Abstract
We assessed the relation between COVID-19 waves in Italy, which was severely affected during the pandemic. We evaluated the hypothesis that a larger impact from the first wave (February–May 2020) predicts a smaller peak during the second wave (September–October 2020), in the absence of local changes in public health interventions and area-specific differences in time trends of environmental parameters. Based on publicly available data on province-specific SARS-CoV-2 infections and both crude and multivariable cubic spline regression models, we found that for provinces with the lowest incidence rates in the first wave, the incidence in the second wave increased roughly in proportion with the incidence in the first wave until an incidence of about 500–600 cases/100,000 in the first wave. Above that value, provinces with higher incidences in the first wave experienced lower incidences in the second wave. It appears that a comparatively high cumulative incidence of infection, even if far below theoretical thresholds required for herd immunity, may provide noticeable protection during the second wave. We speculate that, if real, the mechanism for this pattern could be depletion of most susceptible individuals and of superspreaders in the first wave. A population learning effect regarding cautious behavior could have also contributed. Since no area-specific variation of the national policy against the SARS-CoV-2 outbreak was allowed until early November 2020, neither individual behaviors nor established or purported environmental risk factors of COVID-19, such as air pollution and meteorological factors, are likely to have confounded the inverse trends we observed in infection incidence over time.
Keywords: COVID-19, SARS-CoV-2, Outbreak, Epidemiology, Wave, Immunity, Seroprevalence, Public health
1. Introduction
Coronavirus disease (COVID-19) is a severe and potentially life-threatening disease due to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which spread globally during 2020 after the initial outbreak in late 2019 in China (Docea et al., 2020). Its transmission occurs predominantly through air (Wiersinga et al., 2020). Therapeutic options to treat the infection and its sequelae are limited (CORIST, 2020; Tsatsakis et al., 2020a; Wiersinga et al., 2020). Before a vaccine was available, and in the absence of a highly effective therapy, attempts to control the SARS-CoV-2 pandemic during 2020 relied on public health measures such as donning protective gear, social distancing, and shutdowns that limited population mobility and interaction (Ma and Lipsitch, 2021; Vinceti et al., 2020). Reduced social interaction lowers Rt, and can stem the spread of the virus, but lockdowns cannot be sustained indefinitely. With normal social intercourse, protection from herd immunity is thought to be attainable only at high prevalences of immunity, on the order of at least 50% and likely considerably above that for this virus (Fontanet and Cauchemez, 2020; Omer et al., 2020; Randolph and Barreiro, 2020).
Italy offers a unique opportunity to investigate how successive waves of the infection may interact (Lipsitch et al., 2020). Italy was the first nation with widespread population involvement, with a high mortality from COVID-19 in the spring of 2020, followed by a summer with relatively low infection incidence after easing of the tight lockdown that was in effect from March 8 through May 2, 2020 (Goumenou et al., 2020; Vinceti et al., 2020). The second wave arrived in late August and progressed to a peak in late October 2020. A characteristic of the response to the pandemic in Italy is that the non-pharmacological interventions, including social distancing, mask use and the closing of schools and retail establishments, were evenly adopted throughout the country. There were no important local differences in attempted control until November 6, 2020, when area-specific policies driven by the local spread of the outbreak began (DPCM, 2020). This history allows a relatively unconfounded comparison of the first and second waves of COVID-19 epidemiology during the 2020 February–October period between Italian communities, assuming that environmental and behavioral risk factors for COVID-19 such as outdoor air pollution, meteorological effects, and social interaction are close to uniform across Italy (Ambika et al., 2021; Calina et al., 2021; Copat et al., 2020; Domingo and Rovira, 2020; Filippini et al., 2020, 2021; Marques et al., 2021; Mele et al., 2021; Sidiropoulou et al., 2021; Sunyer et al., 2021; Tsatsakis et al., 2020b). The vaccination campaign did not begin in Italy until December 27, 2020 (Ministry of Health, 2021). Here we consider how the two waves of infection interacted within Italian communities during the 2020 February–October period.
2. Methods
2.1. Study population and outcome
We used data available without cost from public sources. The number of newly diagnosed infections with SARS-CoV-2 was reported on the website of the Civil Protection Agency (CPD - Italian Civil Protection Department, 2020) for two time frames: from February 24-May 31, 2020 (first wave), and from September 1-October 31, 2020 (second wave). These newly diagnosed infections correspond to the new positive tests of infection based on quantitative reverse transcription polymerase chain reaction reported by regional Health Services. Daily data flow from all regions of Italy was mandatory. Based on these data and the population data available at the Italian National Institute of Statistics website at January 1, 2020 (ISTAT, 2020b), we computed wave-specific incidence of SARS-CoV-2 infection during the first and second waves. We also used the regional anti-SARS-CoV-2 antibody seroprevalence data made available by the National Institute of Statistics (ISTAT, 2020a).
