nomenclature. Table 3 presents the selected viral miRNAs or miRNA-like sequences of the SARS-CoV-2 genome that showed potential roles in mediating SARS-CoV-2 infection.
3.4.3. Expression of host miRNAs in SARS-CoV-2 infection
Two studies (#10 and #17) performed miRNA expression analysis in biological samples of COVID-19 patients. Different types of samples were used, however, both studies used RNA sequencing methods to determine the miRNA expression levels.
Li et al., (2020, #17) found 73 miRNAs differentially expressed in peripheral blood from COVID-19 patients when compared to healthy controls: 35 upregulated and 38 downregulated; only six of these miRNAs were described in this study: miR-16-2-3p, miR-6501-5p, miR-618 with a fold change higher than 1.5 in the COVID-19 group, and miR-183-5p, miR-627-5p and miR-144-5p with a fold change of at least 1.3 times lower in the COVID-19 patients.
Chow & Salmena, (2020, #10) characterized the pattern of miRNA expression in normal lung tissue samples from the TCGA-LUAD project, resulting in 128 miRNAs (“candidate miRNAs”). Independently, these authors found 45 miRNAs with altered expression in SARS-CoV-2 infected bronchial adenocarcinoma cells (Calu-3 cells) when compared to mock-infected Calu-3 cells, of which 17 upregulated and 28 downregulated. A comparison with the 128 “candidate miRNAs”, revealed six miRNAs in common: let-7a-3p, miR-135b-5p, miR-16-2-3p, and miR-1275 found upregulated in infected cells, and miR-155-3p, and miR-139-5p found downregulated. MiR-16-2-3p was observed in both studies with up-regulated levels, indicating that it may present with an important role in mediating SARS-CoV-2 infection (Table 2).
3.4.4. Host miRNAs targeting host genes
Three studies (#18 to #20) aimed to better understand the HCoV-host interactome, by focusing on the host miRNAs and their corresponding target genes. Two hundred and thirty-six miRNAs were reported by these studies, with only two of them commonly reported in two studies: miR-326 and miR-922 (#19 and #20). Table 2 presents these two miRNAs and their corresponding predicted targets with potential pathways and biological functions affected by the miRNA/mRNA bindings.
In addition to these two studies, 111 of these 236 miRNAs were also predicted by the studies from the G1 group indicating that these miRNAs can act by regulating host and SARS-CoV-2 genes. Among them, miR-5047 was predicted by four studies (#5, #10, #12, #13), and miR-122-5p, miR-15b-5p, miR-17-5p, miR-34a-5p, miR-3666, miR-424-5p, and miR-8066 were predicted by three independent studies (#2, #3, #5, #6, #9, #10, #11, #12, #13).
3.4.5. Impact of SARS-CoV 2 genome mutations on host miRNA function
Two studies (#21 and #22) analyzed the potential impact of mutations on the SARS-CoV-2 genome on host miRNA function, resulting in the identification of 16 host miRNAs. Rad & McLellan (2020, #22) compared 65 SARS-CoV-2 genome sequences available on NCBI and GISAID databases with the reference sequence from NCBI (NC_045512.2); only miRNAs expressed in SARS-CoV-2 target cells were considered by these authors, which resulted in an initial number of 10 miRNAs. Eight mutations on the SARS-CoV-2 genome that may affect specific binding sites of six miRNAs were observed: four mutations on the ORF1a site affecting the binding sites of miR-197-5p, miR-3935-5p, and miR-18b-5p (two mutations), one mutation on the ORF1b site affecting the binding site of miR-1273d, and three mutations on the S gene affecting the binding sites of miR-4661-3p and miR-338-3p (two mutations) (Table 4).
In the study of Maitra et al., (2020, #21) the SARS-CoV-2 mutation analysis, conducted in biological samples of nasopharyngeal and oropharyngeal swabs from positive cases of SARS-CoV-2 infection, were focused on a specific mutation in the nucleocapsid coding region (28881–3 GGG/AAC). As a result, the authors found that seven miRNAs (miR-3162-3p, miR-6826-3p, miR-5195-5p, miR-24-1-5p, miR-3679-3p, miR-642b-5p, and miR-24-2-5p), depended on the original coding site of this region (GGG) to be able to target this gene. Three other miRNAs (miR-4699-3p, miR-299-5p, and miR-12,132), were, however, found to be able to target the mutated site AAC of the N protein (Table 4).
