Abstract
Background
Neuropsychiatric symptoms (NPS) are frequent in aging and Alzheimer's disease (AD). Here we study the relationship between NPS and AD pathologies in vivo.
Method
Two hundred and twenty‐one individuals from the TRIAD cohort (143 cognitively unimpaired, 52 mild cognitive impairment, and 26 AD) underwent [18F]MK6240‐tau‐positron emission tomography (PET), [18F]AZD4694‐amyloid‐PET, magnetic resonance imaging, and neuropsychological evaluations. Spearman correlations and voxel‐based regression models evaluated the relationship between Neuropsychiatric Inventory Questionnaire (NPI‐Q) scores, and tau‐PET, amyloid‐PET, and voxel‐based morphometry.
Results
Fifty percent of individuals presented NPS; these correlated with tau, not amyloid beta or neurodegeneration. Associations between NPI‐Q score and tau‐PET were stronger in the parietal association area, superior frontal, temporal, and medial occipital lobes. NPI‐Q domains associated with distinct patterns of tau uptake.
Conclusions
NPS are predominantly related to tau in aging and dementia. Regions affected are part of the behavioral circuits, and vulnerable to early AD pathology. Domain‐specific analyses showed NPS are related to the AD pathophysiological processes in a symptom‐specific manner.
Keywords: Alzheimer's disease, amyloid beta, neurodegeneration, neuropsychiatric symptoms, tau
1. INTRODUCTION
Neuropsychiatric symptoms (NPS) are commonly found in persons with dementia such as Alzheimer's disease (AD). 1 More than 80% of demented individuals exhibit at least one NPS since the onset of cognitive impairment. 2 NPS lead to premature institutionalization, increased cost of care, suffering, and significant loss of life quality for patients and caregivers. 3 Symptoms evolve as disease progresses, 4 leading to an increase in number and severity of NPS, in turn requiring implementation of new interventions—pharmacological and non‐pharmacological 5 —during the course of the illness. Furthermore, NPS predict more rapid disease progression. 6 The most common NPS accompanying AD are anxiety, agitation, depression; the least common are hallucinations and euphoria. 7 , 8 Hence, NPS constitute an important clinical characteristic of patients with cognitive impairment; there is therefore a crucial need for research on NPS to find methods to treat or manage them.
Previous research on post mortem AD brains has shown correlations between AD pathological hallmarks and NPS. 9 , 10 , 11 Recent advancements in positron emission tomography (PET) imaging permit the identification of AD pathologies in living individuals. 12 While new PET imaging studies have linked cognitive dysfunction with AD pathologies, it remains unclear whether the presence of NPS are also linked to the core disease process. Previous findings report that cognitively unimpaired (CU) individuals showing mild behavioral impairment present amyloid beta (Aβ), but no presence of tau. 13 Another study focused first on CU individuals at risk of developing dementia, and later, autosomal dominant AD. In both branches, they found different patterns of correlations between Aβ and tau pathologies with personality and neuropsychiatric traits. 14 However, no research has been conducted in aging and AD dementia on how the underlying pathologies are related to NPS appearance and severity.
Here, we investigate the relationship between NPS, using the Neuropsychiatric Inventory Questionnaire (NPI‐Q), and biomarkers of Aβ, tau, and neurodegeneration in vivo. We hypothesize that these biomarkers are associated with increase of NPS emergence and severity.
2. METHODS
2.1. Participants
Data was obtained from the TRIAD cohort. 15 We assessed 221 individuals: 143 CU, 52 individuals with mild cognitive impairment (MCI), and 26 with dementia due to AD, using [18F]MK6240 tau‐PET, [18F]AZD4694 amyloid‐PET, magnetic resonance imaging (MRI), and neuropsychological evaluation. All individuals were over 50 years of age. Participants’ evaluations included a review of their medical history, an assessment from their informant, a neurological examination by a physician, and a neuropsychological examination. More details on the information gathered from participants can be found here: https://triad.tnl‐mcgill.com/. CU individuals are defined as having no cognitive impairment. 16 They were asked to report subjective memory complaints. Consistent with the biological AD research framework from the National Institute on Aging‐Alzheimer's Association, 12 participants without a diagnosis of MCI or AD with subjective memory complaints were analyzed with CU individuals. In addition to standard clinical assessments, Mini‐Mental State Examination (MMSE), and Clinical Dementia Rating (CDR) total scores were used to define operationally MCI as MMSE 26 or above and CDR 0.5, 17 and dementia due to AD as MMSE lower than 26 and CDR above 0.5. 18 No participant met the criteria for another neurological or major neuropsychiatric disorder. Participants with current evidence, or history within 2 years prior to screening, of a psychotic disorder, major depressive disorder, alcoholism, or drug dependency/abuse were excluded from the study to ascertain the absence of comorbidities or psychiatric disorders that could confound the results.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (e.g., PubMed) sources and meeting abstracts and presentations. Recent positron emission tomography imaging studies linked the cognitive dysfunctions with pathological hallmarks of Alzheimer's disease; however, there has been little research regarding the presence of neuropsychiatric symptoms (NPS) and core disease processes.
Interpretation: Our findings suggest that NPS are related to tau only, and that each NPS is linked to a specific tau deposition closely related to the symptom's features.
Future directions: The article proposes a framework for the generation of new hypotheses and the conduct of additional studies. Future directions include longitudinal assessment to study the causality in this relationship, as well as the study of anti‐tau drugs to help decrease or delay NPS, and reduce the caregiver burden.
The study was approved by the Douglas Mental Health Institute Research Ethics Board (Montreal, QC, Canada). Written consent was obtained from all participants. Time between all acquisitions did not exceed 3 months.
