Abstract
The heightened incidence of opioid use during pregnancy has resulted in unprecedented rates of neonates prenatally exposed to opioids. Prenatal opioid exposure (POE) results in significantly adverse medical, developmental, and behavioral outcomes in offspring. Of growing interest is whether POE contributes to future vulnerability to substance use disorders. The effects of POE on brain development is difficult to assess in humans, as the timing, dose, and route of drug exposure together with complex genetic and environmental factors affect susceptibility to addiction. Preclinical models of POE have allowed us to avoid methodological difficulties and confounding factors of POE in humans. Here, we review the effects of maternal opioid exposure on the developing brain with an emphasis on the neurobiological basis of drug addiction and on preclinical models of POE and their limitations. These studies have indicated that POE increases self-administration of drugs, reward-driven behaviors in the conditioned place paradigm, and locomotor sensitization. While addiction is multifaceted and vulnerability to drug addiction is still inconclusive in human studies of prenatally exposed infants, animal studies do provide a noteworthy corroboration of negative behavioral outcomes.
Keywords: prenatal drug, pregnancy, gestation, opioids, opiates, substance use disorder, drug abuse, vulnerability, addiction, behavior, conditioned place preference, locomotor sensitization, self-administration, morphine, long-term outcomes, development
1. Introduction
The opioid epidemic has had disastrous consequences, particularly for women of reproductive age (Azuine et al. 2019; Jantzie et al. 2020). Women aged 18–29 are at highest risk for developing opioid use disorder, which correlates to increased rates of opioid use during pregnancy (Forray 2016). While men still lead women in overall opioid use, this gender gap has steadily been narrowing (Serdarevic et al. 2017). Women, furthermore, progress more rapidly from casual use to drug dependence than men, and exhibit higher rates of drug use and shorter drug free periods (Griffin et al. 1989; Brady and Grice 1993; Westermeyer and Boedicker 2000). Together, this increases their vulnerability to substance use disorders during their reproductive years.
Opioid use disorder (OUDs) during pregnancy is a growing public health concern as both opioids (such as oxycodone, morphine, codeine) and medications used to manage OUD (such as methadone and buprenorphine) cross the placenta, subjecting offspring to the effects of prenatal opioid exposure (POE) (Patrick et al. 2015; McQueen and Murphy-Oikonen 2016; Feder et al. 2019). Most of the attention has focused on the development of neonatal opioid withdrawal syndrome (NOWS), which affects 21–94% of neonates exposed to opioids (McQueen and Murphy-Oikonen 2016). NOWS poses a serious threat to newborns and is characterized by a diverse constellation of symptoms representing neonatal neurobehavioral dysregulation due to opioid exposure. Predicting the severity of NOWS in neonates is currently not possible, but commonly reported symptoms include respiratory distress, tremors, emesis, seizures, irritability, feeding difficulties, and hypertonia after birth (Ross et al. 2015). Collectively, NOWS results in significant neonatal morbidity and long-term impairments, with established deleterious consequences in offspring during the neonatal and childhood periods. For instance, motor and cognitive deficits as well as inattentiveness and hyperactivity have been reported throughout childhood (Ornoy et al. 2001; Hunt et al. 2008). Despite these known costs, the rate of opioid use during pregnancy has nearly quadrupled in the past ten years, ushering in an unprecedented number of babies born with a history of POE (Jantzie et al. 2020).
Research investigating the long-term effects of POE is still in its infancy, but accumulating evidence suggests that POE has detrimental effects on offspring that extends past the neonatal period to adulthood. Of growing interest is how POE affects the developing brain and impacts vulnerability to substance use disorders in adulthood. To date, we have uncovered only one reported clinical study, which was on the development of substance use disorders in Norwegian adolescents and young adults aged 17–21 years old with a prenatal history of heroin and poly-drug exposure (Nygaard et al. 2017). This study found a significantly higher risk of ongoing substance misuse or addiction in the risk group (n = 6, 13%) than in the non-exposed group (n = 1, 2%; chi-square = 4.2, p = .04). However, the limitations of this study illustrate the difficulties with longitudinal clinical studies to determine the long-term consequences of POE. Clinical studies of POE are confounded by multiple variables including polysubstance use during pregnancy, maternal initiation and duration of opioid use, and variable postnatal environmental exposures such as family socioeconomic status, forced parental separation, and impaired maternal-infant bonding. The great heterogeneity of study participants which encompass complex genetic, perinatal, and postnatal environmental factors limits assessments of the direct causal effects of POE in human subjects (Nygaard et al. 2017). Animal models of POE, on the other hand, are able to carefully control for prenatal and postnatal exposures, eliminating most of the confounds in human studies. Admittedly, many animal models of POE have had design limitations including initiation of opioids during pregnancy, which is rarely observed in clinical practice; non-physiological maternal and neonatal opioid dosages; and maternal opioid withdrawal during pregnancy. Still, animal models currently provide the most effective way of studying the complex neurodevelopmental consequences of prenatal drug exposure and have regularly been used to assess for the long-term effects of POE (Thompson et al. 2009).
While there are a paucity of clinical studies, some animal models of POE have suggested long-lasting behavioral changes in drug-conditioned place preference, sensitization, and self-administration in adulthood, warranting further exploration into the consistency of such claims. Thus, in this review, we examine the effects of maternal opioid exposure on the developing brain, the neurobiological basis of drug addiction, preclinical models of POE and addictive-like behaviors later in life, and the limitations of these models in predicting vulnerability to substance use disorders in humans; finally, we conclude with future directions in the field.
2. Maternal opioid exposure and the developing brain: lasting neurodevelopmental consequences
Clinical studies suggest that maternal opioid exposure has lasting neurodevelopmental consequences on the growing fetus (Conradt et al. 2019; Welton et al. 2019). Neural development is a protracted process that begins in the third week of gestation; differentiation of neural progenitor cells extends through early adulthood in response to environmental influences and genetically programmed events (Stiles and Jernigan 2010). The long developmental period results in increased vulnerability to in utero insults during pregnancy. Opioids, for instance, rapidly cross the placenta resulting in functional and structural changes to the developing nervous system (Nekhayeva et al. 2005). Furthermore, neonatal diffusion MRI studies have shown microstructural brain injury in major white matter tracts, associated with prenatal methadone exposure (Monnelly et al. 2018). Still, little is known about how these brain changes correspond to long-term neurological impairments in infants exposed to opioids in utero.
Compared to other drugs of abuse, there are relatively few studies on the longitudinal consequences of POE through adulthood (reviewed in Conradt et al., 2019 and Welton et al., 2019). To compound this, many of these studies have shown inconsistent or equivocal results due to polydrug use during pregnancy, complex environmental factors relating to drug abuse, and heterogeneity in research assessment tools. For instance, in some studies, opioid-exposed children under the age of 6 were shown to exhibit normal physical, cognitive, language, sensory, and behavioral development (Messinger et al. 2004; Kaltenbach et al. 2018). In contrast, other studies have shown delays in motor skills and hand-eye coordination, and problems with memory, hyperactivity, impulsivity, and attention in opioid-exposed children of the same age group (Hans and Jeremy 2001; Hunt et al. 2008; Salo et al. 2009; Konijnenberg and Melinder 2013, 2015; Melinder et al. 2013; Wahlsten and Sarman 2013; Welton et al. 2019). Additionally, the lack of uniformity in reporting study outcomes and in adjusting for perinatal covariates adds another layer of difficulty in interpreting study findings. For example, some studies report impairments in opioid-exposed children if scores on various neurodevelopmental tests deviate from population norms, whereas others report impairments if scores reside within population norms but are significantly lower than those from age and gender-matched peers.
The few studies of older children prenatally exposed to opioids generally have shown problems with cognition and attention (Ornoy et al. 2010; Nygaard et al. 2017). In a meta-analysis of opioid-exposed children from birth to 12 years old, authors found impairments in cognitive, psychomotor, and behavioral development compared with peers (Baldacchino et al. 2015). In a case control study of children aged 5–12 years old with a history of POE, higher levels of inattention, hyperactivity, and behavior problems were also reported (Ornoy et al. 2001). Structural MRI studies of opioid-exposed youth aged 10–14 have further suggested that opioids can induce lasting structural brain changes, with volumes of the basal ganglia, thalamus, and cerebellar white matter reduced in school-aged children with POE (Sirnes et al. 2017). Taken together, these studies suggest that early impairments observed in children with POE may not resolve with age.
Notably, in the longitudinal case series of Norwegian children with a prenatal history of drug exposure (primarily heroin) followed from birth to 21 years old, subtle impairments were noted from infancy to early adulthood. At 1–4 years of age, these children had impulse and attention related problems, weakness in visual-motor abilities, and scores significantly worse than their peers on cognitive and motor tests (Moe 2002; Slinning 2004). Reports were primarily based on the Bayley Scales of Infant & Toddler Development, McCarthy Scales of Children’s Abilities, and behavioral questionnaires completed by primary caregivers, preschool teachers, and clinical psychologists. At about 11.3 years old, these same children were found to have lower neuroanatomical volumes than those of their peers (Walhovd et al. 2007). In a follow-up study at 17–21 years old, while these drug-exposed youths had cognitive (Wechsler Abbreviated Scale of Intelligence) and fine-motor functions (Purdue Grooved Pegboard Test) within the normal range compared to population norms, they scored significantly worse than the non-exposed group. Interestingly, most of the study population was adopted at an early age (e.g., younger than 1 year) and raised in a nurturing environment, yet still displayed higher rates of inattention and behavioral problems from school age to late adolescence than matched non-drug-exposed controls (Nygaard et al. 2017).
While the long-term effects of POE on pediatric development is unclear and no definitive opioid effect has been established (Welton et al. 2019), overall these findings highlight the vulnerability of the developing brain to opioids in utero, which may influence brain circuitry. Thus, based on known neurobiological mechanisms involved in the development of addiction, efforts have been made to study molecular and neurochemical changes in brain circuitry in animal models of POE to provide insight into future vulnerabilities to drug addiction.
3. Neurobiological basis of addiction
Addiction is a neurobiological illness whereby repeated substance use disrupts normal reward circuitry via drug-induced neuroplastic changes (Di Chiara and Imperato 1988; Olive et al. 2001). Despite differences in mechanisms of action and pharmacological effects, all drugs of abuse share common actions on brain reward circuits (Nestler 2005). Most findings support that addictive drugs share the common property of enhancing the mesolimbic dopamine pathway, which plays a key role in addiction. This pathway originates with the dopaminergic cell bodies in the ventral tegmental area (VTA) of the midbrain. The primary targets of these dopaminergic axons are in the nucleus accumbens in the ventral striatum, where activation of the mesolimbic pathway is accompanied by the perception of reward (Adinoff 2004). With opioid use, mu opioid receptors (MORs) located on VTA inhibitory γ-aminobutyric acid (GABA)ergic interneurons enhance dopaminergic transmission in the ventral striatum and medial prefrontal cortex to produce reward (Berrettini 2017). In this way, MOR activation inhibits the tonic inhibition of GABAergic inhibitory interneurons, which allows the release of dopamine into the ventral striatum and medial prefrontal cortex to mediate reward. Molecular alterations in key regions involved in the mesolimbic dopamine pathway following POE have been extensively reviewed in Byrnes and Vassoler, 2018, and Grecco and Atwood, 2020.
