Abstract
The coronavirus disease 19 (COVID-19) pandemic has caused significant morbidity and mortality worldwide and an effective treatment is needed. Chloroquine (CQ) and hydroxychloroquine (HCQ) have shown in vitro antiviral activity against SARS-CoV-2 which causes the disease, but the evidence from in vivo studies so far has been inconclusive. Objective: To evaluate the efficacy and safety of CQ and HCQ in the treatment of COVID-19. Data Sources: We systematically searched the PubMed, Embase, MEDLINE, Cochrane CENTRAL, CINAHL, Scopus, Joanna Briggs Institute Database, ClinicalTrials.gov, and Chinese Clinical Trial Registry (ChiCTR) for all articles published between 01 January 2020 to 15 September 2020 on CQ/HCQ and COVID-19 using a predefined search protocol; without any language restrictions. A search of grey literature repositories (New York Academy of Medicine Grey Literature and Open Grey), and pre-publication server deposits (medRxIV and bioRxIV) was also performed. Study Selection: Randomized clinical trials (RCT) which compared CQ/HCQ to standard supportive therapy in treating COVID-19 were included. Data Extraction and Synthesis: Data were extracted from original publications by four independent reviewers. Risk of bias was assessed using the Cochrane Collaboration’s assessment tool. Data were meta-analyzed using a random-effect models. Results are reported according to PRISMA guidelines. Main Outcome(s) and Measure(s): The primary prespecified efficacy outcome was all-cause mortality. The primary safety outcome was any adverse effect attributed to use of CQ/HCQ. Results: Eight RCTs were included and pooled in the mortality meta-analysis (6,592 unique participants; mean age = 59.4 years; 42% women). CQ/HCQ did not show any mortality benefit when compared to standard supportive therapy (Pooled Relative Risk [RR] 1.07; 95% CI = 0.97-1.18; I2 statistic = 0.00%). Sensitivity and sub-group analyses showed similar findings. Any adverse event was significantly higher in patients randomized to CQ/HCQ (RR = 2.51; 95% CI = 1.53-4.12; n = 1,818 patients), but the risk of developing severe adverse event was not statistically significant (RR = 0.99, 95% CI = 0.53-1.86; n = 6,456 patients). Conclusions and Relevance: Evidence from currently published RCTs do not demonstrate any added benefit for the use of CQ or HCQ in the treatment of COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, hydroxychloroquine, chloroquine, randomized controlled trials, systematic review, meta-analysis
Introduction
The ongoing global pandemic of Coronavirus Disease 19 (COVID-19) caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) presently accounts for more than 56 million cases with over 1,600,000 deaths across 188 countries/regions [1]. To contain the pandemic, several countries implemented social restrictions that resulted in an unprecedented global shutdown with huge psychosocial, economic, and political implications. Hence, the urgent need for an effective treatment and/or prophylaxis for this disease. Chloroquine (CQ) and hydroxychloroquine (HCQ) are among the drugs that have gained attention as potential treatment options for COVID-19 [2].
For over eight decades, CQ has been used for the treatment and prophylaxis of malaria and chronic rheumatoid conditions, whereas its less toxic (about 40% less toxic) hydroxyl analogue, HCQ, has mainly been reserved for treating connective tissue disorders including systemic lupus erythematosus and rheumatoid arthritis [2]. Both drugs have anti-inflammatory, immunomodulatory, and antiviral properties [3-6]. CQ is a potent inhibitor of the SARS Coronavirus (SARS-CoV) and SARS-CoV-2 in in vitro studies [2,6-9], and has been suggested to exhibit antiviral activity against Zika virus, poliovirus, HIV, and influenza viruses A & B [3,10-12]. Despite potent in-vitro efficacy of CQ and HCQ on SARS-CoV-2, lethal side effects such as hypoglycaemia and prolongation of the QTc interval have raised safety concerns for their widespread use in this pandemic [13-15], especially when used in combination with other QT prolonging antimicrobials. Nevertheless, given their extensive clinical use for malaria, rheumatoid and autoimmune disorders, anecdotal evidence suggest that CQ and HCQ are generally safe and well-tolerated [2,6,14,15]. Early promising results from clinical trials in France and China prompted research interests in the clinical efficacy of CQ and HCQ for the treatment and prophylaxis for COVID-19 infection [16-18]. Thus, several studies evaluating the efficacy and safety of CQ and HCQ use in COVID-19 patients were conducted using various designs that include observational, non-randomized controlled trials (non-RCTs), and randomized controlled trials (RCTs). Previous systematic reviews of these studies [18-24] show inconsistent findings related to the efficacy of CQ/HCQ in COVID-19 patients, which may be explained in part by the heterogeneous designs and varied levels of methodological rigour among the included studies [18,25,26].
Pooling evidence from RCTs will provide the highest level of evidence on the efficacy and safety of CQ and HCQ in COVID-19 patients. Hence, we conducted a systematic review with meta-analysis of published and unpublished trials to evaluate the efficacy of CQ and HCQ on a broad range of clinical outcomes when used alone or in combination with other drugs in treating COVID-19 patients. We also aimed to evaluate the safety of CQ and HCQ in these patients.
Methods
The protocol for this systematic review was developed according to PRISMA guidelines [27], and prospectively registered in an International register of systematic reviews: PROSPERO CRD42020209075.
Inclusion criteria
We included articles in any language that met the eligibility criteria based on the PICOS strategy: (1) Population (P): Patients diagnosed with COVID-19, including all ranges of severity (mild, moderate, and severe), all ethnic groups, and all age groups. We excluded patients not diagnosed with COVID-19, and patients being given CQ or HCQ for prophylaxis. (2) Intervention (I): Interventions in which CQ or HCQ was used in the treatment of COVID-19 patients. We excluded studies in which patients received CQ or HCQ as prophylaxis. (3) Comparison (C): compared with standard/usual care provided as per existing protocol in the trial hospital or country. We excluded studies in which CQ or HCQ was in the control/comparator arm. (4) Outcome (O): Relevant outcomes included all-cause mortality; Clinical deterioration (defined as progression from mild/moderate to severe disease requiring hospitalization with or without supplemental oxygen but excluding death); time to clinical recovery (defined as the duration from COVID-19 diagnosis to complete resolution of clinical symptoms); time to negative PCR (defined as the amount of time for seroconversion from positive to negative COVID-19 PCR test); length of stay in hospital; and safety including adverse events (defined as the onset of a new symptom or worsening of a pre-existing condition after randomization) and serious adverse events (defined as any adverse event that resulted in hospitalization or death after randomization). (5) Study design (S): published and unpublished randomized controlled trials. Observational studies and non-RCTs were excluded.