2.2. Data analysis
We assessed the association between first-wave incidence and regional seroprevalence data made available in the national survey with a weighted linear regression analysis, weighting observations by the ratio of provincial or regional population size to average size for all Italian provinces or regions. To investigate the relation between first and second wave province-specific incidence rates, we used linear regression to fit a restricted cubic spline model that weighted provincial data by population size and based on three knots at fixed percentiles of first-wave distribution. We also calculated a pointwise 95% confidence interval (CI) for the forecasted second-wave incidence. We used Stata software (Version-16.1 Stata Inc., College Station-TX, 2021) for all analyses, based on a code developed by NO and TF and available on request.
3. Results
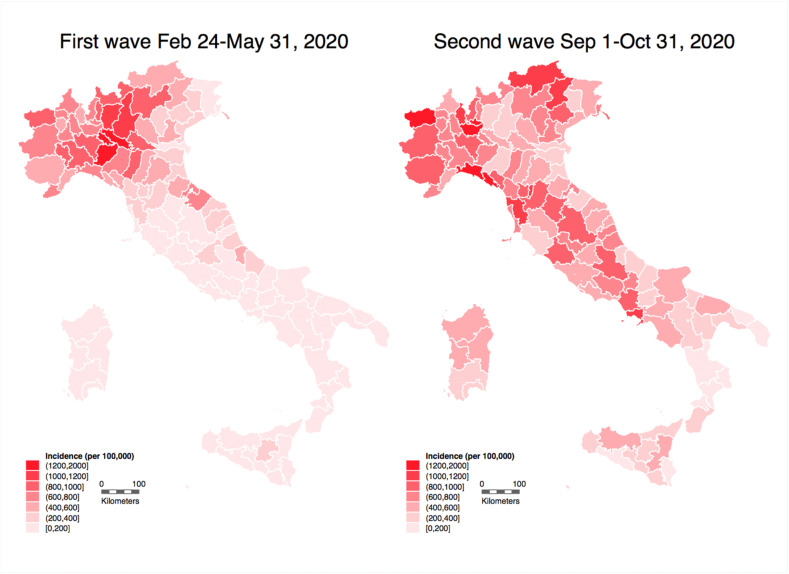
Fig. 1 shows SARS-CoV-2 incidence in the investigated periods. In the first wave, the number of diagnosed cases was 233,019, while in the shorter period of the second wave it was 409,241. Accordingly, the cumulative incidence during the first wave was 387/100,000 as a national average, ranging from 60/100,000 in Calabria region to 943/100,000 in Aosta Valley region. Corresponding figures for the slightly shorter period of the second wave were 679/100,000 for the national average, ranging from 171 (Calabria) to 1571/100,000 (Aosta Valley). The region-specific cumulative incidence and seroprevalence were, as expected, strongly correlated (Supplemental Fig. S1).
Fig. 1.
Incidence of officially diagnosed SARS-CoV-2 infections in the Italian provinces in the two COVID-19 waves.
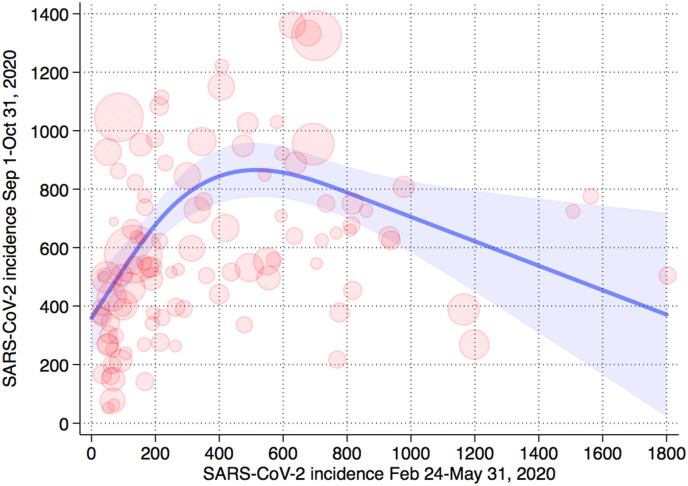
In the spline model we found an inverted U-shaped relation between first-wave incidence and the estimated predicted mean of the second-wave incidence computed on residents uninfected during the first wave, i.e. the susceptible population (Fig. 2 ). If the first-wave incidence was relatively low, the incidence was positively correlated with the incidence in the second wave. If the first-wave incidence, however, was greater than about 500–600 cases/100,000, the incidence was negatively correlated with the second-wave severity. These findings were similar when we computed the incidence in the second wave among all residents, independently of having been infected during the first wave, rather than just in the susceptible population (Supplemental Fig. S2). Adding potential confounders to the regression models such as proportion of elderly people, residents living alone, and an indicator of mobility, also did not appreciably change the results (Supplemental Fig. S3). Based on the relation between estimated seroprevalence and first-wave incidence, and the downward trend on the right side of the inverted U distribution in Fig. 2, we estimated that the seroprevalence threshold for a protective effect in the second wave is around approximately 2.8%–3.6%. Values above this cutpoint, especially much higher levels, were associated with more limited spread during the second wave.