The use of high-performance liquid chromatography (HPLC) analysis to correlate behavioral experiments with neurochemical evidence has provided valuable insight into vulnerability to morphine-induced reward and dependence. Along these lines, Wu et al., 2018, used HPLC to examine levels of dopamine and serotonin metabolites in the terminal regions of the mesolimbic pathway. In rats with prenatal morphine exposure, administration of low-dose morphine in adulthood increased the dopamine and serotonin turnover rates in the nucleus accumbens, implying sensitivity to morphine (Wu et al. 2009). Conversely, co-administration of morphine with dextromethorphan during pregnancy extinguished these changes (Wu et al. 2009). Although the mechanism of dextromethorphan to reduce the sensitivity of the mesocorticolimbic system is unknown, it is believed that dextromethorphan, as a non-competitive antagonist at the glutamatergic N-methyl-D-aspartate (NMDA) receptors, inhibits NMDA receptor activation implicated in regulation of reward in the mesolimbic pathway (Wroblewski and Danysz 1989; Trujillo and Akil 1990; Church et al. 1994; Huang et al. 2003). These results suggest that following prenatal morphine exposure, sensitivity of the mesocorticolimbic system to morphine may contribute to higher liability to morphine-induced reward.
More recent work has established that POE results in long-lasting alterations in mRNA expression of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), N-methyl-d-aspartate receptor (NMDAR), and the postsynaptic architecture of postsynaptic density protein 95 (PSD-95) within the mesocorticolimbic system (Wu et al. 2018). In addition to the mesolimbic dopamine pathway, the glutamate systems AMPAR and NMDAR are critical for opiate addiction and craving, and PSD-95 together with glutamatergic NMDAR-mediated transmission has an important role in drug-dependent synaptic plasticity (Kelley 2004; Yao et al. 2004). Previous reports have shown that prenatal morphine exposure can alter the metabolism kinetics of NMDAR in the CA1 pyramidal neurons in the hippocampus, resulting in deficits in synaptic plasticity and the integration of learning and memory (Yang et al. 2000, 2003). While the dopaminergic axons in the mesolimbic dopamine pathway primarily project into the nucleus accumbens, they also extend into the amygdala, bed nucleus of stria terminalis (BNST), lateral septal area, and lateral hypothalamus, forming the cortico-basal-ganglia reward network (George and Michel Le 2001; Hyman and Malenka 2001; Nestler 2001). Importantly, this highlights that drug-induced neuroplastic changes have detrimental effects on learning, memory, and reward, which are important components of addiction and contribute to the complexity of treating addiction (Sesack and Grace 2010; Lüscher 2016; Cooper et al. 2017). Relatedly, accumulating evidence supports that glutamatergic input onto the mesolimbic system (e.g., AMPAR and NMDAR) and drug-induced changes in glutamate receptors impair reward-dependent learning, which influences synaptic plasticity and the development of addictive behavior (Wu et al. 2018). In a hallmark study, Wu et al., 2018, show that prenatal morphine exposure downregulates gene expression of PSD-95, AMPAR(GluR1-R4), and NMDAR(NR1, NR2A-D) in the nucleus accumbens, VTA, and prefrontal cortex. AMPAR expression levels, however, did vary in the prefrontal cortex. Nevertheless, these findings in rats underscore that prenatal morphine exposure results in long-lasting glutamate dysregulation and altered synaptic plasticity in the mesolimbic system, which might increase vulnerability to the development of addiction later in life (Murray et al. 2007; Lane et al. 2008; Mickiewicz and Napier 2011; Russell et al. 2016). Though this study did not account for sex-based differences, other studies have noted both sex- and strain-based differences in neurochemical alterations in the mesolimbic dopamine pathway (Shoaib et al. 1995), which remain to be studied in the context of POE.
Collectively, these neurochemical data suggest that POE affects neural development and brain circuitry, which calls for studies to assess whether these data are functionally correlated with impairments in opioid-induced reward and other markers of addictive-like behavior in offspring.
4. Pre-clinical models of prenatal opioid exposure and vulnerability to addiction
The alterations in brain neuropeptides involved in reward pathways suggest disruption in the rewarding properties of drugs, though it is unclear if these alterations lead to addictive phenotypes later in life. Therefore, recent and ongoing studies are examining different addiction paradigms. In animals, opioid reward and vulnerability to addiction is measured via drug self-administration, conditioned place preference, and locomotor sensitization paradigms.
Drug self-administration is the gold standard, in which animals are allowed free access to a rewarding drug, testing an animal’s motivation for the drug (Spanagel 2017). On the other hand, drug delivery in conditioned place preference, whereby animals spend more time in an environment paired with a rewarding drug, or sensitization paradigms, whereby repeated opioid exposure induces increased or sensitized locomotor activity, is not contingent on drug-seeking behavior (Kuhn et al. 2019). In this section we examine these three paradigms specifically in preclinical models of POE (summarized in Table 1).
Table 1.
Summary of preclinical models assessing addictive-like behavior following prenatal opioid exposure
| Species | Opioid Exposure | Age at Testing | Reported Findings | Reference |
|---|---|---|---|---|
| Effects on Self-Administration | ||||
| Rats | MOR: 0.4 mg/mL p.o., tapered to 0 from PN1-P14 D: PG-PN14 |
PN90 ♀ | ↑ MOR self-administration | (Glick et al. 1977) |
| Rats | MET: mg./kg, i.p. D: PG-PN22 |
PN85 ♂ | ↑ voluntary MOR consumption No difference in voluntary MET consumption | (Hovious and Peters 1985) |
| Rats | MOR: 10 mg/kg, s.c. (G7-G9), then free access drinking D: G7-birth |
PN63 ♂ | ↑ cocaine and HER self-administration | (Ramsey et al. 1993) |
| Rats | MOR: 5 mg/kg, s.c. (G11, G12) then 10 mg/kg, s.c. (G12-G18) D: G11-G18 |
PN65 ♂ | ↑ MOR self-administration at 1mg/kg No effect on MOR self-administration at 0.3, 2, or 3 mg/kg | (Riley and Vathy 2006) |
| Rats | MOR: 0.1–0.4 mg/mL p.o. (G1-birth), tapered to 0 from (PN1-PN8) D: G1-PN8 |
PN37 ♂ | ↑ voluntary MOR consumption | (Haydari et al. 2014) |
| Rats | MOR: 2 mg/kg, s.c. ↑ by 1 mg/kg q1wk to 4 mg/kg (final dose) D: G3–20 |
PN49 ♂ | Delayed extinction and stronger drug-primed reinstatement of MA self-administration | (Shen et al. 2016) |
| Rats | MOR: 5 mg/kg, s.c. (G11–13) then 10 mg/kg, s.c. (G14-G18) tapered to 0 (G19-G22) d: G11-birth |
PN35 ♂ and ♀ | ↑ voluntary MOR consumption | (Torabi et al. 2017) |
| Effects on Conditioned Place Preference (CPP) | ||||
| Rats | MOR: 0.75–12 mg/kg, s.c. D: G12-G18 |
PN70 ♂ and ♀ | ↑ CPP to MOR | (Gagin et al. 1997) |
| Rats | MOR: 5 mg/kg, s.c. (G11, G12) then 10 mg/kg, s.c. (G12-G18) D: G11-G18 |
PN65 ♂ | ↑ CPP to MOR at 0.1 mg/kg s.c. No difference in CPP to MOR at 0.3, 1, 3, and 5 mg/kg, s.c. |
(Riley and Vathy 2006) |
| Rats | MOR: 2 mg/kg, s.c. ↑ by 1 mg/kg q1wk until birth D: PG-birth |
PN60 ♂ | ↑ CPP to MOR | (Wu et al. 2009) |
| Rats | MOR: 5 mg/kg, s.c. (G1–2) then 10 mg/kg, s.c. (G3-PN21) D: G1-PN21 |
PN90 ♂ and ♀ | ↑ CPP to MOR | (Timár et al. 2010) |
| Chicks | MOR: 1 mg/kg/egg weight D: E5–8, E9–12, E13–16, or E17–20 |
PN1 (sex unspecified) | ↑ CPP to MOR in E9–12 and E17–20 groups | (He et al. 2010) |
| Rats | MET: 5 mg/kg, s.c. (G3), then 7 mg/kg, s.c. (G4-G20). D: G3-G20 |
PN64 ♂ | ↑ CPP to MET | (Chiang et al. 2015) |
| Rats | MOR: 2 mg/kg, s.c. ↑ by 1 mg/kg q1wk to 4 mg/kg (final dose) D: G3–20 |
PN49 ♂ | Delayed extinction and stronger drug-primed reinstatement of MA CPP | (Shen et al. 2016) |
| Chicks | MOR: 1 mg/kg/egg weight D: E17-E20 |
PN1 (sex unspecified) | ↑ CPP to MOR | (Wang et al. 2017a) |
| Effects on Locomotor Sensitization | ||||
| Rats | MOR: 2 mg/kg, s.c. ↑ by 1 mg/kg q1wk D: PG-birth |
PN60 ♂ | ↑ MOR-induced behavioral sensitization | (Wu et al. 2009) |
| Rats | MOR: 2 mg/kg, s.c. ↑ by 1 mg/kg q1wk MET: 5 mg/kg, s.c. at G3, then 7 mg/kg, s.c. BUP: 3 mg/kg s.c. D: G3-G20 |
PN56 ♂ | ↑ MA-induced behavioral sensitization in prenatally BUP-exposed animals | (Chiang et al. 2013) |
| Chicks | MOR: 1 mg/kg egg weight D: E17-E20 |
PN1 (sex unspecified) | ↑ locomotor activity during the MOR CPP posttest phase | (Wang et al. 2017a) |
| Mice | MOR: 10 mg/kg s.c. D: PN1-PN14 |
Adult ♂ and ♀ | ↑ MOR-induced locomotor sensitization in OPRM1 A118A ♂ ↓ MOR-induced locomotor sensitization in A118A ♀ |
(Robinson et al. 2019) |
| Mice | HER: 1.05 mg/kg s.c. D: G12, G15, G18 |
PN28 ♂ and ♀ | ↑ heroin-induced hyperactivity and drug sensitization in adolescent ♀ | (Kvello et al. 2018) |
Abbreviations: G (gestation day); E (embryonic day); PG (pre-gestation); PN (postnatal day); D (duration); i.p. (intraperitoneal); s.c. (subcutaneous); p.o. (oral); q (every); BUP (buprenorphine); MOR (morphine); MET (Methadone); HER (heroin); MA (methamphetamine)
4.1. Self-Administration
Self-administration-based models of POE have greatly increased our understanding of the molecular and neurobiological mechanisms of drug-related behavior (Spanagel 2017). Yet studies examining how POE affects future drug self-administration in offspring are relatively few. Most of these studies investigate morphine as the opioid of choice during pregnancy, though the timing and route of exposure are considerably different between studies. For example, Haydari et al., 2014, and Ramsey et al., 1993, allowed free access to morphine via drinking water in pregnancy; additionally, Torabi et al., 2017, gradually tapered down the dose of morphine during pregnancy to avoid sudden withdrawal, which often confounds these analyses. In contrast, other investigators have more constrictive regimens of POE, initiate drug exposure solely during pregnancy, and do not parse out the effects of maternal opioid withdrawal.