Search strategy
We searched the National Library of Medicine, Embase, MEDLINE, Cochrane CENTRAL, CINAHL, Scopus, Joanna Briggs Institute Database, ClinicalTrials.gov, and Chinese Clinical Trial Registry (ChiCTR) for eligible studies published between 01 January 2020 and 15 September 2020. Search terms included hydroxychloroquine, hydroxychloroquine sulphate, chloroquine, chloroquine phosphate, chloroquine diphosphate, clinical trial, and randomized controlled trial. We also searched grey literature websites (e.g. New York Academy of Medicine Grey Literature and Open Grey) and pre-publication server deposits (e.g. medRxIV and bioRxIV). Additionally, we sought relevant articles from the references of studies identified through the database search. There was no language restriction and non-English studies were translated into English using a translation service.
Data extraction
Data on participants’ demographic and clinical characteristics were retrieved using a data extraction form, including age; sex; ethnicity; country of origin; pre-existing comorbidities (e.g. CAD, CHF, arrhythmia, hypertension, diabetes, dyslipidaemia, COPD, CKD, liver diseases, cancer, and immune system disorders); smoker status (e.g. ever smokers, never smokers); regular medications (e.g. anticoagulants, ACE inhibitors, ARBs, statins, antivirals); and COVID-19 severity. We also extracted the data on the study outcomes for each treatment arm.
Risk of bias assessment
Two reviewers independently performed risk of bias assessments using the Cochrane Collaboration’s Tool for assessing risk of bias in five domains: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (income outcome data reporting), and reporting bias (selective reporting) [28]. If any of the five domains was found to be associated with some concerns of risk of bias or high risk of bias, the overall risk of bias was rated as ‘some concern’ or ‘high risk’, respectively. Otherwise, the RCT was rated as ‘low risk’. Any discrepancies in these assessments were resolved by discussion with a third reviewer.
Data synthesis
Descriptive statistics and narrative synthesis were used to summarize the characteristics of included studies. Pairwise meta-analysis using the conventional random-effects model were performed to pool individual results. Sensitivity and subgroup analyses were performed for the primary study outcome (all-cause mortality). Risk ratios (RR) and 95% confidence interval (CI) were estimated for categorical outcomes, and mean differences (MD) and 95% CI for continuous outcomes. Analyses were conducted using Stata version 16.1 (STATA Corp, College Station, TX).
Results
Selection of studies
Our search yielded a total of 1,639 studies, of which 387 duplicates were removed, leaving 1,252 studies. After screening by titles and abstracts, a further 1,232 studies were excluded, leaving 20 full-text articles for review. Eleven of these 20 studies met inclusion criteria [29-39], while the remaining nine studies were excluded for the following reasons: use of CQ or HCQ for prophylaxis [40-42], non-RCTs and quasi-randomized trials [16,43,44], the comparator arm was not usual care or placebo [45,46], and pre-print of a published study that already met inclusion criteria [31]. The study selection process is illustrated in a PRISMA flow diagram (Figure 1).
Figure 1.
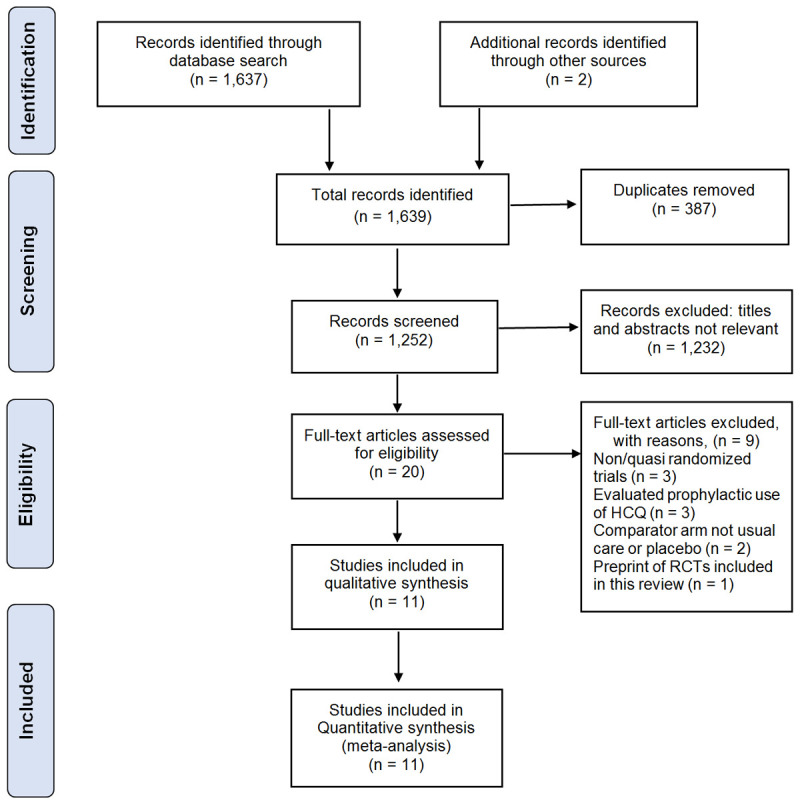
PRISMA Flow Diagram showing the process of selection of the included studies (PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses).