Fig. 2.
Population-weighted cubic spline regression analysis of the relation between first and second wave incidence of SARS-CoV-2 infection in the Italian provinces based on susceptible population (removing subjects with a previous positive swab test up to August 31, 2020). Shadow area, forecasted interval of the mean incidence point estimate; circles, province-specific values weighted for population size.
4. Discussion
We saw an inverted U-shaped relation between first-wave and second-wave incidence. Above 500–600 infection cases/100,000, greater first-wave incidence predicted progressively smaller second-wave incidence. This inflection point corresponded with a population-based anti-SARS-CoV-2 antibody prevalence of approximately 3%. The official case numbers from which we computed first and second wave infection rates underestimated the actual incidence, since they mostly comprised symptomatic cases, particularly during the first wave. Nonetheless, these figures were highly correlated with the seroprevalence data at the regional level.
The seroprevalence levels corresponding to these first-wave incidences are much lower than those expected to bring about herd immunity, which for COVID-19 is calculated to be above 50% (Fontanet and Cauchemez, 2020). The Rt and the corresponding level for herd immunity could be affected by heterogeneity of behavior, if high-risk people are more likely to have contracted and spread the disease in the first wave (such as superspreaders, frequent travelers, nursing home and hospital personnel) (Calo et al., 2020; Kault, 2020; Kochanczyk et al., 2020; Lewis, 2021; Ma and Lipsitch, 2021; Signorelli et al., 2020) and therefore play a smaller role in the spread of the virus during a second wave. In fact, individuals most likely to contract and to spread the virus were also most likely to be infected early and after that to show immunity, diminishing Rt during later waves on the presumption that immunity persists during the interval between waves (Braun et al., 2020; Long et al., 2020; Wajnberg et al., 2020). This presumption seems reasonable, as a study from China suggested that the duration of neutralizing antibodies against SARS-CoV-2 persisted for at least nine months (He et al., 2021). In addition, recent evidence indicates that seroprevalence estimates, usually based on anti-SARS-CoV-2 antibody prevalence as was the case in Italy and other countries (Deeks et al., 2020; ISTAT, 2020a; Pollan et al., 2020), considerably underestimate the prevalence of effective immunity. T-cell mediated immunity may occur even in anti-SARS-CoV-2 antibody negative individuals (Braun et al., 2020; Sekine et al., 2020), perhaps from cross-reactivity from other human coronavirus infections (Calina et al., 2020; Kostoff et al., 2020), such as those accounting for 20% of upper respiratory tract infections, and might last for years (Braun et al., 2020; Callow et al., 1990; Hope and Bradley, 2021; Jarjour et al., 2021; Ledford, 2021; Neagu et al., 2021; Ng et al., 2016). The effect of this unmeasured immunity could depress the Rt closer to 1 in populations with high first-wave infection incidence.