In most of these studies, prenatal morphine exposure results in increased self-administration of morphine (Glick et al. 1977; Hovious and Peters 1985; Riley and Vathy 2006; Haydari et al. 2014; Torabi et al. 2017), depending on the dosage of morphine self-administered. Collectively, the studies show that in utero morphine exposure facilitates the rate at which morphine self-administrative behavior is learned. These works also support the hypothesis that opioid exposure during pregnancy predisposes offspring to develop morphine-seeking behavior compared to offspring of non-exposed dams. However, discordance between prenatal and postnatal opioid exposure can give markedly different results (Hovious and Peters 1985; Vathy et al. 2007). For example, prenatal methadone exposure enhances morphine, but not methadone self-administration (Hovious and Peters 1985). Similarly, prenatal morphine exposure results in increased cocaine self-administration in some studies, but not in others (Vathy et al. 2007). These differences may be explained by differences in the taste of drugs administered (e.g., sucrose supplementation with maternal opioid administration), differences in the development of opiate receptors, and the characteristics of the rewarding drug (e.g., reinforcement characteristics, potency, disposition, pharmacokinetics) (Hovious and Peters 1985). Additionally, sex-based differences play a key role in discrepancies in drug self-administration. Despite constant brain levels of rewarding drugs, response of females to psychostimulants varies with their estrous cycle (Vathy et al. 2007). This is consistent with reports of sex-based differences in addiction in human studies (Griffin et al. 1989; Brady and Grice 1993; Westermeyer and Boedicker 2000).
Overall, the majority of these studies have limited environmental exposures to just prenatal or perinatal drug exposure. However, it is also important to consider and control for other etiological factors that play a role in drug dependence. For example, oral opiate self-administration in offspring can be influenced by maternal housing conditions and socialization (Alexander et al. 1981). Similarly, amphetamine and cocaine self-administration can be enhanced by repeated tail pinching (Piazza et al. 1989) or emotional, but not physical, stress (Ramsey and van Ree 1990). Interestingly, Haydari et al., 2014, showed that voluntary exercise in morphine-dependent mothers during pregnancy can reduce morphine consumption in offspring. These findings are consistent with exercise activating the same rewarding pathways as morphine (Greenwood et al. 2003), which in turn may lead to neuroplastic changes in the mesolimbic reward pathway and decrease morphine sensitivity in opioid dependent mothers during pregnancy (Smith and Lyle 2006; Hosseini et al. 2009; Haydari et al. 2014). Future studies will need to further investigate environmental cues that may influence later addiction as potential therapeutic targets.
4.2. Conditioned Place Preference
Self-administration of addictive drugs is arguably the most valid preclinical model of addictive-like behavior. However, the conditioned place preference (CPP) paradigm to study the rewarding and aversive effects of drugs is more commonly used due to its simplicity (Lynch et al. 2010). Although there is much variation in the design and apparatuses used in this model, the standard CPP paradigm consists of the repeated pairing of a distinctive environment with the rewarding or aversive characteristics of the drug being tested (Gagin et al. 1997). Increased time spent on the drug paired side correlates with a positively reinforcing effect from the drug. The CPP paradigm confers important advantages, among which is capturing aversion, which the self-administration paradigm lacks (Kuhn et al. 2019). Additionally, the drug of interest is not administered on testing day, which allows determination of enduring neurobiological changes induced by the rewarding drug in the absence of the massive neurotransmitter release with recent drug administration (Kuhn et al. 2019).
Opiate drugs and endogenous opiate peptides can induce CPP, whereas conditioning with opioid antagonists results in conditioned place aversion (Mucha et al. 1982; Mucha 1987). As POE results in long-term alterations in the reward circuitry, this has motivated studies investigating the effect of POE on drug-associated CPP in adulthood.
Gagin et al., 1997, were the first to show that prenatal morphine exposure increases preference for morphine in adulthood. Further studies have confirmed these findings (Gagin et al., 1997; He et al., 2010; Riley and Vathy, 2006; Timár et al., 2010; Wang et al., 2017; Wu et al., 2009). However, variations in maternal administration of opioids during pregnancy has led to contention over an appropriate balance between 1) the physiological relevance of models of maternal opioid use during pregnancy, and 2) the elimination of confounders such as maternal withdrawal from POE. For instance, Gagin et al., 1997, used slow-release emulsion to ensure continuous presence of the drug in circulation. In contrast, others have used injection schedules, which result in fluctuations in drug levels and introduce withdrawal between injections (Wu et al. 2009; Chiang et al. 2015). While intermittent, repeated injections may more closely simulate drug abuse in pregnant women; achieving constant drug levels in maternal circulation also has merit in simplifying investigations of fetal opiate exposure, without the added complexity of maternal withdrawal.
While most CPP models to test reward following POE utilize rats, expansion to other animal models is necessary to validate findings. Notably, He et al., 2010, and Wang et al., 2017, confirm increased preference to morphine following prenatal morphine exposure in chicks. He et al., 2010 additionally showed morphine CPP in chicks prenatally exposed to morphine at both E9–12 and E17–20. The mechanism underlying these changes in drug-induced reward at different embryonic periods may be due to changes in the density, affinity, or distribution of opiate receptors following POE (Gagin et al. 1997; Vathy et al. 2003). For instance, MORs display higher affinity to opioids in the late developmental stage of the chick embryo (Geladopoulos et al. 1987). In agreement with this finding, many other studies have found sensitivity to morphine reward in adulthood with maternal opioid use at various timepoints throughout gestation. However, Riley and Vathy, 2006, have claimed there is no sensitivity to morphine reward during mid-late gestation. These claims are based on failure of escalating doses of the rewarding drug to produce dose-dependent CPP of morphine, though the lowest dose morphine administration did induce CPP in offspring with prenatal morphine exposure (Riley and Vathy 2006). Different sensitivities of CPP paradigms across studies and alterations in maternal care (e.g., cross fostering procedures, maternal stress) may have played a role in failure to obtain CPP at higher doses.
While most studies have shown prenatal morphine exposure can induce morphine CPP in adulthood, other studies have concomitantly focused on extinguishing this propensity to morphine-induced CPP. Both Chiang et al., 2015, and Wu et al., 2009, have shown that dextromethorphan can attenuate drug-induced CPP in models of prenatal methadone and prenatal morphine exposure, respectively. Notably, Wu et al., 2009, corroborated their finding of increased preference to morphine following prenatal exposure with neurochemical evidence showing increased dopamine turnover in the nucleus accumbens (Wu et al. 2009). Their study highlights associations between molecular alterations in the mesolimbic dopamine pathway with functional alterations in addictive-like behavior, following prenatal exposure in rats. These functional changes are not likely the result of improved learning, since deficits in learning and memory induced by prenatal morphine exposure have been reported (Šlamberová et al. 2001). Rather, this study, in accordance with the other studies described in this section, suggests that offspring from opioid-addicted mothers may be more sensitive to morphine-induced reward after postnatal morphine exposure.
4.3. Locomotor sensitization
In rodents, locomotor sensitization is another preclinical model of addiction, whereby repeated, intermittent exposure to abused drugs results in augmented motor-stimulant responses (Kalivas and Stewart 1991; Steketee and Kalivas 2011). Accordingly, in this model, potentiation of drug-induced locomotion is commonly assessed via locomotor activity (Kuhn et al. 2019). In preclinical animal models of addiction, sensitization correlates with increased propensity to both drug self-administration and reinstatement of extinguished self-administration (De Vries 1998; Valjent et al. 2010). However, the face validity of locomotor sensitization in preclinical models is often questioned as sensitization in humans is challenging to demonstrate (Kuhn et al. 2019). Still, others have posited that locomotor sensitization corresponds to certain aspects of drug addiction such as compulsive drug-seeking behavior (Robinson and Berridge 1993; Vanderschuren and Kalivas 2000; Vezina and Leyton 2009; Valjent et al. 2010). Additionally, while animals and humans may differ in the number of exposures that precede sensitization or abuse, the sensitization model is still an important model to aid in our understanding of the initial phases of drug intake that may influence addiction (Vanderschuren and Pierce 2010).
The few preclinical studies of locomotor sensitization have all shown that exposure to intrauterine opioids results in drug sensitization, consistent with previous reports of alterations in brain reward circuitry (Wu et al. 2009; Chiang et al. 2013; Wang et al. 2017a; Kvello et al. 2018; Robinson et al. 2019). These studies have been the most varied, conducted in rats (Wu et al. 2009; Chiang et al. 2013), mice (Kvello et al. 2018; Robinson et al. 2019), and chicks (Wang et al. 2017a). Collectively, major themes in these works have centered on the ability of rewarding drugs to produce cross-sensitization and the influence of sex and genetics in vulnerability to addiction.
Multiple studies have shown cross-sensitization to the neural and behavioral effects of drugs of abuse, whereby sensitization to the locomotor responses of opioids can result in cross-sensitization to the motor-activating effects of other drugs in addition to opioids (Kuhn et al. 2019). Thus, Chiang et al., 2013, used male rats to investigate whether prenatal exposure to methadone, morphine, or buprenorphine could produce cross-sensitization to methamphetamine. Interestingly, of all prenatal drug exposures, buprenorphine-exposed animals had significantly increased rates of development of locomotor activity induced by methamphetamine compared to other groups tested (Chiang et al. 2013). Molecular analysis shows that downregulation of both mRNA expression of D1R in the nucleus accumbens and cAMP signals in these animals is involved in enhancing methamphetamine-induced behavioral sensitization in prenatally buprenorphine-exposed offspring (Chiang et al. 2013).
While Chiang et al., 2013, only used male offspring in their studies, Kvello et al., 2018, further highlighted sex-based vulnerabilities to addiction in their locomotor sensitization studies in animals prenatally exposed to heroin. While the total run distance in female offspring exposed to heroin was doubled compared with females exposed to saline, no sensitized heroin-induced behavioral responses were observed in male adolescent offspring exposed to heroin (Kvello et al. 2018). Collectively, these results imply increased vulnerability to drug sensitization in adolescent female offspring. Of great concern, very low heroin concentrations were sufficient to result in drug sensitization in females. That being said, there has been much inconsistency in human data regarding sex-based addiction vulnerabilities. Nevertheless, preclinical models appear to trend towards increased vulnerability in females to the reinforcing effects of opiates during many phases of the addiction process (Lynch et al. 2002; Timár et al. 2010).
Both genetic and environmental factors contribute to the complexity of addiction. To date, very few studies have investigated the influence of genetics in combination with prenatal drug exposure on vulnerability to drug addiction in adulthood. Recently, Robinson et al., 2019, examined the impact of single nucleotide polymorphisms (SNP) in the MOR gene on locomotor sensitization to morphine in offspring prenatally exposed to morphine. Authors studied the OPRM1 A118G SNP, present in 25% of the human population; this adenine‐to‐guanine substitution (A118G) results in the substitution of asparagine to aspartic acid in exon 1 of the gene. In neonates, this SNP is reported to influence NOWS severity (Mague and Blendy 2010). Interestingly, Robinson et al., 2019, have shown that early postnatal morphine exposure (corresponding to the third trimester of human pregnancy) alters the development of adult morphine locomotor sensitization in a sex‐ and genotype‐dependent manner. Of all the SNPs examined, only the A118A SNP influenced locomotor sensitization. Specifically, while A118A males exhibited enhanced locomotor sensitization to morphine, females instead showed reduced locomotor sensitization (Robinson et al. 2019). Collectively, these studies highlight the importance of investigating sex and genetic variability in the development of locomotor sensitization to morphine.