Description of included RCTs
Eleven RCTs, presented in Table 1, include six peer-reviewed published studies and five preprints comprising a total of 7,184 patients (Mean age = 57.6 years, SD = 18.5 years, 39.6% women) across nine countries. The most recent study, Abd-Elsalam et al., 2020 was published on 14 August 2020 [29]. Nine RCTs were open-label RCTs and the other two were double-blinded studies. Efficacy and safety of HCQ in COVID-19 patients was evaluated in all 11 RCTs, HCQ in combination with Azithromycin in one RCT, and CQ alone in one RCTs. Participants in the RCTs were patients with mild COVID-19 in three RCTs [35,37,38], mild-to-moderate patients in four RCTs [30-32,39], patients with moderate COVID-19 symptoms in one RCT [33], moderate-to-severe COVID-19 patients in one RCTs [34], and all severity of COVID-19 in two RCTs [29,36]. Based on different study endpoint periods, included RCTs evaluated outcomes on mortality, viral clearance, improvement of clinical status, time to clinical recovery (TTCR), utilization of mechanical ventilation and adverse events due to the use of CQ alone, HCQ alone, or HCQ in combination with Azithromycin.
Table 1.
Summary of RCTs evaluating use of CQ and HCQ for treatment of COVID-19 patients
| Study, Publication status | Trial Registry Identifier | Country | Design | No. of Participants | Participants | Study (trial) arms | Outcomes |
|---|---|---|---|---|---|---|---|
| Abd-Elsalam et al., 2020; Published | NCT04353336 | Egypt | RCT, Open label | 194 | Severity: Mild, moderate, and severe | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 40.7 ± 19.3 yrs | Arm 2: Usual care (Control group) | 1). Clinical recovery | |||||
| Sex, Female: 41.2% | 2). Need for mechanical ventilation | ||||||
| 3). Mortality within 28 days | |||||||
| Secondary | |||||||
| Adverse/Side effects | |||||||
| Cavalcanti et al., 2020; Published | NCT04322123 | Brazil | RCT, Open label | 667 | Severity: Mild to moderate | Arm 1: HCQ and Azithromycin | Primary |
| Age, Mean ± SD: 50.3 ± 14.6 yrs | Arm 2: HCQ alone | Clinical status on Day 15 | |||||
| Sex, Female: 41.7% | Arm 3: Usual care (Control) | Secondary | |||||
| 1). Clinical status at 7 days | |||||||
| 2). An indication for intubation within 15 days | |||||||
| 3). Receipt of supplemental oxygen between randomization and 15 days | |||||||
| 4). Duration of hospital stay | |||||||
| 5). In-hospital death | |||||||
| C. Chen et al., 2020; Preprint | NCT04384380 | Taiwan | RCT, Open label | 33 | Severity: Mild to moderate | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 32.9 ± 10.7 yrs | Arm 2: Usual care (Control) | Time to negative rRT-PCR assessments from randomization up to 14 days | |||||
| Sex, Female: 42.4% | Secondary | ||||||
| 1). Proportion of negative viral PCR on Day 14 | |||||||
| 2). Time to clinical recovery | |||||||
| 3). Proportion of discharges by Day 14 | |||||||
| 4). Mortality rate | |||||||
| 5). Safety and tolerability | |||||||
| J. Chen et al., 2020; Published | NCT04261517 | China | RCT, Open label | 30 | Severity: Moderate | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 48.6 ± 3.6 yrs | Arm 2: Usual care (Control) | Proportion of patients with negative rRT-PCR in pharyngeal swab on Day 7 | |||||
| Sex, Female: 30% | Secondary | ||||||
| 1). Occurrence of severe drug toxicity | |||||||
| L. Chen et al., 2020; Preprint | ChiCTR2000030054 | China | RCT, Open label | 48 | Severity: Moderate to severe hospitalized | Arm 1: CQ Phosphate | Primary |
| Age, Mean ± SD: 46.9 ± 14.6 yrs | Arm 2: HCQ | Time to clinical recovery | |||||
| Sex, Female: 54.2% | Arm 3: Usual care | Secondary | |||||
| 1). Time to negative rRT-PCR assessments | |||||||
| 2). Length of hospital stay | |||||||
| 3). Duration (days) of supplemental oxygenation | |||||||
| 4). Adverse events | |||||||
| 5). All-cause mortality | |||||||
| Z. Chen et al., 2020; Preprint | ChiCTR2000029559 | China | RCT, double blind | 62 | Severity: Mild hospitalized | Arm 1: HCQ Sulphate | Primary |
| Age, Mean ± SD: 44.7 ± 15.3 yrs | Arm 2: Usual care (Control) | Time to clinical recovery | |||||
| Sex, Female: 53.2% | Secondary | ||||||
| Adverse effects | |||||||
| Horby et al., 2020; Preprint | NCT04381936 | UK | RCT, Open label | 4,716 | Severity: Mild, moderate, and severe hospitalized | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 65.3 ± 15.3 yrs | Arm 2: Usual care (Control) | All-cause mortality by Day 28 | |||||
| Sex, Female: 38.8% | Secondary | ||||||
| 1). Time to discharge from hospital and | |||||||
| 2). Invasive mechanical ventilation | |||||||
| 3). Cause-specific mortality | |||||||
| 4). Major cardiac arrhythmia (recorded in a subset), | |||||||
| 5). Receipt and duration of ventilation. | |||||||
| Kamran et al., 2020; Preprint | NCT04491994 | Pakistan | RCT, Open label | 500 | Severity: Mild | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 35.9 ± 11.2 yrs | Arm 2: Usual care (Control) | Clinical progression of disease as per WHO criteria | |||||
| Sex, Female: 6.8% | Secondary | ||||||
| PCR negativity on Day 7 and Day 14 | |||||||
| Mitja et al., 2020; Published | NCT04304053 | Spain | RCT, Open label | 293 | Severity: Mild non-hospitalized | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 41.7 ± 12.5 yrs | Arm 2: Usual care (Control) | Reduction of viral RNA load in nasopharyngeal swabs at day 3 and day 7 after treatment start | |||||
| Sex, Female: 68.6% | Secondary | ||||||
| 1). Clinical progression up to 28 days | |||||||
| 2). TTCR of symptoms within 28 days | |||||||
| 3). Adverse events up to Day 28 | |||||||
| Skipper et al., 2020; Published | NCT04308668 | US, Canada | RCT, double blind | 491 | Severity: Mild to moderate non-hospitalized | Arm 1: HCQ | Primary |
| Age, Median (IQR): 40.0 (32 to 50) yrs | Arm 2: Placebo (Control) | 1). Presence and severity of COVID-19 symptoms | |||||
| Sex, Female: 56% | 2). Hospitalization status | ||||||
| Secondary | |||||||
| 1). Medication adherence | |||||||
| 2). Adverse effects | |||||||
| Tang et al., 2020; Published | ChiCTR2000029868 | China | RCT, Open label | 150 | Severity: Mild to moderate hospitalized | Arm 1: HCQ | Primary |
| Age, Mean ± SD: 46.1 ± 14.7 yrs | Arm 2: Usual care (Control) | 1). Negative conversion of SARS-CoV-2 by Day 28 | |||||
| Sex, Female: 45% | 2). Clinical improvement in severity symptoms by Day 28 | ||||||
| Secondary | |||||||
| 1). Alleviation of clinical symptoms | |||||||
| 2). All-cause mortality | |||||||
| 3). Disease progression in patients |
Risk of bias assessment
Table 2 describes the risks of bias in the included RCTs. Of the 11 RCTs included in this study, 10 were assessed to have a high risk of bias in one or more domains [29-34,36-39], and one RCTs was assessed to have a low risk of bias across all domains [35]. Of note, analyses were intention-to-treat (ITT) in eight of the 11 RCTs, hence attrition bias was deemed to be low risk in these studies [29-31,33,35-38].
