Abstract
Introduction
Subjects exhibiting subjective cognitive decline (SCD) are at an increased risk for mild cognitive impairment and dementia. Given the delay between risk exposure and disease onset, SCD individuals are increasingly considered a good target population for cost‐effective lifestyle‐based Alzheimer's disease prevention trials.
Methods
The PENSA study is a randomized, double‐blind, controlled clinical trial that aims to evaluate the efficacy of a personalized multimodal intervention in lifestyle (diet counseling, physical activity, cognitive training, and social engagement) combined with the use of epigallocatechin gallate (EGCG) over 12 months, in slowing down cognitive decline and improving brain connectivity. The study population includes 200 individuals meeting SCD criteria and carrying the apolipoprotein E ε4 allele, who will be randomized into four treatment arms (multimodal intervention + EGCG/placebo, or lifestyle recommendations + EGCG/placebo). The primary efficacy outcome is change in the composite score for cognitive performance measured with the Alzheimer's Disease Cooperative Study Preclinical Alzheimer Cognitive Composite (ADCS‐PACC‐like) adding to the original version the Interference score from the Stroop Color and Word Test and the Five Digit Test. Secondary efficacy outcomes are (1) change in functional magnetic resonance imaging (fMRI) and structural neuronal connectivity (structural MRI) and (2) the safety assessment of the EGCG compound. This study is framed within the WW‐FINGERS consortium.
Discussion
The use of new technologies (i.e., mobile ecological momentary assessments [EMAs], activity tracker) in the PENSA study allows the collection of continuous data on lifestyle behaviors (diet and physical activity) and mood, enabling a personalized design as well as an intensive follow‐up of participants. These data will be used to give feedback to participants about their own performance along the intervention, promoting their involvement and adherence. The results of the study may aid researchers on the design of future clinical trials involving preventive lifestyle multicomponent interventions.
Keywords: apolipoprotein E ε4, epigallocatechin gallate, lifestyle multimodal intervention, PENSA study, preclinical Alzheimer's disease, prevention, randomized clinical trial, subjective cognitive decline
1. INTRODUCTION
Dementia affected nearly 50 million people worldwide in 2018, with Alzheimer's disease (AD) the most common cause. Its prevalence has risen over the past decades mainly due to the increase in life expectancy, and is expected to triple by 2050. 1 , 2 Currently, there is no effective treatment to slow down or ameliorate AD neuropathological changes, symptomatic approaches being the only therapeutic option. Therefore, it is a research priority to find effective interventions to prevent or delay the onset of cognitive impairment and AD. 3
AD is understood as a continuum in which preclinical physio‐pathological events may occur even 20 years before the appearance of the first symptoms. 4 In this continuum, there are a number of cognitively unimpaired individuals, who are concerned that they have reduced cognitive function. This condition is known as subjective cognitive decline (SCD). Subjects with SCD are at an increased risk for mild cognitive impairment (MCI) and dementia. 5 Given the delay between risk exposure and disease onset, SCD individuals with biomarkers of neurodegeneration are increasingly considered a good target population for cost‐effective lifestyle‐based AD prevention trials. 6 Furthermore, the presence of the ɛ4 allele in apolipoprotein E (APOE) represents the strongest genetic risk factor for late onset AD, with about a 3‐fold increased risk for heterozygotes and 8‐ to 12‐fold for homozygotes compared to the ε3/ε3 genotype. 7 , 8 APOE is the major cholesterol transporter to the central nervous system. APOE ɛ4 contributes to AD pathology by different mechanisms. 7 , 9
International research agencies have underlined the importance of prioritizing well‐designed randomized controlled clinical trials (RCTs) involving interventions that have already shown promising effects on the prevention of cognitive decline such as cognitive training and physical activity 10 and healthy dietary patterns such as the Mediterranean diet. 11 Given the multifactorial causes of AD, the report also recommends testing interventions that target several risk factors and mechanisms simultaneously. These risk factors, including diabetes, midlife hypertension, midlife obesity, smoking, depression, cognitive inactivity, and physical inactivity, explain ≈50% of AD cases. 12 Nonetheless, these are potentially modifiable by altering some components of individual's lifestyle (e.g., eating a healthy diet and performing physical activity or cognitive training/stimulation). Although evidence is not conclusive, studies suggest that strategies involving multifactorial interventions including regular exercise, healthy diet, and vascular risk management may be promising for preventing cognitive decline. 12
Accordingly, some RCTs focusing on multiple risk factors and involving multicomponent interventions have been initiated in the last years. The Finnish Geriatric Intervention Study to Prevent Cognitive Decline (FINGER) trial evidenced a beneficial effect on cognition for at‐risk old individuals following a multicomponent lifestyle intervention. 13 Globally, this RCT suggests that secondary prevention strategies to reduce modifiable risk factors correlate with an improvement in some cognitive functions and a decrease in the rate of progression to MCI and symptomatic AD, especially among APOE ɛ4 carriers. The FINGER model is now tested and adapted in several ongoing preventive trials, and the World‐Wide‐FINGERS (WW‐FINGERS) network (https://alz.org/wwfingers) was launched to support these joint initiatives. 14
The PENSA study is framed within the WW‐FINGERS consortium, but differentially from the original FINGER study, the multimodal intervention in lifestyle (diet counseling, physical activity, cognitive training, and social engagement) is combined with the use of a dietary supplement, epigallocatechin gallate (EGCG), in a population meeting SCD criteria and carrying the APOE ε4 allele.