5. Limitations of preclinical models in predicting vulnerability to substance use disorders in humans
There is a major gap in our understanding of neuroadaptive processes that play a key role in opioid dependence and withdrawal in neonates prenatally exposed to opioids (Le Merrer et al. 2009; Robinson et al. 2019). Overall, animal studies have shown that intrauterine opioid exposure dysregulates neural machinery underlying drug-seeking and drug-taking (Wu et al. 2009, 2018). Furthermore, they show that intrauterine opioid exposure may predispose animals to increased locomotor sensitization, reward-driven behavior, and self-administration of drugs, paralleling findings of other intrauterine drug exposures in animals (Spano et al. 2007; Barbier et al. 2009; Šlamberová et al. 2012; Wang et al. 2019; Cantacorps et al. 2020). Although the exact mechanisms underlying this predisposition is unclear, collectively, these studies suggest that prenatal opioid exposure increases drug-reinforcement related behavior, independent of other environmental influences.
Thus, the question arises as to the translation of these animal studies in humans—specifically, whether individuals prenatally exposed to opioids may have increased vulnerability to substance use disorders. That is a complex and difficult research question to answer, since drug addiction is a chronic, relapsing disease, strongly influenced by the complex interaction of genetic, familial, environmental, and parental influences (see Figure 1). Thus, animal studies are inherently limited in their ability to distinguish the consequences of in utero drug exposure from genetic and environmental influences in addition to polysubstance use during pregnancy (Glantz and Chambers 2006; Spanagel 2017; Müller 2018). The limited number of studies of POE on drug self-administration, CPP, and locomotor sensitization does not help to clarify long-term consequences, instead preventing generalization of study findings. Furthermore, of the existing studies, major limitations in the methodology of drug reward and/or motivation paradigms prevent appreciation of the complex neurocircuitry involved in addiction (Everitt and Robbins 2016). For instance, in animal models of locomotor sensitization, a single drug injection can induce sensitization, whereas in humans, repeated exposures over a long period of time precede drug abuse. As such, a major shortcoming of the locomotor sensitization model has been its ability to provide information past the initial phase of drug intake, leaving questions unanswered about the transition to substance use disorder (Vanderschuren and Pierce 2010; Kuhn et al. 2019). Furthermore, neither this nor the CPP model allows for examination of animal-driven behavior, and at high doses may produce results inconsistent with those that would be seen in humans. Overall, careful attention needs to be given to these behavioral procedures while testing for addictive-like behavior, as environmental stressors such as housing conditions, foot shock, restrain stress, and tail pinch influence self-administration, CPP, and locomotor sensitization, which may confound analyses (Alexander et al. 1981; Piazza et al. 1989; Ramsey et al. 1993).
Figure 1. Complexity of predicting vulnerability to addiction in individuals prenatally exposed to opioids.
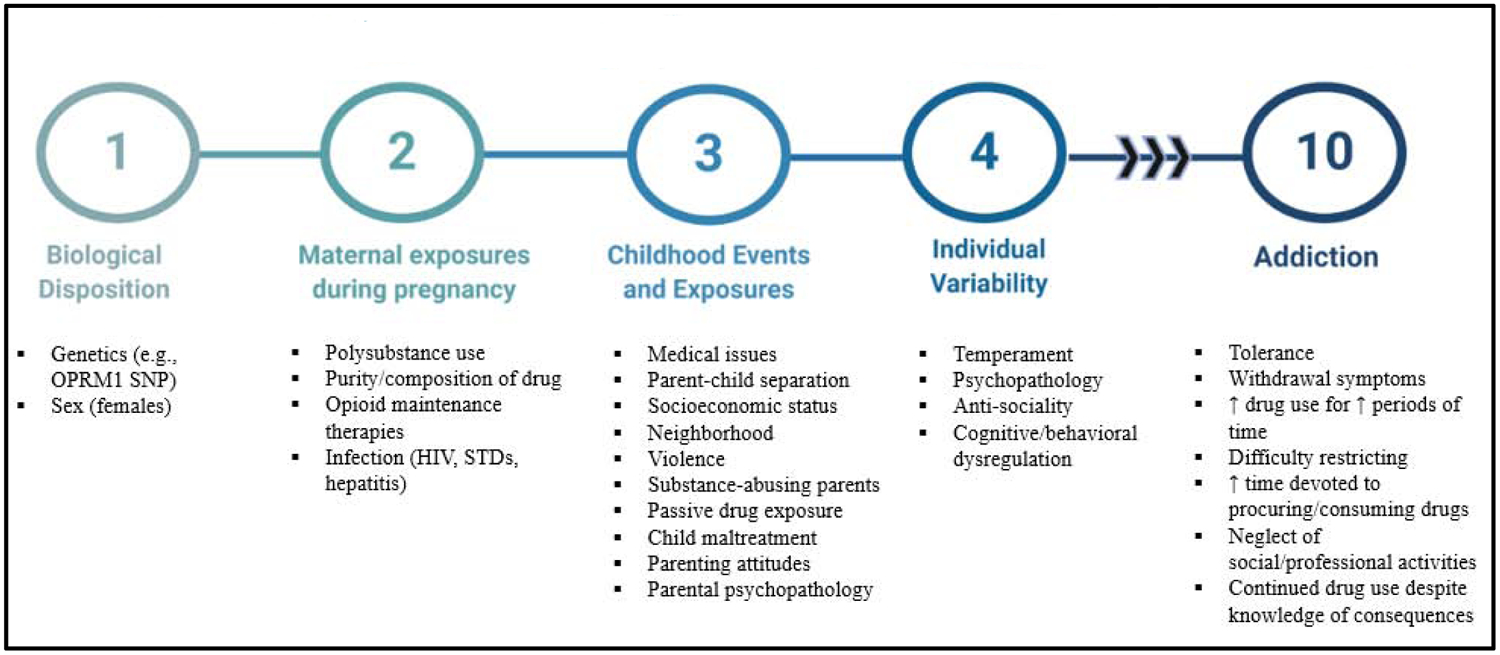
Vulnerability to addiction depends on the complex interaction between genetic and environmental conditions. Each of these factors differentially affects an individual’s predisposition to substance use disorders. Additionally, protective factors throughout a child’s lifetime can greatly offset adverse behavioral effects of prenatal opioid exposure. This complicates both animal and human studies investigating susceptibility to addiction.
Whether these animal models measure addiction in itself is a major source of contention. Some have argued that studying drug intake in animal models does not equate to studying genuine addiction, which is marked by loss of control over drug use (Vanderschuren and Ahmed 2013). Indeed, the DSM-V criteria for substance use disorder is based on impaired control (e.g., using more substance than intended, difficulty restricting substance use), social problems (e.g., neglecting social and professional responsibilities), risky use (e.g., drug use in risky settings and continued drug use despite problems), and physical dependence (e.g., tolerance, withdrawal symptoms) (Hasin et al. 2013). Over the course of addiction, drug use escalates from casual use to inappropriate abuse, whereby individuals lose control over drug seeking and taking. Important consideration in modeling addiction-related behavior must be given to incorporating DSM-V diagnostic criteria for substance use disorder (Kuhn et al. 2019). It is unknown whether animal models can meet all diagnostic criteria, or whether a certain subset of these criteria is specific to humans. However, researchers have found some behavioral equivalents. In rodents, some of these criteria are applied by measuring tolerance, drug preference over nondrug rewards, resistance to punishment, increased motivation for drugs, resistance to drug extinction, and impaired control or neurocognitive deficits (Vanderschuren and Ahmed 2013). In this way, it is possible to quantitatively assess animals meeting criteria for substance use disorders. Interestingly, the percentage of rats that meet all diagnostic criteria has strong clinical correlation to the percentage of human drug addicts that meet DSM criteria for substance use disorder (indicated by two or more symptoms in a given year) (Deroche-Gamonet et al. 2004; Kuhn et al. 2019). That being said, this multi-symptomatic approach is rarely used in animal studies of addiction, and findings from POE studies have not been described in terms of diagnostic criteria but rather solely in terms of behavioral measures. This creates difficulties in distinguishing casual from compulsive drug use.
Even if these preclinical models of addiction hold true, results are still contingent on initiation of drug use and, subsequently, whether alterations in brain circuitry from POE predisposes towards addiction. For example, Chiang et al., 2013, found no changes in basal locomotor activities in prenatally opioid-exposed offspring at adulthood. However, following methamphetamine exposure, significant changes in locomotor sensitization were observed in buprenorphine exposed offspring (Chiang et al. 2013). These results emphasize the role of postnatal drug exposure, but like other studies have not been able to suggest whether prenatally exposed offspring display increased propensity to initiate substance use. In addition, it is increasingly important to recognize that drug addiction only occurs in genetically susceptible individuals, despite prolonged drug exposure. This further underscores the need to examine genetic, neural, and environmental factors that play a key role in the development of addictive behavior (Vanderschuren and Ahmed 2013). For instance, polysubstance use is common in mothers who abuse drugs during pregnancy, which is an issue that has hardly been tackled in animal studies. Furthermore, the effect of polysubstance use is not necessarily the sum of the consequences of each substance used, but more likely represents an interaction. Even when polysubstance use is studied, the drugs used in experimental studies do not necessarily reflect the purity and composition of illegally obtained drugs (Glantz and Chambers 2006). How this, as well as childhood environmental exposures, affects study outcomes is unknown.
Once again, addiction is an accumulation of environmental risks and lower protective factors in genetically susceptible individuals in a developmental and transactional fashion (reviewed in Glantz and Chambers, 2006). Animal models of chronic opioid exposure have demonstrated a genetic component to drug abuse etiology and have identified neurobiological mechanisms underlying escalating and compulsive drug use (Adinoff 2004; Berrettini 2017). As discussed previously, single nucleotide polymorphisms in the OPRM1 gene have also contributed different genetic profiles of drug susceptibilities. Additionally, experimental models have shown significant vulnerability to later addictive-like behavior in females (Lynch et al. 2002; Vathy et al. 2007; Timár et al. 2010; Kvello et al. 2018). Furthermore, there is a strong genetic component of psychopathology, with parental psychopathology associated with significantly higher rates of substance use disorders and psychopathology in offspring (Wilens et al. 2002; Chassin et al. 2004; Elkins et al. 2004; Whitesell et al. 2013).
