Abstract
Objective
The aim was to characterize the electrophysiological features and plasma biomarkers of critical illness polyneuropathy (CIN) and myopathy (CIM) in coronavirus disease 2019 (COVID-19) patients with intensive care unit acquired weakness (ICUAW).
Methods
An observational ICU cohort study including adult patients admitted to the ICU at Uppsala University Hospital, Uppsala, Sweden, from March 13th to June 8th 2020. We compared the clinical, electrophysiological and plasma biomarker data between COVID-19 patients who developed CIN/CIM and those who did not. Electrophysiological characteristics were also compared between COVID-19 and non-COVID-19 ICU patients.
Results
111 COVID-19 patients were included, 11 of whom developed CIN/CIM. Patients with CIN/CIM had more severe illness; longer ICU stay, more thromboembolic events and were more frequently treated with invasive ventilation for longer than 2 weeks. In particular CIN was more frequent among COVID-19 patients with ICUAW (50%) compared with a non-COVID-19 cohort (0%, p = 0.008). Neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAp) levels were higher in the CIN/CIM group compared with those that did not develop CIN/CIM (both p = 0.001) and correlated with nerve amplitudes.
Conclusions
CIN/CIM was more prevalent among COVID-19 ICU patients with severe illness.
Significance
COVID-19 patients who later developed CIN/CIM had significantly higher NfL and GFAp in the early phase of ICU care, suggesting their potential as predictive biomarkers for CIN/CIM.
Keywords: Critical illness neuropathy, Myopathy, COVID-19, SARS-CoV-2, NFL
1. Introduction
Patients with severe coronavirus disease 2019 (COVID-19) infections are at risk of developing neurological complications (Ellul et al., 2020, Mao et al., 2020). Symptoms from the peripheral nervous system have been less frequently reported and their characterization requires electrophysiological investigations that are not always readily available in the setting of the ongoing COVID-19 pandemic. In the 2004 SARS (severe acute respiratory syndrome) pandemic, a case series of four patients highlighted the possible coexistence of critical-illness polyneuropathy and/or myopathy in SARS patients, highlighting the neeed for further studies to determine a potential relationship between SARS coronavirus and neuromuscular problems (Tsai et al., 2004).
Acute inflammatory polyneuropathy, e.g., Guillain-Barré syndrome (GBS), has been described in 19 COVID-19 patients worldwide (Camdessanche et al., 2020, Toscano et al., 2020, Zhao et al., 2020); however intensive care unit acquired weakness (ICUAW) has not yet been prospectively characterized in COVID-19 patients.
Important pathophysiological mechanisms of ICUAW are critical illness neuropathy (CIN) and myopathy (CIM), where patients present with flaccid limb muscle weakness and failure to wean from the ventilator (Latronico and Bolton, 2011). A very recent retrospective report of 12 COVID-19 patients with intensive care unit acquired weakness (ICUAW) showed that four patients had sensory-motor axonal polyneuropathy and seven patients had myopathy (Cabanes-Martinez et al., 2020). Furthermore, a single case report of a COVID-19 patient with CIM has been published (Tankisi et al., 2020). One of the medical challenges in treating COVID-19 patients is the high number of patients requiring prolonged mechanical ventilation in the intensive care unit (ICU) in combination with unusually high sedation requirements, which predisposes to ICUAW(Hanidziar and Bittner, 2020). CIN and CIM are important to identify, since survivors often present with severe residual disability and persistent exercise limitations several years afterwards (Herridge et al., 2011, Iwashyna, 2010, Leijten et al., 1995). There is furthermore a clear distinction between outcome in CIN versus CIM, where patients with CIN have a slower or incomplete recovery, and higher mortality rate, whereas patients with CIM often show complete recovery within 6 months (Guarneri et al., 2008). Thus, it would be of value to first be able to predict COVID-19 patients at risk of developing CIN/CIM and second to distinguish between the two. Increased plasma concentration of the axonal injury marker neurofilament has been described in patients with ICUAW (Wieske et al., 2014).
The aim of this study was to investigate the occurrence of ICUAW in COVID-19 patients admitted to the ICU and characterize the ICUAW into CIN and CIM. Furthermore, to correlate ICUAW with known clinical risk factors and to determine potential correlating plasma biomarkers for neuronal injury.
2. Materials and methods
2.1. Study design
The study was approved by the National Ethical Review Authority (EPM; No. 2020-01623). Informed consent was obtained from the patient, or next of kin if the patient was unable give consent. The Declaration of Helsinki and its subsequent revisions were followed and the study protocol was registered (ClinicalTrials ID: NCT04316884). Electrophysiological data collected from COVID-19 patients and ICU controls were approved by the Ethical Review Board (case no 2015/105 and 2020-03377).
2.2. Subjects
Patients were screened from an ongoing prospective study. This observational cohort study was performed at the general ICU at Uppsala University Hospital, Uppsala, Sweden. All adult patients admitted to the ICU between March 13, and June 8, 2020 were screened for inclusion (Fig. 1 ). The COVID-19 cohort (COVID-19) was defined as hospitalized patients with PCR confirmed positive SARS-CoV-2 (Corman et al., 2020). Clinical data and medical history as well as blood samples of the cohort was collected.