3.4.6. Host miRNAs targeting ACE2 expression
Six studies (#23 to #28) focused on determining the role of ACE2 protein in SARS-CoV-2 infection, including the prediction analysis of miRNAs that potentially regulate its gene expression. Fifty-five miRNAs were predicted to directly target ACE2 mRNA or ACE2 associated pathways. The regulatory mechanisms of three of these miRNAs (miR-1246, miR-200c-3p, and miR-125a-5p) (Table 2) were independently explored in these studies.
MiR-1246 was predicted to target the 3’UTR sequence of ACE2 mRNA, inhibiting its translation by two independent studies (#23 and #25). Altered expression of this miRNA was reported in the respiratory tract with opposite expression levels: increased levels of miR-1246 was associated with Acute Respiratory Distress Syndrome (ARDS) (#23), while low levels of miR-1246 were found in the small airway epithelium of smokers when compared to non-smokers (#25), indicating a possible role for this miRNA in the respiratory tract disorders.
MiR-200c-3p interaction with ACE2 mRNA was predicted by three independent studies (#24, #26, #28) and experimentally validated in human embryonic kidney cells (HEK-293T) through luciferase reporter assays (Lu et al., 2020). Also, induced overexpression of miR-200c-3p in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) resulted in lower levels of ACE2 expression (#24).
Finally, one study (#28) showed that lower levels of miR-125a-5p resulting from the transcription repression of JARID1B, a protein with H3K4 histone demethylase activity, can increase ACE2 expression levels. Loss of this epigenetic regulation can be the cause of the ACE2 protein dysregulated expression observed in COVID-19 patients, through alterations in the miR-125a-5p expression levels.
3.4.7. Host miRNAs targeting TMPRSS2 mRNA expression
The other critical enzyme, TMPRSS2, that mediates the SARS-CoV-2 insertion in the cell was evaluated by four studies (#26 to #29). Three of them (#26 to #28) focused on the identification of the miRNAs that could potentially target the TMPRSS2 mRNA and modulate its expression. These studies resulted in the identification of 58 miRNAs. Let-7a-5p and let-7d-5p were reported by two studies (#26 and #28); an independent study showed that their expression was negatively correlated with TMPRSS2 expression (#27), however, their predicted interaction with TMPRSS2 was not experimentally validated (#26, #28). Another seven miRNAs, miR-30a, miR-30c, miR-127, miR-194-3p, miR-200c, miR-361, and miR-423, were identified with expression levels negatively correlated with TMPRSS2 protein in the same study (#27), however, they were not predicted to target TMPRSS2 mRNA by any other study.
Paniri et al., (2020, #29) described SNPs that can alter the biogenesis and function of specific miRNAs that target the TMPRSS2 mRNA. Six of these SNPs were predicted by PolymiRTS and miRSNPs and may impact the affinity of the miRNA-mRNA interaction, create, or break miRNA target binding sites (Table 4).
4. Discussion
This systematic review provides a comprehensive analysis of the potential roles, functions, and associations of host and viral miRNAs in the modulation of SARS-CoV-2 infection in humans. Although a limited number of studies on these subjects were found, it is evident the impact of miRNAs on SARS-CoV-2 infection and COVID-19.
MiRNAs present the unique ability to regulate the expression of hundreds of mRNAs targets that act in several biological processes (Bartel, 2004; He and Hannon, 2004). Dysregulation of their expression levels has been associated with several diseases, including the ones caused by viral infections (Lu et al., 2008; Vidigal and Ventura, 2015; Drury et al., 2017; Saliminejad et al., 2019). Although the mechanisms of action of the miRNAs upon a viral infection are not completely known, it involves a complex interaction with the viral and host cells and their corresponding gene targets. It is suggested that host cells can regulate the expression of miRNAs and inhibit virus replication through the direct interaction with the viral genome or through the regulation of innate immunity pathways (Chen et al., 2018). On the other hand, virus-encoded miRNAs can alter the expression of host genes, creating environments conducive to their replication and preventing the anti-viral host's immune response (Sharma et al., 2015; Piedade and Azevedo-Pereira, 2016).