PET scans were obtained with a Siemens High Resolution Research Tomograph (HRRT). Acquisition time was 90 to 110 minutes post‐injection for [18F]MK6240, and 40 to 70 minutes post‐injection for [18F]AZD4694. Participants were also subjected to an MRI scan, which underwent an optimized Voxel‐Based Morphometry (VBM) protocol. More detailed information regarding PET and MRI imaging processing can be found in the supporting information.
2.2. Neuropsychiatric symptoms assessment
Participants were asked to name an informant to complete questionnaires. They primarily named their partner or an adult child.
The informants completed English or French versions of the NPI‐Q. 19 Based on the Neuropsychiatric Inventory (NPI), 20 which requires an interview between the informant and a clinician, the NPI‐Q has been accepted as a brief, reliable, informant‐based assessment of NPS. 19 The NPI‐Q has been validated in clinical and research settings, 19 and is now widely used in studies investigating NPS. 21 It assesses 12 NPS from the informant's perspective: hallucinations, delusions, agitation, depression, anxiety, elation, apathy, disinhibition, irritability, motor disturbance, night‐time behavior, and appetite. Respondents first identify presence of changes regarding the symptoms in the last 4 weeks, by answering “yes” or “no,” and subsequently rate their severity, defined as how they think these changes affect the participant, on a scale of 1 to 3.
By summing the severity scores of the NPI‐Q domains, we obtain an overall numerical severity score (with symptoms rated as “no” having a score of 0). The possible total scores for the NPI‐Q range from 0 to 36.
2.3. Statistical analyses
Statistical analyses were performed using R Statistical Software Package, neuroimaging analyses were conducted using the VoxelStats 22 (Matlab 9.4).
Demographics underwent multiple t‐tests for continuous variables and χ2 tests for categorical variables. NPI‐Q scores were considered continuous variables in all tests.
Voxel‐wise regression models were conducted to explore the relationship between NPI‐Q score and brain Aβ deposition, tau deposition, and neurodegeneration, as shown by [18F]AZD4694 amyloid‐PET, [18F]MK6240 tau‐PET uptake, and VBM, respectively. Regression analyses for each voxel were executed. Due to recent studies implicating sex differences in the topographical distribution of AD pathology, 23 we corrected amyloid‐PET, tau‐PET, and VBM voxel‐wise analyses for sex. Analyses were also corrected for age, years of education, and diagnosis. We subsequently conducted domain analyses for the 12 NPI‐Q domains, again, all corrected for sex, age, years of education, and diagnosis. Finally, the parametric images were corrected for multiple comparisons using a random field theory (RFT) correction with a voxel‐level correction of P < .001 and cluster‐level correction of P < .05.
Using the RFT‐corrected images, we conducted a cluster‐based regression analysis for the NPI‐Q severity score. Details can be found in the supporting information.
Exploratory analyses were conducted on CU individuals only, cognitively impaired individuals only, Aβ‐positive individuals only. The same voxel‐based regression model as above was conducted, adding either Aβ status or VBM images as a covariate. Individuals were classified as Aβ positive if their neocortical [18F]AZD4694 uptake exceeded a published threshold of 1.55. 15 Analyses were also conducted in subsamples of amyloid‐positive in each diagnostic group, as well as the whole sample removing MCI Aβ‐negative individuals. Finally, we looked at the relationship between NPI‐Q subsyndromes and AD pathophysiologies. 24
3. RESULTS
Two hundred and twenty‐one individuals (143 CU, 52 MCI, and 26 dementia due to AD) were included in the study, aged between 51 and 85 years; 108 (48.87%) had changes in NPS over the last 4 weeks, among which 44 CU (30.77%), 39 MCI (75.99%), and 25 (96.15%) dementia due to AD. There were no significant differences between the diagnostic groups regarding age and years of education. However, significant differences between groups were observed in MMSE, CDR, Aβ and tau status, and atrophy score, as well as [18F]MK6240 cluster‐based standardized uptake value ratio (SUVR) and [18F]MK6240 Braak stages SUVR (Braak stages 1–2, 3–4, and 5–6). A weak difference was found regarding sex (Table 1).
TABLE 1.
Demographic characteristics of the sample
| Overall | CU | MCI | AD | P | |
|---|---|---|---|---|---|
| N | 221 | 143 | 52 | 26 | |
| N symptomatic (%) | 108 (48.87) | 44 (30.77) | 39 (75.99) | 25 (96.15) | <0.001 |
| Age, mean (SD) | 71.59 (6.45) | 72.15 (5.68) | 71.75 (7.49) | 68.21 (7.40) | 0.016 |
| Male, n (%) | 92 (41.63) | 49 (34.27) | 30 (57.69) | 13 (50.00) | 0.011 |
| Education, mean (SD) | 15.25 (3.62) | 15.44 (3.55) | 15.14 (3.99) | 14.42 (3.26) | 0.409 |
| MMSE, mean (SD) | 27.59 (4.09) | 29.01 (1.21) | 27.75 (1.87) | 19.32 (7.15) | <0.001 |
| CDR, mean (SD) | 0.25 (0.41) | 0.00 (0.00) | 0.50 (0.00) | 1.08 (0.51) | <0.001 |
| Amyloid positivity (%)a | 89 (40.3) | 30 (21.0) | 33 (63.5) | 26 (100.0) | < 0.001 |
| Tau positivity (%)b | 80 (37.9) | 27 (18.9) | 29 (55.8) | 24 (92.3) | < 0.001 |
|
[18F]MK6240 Parietal/occipital/temporal/ frontal SUVR, mean (SD) |
1.23 (0.35) | 1.12 (0.13) | 1.19 (0.21) | 1.89 (0.61) | < 0.001 |
| [18F]MK6240 Braak 1 and 2 SUVR, mean (SD) | 1.14 (0.38) | 0.97 (0.18) | 1.35 (0.46) | 1.63 (0.40) | < 0.001 |
| [18F]MK6240 Braak 3 and 4 SUVR, mean (SD) | 1.33 (0.65) | 1.07 (0.12) | 1.44 (0.69) | 2.51 (0.91) | < 0.001 |
| [18F]MK6240 Braak 5 and 6 SUVR, mean (SD) | 1.28 (0.51) | 1.10 (0.14) | 1.28 (0.38) | 2.24 (0.87) | < 0.001 |
| Atrophy score, mean (SD)c | 2.87 (0.19) | 2.94 (0.13) | 2.84 (0.18) | 2.57 (0.21) | < 0.001 |
Abbreviations: AD, Alzheimer's disease; CDR, Clinical Dementia Rating; CU, cognitively unimpaired; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; SD, standard deviation; r SUVR, standardized uptake value ratios.
aBased on Therriault et al. 15
Based on visual rating.