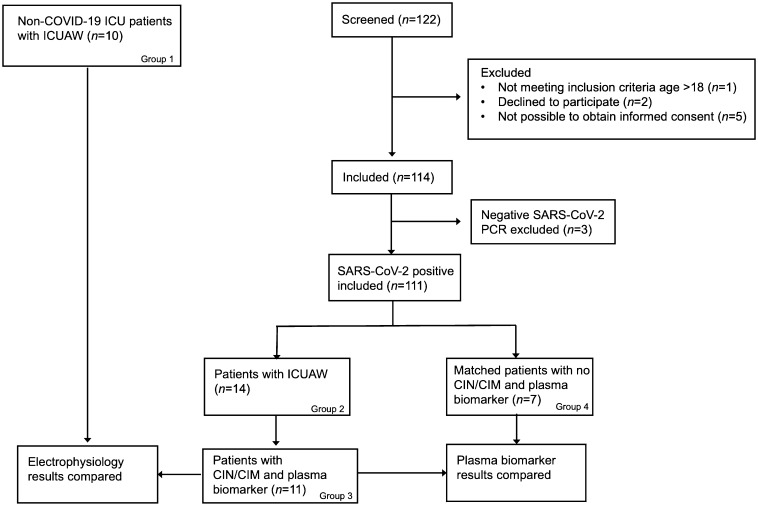
Fig. 1.
Flow chart of the screening and inclusion of patients in the study.
All non-COVID-19 ICU patients who were referred for electrophysiological evaluation due to ICUAW during the same time period were used as ICU controls (ICU ctrl; Group 1). These non-COVID-control patients were treated in the ICU for other disorders than COVID-19 [subarachnoid hemorrhage (N = 4), sepsis (N = 2), pneumonia (N = 1), herpes encephalitis (N = 1), meningitis (N = 1) and subdural hematoma (N = 1)]. Blood samples were not routinely collected from these patients and were not analyzed for biomarkers. However, the results from electrophysiological evaluation could be used to compare distinct characteristics of ICUAW in COVID-19 patients (Fig. 1).
2.3. Electrophysiological examinations
All patients with ICUAW (Fig. 1, group 1 = 10 non-COVID-19 patients; group 2 = 14 COVID-19 patients) were referred to the department of clinical neurophysiology with the question of CIN/CIM and electrophysiological examinations were done in the ICU. Motor nerve conduction studies (NCS) were performed in the median and ulnar nerves of one arm and in the fibular and tibial nerves of both legs. Orthodromic sensory NCS were done for the ulnar and radial nerves of one arm as well as the sural and superficial fibular nerves in both legs. Reduced amplitude of the compound muscle action potential (CMAP) and sensory nerve action potential (SNAP) is the predominant finding in CIN (Bolton et al., 1986); therefore, combined motor and sensory amplitude scores were calculated as the mean amplitude of all recorded CMAPs and SNAPs respectively divided by number of nerves examined. Low frequency (3 Hz) repetitive nerve stimulation (RNS) was performed in the ulnar nerve with recording over the abductor digiti quinti muscle, to rule out decremental response as a lingering neuromuscular blocking effect of medications. Electromyography (EMG) with a concentric needle electrode was performed in upper and lower limb muscles and in most cases one facial skeletal muscle (often spared in CIM), in order to detect abnormal spontaneous activity at rest (fibrillations and/or positive sharp waves) and if possible, if the patients could collaborate, analyze the presence of myopathic (polyphasic, short duration, low amplitude) motor unit potentials (Lacomis et al., 2000). Presence of edema, primarily of the lower limbs, was regularly evaluated, and if present reported, since this could influence the NCS studies.
2.4. Diagnostic criteria of CIN and CIM
Diagnostic criteria for both CIN and CIM were based on international guidelines (Bolton, 2005, Lacomis et al., 2000) as follows:
The patient was i) critically ill and had ii) limb weakness or difficulty weaning from ventilator after non-neuromuscular causes such as heart and lung disease have been excluded.
For CIN, additional diagnostic criteria included: iii) electrophysiological evidence of axonal motor and sensory polyneuropathy, iv) absence of a decremental response on RNS (Bolton, 2005).
For CIM, additional criteria included: iii) CMAP amplitudes < 80% of the lower limit of normal in ≥ 2 nerves without conduction block; iv) SNAP amplitudes > 80% of the lower normal limit in ≥ 2 nerves; v) Needle electromyography with short duration, low-amplitude motor unit potentials with early or normal full recruitment, with or without fibrillation potentials in conscious and collaborative patients; vi) absence of a decremental response on RNS (Lacomis et al., 2000). Prolonged CMAP duration, especially in lower limb nerves, would additionally be in support of CIM (Goodman et al., 2009).
2.5. Plasma biomarkers for neuronal injury
Plasma samples were collected at different time points in the ICU ward, early (<9 days from admission) and at a later stage (>11 days from admission) and could be analyzed for biomarkers in eleven COVID-19 patients with ICUAW (Group 3) and a matched group of seven COVID-19 patients who did not develop CIN/CIM (Group 4). Levels of neurofilament light chain (NfL), tau and glial fibrillary acidic protein (GFAp) were measured using Single molecule array (Simoa) technology (Quanterix, Billerica, MA), as previously described (Kanberg et al., 2020) using a single batch of reagents. Intra-assay coefficients for internal control samples were below 7% for all analytes.
2.6. Statistical analysis
Data are represented as median (IQR) or n (%). Mann-Whitney U test was used for continuous parameters and the chi2 test for categorical parameters.
The plasma biomarker data were log transformed to achieve near normal distribution and analyzed with two-way repeated measures analysis of variance (ANOVA; CIN/CIM y/n × time) followed by Fishers LSD test. Correlation analysis between plasma biomarkers (tau, GFAp, NfL) with NCS parameters were done with Spearman Rank correlation. Statistical comparison of descriptive variables (diagnoses) between the groups was done with Chi2-test. A p-value of < 0.05 was considered significant.
