Abstract
Deep vein thrombosis (DVT), a subset of venous thromboembolism (VTE), is a major preventable cause of morbidity and mortality worldwide. We aim to investigate changes in oxidative injury markers and the levels of antioxidant parameters in DVT patients. 50 DVT patients who were admitted to the cardiovascular surgery clinic with acute (<2 weeks) or chronic (≥4 weeks) DVT and 100 healthy individuals were included. As oxidative injury marker malondialdehyde and the antioxidant parameters including reduced glutathione, glutathione peroxidase, catalase, and superoxide dismutase were analysed in the collected peripheral blood samples. Demographic characteristics of the acute and chronic DVT patients were similar (P>0.05). In all DVT patients having thrombosis in the distal and proximal leg veins the mean malondialdehyde and glutathione peroxidase activity were significantly increased, but superoxide dismutase activity significantly decreased, compared to the healthy controls (P<0.05). No difference was observed between distal and proximal DVT patient groups. The increased levels of malondialdehyde in our study is considered to result from the significant decrease in the antioxidant enzyme activity of superoxide dismutase. Furthermore, we are of the opinion that the increased levels of glutathione peroxidase enzyme activity observed in all patients could not compensate the reduction in the superoxide dismutase activity, thereby being insufficient in preventing the increase in the malondialdehyde levels.
Keywords: DVT, SOD, vein thrombosis, oxidative damage, MDA
Introduction
Deep vein thrombosis (DVT) is the most common clinical form of venous thromboembolism (VTE), various etiologic factors play severe role and make it complex vascular disease. It is a major cardiovascular disease holding the third rank after ischemic heart disease and stroke [1]. Oxidative stress is associated with the impaired balance between the pro-oxidant and antioxidant systems [2]. Several antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPx), heme oxygenase, thioredoxin system and small molecule antioxidants (glutathione, vitamin A, C and E) are produced to scavenge ROS, thereby limiting these detrimental effects. For normal physiological homeostasis these redox mechanisms are essential, but their dysregulation may have pathological consequences [3]. Reactive oxygen species (ROS) orchestrate various components of the thrombotic process including platelet activation [4]. The levels of circulating inflammatory markers increase in superficial vein thrombosis, directly and negatively affecting the recanalization rate and time [5].
There is increasing evidence that platelets have a role in the formation of venous thrombi, and changes in platelet reactivity affect the risk of DVT. Platelet function is regulated by ROS and impairments in these processes might be responsible for adverse outcomes in patients at risk of developing a DVT [3]. Arachidonic acid and glutathione metabolism can be the potential origin of free radicals, or which cause platelet activation and enhanced thrombosis. Also free radicals and antioxidants (NO, prostacyclin) produced by fibroblasts and endothelial cells of vascular wall may influence platelets. These are important modulators of platelet function by the influence on their redox status. During the oxygen explosion in generalised inflammatory conditions platelets are exposed to the free radicals released by phagocytes [6]. Increased platelet aggregation is involved in the development of DVT [5].
Although there are many studies reporting that the activation of the oxidative pathways and increased synthesis of ROS were involved in vascular diseases, the number of studies investigating their involvement in thrombus formation is limited [7]. Under the circumstances of oxidative stress, the superoxide radical reacts with the platelets or endothelium, converting NO to peroxynitrite (ONOO-), thereby affecting the vascular thrombus formation. Several studies report that the superoxide radical is involved in inducing the aggregation of platelets [8]. Protection from excess levels of superoxide by SOD is a critical component of antioxidant defence. Although superoxide will spontaneously dismutate to form hydrogen peroxide (H2O2), in the presence of superoxide dismutase, the removal of superoxide is dramatically accelerated [9].
In recent studies; increased levels of ROS have been reported to be significantly involved in hypertension, atherosclerosis, abdominal aortic aneurysm, and venous pathologies [9]. However, in the literature review we performed, we found limited number of studies investigating the association of DVT, oxidative stress, and the antioxidant system. Therefore, in this non-invasive prospective study, we aimed to investigate the association of DVT with oxidative injury and antioxidant parameters. Innovation of this study is searching a clinical parameter which can measurable in standard laboratories for DVT diagnosis for daily practice. Accordingly, we looked for an antioxidant enzyme which can be related to DVT formation.