In this systematic review, 29 original articles were identified applying the selected search strategy, using the microRNA, SARS-CoV-2, COVID-19, and synonyms words in the articles' titles and abstracts. These studies showed that, as suggested above, miRNAs may present a role in the modulation of SARS-CoV-2 infection, through complex and diverse virus-host miRNA and mRNA targets mechanisms of interaction.
These mechanisms were determined in these studies with different methodological approaches. One of them involves the identification of miRNAs that can specifically target the SARS-CoV-2 genome. This can be achieved by implementing a workflow based on a pipeline of bioinformatic analysis that provides a list of known miRNAs (eg. miRbase, VIRmiRNA), predicted and experimentally validated miRNA targets (eg. TargetScan, miRDB, miRTarbase), Gene Ontology and Pathway enrichment analysis (eg. Diana miRpath, Wikipathways), and online available databases with genome sequences (eg. NCBI, GISAID) and gene expression data (eg. TCGA). As a result of this type of approach, which in this systematic review was performed by 14 studies, 1266 human miRNAs were predicted to target the SARS-CoV-2 genome. Of these, 13 miRNAs were commonly reported by four independent studies (miR-1202, miR-125a-3p, miR-1307-3p, miR-138-5p, miR-196a-5p, miR-197-5p, miR-21-3p, miR-323a-5p, miR-3935, miR-4758-5p, miR-5047, miR-506-3p, and miR-6838-5p), suggesting a high potential for the functional interaction of these miRNAs and SARS-CoV-2 viral activity. One example is the miR-1307-3p, which was found to be expressed in high levels in the lung tissue. This miRNA was predicted to target the 3’UTR site of the SARS-CoV-2 genome, and human genes (AP2, PIP5k, ACTB) critical for the virus entry and spread in the cells, as well as, genes (BCL2, PI3K/AKT pathway activators) associated with the host cell survival and proliferation (Balmeh et al., 2020).
As a response to viral infection, host cells can also produce antiviral miRNAs that seem to adapt to target critical genes for virus survival, playing an important role in the host antiviral defense (Barbu et al., 2020). AVIRmiR is an online database that contains 542 antiviral miRNAs reported to act against 26 different types of viruses; some of these miRNAs are not currently reported on the miRbase database and therefore present different nomenclature. Among them, are the miR-A2r and miR-B1r, which were previously found to affect the replication processes in HIV-infected cells (Zhang et al., 2012). These miRNAs were predicted to target three sites of the SARS-CoV-2 genome, indicating a potential antiviral role for these miRNAs in COVID-19 patients (Sardar et al., 2020).
Of similar importance, viruses can produce miRNA-like sequences or viral miRNAs that can target host genes, which can disrupt the host gene expression levels and enable the virus to create a propagation environment in the host cell (Barbu et al., 2020). In this systematic review, five studies found these sequences predicted to target host immune response biological pathways, including the ones associated with the immune system, T cell-mediated immunity, response to cytokines, biological adhesion, and autophagy, as well as the regulation of signaling pathways, such as the WNT, MAPK, and TGF-beta signaling (Demirci and Adan, 2020, Demongeot and Seligmann, 2020, Khan et al., 2020b, Saini et al., 2020, Sarma et al., 2020).
Another methodological approach utilized in two of the miRNA studies (Chow and Salmena, 2020; Lu et al., 2020) of this systematic review, was the detection of the dysregulated expression level of miRNAs in pathological processes. In these two studies, higher levels of miR-16-2-3p were commonly reported in samples infected with SARS-CoV-2, indicating a potential role for this miRNA in the infection. Interestingly, this miRNA was also predicted by Fulzele et al. (2020) and found to target all the SARS-CoV-2 genome isolates analyzed by this group (29 in total), but not the SARS genomes (four in total). Despite the genomic similarity between SARS and SARS-CoV-2, these findings may indicate a unique role for miR-16-2-3p in SARS-CoV-2 infection.