3. Results
3.1. Characteristics of the ICU COVID-19 cohort
After screening of 122 patients, a total of 111 COVID-19 patients were included in the study (Fig. 1). Out of these patients, 14 patients (group 2) developed ICUAW out of which 11 patients were diagnosed with CIN/CIM (group 3) and three patients did not receive a diagnosis of neuromuscular disorder (one patient had normal MRI, one had cerebral infarctions and one had acute necrotizing encephalopathy). Out of the surviving COVID-19 patients who remained intubated for > 2 weeks, 8 patients (62%) developed ICUAW. One hundred patients were not diagnosed with CIN/CIM (“non-CIN/CIM”). Demographic characteristics and comorbidities of these patients are displayed in Table 1 . There were significantly more male patients with high BMI in the CIN/CIM patient cohort (group 3, Table 1), whereas age, blood group or preexisting comorbidities did not differ between the groups (Table 1; p > 0.05). On admission, there were no significant differences in vital parameters or number of days with the COVID-19 infection (Table 2 ; p > 0.05). The COVID-19 cohort that developed CIN/CIM had more severe illness, defined as a longer duration of stay in the ICU (median: 27 versus 7 days; p < 0.001), more thromboembolic events (p = 0.006), more days with invasive ventilation (median: 18 versus 8; p < 0.001), more frequent vasoactive medication (median: 14 versus 5; p < 0.001) and renal replacement therapy (median: 21 versus 6; p = 0.006; Table 2). Renal replacement therapy was defined as continuous veno-venous hemodialysis. Additionally, patients who developed CIN/CIM were more frequently treated with anesthetics, opioid analgesics and neuromuscular blocking agents (Table 2; p < 0.001). No difference was seen in ICU mortality or 30-day mortality between the groups (Table 2; p > 0.05).
Table 1.
Demographic characteristics and comorbidities of 111 patients admitted to the intensive care unit (ICU) due to severe COVID-19 divided by the development of critical illness neuropathy (CIN) or myopathy (CIM) or not. ACEi – Angiotensin Converting Enzyme inhibitor; ARB – Angiotensin Receptor Blocker. Values are represented as median (IQR) or n (%). The p-value originates from the Mann-Whitney U test for continuous parameters and the chi2 test for categorical parameters.
| n | COVID-19 ICU non-CIN/CIM | n | COVID-19 ICU CIN/CIM | P | |
|---|---|---|---|---|---|
| Gender, male n (%) | 100 | 74 (74) | 11 | 11 (100) | 0.01 |
| Age, yrs | 100 | 61 (51–73) | 11 | 64 (55–70) | 0.97 |
| Body mass index (kg/m2) | 87 | 28 (25–32) | 10 | 35 (30–38) | 0.006 |
| ACEi/ARB treatment (%) | 99 | 36 (36) | 11 | 5 (46) | 0.56 |
| Comorbidities, n (%) | |||||
| Pulmonary disease | 100 | 27 (27) | 11 | 2 (18) | 0.52 |
| Hypertension | 100 | 51 (51) | 11 | 7 (64) | 0.42 |
| Heart failure | 100 | 3 (3) | 11 | 2 (18) | 0.07 |
| Peripheral vessel disease | 100 | 16 (16) | 11 | 3 (27) | 0.37 |
| Diabetes mellitus | 100 | 25 (25) | 11 | 4 (36) | 0.43 |
| Neurological disease | 100 | 6 (6) | 11 | 0 (0) | 0.25 |
| Malignancy | 100 | 8 (8) | 11 | 0 (0) | 0.19 |
Table 2.
Patient characteristics and intensive care unit (ICU) treatment of 111 patients due to severe COVID-19, divided by the development of critical illness neuropathy (CIN) or myopathy (CIM) or not. Intravenous anesthesia includes propofol and/or midazolam. Opioid analgesia includes morphine, oxycodone, remifentanil and/or fentanyl. Neuromuscular blockade includes rocuronium or atracurium. Values are represented as median (IQR) or n (%). The p-value originates from the Mann-Whitney U test for continuous parameters and the chi2 test for categorical parameters. Values are represented as median (IQR) or n (%). The p-value is calculated for continuous parameters with the Mann-Whitney U test, and for categorical parameters the chi2 test; p < 0.05 is considered significant. SAPS, Simplified Acute Physiology Score.
| n | COVID-19 ICU non-CIN/CIM | n | COVID-19 ICU CIN/CIM | P | |
|---|---|---|---|---|---|
| On admission | |||||
| SAPS | 97 | 53 (47–57) | 11 | 50 (46–55) | 0.40 |
| Respiratory rate (breaths per minute) | 97 | 28 (23–36) | 11 | 31 (26–36) | 0.51 |
| Mean arterial blood pressure (mmHg) | 97 | 90 (80–98) | 11 | 86 (79–96) | 0.53 |
| Heart rate (beats per minute) | 99 | 89 (78–100) | 11 | 89 (79–101) | 0.87 |
| Body temperature (degrees Celsius) | 99 | 38.0 (37.4–38.7) | 11 | 38.0 (37.9–38.6) | 0.60 |
| Days between symptom onset and admission | 95 | 10 (9–13) | 11 | 8 (8–10.5) | 0.11 |
| In the ICU | |||||
| Length of stay | 100 | 7 (4–12) | 11 | 27 (18–41) | <0.001 |
| Thromboembolic events | 100 | 10 (10) | 11 | 5 (46) | 0.05 |
| Invasive ventilation (%) | 100 | 52 (52) | 11 | 11 (100) | 0.001 |
| Ventilator free days | 52 | 20 (14–24) | 11 | 3 (0–12) | <0.001 |
| Vasoactive medication (%) | 100 | 40 (40) | 11 | 11 (100) | 0.002 |
| Vasoactive medication free days | 40 | 20 (0–24) | 11 | 13 (0–17) | 0.12 |
| Renal replacement therapy (%) | 100 | 8 (8) | 11 | 7 (64) | 0.003 |