EGCG is a flavanol from green tea that already has been shown to be safe in humans at the doses proposed. 15 According to previous studies, the underlying mechanism of action of EGCG is the improvement of synaptic plasticity and brain connectivity. In a phase 2 clinical trial with Down syndrome adults, it has been shown that EGCG combined with cognitive training improves cognitive performance (executive functions), adaptive functionality, and brain connectivity (monitored with functional magnetic resonance imaging [fMRI] and transcranial magnetic stimulation). 16 The most relevant additional mechanisms 17 , 18 by which EGCG may confer neuroprotection to different AD insults are: (2) antioxidant activity via the Nrf2‐pathway, 19 (2) protection in neuroinflammation via the brain‐derived neurotrophic factor (BDNF), 20 (3) regulation of insulin signaling, 21 and (4) targeting amyloidogenesis and tau hyperphosphorylation. EGCG modulates amyloid precursor protein (APP) processing favoring the non‐amyloidogenic pathway by activating ADAM10, and inhibiting BACE‐1. 22 EGCG inhibits DYRK1A activity, a gene associated with AD, involved in the phosphorylation of both APP and tau. 23 , 24 All of this is expected to be translated into an improvement of cognitive performance in the PENSA study participants. 25
It is expected that EGCG combined with a lifestyle multimodal intervention will result in longer lasting and sustainable effects than those derived from each component evaluated separately. Moreover, the PENSA study also includes a personalized design, as well as an intensive personal follow‐up mainly based on the use of new technologies. This approach allows the continuous monitoring of a participant's engagement on the different lifestyle interventions.
The main objective of the PENSA study is to evaluate the efficacy of a multimodal intervention (dietary, physical activity, and cognition) combined with EGCG in slowing down cognitive decline in APOE ε4 carriers with SCD. Secondary objectives are two‐fold: (1) to evaluate the efficacy of the study intervention in improving brain connectivity, and (2) to evaluate the safety of the EGCG compound. Exploratory objectives encompass the study of several underlying mechanisms that could explain the efficacy of the intervention: (i) changes in gut microbiota composition and in the metabolome derived by the action of microorganisms, (ii) changes in AD biomarkers, and (iii) changes in biomarkers of inflammation. The purpose of this report is to present the PENSA study design.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using the traditional sources. Multimodal lifestyle interventions targeting multiple risk factors have shown to be a promising strategy to prevent or delay the onset of cognitive impairment and dementia, especially in at‐risk individuals (apoliprotein E [APOE] ε4 carriers). Studies assessing the efficacy of dietary supplements (e.g., vitamins, fatty acids) have shown they might be useful in improving cognitive functioning in the context of healthy dietary patterns. Additional long‐term clinical trials are needed to further substantiate their effects and interactions with lifestyle interventions.
Interpretation: The PENSA study is a 12‐month plus 3‐month follow‐up, double‐blind, personalized, placebo‐controlled clinical trial enrolling APOE ε4 carriers exhibiting subjective cognitive decline aimed at assessing the effects of a multimodal lifestyle intervention complemented with epigallocatechin gallate in their cognitive functioning.
Future directions: Results from the present study and other randomized controlled trials involving preventive multimodal lifestyle interventions should determine the effectiveness of these strategies.
2. METHODS
2.1. Global study design
This is a randomized, double‐blind, personalized, controlled clinical trial enrolling 200 APOE ε4 carriers with SCD. The project uses a web‐based recruitment system (https://www.pensaalzheimer.org/). Registered individuals are filtered by a first step‐screening algorithm. Candidates are then referred to the Neurology Department of the Hospital del Mar (Barcelona, Spain) and the Barcelonaβeta Brain Research Center (BBRC) for further explorations. The study includes four treatment arms: (1) EGCG + multimodal intervention, (2) placebo + multimodal intervention (multimodal intervention groups), (3) EGCG + lifestyle recommendations, or (4) placebo + lifestyle recommendations (control groups). Investigators and participants are blinded to EGCG or placebo. To ensure the blindness regarding the multimodal/control intervention, the investigators assessing the main outcome measures are not involved in the follow‐up of subjects nor are they aware of the randomization of the participants. The duration of the interventions is 12 months. An additional evaluation will be carried out 3 months after discontinuing the treatment, to evaluate effects’ sustainability (Figure 1).