As previously mentioned, there are a limited number of studies, that the investigation of the miRNA expression patterns was actually conducted in biological samples obtained from COVID-19 patients. This can be related to the difficulty in the collection and subsequent handling of these samples. A study performed by our group, using post-mortem lung biopsies of COVID-19 patients, circumvented these limitations and was able to determine the expression of miRNAs involved in lung endothelial dysfunction (Centa et al., 2020). The analysis of six post-mortem lung biopsies, revealed lower expression levels of miR-26a-5p, miR-29b-3p, and miR-34a-5p in comparison to controls (post-mortem lung biopsies from unaffected individuals). Also, a correlation with these miRNAs and inflammatory markers associated with the cytokine storm was observed, substantiating their roles in SARS-CoV-2 respiratory cell infection.
Another mechanism associated with the virus and host miRNA interaction is the presence of alterations in the genome sequence of the virus that can modify the miRNA-mRNA target binding (Mishra et al., 2020). These alterations can result in either loss of function (when the miRNA can no longer target the mRNA target sequence) or gain of function (a new miRNA site is created in the mRNA target sequence). Indeed, two studies in this systematic review reported break/decrease and/or creation/increase of miRNA-mRNA pairings when comparing original SARS-CoV-2 genome sequences with mutated sequences (Maitra et al., 2020; Paniri et al., 2020). These alterations could be one of the mechanisms that result in the evasion of immunity by SARS-CoV-2 and other viruses, by impeding proper miRNA function (Prompetchara et al., 2020), and could explain differences in the pathogenicity of specific SARS-CoV-2 strains.
The SARS-CoV-2 virus frequently depends on the angiotensin-converting enzyme 2 (ACE2) for entering the host cells (Hoffmann et al., 2020). However, ACE2 is not sufficient for SARS-CoV-2 infection and requires other enzymes, such as the transmembrane protease serine 2 (TMPRSS2) enzyme that cleaves the spike (S) protein of the virus into various residues, facilitating its entry into the host cells (Hoffmann et al., 2020; Paniri et al., 2020). Therefore, differences in the expression of these and other proteins, associated with SARS-CoV-2 entrance have been the subject of most of the genetic studies in COVID-19 patients. Indeed, several mutations and/or polymorphisms have been identified in these enzymes in both distinct patients and populations (Gemmati et al., 2020; Hoffmann et al., 2020; Paniri et al., 2020). As expected, miRNAs that target ACE2 and TMPRSS2 can regulate their expression and function. Among these miRNAs, miR-1246, miR-200c-3p, and miR-125a-5p were predicted as potential regulators of ACE2 expression, with their interactions functionally validated or expressions negatively correlated (Khan et al., 2020a, Lu et al., 2020, Nersisyan et al., 2020b, Nersisyan et al., 2020c, Zhang et al., 2020). Let-7a-5p and let-7d-5p were predicted to target TMPRSS2, however, its interaction remains to be validated (Mukhopadhyay and Mussa, 2020; Nersisyan et al., 2020b).
In summary, the studies of this systematic review indicated several mechanisms that can mediate the interaction of SARS-CoV-2 virus-human host miRNAs and their targets. In most of these studies, the identified miRNAs' roles in SARS-CoV-2 infection were based on computational prediction analysis. Studies focusing on patients' biological samples are therefore imperative to determine the expression patterns of miRNAs and their association with the clinical characteristics of the COVID-19 patients, including their comorbidities and severity of the symptoms. In addition, in vitro analyzes in well-established cell models, such as pneumocytes and other susceptible cells to SARS-COV-2 infection, are necessary to validate the biological action of these miRNAs on target cells and their impact on the inflammatory and/or immune responses. These analyses will contribute to the understanding of the interaction of host and virus miRNAs and their effective involvement in the cellular activities and host cell responses upon SARS-CoV-2 infection.