| Renal replacement therapy free days | 8 | 18 (0–22) | 7 | 9 (0–10) | 0.19 |
| Continuous propofol anesthesia (%) | 100 | 52 (52) | 11 | 11 (100) | <0.001 |
| Propofol anesthesia free days | 52 | 21 (17–23) | 11 | 13 (0–15) | <0.001 |
| Iv Opioid analgesia (%) | 100 | 74 (74) | 11 | 11 (100) | 0.01 |
| Iv Opioid analgesia free days | 74 | 19 (14–24) | 11 | 6 (0–10) | <0.001 |
| Continuous iv neuromuscular blockade (%) | 100 | 45 (45) | 11 | 11 (100) | <0.001 |
| Iv neuromuscular blockade free days | 45 | 25 (21–27) | 11 | 22 (0–23) | <0.001 |
| ICU-mortality (%) | 100 | 20 (20) | 11 | 3 (27) | 0.58 |
| 30-day mortality (%) | 100 | 25 (25) | 11 | 2 (18) | 0.60 |
3.2. Characteristics of the ICUAW in COVID-19 compared to non-COVID-19 patients
Fourteen COVID-19 patients (Group 2) and 10 non-COVID ICU control patients (Group 1) underwent electrophysiological examination due to ICUAW. All of these patients had reduced muscle tone and none had any signs of decrement at low frequency RNS, thus eliminating neuromuscular blockade as a cause of the muscle weakness. There were significantly more men (94%) in the COVID-19 cohort compared to the ICU control group (30%; p < 0.001; Table 3 ), whereas age did not differ (p = 0.42). A neurophysiological pattern of CIN/CIM was seen in 11 (79%) of the COVID-19 cohort and in seven ICU control patients (70%, all CIM; Table 1). A clear CIN pattern with axonal sensorimotor polyneuropathy was seen in as many as seven (50%) patients in the COVID-19 cohort, whereas this was not seen in any patients in the ICU control cohort (group 1; p = 0.008). Instead, a distinct CIM pattern was slightly more commonly encountered among the ICU control patients in group 1 (Table 3; p = 0.05). CMAP duration was significantly longer in CIM patients in the median (p = 0.00046) and fibular (p = 0.017) nerves.
Table 3.
Comparison of the cohort of COVID-19 patients (N = 14; group 2 in Fig. 1) and the non-COVID-19 intensive care unit control group (ICU-ctrl, N = 10; group 1 in Fig. 1) with regards to nerve conduction studies and electromyography (EMG) due to ICU acquired weakness. CIN, critical illness polyneuropathy, ICD-10-code G63.9; CIM, critical illness myopathy, ICD-code G72.9; PNP, polyneuropathy of other cause, ICD-code G62.9; normal findings ICD-code Z01.9; abnormal, unspecified abnormal findings. EMG fib/psw, abnormal spontaneous activity including fibrillations and positive sharp waves. n.a., not assessed due to low number of cases.
| Cohort |
|||
|---|---|---|---|
|
COVID-19 n = 14 |
ICU-ctrl n = 10 |
P | |
| Male N (%) | 13 (93%) | 3 (30%) | <0.001 |
| Age (mean ± SD) | 60.5 ± 11 | 66 ± 13 | 0.43 |
| CIM | 4 (29%) | 7 (70%) | 0.05 |
| CIN | 7 (50%) | 0 (0%) | 0.008 |
| PNP | 0 | 2 | n.a. |
| Normal findings | 2 | 1 | n.a. |
| Abnormal findings | 1 | 0 | n.a. |
| EMG fib/psw | 9 (64%) | 7 (88%) | 0.25 |
Thirteen patients in the COVID-19 ICUAW cohort (group 2) were examined with a computer tomography scan of the head at least once, and seven of these were also examined with a magnetic resonance imaging of the brain. Four patients had pathological neuroradiological findings that did not explain the peripheral weakness: acute necrotizing encephalopathy (N = 1), bilateral infarcts in deep white matter (N = 1), unspecific white matter changes with microhemorrhage (N = 1), and numerous bilateral microhemorrhages in white matter (N = 1). The remaining patients had normal neuroradiological findings.
3.3. Higher levels of plasma biomarkers for neuronal injury in CIN/CIM patients
Analysis of biomarkers for neuronal injury and astrocytic activation was performed from early (median: 4, range: 3–9 days) and late (median: 16, range: 11–42 days) plasma samples. All analyzed COVID-19 patients required invasive ventilation and were treated in the ICU for > 12 days. Seven COVID-19 patients (group 4), who did not develop CIN/CIM, had fewer days with invasive ventilation and renal replacement therapy compared with the CIN/CIM group. Otherwise, there were no significant differences regarding demography and clinical parameters (Supplementary Tables 1 and 2). NfL levels were significantly higher in the CIN/CIM group (group 3) both at the early and late time points (p < 0.001 and p = 0.03, respectively; Fig. 2 A). NfL levels were also compared with time spent in ICU (Supplementary Fig. 1). Higher NfL concentration correlated with longer ICU time both for the CIN/CIM group (group 3, p = 0.02; R = 0.4) and for the non-CIN/CIM group (group 4, p = 0.005; R = 0.62). GFAp levels were significantly higher in the CIN/CIM group (group 3) at both early (p = 0.04) and late (p = 0.02) timepoints (Fig. 2B), with no significant effect of time in the ICU (data not shown). Tau was significantly increased in the CIN/CIM cohort compared to patients with non-CIN/CIM (p = 0.04) when considering all samples but not when divided in early and late timepoints (p = 0.08 and p = 0.06, respectively; Fig. 2C).
Fig. 2.