FIGURE 1.
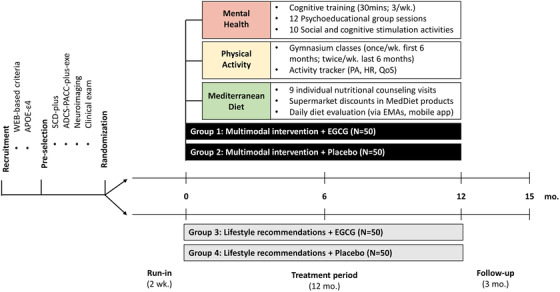
Schematic diagram of the trial design
The study protocol has been approved by the local institutional review board (CEIM‐PSMAR) and adheres to standards of the WAMA Declaration of Helsinki (Brazil, October 2013) as well as the European Union standards for conducting clinical trials of and good clinical practices (Directives 2001/20/EC and 2005/28/EC) and the General Data Protection Regulation (GDPR UE 2016/976). The clinical trial is registered in ClinicalTrials.gov (NCT03978052). The safety of the interventions is closely monitored.
2.2. Selection of study population
Inclusion criteria include male or female individuals aged 60 to 80 years with SCD 26 (based on a positive answer to the question Have you experienced a decrease in your cognitive ability [e.g., memory, concentration, planning, orientation, or language) compared to a few years ago]?) and APOE ε4 carriers (either hetero or homozygotes), fulfilling at least two additional SCD “plus” criteria (memory complaints rather than other domains of cognition, onset of symptoms within the last 5 years, concern about symptoms, perception of lower performance compared to same age group and/or confirmation of symptoms by an informant). 26 , 27
Exclusion criteria include (1) history of neurological or psychiatric conditions according to DSM‐5 criteria, (2) clinically significant abnormalities in laboratory test, (3) any contraindication for brain MRI, (4) presence of mild to moderate leukoaraiosis (scoring <3 on Fazekas scale, 28 and/or less than three lacunar infarcts not localized on strategic territory [e.g., bilateral thalamic]), (5) primary or recurrent malignant disease treated within the last 2 years, (6) evidence of medical conditions/medications that may interfere with study assessments, (7) body mass index < 18.5 or ≥35 kg/m2, or (8) current intake of vitamins or products containing EGCG supplements for at least 3 months previous to the screening visit.
2.3. Study flow
As a general procedure, all participants have to fill out a web‐based questionnaire including information about their subjective cognitive performance designed for study recruitment. Based on SCD status, a priori eligible participants are invited to perform a short face‐to‐face pre‐screening visit to allow the collection of a buccal swab for APOE genotyping. In this visit, researchers explain the implications of knowing their APOE genotype (i.e., that the presence of the allele ε4 increases the risk of AD but it is not a diagnostic factor). To ensure the participants’ ability to receive and understand the results of genotyping, the absence of anxious and depressive symptomatology is assessed before the APOE status disclosure. Disclosure of APOE status is postponed in those individuals with relevant anxiety symptoms. APOE ɛ4 carriers undergo a second screening that includes neurological and neuropsychological examination 29 , 30 and neuroimaging tests. After this assessment procedure, only participants with cognitive performance within normal values (normal scoring on psychometric evaluation, adjusted for age and education) and no abnormalities in the neuroimaging tests are eligible. Table 1 summarizes the details of study assessments in each visit.
TABLE 1.
Summary of assessments
| Period | Pre‐screening | Screening | RZ | Run‐in | Treatment | Follow‐up | ||
|---|---|---|---|---|---|---|---|---|
| Procedure/month | M‐1 | M0 | M6 | M12 | M15 | |||
| APOE genotyping | x | |||||||
| SCD‐Plus criteria | X | |||||||
| Medical, neurological exploration and interview | X | x | ||||||
| Clinical exam, vital signs, and adverse events | X | X | x | x | x | |||
| ECG, blood chemistry, hematology, and coagulation | X | x | x | |||||
| ADCS‐PACC‐Plus‐exe and additional neuropsychological tests | X | x | x | x | ||||
| Neuroimaging acquisitions (MRI, fMRI) | X | x | ||||||
| Safety assessment of EGCG and biomarkers of treatment compliance | x | x | ||||||
| Blood sampling for AD biomarkers, microbiota, metabolomics, genetics, and epigenetics | X | x | x | |||||
| Urine sampling for dietary patterns (metabolomics) | X | x | x | |||||
| Fecal sampling for microbiota | X | x | x | |||||
| Plasma neuron‐derived exosomes and oral fluid | X | x | ||||||
| Olfactory function | X | x | ||||||
| Adaptive behavior | X | x | x | x | ||||
| Quality of life and general health | X | x | x | x | ||||
| Lifestyle habits (questionnaires) | X | x | x | x | ||||
| Lifestyle habits (continuous assessments)* | ||||||||
Abbreviations: AD, Alzheimer's disease; ADCS‐PACC‐Plus‐exe, Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite plus executive functioning tests; APOE, apolipoprotein E; ECG, electrocardiogram; EGCG, epigallocatechin gallate; MRI, magnetic resonance imaging; RZ, randomization; SCD, subjective cognitive decline.