Table 2.
Summary of risks of bias assessment using the Cochrane collaboration risk of bias assessment tool
| Study | Doman 1: Selection bias Random sequence generation | Domain 1: Selection bias Allocation concealment | Domain 2: Performance bias Blinding of participants & personnel | Domain 3: Detection bias Blinding of outcome assessment | Domain 4: Attrition bias Incomplete outcome data | Domain 5: Reporting bias Selective reporting | Overall Judgement of Risk |
|---|---|---|---|---|---|---|---|
| Abd-Elsalam et al., 2020 | Low risk | Unclear | High risk | High risk | Low risk | Low risk | High risk |
| Cavalcanti et al., 2020 | Low risk | Low risk | High risk | High risk | High risk | Unclear | High Risk |
| C. Chen et al., 2020 | Low risk | Low risk | High risk | High risk | Low risk | Low risk | High risk |
| J. Chen et al., 2019 | Some concern | Some concern | High risk | High risk | Low risk | Low risk | High risk |
| L. Chen et al., 2020 | Low risk | Unclear | High risk | High risk | High risk | Unclear | High risk |
| Z. Chen et al., 2020 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Horby et al., 2020 | Low risk | Low risk | High risk | High risk | Low risk | Low risk | High Risk |
| Kamran et al., 2020 | Unclear | Unclear | High risk | High risk | Low risk | Low risk | High Risk |
| Mitja et al., 2020 A | Low risk | Low risk | High risk | High risk | Low risk | Low risk | High risk |
| Skipper et al., 2020 | Low risk | Low risk | Low risk | Low risk | High risk | Low risk | High risk |
| Tang et al., 2020 | Low risk | Low risk | High risk | High risk | Low risk | Low risk | High risk |
Mortality
Eight RCTs [29-32,36,38,39] compared mortality outcomes among 6,592 patients (Mean = 59.4 years, SD = 17.9 years, 42% women) randomized to CQ/HCQ or control arms. Of note, all deaths occurred within 28 days of COVID-19 diagnosis. The pooled results showed no significant difference in mortality rates between treatment arms (RR = 1.07, 95% CI = 0.97-1.18) (Figure 2A). Given that Horby et al. [36] accounted for 97% of the combined weight of the eight RCTs, we performed a sensitivity analysis excluding this study from the meta-analysis. The pooled results excluding Horby et al. [36] also showed no statistically significant difference in mortality rates between HCQ/CQ and control [RR = 0.94, 95% CI = 0.49-1.80) (Figure 2B). Sub-group analysis showed mortality outcomes did not significantly vary with the pharmaceutical agent used (CQ vs HCQ vs HCQ+AZM), the categories of patients assessed (mild vs mild to moderate vs Moderate to severe vs All severity combined) nor with the duration of follow-up (≤ 15 days vs > 15 days) (Table 3).
Figure 2.
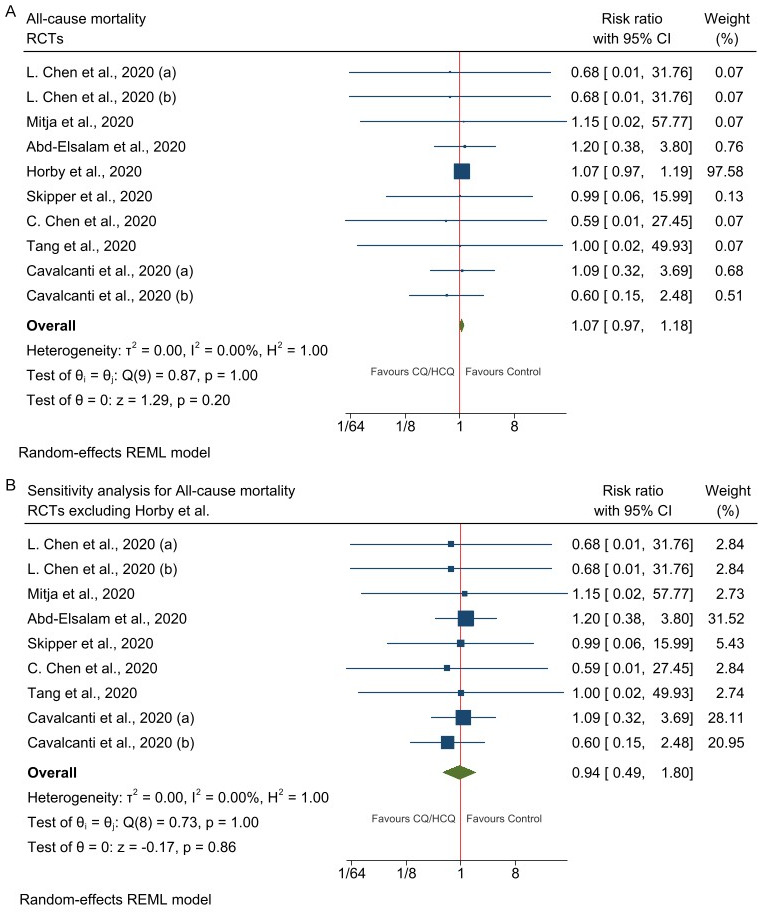
Forest plot of studies assessing mortality. A. All-cause mortality in patients randomized to CQ/HCQ vs Control (Usual care). B. Sensitivity analysis for all-cause mortality in patients randomized to CQ/HCQ vs Control (Usual care) excluding Horby et al., 2020. Legend: L. Chen et al., 2020 (a) represents the trials arm CQ vs Control; L. Chen et al., 2020 (b) represents the trials arm HCQ vs Control; Cavalcanti et al., 2020 (a) represents the trials arm HCQ vs Control; Cavalcanti et al., 2020 (a) represents the trials arm HCQ+ZAM vs Control.