It has been suggested that while drug involvement, abuse, and dependence are more associated with biological and psychiatric factors, the initiation of drug use is heavily dependent on social and environmental factors (Glantz and Chambers 2006; Burstein et al. 2012; Zimić and Jukić 2012). Even children who do recover from early functional impairments are still at increased risk for later cognitive and behavioral problems exacerbated by familial and environmental factors (reviewed in Glantz and Chambers 2006). Contributing childhood environmental exposures include forced parent-child separation, high-crime neighborhoods, neighborhood and household violence, passive drug exposure, child maltreatment, and parenting attitudes (Glantz and Chambers 2006). For instance, although the majority of children with drug-abusing mothers reside in the same household, they are at high risk of forced parental separation as a result of parental incarceration, drug-related parent death, or drug exposure in children (Nair et al. 1997; Pilowsky et al. 2001). Indeed, mothers who abuse drugs during pregnancy often continue drug use after the child is born, subjecting offspring to significant postnatal drug exposures which also play a role in maternal-infant interactions (Johnson et al. 2002; Schuler et al. 2002; Pulsifer et al. 2004). Continued passive exposure to drugs from drugs smoked in the presence of a child (e.g., marijuana, cocaine) or living in a home where drugs are stored and processed (e.g., home-based methamphetamine labs) contribute to periodic placement of these children in other homes by child protective services (Glantz and Chambers 2006). This forced separation has been linked with a number of adverse outcomes, including psychopathology and increased rates of substance abuse (Zlotnick et al. 1999). Families affected by drug abuse are also more likely to experience high levels of domestic conflict (Dunn et al. 2002), which is often exacerbated by living in neighborhoods with high rates of crime, poverty, and substandard housing (Luthar and Cushing 1999). Children living in these environmental conditions have been reported to be more likely to use illegal drugs, with some reports indicating that these neighborhood characteristics more heavily influence propensity to drug addiction than their mother’s attributes (e.g., psychiatric illness) (Brook et al. 2001).
Additionally, individual variability in temperament and cognitive function also contributes to increased susceptibility to substance use disorders in adolescence and adulthood. Difficult temperament, frequently observed in prenatally exposed children, has been linked with poor parenting practices, which predisposes towards emotional dysregulation, deficits in attention and impulse control, sensation seeking, and psychological disorders—all of which are drug abuse vulnerability factors (Young Mun et al. 2001; Dalley et al. 2007; Belin et al. 2008). While it may be possible to parse out individual influences of POE alone from the multiple genetic and environmental risk factors, vulnerability to drug abuse is likely the potentiation of POE and maladaptive environmental settings. It must be noted that at every step in the development of addiction, protective factors may offset negative behavioral changes that predispose towards the development of a substance use disorder (Glantz and Chambers 2006). For instance, in many cases a nurturing home environment and positive attention can promote resilience in children, which may help mitigate the adverse consequences of POE (Lewis et al. 2004). That being said, more often than not children of mothers who use opioids while pregnant continue to face multiple negative environmental exposures throughout their childhood.
Together, these various risk factors add to the complexity of predicting vulnerability to addiction in human studies of prenatally opioid-exposed infants. As such, we are unable to disprove or verify reported findings of in utero drug exposure in preclinical studies in humans. Animal studies have thus been the only way to understand dysregulation in the neural reward circuitry underlying drug-seeking in the absence of the multiple genetic and environmental confounders in human studies. Collectively, these studies of drug self-administration, conditioned place preference, and locomotor sensitization have been pivotal in our understanding of the neurobiological factors underlying drug addiction. While it is unknown whether in utero opioid exposure substantially increases vulnerability to drug abuse in offspring, these preclinical studies point to increased vulnerability beyond having substance abusing parents (Glantz and Chambers 2006). At the very least, these studies have presented negative neurodevelopmental findings with POE, consistent with human studies.
6. Future Directions
Here, we have reviewed the literature on animal models of prenatal opioid exposure (POE), paying specific attention to studies assessing vulnerability to addiction later in life. While it is difficult to generate definitive conclusions based on the limited number of studies, the available literature indicates that POE may dysregulate neural reward circuitry that may confer susceptibility to drug use in animal models. Overall, we have highlighted that vulnerability to addiction is a complex interaction of genetic and environmental factors. This has limited interpretation of findings in animal studies of POE and has made investigations of the long-term effects of POE in humans particularly challenging. Additionally, we have assessed the merit of animal models of POE in measuring addiction; indeed, loss of control is pathognomonic of substance use disorders in DSM-V criteria, but hardly recapitulated in animal models. From here, we provide a brief discussion on 1) measures to prevent substance use during pregnancy and/or limit fetal drug exposure, 2) considerations for improving animal models of POE, and 3) current strategies underway to predict propensity towards addiction.
6.1. Measures to prevent substance use during pregnancy and/or limit fetal drug exposure
First and foremost, progress in this field will require increased measures to reduce substance use during pregnancy and to limit fetal drug exposure, while preventing fetal drug withdrawal. Although treating substance use disorders is difficult and marked by high rates of relapse, drug prevention efforts may be more successful in pregnant women who abuse drugs compared to non-pregnant, drug-abusing females because of some concern over possible harm to the developing fetus (Glantz and Chambers 2006). Volkow and Boyle, 2018, have reviewed many of these interventions, which are based on epidemiological data of both adverse and protective risk factors in the development and maintenance of substance use disorders. While this does not imply that prevention programs are more likely to result in drug abstinence during pregnancy, a decrease in drug use, even with some degree of relapse, may result in less harm to the fetus than continued drug abuse during pregnancy, though this has not been verified. In drug-abusing women, the main focus has been to limit abrupt opioid reduction, which is associated with increased central nervous stress in the fetus, stillbirth, and fetal death, rather than to encourage abstinence (Park et al. 2012). Hence, the rationale behind opioid-agonist maintenance therapies has been to minimize the fluctuations in maternal serum opioid levels with repeated use of short-acting opioids (e.g., heroin) in order to reduce repeated intoxication and withdrawal to the fetus (Jones et al. 2008). However, these therapies have several disadvantages, among which are continued fetal drug exposure as well as the risk of fetal drug withdrawal. As discussed previously, though buprenorphine is gaining popularity over methadone due to its availability in office-based settings, diminished risk of overdose, and decreased rates of abrupt withdrawal (Park et al. 2012; Stover and Davis 2015), animal models of POE have shown significantly adverse effects in buprenorphine-exposed offspring compared to offspring prenatally exposed to morphine (Chiang et al. 2013). Thus, there has been a shift in focus to investigations of non-pharmacological therapies in pregnant women to both limit fetal drug exposure and prevent fetal drug withdrawal.
To date, very few preclinical models (summarized in Table 2) have investigated maternal interventions that influence behavioral outcomes in prenatally opioid-exposed infants. Promising non-pharmacological strategies have included passive immunization with monoclonal antibodies and anti-drug abuse vaccinations, which work by creating immune complexes that trap substances of abuse in circulation, preventing them from crossing the blood brain barrier (reviewed in Volkow and Boyle, 2018). Excitingly, monoclonal antibodies targeting heroin’s first metabolite, 6-acetylmorphine (6-AM), have been demonstrated to reduce the distribution of active heroin metabolites in the fetal brain and prevent hyperactivity and drug sensitization in adolescent female offspring prenatally exposed to heroin (Kvello et al. 2018). Future studies on non-pharmacological interventions will need to focus on ensuring the production of long-lasting monoclonal or polyclonal antibodies, using booster injections to prolong antibody effects, and combining immunotherapy with behavioral interventions to collectively lower the risk of fetal drug exposure and withdrawal (Volkow and Boyle 2018).
Table 2.
Summary of interventions used to prevent addictive-like behavior in preclinical models of prenatal opioid exposure
| Intervention | POE Model | Timing of Intervention | Reported Findings | Reference |
|---|---|---|---|---|
| mAb targeting heroin’s first metabolite, 6-acetylmorphine (6-AM) | HER | s.c. mAb injections on G11 (50 mg/kg), G14 (10 mg/kg), and G17 (10 mg/kg), and single s.c. HER injections (2.5 mmol/kg) on G12, G15, and G18. | ↓ HER-induced hyperactivity, and ↓ HER-induced drug sensitization compared with adolescent ♀ offspring prenatally exposed to HER |
(Kvello et al. 2018) |
| Dextromethorphan | MET | MET injection (5 mg/kg, s.c. at G3; then 7 mg/kg, s.c. G4-G20) followed by DEX injection (3 mg/kg, s.c. G3-G20) | ↓ CPP to MET (4 mg/kg, s.c.) compared with adult ♂ offspring prenatally exposed to MET | (Chiang et al. 2015) |
| Dextromethorphan | MOR | Both DEX and MOR injections (2 mg/kg, s.c. ↑ by 1 mg/kg q1wk) initiated during mating and continued throughout pregnancy until birth of pups | ↓ CPP to MOR (1 mg/kg, i.p.), ↓ MOR-induced behavioral sensitization, ↓ dopamine and serotonin turnover rates in nucleus accumbens compared with adult ♂ offspring prenatally exposed to MOR |
(Wu et al. 2009) |
| Exercise | MOR | Free access to running wheel G1-PN1 | ↓ voluntary consumption of MOR compared to ♂ offspring from sedentary morphine-dependent mothers. | (Haydari et al. 2014) |
| Exercise | MOR | Swimming (30–45 min/day, 3 days per week) on G11–18. | ↓ voluntary consumption of MOR compared to ♂ and ♀ offspring from sedentary morphine-dependent mothers | (Torabi et al. 2017) |
Abbreviations: G (gestation day); PN (postnatal day); s.c. (subcutaneous); mAb (monoclonal antibody); DEX (dextromethorphan); MOR (morphine); MET (methadone); HER (heroin).
6.2. Considerations for improving animal models of POE
Additionally, improving current animal models of POE will be crucial in elucidating neurobiological mechanisms that contribute to addiction-related behaviors. Throughout this review we have discussed limitations of animal models of POE. The most notable are whether these models mirror human addiction and the failure of these models to account for the complex interaction of genetic predispositions and environmental conditions.
The validity of these animal models in studying addiction is often contended, as human addiction is marked by loss of control over drug use (Vanderschuren and Ahmed 2013); this phenomenon is lacking in many animal models. However, this limitation can be addressed by utilizing multi-symptomatic training paradigms, which incorporate DSM-V diagnostic criteria for substance use disorders; or behavioral/demand curve economics, which allows for the identification of multiple components of motivation for drug-taking within a single session (Kuhn et al. 2019).
Recent advancements in genetic technology have further provided a variety of ways to introduce genetic variability into rodent models. For instance, the availability of heterogeneous stock rats, which represents a genetically random mosaic of founding animal chromosomes due to multiple generations of recombination, has presented a model which more accurately reflects genetic and behavioral heterogeneity in the human population (Woods and Mott 2017). Other approaches to understanding the genetic differences that may contribute to certain phenotypic traits in animal models are reviewed in Kuhn et al., 2019. Additional consideration must be given to the effect of adverse environmental exposures following POE, which reflect those commonly experienced by humans, such as forced maternal-child separation, stress, trauma, and passive drug-exposure. Overall, understanding this balance between genetic and environmental factors is crucial to inform about the individual differences predisposing towards drug abuse and substance use disorders.