Activity tracker measures and ecological momentary assessments.
Participants are randomized to one of the four treatment arms following a randomization list balanced by sex. The electronic Case Report Form (eCRF, SAIL Biometria SA) automatically performs treatment allocation based on randomization. Treatment allocation will be concealed until the end of the study.
After randomization, individuals are pooled in groups of 10 to 12 people allocated to the multimodal intervention or to the control groups. The group size is based on optimal ratios for behavior‐change group interventions, which allows optimal interactions between participants as well as promotes social change processes. Every group participates in an induction session providing detailed information on study procedures and training on the digital technologies used in each intervention. Thereafter, 15 to 30 days before starting the intervention, dietary, physical activity, and sleep habits data are collected (run‐in period). Data are used to personalize the dietary and physical activity interventions (Figure 2). After the run‐in period, participants attend a baseline session, which represents the beginning of the 12‐month intervention.
FIGURE 2.
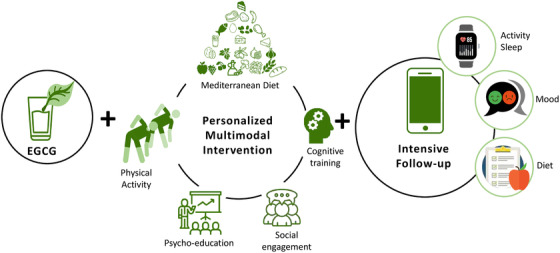
Graphic summary of the multimodal intervention
2.4. Intervention
2.4.1. EGCG dietary supplement
A dietary supplement (powder sachets), suitable for diabetic patients, containing EGCG (400 to 600 mg/day prior to meals) or placebo, is administered dissolved in 100 mL of water. This preparation was developed specifically by Laboratoires Grand Fontaine based on the FontUp product (http://www.grandfontaine.eu/). The packaging of the sachets is the same for both the active and the placebo groups without any reference to their EGCG content.
2.4.2. Lifestyle recommendations to control groups
At the beginning of the study, participants in the control groups receive personalized advice on diet, physical activity, cognitive training, and social stimulation activities. Dietary recommendations are based on the Mediterranean Diet (MedDiet) adapted to the dietary habits observed during the run‐in period, the nutritional status, and concomitant health problems and medication.
2.4.3. Multimodal intervention groups
2.4.3.1. Digital platform
To continuously monitor the adherence to the multimodal intervention, motivate participants, and promote engagement in healthy behaviors, a digital platform collects information on participants’ diet (daily) and mood and perceived mental health (weekly) using ecological momentary assessments (EMAs), and registers the attendance at social engagement activities and gymnasium classes. Continuous measures of physical activity, heart rate, and sleep quality are collected from an activity tracker (Fitbit© Charge 3). All data tracked through the platform are accessible from the online platform for researchers. Throughout the intervention, participants receive personalized recommendations based on the collected data, as well as feedback on their behaviors. See Table S1 in supporting information for details on the schedule of continuous data collection.
2.4.3.2. Diet
The multimodal intervention includes a personalized nutritional program, providing tailored dietary recommendations at the beginning of the study based on participants’ dietary habits (food preferences, allergies or intolerances, frequency and schedules of the meals, type and amount of fat and salt intake, coffee and alcohol intake, deposition habits), nutritional status, concomitant health comorbidities (obesity, diabetes, hypertension, and hyperlipidemia), and medication. A total of nine individual counselling sessions reinforce, revise, and readjust the nutritional intervention. The recommended diet is based on MedDiet. The energy intake of the diet is adjusted to the energy expenditure to achieve a healthy weight. The nutritional profile of the MedDiet prescribed is as follows: 30% to 35% of the energy as fat (<7% of saturated fatty acids, >20% of monounsaturated fatty acids, and >10% polyunsaturated fatty acids), 15% of the energy in the form of protein, and 50% to 55% as carbohydrates. Periodical dietary assessments are performed via 3‐day food records.
An additional follow‐up is performed via EMAs that enquires about the consumption of key food groups of the MedDiet. Participants’ adherence to the MedDiet is calculated and periodical individualized reports providing dietary strategies to improve MedDiet adherence are sent to participants.
2.4.3.3. Physical activity
Participants are invited to 60‐minute gymnasium sessions combining aerobic, strength, and balance activities (minimum of one class/week the first 6 months, and two classes/week from month 7 to 12). Sessions are guided by a trained physiotherapist and personalized based on participants’ baseline physical condition status.