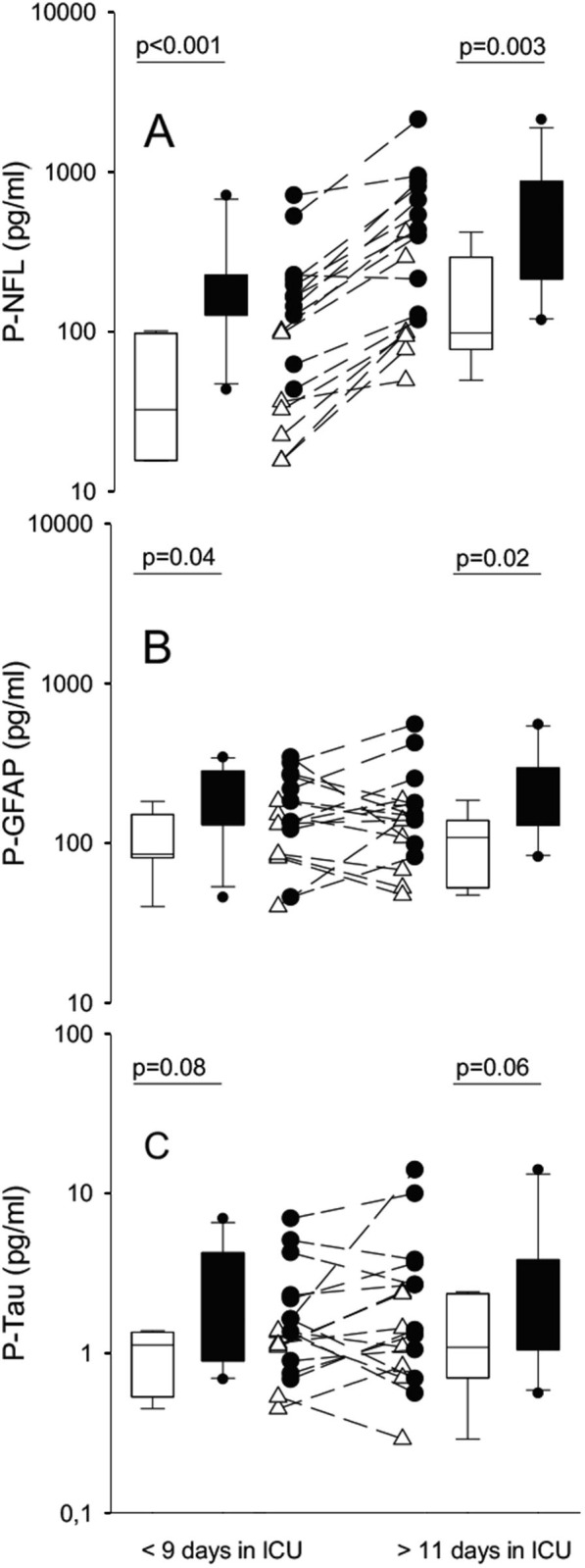
Levels of plasma A) neurofilament light chain (NfL), B) glial fibrillary acidic protein (GFAp) and C) Tau in pg/ml (logarithmic scale) comparing patients with critical illness neuropathy/myopathy (CIN/CIM; filled circles) with the patients without CIN/CIM (open triangles) at the early (median: 4 days, range: 3–9 days) and late timepoint (median: 16 days, range: 11–42 days).
3.4. Plasma biomarkers correlate with NCS parameters
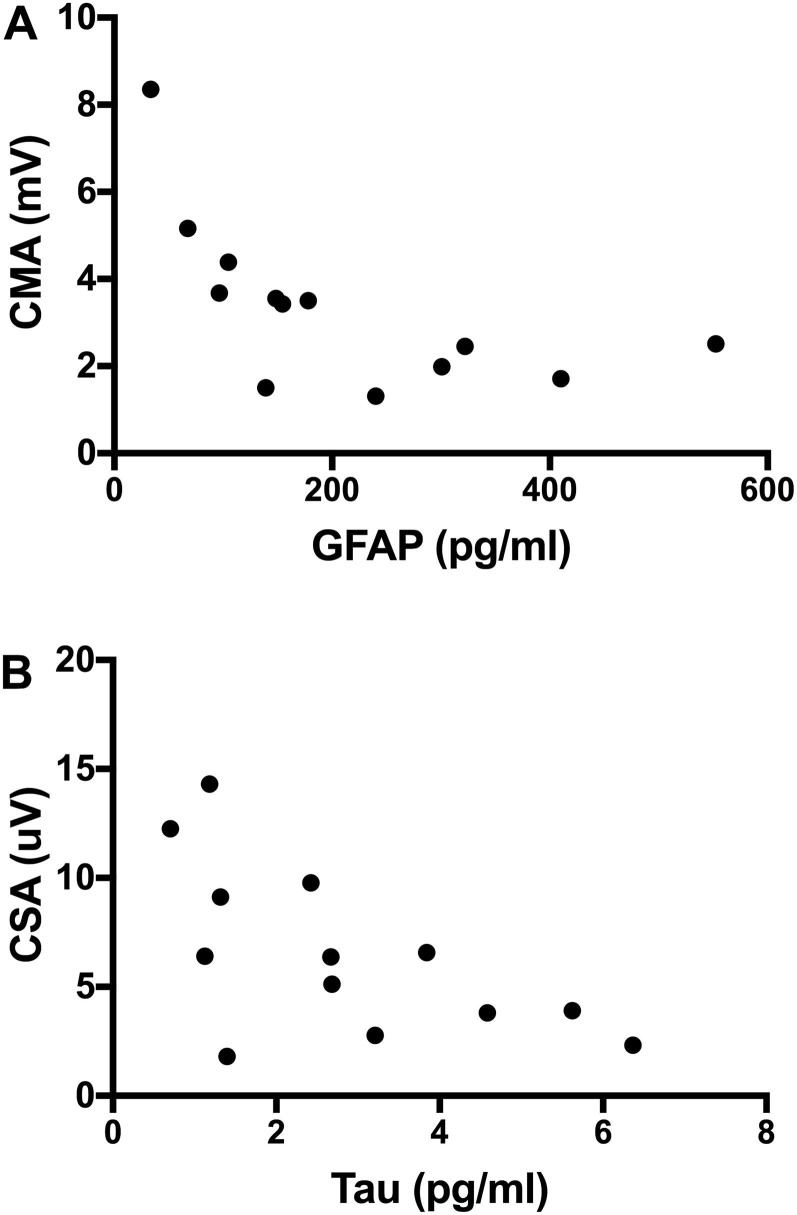
In order to correlate the findings in NCS parameters and plasma biomarkers we selected the plasma analyses that coincided at the same timepoint as the electrophysiological work-up. This was the case for 13 COVID-19 patients (selected both from group 3 and 4). Six patients were diagnosed with CIN, four patients were diagnosed with CIM and three patients had normal electrophysiological data. The combined motor amplitude (CMA) score was significantly lower in the CIN/CIM cohort (median: 2.5, range 1.3–3.5) than in the non-CIN/CIM cohort (n = 7) (median: 5.2, range 4.4–8.4; p = 0.0070; Fig. 3 A). In addition, the combined sensory amplitude (CSA) score was lower in the CIN/CIM cohort (median: 4.5, range 2.7–6.4) than the non-CIN/CIM group (median: 12.3, range 9.8–14.3; p = 0.007; Fig. 3B). A correlation analysis was done between levels of GFAp, NfL and tau with sensory and motor nerve parameters from the NCS data. For GFAp, there was a significant correlation with CMA (R = -0.72; p = 0.007), i.e., the more severe axonal motor neuropathy (lower CMA score), the higher the GFAp levels (Fig. 4 A). For NfL, there was a similar trend towards significant correlation with CMA (R = -0.511; p = 0.078) and a correlation with fibular nerve motor amplitude (R = -0.64; p = 0.022). For tau, there was a significant correlation with sural nerve sensory amplitude (R = -0.59; p = 0.036) and CSA (R = -0.63; p = 0.024; Fig. 4B).
Fig. 3.
A) Combined motor amplitude (CMA) of all examined motor nerves (ulnar, median, fibular and tibial) and B) combined sensory amplitude (CSA) of all examined sensory nerves (ulnar, radial, sural) in the group of patients without critical illness neuropathy (CIN) or myopathy (CIM) (N = 3) and among patients with CIN/CIM (N = 11).
Fig. 4.
A) Correlation between compound motor amplitude score (CMA) and glial fibrillary acidic protein (GFAp). Spearman R = -0.72; p = 0.007. B) Correlation between compound sensory amplitude score (CSA) and Tau. Spearman R = -0.63; p = 0.024.
4. Discussion