Material and methods
Subjects
A total of 50 patients with DVT (29 females, 21 males; mean age 48 years; range, 20 to 75 years) who were admitted to the Cardiovascular Surgery Clinic of Elazığ Fethi Sekin City Hospital with acute (<2 weeks) or chronic (≥4 weeks) DVT (Table 1) and 100 healthy individuals (54 females, 46 males; mean age 46 years; range, 18 to 69 years) between January 2018 and July 2018 were included in this study. Inclusion criteria is patients who had a DVT diagnosis in lower extremity in any time of life span. Those with chronic renal insufficiency and advanced age (≥75 years), those receiving active cancer treatment, and long-bedridden patients were excluded. Acute DVT diagnosis was made in the outpatient or emergency setting based on the medical history, presence of positive Homans’ sign and lower extremity venous Doppler ultrasound (DUS). Their DVT treatment during taking blood samples was recorded. Venous DUS results were obtained from the digital recording systems or from the patients themselves. The control group was selected by the treating physicians, nurses, and other health care personnel working in the hospital. A written informed consent was obtained from each participant. For sample size detection power analysis done by using Kelsey et al. and Fleiss methods [10,11] (Table 2). The study protocol was approved by the Ethics Committee of Firat University, Faculty of Medicine (local university of the city) (No. 2017-25). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Table 1.
Distribution of DVT patients by gender and the anticoagulants used in the treatment
| Female (number) | Male (Number) | |
|---|---|---|
| Chronic Distal DVT | 2 | 7 |
| Chronic Proximal DVT | 12 | 12 |
| Acute Distal DVT | 2 | 2 |
| Acute Proximal DVT | 2 | 11 |
| History of Pulmonary Embolism | 4 | |
| Anticoagulant Treatment | ||
| Low Molecular Weight Heparin + Warfarin | 2 | |
| Warfarin | 11 | 7 |
| Non-warfarin Oral Anticoaguant | 13 | 15 |
| Low Molecular Weight Heparin | 2 | 4 |
| ASA (acetylsalicylic acid) | 4 | 4 |
(Two patients used ASA with Warfarin).
Table 2.
Power analysis of patient and control groups
| Sample Size: X-Sectional, Cohort, & Randomized Clinical Trials | |||
|
| |||
| Two-sided significance level (1-alpha): | 95 | ||
| Power (1-beta, % chance of detecting): | 80 | ||
| Ratio of sample size, Unexposed/Exposed: | 1 | ||
| Percent of Unexposed with Outcome: | 35 | ||
| Percent of Exposed with Outcome: | 65 | ||
| Odds Ratio: | 3.5 | ||
| Risk/Prevalence Ratio: | 1.9 | ||
| Risk/Prevalence difference: | 30 | ||
|
| |||
| Kelsey | Fleiss | Fleiss with CC | |
|
| |||
| Sample Size-Exposed | 44 | 43 | 49 |
| Sample Size-Nonexposed | 44 | 43 | 49 |
| Total sample size: | 88 | 86 | 98 |
CC = continuity correction. Results are rounded up to the nearest integer. Print from the browser menu or select, copy, and paste to other programs. Results from OpenEpi, Version 3, open source calculator-SS Cohort. Print from the browser with ctrl-P. Or select text to copy and paste to other programs.
Blood sampling and biochemical analyses
The collected venous peripheral blood samples were kept in two different test tubes containing heparin. The heparinised blood in one of these two tubes was used as a whole blood sample. The blood in the other tube was centrifuged at 3000 rpm for 5 minutes and its plasma was separated. Then, it was washed three times with physiological saline. The tubes were kept at the deep freezer at -80°C before the biochemical analyses. All collected samples, both patient and control groups are studied in the laboratories of local university veterinary faculty biochemistry department by manually at the same day and same conditions for standardisation. As oxidative injury marker, lipid peroxidation product, malondialdehyde (MDA) and the levels of antioxidant parameters including reduced glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD) were analysed in the collected peripheral blood samples.