Participants are also encouraged to achieve a physically active lifestyle according to the classification system based on steps‐per‐day categories. 31 Healthy older adults should take 10,000 steps/day and individuals living with disability or chronic illness should achieve about 8,500 steps/day. At the same time, participants are encouraged to reach the minimum recommendations of moderate physical exercise in older adults, which are 150 to 210 minutes/week (90 to 150 minutes/week in those with a history of cardiovascular disease, osteoporosis, or mobility problems such as osteoarthritis). 32
To evaluate the adherence and progress in physical activity and sleep throughout the study, participants receive a wristband activity tracker (Model: Fitbit® Charge 3: Fitbit Inc), which can estimate steps, activity minutes, heart rate, and sleep duration and stages.
Individualized reports are periodically given to participants showing their weekly and monthly evolution in physical activity, steps, and sleep quality. Different scores are calculated, based on whether they exceed their own baseline values recorded during the run‐in period and also whether they achieve the minimum recommendations of moderate physical activity. Accordingly, strategies to improve and reinforce their behavior are provided.
2.4.4. Cognitive training
Cognitive training includes the performance of specific exercises to optimize and strengthen cognitive functioning and improve the cognitive reserve of the brain. The training is carried out through the web‐based platform NeuronUP (https://www.neuronup.com/en/neuronup2go‐braintraning‐home). It consists of thrice weekly, 30‐minute sessions along the intervention. The planning of the specific exercises and cognitive functions involved in each session has been predefined by a team of neuropsychologists. As long as participants do not have relevant cognitive impairments, the training includes a wide range of cognitive domains targeting the pathognomonic neuropsychological features of AD. All participants follow the same plan; however, the difficulty level of tasks is automatically adjusted on a trial‐by‐trial basis allowing for personalization.
2.4.5. Psychoeducation
The intervention consists of ten 90‐minute sessions, led by experienced psychologists. Sessions have been designed to motivate and empower participants to elongate the impact of the lifestyle intervention, by teaching them the nature of cognitive decline (genetic, lifestyle, and environmental factors), promoting behavioral change strategies, discussing what to expect during and after the intervention, and detailing how changes in lifestyle habits can help them in dealing with cognitive decline.
2.4.6. Social stimulation activities
Social stimulation activities refer to the participant's involvement in group activities, designed to increase cognitive and social functioning in a nonspecific manner. The intervention consists of ten to twelve 90‐ to 120‐minute monthly sessions, led by specific professionals according to the topic of the month.
The purpose of these sessions is to provide opportunities for participants to interact with their groupmates, as well as to provide them access to environments and resources that will help keep themselves cognitively stimulated.
3. FOLLOW‐UP AND OUTCOME MEASURES
Efficacy will be evaluated measuring the effect of the multimodal intervention versus the control intervention on cognitive decline measured at baseline and months 6, 12, and 15 (3 months after intervention discontinuation).
Safety is evaluated at months 6 and 12 with adverse events (AEs) and serious adverse events (SAEs) reported according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice definitions, as well as with clinically significant changes in heart rate, blood pressure, and in blood analyses (serum creatinine, liver function, thyroid hormones, and serum cholesterol). Additionally, AE of special interest for this study include the following: changes in heart rate (HR) and blood pressure (BP) relative to baseline; observed or suspected clinical seizure that resolves within 3 minutes and does not require treatment, hospitalization, or intervention; cases of elevated alanine transaminase (ALT) or aspartate transaminase; elevated bilirubin or clinical jaundice; and cases of elevated thyroid‐stimulating hormone (TSH) and decreased free T4.
Compliance will be assessed by measuring EGCG in plasma, at 6 and 12 months. Additionally, participants will return the empty medication sachets.
3.1. Primary outcome measure
Change in global cognition will be evaluated with a composite measure designed for clinical trials conducted at the asymptomatic phase of AD, the Preclinical Alzheimer Cognitive Composite, which combines tests that evaluate episodic memory, timed executive function, and global cognition (Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite [ADCS‐PACC]). 30 However, we added to the original version the Interference score from the Stroop Color and Word Test (SCWT) 33 and the Five Digit Test, 34 resulting in the Modified Preclinical Alzheimer Cognitive Composite‐Plus exe.
3.2. Secondary outcome measures
Change in brain connectivity will be evaluated with functional neuronal connectivity (fMRI) and structural connectivity (structural MRI; see supporting information). The safety assessment of the EGCG compound will be based on thyroid, liver, and renal function; TSH; freeT4; alkaline phosphatase (ALP); ALT; and creatinine.
3.3. Exploratory outcome measures
There are a number of exploratory outcome measures (see supporting information) evaluating additional cognitive performance scores, anxiety and depression, adaptive behavior, quality of life and general health, lifestyle habits (quality of sleep, physical activity and functional capacity, MedDiet adherence), microbiota composition and metabolome derived from gut microorganisms’ activity, dietary patterns (metabolomics), olfactory function, plasma AD biomarkers, plasma neuron‐derived exosomes, biological age biomarkers (epigenetics, biological aging), and a number of biomarkers.
