Abstract
Background
Patients hospitalized with coronavirus disease 2019 (COVID-19) often have abnormal findings on transthoracic echocardiography (TTE). However, although not all abnormalities on TTE result in changes in clinical management, performing TTE in recently infected patients increases disease transmission risks. It remains unknown whether common biomarker tests, such as troponin and B-type natriuretic peptide (BNP), can help distinguish in which patients with COVID-19 TTE may be safely delayed until infection risks subside.
Methods
Using electronic health records data and chart review, the authors retrospectively studied all patients hospitalized with COVID-19 in a multisite health care system from March 1, 2020, to January 15, 2021, who underwent TTE within 14 days of their first positive COVID-19 result and had BNP and troponin measured before or within 7 days of TTE. The primary outcome was the presence of one or more urgent echocardiographic findings, defined as left ventricular ejection fraction ≤ 35%, wall motion score index ≥ 1.5, moderate or greater right ventricular dysfunction, moderate or greater pericardial effusion, intracardiac thrombus, pulmonary artery systolic pressure > 50 mm Hg, or at least moderate to severe valvular disease. Stepwise logistic regression was conducted to determine biomarkers and comorbidities associated with the outcome. The performance of a rule for classifying TTE using troponin and BNP was evaluated.
Results
Four hundred thirty-four hospitalized and 151 intensive care unit patients with COVID-19 were included. Urgent findings on TTE were present in 105 patients (24.2%). Troponin and BNP were abnormal in 311 (71.7%). Heart failure (odds ratio, 5.41; 95% CI, 2.61–11.68), troponin > 0.04 ng/mL (odds ratio, 4.40; 95% CI, 2.05–10.05), and BNP > 100 pg/mL (odds ratio, 5.85; 95% CI, 2.35–16.09) remained significant predictors of urgent findings on TTE after stepwise selection. No urgent findings on TTE were seen in 95.1% of all patients and in 91.3% of intensive care unit patients with normal troponin and BNP.
Conclusions
Troponin and BNP were highly associated with urgent echocardiographic findings and may be used in triaging algorithms for determining in which patients TTE can be safely delayed until after their peak infectious window has passed.
Keywords: Transthoracic echocardiography, Safety, Risk stratification, COVID-19, Troponin, B-type natriuretic peptide
Abbreviations: BNP, B-type natriuretic peptide; COVID-19, Coronavirus disease 2019; ICU, Intensive care unit; LV, Left ventricular; TTE, Transthoracic echocardiography
The coronavirus disease 2019 (COVID-19) pandemic has resulted in >100 million infections and 2 million deaths worldwide as of January 2021.1 Cardiovascular complications of COVID-19 have been well described and include arrhythmias, pericarditis and myocarditis, cardiomyopathy, right ventricular failure, acute coronary syndrome, vascular thrombus, and pulmonary embolism.2, 3, 4
Transthoracic echocardiography (TTE) is a noninvasive and widely accessible method of evaluating patients for COVID-19-related cardiac complications. Previous studies have shown that up to 55% of patients with COVID-19 may have abnormal echocardiographic findings and that in some cases these abnormalities are associated with worse clinical outcomes.5 , 6 Although echocardiography can be informative, its value must be weighed against the costs and potential risks associated with performing TTE in patients with COVID-19.7 Specifically, overusing TTE in patients unlikely to have findings that will change clinical management may unnecessarily place sonographers and physicians performing studies at risk for severe acute respiratory syndrome coronavirus-2 infection. This risk is especially amplified during the early stages of disease, when patients may be most infectious.8 , 9 A thoughtful approach to the proper timing and use of TTE in recently infected patients with COVID-19 is therefore needed to minimize exposure and viral spread. However, there are limited data on the utility of clinical markers for determining if there is an urgent need to perform TTE.
Recent studies have clarified that certain patient comorbidities and biomarkers are associated with more severe COVID-19 disease.10 , 11 Some of these markers, such as troponin I and B-type natriuretic peptide (BNP), which reflect myocardial injury and elevated filling pressures, respectively, were associated with worse outcomes and were indicative of abnormal echocardiographic findings.5 , 6 , 12 However, these studies were limited by small patient numbers or were based on surveys with unavailable echocardiographic details.5 , 6
In this retrospective study of hospitalized patients with COVID-19, we sought to understand whether widely available biomarkers could be used to determine which patients may benefit most from urgent TTE. Moreover, we sought to determine in which patients TTE would be unlikely to change management and could thus be safely postponed until their infection risk subsided. We hypothesized that in patients with normal biomarkers such as troponin and BNP, it is unlikely that TTE performed within 14 days of COVID-19 diagnosis would reveal echocardiographic findings leading to an urgent change in clinical management.