One recent case of CIM (Tankisi et al., 2020) as well as a retrospective case series of ICUAW COVID-19 patients with four polyneuropathy and seven myopathy patients (Cabanes-Martinez et al., 2020) as likely consequences of SARS-CoV-2 infection have been reported. However, to our knowledge, no prospective systematic reports of CIN/CIM in COVID-19 ICU patients have been published. The major risk factors associated with CIN/CIM are multiple organ failure, muscle inactivity, hyperglycemia and the use of corticosteroids, sedative and neuromuscular blocking agents (Yang et al., 2018). Speculations have also been made regarding increased number of CIM patients in COVID-19 cohorts as propofol is considered a risk factor for CIM (Lonnqvist et al., 2020).
4.1. Higher incidence of CIN in COVID-19 ICU patients with ICUAW
We found a higher incidence of CIN in the COVID-19 ICU cohort compared with the non-COVID-19 ICU control cohort in this study. This is in contrast to most published literature, which states that CIM is more common than CIN (Z'Graggen and Tankisi, 2020). Although higher incidence in our study than the previous publication from Cabanes-Martinez, we found equally high number of male COVID-19 ICU patients with CIN/CIM (Cabanes-Martinez et al., 2020). Since patients with CIN have slower recovery, or do not recover and have higher mortality rates, this is an important consequence to consider for the ICU treated COVID-19 patients. Clinically, it is very difficult to distinguish CIN from CIM in patients with ICUAW; hence, detailed NCS and EMG studies are essential for this diagnostic classification. Immobility has severe effects on skeletal muscle, and is a risk factor for muscle weakness and wasting; however this is usually characterized by normal motor and sensory nerve conduction and EMG studies (Latronico and Bolton, 2011). Prolonged CMAP duration has been suggested as an additional discriminative parameter for CIM (Goodman et al., 2009), also supported by our findings in this study. One interesting aspect of COVID-19 is the large number of reports of neurological symptoms associated with this disease (Battaglini et al., 2020, Helms et al., 2020). A recent report from our study group showed significantly higher levels of NfL in CSF of COVID-19 patients with neurological symptoms, which also correlated with disease severity (Virhammar et al., 2020). Nevertheless, potential peripheral nerve damage caused by neurotropism of SARS-CoV-2 remains to be elucidated in mechanistic studies. The high incidence of CIN in the COVID-19 patients in our study may also have other plausible explanation, such as increased number of days with mechanical ventilation, requiring an accumulated higher amount of anesthesia and analgesia. In fact, almost all COVID-19 patients who were intubated for > 2 weeks developed ICUAW and the majority of them developed CIN/CIM. Another possible explanation is severe respiratory failure prompting the use of neuromuscular blocking agents to improve oxygenation during invasive ventilation. Although these factors may explain the higher numbers of CIN/CIM in COVID-19 patients, further studies are needed to properly explore the underlying cause of higher rates of CIN compared to CIM in patients infected with SARS-CoV-2.