3.3.1. Biomarker analysis
For the analysis of biomarkers, blood, plasma, urine, and feces are collected (see Table 1 for more details). Plasma neuron‐derived exosomes and oral fluid are also collected as exploratory matrices to test their usefulness to derive surrogate biomarkers of efficacy.
Blood samples are collected to assess blood chemistry and safety parameters that focus on liver, renal, and thyroid function; as well as core AD, neuroinflammation, and inflammatory biomarkers; microbiota‐related biomarkers; and metabolomics profiles. Plasma for EGCG analysis is collected as a biomarker of treatment compliance. Finally, buffy coat from these samples are used to evaluate genetic and epigenetic biomarkers. Metabolomics and dietary profiles are explored in 24‐hour urine samples.
Fecal samples are collected for the study of taxonomy and phylogenetics of gut microbiota. The analysis of microbiota‐related metabolites will be quantified in the same samples and in an additional sample collected at the 6‐month visit.
4. STATISTICAL CONSIDERATIONS
Sample size calculations were based on the expected minimum difference in change in the ADCS‐PACC score between groups. Considering previous observational studies in clinically normal elderly individuals with evidence of AD pathology and APOE ε4 carriers, 30 a difference in ADCS‐PACC of ≈1.5 units with a standard deviation (SD) of 2.4 is expected between any pair of groups, and a correlation between visits of 0.5. With 5% significance level and 80% power, the sample size required is 45 participants in each group, so 50 participants in each group will be included, 200 in total. A drop‐out rate of 10% has been anticipated.
An exhaustive descriptive analysis will be carried out for all variables of interest at all study times. Primary and secondary outcomes will be analyzed with linear mixed models to study their evolution over time in each study group and the differences among them. The effect size measure of interest will be the adjusted mean differences among treatments and pairwise post hoc comparisons controlling a family‐wise error rate of 0.05 will be performed if statistically significant differences among the study groups are detected.
5. DATA MANAGEMENT PROCESS
The process of collection, cleaning, and management of subject data will be compliant with regulatory standards in clinical research. Monitoring is provided along the trial to collect and keep high‐quality data. The eCRF will maintain an audit trial and provide easy identification and resolution of data discrepancies.
6. DISCUSSION
The PENSA study is an initiative framed on the WW‐FINGERS global network, aimed at assessing the efficacy of a multimodal intervention on lifestyle habits complemented with a dietary supplement containing EGCG in slowing down cognitive decline and improving brain connectivity in subjects with SCD and APOE ε4 carriers.
One of the differential aspects of the PENSA study is the administration of a dietary supplement containing EGCG. Although EGCG has been tested in different pathological conditions 35 our main experience with this compound has been its preclinical and clinical development for the treatment of cognitive deficits in intellectual disabilities (e.g., Down and Fragile‐X syndromes). The most used mouse model of Down syndrome, the Ts65Dn trisomic mouse, displays poor memory abilities and disordered prefrontal‐hippocampal functional connectivity. Both memory performance and key neurophysiological alterations are improved after 1 month of chronic administration of a green tea extract containing EGCG. 36 Further studies in middle‐aged Ts65Dn mice have shown that environmental enrichment has a similar effect to that of EGCG, but more interesting is that the combination of both has synergistic effects being the impact on cognition lasts much longer than with treatments administered separately. Combined environmental enrichment and EGCG treatment improves corticohippocampal‐dependent learning and memory. 37 , 38 This interaction prompted translationally in clinical trials to combine EGCG with cognitive training. 16 , 39 Effects on cognitive functioning were sustained after 6 months of discontinuing the combined treatment, while in an early study administering EGCG only, effects vanished in 3 months. 40
The complexity of the multimodal intervention as well as the procedures involved in the study required the inclusion of multiple agents in its design. In this context, we used a co‐creation approach in which healthy volunteers at risk of cognitive decline expressed their views and needs about the different interventions and procedures. As a result of this collaborative process, several adaptations and improvements have been made to the study design, which are already included in the present protocol.
Additional adaptations to the protocol have been made given the current COVID‐19 pandemic situation. On the one hand, after the web‐based initial screening, a first phone‐based screening (involving medical and cognitive information) is performed to assess the suitability for further explorations and APOE genotyping. This avoids unnecessary visits of those individuals that fulfil some basic exclusion criteria related to medication or to inability to undergo an MRI scan. This also streamlines the face‐to‐face visit aimed at collecting a buccal swab for APOE genotyping. On the other hand, some intervention components have been moved to online individual or group meetings. Accordingly, all the psychoeducation sessions, some social stimulation activities (e.g., cooking lessons), some individual nutritional counselling visits, and some gymnasium classes (only when gymnasiums are closed) are performed online. Moreover, gymnasium sessions take place in a pavilion of large dimensions and in small groups, fully respecting all the social distancing measures.