Based on Jack et al. 25
3.1. NPI‐Q results
NPI‐Q results were collected for all individuals. The mean severity score across groups was 1.90 ± 2.93. A summary of the NPI‐Q results can be found in Table 2. The most common symptoms were night‐time behavior (person waking up at night, taking excessive naps), followed by irritability (such as having difficulty coping with delays), depression (participant seemingly sad), appetite (such as if person lost or gained weight), anxiety (participant upset when separated from the primary informant), apathy (participant less interested in their activities), agitation (resistant to help), disinhibition (impulsive acts), motor disturbance (such as pacing around the house), elation (participant acting excessively happy), hallucinations (false visions and voices), and delusions (false beliefs). All domain scores were significantly different across groups (P < .005). A correlation matrix was created to assess the relationship among the different NPS (Figure S1 in supporting information).
TABLE 2.
NPI‐Q severity scores
| NPI‐Q scores, mean (SD) | Overall | CU | MCI | AD | P |
|---|---|---|---|---|---|
| NPI‐Q Severity | 1.90 (2.93) | 0.85 (1.88) | 3.09 (3.02) | 5.31 (3.95) | <0.001 |
| Delusions severity | 0.02 (0.18) | 0.00 (0.00) | 0.02 (0.14) | 0.15 (0.46) | <0.001 |
| Hallucinations severity | 0.03 (0.22) | 0.01 (0.08) | 0.02 (0.14) | 0.19 (0.57) | <0.001 |
| Agitation severity | 0.12 (0.43) | 0.04 (0.32) | 0.17 (0.47) | 0.42 (0.70) | <0.001 |
| Depression severity | 0.27 (0.59) | 0.15 (0.46) | 0.37 (0.60) | 0.77 (0.91) | <0.001 |
| Anxiety severity | 0.19 (0.52) | 0.06 (0.29) | 0.37 (0.69) | 0.62 (0.80) | <0.001 |
| Elation severity | 0.02 (0.16) | 0.01 (0.16) | 0.06 (0.23) | 0.08 (0.27) | 0.039 |
| Apathy severity | 0.14 (0.42) | 0.03 (0.22) | 0.17 (0.43) | 0.65 (0.75) | <0.001 |
| Disinhibition severity | 0.09 (0.37) | 0.02 (0.19) | 0.15 (0.50) | 0.35 (0.63) | <0.001 |
| Irritability severity | 0.29 (0.63) | 0.17 (0.51) | 0.50 (0.80) | 0.54 (0.71) | <0.001 |
| Motor disturbance severity | 0.08 (0.38) | 0.01 (0.08) | 0.13 (0.44) | 0.38 (0.85) | <0.001 |
| Night‐time behavior severity | 0.40 (0.74) | 0.27 (0.63) | 0.75 (0.95) | 0.46 (0.65) | <0.001 |
| Appetite severity | 0.23 (0.55) | 0.08 (0.37) | 0.38 (0.66) | 0.69 (0.78) | <0.001 |
Abbreviations: CU, cognitively unimpaired; NPI‐Q, Neuropsychiatric Inventory Questionnaire.
3.2. Spearman correlations and voxel‐based regression models
Spearman correlations were conducted among NPI‐Q severity score with [18F]AZD4694 global SUVRs. A significant correlation was found between [18F]AZD4694 uptake and NPI‐Q severity score (R = 0.36, P = < .001; Figure 1).
FIGURE 1.
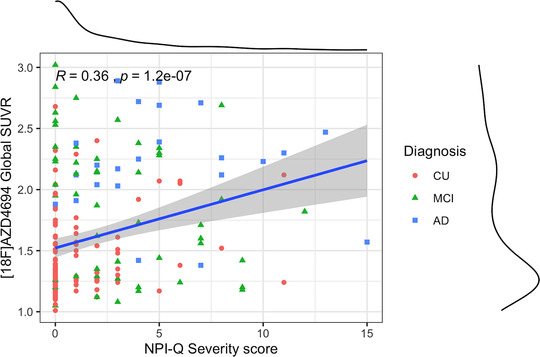
Spearman correlations using [18F]AZD4694 global standardized uptake value ratio (SUVR) with Neuropsychiatric Inventory Questionnaire (NPI‐Q) global severity score. [18F]AZD4694 showed significant correlation with NPI‐Q global severity score (R = 0.36 and P < .001)
Voxel‐based regression models were conducted to evaluate the relationship between global NPI‐Q severity and amyloid‐PET with [18F]AZD4694. No results survived correction for multiple comparisons using RFT. When studying the NPI‐Q domains in relation to [18F]AZD4694 scans, most results did not survive RFT correction, except anxiety and motor disturbance. The anxiety domain presented [18F]AZD4694 uptake in the medial superior frontal gyrus, while motor disturbance uptake was in the pre/postcentral gyrus (Figure S2 in supporting information).
Results from voxel‐based regression models between NPI‐Q global and domain severity scores, and neurodegeneration using VBM images, did not survive RFT correction.