6.3. Current strategies underway to predict propensity to addiction
We conclude by briefly discussing emerging strategies to predict propensity towards addiction. Pharmacogenetics, for example, has a key role in informing how genetic factors influence drug response and toxicity. What continues to be missing in our current understanding of the factors predisposing to addiction is an assessment of interpersonal genetic and environmental variation (reviewed in Hurd and O’Brien, 2018). Candidate gene studies and genome-wide association (GWA) analyses can be used to identify genetic influences on drug abuse and dependence with the added advantage of discovering novel genetic variants and biomarkers that associate with particular phenotypes (Mroziewicz and Tyndale 2010). For instance, GWA studies have implicated genes involved with cell adhesion, enzymatic activities, and transcriptional regulation in dependence phenotypes (Ishiguro et al. 2008; Uhl et al. 2008; Mroziewicz and Tyndale 2010). In addition to genetics, increased awareness of the epigenetic mechanisms induced by both intrauterine opioid exposures and adverse childhood exposures is of great importance in advancing our understanding of individual vulnerability to addiction. Such knowledge and integration of both genetic and epigenetic data can provide a strong biological foundation of the development of addiction (Hurd and O’Brien 2018).
A focus on biomarkers that have high predictive and explanatory power would also greatly contribute to classifying individuals with different propensities towards addiction into biologically-based categories (Kwako et al. 2018; Volkow and Boyle 2018). Neuroimaging has the great potential to facilitate our identification of addiction biomarkers by providing a multidimensional representation of human functioning, including cue reactivity, impulsivity, and cognitive control, that relate to addiction (reviewed in Garrison and Potenza, 2014). These biomarkers represent molecular or biochemical alterations that can be objectively scored and assessed as an indicator of disease diagnoses, pathology, or pharmacologic response (Naylor 2003; Wang et al. 2016). The development and advancement of “omics” research in particular has ushered in a number of proteomic and metabolomic approaches that can be used to facilitate our understanding of the underlying etiology of drug addiction (reviewed in Wang et al., 2016). Indeed, many have argued that metabolic dysregulation following the first instance of narcotic drug use contributes to the initiation step of addiction (reviewed in Ghanbari and Sumner, 2018). Thus, mapping metabolic perturbations can improve our knowledge of addiction profiles and provide clinically relevant biomarkers (Ghanbari and Sumner 2018). The view of addiction as an overall metabolic disturbance brings up the exciting possibility of reducing the risk of intrauterine opioid exposure through nutritional interventions. For instance, in the same way that folic acid supplementation during pregnancy has been used to reduce neural tube defects in neonates and probiotic administration following chronic opioid exposure has been used to attenuate gastrointestinal pathology exacerbated by bacterial metabolites (Ghanbari and Sumner 2018; Zhang et al. 2019), identifying metabolic disturbances in individuals with a history of POE may provide targeted nutritional interventions to mitigate adverse intrauterine opioid exposures and concomitant environmental risk factors.
7. Conclusion
Ultimately, much work remains to be done in terms of characterizing the genetic and environmental factors underlying vulnerability to addiction in individuals with prenatal opioid exposure (POE). Improving our current animal models of POE as well as our paradigms for assessing addictive-like behavior will be imperative in providing robust preventative and therapeutic measures moving forward. Future studies should also be directed towards identifying non-pharmacological therapies to limit fetal drug exposures and withdrawal, as well as towards identifying biomarkers in the development of addiction using pharmacogenetic, neuroimaging, and metabolomic approaches.
Key Points:
One consequence of the opioid epidemic has been an increased number of neonates with prenatal opioid exposure (POE), which results in significantly adverse medical, developmental, and behavioral outcomes in offspring.
Of growing interest is whether POE contributes to later vulnerability to substance use disorders.
Overall, animal studies have suggested that POE dysregulates neural machinery underlying drug-seeking and drug-taking, and may predispose animals to increased locomotor sensitization, reward-driven behavior, and self-administration of drugs.
How these findings relate to increased vulnerability to addiction in opioid-exposed infants is an incredibly complex question, and likely the result of the complex interaction of genetic, familial, environmental, and parental influences.
Animal studies have greatly enhanced our understanding of the molecular and neurobiological mechanisms of drug-related behavior, but improving animal models of POE and addiction paradigms will be essential to providing robust preventative and therapeutic measures against the adverse effects of POE.
Future research will need to be directed towards developing non-pharmacological therapies to limit fetal drug exposure and withdrawal as well as towards developing effective strategies to determine propensity to addiction.
Acknowledgments
This work was supported by the National Institutes of Health Grants (R01 DA043252, R01 DA037843, R01 DA044582, R01 DA047089 and R01 DA050542). Additionally, we thank Dr. Valerie Gramling from the University of Miami Writing Center for help with reading and revising this manuscript.
List of Abbreviations:
- POE
prenatal opioid exposure
- OUD
opioid use disorder
- NOWS
neonatal opioid withdrawal syndrome
- VTA
ventral tegmental area
- MOR
mu opioid receptors
- GABA
γ-aminobutyric acid
- HPLC
high-performance liquid chromatography
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- NMDAR
N-methyl-d-aspartate receptor
- PSD
postsynaptic density protein 95
- CPP
conditioned place preference
- GWA
genome-wide association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflict of interest in this manuscript.
References
- Adinoff B (2004) Neurobiologic Processes in Drug Reward and Addiction. Harv Rev Psychiatry 12:305–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BK, Beyerstein BL, Hadaway PF, Coambs RB (1981) Effect of early and later colony housing on oral ingestion of morphine in rats. Pharmacol Biochem Behav 15:571–576. 10.1016/0091-3057(81)90211-2 [DOI] [PubMed] [Google Scholar]
- Azuine RE, Ji Y, Chang HY, et al. (2019) Prenatal Risk Factors and Perinatal and Postnatal Outcomes Associated with Maternal Opioid Exposure in an Urban, Low-Income, Multiethnic US Population. JAMA Netw Open 2:1–14. 10.1001/jamanetworkopen.2019.6405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacchino A, Arbuckle K, Petrie D, McCowan C (2015) Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: A systematic review and meta-analysis’: Erratum. BMC Psychiatry 14: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier E, Houchi H, Warnault V, et al. (2009) Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience 161:427–440. 10.1016/j.neuroscience.2009.03.076 [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, et al. (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science (80-). 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W (2017) A brief review of the genetics and pharmacogenetics of opioid use disorders. Dialogues Clin Neurosci 19:229–236. 10.1111/clr.125_12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady T, Grice E (1993) Gender in Substance Use Disorders. Psychiatry Interpers Biol Process 1707–1711 [DOI] [PubMed] [Google Scholar]
- Brook JS, Zheng L, Whiteman M, Brook DW (2001) Aggression in toddlers: Associations with parenting and marital relations. J Genet Psychol. 10.1080/00221320109597963 [DOI] [PubMed] [Google Scholar]
- Burstein M, Stanger C, Dumenci L (2012) Relations between parent psychopathology family functioning and adolescent problems in substance-abusing families: Disaggregating the effects of parent gender. Child Psychiatry Hum Dev. 10.1007/s10578-012-0288-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Vassoler FM (2018) Modeling prenatal opioid exposure in animals: Current findings and future directions. Front Neuroendocrinol 51:1–13. 10.1016/j.yfrne.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantacorps L, Montagud-Romero S, Luján MÁ, Valverde O (2020) Prenatal and postnatal alcohol exposure increases vulnerability to cocaine addiction in adult mice. Br J Pharmacol 177:1090–1105. 10.1111/bph.14901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Flora DB, King KM (2004) Trajectories of alcohol and drug use and dependence from adolescence to adulthood: The effects of familial alcoholism and personality. J Abnorm Psychol. 10.1037/0021-843X.113.4.483 [DOI] [PubMed] [Google Scholar]
- Chiang YC, Hung TW, Ho IK (2013) Development of sensitization to methamphetamine in offspring prenatally exposed to morphine, methadone and buprenorphine. Addict Biol 19:676–686. 10.1111/adb.12055 [DOI] [PubMed] [Google Scholar]
- Chiang YC, Ye LC, Hsu KY, et al. (2015) Beneficial effects of co-treatment with dextromethorphan on prenatally methadone-exposed offspring. J Biomed Sci 22:1–12. 10.1186/s12929-015-0126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J, Sawyer D, McLarnon JG (1994) Interactions of dextromethorphan with the N-methyl-d-aspartate receptor-channel complex: single channel recordings. Brain Res 666:189–194. 10.1016/0006-8993(94)90771-4 [DOI] [PubMed] [Google Scholar]
- Conradt E, Flannery T, Aschner JL, et al. (2019) Prenatal opioid exposure: Neurodevelopmental consequences and future research priorities. Pediatrics 144:. 10.1542/peds.2019-0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Robison AJ, Mazei-Robison MS (2017) Reward Circuitry in Addiction. Neurotherapeutics 14:687–697. 10.1007/s13311-017-0525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Lääne K, Theobald DEH, et al. (2007) Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: Results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology 10.1038/sj.npp.1301220 [DOI] [PubMed] [Google Scholar]
- De Vries TJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci. 10.1046/j.1460-9568.1998.00368.x [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science (80-). 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278. 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MG, Tarter RE, Mezzich AC, et al. (2002) Origins and consequences of child neglect in substance abuse families. Clin Psychol Rev. 10.1016/S0272-7358(02)00132-0 [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Malone S, Iacono WG (2004) The Effect of Parental Alcohol and Drug Disorders on Adolescent Personality. Am J Psychiatry. 10.1176/appi.ajp.161.4.670 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2016) Drug addiction: Updating actions to habits to compulsions ten years on. Annu Rev Psychol 67:23–50. 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Feder KA, Letourneau EJ, Brook J (2019) Children in the opioid epidemic: Addressing the next generation’s public health crisis. Pediatrics 143:1–3. 10.1542/peds.2018-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A (2016) Substance use during pregnancy. F1000Research 5:1–9. 10.12688/F1000RESEARCH.7645.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagin R, Kook N, Cohen E, Shavit Y (1997) Prenatal morphine enhances morphine-conditioned place preference in adult rats. Pharmacol Biochem Behav 58:525–528. 10.1016/S0091-3057(97)00281-5 [DOI] [PubMed] [Google Scholar]
- Garrison KA, Potenza MN (2014) Neuroimaging and Biomarkers in Addiction Treatment. Curr Psychiatry Rep 16:. 10.1007/s11920-014-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geladopoulos T, Sakellaridis N, Vernadakis A (1987) Differential maturation of μ and δ opioid receptors in the chick embryonic brain. Neurochem Res 12:279–288. 10.1007/BF00972138 [DOI] [PubMed] [Google Scholar]