Finally, the use and acceptance of new technologies among elderly people is heterogeneous. In the context of health programs, implementation may aid them in keeping active, healthy, and monitored, favoring their independence for longer. An important strength of the PENSA study is the commitment to the use of these tools in the elderly. The remarkable use of new technologies through EMAs and an activity tracker allows the collection of continuous data on lifestyle behaviors (diet and physical activity) and mood, enabling an intensive follow‐up of participants. These data will also be used to give feedback to participants about their own performance along the intervention, promoting their involvement and adherence. Furthermore, the analyses of these continuous data will allow for the identification of different cognitive performance trajectories based on the lifestyle habits of each participant and their adherence to the intervention. Accordingly, we expect to identify responders and non‐responders to the intervention and characterize which factors are associated with such response profiles.
In summary, we expect the data gathered from this study and the innovative analyses of the results may aid researchers in the design of future clinical trials involving preventive lifestyle multicomponent interventions as well as to shed light on effective precision medicine approach strategies.
SOURCES OF FUNDING
This project is primarily supported by the Alzheimer Association (18PTC‐R‐592192 The PART THE CLOUD to RESCUE Brain Cell Degeneration in Alzheimer's disease Program) and secondarily by Instituto de Salud Carlos III (ISCIII PI17/00223). Thais Lorenzo is supported by a predoctoral fellowship PFIS‐ISCIII (FI18/00041). CIBER de Fragilidad y Envejecimiento Saludable (CIBERFES), and Fisiopatología de la Obesidad y Nutrición (CIBEROBN) are initiatives of the Instituto de Salud Carlos III, Madrid, Spain, and funded by the European Regional Development Fund. The Government of Catalonia through the Agency for Management of University and Research Grants [2017 SGR 138]
CONFLICTS OF INTEREST
The authors declare no conflicts of interest or other relevant disclosures.
Supporting information
Supplementary Information
Forcano L, Fauria K, Soldevila‐Domenech N, et al. Prevention of cognitive decline in subjective cognitive decline APOE ε4 carriers after EGCG and a multimodal intervention (PENSA): Study design. Alzheimer's Dement. 2021;7:e12155. 10.1002/trc2.12155
Laura Forcano and Karine Fauria share first co‐authorship.
REFERENCES
- 1. ADI. Alzheimer's Disease International . World Alzheimer Report 2018: The State of the Art of Dementia Research: New frontiers: Alzheimer's Disease International; 2018. [Google Scholar]
- 2. Szoeke CEI, Vollset SE, Abbasi N, et al, GBD 2016 Dementia Collaborators E . Global, regional, and national burden of Alzheimer's disease and other dementias, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734.Lancet Publishing Group. [DOI] [PubMed] [Google Scholar]
- 4. Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimer's Dement. 2016;12:292‐323.Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng Y‐W, Chen T‐F, Chiu M‐J. From mild cognitive impairment to subjective cognitive decline: conceptual and methodological evolution. Neuropsychiatr Dis Treat. 2017;13:491‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein e and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta‐analysis. JAMA Neurol. 2017;74(10):1178‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol Dis. 2014;72:3‐12.Academic Press Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kane RL, Butler M, Fink HA, et al. Interventions to prevent age‐related cognitive decline, mild cognitive impairment, and clinical Alzheimer's‐type dementia. Comparative Effectiveness Reviews. Rockville: Agency for Healthcare Research and Quality (US); 2017. [PubMed] [Google Scholar]
- 11. Valls‐Pedret C, Sala‐Vila A, Serra‐Mir M, et al. Mediterranean diet and agerelated cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175(7):1094‐1103. [DOI] [PubMed] [Google Scholar]
- 12. Hankey GJ. Public health interventions for decreasing dementia risk. JAMA Neuro. 2018;75(1):11‐12.American Medical Association. [DOI] [PubMed] [Google Scholar]
- 13. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653‐666. [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain interventions to prevent cognitive impairment, Alzheimer's disease, and dementia: from FINGER to World‐Wide FINGERS. J Prev Alzheimer's Dis. 2020;7(1):29‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Younes M, Aggett P, Aguilar F, et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018;16(4):e05239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de la Torre R, de Sola S, Hernandez G, et al. Safety and efficacy of cognitive training plus epigallocatechin‐3‐gallate in young adults with Down's syndrome (TESDAD): a double‐blind, randomised, placebo‐controlled, phase 2 trial. Lancet Neurol. 2016;15(8):801‐810. [DOI] [PubMed] [Google Scholar]