Table 3.
Sub-group analysis for mortality in included RCTs
| Sub-group | No. of RCTs | Sample size | Pooled RR | 95% CI | Comment(s) |
|---|---|---|---|---|---|
| Pharmacological agent | |||||
| CQ | 1 | 30 | 0.68 | 0.01-31.76 | P = 0.846 |
| HCQ | 8 | 6,513 | 1.07 | 0.97-1.19 | I2 statistic = 0.00% |
| HCQ+AZM | 1 | 345 | 0.60 | 0.15-2.48 | P = 0.485 |
| Severity of COVID-19 patients | |||||
| Mild | 1 | 635 | 1.15 | 0.02-57.74 | P = 0.943 |
| Mild to moderate | 4 | 1,110 | 0.85 | 0.37-1.96 | I2 statistic = 0.00% |
| Moderate to Severe | 1* | 48 | 0.68 | 0.05-10.32 | I2 statistic = 0.00% |
| Mild, Moderate & Severe | 2 | 4910 | 1.07 | 0.97-1.19 | I2 statistic = 0.00% |
| Duration of follow-up | |||||
| ≤15 days | 3 | 960 | 0.84 | 0.36-1.98 | I2 statistic = 0.00% |
| >15 days | 5 | 5743 | 1.07 | 0.97-1.19 | I2 statistic = 0.00% |
Two comparisons: CQ vs Usual care and HCQ vs Usual care, of the same study were pooled together.
Clinical deterioration excluding mortality
Data from eight RCTs [30,33-39], including 6,630 patients showed no difference between CQ/HCQ and control in the proportions of patients who experienced deterioration of symptoms (RR 1.06, 95% CI = 0.90-1.26) (Supplementary Figure 1).
Time to clinical recovery
Data from four RCTs [29,33-35] comprising 328 patients, showed that patients in the control arm recovered on average 8 hours earlier than patients in the CQ/HCQ arm, however this difference was not statistically significant (MD -0.34, 95% CI = -0.75-0.08) (Supplementary Figure 2).
Viral clearance
Five RCTs [29,31-34] comprising data on 467 patients, compared the average time to negative PCR between HCQ/CQ and Usual Care. The results show that COVID-19 patients who received usual care achieved seroconversion approximately 11 hours earlier than patients who received HCQ/CQ, however the difference was not statistically significant (MD = -0.45, 95% CI = -1.02-0.11) (Supplementary Figure 3).
There was no difference in the viral load reduction between HCQ and controls on the 3rd day (mean reduction = -1.41 Log10 copies/mL, Standard Error (SE) = 0.15 vs mean reduction = -1.41 Log10 copies/mL, SE = 0.14) and on the 7th day (mean reduction = -3.44 Log10 copies/mL, SE = 0.18 vs mean reduction = -3.37 Log10 copies/mL, SE = 0.18) [38].
Length of stay in hospital
The mean duration of hospitalization did not differ between CQ/HCQ and control (MD = 0.03 (95% CI = -0.11-0.16, n = 698 patients)) [29,30] (Supplementary Figure 4). Likewise, the proportions of patients discharged by the end of the study period were comparable between CQ/HCQ and control (RR 0.96, 95% CI = 0.93-1.00, n = 5,220 patients) [30,36] (Supplementary Figure 5).
Safety
The risk of developing any adverse event was significantly higher in patients treated with CQ/HCQ than in those on usual care (RR = 2.25; 95% CI = 1.41-3.60; n = 1,818 patients) [30-35,38,39]-Figure 3A. However, the risk of developing serious adverse event (RR = 0.99, 95% CI = 0.53-1.86; n = 6,456 patients) [30-32,35,36,38,39] was the same in patients randomized to CQ/HCQ versus usual care (Figure 3B).
Figure 3.