Methods
Study Design
We performed a retrospective observational study of all patients with COVID-19, confirmed by reverse transcriptase polymerase chain reaction of ribonucleic acid extracted from nasopharyngeal swabs, hospitalized in our US multisite health care system from March 1, 2020, to January 15, 2021. We included only those patients who underwent TTE within 14 days of their first positive COVID-19 result and had BNP and troponin measured either before or within 7 days of TTE. Our institutional review board approved this retrospective study and waived the requirement to obtain individual informed consent.
Data Collection and End Points
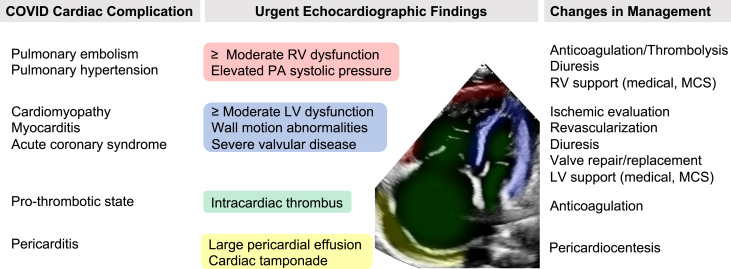
Data were collected from the electronic health records system and included patient demographic and comorbidity information, hospital stay characteristics, laboratory values, and echocardiographic findings. All patient comorbidities were identified by International Classification of Diseases coding according to standard Elixhauser comorbidity definitions.13 We extracted the highest laboratory value (or lowest for hemoglobin) during the patient's admission occurring before or within 7 days of TTE. All laboratory values were classified according to our medical center's standard upper and lower limits for normal values. On the basis of discussion with several TTE experts, we outlined common COVID-19 cardiovascular complications and the corresponding clinically significant transthoracic echocardiographic findings that might result in an immediate change in clinical management (Figure 1 ). According to this outline, we defined urgent echocardiographic findings as severe left ventricular (LV) dysfunction (LV ejection fraction ≤ 35%), wall motion score index ≥ 1.5, moderate or severe right ventricular dysfunction, moderate or large pericardial effusion, presence of any intracardiac thrombus, pulmonary artery systolic pressure > 50 mm Hg, or moderate to severe or severe valvular stenosis or regurgitation. The outcome of interest was the presence of at least one urgent echocardiographic finding on the first transthoracic echocardiographic examination performed within 14 days of the first positive COVID-19 result.
Figure 1.
Urgent echocardiographic findings in patients with COVID-19 that may change clinical management. MCS, Mechanical circulatory support; PA, pulmonary artery; RV, right ventricular.
Statistical Analysis
Patient demographics and comorbidities, laboratory values, and echocardiographic findings are expressed as frequency counts and percentages for categorical values and as mean ± SD for continuous values. We additionally stratified patients by whether they had preexisting cardiovascular disease, defined as having a history of hypertension, prior myocardial infarction, heart failure, peripheral artery disease, and/or diabetes. We compared patients with preexisting cardiovascular disease with those without using t tests for continuous variables and χ2 tests for categorical variables. We performed univariate logistic regression and χ2 tests to assess the individual associations of patient characteristics and laboratory values with the presence of at least one urgent finding on TTE. We then performed multivariate logistic regression with the same outcome using only patient characteristics and laboratory values selected by backward stepwise predictor selection by Akaike information criterion. Using our regression results, we created a simple TTE triaging rule using troponin and BNP and described its test characteristics, including negative predictive value and false-negative rate when applied to all patients in our cohort as well as a subgroup consisting of only patients admitted to the intensive care unit (ICU) at the time of TTE. All analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria). We used the MASS package for stepwise predictor selection by Akaike information criterion.14
Results
We identified 2,956 patients who were hospitalized with confirmed COVID-19 during the study period. Of these patients, 434 (14.7%) underwent TTE within 14 days of their first positive COVID-19 result and had BNP and troponin drawn either before or within 7 days of TTE. Patients had a mean age of 66 ± 16 years, were more often male (65.2%), were racially and ethnically diverse (7.6% Asian, 18.2% Black, 31.6% Hispanic, 25.1% non-Hispanic White), and had a high prevalence of cardiovascular comorbidities (Table 1 ). The average time between positive COVID-19 result and TTE was 3.4 ± 3.6 days, and 151 (34.8%) of patients were admitted to the ICU at the time of TTE. At least one urgent finding on TTE was present in 105 patients (24.2%), with the most common findings being a wall motion score index ≥ 1.5 (16.6%) and LV ejection fraction ≤ 35% (12.7%). These urgent transthoracic echocardiographic findings were not previously known in 99 of the 105 patients on the basis of TTE performed before hospitalization. Laboratory markers were also frequently abnormal, with 311 patients (71.7%) having troponin > 0.04 ng/mL or BNP > 100 pg/mL.