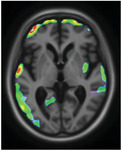
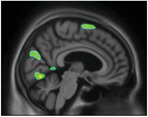
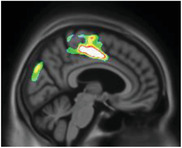
However, voxel‐based regression models demonstrated that NPI‐Q severity score was positively associated with [18F]MK6240 uptake. The regions in which the associations were the strongest were the cuneus, parietal association area, superior temporal lobe, and superior frontal gyrus (Figure 2). There were strong correlations between NPI‐Q severity score and [18F]MK6240 SUVR in the parietal, occipital, temporal, and frontal clusters (R = 0.36, P = < .001; Figure 3). Additionally, NPI‐Q global severity score correlated positively with [18F]MK6240 SUVR values at Braak stages 1–2, Braak stages 3—4, and Braak stages 5–6 (Figure S3 in supporting information).
FIGURE 2.
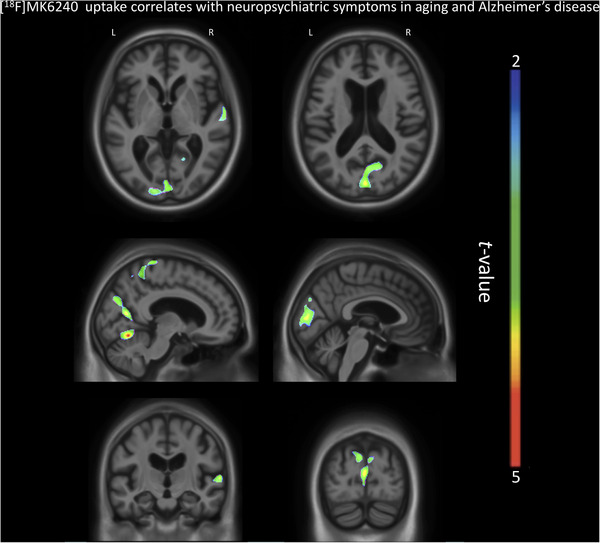
[18F]MK6240 SUVR correlates with Neuropsychiatric Inventory Questionnaire (NPI‐Q) severity score in aging and Alzheimer's disease. The regions showing strong associations with [18F]MK6240 uptake were the cuneus, the parietal association area, the occipital and temporal lobes, and the superior frontal gyrus
FIGURE 3.
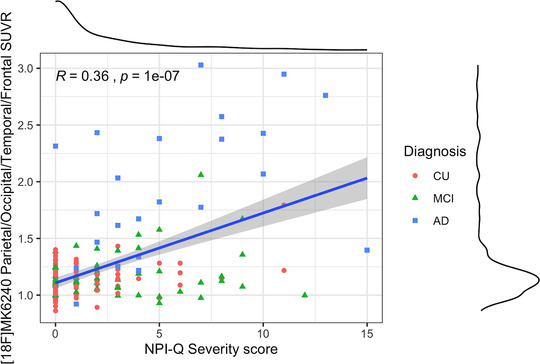
Cluster‐based regression analysis with Neuropsychiatric Inventory Questionnaire (NPI‐Q) severity score. The linear regression showed a strong correlation in aging and Alzheimer's disease (AD). CU, cognitively unimpaired; MCI, mild cognitive impairment; SUVR, standardized uptake value ratio
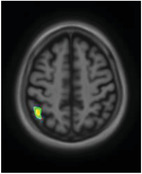
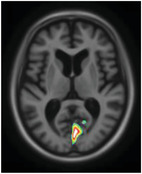
We subsequently observed differences in [18F]MK6240 uptake along the various domains of the NPI‐Q (Figure 4). Agitation was related to tau‐PET uptake in the orbitofrontal, ventromedial prefrontal cortices, temporal lobes, and precuneus. The anxiety domain showed a positive correlation with uptake in the cuneus, superior frontal gyrus, and parahippocampal gyrus. Apathy correlated with [18F]MK6240 uptake in the superior parietal association area. Delusions showed cuneus/occipital uptake. Depression presented uptake in the superior temporal gyrus and superior parietal association area. Moreover, disinhibition was positively related with uptake in the medial occipital, superior temporal lobes, and parahippocampal gyrus. Elation presented uptake in the precuneus, anterior cingulate, medial frontal, and parahippocampal regions. Hallucinations were related to [18F]MK6240 uptake in the medial occipital cortex. Irritability was related with frontal pole uptake, while motor disturbance associated with uptake in the pre/postcentral region, parietal association area, medial occipital lobe, and cingulate gyrus. Finally, night‐time behavior was associated with medial frontal uptake. Appetite was the only domain which did not show a significant correlation with [18F]MK6240 uptake. A summary of the topographical relationships between [18F]MK6240 and various NPS domains can be found Table 3. Results were similar when analyses were repeated with images that underwent partial volume correction. Moreover, no significant negative relationship was observed between [18F]MK6240 and NPI‐Q results.
FIGURE 4.
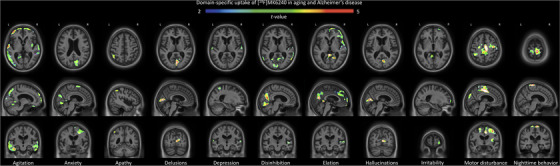
[18F]MK6240 uptake in domain‐specific analyses of the Neuropsychiatric Inventory Questionnaire (NPI‐Q). There were different patterns of association of [18F]MK6240 uptake with NPI‐Q domains; agitation was related to tau‐positron emission tomography uptake in the orbitofrontal cortex, ventromedial prefrontal cortex, the temporal lobes, and the precuneus. The anxiety domain showed a positive correlation with uptake in the cuneus, the superior frontal gyrus, and the parahippocampal gyrus. Apathy correlated with [18F]MK6240 uptake in the parietal association area. Delusions, however, showed cuneus/occipital uptake. Depression presented uptake in the superior temporal gyrus and the parietal association area. Moreover, disinhibition was positively related with uptake in the occipital and temporal lobes and parahippocampal gyrus. Elation on the other end presented uptake in the precuneus, anterior cingulate, medial frontal, and parahippocampal regions. In the case of hallucinations, [18F]MK6240 presented signals in the occipital lobe. Irritability was related to frontal pole uptake, while motor disturbance was associated with uptake in pre/postcentral region, as well as parietal association area, occipital, and cingulate. Finally, regarding night‐time behavior, the severity score was associated with uptake of the tracer in the medial frontal region
TABLE 3.