The MDA assay in plasma was carried out based on the method of Placer et al. with slight modifications. MDA formed a pink complex with thiobarbituric acid and the absorbance was read in 532 nm. The quantity of MDA in the plasma was expressed as nmol/ml [12].
Being determined in accordance with the method of Chavan et al. the quantity of GSH was expressed as µmol/ml [13].
The Góth method was used to measure CAT activity. The yellow complex of molybdate and hydrogen peroxide was measured at 405 nm. The CAT activity was expressed as U/g Hb [14].
The SOD enzyme activity was measured based on the degradation of nitroblue tetrazolium by the superoxide radical, which was produced by the xanthine-xanthine oxidase system. The blue coloured Formosan obtained at the end of the reactions was maximally absorbed in 560 nm [15]. The SOD enzyme activity was expressed as U/g Hb.
The Beutler method was used for determining the activity of GPx, which was calculated with spectrophotometry by measuring the reduction in the optic density of the system in 340 nm following the NADPH oxidation [17]. The GPx activity was expressed as U/g Hb.
Statistical analyses
The SPSS package programme (15.0 for Windows) was used for the statistical analyses. The independent samples t-test was used for comparing the data of DVT patients to those of healthy individuals. The Mann-Whitney U test was used for comparing the distal DVT patient group (DVT involving the calf veins only) to the proximal DVT group (DVT involving the popliteal and proximal veins). The Mann-Whitney U test was also used for the individual comparisons of these two groups of patients to the healthy individual group. In addition, the demographic data of the DVT patients were analysed with the independent samples t-test.
All results were shown as mean ± standard deviation. A p-value of less than 0.05 was considered statistically significant.
Results
In the control group consisting of healthy individuals; three were under the age of 20, 25 people were between 21 and 30 years, 38 were between 31 and 40 years, 25 were between 41 and 50 years, seven were between 51 and 60 years, and two were between 61 and 70 years. Six individuals in the control group had diabetes mellitus, four had hypertension, and 35 were smokers. Demographic characteristics were similar between the acute and chronic DVT patients. Distribution of DVT patients according to gender and DVT therapy is summarized in Table 1.
Examination of the parameters of oxidative injury and antioxidant processes revealed that; i) The level of MDA and the GPx activity were significantly high (P=0.000 and P=0.000) and the SOD activity was significantly reduced (P=0.004) in both patient groups compared to the control group (Figure 1; Table 3). ii) When the comparison of distal and proximal DVT patient groups no statistically significant differences were found out in these parameters (P=0.807) (Table 4). iii) The comparison of the control group to the distal DVT patient group showed that the level of MDA and the activity of GPx were high (P=0.000 and P=0.01) and the activity of SOD (P=0.02) was reduced in the patient group, and these results were statistically significant (Figure 2; Table 5). iv) When the proximal DVT and healthy control groups were compared, it was determined that the level of MDA and the GPx activity were significantly high (P=0.000 and P=0.000) and the activities of GSH and SOD were significantly reduced (P<0.05) in the patient group (Figure 3; Table 6).
Figure 1.
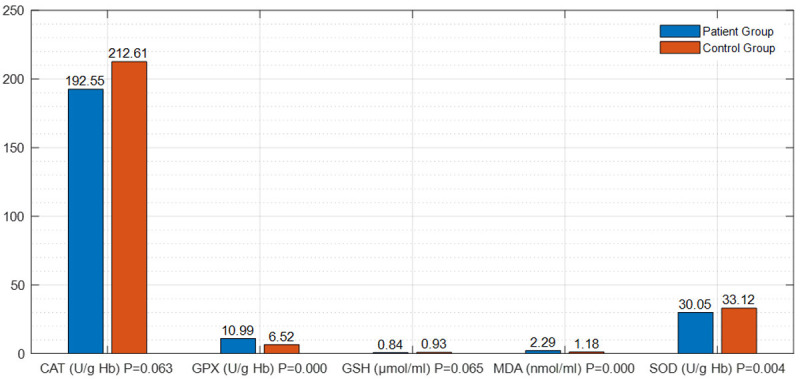
The differences in the parameters of oxidative injury and the antioxidant process between the group of both DVT patients and the control group.