5. Conclusion
In conclusion, this systematic review, using specific searched words and articles' selection criteria, reports on 29 studies on miRNAs and SARS-CoV-2 infection. The reported interactions of virus and host miRNAs were obtained mostly through in silico prediction analysis of miRNAs and gene targets conducted in SARS-CoV-2 sequence isolates obtained worldwide. However, these studies provide evidence of the relevance of this interaction for the viral activity and host responses and the potential use of miRNAs as therapeutic targets, not only against COVID-19 but to other viral human threats.
The following are the supplementary data related to this article.
Risk of bias assessment results of the nine articles selected (QUIPS tool).
Summary of the samples and methodologies used for each study.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed S.S.S.J., Paramasivam P., Raj K., Kumar V., Murugesan R., Ramakrishnan V. Regulatory cross talk between SARS-CoV-2 receptor binding and replication machinery in the human host. Front. Physiol. 2020;11:802. doi: 10.3389/fphys.2020.00802. (eCollection 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisan E.D., Dart A., Grant G.H., Arisan S., Cuhadaroglu S., Lange S., Uysal-Onganer P. The prediction of miRNAs in SARS-CoV-2 genomes: hsa-mir databases identify 7 key miRs linked to host responses and virus pathogenicity-related KEGG pathways significant for comorbidities. Viruses. 2020;12(6):614. doi: 10.3390/v12060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmeh N., Mahmoudi S., Mohammadi N., Karabedianhajiabadi A. Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors. Inform. Med. Unlocked. 2020;20:100407. doi: 10.1016/j.imu.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbu M.G., Condrat C.E., Thompson D.C., Bugnar O.L., Cretoiu D., Toader O.D., Suciu N., Voinea S.C. MicroRNA involvement in signaling pathways during viral infection. Front. Cell Dev. Biol. 2020;8:143. doi: 10.3389/fcell.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bernier A., Sagan S.M. The diverse roles of microRNAs at the host-virus interface. Viruses. 2018;10(8):440. doi: 10.3390/v10080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canatan D., Sanctis V.D. The impact of microRNAs (miRNAs) on the genotype of coronaviruses. Acta Biomed. 2020;91(2):195–198. doi: 10.23750/abm.v91i2.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centa A., Fonseca A.S., Ferreira S.G.S., Azevedo M.L.V., de Paula C.B.V., Nagashima S., Machado-Souza C., Miggiolaro A.F.R.S., Baena C.P., Noronha L., Cavalli R., R. L. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am. J. Phys. Lung Cell. Mol. Phys. 2020 doi: 10.1152/ajplung.00457.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhong L. Genomics functional analysis and drug screening of SARS-CoV-2. Genes Dis. 2020 doi: 10.1016/j.gendis.2020.04.002. 2020 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhou Y., Li H. LncRNA, miRNA and lncRNA-miRNA interaction in viral infection. Virus Res. 2018;15(257):25–32. doi: 10.1016/j.virusres.2018.08.018. [DOI] [PubMed] [Google Scholar]
- Chow J.T.S., Salmena L. Prediction and analysis of SARS-CoV-2 targeting microRNA in human lung epithelium. Genes (Basel) 2020;11(9):1002. doi: 10.3390/genes11091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci M.D.S., Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ. 2020;8 doi: 10.7717/peerj.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demongeot J., Seligmann H. SARS-CoV-2 and miRNA-like inhibition power. Med. Hypotheses. 2020;144:110245. doi: 10.1016/j.mehy.2020.110245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury R.E., O’Connor D., Pollard A.J. The clinical application of microRNAs in infectious disease. Front. Immunol. 2017;8:1182. doi: 10.3389/fimmu.2017.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulzele S., Sahay B., Yusufu I., Lee T.J., Sharma A., Kolhe R., Isales C.M. COVID-19 virulence in aged patients might be impacted by the host cellular microRNAs abundance/profile. Aging Dis. 2020;11(3):509–522. doi: 10.14336/AD.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmati D., Bramanti B., Serino M.L., Secchiero P., Zauli G., Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double x-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int. J. Mol. Sci. 2020;21(10):3474. doi: 10.3390/ijms21103474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi E., López P., Pfeffer S. On the importance of host microRNAs during viral infection. Front. Genet. 