Table 1.
Characteristics of hospitalized patients who underwent TTE within 2 weeks of testing positive for COVID-19
| Characteristic | All patients |
Patients with no CV disease |
Patients with CV disease |
P value, no CV disease vs CV disease |
|---|---|---|---|---|
| (N = 434) | (n = 129) | (n = 305) | ||
| Age, y | 65.8 ± 16.0 | 58.9 ± 17.3 | 68.7 ± 14.5 | <.001 |
| Gender, male | 283 (65.2) | 85 (65.9) | 198 (64.9) | .93 |
| Race/ethnicity | .08 | |||
| Asian | 33 (7.6) | 6 (4.7) | 27 (8.9) | |
| Black | 79 (18.2) | 24 (18.6) | 55 (18.0) | |
| Hispanic | 137 (31.6) | 46 (35.7) | 91 (29.8) | |
| White | 109 (25.1) | 17 (13.2) | 37 (12.1) | |
| Other | 54 (12.4) | 11 (8.5) | 11 (3.6) | |
| Unknown | 22 (5.1) | 25 (19.4) | 84 (27.5) | |
| Diabetes | 163 (37.6) | 0 (0.0) | 163 (53.4) | <.001 |
| Hypertension | 224 (51.6) | 0 (0.0) | 224 (73.4) | <.001 |
| Prior myocardial infarction | 101 (23.3) | 0 (0.0) | 101 (33.1) | <.001 |
| Congestive heart failure | 147 (33.9) | 0 (0.0) | 147 (48.2) | <.001 |
| Chronic obstructive pulmonary disease | 69 (15.9) | 7 (5.4) | 62 (20.3) | <.001 |
| Chronic kidney disease | 132 (30.4) | 7 (5.4) | 125 (41.0) | <.001 |
| Peripheral artery disease | 74 (17.1) | 0 (0.0) | 74 (24.3) | <.001 |
| Obesity | 82 (18.9) | 7 (5.4) | 75 (24.6) | <.001 |
| Days from positive COVID-19 result to TTE | 3.4 ± 3.6 | 3.8 ± 3.5 | 3.2 ± 3.6 | .12 |
| ICU at time of TTE | 151 (34.8) | 55 (42.6) | 96 (31.5) | .03 |
| One or more urgent findings on TTE | 105 (24.2) | 18 (14.0) | 86 (28.2) | .002 |
| LV ejection fraction ≤ 35% | 55 (12.7) | 5 (3.9) | 50 (16.4) | .001 |
| Wall motion score index ≥ 1.5 | 72 (16.6) | 7 (5.4) | 65 (21.3) | <.001 |
| Cardiac thrombus | 2 (0.5) | 0 (0.0) | 2 (0.7) | .88 |
| Right ventricular dysfunction moderate or greater | 38 (8.8) | 9 (7.0) | 29 (9.5) | .51 |
| Pericardial effusion moderate or greater | 1 (0.2) | 0 (0.0) | 1 (0.3) | 1.0 |
| Pulmonary artery systolic pressure > 50 mm Hg | 16 (3.7) | 4 (3.1) | 12 (3.9) | .89 |
| Moderate to severe or severe valvular stenosis/regurgitation | 10 (2.3) | 2 (1.6) | 8 (2.6) | .74 |
| Troponin > 0.04 ng/mL or BNP > 100 pg/mL | 311 (71.7) | 72 (55.8) | 239 (78.4) | <.001 |
| Troponin > 0.04 ng/mL | 228 (52.5) | 49 (38.0) | 179 (58.7) | <.001 |
| BNP > 100 pg/mL | 254 (58.5) | 50 (38.8) | 204 (66.9) | <.001 |
| Creatinine > 1.25 mg/dL | 247 (57.0) | 55 (42.6) | 192 (63.2) | <.001 |
| C-reactive protein > 5 mg/L | 311 (96.9) | 97 (97.0) | 214 (96.8) | 1.0 |
| d-dimer > 0.5 μg/mL FEU | 342 (94.0) | 99 (91.7) | 243 (94.9) | .34 |
| Ferritin > 275 ng/mL | 306 (88.7) | 97 (89.0) | 209 (88.6) | 1.0 |
| Hemoglobin < 13 g/dL | 197 (45.4) | 49 (38.0) | 148 (48.5) | .06 |
| Platelet count > 450 × 1,000/μL | 64 (14.7) | 30 (23.3) | 34 (11.1) | .002 |
| WBC count > 11 × 1,000/μL | 260 (66.3) | 85 (71.4) | 175 (64.1) | .20 |
Data are expressed as mean ± SD or as number (percentage). All laboratory test thresholds are the standard upper limits of normal.