Summary of the NPI‐Q domain results with [18F]MK6240 uptake, and relation with brain regions
| Symptom | Brain image | Region | Role of region |
|---|---|---|---|
| Agitation |
|
Ventromedial prefrontal cortex Orbitofrontal cortex Precuneus Temporal lobe |
Processing of risk, fear and emotional responses Emotion and memory Recollection and memory Emotion association |
| Anxiety |
|
Cuneus Superior frontal Parahippocampal gyrus |
Vision‐related region, inhibition Working memory and executive processing Memory formation |
| Apathy |
|
Parietal association area | Association of sensory information |
| Delusions |
|
Cuneus | Vision‐related region, inhibition |
| Depression |
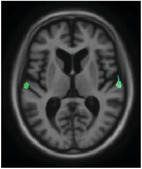
|
Parietal association area Superior temporal gyrus |
Association of sensory information Emotional processing and social cognition |
| Disinhibition |
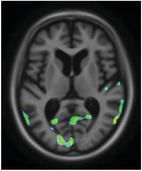
|
Superior temporal gyrus Cuneus Medial parietal Parahippocampus |
Emotional processing and social cognition Vision related, inhibition Sensory information processing Memory formation |
| Elation |
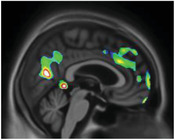
|
Precuneus Cingulate Medial frontal Parahippocampus |
Recollection and memory Attention, decision making, and emotions Behavior regulation Memory formation |
| Hallucinations |
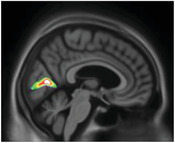
|
Cuneus | Vision‐related region, inhibition |
| Irritability |
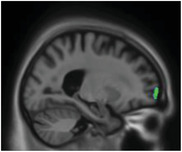
|
Frontal pole | Behavior regulation |
| Motor disturbance |
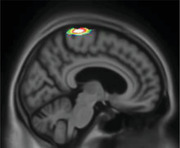
|
Pre‐/post‐central gyrus Parietal association area Occipital pole Cingulate |
Motor‐relation region Association of sensory information Information processing Attention, decision making, and emotions |
| Night‐time behavior |
|
Superior frontal gyrus | Behavior regulation |
Abbreviation: NPI‐Q, Neuropsychiatric Inventory Questionnaire.
Exploratory analyses were conducted on cognitively unimpaired individuals only (Figure S4 in supporting information); showing tau‐PET uptake in regions each associated to symptomatic networks. Analyses on 6 out of 12 NPS did not survive correction for multiple comparison. Results from cognitively impaired individuals (MCI and AD), showed [18F]MK6240 uptake in broader regions compared to the main findings, including the temporal, medial frontal, precuneus, and cuneus (Figure S5 in supporting information). Results from analyses on Aβ‐positive individuals were similar to the main findings; they slightly differed for two NPS, probably because of lower statistical power (Figure S6 in supporting information). When correcting for Aβ status, results were similar to the main findings, except for two NPS (Figure S7 in supporting information). When correcting for VBM images, results were identical to the main findings. The analyses conducted in the amyloid‐positive individuals of each diagnostic group presented patterns of tau uptake related to each NPI‐Q domain (Figure S8 in supporting information). In the sample removing MCI Aβ‐negative individuals, the results were similar except for two domains (Figure S9 in supporting information). Finally, NPI‐Q subsyndromes correlated with tau‐tracer uptake, rather than amyloid‐tracer or neurodegeneration (Figure S10 in supporting information).
4. DISCUSSION
Our study provides evidence that NPS seem to be predominantly related to tau pathology, rather than Aβ or neurodegeneration, in aging and dementia due to AD. It builds on previous studies presenting relationships between NPS and CSF phosphorylated‐tau 26 by identifying the regional contributions of cerebral tau pathology to specific NPS. Other studies were conducted regarding NPS in CU individuals 13 and AD patients 14 separately, with additional investigations showing conflicting results between CSF and NPS. 27 However, our research included participants that were AD‐biomarker‐negative CU, AD‐biomarker‐positive CU, MCI, and AD patients, and using the latest advances in neuroimaging and pathophysiology assessment. These findings highlight the importance of assessing NPS, which can appear before cognitive alterations 4 and may predict the onset of cognitive symptoms; which is why focusing on a broad spectrum of individuals is an advantage.
We observed that tau predominantly is related to NPS, not Aβ or neurodegeneration, in aging and dementia due to AD. The results fit with the evolving framework that tau appears to be closely related to cognitive symptoms in AD, 28 rather than Aβ. Correcting for Aβ status did not alter the results, suggesting that it does not interfere with the presence of NPS. Associations with tau deposition have been found in brain regions vulnerable to early AD pathophysiologies, and to behavior problems. 29 Regions such as precuneus and medial frontal cortex have similarly been correlated with neuropsychological symptoms such as depression and anxiety, using functional MRI and diffusion tensor imaging studies. 9 Research on AD patients showed that NPS were associated with glucose metabolism changes, in core regions of behavioral control, as the frontal, temporal lobes, and paralimbic regions. 30 The finding that NPS are related to tau deposition in these brain regions suggests a link between specific brain areas affected by early AD pathophysiologies and regions involved in behavioral control.