4.2. Correlation of neuronal blood biomarkers and NCS parameters in CIN/CIM patients
In previous studies comparing CIN patients to controls, the only statistically significant NCS abnormality was reduced CMAP and SNAP amplitudes, whereas motor and sensory conduction velocities, distal latencies, and F wave latencies were only mildly abnormal (Bolton et al., 1986). However, there are no available blood biomarkers to distinguish CIN or CIM from normal NCS in patients with ICUAW. A previous pilot study observed that peak NfL levels had good discriminative power for ICUAW, although at a later stage and first after the muscle strength assessment (Wieske et al., 2014). In a recent study higher plasma levels of NfL and GFAp were correlated with COVID-19 severity (Kanberg et al., 2020). All our included subjects suffered from severe COVID-19, but still the CIN/CIM patients had higher plasma NfL levels both at the early and late time points, prior to onset of ICUAW. Therefore, the increase in biomarkers is most likely related to development of CIN/CIM and not to COVID-19 itself. NfL is a neuronal cytoplasmic protein, which is particularly highly expressed in myelinated axons. Levels of NfL in the blood proportionally increase in relation to the degree of axonal damage in several neurological disorders, including multiple sclerosis, amyotrophic lateral sclerosis and Alzheimer’s disease (Gaetani et al., 2018), but also in peripheral neuropathies (Chelban et al., 2019, Kapoor et al., 2019, Sandelius et al., 2018). Several patients in our control group had underlying cerebral insults that could affect the biomarker levels but still the CIN/CIM showed significantly higher levels of NfL. A larger study would however be required to confirm the potential of NfL to determine peripheral axonal loss in CIN or other polyneuropathies. GFAp is a suggested astrocyte biomarker for glial activation, with elevated levels seen in Alzheimer’s disease (Olsson et al., 2016). Early high levels of GFAp were also found in the CIN/CIM group, which also correlated with motor amplitude score, indicating that GFAP could have potential as marker for axonal pathology in myelinated nerves. Tau levels were slightly higher among CIN/CIM patients and correlated with the sensory amplitude score, which is in line with a recent study indicating that serum levels of phosphorylated tau may be a useful biomarker for severe axonal injury (Caprelli et al., 2018).
4.3. Limitations and future directions
Our study has several limitations. First, referrals to NCS and EMG were strictly selected to ICU patients with quadriplegia, and not all patients were assessed systematically for quantitative muscle strength, which could have resulted in a false low incidence of CIN/CIM. Second, due to the COVID-19 related restrictions, imposed to isolate and protect personnel and equipment, neither quantitative muscle strength grading nor muscle biopsy were performed in these patients. Theoretically, sensory nerve amplitudes could be lower in cases of edema in the ICU, however in our COVID-19 cohort fluid balance was generally slightly negative and edema was not frequently observed. Edema was also not described by staff in the electrophysiological examination, edema is commonly stated both by the technician and/or the physician if present. Due to the higher number of men and the small COVID-19 cohort with ICUAW, we cannot draw firm conclusions about higher incidence of CIN/CIM in COVID-19 patients.
In summary, we found a high proportion of COVID-19 patients with ICUAW who developed CIN and the most likely explanation is the lengthy ICU care. This is important to consider in the differential diagnostic workup as well as the further rehabilitation of these patients. Further larger studies are needed to explore why CIN is more common than CIM in COVID-19 patients compared to non-COVID-19 patients. The plasma levels of NfL, GFAp and tau could play a role in diagnosing and predicting axonal nerve pathology and deserves to be further explored. Until then, however, NCS and EMG are crucial in optimizing the diagnostic workup for all patients with ICUAW.
CRediT authorship contribution statement
Robert Frithiof: Study design Data collection, Data analysis, Data interpretation, Figures, Lterature search, Writing. Elham Rostami: Study design Data collection, Data analysis, Data interpretation, Figures, Lterature search, Writing. Eva Kumlien: Data collection, Data interpretation, Writing. Johan Virhammar: Data collection, Writing. David Fällmar: Data collection, Data interpretation, Writing. Michael Hultström: Data collection, Data interpretation, Writing. Miklós Lipcsey: Data collection, Writing; Nicholas Ashton: Data collection, Data analysis, Writing. Kaj Blennow: Data analysis, Data interpretation, Writing; Henrik Zetterberg: Data analysis, Data interpretation, Writing; Anna Rostedt Punga: Data collection, Data analysis, Data interpretation, Figures, Literature search, Writing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (all outside submitted work). The other authors report no disclosures.
Acknowledgments
Acknowledgements
The authors thank research nurses Joanna Wessbergh and Elin Söderman, and the biobank assistants Erik Danielsson and Philip Karlsson for their expertise in compiling the study. The authors thank the physicians and technicians at the department of Clinical Neurophysiology, for excellent electrophysiological work up in the ICU and Dr Laura ÓConnor for proof-reading.
Funding
The study was funded by the SciLifeLab/KAW national COVID-19 research program project (MH and ER), the Swedish Research Council (RF:2014-02569, 2014-07606; ARP:#2014-02048 and 2014-07603; KB:#2017-00915; HZ:#2018-02532); Hjärnfonden (ARP:#FO FO2020-0153 and KB:#FO2017-0243), Alzheimer Drug Discovery Foundation, USA (KB:#RDAPB-201809-2016615 and HZ:#201809-2016862), the Swedish Alzheimer Foundation (#AF-742881), European Union Joint Program for Neurodegenerative Disorders (KB:JPND2019-466-236), the European Research Council (#HZ:681712) and the UK Dementia Research Institute at UCL.
See Editorial, pages 1716–1717
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clinph.2021.03.016.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Plasma neurofilament light chain (NfL) levels plotted against number of days treated in the intensive care unit divided by critical illness neuropathy/myopathy (CIN/CIM; filled circles) and no CIN/CIM (open circles).