CV, Cardiovascular; FEU, fibrinogen-equivalent units; WBC, white blood cell.
We found that compared with patients without preexisting cardiovascular disease, those with cardiovascular disease were older (mean age, 68.7 ± 14.5 vs 58.9 ± 17.3 years) and had higher rates of chronic obstructive pulmonary disease (20.3% vs 5.4%), chronic kidney disease (41.0% vs 5.4%), and obesity (24.6% vs 5.4%; P < .001 for all comparisons). Patients with cardiovascular disease were less frequently admitted to the ICU at the time of TTE (31.5% vs 42.6%, P = .03), but had higher rates of having an urgent findings on TTE (28.2% vs 14.0%, P = .002) and were more likely to have elevated troponin (78.4% vs 55.8%, P < .001) or BNP (66.9% vs 38.8%, P < .001).
By univariate analysis, prior myocardial infarction, congestive heart failure, chronic obstructive pulmonary disease, chronic kidney disease, and peripheral artery disease, as well as abnormal troponin, BNP, creatinine, and platelets, were all significantly associated with having an urgent finding on TTE (Table 2 ). After stepwise predictor selection and multivariate modeling, only a history of congestive heart failure and abnormal troponin and BNP remained significant (Table 2).
Table 2.
Characteristics associated with urgent findings on TTE∗
| Univariate models |
Multivariate model† |
||||
|---|---|---|---|---|---|
| Or (95% CI) | P | Or (95% CI) | P | ||
| Age (per 10 y) | 1.00 (0.99–1.02) | .69 | 0.97 (0.95–1.00) | .03 | |
| Gender, male | 1.10 (0.69–1.76) | .69 | |||
| Race/ethnicity | |||||
| Asian | 1.20 (0.48–2.83) | .69 | |||
| Black | 0.99 (0.50–1.95) | .99 | |||
| Hispanic | 1.05 (0.59–1.91) | .86 | |||
| Other | 0.82 (0.36–1.77) | .62 | |||
| White | Reference | — | |||
| Diabetes | 1.04 (0.66–1.62) | .88 | |||
| Hypertension | 0.90 (0.58–1.40) | .64 | 0.56 (0.26–1.16) | .12 | |
| Prior myocardial infarction | 2.13 (1.30–3.45) | <.01 | |||
| Congestive heart failure | 7.42 (4.60–12.18) | <.01 | 5.41 (2.61–11.68) | <.01 | |
| COPD | 2.03 (1.16–3.50) | .01 | |||
| Chronic kidney disease | 2.21 (1.40–3.49) | <.01 | |||
| Peripheral arterial disease | 2.60 (1.52–4.40) | <.01 | |||
| Obesity | 1.10 (0.62–1.89) | .73 | |||
| Troponin > 0.04 ng/mL | 4.15 (2.54–6.99) | <.01 | 4.40 (2.05–10.05) | <.01 | |
| BNP > 100 pg/mL | 6.58 (3.71–12.49) | <.01 | 5.85 (2.35–16.09) | <.01 | |
| Creatinine > 1.25 mg/dL | 1.92 (1.21–3.09) | .01 | |||
| C-reactive protein > 5 mg/L | 1.01 (0.62–1.67) | .98 | |||
| d-dimer > 0.5 μg/mL FEU | 2.02 (0.67–8.76) | .27 | |||
| Ferritin > 275 ng/mL | 0.97 (0.47–2.18) | .95 | |||
| Hemoglobin < 13 g/dL | 1.32 (0.85–2.05) | .22 | 0.55 (0.27–1.10) | .10 | |
| Platelet count > 450 × 1,000/μL | 0.34 (0.14–0.73) | .01 | |||
| WBC count > 11 × 1,000/μL | 1.12 (0.69–1.83) | .65 | |||
All laboratory test thresholds are the standard upper limits of normal. Characteristics in boldface type were significant in both univariate and multivariate models.
COPD, Chronic obstructive pulmonary disease; FEU, fibrinogen-equivalent units; OR, odds ratio; WBC, white blood cell.
Urgent findings on TTE include LV ejection fraction ≤ 35%, wall motion score index ≥ 1.5, presence of cardiac thrombus, moderate or greater right ventricular dysfunction, moderate or greater pericardial effusion, and pulmonary artery systolic pressure > 50 mm Hg.
To avoid model overfitting, covariates included in the multivariate model were selected using stepwise backward selection by Akaike information criterion.