Results indicated that NPS were associated with tau deposition in the parietal association area, precuneus, and temporal cortex, regions related to memory formation and retrieval. AD patients present lower glucose consumption in those areas, 29 usually starting with a slight decrease in mildly impaired patients, and larger reductions as disease progresses. It has been noted that disruptions in these regions begin years before clinical signs of dementia. 29 The regions are first affected by Aβ build‐up and later on other AD‐related pathophysiologies converge. Knowing that tau predominantly is related to NPS can help in disease management, and the study of anti‐tau drugs to prevent, or delay, NPS appearance.
Elevated tau‐PET was observed in brain areas subserving specific functional processes interrogated by each NPI‐Q domain. Some regions affected have been previously shown to be impaired with other imaging methods. 9 , 10 , 31 Emerging data support the idea that specific tau patterns are related to specific cognitive symptoms, 28 with previous research linking distinct NPS to patterns of tau uptake. 11 , 32 Our study extends these findings by providing evidence that regional patterns of tau aggregation are related to different NPS. Taken together, these studies support a framework in which the topographical distribution of tau pathology is related to heterogeneity of symptoms in AD. Ossenkoppele et al. discovered that regions presenting the highest tau‐PET uptake corresponded to the nature of the symptoms: worse visuospatial performances correlated with tau deposition in the occipital lobe, lower memory performances with higher tau deposition in the hippocampus. 28 Our research focused on the different types of NPS individuals in aging and AD suffer from, rather than neuropsychological presentations. We discovered that tau deposition patterns seemed to be closely related to the symptoms’ features.
The agitation domain showed tau uptake in paralimbic regions, where an association was observed with post mortem studies. 10 Another investigation found lower glucose metabolism in the temporal and frontal regions of AD patients. 33 Our study follows the framework, adding in vivo anatomical correlates of [18F]MK6240 uptake when participants present agitation symptoms.
Agitation and anxiety evolve closely together in AD, 9 and have already been related to cuneus impairment. 31 , 34 The superior medial frontal gyrus showed an uptake of both tau and amyloid‐PET when individuals presented anxiety symptoms. This region is known to be important in the frontoparietal‐executive network, related to anxiety as well. 35 The combination of both Aβ deposition and tau might aggravate the anxiety symptoms.
Apathy, a late AD symptom, 7 presents different findings. The parietal lobe is related to apathetic symptoms, 36 and more specifically negative mood. 37 This corroborates the idea that tau deposition in parietal regions is linked to a negative symptom such as apathy.
Delusions are related to lower glucose metabolism in the occipital/cuneus region of AD patients. 38 , 39 It has been proposed as a core part of emotional processing; impairment of the brain region due to tau might lead to the symptoms’ development.
Depression correlated with tau in the lateral superior temporal gyrus, crucial in emotional processing and social cognition, and previously related to GM atrophy. 40 The parietal association area is commonly affected in AD, and was not previously linked to depressive symptoms. These findings, however, corroborate with recent research showing an association between depressive symptoms and CSF phosphorylated‐tau. 41
Disinhibition has previously been associated with hypometabolism in temporal and occipital lobes. 42 Moreover, the cuneus is heavily impacted in bipolar patients showing strong disinhibition, with a decrease in GM volume. 43 Finally, the parahippocamal gyrus is a core AD region affected by the disease's pathophysiological changes.
Elation is rare in AD, 20 and poorly understood. However, brain circuits have been studied with regard to elation in schizophrenic patients. These studies show parahippocampal and cingulate volume reduction, 44 as well as frontal abnormalities. 45 These regions are crucial for emotional processing. Elation symptoms in our study were related to tau deposition in these regions.
Hallucinations have previously been related to occipital lobe atrophy, 39 , 46 a core region for the visual system. Tau deposition in this region might cause further impairments.
Anatomical correlates of irritability have been elusive, linking it to decreased volume of the insula only. 47 We report that tau pathology in the frontal pole was related to irritability, a region involved in complex behavior; 48 impairment due to tau deposition might lead to irritable traits.
Motor disturbance showed tau uptake in the cingulate cortex and motor‐related regions. The latter were previously shown to exhibit tissue loss in individuals with aberrant motor behaviors. 49 The occipital and parietal association areas are critical for sensory information processing. In our study, amyloid‐PET also showed uptake in the motor regions of the brain. Tau deposition might impair more broadly all those brain areas, and the combination with Aβ exacerbates motor disturbances.
There is no previous research studying sleep disturbances anatomically in AD. However, it was observed that the metabolism of the medial frontal cortex is decreased after sleep deprivation, 50 which is the region showing correlation between sleep symptoms and tau deposition in this study.
To summarize, the present study reveals the predominant link between tau and NPS, and supplies additional information regarding the anatomical correlates of common NPS found in aging and dementia due to AD. Indeed, the relationship is seen across a cohort of AD‐biomarker‐negative individuals suffering from AD. The different patterns of tau deposition might explain the heterogeneity of NPS, similar to tau deposition explaining the different neuropsychological disturbances of AD. 28 Additional studies conducted on NPS showed the involvement of other brain regions. 39 The complexity of the NPS networks does not allow us to study the whole array of biological underpinnings associated with them. 51 However, we relate here the current findings to previous studies, which corroborate the idea of tau predominance in the emergence and severity of NPS, with distinct patterns related to specific NPS.
We were first surprised to find little to no correlation between NPS and Aβ deposition or neurodegeneration. However, growing evidence presents tau as being the cause of clinical symptoms. 28 When we conducted similar analyses adding Aβ status or VBM images as a covariate, results did not differ; even though Aβ is known to appear before tau along the AD spectrum. 12 It is important to note that CU individuals are still known to show a certain amount of neurofibrillary tangles. 52 NPS have already been linked to tau deposition in the brain of CU individuals. 14 , 32 The levels of pathology might not be sufficient to cause cognitive problems observed in MCI and AD; however, it might lead to mild NPS in some individuals. Finally, NPS are of heterogeneous origin. In CU elderly individuals, the appearance of NPS cannot be immediately interpreted as the presence of neocortical tau pathology.