Table 3.
The differences in the parameters of oxidative injury and the antioxidant process between the group of DVT patients and the healthy control group
| Parameter (unit) | Patient Group | Control Group | P-Value |
|---|---|---|---|
| MDA (nmol/ml) | 2.29±0.50a | 1.18±0.44b | P<0.05 |
| (P=0.000) | |||
| GSH (μmol/ml) | 0.84±0.33 | 0.93±0.26 | P>0.05 |
| (P=0.065) | |||
| GPX (U/g Hb) | 10.99±3.98a | 6.52±5.67b | P<0.05 |
| (P=0.000) | |||
| CAT (U/g Hb) | 192.55±63.80 | 212.61±55.61 | P>0.05 |
| (P=0.063) | |||
| SOD (U/g Hb) | 30.05±6.35a | 33.12±5.51b | P<0.05 |
| (P=0.004) |
a, b: Different letters in the same row indicate a statistical difference.
Table 4.
Comparison of the parameters of oxidative damage and antioxidant process in the distal DVT (involving the calf veins only) patient group to the proximal DVT (involving the popliteal vein along with the cranial veins) patient group
| Parameter (unit) | Mean Patient/Patient | Mean ± Std. Deviation | P-Value |
|---|---|---|---|
| MDA (nmol/ml) | 21.25/22.29 | 2.29±0.50 | P>0.05 |
| (P=0.807) | |||
| GSH (μmol/ml) | 25.00/20.84 | 0.83±0.33 | P>0.05 |
| (P=0.329) | |||
| GPX (U/g Hb) | 16.83/24.00 | 10.98±3.98 | P>0.05 |
| (P=0.09) | |||
| CAT (U/g Hb) | 20.63/22.53 | 192.55±63.80 | P>0.05 |
| (P=0.65) | |||
| SOD (U/g Hb) | 19.71/22.89 | 30.04±6.35 | P>0.05 |
| (P=0.45) |
Figure 2.
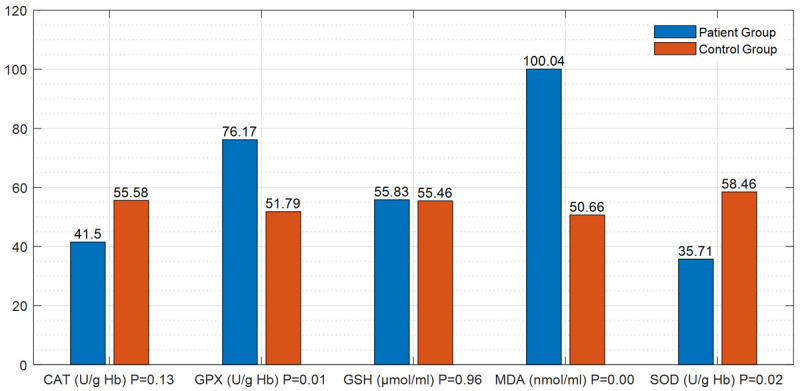
Differences in the parameters of oxidative injury and antioxidant process between the patient group with distal DVT (isolated calf veins) and the control group.
Table 5.
Differences in the parameters of oxidative injury and antioxidant process between the patient group with distal DVT (isolated calf veins) and the healthy control group
| Parameter (unit) | Mean Patient/Control | Mean ± Std. Deviation | P-Value |
|---|---|---|---|
| MDA (nmol/ml) | 100.04/50.66 | 1.51±0.68 | P<0.05 |
| (P=0.000) | |||
| GSH (μmol/ml) | 55.83/55.46 | 0.90±0.28 | P>0.05 |
| (P=0.96) | |||
| GPX (U/g Hb) | 76.17/51.79 | 7.90±5.59 | P<0.05 |
| (P=0.01) | |||
| CAT (U/g Hb) | 41.50/55.58 | 206.36±58.79 | P>0.05 |
| (P=0.13) | |||
| SOD (U/g Hb) | 35.71/58.46 | 32.18±5.93 | P<0.05 |
| (P=0.02) |
Figure 3.