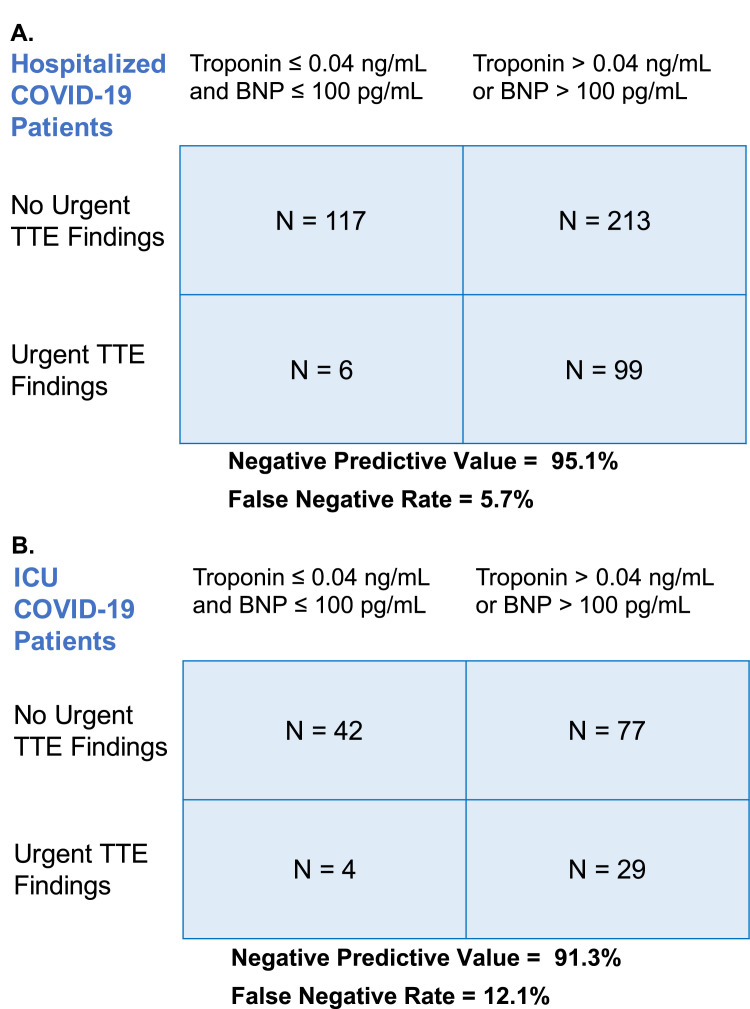
We found that 88.3% of patients with troponin ≤ 0.04 ng/mL and 92.2% of patients with BNP ≤ 100 pg/mL had no urgent findings on TTE. When considering both biomarkers together, 95.1% of patients with both troponin ≤ 0.04 ng/mL and BNP ≤ 100 pg/mL had no urgent findings TTE, with a false-negative rate of 5.7% (Figure 2 A). When applying this same rule to 151 patients admitted to the ICU during TTE, we found that the negative predictive value was 91.3% and the false-negative rate was 12.1% (Figure 2B).
Figure 2.
Use of troponin and BNP to predict presence of urgent findings on TTE in hospitalized and ICU patients with COVID-19. Urgent findings on TTE include LV ejection fraction ≤ 35%, wall motion score index ≥ 1.5, presence of cardiac thrombus, moderate or greater right ventricular dysfunction, moderate or greater pericardial effusion, pulmonary artery systolic pressure > 50 mm Hg, and moderate to severe or severe valvular disease.
Six patients (5%) had urgent abnormal echocardiographic findings despite negative troponin and BNP levels (Table 3 ). All patients had either significant LV or right ventricular dysfunction. In two of these patients, the findings were already known from TTE performed before COVID-19 diagnosis, and in one patient the urgent diagnosis (pulmonary embolism) was already suspected by other testing (lower extremity venous thromboembolism seen on vascular ultrasound). In only one patient did the findings on TTE result in an urgent change in clinical management (anticoagulation for presumed pulmonary embolism).
Table 3.
Summary of patients with COVID-19 with normal troponin and BNP levels who had urgent findings on TTE
| Patient | Finding on TTE | Finding already known or suspected | Change in management during hospitalization |
|---|---|---|---|
| 1 | Moderate RV dysfunction | Suspected given lower extremity VTE by ultrasound | Anticoagulated for presumed pulmonary embolism |
| 2 | LVEF 28%, WMSI 1.71 | No | Delayed coronary angiography after patient recovered from COVID-19, which showed no obstructive coronary disease |
| 3 | LVEF 25%, WMSI 2.29 | Yes; prior TTE showed LVEF 28%, WMSI > 2 | None |
| 4 | LVEF 30% | No | None |
| 5 | LVEF 39%, WMSI 1.65 | Yes; history of myocardial infarction with LV dysfunction (LVEF unknown) | None |
| 6 | Severe RV dysfunction | No | Anticoagulated for presumed pulmonary embolism |
LVEF, LV ejection fraction; RV, right ventricular; VTE, venous thromboembolism; WMSI, wall motion score index.