Populations of the provinces more severely hit by the epidemic during the first wave may have undergone a more profound behavioral change during the summer and the second wave, by voluntarily adhering to prudent behaviors protective against contracting COVID-19. On the other hand, this theory is unlikely to explain the association we observed, because all of Italy engaged in the same set of public health measures adopted to curb the COVID-19 outbreak, including the light and tight lockdowns after the beginning of the outbreak until October 2020, as well as the prescriptions for public health measures such as systematic mask wearing (Vinceti et al., 2020). It was not until November 6, 2020 that the Italian government activated area-specific differential approaches for COVID-19 prevention and control, including bar, restaurant, shop and school closure, and mobility restrictions, adapted to the spread of the COVID-19 outbreak in each region. Furthermore, neither the SARS-CoV-2 variant B.1.1.7, a variant first detected in the UK in late 2021, nor the South African and Brazilian variants, were detected in Italy during the period investigated in this study (ISS, 2021), making it unlikely that there was any confounding related to lineage-specific transmission dynamics of the virus (Davies et al., 2021). It appears that the severity of the first wave of the COVID-19 outbreak may have been a determinant of the severity of the subsequent wave, even with comparatively low levels of immunity at the population level.
Funding
This study was supported by grants ‘UNIMORE FAR 2019 and 2020 Interdisciplinare Linea FCRMO - Fondazione Cassa di Risparmio di Modena’.
Credit author statement
MV and TF designed the original study, and with KJR analyzed and interpreted the data, and wrote the manuscript. NO designed and carried out data analysis with TF and SDF. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111097.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ambika S., Basappa U., Singh A., Gonugade V., Tholiya R. Impact of social lockdown due to COVID-19 on environmental and health risk indices in India. Environ. Res. 2021;196:110932. doi: 10.1016/j.envres.2021.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., Baysal E., Mangold M., Henze L., Lauster R., Mall M.A., Beyer K., Rohmel J., Voigt S., Schmitz J., Miltenyi S., Demuth I., Muller M.A., Hocke A., Witzenrath M., Suttorp N., Kern F., Reimer U., Wenschuh H., Drosten C., Corman V.M., Giesecke-Thiel C., Sander L.E., Thiel A. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Calina D., Docea A.O., Petrakis D., Egorov A.M., Ishmukhametov A.A., Gabibov A.G., Shtilman M.I., Kostoff R., Carvalho F., Vinceti M., Spandidos D.A., Tsatsakis A. Towards effective COVID19 vaccines: updates, perspectives and challenges (Review) Int. J. Mol. Med. 2020;46:3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calina D., Hartung T., Mardare I., Mitroi M., Poulas K., Tsatsakis A., Rogoveanu I., Docea A.O. COVID-19 pandemic and alcohol consumption: impacts and interconnections. Toxicol. Rep. 2021;8:529–535. doi: 10.1016/j.toxrep.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo F., Russo A., Camaioni C., De Pascalis S., Coppola N. Burden, risk assessment, surveillance and management of SARS-CoV-2 infection in health workers: a scoping review. Infect. Dis. Poverty. 2020;9:139. doi: 10.1186/s40249-020-00756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copat C., Cristaldi A., Fiore M., Grasso A., Zuccarello P., Signorelli S.S., Conti G.O., Ferrante M. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: a systematic review. Environ. Res. 2020;191:110129. doi: 10.1016/j.envres.2020.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORIST CORVID-19 RISK and Treatments (CORIST) Collaboration. Use of hydroxychloroquine in hospitalised COVID-19 patients is associated with reduced mortality: findings from the observational multicentre Italian CORIST study. Eur. J. Intern. Med. 2020;82:38–47. doi: 10.1016/j.ejim.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPD Italian Civil protection department, COVID-19 data. 2020. https://github.com/pcm-dpc/COVID-19 Access November 15, 2020. [DOI] [PMC free article] [PubMed]
- Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., Wenseleers T., Gimma A., Waites W., Wong K.L.M., van Zandvoort K., Silverman J.D., Group C.C.-W., Consortium C.-G.U., Diaz-Ordaz K., Keogh R., Eggo R.M., Funk S., Jit M., Atkins K.E., Edmunds W.J. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., Adriano A., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Dittrich S., Emperador D., Hooft L., Leeflang M.M., Van den Bruel A., Cochrane C.-D.T.A.G. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docea A.O., Tsatsakis A., Albulescu D., Cristea O., Zlatian O., Vinceti M., Moschos S.A., Tsoukalas D., Goumenou M., Drakoulis N., Dumanov J.M., Tutelyan V.A., Onischenko G.G., Aschner M., Spandidos D.A., Calina D. A new threat from an old enemy: reemergence of coronavirus (Review) Int. J. Mol. Med. 2020;45:1631–1643. doi: 10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J.L., Rovira J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ. Res. 2020;187:109650. doi: 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DPCM . 2020. [Decreto del Presidente del Consiglio dei Ministri. Ulteriori disposizioni attuative del decreto-legge 25 marzo 2020, n. 19, convertito, con modificazioni, dalla legge 25 maggio 2020, n. 35, recante «Misure urgenti per fronteggiare l'emergenza epidemiologica da COVID-19», e del decreto-legge 16 maggio 2020, n. 33, convertito, con modificazioni, dalla legge 14 luglio 2020, n. 74.https://www.gazzettaufficiale.it/eli/id/2020/11/04/20A06109/sg recante «Ulteriori misure urgenti per fronteggiare l'emergenza epidemiologica da COVID-19»] Accessed March 24, 2021]. GU Serie Generale n.275 275 04 Nov 2020 - Suppl. Ordinario n. 41. [Google Scholar]