- 17. Xicota L, Rodriguez‐Morato J, Dierssen M, de la Torre R. Potential role of (‐)‐epigallocatechin‐3‐gallate (egcg) in the secondary prevention of Alzheimer disease. Curr Drug Targets. 2016;18(2):174‐195. [DOI] [PubMed] [Google Scholar]
- 18. Polito CA, Cai ZY, Shi YL, Li XM, Yang R, Shi M, et al. Association of tea consumption with risk of Alzheimer's disease and anti‐beta‐amyloid effects of tea. Nutrients. 2018;10(5):655. MDPI AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Na HK, Kim EH, Jung JH, et al. (‐)‐Epigallocatechin gallate induces Nrf2‐mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys [Internet]. 2008;476(2):171‐177. [DOI] [PubMed] [Google Scholar]
- 20. Lai SW, Chen JH, Lin HY, et al. Regulatory effects of neuroinflammatory responses through brain‐derived neurotrophic factor signaling in microglial cells. Mol Neurobiol. 2018;55(9):7487‐7499. [DOI] [PubMed] [Google Scholar]
- 21. Jia JJ, Zeng XS, Song XQ, Zhang P, peng ChenL. Diabetes mellitus and Alzheimer's disease: the protection of epigallocatechin‐3‐gallate in streptozotocin injection‐induced models. Front Pharmacol. 2017;8:834. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandez JW, Rezai‐Zadeh K, Obregon D, Tan J. EGCG functions through estrogen receptor‐mediated activation of ADAM10 in the promotion of non‐amyloidogenic processing of APP. FEBS Lett. 2010;584(19):4259‐4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Branca C, Shaw DM, Belfiore R, et al. Dyrk1 inhibition improves Alzheimer's disease‐like pathology. Aging Cell. 2017;16(5):1146‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrer I, Barrachina M, Puig B, et al. Constitutive Dyrk1A is abnormally expressed in Alzheimer disease, down syndrome, pick disease, and related transgenic models. Neurobiol Dis. 2005;20(2):392‐400. [DOI] [PubMed] [Google Scholar]
- 25. Li Q, Zhao HF, Zhang ZF, et al. Long‐term green tea catechin administration prevents spatial learning and memory impairment in senescence‐accelerated mouse prone‐8 mice by decreasing Aβ1‐42 oligomers and upregulating synaptic plasticity‐related proteins in the hippocampus. Neuroscience. 2009;163(3):741‐749. [DOI] [PubMed] [Google Scholar]
- 26. Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease and Subjective Cognitive Decline Initiative (SCD‐I) working group. Alzheimers Dement. 2014;10(6):844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer's and Dementia. 2017;13:296‐311.Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. 1987;149(2):351‐356. [DOI] [PubMed] [Google Scholar]
- 29. Rami L, Mollica MA, García‐Sanchez C, et al. The Subjective Cognitive Decline Questionnaire (SCD‐Q): a validation study. J Alzheimer's Dis. 2014;41(2):453‐466. [DOI] [PubMed] [Google Scholar]
- 30. Donohue MC, Sperling RA, Salmon DP, et al. The preclinical Alzheimer cognitive composite: measuring amyloid‐related decline. JAMA Neurol. 2014;71(8):961‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tudor‐Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American college of sports medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435‐1445. [DOI] [PubMed] [Google Scholar]
- 33. Golden CJ. Stroop Color and Word Test. Wood Dale, IL: Stoelting Co.; 1975. [Google Scholar]
- 34. Sedó MA. Five Digit Test. Madrid. TEA Ediciones; 2007. [Google Scholar]
- 35. Xicota L, Rodriguez‐Morato J, Dierssen M, de la Torre R. Potential role of (‐)‐Epigallocatechin‐3‐Gallate (EGCG) in the secondary prevention of Alzheimer disease. Curr Drug Targets. 2015;18(2):174‐195. [DOI] [PubMed] [Google Scholar]
- 36. Alemany‐González M, Gener T, Nebot P, Vilademunt M, Dierssen M, Puig MV. Prefrontal‐hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc Natl Acad Sci U S A. 2020;117(21):11788‐11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Catuara‐Solarz S, Espinosa‐Carrasco J, Erb I, et al. Combined treatment with environmental enrichment and (‐)‐epigallocatechin‐3‐gallate ameliorates learning deficits and hippocampal alterations in a mouse model of down syndrome. eNeuro. 2016;3(5):103‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Catuara‐Solarz S, Espinosa‐Carrasco J, Erb I, et al. Principal component analysis of the effects of environmental enrichment and (‐)‐epigallocatechin‐3‐gallate on age‐associated learning deficits in a mouse model of down syndrome. Front Behav Neurosci. 2015;9:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de la Torre R, de Sola S, Farré M, et al. A phase 1, randomized double‐blind, placebo controlled trial to evaluate safety and efficacy of epigallocatechin‐3‐gallate and cognitive training in adults with Fragile X syndrome. Clin Nutr. 2020;39(2):378‐387. [DOI] [PubMed] [Google Scholar]
- 40. De la Torre R, De Sola S, Pons M, et al. Epigallocatechin‐3‐gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol Nutr Food Res. 2014;58(2):278‐288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