Discussion
To the best of our knowledge, this is one of the first and largest studies to use commonly available laboratory values to risk-stratify and prioritize echocardiographic studies in patients hospitalized with recent COVID-19. Data from 434 patients hospitalized with COVID-19 with TTE performed within 14 days of COVID-19 diagnosis demonstrated that using troponin and BNP together resulted in a 95% negative predictive value for ruling out an urgent finding on TTE when both cardiac biomarkers were normal. The negative predictive value remained high at 91% when only patients in the ICU were included. These findings illustrate that the use of troponin and BNP may serve as a simple screening tool in helping determine which patients with COVID-19 are unlikely to have urgent findings on TTE, thereby helping reduce unnecessary TTE-related costs and coronavirus exposure.
Patients with COVID-19 are known to present frequently with echocardiographic abnormalities, including LV systolic dysfunction, wall motion abnormalities, and diastolic dysfunction, as well as signs of right ventricular dysfunction and elevated pulmonary pressures.6 , 12 Although detection of many of these structural abnormalities may be important for long-term care, for recently infected patients with COVID-19, a narrower definition of clinically relevant echocardiographic findings may be appropriate given the costs and exposure risks associated with performing TTE in this population. The American Society of Echocardiography recommends categorizing all clinically indicated transthoracic echocardiographic examinations in patients with COVID-19 as elective or nonelective, further advocating that all elective studies be deferred and all nonelective studies be performed using limited and problem-focused assessments.7 For this study, in considering when TTE was most indicated, we defined urgent echocardiographic abnormalities (Figure 1) as including only findings that might lead to an immediate change in clinical management. Previous studies have identified a similar set of urgent findings in patients with COVID-19.15 , 16 We chose not to include more common findings, such as mild LV ejection fraction reduction, LV diastolic dysfunction, focal LV wall motion abnormalities, and small pericardial effusions, given that these results were unlikely to alter short-term care plans. In fact, it is even possible that many of these findings were related to acute infection and may have resolved with time, although more studies are needed to evaluate the persistence of structural abnormalities after recovery from COVID-19.17 , 18
We found that although urgent echocardiographic abnormalities were found in nearly one quarter of all patients with COVID-19, in those with normal troponin and BNP levels, 95% had no urgent echocardiographic abnormalities. Of the six patients who did have findings despite normal troponin and BNP levels, in only one patient was there an urgent change in management, and in three patients, the findings were previously known or suspected on the basis of other clinical and imaging information. Indeed, thorough history taking as well as selective physical examination may thus further complement and improve the utility of using troponin and BNP levels for screening for urgent TTE. The troponin and BNP screening rule also performed well among ICU patients, although with a slightly lower negative predictive value of 91%, likely because of the higher pretest probability for cardiac abnormalities in critically ill patients. We considered a range of other comorbidities and laboratory tests as screening criteria, but after stepwise predictor selection, troponin and BNP were the two markers that persisted as the main predictors of urgent echocardiographic findings. Heart failure was also a significant predictor, which is not surprising given that many of the echocardiographic abnormalities we considered urgent are found with high frequency at baseline in patients with heart failure. We did not include heart failure in our screening criteria given that it did not substantially add to the performance of the screening rule when using troponin and BNP alone.
The excellent performance of troponin and BNP in identifying patients without echocardiographic abnormalities is consistent with the findings of prior studies using these biomarkers. Because of their sensitivity for myopathic disease as well as their widespread availability, troponin and BNP levels have long been used as screening and prognostic tools for several cardiac conditions, including acute myocardial infarctions, congestive heart failure, and specific cardiomyopathic diseases such as amyloid light-chain amyloidosis and cardiotoxicity from chemotherapy.19, 20, 21, 22, 23 For patients with COVID-19 in particular, troponin and BNP are known to be associated with more severe illness and increased mortality.12 , 24 , 25 Abnormal levels of these biomarkers indicate myocardial stress, ischemia, and injury, which can be due to direct viral infection (myocarditis), virus-related acute coronary syndrome or microvascular disease, stress cardiomyopathy, and increased cardiovascular demand from pulmonary embolism, severe systemic stress, or hypoxic lung disease.26, 27, 28, 29 We showed that for likely the same reason that troponin and BNP have performed well as indicators of disease in other cardiac processes, these biomarkers may also be useful for predicting echocardiographic abnormalities in recently infected patients with COVID-19.