- Filippini T., Rothman K.J., Cocchio S., Narne E., Mantoan D., Saia M., Goffi A., Ferrari F., Maffeis G., Orsini N., Baldo V., Vinceti M. Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide. Sci. Total Environ. 2021;760:143355. doi: 10.1016/j.scitotenv.2020.143355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T., Rothman K.J., Goffi A., Ferrari F., Maffeis G., Orsini N., Vinceti M. Satellite-detected tropospheric nitrogen dioxide and spread of SARS-CoV-2 infection in Northern Italy. Sci. Total Environ. 2020;739:140278. doi: 10.1016/j.scitotenv.2020.140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A., Cauchemez S. COVID-19 herd immunity: where are we? Nat. Rev. Immunol. 2020;20:583–584. doi: 10.1038/s41577-020-00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea A.O., Petrakis D., Tsoukalas D., Kostoff R., Rakitskii V., Spandidos D.A., Aschner M., Calina D. COVID19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020;22:20–32. doi: 10.3892/mmr.2020.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Ren L., Yang J., Guo L., Feng L., Ma C., Wang X., Leng Z., Tong X., Zhou W., Wang G., Zhang T., Guo Y., Wu C., Wang Q., Liu M., Wang C., Jia M., Hu X., Wang Y., Zhang X., Hu R., Zhong J., Yang J., Dai J., Chen L., Zhou X., Wang J., Yang W., Wang C. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397:1075–1084. doi: 10.1016/S0140-6736(21)00238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope J.L., Bradley L.M. Lessons in antiviral immunity. Science. 2021;371:464–465. doi: 10.1126/science.abf6446. [DOI] [PubMed] [Google Scholar]
- ISS ISS first report on SARS-CoV-2 variants. 2021. www.covid19dataportal.it/highlights/highlight6/ Accessed March 24, 2021.
- ISTAT . Italian National Institute of Statistics; 2020. First Results of the Seroprevalence Investigation on SARS-COV-2.https://www.istat.it/it/files//2020/08/ReportPrimiRisultatiIndagineSiero.pdf Accessed March 24, 2021. [Google Scholar]
- ISTAT . Italian National Institute of Statistics; 2020. Populations and Families.www.istat.it Accessed March 24, 2021. [Google Scholar]
- Jarjour N.N., Masopust D., Jameson S.C. T cell memory: understanding COVID-19. Immunity. 2021;54:14–18. doi: 10.1016/j.immuni.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kault D. Superspreaders, asymptomatics and COVID-19 elimination. Med. J. Aust. 2020;213:447–448. doi: 10.5694/mja2.50835. e441. [DOI] [PubMed] [Google Scholar]
- Kochanczyk M., Grabowski F., Lipniacki T. Super-spreading events initiated the exponential growth phase of COVID-19 with R0 higher than initially estimated. R. Soc. Open Sci. 2020;7:200786. doi: 10.1098/rsos.200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostoff R.N., Kanduc D., Porter A.L., Shoenfeld Y., Calina D., Briggs M.B., Spandidos D.A., Tsatsakis A. Vaccine- and natural infection-induced mechanisms that could modulate vaccine safety. Toxicol. Rep. 2020;7:1448–1458. doi: 10.1016/j.toxrep.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. How 'killer' T cells could boost COVID immunity in face of new variants. Nature. 2021;590:374–375. doi: 10.1038/d41586-021-00367-7. [DOI] [PubMed] [Google Scholar]
- Lewis D. Superspreading drives the COVID pandemic - and could help to tame it. Nature. 2021;590:544–546. doi: 10.1038/d41586-021-00460-x. [DOI] [PubMed] [Google Scholar]
- Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of covid-19 - studies needed. N. Engl. J. Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., Su K., Zhang F., Gong J., Wu B., Liu X.M., Li J.J., Qiu J.F., Chen J., Huang A.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Ma K.C., Lipsitch M. Big data and simple models used to track the spread of COVID-19 in cities. Nature. 2021;589:26–28. doi: 10.1038/d41586-020-02964-4. [DOI] [PubMed] [Google Scholar]
- Marques M., Rovira J., Nadal M., Domingo J.L. Effects of air pollution on the potential transmission and mortality of COVID-19: a preliminary case-study in Tarragona Province (Catalonia, Spain) Environ. Res. 2021;192:110315. doi: 10.1016/j.envres.2020.110315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M., Magazzino C., Schneider N., Strezov V. NO2 levels as a contributing factor to COVID-19 deaths: the first empirical estimate of threshold values. Environ. Res. 2021;194:110663. doi: 10.1016/j.envres.2020.110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Anti-Covid vaccination campaign. 2021. www.salute.gov.it Accessed March 24, 2021.