- George FK, Michel Le M (2001) Drug Addiction, Dysregulation of Reward, and Allostasis. Neuropsychopharmacology 24:97. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, Sumner S (2018) Using Metabolomics to Investigate Biomarkers of Drug Addiction. Trends Mol Med 24:197–205. 10.1016/j.molmed.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Glantz MD, Chambers JC (2006) Prenatal drug exposure effects on subsequent vulnerability to drug abuse. Dev Psychopathol 18:893–922. 10.1017/S0954579406060445 [DOI] [PubMed] [Google Scholar]
- Glick SD, Strumpf AJ, Zimmerberg B (1977) Effect of in utero administration of morphine on the subsequent development of self-administration behavior. Brain Res 132:194–196 [DOI] [PubMed] [Google Scholar]
- Grecco GG, Atwood BK (2020) Prenatal Opioid Exposure Enhances Responsiveness to Future Drug Reward and Alters Sensitivity to Pain: A Review of Preclinical Models and Contributing Mechanisms. Eneuro ENEURO.0393–20.2020. 10.1523/eneuro.0393-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HEW, et al. (2003) Freewheel running prevents learned helplessness/behavioral depression: Role of dorsal raphe serotonergic neurons. J Neurosci 23:2889–2898. 10.1523/jneurosci.23-07-02889.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A Comparison of Male and Female Cocaine Abusers. Arch Gen Psychiatry 46:122–126. 10.1001/archpsyc.1989.01810020024005 [DOI] [PubMed] [Google Scholar]
- Hans SL, Jeremy RJ (2001) Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Ment Health J 22:300–315. 10.1002/imhj.1003 [DOI] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, et al. (2013) DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydari S, Miladi-Gorji H, Mokhtari A, Safari M (2014) Effects of voluntary exercise on anxiety-like behavior and voluntary morphine consumption in rat pups borne from morphine-dependent mothers during pregnancy. Neurosci Lett 578:50–54. 10.1016/j.neulet.2014.06.026 [DOI] [PubMed] [Google Scholar]
- He X, Bao Y, Li Y, Sui N (2010) The effects of morphine at different embryonic ages on memory consolidation and rewarding properties of morphine in day-old chicks. Neurosci Lett 482:12–16. 10.1016/j.neulet.2010.06.074 [DOI] [PubMed] [Google Scholar]
- Hosseini M, Alaei HA, Naderi A, et al. (2009) Treadmill exercise reduces self-administration of morphine in male rats. Pathophysiology 16:3–7. 10.1016/j.pathophys.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Hovious JR, Peters MA (1985) Opiate self-administration in adult offspring of methadone-treated female rats. Pharmacol Biochem Behav 22:949–953. 10.1016/0091-3057(85)90301-6 [DOI] [PubMed] [Google Scholar]
- Huang EYK, Liu TC, Tao PL (2003) Co-administration of dextromethorphan with morphine attenuates morphine rewarding effect and related dopamine releases at the nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol 368:386–392. 10.1007/s00210-003-0803-7 [DOI] [PubMed] [Google Scholar]
- Hunt RW, Tzioumi D, Collins E, Jeffery HE (2008) Adverse neurodevelopmental outcome of infants exposed to opiate in-utero. Early Hum Dev 84:29–35. 10.1016/j.earlhumdev.2007.01.013 [DOI] [PubMed] [Google Scholar]
- Hurd YL, O’Brien C (2018) Molecular genetics and new medication strategies for opioid addiction. Am J Psychiatry 175:935–942. 10.1176/appi.ajp.2018.18030352.Molecular [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC (2001) Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci 2:695–703. 10.1038/35094560 [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Gong JP, Hall FS, et al. (2008) Association of PTPRB gene polymorphism with drug addiction. Am J Med Genet Part B Neuropsychiatr Genet. 10.1002/ajmg.b.30742 [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Maxwell JR, Newville JC, et al. (2020) Prenatal opioid exposure: The next neonatal neuroinflammatory disease. Brain Behav Immun 84:45–58. 10.1016/j.bbi.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AL, Morrow CE, Accornero VH, et al. (2002) Maternal cocaine use: Estimated effects on mother-child play interactions in the preschool period. J Dev Behav Pediatr. 10.1097/00004703-200208000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Martin PR, Heil SH, et al. (2008) Treatment of opioid-dependent pregnant women: Clinical and research issues. J Subst Abuse Treat. 10.1016/j.jsat.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Rev [DOI] [PubMed] [Google Scholar]
- Kaltenbach K, O’Grady KE, Heil SH, et al. (2018) Prenatal exposure to methadone or buprenorphine: Early childhood developmental outcomes. Drug Alcohol Depend 185:40–49. 10.1016/j.drugalcdep.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE (2004) Memory and addiction: Shared neural circuitry and molecular mechanisms. Neuron 44:161–179. 10.1016/j.neuron.2004.09.016 [DOI] [PubMed] [Google Scholar]
- Konijnenberg C, Melinder A (2013) Neurodevelopmental investigation of the mirror neurone system in children of women receiving opioid maintenance therapy during pregnancy. Addiction 108:154–160. 10.1111/j.1360-0443.2012.04006.x [DOI] [PubMed] [Google Scholar]
- Konijnenberg C, Melinder A (2015) Executive function in preschool children prenatally exposed to methadone or buprenorphine. Child Neuropsychol 21:570–585. 10.1080/09297049.2014.967201 [DOI] [PubMed] [Google Scholar]
- Kuhn BN, Kalivas PW, Bobadilla AC (2019) Understanding Addiction Using Animal Models. Front Behav Neurosci 13:1–24. 10.3389/fnbeh.2019.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvello AMS, Andersen JM, Øiestad EL, et al. (2018) A monoclonal antibody against 6-acetylmorphine protects female mice offspring from adverse behavioral effects induced by prenatal heroin exposure. J Pharmacol Exp Ther 368:106–115. 10.1124/jpet.118.251504 [DOI] [PubMed] [Google Scholar]
- Kwako L, Bickel W, Goldman D (2018) Addiction Biomarkers: Dimensional Approaches to Understanding Addiction. Physiol Behav 24:121–128. 10.1016/j.molmed.2017.12.007.Addiction [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Lessard AA, Chan J, et al. (2008) Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci 28:9670–9681. 10.1523/JNEUROSCI.2151-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol. Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BA, Singer LT, Short EJ, et al. (2004) Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicol Teratol. 10.1016/j.ntt.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Lüscher C (2016) The Emergence of a Circuit Model for Addiction. Annu Rev Neurosci 39:257–276. 10.1146/annurev-neuro-070815-013920 [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cushing G (1999) Neighborhood influences and child development: A prospective study of substance abusers’ offspring. Dev Psychopathol. 10.1017/S095457949900231X [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, et al. (2010) Animal models of substance abuse and addiction: Implications for science, animal welfare, and society. Comp Med 60:177–188 [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Mague SD, Blendy JA (2010) OPRM1 SNP (A118G): Involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen K, Murphy-Oikonen J (2016) Neonatal abstinence syndrome. N Engl J Med 375:2468–2479. 10.1056/NEJMra1600879 [DOI] [PubMed] [Google Scholar]
- Melinder A, Konijnenberg C, Sarfi M (2013) Deviant smooth pursuit in preschool children exposed prenatally to methadone or buprenorphine and tobacco affects integrative visuomotor capabilities. Addiction 108:2175–2182. 10.1111/add.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger DS, Bauer CR, Das A, et al. (2004) The maternal lifestyle study: Cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics 113:1677–1685. 10.1542/peds.113.6.1677 [DOI] [PubMed] [Google Scholar]
- Mickiewicz AL, Napier TC (2011) Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur J Neurosci 33:259–265. 10.1111/j.1460-9568.2010.07502.x [DOI] [PubMed] [Google Scholar]
- Moe V (2002) Foster-placed and adopted children exposed in utero to opiates and other substances: Prediction and outcome at four and a half years. J Dev Behav Pediatr 23:330–339. 10.1097/00004703-200210000-00006 [DOI] [PubMed] [Google Scholar]
- Monnelly VJ, Anblagan D, Quigley A, et al. (2018) Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage Clin 18:9–14. 10.1016/j.nicl.2017.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroziewicz M, Tyndale RF (2010) Pharmacogenetics: a tool for identifying genetic factors in drug dependence and response to treatment. Addict Sci Clin Pract 5:17–29 [PMC free article] [PubMed] [Google Scholar]
- Mucha RF (1987) Is the motivational effect of opiate withdrawal reflected by common somatic indices of precipitated withdrawal? A place conditioning study in the rat. Brain Res 418:214–220. 10.1016/0006-8993(87)90088-6 [DOI] [PubMed] [Google Scholar]
- Mucha RF, Van Der Kooy D, O’Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105. 10.1016/0006-8993(82)91123-4 [DOI] [PubMed] [Google Scholar]
- Müller CP (2018) Animal models of psychoactive drug use and addiction - Present problems and future needs for translational approaches. Behav. Brain Res [DOI] [PubMed] [Google Scholar]
- Murray F, Harrison NJ, Grimwood S, et al. (2007) Nucleus accumbens NMDA receptor subunit expression and function is enhanced in morphine-dependent rats. Eur J Pharmacol 562:191–197. 10.1016/j.ejphar.2007.01.027 [DOI] [PubMed] [Google Scholar]
- Nair P, Black MM, Schuler M, et al. (1997) Risk factors for disruption in primary caregiving among infants of substance abusing women. Child Abus Negl. 10.1016/S0145-2134(97)00064-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S (2003) Biomarkers: Current perspectives and future prospects. Expert Rev. Mol. Diagn [DOI] [PubMed] [Google Scholar]
- Nekhayeva IA, Nanovskaya TN, Deshmukh SV., et al. (2005) Bidirectional transfer of methadone across human placenta. Biochem Pharmacol 69:187–197. 10.1016/j.bcp.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8:1445–1449. 10.1038/nn1578 [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Nygaard E, Slinning K, Moe V, Walhovd KB (2017) Cognitive function of youths born to mothers with opioid and polysubstance abuse problems during pregnancy. Child Neuropsychol 23:159–187. 10.1080/09297049.2015.1092509 [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW (2001) Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci 21:1–5. 10.1523/jneurosci.21-23-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Daka L, Goldzweig G, et al. (2010) Neurodevelopmental and psychological assessment of adolescents born to drug-addicted parents: Effects of SES and adoption. Child Abus Negl 34:354–368. 10.1016/j.chiabu.2009.09.012 [DOI] [PubMed] [Google Scholar]
- Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C (2001) Developmental outcome of school-age children born to mothers with heroin dependency: Importance of environmental factors. Dev Med Child Neurol 43:668–675. 10.1111/j.1469-8749.2001.tb00140.x [DOI] [PubMed] [Google Scholar]
- Park EM, Meltzer-Brody S, Suzuki J (2012) Evaluation and Management of Opioid Dependence in Pregnancy. Psychosomatics 53:424–432. 10.1016/j.psym.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SW, Dudley J, Martin PR, et al. (2015) Prescription opioid epidemic and infant outcomes. Pediatrics 135:842–850. 10.1542/peds.2014-3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science (80-) 245:1511–1513. 10.1126/science.2781295 [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Lyles CM, Cross SI, et al. (2001) Characteristics of injection drug using parents who retain their children. Drug Alcohol Depend. 10.1016/S0376-8716(00)00130-7 [DOI] [PubMed] [Google Scholar]
- Pulsifer MB, Radonovich K, Belcher HME, Butz AM (2004) Intelligence and school readiness in preschool children with prenatal drug exposure. Child Neuropsychol. 10.1080/09297040490911104 [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Niesink RJM, Van Ree JM (1993) Prenatal exposure to morphine enhances cocaine and heroin self-administration in drug-naive rats. Drug Alcohol Depend 33:41–51. 10.1016/0376-8716(93)90032-L [DOI] [PubMed] [Google Scholar]