The main limitation of the study is the use of the NPI‐Q and not the NPI; the latter enables observation of more subtle NPS changes from the informant's perspective. In the case of the NPI‐Q the informant could over‐ or under‐report NPS, though previous research supports that it is highly accurate. 19 Second, while the NPI‐Q evaluates a wide spectrum of NPS seen in AD, it does not sample all abnormal behaviors. Finally, PET research always involves off‐target binding due to the tracers as well as artefacts; in our study it is unlikely to that results are driven by them as the regions mentioned in our article are not known off‐target binding sites for [18F]MK6240. 53
Considering these limitations, the study provides empirical evidence that NPS are predominantly related to tau. Correspondingly, there is hope that disease‐modifying interventions will not only aid in the cognitive symptoms related to AD but also aid in NPS. Another important aspect is the idea that different NPS show distinct patterns of tau uptake. Indeed, increased tau‐PET is seen in regions subserving the specific functional processes of each NPS. However, other brain regions might also be involved in the complex biological underpinnings of each NPS. As NPS cause a great burden on the caregivers, and are also a marker of disease progression, understanding their origin and focusing on treatments to prevent or delay them is crucial. The next step is studying NPS longitudinally, to make a causal relationship between tau and the emergence of neuropsychiatric problems.
CONFLICTS OF INTEREST
C. Tissot, J. Therriault, T.A. Pascoal, M. Chamoun, F. Lussier, M. Savard, S. Mathotaarachchi, A.L. Benedet, E.M. Thomas, M. Parsons, Z. Nasreddine, P. Rosa‐Neto, and S. Gauthier report no disclosures relevant to the article.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank all participants of the present study as well as all members of staff of the McGill Center for Studies in Aging for their role in data collection. The authors would also like to thank Dean Jolly, Alexey Kostikov, Monica Samoila‐Lactatus, Karen Ross, Mehdi Boudjemeline, and Sandy Li for their assistance with radiochemistry production. PR‐N is supported by the Weston Brain Institute, the Canadian Institutes of Health Research (CIHR; MOP‐11‐51‐31, PR N), the Alzheimer's Association (NIRG‐12‐ 92090, NIRP‐12‐259245, PR‐N), Fonds de Recherche du Québec—Santé (FRQS; Chercheur Boursier, 2020‐VICO‐279314). TAP, PR‐N, and SG are members of the CIHR‐CCNA Canadian Consortium of Neurodegeneration in Aging.
Tissot C, Therriault J, Pascoal TA, et al. Association between regional tau pathology and neuropsychiatric symptoms in aging and dementia due to Alzheimer's disease.. Alzheimer's Dement. 2021;7:e12154. 10.1002/trc2.12154
REFERENCES
- 1. Steinberg M, Shao H, Zandi P, et al. Point and 5‐year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyketsos CG, Lopez OL, Jones B, Fitzpatrick AL, Breitner J, DeKosky ST. Prevalence of neuropsychiatric symptoms results from the cardiovascular health study. Jama. 2002;288:1475‐1483. [DOI] [PubMed] [Google Scholar]
- 3. Herrmann N, Lanctôt KL. The contribution of neuropsychiatric symptoms to the cost of dementia care. Int J Geriatr Psychiatry. 2007:874‐882. [DOI] [PubMed] [Google Scholar]
- 4. Petry S, Cummings JL, Hill MA, Shapira J. Personality Alterations in Dementia of the Alzheimer Type. JAMA Neurol. 1988;45:1187‐1190. [DOI] [PubMed] [Google Scholar]
- 5. Gauthier S, Cummings JL, Ballard C, et al. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatrics. 2010;22:346‐372. [DOI] [PubMed] [Google Scholar]
- 6. Peters ME, Schwartz S, Han D, et al. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer's dementia and death: the cache county dementia progression study. Am J Psychiatry. 2015;172:460‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reisberg B, Borenstein J, Salob SP, Ferris SH. Behavioral symptoms in Alzheimer's disease: phenomenology and treatment. J Clin Psychiatry. 1987;48:9‐15. [PubMed] [Google Scholar]
- 8. Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer's disease. I: disorders of thought content. Br J Psychiatry. 1990;157:72‐76. [DOI] [PubMed] [Google Scholar]
- 9. Rosenberg PB, Nowrangi MA, Lyketsos CG. Neuropsychiatric symptoms in Alzheimer's disease: what might be associated brain circuits?. Mol Aspects Med. 2015;43‐44:25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tekin S, Mega MS, Masterman DM, et al. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49:355‐361. [PubMed] [Google Scholar]
- 11. Guadagna S, Esiri MM, Williams RJ, Francis PT. Tau phosphorylation in human brain: relationship to behavioral disturbance in dementia. Neurobiol Aging. 2012;33:2798‐2806. [DOI] [PubMed] [Google Scholar]
- 12. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's. Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lussier FZ, Pascoal TA, Chamoun M, et al. Mild behavioral impairment is associated with β‐amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimer's. Dement. 2020;16:192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pichet Binette A, É Vachon‐Presseau, Morris JC, et al. Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms, and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer's disease. Biol Psychiatry. 2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Therriault J, Benedet AL, Pascoal TA, et al. Determining Amyloid‐Beta positivity using [18F]AZD4694 PET imaging. J Nucl Med. 2020;62(2):247–252. 10.2967/jnumed.120.245209. [DOI] [PubMed] [Google Scholar]
- 16. Jack CR, Wiste HJ, Schwarz CG, et al. Longitudinal tau PET in ageing and Alzheimer's disease. Brain. 2018;141:1517‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183‐194. [DOI] [PubMed] [Google Scholar]
- 18. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's. Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233‐239. [DOI] [PubMed] [Google Scholar]