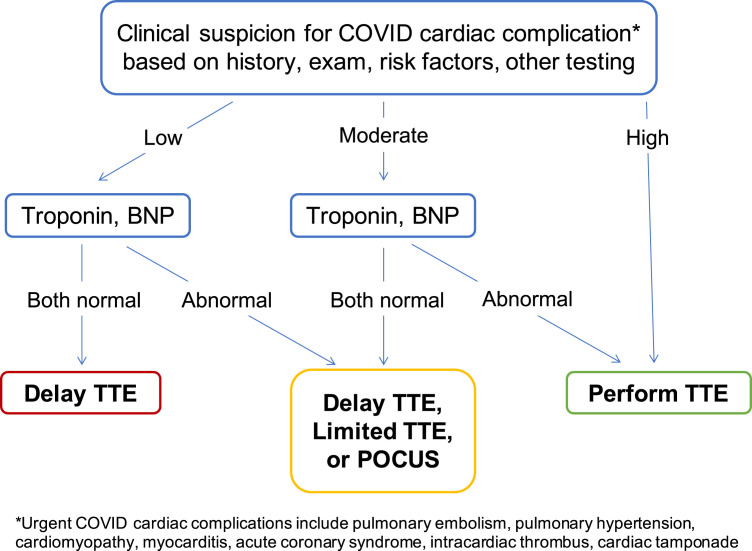
On the basis of our findings, we propose that troponin and BNP might be used as part of a formal triaging process for urgent TTE in patients with COVID-19 (Figure 3 ). Prior studies have shown that clinical judgment by cardiologists may be helpful as a first step for establishing a patient's baseline risk for having urgent findings.30 , 31 BNP and troponin might then be used to further improve decision-making when integrated with findings from history, physical examination, and other condition-specific studies. In addition to delaying or performing full TTE, other options to consider in such an algorithm might include using limited TTE or even focused handheld echocardiography for patients who have normal or borderline biomarkers but for whom clinical suspicion for an urgent cardiac condition remains.32 , 33
Figure 3.
Proposed integrated algorithm for triaging urgent TTE requests in hospitalized patients with COVID-19. POCUS, Point-of-care ultrasound.
Several limitations to this study merit consideration. Although the strengths of this study include its relatively large sample size and the completeness of the data, we studied patients from one medical system and geographic location. Data from more patients from additional sites would be helpful for confirming our findings. Echocardiographic findings were based on reads by clinicians at our academic medical center who were not blinded to clinical history or laboratory values, which may have introduced bias, especially with more subjective findings such as right ventricular dysfunction. As this study was retrospective, we were unable to control for which patients with COVID-19 underwent TTE and which underwent troponin and BNP assessment. Fortunately, it is most likely that this resulted in an enrichment of our cohort with generally sicker patients, which would have depressed the negative predictive value of our screening rule and made our results more conservative. Although we believe our proposed screening criteria rule out the vast majority of COVID-19-related urgent echocardiographic abnormalities, there may be less frequent important indications for echocardiography that we did not consider, such as aortic disease and evaluation of mechanical support devices. Last, it should be noted that we studied the performance of our screening rule only in acutely infected patients with COVID-19 who were within 14 days of diagnosis. Our findings may not be generalizable to patients who are outside this window.
Conclusion
In recently infected patients with COVID-19, the benefits of performing echocardiography must be weighed against the risks for coronavirus exposure and transmission. After evaluation of multiple comorbidities and biomarkers, we found that troponin and BNP were highly sensitive for echocardiographic abnormalities. More than 95% of patients with normal troponin and BNP had no urgent echocardiographic findings. We thus propose that troponin and BNP may be used as part of a simple triaging tool for determining in which patients TTE may be safely delayed until after their peak infectious window has passed.
Acknowledgments
We thank Joseph E. Ebinger, MD, MS, and Patrick Botting for help with data extraction.
Footnotes
Dr. Yuan was supported by the National Heart, Lung, and Blood Institute (grant T32 5T32HL116273-07). The funding organizations had no involvement in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of interest: None.