- Ramsey NF, van Ree JM (1990) Chronic pretreatment with naltrexone facilitates acquisition of intravenous cocaine self-administration in rats. Eur Neuropsychopharmacol 1:55–61. 10.1016/0924-977X(90)90012-Y [DOI] [PubMed] [Google Scholar]
- Riley MA, Vathy I (2006) Mid- to late gestational morphine exposure does not alter the rewarding properties of morphine in adult male rats. Neuropharmacology 51:295–304. 10.1016/j.neuropharm.2006.03.022 [DOI] [PubMed] [Google Scholar]
- Robinson SA, Jones AD, Brynildsen JK, et al. (2019) Neurobehavioral effects of neonatal opioid exposure in mice: Influence of the OPRM1 SNP. Addict Biol 25:. 10.1111/adb.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev [DOI] [PubMed] [Google Scholar]
- Ross EJ, Graham DL, Money KM, Stanwood GD (2015) Developmental consequences of fetal exposure to drugs: What we know and what we still must learn. Neuropsychopharmacology 40:61–87. 10.1038/npp.2014.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Puttick DJ, Sawyer AM, et al. (2016) Nucleus accumbens AMPA receptors are necessary for morphine-withdrawal-induced negative-affective states in rats. J Neurosci 36:5748–5762. 10.1523/JNEUROSCI.2875-12.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo S, Kivistö K, Korja R, et al. (2009) Emotional availability, parental self-efficacy beliefs, and child development in caregiver-child relationships with buprenorphine-exposed 3-year-olds. Parenting 9:244–259. 10.1080/15295190902844563 [DOI] [Google Scholar]
- Schuler ME, Nair P, Black MM (2002) Ongoing maternal drug use, parenting attitudes, and a home intervention: Effects on mother-child interaction at 18 months. J Dev Behav Pediatr. 10.1097/00004703-200204000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdarevic M, Striley CW, Cottler LB (2017) Sex differences in prescription opioid use. Curr Opin Psychiatry 30:238–246. 10.1097/YCO.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology 35:27–47. 10.1038/npp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YL, Chen ST, Chan TY, et al. (2016) Delayed extinction and stronger drug-primed reinstatement of methamphetamine seeking in rats prenatally exposed to morphine. Neurobiol Learn Mem 128:56–64. 10.1016/j.nlm.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R, Stohr T, Shippenberg TS (1995) Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology (Berl) 117:240–247. 10.1007/BF02245193 [DOI] [PubMed] [Google Scholar]
- Sirnes E, Oltedal L, Bartsch H, et al. (2017) Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum Dev 106–107:33–39. 10.1016/j.earlhumdev.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Pometlová M, Schutová B, et al. (2012) Do prenatally methamphetamine-exposed adult male rats display general predisposition to drug abuse in the conditioned place preference test? Physiol Res 61: [DOI] [PubMed] [Google Scholar]
- Šlamberová R, Schindler CJ, Pometlová M, et al. (2001) Prenatal morphine exposure differentially alters learning and memory in male and female rats. Physiol Behav 73:93–103. 10.1016/S0031-9384(01)00469-3 [DOI] [PubMed] [Google Scholar]
- Slinning K (2004) Foster placed children prenatally exposed to poly-substances: Attention-related problems at ages 2 and 4 1/2. Eur Child Adolesc Psychiatry 13:19–27. 10.1007/s00787-004-0350-x [DOI] [PubMed] [Google Scholar]
- Smith MA, Lyle MA (2006) Chronic exercise decreases sensitivity to mu opioids in female rats: Correlation with exercise output. Pharmacol Biochem Behav 85:12–22. 10.1016/j.pbb.2006.06.020 [DOI] [PubMed] [Google Scholar]
- Spanagel R (2017) Animal models of addiction. Dialogues Clin Neurosci 19:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano MS, Ellgren M, Wang X, Hurd YL (2007) Prenatal Cannabis Exposure Increases Heroin Seeking with Allostatic Changes in Limbic Enkephalin Systems in Adulthood. Biol Psychiatry 61:554–563. 10.1016/j.biopsych.2006.03.073 [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011) Drug wanting: Behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365. 10.1124/pr.109.001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, Jernigan TL (2010) The basics of brain development. Neuropsychol Rev 20:327–348. 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover MW, Davis JM (2015) Opioids in pregnancy and neonatal abstinence syndrome. Semin Perinatol 39:561–565. 10.1053/j.semperi.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BL, Levitt P, Stanwood GD (2009) Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat Rev Neurosci 10:303–312. 10.1038/nrn2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timár J, Sobor M, Király KP, et al. (2010) Peri, pre and postnatal morphine exposure: Exposure-induced effects and sex differences in the behavioural consequences in rat offspring. Behav Pharmacol 21:58–68. 10.1097/FBP.0b013e3283359f39 [DOI] [PubMed] [Google Scholar]
- Torabi M, Pooriamehr A, Bigdeli I, Miladi-Gorji H (2017) Maternal swimming exercise during pregnancy attenuates anxiety/depressive-like behaviors and voluntary morphine consumption in the pubertal male and female rat offspring born from morphine dependent mothers. Neurosci Lett 659:110–114. 10.1016/j.neulet.2017.08.074 [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H (1990) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science (80-) 251:85–87. 10.1126/science.1824728 [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, et al. (2008) “Higher order” addiction molecular genetics: Convergent data from genome-wide association in humans and mice. Biochem Pharmacol. 10.1016/j.bcp.2007.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, et al. (2010) Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35:401–415. 10.1038/npp.2009.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ Ahmed SH (2013) Animal studies of addictive behavior. Cold Spring Harb Perspect Med 3:1–14. 10.1101/cshperspect.a011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: A critical review of preclinical studies. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ Pierce RC (2010) Sensitization processes in drug addiction. Curr. Top. Behav. Neurosci [DOI] [PubMed] [Google Scholar]
- Vathy I, Šlamberová R, Liu X (2007) Foster mother care but not prenatal morphine exposure enhances cocaine self-administration in young adult male and female rats. Dev Psychobiol 49:463–473. 10.1002/dev.20240 [DOI] [PubMed] [Google Scholar]
- Vathy I, Šlamberová R, Rimanóczy Á, et al. (2003) Autoradiographic evidence that prenatal morphine exposure sex-dependently alters μ-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog Neuro-Psychopharmacology Biol Psychiatry 27:381–393. 10.1016/S0278-5846(02)00355-X [DOI] [PubMed] [Google Scholar]
- Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Boyle M (2018) Neuroscience of addiction: Relevance to prevention and treatment. Am J Psychiatry 175:729–740. 10.1176/appi.ajp.2018.17101174 [DOI] [PubMed] [Google Scholar]
- Wahlsten VS, Sarman I (2013) Neurobehavioural development of preschool-age children born to addicted mothers given opiate maintenance treatment with buprenorphine during pregnancy. Acta Paediatr Int J Paediatr 102:544–549. 10.1111/apa.12210 [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, et al. (2007) Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. Neuroimage 36:1331–1344. 10.1016/j.neuroimage.2007.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu N, Zhao TY, Li J (2016) The potential biomarkers of drug addiction: proteomic and metabolomics challenges. Biomarkers 21:678–685. 10.1080/1354750X.2016.1201530 [DOI] [PubMed] [Google Scholar]
- Wang R, Shena Y-L, Hausknecht KA, et al. (2019) Prenatal Ethanol Exposure Increases Risk of Psychostimulant Addiction. Behav Brain Res 356:51–61. 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yao Y, Li Y, et al. (2017a) Prenatal morphine exposure during late embryonic stage enhances the rewarding effects of morphine and induces the loss of membrane-bound protein kinase C-α in intermediate medial mesopallium in the chick. Neurosci Lett 639:25–30. 10.1016/j.neulet.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yao Y, Nie H, He X (2017b) Implication of protein kinase C of the left intermediate medial mesopallium in memory impairments induced by early prenatal morphine exposure in one-day old chicks. Eur J Pharmacol 795:94–100. 10.1016/j.ejphar.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Welton S, Blakelock B, Madden S, Kelly L (2019) Systematic review Effects of opioid use in pregnancy on pediatric development and behaviour in children older than age 2 Revue systématique Effets de l ‘ utilisation d ‘ opioïdes durant la grossesse sur le développement et les comportements pédiatriques c. Can Fam Physician 65:544–551 [PMC free article] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE (2000) Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse 26:523–535. 10.1081/ADA-100101893 [DOI] [PubMed] [Google Scholar]
- Whitesell M, Bachand A, Peel J, Brown M (2013) Familial, Social, and Individual Factors Contributing to Risk for Adolescent Substance Use. J Addict. 10.1155/2013/579310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Licsw EB, et al. (2002) A family study of the high-risk children of opioid- and alcohol-dependent parents. Am J Addict. 10.1080/10550490252801620 [DOI] [PubMed] [Google Scholar]
- Woods LCS, Mott R (2017) Heterogeneous stock populations for analysis of complex traits. Methods Mol Biol 1488:31–44. 10.1007/978-1-4939-6427-7_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewski JT, Danysz W (1989) Receptors : Molecular Mechanisms and Functional [DOI] [PubMed]
- Wu LY, Chen JF, Tao PL, Huang EYK (2009) Attenuation by dextromethorphan on the higher liability to morphine-induced reward, caused by prenatal exposure of morphine in rat offspring. J Biomed Sci 16:106. 10.1186/1423-0127-16-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PL, Yang YN, Suen JL, et al. (2018) Long-Lasting Alterations in Gene Expression of Postsynaptic Density 95 and Inotropic Glutamatergic Receptor Subunit in the Mesocorticolimbic System of Rat Offspring Born to Morphine-Addicted Mothers. Biomed Res Int 2018:1–9. 10.1155/2018/5437092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Huang LT, Wang CL, et al. (2003) Prenatal administration of morphine decreases CREBSerine-133 phosphorylation and synaptic plasticity range mediated by glutamatergic transmission in the hippocampal CA1 area of cognitive-deficient rat offspring. Hippocampus 13:915–921. 10.1002/hipo.10137 [DOI] [PubMed] [Google Scholar]
- Yang SN, Yang JM, Wu JN, et al. (2000) Prenatal exposure to morphine alters kinetic properties of NMDA receptor-mediated synaptic currents in the hippocampus of rat offspring. Hippocampus 10:654–662. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, et al. (2004) Identification of PSD-95 as a Regulator of Dopamine-Mediated Synaptic and Behavioral Plasticity. Neuron 41:625–638. 10.1016/S0896-6273(04)00048-0 [DOI] [PubMed] [Google Scholar]
- Young Mun E, Fitzgerald HE, Von Eye A, et al. (2001) Temperamental characteristics as predictors of externalizing and internalizing child behavior problems in the contexts of high and low parental psychopathology. Infant Ment Health J. 10.1002/imhj.1008 [DOI] [Google Scholar]
- Zhang L, Meng J, Ban Y, et al. (2019) Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A 116:13523–13532. 10.1073/pnas.1901182116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimić JI, Jukić V (2012) Familial risk factors favoring drug addiction onset. J Psychoactive Drugs. 10.1080/02791072.2012.685408 [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Robertson MJ, Wright MA (1999) The impact of childhood foster care and other out-of-home placement on homeless women and their children. Child Abus Negl. 10.1016/S0145-2134(99)00082-4 [DOI] [PubMed] [Google Scholar]