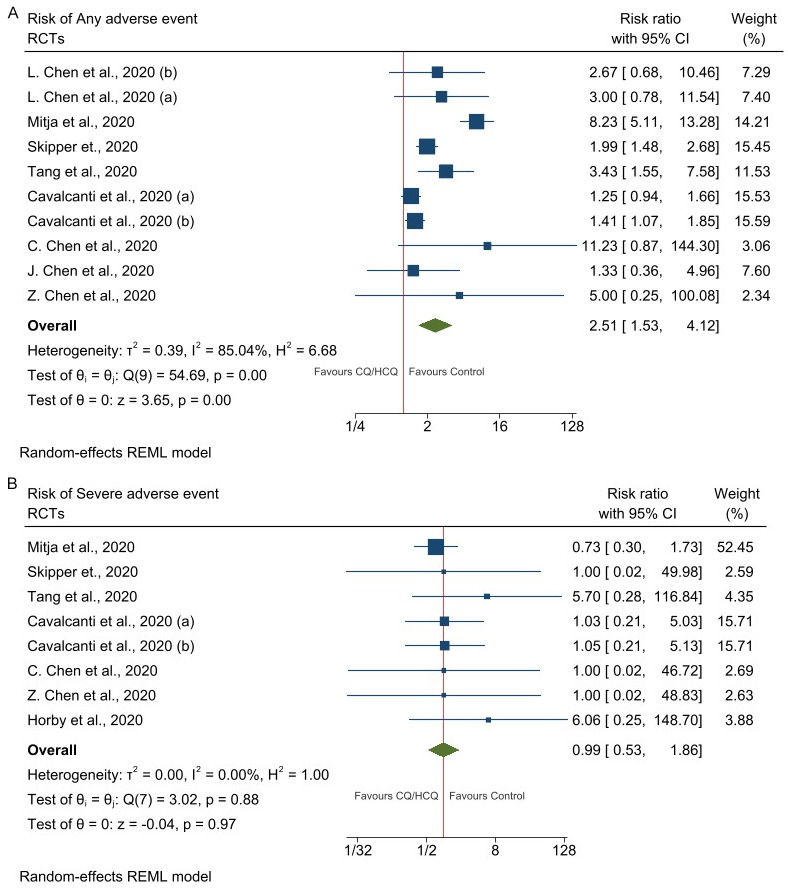
Forest plots of studies assess adverse events. A. Any adverse events in patients randomized to CQ/HCQ vs Control (Usual care). B. Severe adverse events in patients randomized to CQ/HCQ vs Control (Usual care). Legend: L. Chen et al., 2020 (a) represents the trials arm CQ vs Control; L. Chen et al., 2020 (b) represents the trials arm HCQ vs Control; Cavalcanti et al., 2020 (a) represents the trials arm HCQ vs Control; Cavalcanti et al., 2020 (a) represents the trials arm HCQ+ZAM vs Control.
The most frequent adverse events were transient, non-threatening insignificant treatment-related gastrointestinal (e.g., diarrhea, nausea, and abdominal pain) and nervous system disorders (e.g., drowsiness, headache, and metallic taste) [31,34,35,38,39]. Generally, patients who were randomized to HCQ-azithromycin combination reported a few more adverse events compared to patients who received HCQ alone [30]. Overall, CQ and HCQ were generally safe and well tolerated [31,34,38].
Electrocardiograph (ECG) monitoring for prolongation of QTc interval and serial cardiac enzyme testing showed no evidence of CQ/HCQ-related cardiotoxicity [31,34,38]. However, QTc prolongation was more common in patients randomized to HCQ plus azithromycin combination or HCQ alone compared to patients randomized to usual care [30]. CQ did not cause any ECG changes or abnormal levels of cardiac enzymes, though patients with prior history of cardiac diseases were excluded in this study [34]. Concurrent use of CQ with other medications was not evaluated [34].
Discussion
Our meta-analysis demonstrated that there is no mortality benefit in treatment with chloroquine or hydroxychloroquine in either mild, moderate, or severe COVID-19 disease. Clinical recovery, viral clearance and duration of hospital stay did not differ between treatment groups and controls in pooled analysis. This is a frequent feature of respiratory viral infections which are usually mostly self-limiting and do not have effective treatments [47-49]. Previous observational data that showed benefit for chloroquine or hydroxychloroquine in the treatment of COVID-19 are likely affected by confounding and selection bias [50]. For example, the observational study from the Henry Ford Hospital in Detroit, Michigan reported benefit for patients who received hydroxychloroquine; however, a significant proportion of the patients in this study also received steroids, which were recently reported to benefit a subset of patients with COVID-19 [50-52]. Additionally, some observational data also do not demonstrate improved clinical outcomes for use of chloroquine or hydroxychloroquine further illustrating this issue of inconsistent selection bias and confounding [53].
Our study also showed that chloroquine and hydroxychloroquine did not significantly cause severe adverse events to the patients in the treatment groups compared to those treated with usual care or placebo. This aligns with reports of a good safety profile and low risk/benefit balance of these drugs especially with short term usage [54]. Although this finding may help assuage fears and lay credence to the persistent use by some health institutions in various countries who may not have access to other treatment options, our study does not demonstrate any obvious benefit in morbidity or hard outcomes.
Prevention efforts centered around viral transmission risk mitigation, case containment, and treatment efforts based on high quality supportive care are likely the most important key efforts that are currently available in limiting the morbidity and mortality from COVID-19 [55-57]. Novel mRNA vaccines have also shown promise for reducing severity of disease, and possibly reducing viral transmission in early clinical trials. As such, research efforts should be channeled towards these initiatives and other ongoing therapeutic options. Finally, future clinical trials evaluating CQ, HCQ and other potential drugs for COVID-19 should address methodological quality gaps identified in this review, recruit adequate sample size of participants including children and should preferably be multi-centric.
Strengths and limitations
Randomized controlled trials (RCTs) are the best study design to test the efficacy of interventions as they are not subject to known and unknown confounders [58,59]. However, the extent to which their results can be extrapolated to a wider population is debatable because standardized and controlled study conditions may not always adequately reflect clinical reality [58,59]. Notwithstanding, RCTs are considered the gold standard and our systematic review only shortlisted RCTs as this increases the internal validity of the findings.
Most RCTs included in our study utilized open-label randomization. As such, we cannot exclude the possibility of any residual confounding in these studies. However, recent study showed no difference in estimated treatment effect between trials with and without blinded patients, healthcare providers, or outcome assessors [60]. Few of the included RCTs had relatively small sample size so it is not impossible that a true therapeutic effect and difference may have been undetected. Although we included all eligible published and unpublished RCTs as of today in our study, our findings may not be considered conclusive since there are still other ongoing RCTs that are underway whose results are pending and have not been considered in our current meta-analysis. Albeit these limitations, our study summarizes the most recent and robust available RCTs at this time.