References
- 1.World Health Organization WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int Available at:
- 2.Ranard L.S., Fried J.A., Abdalla M., Anstey D.E., Givens R.C., Kumaraiah D. Approach to acute cardiovascular complications in COVID-19 infection. Circ Heart Fail. 2020;13:e007220. doi: 10.1161/CIRCHEARTFAILURE.120.007220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.F., Banerjee S., Umar S. In the eye of the storm: the right ventricle in COVID-19. Pulm Circ. 2020;10 doi: 10.1177/2045894020936660. 2045894020936660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkpatrick J.N., Mitchell C., Taub C., Kort S., Hung J., Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X. Author correction: temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:1491–1493. doi: 10.1038/s41591-020-1016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To K.K.-W., Tsang O.T.-Y., Leung W.-S., Tam A.R., Wu T.-C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giustino G., Croft L.B., Stefanini G.G., Bragato R., Silbiger J.J., Vicenzi M. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76:2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Venables W.N., Ripley B.D. 4th ed. Springer-Verlag; New York: 2002. Modern applied statistics with S. [Google Scholar]
- 15.Jain S.S., Liu Q., Raikhelkar J., Fried J., Elias P., Poterucha T.J. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33:1278–1284. doi: 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sud K., Vogel B., Bohra C., Garg V., Talebi S., Lerakis S. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J Am Soc Echocardiogr. 2020;33:1054–1055. doi: 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catena C., Colussi G., Bulfone L., Da Porto A., Tascini C., Sechi L.A. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J Am Soc Echocardiogr. 2021;34:193–195. doi: 10.1016/j.echo.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassan R., Potsch A., Maisel A., Tura B., Villacorta H., Nogueira M.V. B-type natriuretic peptide: a novel early blood marker of acute myocardial infarction in patients with chest pain and no ST-segment elevation. Eur Heart J. 2005;26:234–240. doi: 10.1093/eurheartj/ehi033. [DOI] [PubMed] [Google Scholar]
- 20.Patterson C.C., Blankenberg S., Ben-Shlomo Y., Heslop L., Bayer A., Lowe G. Troponin and BNP are markers for subsequent non-ischaemic congestive heart failure: the Caerphilly Prospective Study (CaPS) Open Heart. 2018;5:e000692. doi: 10.1136/openhrt-2017-000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shemisa K., Bhatt A., Cheeran D., Neeland I.J. Novel biomarkers of subclinical cardiac dysfunction in the general population. Curr Heart Fail Rep. 2017;14:301–310. doi: 10.1007/s11897-017-0342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvanayagam J.B., Hawkins P.N., Paul B., Myerson S.G., Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Michel L., Mincu R.I., Mahabadi A.A., Settelmeier S., Al-Rashid F., Rassaf T. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail. 2020;22:350–361. doi: 10.1002/ejhf.1631. [DOI] [PubMed] [Google Scholar]
- 24.Stefanini G.G., Chiarito M., Ferrante G., Cannata F., Azzolini E., Viggiani G. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106:1512–1518. doi: 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 25.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavishi C., Bonow R.O., Trivedi V., Abbott J.D., Messerli F.H., Bhatt D.L. Special article—acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis. 2020;63:682–689. doi: 10.1016/j.pcad.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhmerov A., Marbán E. COVID-19 and the heart. Circ Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajwa E.K., Boyce P.D., Januzzi J.L., Gong M.N., Thompson B.T., Christiani D.C. Biomarker evidence of myocardial cell injury is associated with mortality in acute respiratory distress syndrome. Crit Care Med. 2007;35:2484–2490. doi: 10.1097/01.ccm.0000281852.36573.22. [DOI] [PubMed] [Google Scholar]
- 29.Bajwa E.K., Januzzi J.L., Gong M.N., Thompson B.T., Christiani D.C. Prognostic value of plasma N-terminal probrain natriuretic peptide levels in the acute respiratory distress syndrome. Crit Care Med. 2008;36:2322–2327. doi: 10.1097/CCM.0b013e318181040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennessey K.C., Shah N., Soufer A., Wang Y., Agarwal V., McNamara R.L. Inpatient transthoracic echocardiography during the COVID-19 pandemic: evaluating a new triage process. J Am Soc Echocardiogr. 2020;33:1418–1419. doi: 10.1016/j.echo.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward R.P., Lee L., Ward T.J., Lang R.M. Utilization and appropriateness of transthoracic echocardiography in response to the COVID-19 pandemic. J Am Soc Echocardiogr. 2020;33:690–691. doi: 10.1016/j.echo.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaminski A., Payne A., Roemer S., Ignatowski D., Khandheria B.K. Answering to the call of critically ill patients: limiting sonographer exposure to COVID-19 with focused protocols. J Am Soc Echocardiogr. 2020;33:902–903. doi: 10.1016/j.echo.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon S.R., De Francis G., Schwartz S., Duvall W.L., Arora B., Silverman D.I. Tablet-based limited echocardiography to reduce sonographer scan and decontamination time during the COVID-19 pandemic. J Am Soc Echocardiogr. 2020;33:895–899. doi: 10.1016/j.echo.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]