- Neagu M., Calina D., Docea A.O., Constantin C., Filippini T., Vinceti M., Drakoulis N., Poulas K., Nikolouzakis T.K., Spandidos D.A., Tsatsakis A. Back to basics in COVID-19: antigens and antibodies-Completing the puzzle. J. Cell Mol. Med. 2021 doi: 10.1111/jcmm.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng O.W., Chia A., Tan A.T., Jadi R.S., Leong H.N., Bertoletti A., Tan Y.J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34:2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer S.B., Yildirim I., Forman H.P. Herd immunity and implications for SARS-CoV-2 control. J. Am. Med. Assoc. 2020;324:2095–2096. doi: 10.1001/jama.2020.20892. [DOI] [PubMed] [Google Scholar]
- Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M., Sanmartin J.L., Fernandez-Garcia A., Cruz I., Fernandez de Larrea N., Molina M., Rodriguez-Cabrera F., Martin M., Merino-Amador P., Leon Paniagua J., Munoz-Montalvo J.F., Blanco F., Yotti R., Group E.-C.S. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., Wullimann D.J., Kammann T., Emgard J., Parrot T., Folkesson E., Karolinska C.-S.G., Rooyackers O., Eriksson L.I., Henter J.I., Sonnerborg A., Allander T., Albert J., Nielsen M., Klingstrom J., Gredmark-Russ S., Bjorkstrom N.K., Sandberg J.K., Price D.A., Ljunggren H.G., Aleman S., Buggert M. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou P., Docea A.O., Nikolaou V., Katsarou M.S., Spandidos D.A., Tsatsakis A., Calina D., Drakoulis N. Unraveling the roles of vitamin D status and melanin during Covid19 (Review) Int. J. Mol. Med. 2021;47:92–100. doi: 10.3892/ijmm.2020.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli C., Zucchi A., Tersalvi C.A., Ciampichini R., Beato E., Balzarini F., Odone A., Middleton J. High seroprevalence of SARS_COV-2 in Bergamo: evidence for herd immunity or reason to be cautious? Int. J. Publ. Health. 2020;65:1815–1817. doi: 10.1007/s00038-020-01524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J., Dadvand P., Foraster M., Gilliland F., Nawrot T. Environment and the COVID-19 pandemic. Environ. Res. 2021;195:110819. doi: 10.1016/j.envres.2021.110819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A., Calina D., Falzone L., Petrakis D., Mitrut R., Siokas V., Pennisi M., Lanza G., Libra M., Doukas S.G., Doukas P.G., Kavali L., Bukhari A., Gadiparthi C., Vageli D.P., Kofteridis D.P., Spandidos D.A., Paoliello M.M.B., Aschner M., Docea A.O. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A., Petrakis D., Nikolouzakis T.K., Docea A.O., Calina D., Vinceti M., Goumenou M., Kostoff R.N., Mamoulakis C., Aschner M., Hernandez A.F. COVID-19, an opportunity to reevaluate the correlation between long-term effects of anthropogenic pollutants on viral epidemic/pandemic events and prevalence. Food Chem. Toxicol. 2020;141:111418. doi: 10.1016/j.fct.2020.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M., Filippini T., Rothman K.J., Ferrari F., Goffi A., Maffeis G., Orsini N. Lockdown timing and efficacy in controlling COVID-19 using mobile phone tracking. EClinicalMedicine. 2020;25:100457. doi: 10.1016/j.eclinm.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., Stadlbauer D., Stone K., Strohmeier S., Simon V., Aberg J., Reich D.L., Krammer F., Cordon-Cardo C. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.