Conclusion
Evidence from currently published RCTs do not demonstrate any added benefit for the use of CQ or HCQ in the treatment of COVID-19 patients. Unless future clinicals trials prove otherwise, our findings suggest that research efforts should be directed towards other potential treatment options to control this and future coronavirus outbreaks.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shippey EA, Wagler VD, Collamer AN. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85:459–467. doi: 10.3949/ccjm.85a.17034. [DOI] [PubMed] [Google Scholar]
- 5.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A. A pharmacological perspective of chloroquine in SARS-CoV-2 infection: An old drug for the fight against a new coronavirus? Int J Antimicrob Agents. 2020;56:106078. doi: 10.1016/j.ijantimicag.2020.106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savarino A. Use of chloroquine in viral diseases. Lancet Infect Dis. 2011;11:653–654. doi: 10.1016/S1473-3099(11)70092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piconi S, Parisotto S, Rizzardini G, Passerini S, Terzi R, Argenteri B, Meraviglia P, Capetti A, Biasin M, Trabattoni D, Clerici M. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 12.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keshtkar-Jahromi M, Bavari S. A call for randomized controlled trials to test the efficacy of chloroquine and hydroxychloroquine as therapeutics against novel coronavirus disease (COVID-19) Am J Trop Med Hyg. 2020;102:932–933. doi: 10.4269/ajtmh.20-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192:E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Food and Drug Administration (USFDA) Hydroxychloroquine or Chloroquine for COVID-19: Drug Safety Communication - FDA Cautions Against Use Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems. Drug Safety Communication. 2020 [Google Scholar]
- 16.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 18.Chacko J, Brar G, Premkumar R. Hydroxychloroquine in COVID-19: a systematic review and meta-analysis. medRxiv. 2020;14:589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortegiani A, Ippolito M, Ingoglia G, Iozzo P, Giarratano A, Einav S. Update I. A systematic review on the efficacy and safety of chloroquine/hydroxychloroquine for COVID-19. J Crit Care. 2020;59:176–190. doi: 10.1016/j.jcrc.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Million M, Gautret P, Colson P, Roussel Y, Dubourg G, Chabriere E, Honore S, Rolain JM, Fenollar F, Fournier PE, Lagier JC, Parola P, Brouqui P, Raoult D. Clinical efficacy of chloroquine derivatives in COVID-19 infection: comparative meta-analysis between the big data and the real world. New Microbes New Infect. 2020;38:100709. doi: 10.1016/j.nmni.2020.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel TK, Barvaliya M, Kevadiya BD, Patel PB, Bhalla HL. Does adding of hydroxychloroquine to the standard care provide any benefit in reducing the mortality among COVID-19 patients?: a systematic review. J Neuroimmune Pharmacol. 2020;15:350–358. doi: 10.1007/s11481-020-09930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah S, Das S, Jain A, Misra DP, Negi VS. A systematic review of the prophylactic role of chloroquine and hydroxychloroquine in coronavirus disease-19 (COVID-19) Int J Rheum Dis. 2020;23:613–619. doi: 10.1111/1756-185X.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AK, Singh A, Singh R, Misra A. “Hydroxychloroquine in patients with COVID-19: a systematic review and meta-analysis”. Diabetes Metab Syndr. 2020;14:589–596. doi: 10.1016/j.dsx.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarma P, Kaur H, Kumar H, Mahendru D, Avti P, Bhattacharyya A, Prajapat M, Shekhar N, Kumar S, Singh R, Singh A, Dhibar DP, Prakash A, Medhi B. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: a systematic review and meta-analysis. J Med Virol. 2020;92:776–785. doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander PE, Debono VB, Mammen MJ, Iorio A, Aryal K, Deng D, Brocard E, Alhazzani W. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020;123:120–126. doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosendaal FR. Review of: “Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al 2010, DOI:10.1016/j.ijantimicag.2020.105949. Int J Antimicrob Agents. 2020;56:106063. doi: 10.1016/j.ijantimicag.2020.106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abd-Elsalam S, Esmail ES, Khalaf M, Abdo EF, Medhat MA, Abd El Ghafar MS, Ahmed OA, Soliman S, Serangawy GN, Alboraie M. Hydroxychloroquine in the treatment of COVID-19: a multicenter randomized controlled study. Am J Trop Med Hyg. 2020;103:1635–1639. doi: 10.4269/ajtmh.20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CP, Lin YC, Chen TC, Tseng TY, Wong HL, Kuo CY, Lin WP, Huang SR, Wang WY, Liao JH, Liao CS, Hung YP, Lin TH, Chang TY, Hsiao CF, Huang YW, Chung WS, Cheng CY, Cheng SH Taiwan HCQ Study Group. A Multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19) PLoS One. 2020;15:e0242763. doi: 10.1371/journal.pone.0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L, Ling Y, Huang D, Song S, Zhang D, Qian Z, Li T, Shen Y, Lu H. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Journal of Zhejiang University (Medical Sciences) 2019;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Zhang ZY, Fu JG, Feng ZP, Zhang SZ, Han QY, Zhang XB, Xiao X, Chen HM, Liu LL, Chen XL, Lan YP, Zhong DJ, Hu L, Wang JH, Yu XH, She DY, Zhu YH, Yin ZY. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. medRxiv. 2020 [Google Scholar]
- 35.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, Zhuang R, Hu B, Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 [Google Scholar]
- 36.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse A, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell L, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 [Google Scholar]