- 20. Cummings JL, Carusi DA, Mega MS, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308‐2314. [DOI] [PubMed] [Google Scholar]
- 21. Trzepacz PT, Saykin A, Yu P, et al. Subscale validation of the neuropsychiatric inventory questionnaire: comparison of alzheimer's disease neuroimaging initiative and national alzheimer's coordinating center cohorts. Am J Geriatr Psychiatry. 2013;21:607‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathotaarachchi S, Wang S, Shin M, et al. VoxelStats: a MATLAB package for multi‐modal voxel‐wise brain image analysis. Front Neuroinform. 2016;10:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76:542‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aalten P, Verhey FRJ, Boziki M, et al. Neuropsychiatric syndromes in dementia: results from the European Alzheimer's disease Consortium: part I. Dement Geriatr Cogn Disord. 2007;24:457‐463. [DOI] [PubMed] [Google Scholar]
- 25. Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, Petersen RC. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's Dement. 2017;13(3):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinosaki K, Nishikawa T, Takeda M. Neurobiological basis of behavioral and psychological symptoms in dementia of the Alzheimer's type. Psychiatry Clin Neurosci. 2000;54:611‐620. [DOI] [PubMed] [Google Scholar]
- 27. Banning LCP, Ramakers IHGB, Deckers K, Verhey FRJ, Aalten P. Affective symptoms and AT(N) biomarkers in mild cognitive impairment and Alzheimer's disease: a systematic literature review. Neurosci Biobehav Rev. 2019;107:346‐359. [DOI] [PubMed] [Google Scholar]
- 28. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016;139:1551‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buckner RL, Andrews‐Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1‐38. [DOI] [PubMed] [Google Scholar]
- 30. Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer's disease. Hum Brain Mapp. 2016;37:4234‐4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 2010;167(5):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Babulal G, Roe CM, Stout SS, et al. Depression is associated with tau and not amyloid positron emission tomography in cognitively normal adults. J Alzheimer's Dis. 2020;74:1045‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weissberger GH, Melrose RJ, Narvaez TA, Harwood D, Mandelkern MA, Sultzer DL. 18F‐Fluorodeoxyglucose Positron emission tomography cortical metabolic activity associated with distinct agitation behaviors in Alzheimer's disease. Am J Geriatr Psychiatry. 2017;25:569‐579. [DOI] [PubMed] [Google Scholar]
- 34. Kimbrell TA, George MS, Parekh PI, et al. Regional brain activity during transient self‐induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454‐465. [DOI] [PubMed] [Google Scholar]
- 35. Liao W, Chen H, Feng Y, et al. Selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 2010;52:1549‐1558. [DOI] [PubMed] [Google Scholar]
- 36. Ota M, Sato N, Nakata Y, Arima K, Uno M. Relationship between apathy and diffusion tensor imaging metrics of the brain in Alzheimer's disease. Int J Geriatr Psychiatry. 2012;27:722‐726. [DOI] [PubMed] [Google Scholar]
- 37. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic‐cortical function and negative mood: converging PET findings in depression and normal sadness. Depress Sci Ment Heal. 2013;6:245‐253. [DOI] [PubMed] [Google Scholar]
- 38. Hirono N, Mori E, Ishii K, et al. Alteration of regional cerebral glucose utilization with delusions in Alzheimer's disease. J Neuropsychiatry Clin Neurosci 1998;10:433–9. [DOI] [PubMed] [Google Scholar]
- 39. Boublay N, Schott AM, Krolak‐Salmon P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer's disease: a review of 20 years of research. Eur J Neurol. 2016;23:1500‐1509. [DOI] [PubMed] [Google Scholar]
- 40. Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roe CM, Fagan AM, Grant EA, Holtzman DM, Morris JC. CSF biomarkers of Alzheimer disease: “Noncognitive” outcomes. Neurology. 2013;81:2028‐2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levin HS, Goldstein FC, Williams DH, Eisenberg HM. The contribution of frontal lobe lesions to the neurobehavioral outcome of closed head injury. New York, NY, US: Oxford University Press; 1991:318‐338. [Google Scholar]
- 43. Haldane M, Cunningham G, Androutsos C, Frangou S. Structural brain correlates of response inhibition in Bipolar Disorder I. J Psychopharmacol. 2008;22:138‐143. [DOI] [PubMed] [Google Scholar]
- 44. Brown R, Colter N, Corsellis N, et al. Postmortem evidence of structural brain changes in schizophrenia. Arch Gen Psychiatry. 1986;43:36. [DOI] [PubMed] [Google Scholar]
- 45. Chan WY, Yang GL, Chia MY, et al. Cortical and subcortical white matter abnormalities in adults with remitted first‐episode mania revealed by tract‐based spatial statistics. Bipolar Disord. 2010;12:383‐389. [DOI] [PubMed] [Google Scholar]
- 46. Holroyd S, Shepherd ML, Downs JH. Occipital atrophy is associated with visual hallucinations in Alzheimer's disease. J Neuropsychiatr. 2000:25‐28. [DOI] [PubMed] [Google Scholar]
- 47. Moon Y, Moon WJ, Kim H, Han SH. Regional atrophy of the insular cortex is associated with neuropsychiatric symptoms in alzheimer's disease patients. Eur Neurol. 2014;71:223‐229. [DOI] [PubMed] [Google Scholar]
- 48. Koechlin E, Hyafil A, Anterior prefrontal function and the limits of human decision‐making. Science 2007;318(5850):594‐598. [DOI] [PubMed] [Google Scholar]
- 49. Rosen HJ, Allison SC, Schauer GF, Gorno‐Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783‐2792. [DOI] [PubMed] [Google Scholar]
- 51. Darby RR, Joutsa J, Fox MD. Network localization of heterogeneous neuroimaging findings. Brain. 2019;142:70‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19:687‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pascoal TA, Shin M, Kang MS, et al. In vivo quantification of neurofibrillary tangles with [ 18 F]MK‐6240. Alzheimer's Res Ther. 2018;10:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