References
- Battaglini D., Brunetti I., Anania P., Fiaschi P., Zona G., Ball L. Neurological manifestations of severe SARS-CoV-2 infection: potential mechanisms and implications of individualized mechanical ventilation settings. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C.F. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32(2):140–163. doi: 10.1002/mus.20304. [DOI] [PubMed] [Google Scholar]
- Bolton C.F., Laverty D.A., Brown J.D., Witt N.J., Hahn A.F., Sibbald W.J. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 1986;49(5):563–573. doi: 10.1136/jnnp.49.5.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabañes-Martínez L., Villadóniga M., González-Rodríguez L., Araque L., Díaz-Cid A., Ruz-Caracuel I. Neuromuscular involvement in COVID-19 critically ill patients. Clin Neurophysiol. 2020;131(12):2809–2816. doi: 10.1016/j.clinph.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camdessanche J.P., Morel J., Pozzetto B., Paul S., Tholance Y., Botelho-Nevers E. COVID-19 may induce Guillain-Barre syndrome. Rev Neurol (Paris) 2020;176:516–518. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprelli M.T., Mothe A.J., Tator C.H. Hyperphosphorylated tau as a novel biomarker for traumatic axonal injury in the spinal cord. J Neurotrauma. 2018;35(16):1929–1941. doi: 10.1089/neu.2017.5495. [DOI] [PubMed] [Google Scholar]
- Chelban V., Wilson M.P., Warman Chardon J., Vandrovcova J., Zanetti M.N., Zamba-Papanicolaou E. PDXK mutations cause polyneuropathy responsive to pyridoxal 5'-phosphate supplementation. Ann Neurol. 2019;86:225–240. doi: 10.1002/ana.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25; 2020. [DOI] [PMC free article] [PubMed]
- Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani L., Höglund K., Parnetti L., Pujol-Calderon F., Becker B., Eusebi P. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimers Res Ther. 2018;10(1) doi: 10.1186/s13195-018-0339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman B.P., Harper C.M., Boon A.J. Prolonged compound muscle action potential duration in critical illness myopathy. Muscle Nerve. 2009;40(6):1040–1042. doi: 10.1002/mus.21445. [DOI] [PubMed] [Google Scholar]
- Guarneri B., Bertolini G., Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79(7):838–841. doi: 10.1136/jnnp.2007.142430. [DOI] [PubMed] [Google Scholar]
- Hanidziar D., Bittner E.A. Sedation of mechanically ventilated COVID-19 patients: challenges and special considerations. Anesth Analg. 2020;131:e40–e41. doi: 10.1213/ANE.0000000000004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge M.S., Tansey C.M., Matté A., Tomlinson G., Diaz-Granados N., Cooper A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- Iwashyna T.J. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153(3):204. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- Kanberg N., Ashton N.J., Andersson L.-M., Yilmaz A., Lindh M., Nilsson S. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020;95(12):e1754–e1759. doi: 10.1212/WNL.0000000000010111. [DOI] [PubMed] [Google Scholar]
- Kapoor M., Foiani M., Heslegrave A., Zetterberg H., Lunn M.P., Malaspina A. Plasma neurofilament light chain concentration is increased and correlates with the severity of neuropathy in hereditary transthyretin amyloidosis. J Peripher Nerv Syst. 2019;24(4):314–319. doi: 10.1111/jns.12350. [DOI] [PubMed] [Google Scholar]
- Lacomis D., Zochodne D.W., Bird S.J. Critical illness myopathy. Muscle Nerve. 2000;23(12):1785–1788. doi: 10.1002/1097-4598(200012)23:12<1785::aid-mus1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Latronico N., Bolton C.F. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- Leijten F.S., Harinck-de Weerd J.E., Poortvliet D.C., de Weerd A.W. The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA. 1995;274:1221–1225. [PubMed] [Google Scholar]
- Lönnqvist P.-A., Bell M., Karlsson T., Wiklund L., Höglund A.-S., Larsson L. Does prolonged propofol sedation of mechanically ventilated COVID-19 patients contribute to critical illness myopathy? Br J Anaesth. 2020;125(3):e334–e336. doi: 10.1016/j.bja.2020.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y.u., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Lautner R., Andreasson U., Öhrfelt A., Portelius E., Bjerke M. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- Sandelius Å., Zetterberg H., Blennow K., Adiutori R., Malaspina A. Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology. 2018;90(6):e518–e524. doi: 10.1212/WNL.0000000000004932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankisi H., Tankisi A., Harbo T., Markvardsen L.K., Andersen H., Pedersen T.H. Critical illness myopathy as a consequence of Covid-19 infection. Clin Neurophysiol. 2020;131(8):1931–1932. doi: 10.1016/j.clinph.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.-K., Hsieh S.-T., Chao C.-C., Chen Y.-C., Lin Y.-H., Chang S.-C. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11):1669. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- Virhammar J, Naas A, Fallmar D, Cunningham JL, Klang A, Ashton NJ, et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol. 2020; 10.1111/ene.14703 [DOI] [PMC free article] [PubMed]
- Wieske L., Witteveen E., Petzold A., Verhamme C., Schultz M.J., van Schaik I.N., Horn J. Neurofilaments as a plasma biomarker for ICU-acquired weakness: an observational pilot study. Crit Care. 2014;18(1):R18. doi: 10.1186/cc13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Li Z., Jiang L.i., Wang Y., Xi X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol Scand. 2018;138(2):104–114. doi: 10.1111/ane.12964. [DOI] [PubMed] [Google Scholar]
- Z'Graggen W.J., Tankisi H. Critical illness myopathy. J Clin Neurophysiol. 2020;37:200–204. doi: 10.1097/WNP.0000000000000652. [DOI] [PubMed] [Google Scholar]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plasma neurofilament light chain (NfL) levels plotted against number of days treated in the intensive care unit divided by critical illness neuropathy/myopathy (CIN/CIM; filled circles) and no CIN/CIM (open circles).