- 37.Kamran SM, Mirza Z-e-H, Naseem A, Azam R, Ullah N, Saeed F, Alamgir W, Saleem S, Nisar S, Hussain M. Clearing the fog: is HCQ effective in reducing COVID-19 progression: a randomized controlled trial. medRxiv. 2020 doi: 10.7759/cureus.14186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitjà O, Corbacho-Monné M, Ubals M, Tebe C, Peñafiel J, Tobias A, Ballana E, Alemany A, Riera-Martí N, Pérez CA, Suñer C, Laporte P, Admella P, Mitjà J, Clua M, Bertran L, Sarquella M, Gavilán S, Ara J, Argimon JM, Casabona J, Cuatrecasas G, Cañadas P, Elizalde-Torrent A, Fabregat R, Farré M, Forcada A, Flores-Mateo G, Muntada E, Nadal N, Narejos S, Gil-Ortega AN, Prat N, Puig J, Quiñones C, Reyes-Ureña J, Ramírez-Viaplana F, Ruiz L, Riveira-Muñoz E, Sierra A, Velasco C, Vivanco-Hidalgo RM, Sentís A, G-Beiras C, Clotet B, Vall-Mayans M BCN PEP-CoV-2 RESEARCH GROUP. Hydroxychloroquine for early treatment of adults with mild covid-19: a randomized-controlled trial. Clin Infect Dis. 2020:ciaa1009. doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MR, Nascene AA, Hullsiek KH, Cheng MP, Luke D, Lother SA, MacKenzie LJ, Drobot G, Kelly LE, Schwartz IS, Zarychanski R, McDonald EG, Lee TC, Rajasingham R, Boulware DR. Hydroxychloroquine in nonhospitalized adults with early COVID-19 : a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as post-exposure prophylaxis for COVID-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lofgren SM, Nicol MR, Bangdiwala AS, Pastick KA, Okafor EC, Skipper CP, Pullen MF, Engen NW, Abassi M, Williams DA, Nascene AA, Axelrod ML, Lother SA, MacKenzie LJ, Drobot G, Marten N, Cheng MP, Zarychanshi R, Schwartz IS, Silverman M, Chagla Z, Kelly LE, McDonald EG, Lee TC, Hullsiek KH, Boulware DR, Rajasingham R. Safety of hydroxychloroquine among outpatient clinical trial participants for COVID-19. medRxiv. 2020 doi: 10.1093/ofid/ofaa500. 2020.07.16.20155531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitja O, Ubals M, Corbacho-Monne M, Alemany A, Suner C, Tebe C, Tobias A, Penafiel J, Ballana E, Perez CA, Admella P, Riera-Marti N, Laporte P, Mitja J, Clua M, Bertran L, Gavilan S, Ara J, Sarquella M, Argimon JM, Cuatrecasas G, Canadas P, Elizalde-Torrent A, Fabregat R, Farre M, Forcada A, Flores-Mateo G, Lopez C, Muntada E, Nadal N, Narejos S, Gil-Ortega AN, Prat N, Puig J, Quinones C, Ramirez-Viaplana F, Reyes-Urena J, Riveira-Munoz E, Ruiz L, Sanz S, Sentis A, Sierra A, Velasco C, Vivanco-Hidalgo RM, Zamora J, Casabona J, Vall-Mayans M, Beiras CG, Clotet B. A cluster-randomized trial of hydroxychloroquine as prevention of covid-19 transmission and disease. N Engl J Med. 2021;384:417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa J, Kaitis D, Freedman R, Le K, Lin X. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID-19: a quasi-randomized comparative study. New England Journal of Medicine. 2020 [Google Scholar]
- 44.Komissarov A, Molodtsov I, Ivanova O, Maryukhnich E, Kudryavtseva S, Mazus A, Nikonov E, Vasilieva E. Hydroxychloroquine has no effect on SARS-COV-2 load in nasopharynx of patients with mild form of COVID-19. MedRxiv. 2020 doi: 10.1371/journal.pone.0246396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 46.Huang M, Tang T, Pang P, Li M, Ma R, Lu J, Shu J, You Y, Chen B, Liang J, Hong Z, Chen H, Kong L, Qin D, Pei D, Xia J, Jiang S, Shan H. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papadopoulos NG, Megremis S, Kitsioulis NA, Vangelatou O, West P, Xepapadaki P. Promising approaches for the treatment and prevention of viral respiratory illnesses. J Allergy Clin Immunol. 2017;140:921–932. doi: 10.1016/j.jaci.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abed Y, Boivin G. Treatment of respiratory virus infections. Antiviral Res. 2006;70:1–16. doi: 10.1016/j.antiviral.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brendish NJ, Clark TW. Antiviral treatment of severe non-influenza respiratory virus infection. Curr Opin Infect Dis. 2017;30:573–578. doi: 10.1097/QCO.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 50.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE, O’Neill W, Zervos M Henry Ford COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. [Google Scholar]
- 52.Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ. 2020;369:m2512. doi: 10.1136/bmj.m2512. [DOI] [PubMed] [Google Scholar]
- 53.Ip A, Berry DA, Hansen E, Goy AH, Pecora AL, Sinclaire BA, Bednarz U, Marafelias M, Berry SM, Berry NS, Mathura S, Sawczuk IS, Biran N, Go RC, Sperber S, Piwoz JA, Balani B, Cicogna C, Sebti R, Zuckerman J, Rose KM, Tank L, Jacobs LG, Korcak J, Timmapuri SL, Underwood JP, Sugalski G, Barsky C, Varga DW, Asif A, Landolfi JC, Goldberg SL. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study. PLoS One. 2020;15:e0237693. doi: 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicola M, O’Niell N, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic - review article. Int J Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X, Ong YK, Wang Y. Role of adjunctive treatment strategies in COVID-19 and a review of international and national clinical guidelines. Mil Med Res. 2020;7:22. doi: 10.1186/s40779-020-00251-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bothwell LE, Podolsky SH. The emergence of the randomized, controlled trial. N Engl J Med. 2016;375:501–504. doi: 10.1056/NEJMp1604635. [DOI] [PubMed] [Google Scholar]
- 59.Bhide A, Shah PS, Acharya G. A simplified guide to randomized controlled trials. Acta Obstet Gynecol Scand. 2018;97:380–387. doi: 10.1111/aogs.13309. [DOI] [PubMed] [Google Scholar]
- 60.Moustgaard H, Clayton GL, Jones HE, Boutron I, Jorgensen L, Laursen DRT, Olsen MF, Paludan-Muller A, Ravaud P, Savovic J, Sterne JA, Higgins JP, Hrobjartsson A. Impact of blinding on estimated treatment effects in randomized clinical trials: meta-epidemiological stdy. BMJ. 2020;368:l6802. doi: 10.1136/bmj.l6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