2018;9:439. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad H., Al-Zyoud W. miRNA target prediction might explain the reduced transmission of SARS-CoV-2 in Jordan, middle east. Noncoding RNA Res. 2020;5(3):135–143. doi: 10.1016/j.ncrna.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden J.A., Côté P., Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 2006;144:427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.T.A., Khalid Z., Zahid H., Yousaf M.A., Shakoori A.R. A computational and bioinformatic analysis of ACE2: an elucidation of its dual role in COVID-19 pathology and finding its associated partners as potential therapeutic targets. J. Biomol. Struct. Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1833760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.A.K., Sany R.U., Islam S., Islam A.B.M. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front. Genet. 2020;11:765. doi: 10.3389/fgene.2020.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R.P., Sullivan C.S. Virus-encoded microRNAs: an overview and a look to the future. PLoS Pathog. 2012;8(12) doi: 10.1371/journal.ppat.1003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Hu X., Li L., Li J.H. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J. Clin. Lab. Anal. 2020;34(10) doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Zhang Q., Deng M., Miao J., Guo Y., Gao W., Cui Q. An analysis of human microRNA and disease associations. PLoS One. 2008;3(10) doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Chatterjee S., Xiao K., Riedel I., Wang Y., Foo R., Bär C., Thum T. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J. Mol. Cell. Cardiol. 2020;148:46–49. doi: 10.1016/j.yjmcc.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A., Sarkar M.C., Raheja H., Biswas N.K., Chakraborti S., Singh A.K., Ghosh S., Sarkar S., Patra S., Mondal R.K., Ghosh T., Chatterjee A., Banu H., Majumdar A., Chinnaswamy S., Srinivasan N., Dutta S., Saumitra D.A.S. Mutations in SARS-CoV-2 viral RNA identified in Eastern India: possible implications for the ongoing outbreak in India and impact on viral structure and host susceptibility. J. Biosci. 2020;45(1):76. doi: 10.1007/s12038-020-00046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R., Kumar A., Ingle H., Kumar H. The interplay between viral-derived miRNAs and host immunity during infection. Front. Immunol. 2020;10:3079. doi: 10.3389/fimmu.2019.03079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M., Goswami S. Global cataloguing of variations in untranslated regions of viral genome and prediction of key host RNA binding protein-microRNA interactions modulating genome stability in SARS-CoV-2. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D., Mussa B.M. Identification of novel hypothalamic microRNAs as promising therapeutics for SARS-CoV-2 by regulating ACE2 and TMPRSS2 expression: an in silico analysis. Brain Sci. 2020;10(10):666. doi: 10.3390/brainsci10100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersisyan S.A., Yu S.M., Osipyants A.I., Vechorko V.I. Role of ACE2/TMPRSS2 genes regulation by intestinal microRNA isoforms in the COVID-19 pathogenesis. Bull. Rsmu. 2020:16–18. doi: 10.24075/brsmu.2020.024. [DOI] [Google Scholar]
- Nersisyan S., Engibaryan N., Gorbonos A., Kirdey K., Makhonin A., Tonevitsky A. Potential role of cellular miRNAs in coronavirus-host interplay. PeerJ. 2020;8 doi: 10.7717/peerj.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersisyan S., Shkurnikov M., Turchinovich A., Knyazev E., Tonevitsky A. Integrative analysis of miRNA and mRNA sequencing data reveals potential regulatory mechanisms of ACE2 and TMPRSS2. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniri A., Hosseini M.M., Akhavan-Niaki H. First comprehensive computational analysis of functional consequences of TMPRSS2 SNPs in susceptibility to SARS-CoV-2 among different populations. J. Biomol. Struct. Dyn. 2020:1–18. doi: 10.1080/07391102.2020.1767690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedade D., Azevedo-Pereira J.M. The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses. 2016;8 doi: 10.3390/v8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politano G., Benso A. IL6-mediated HCoV-host interactome regulatory network and GO/pathway enrichment analysis. PLoS Comput. Biol. 2020;16(9) doi: 10.1371/journal.pcbi.1008238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- Rad A.H., McLellan A.D. Implications of SARS-CoV-2 mutations for genomic RNA structure and host microRNA targeting. Int. J. Mol. Sci. 2020;21(13):4807. doi: 10.3390/ijms21134807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Saini A., Thakur C.J., Kumar V., RGupta R.D., Sharma J.K. Genome-wide computational prediction of miRNAs in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed target genes involved in pulmonary vasculature and antiviral innate immunity. Mol. Biol. Res. Commun. 2020;9(2):83–91. doi: 10.22099/mbrc.2020.36507.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliminejad K., Khorshid H.R.K., Fard S.F., Ghaffari S.H. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019;234(5):5451–5465. doi: 10.1002/jcp.27486. [DOI] [PubMed] [Google Scholar]
- Sardar R., Satish D., Birla S., Gupta D. Integrative analyses of SARS-CoV-2 genomes from different geographical locations reveal unique features potentially consequential to host-virus interaction, pathogenesis and clues for novel therapies. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma A., Phukan H., Halder N., Madanan M.G. An in-silico approach to study the possible interactions of miRNA between human and SARS-CoV2. Comput. Biol. Chem. 2020;88:107352. doi: 10.1016/j.compbiolchem.2020.107352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel T.K.H., Luna J.M., Liniger M., Nishiuchi E., Rozen-Gagnon K., Shlomai A., Auray G., Gerber M., Fak J., Keller I., Bruggmann R., Darnell R.B., Ruggli N., Rice C.M. A broad RNA virus survey reveals both miRNA dependence and functional sequestration. Cell Host Microbe. 2016;19(3):409–423. doi: 10.1016/j.chom.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., Verma R., Kumawat K.L., Basu A., Singh S.K. miR146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells. J. Neuroinflammation. 2015;12:30. doi: 10.1186/s12974-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Daulatabad S.V., Srivastava M., Janga S.C. Role of SARS-CoV-2 in altering the RNA-binding protein and miRNA-directed post-transcriptional regulatory networks in humans. Int. J. Mol. Sci. 2020;21(19):7090. doi: 10.3390/ijms21197090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., Liu W., Bi Y., Gao G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taz T.A., Ahmed K., Paul B.K., Kawsar, Aktar N., Mahmud S.M.H., Moni M.A. Network-based identification genetic effect of SARS-CoV-2 infections to Idiopathic pulmonary fibrosis (IPF) patients. Brief. Bioinform. 2020:bbaa235. doi: 10.1093/bib/bbaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobaugh D.W., Klimstra W.B. MicroRNA regulation of RNA virus replication and pathogenesis. Trends Mol. Med. 2017;23(1):80–93. doi: 10.1016/j.molmed.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastrad B., Vastrad C., Tengli A. Identification of potential mRNA panels for severe acute respiratory syndrome coronavirus 2 (COVID-19) diagnosis and treatment using microarray dataset and bioinformatics methods. 3 Biotech. 2020;10(10):422. doi: 10.1007/s13205-020-02406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidigal J.A., Ventura A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 2015;25(3):137–147. doi: 10.1016/j.tcb.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int/
- Woo P.C.Y., Lau S.K.P., Chu C.M., Chan K.H., Tsoi H.W., Huang Y., Wong B.H.L., Poon R.W.S., Cai J.J., Luk W.K., Poon L.L.M., Wong S.S.Y., Guan Y., Peiris J.S.M., Yuen K.Y. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 2005:884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Cheng T., Wei L., Cai Y., Yeo A.T., Han J., Yuan Y.A., Zhang J., Xia N. Efficient inhibition of HIV-1 replication by an artificial polycistronic miRNA construct. Virol. J. 2012;9:118. doi: 10.1186/1743-422X-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Rostami M.R., Leopold P.L., Mezey J.G., O’Beirne S.L., Strulovici-Barel Y., Crystal R.G. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am. J. Respir. Crit. Care Med. 2020;202(2):219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of bias assessment results of the nine articles selected (QUIPS tool).
Summary of the samples and methodologies used for each study.