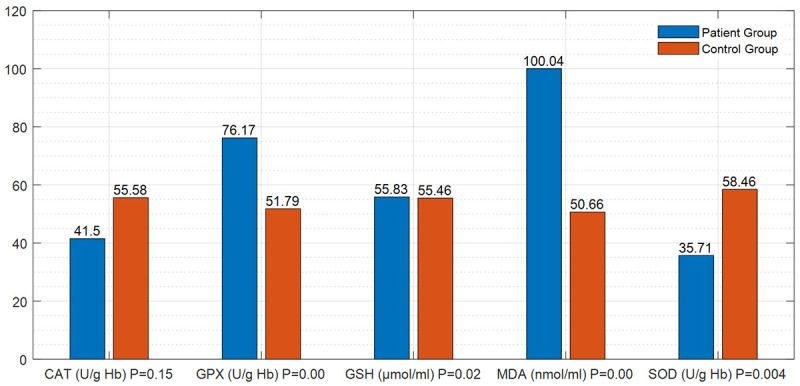
Differences in the parameters of oxidative injury and antioxidant process between the proximal DVT patient group (DVT involving the popliteal vein and proximal) and the control group.
Table 6.
Differences in the parameters of oxidative injury and antioxidant process between the proximal DVT patient group (DVT involving the proximal popliteal vein) and the healthy control group
| Parameter (unit) | Mean Patient/Control | Mean ± Std. Deviation | P-Value |
|---|---|---|---|
| MDA (nmol/ml) | 100.04/50.66 | 1.51±0.68 | P<0.05 |
| (P=0.000) | |||
| GSH (μmol/ml) | 55.83/55.46 | 0.90±0.28 | P<0.05 |
| (P=0.02) | |||
| GPX (U/g Hb) | 76.17/51.79 | 7.90±5.59 | P<0.05 |
| (P=0.000) | |||
| CAT (U/g Hb) | 41.50/55.58 | 206.36±58.79 | P>0.05 |
| (P=0.15) | |||
| SOD (U/g Hb) | 35.71/58.46 | 32.18±5.93 | P<0.05 |
| (P=0.004) |
Comparison of gender groups is not reliable because the discordance in the group populations.
Discussion
In 1856 Virchow’s Triad was described, implicates three contributing factors in the formation of thrombosis: vascular injury, venous stasis and hypercoagulability. Venous stasis is the most consequential of the three factors, but stasis alone appears to be insufficient to cause thrombus formation [17]. Surgery or trauma, malignancy, prolonged immobility, pregnancy, congestive heart failure, varicose veins, obesity, advancing age, and a history of DVT are fundamental clinical conditions related to the elements of Virchow’s Triad [18].
A study published in 2020 evaluated the relations between roles of oxidative stress in red blood cells in venous thrombosis and aging. Aging is associated with overproduction of ROSs, which could evoke a series of physiological changes that create a discrepancy between thrombosis and haemorrhage. Oxidative stress and antioxidant status are associated with aging. Aging leads to up regulation of ROS-producing enzymes and also down-regulation of antioxidant enzymes, such as SOD and GPx. However, an imbalance between the generation of ROS and antioxidant systems, excessive ROS generation or a defect in the antioxidant defence system (which impacts a wide variety of biological molecules, lipids in the plasma and mitochondrial membranes, causing lipid peroxidation that impairs membrane selective permeability, proteins, resulting in structural instability and damage to their enzymatic function, and nucleic acids) causes endothelial dysfunction and damage to endothelial cell lining. It has been postulated that the pathologic process of thrombosis begins with endothelial injury, and subsequently depends largely on a function of platelets, coagulation factors, and antithrombotic and fibrinolytic systems [19].
In our study, we investigated MDA as a marker of oxidative stress and found that, compared to the healthy individuals, the levels of MDA were elevated in both groups of patients with DVT; namely “the patients with distal DVT (calf veins) and proximal DVT (the popliteal and proximal veins)”. We also determined that, compared to the control, the antioxidant SOD enzyme activity was significantly reduced in the patients with both distal and proximal DVT. Another antioxidant parameter investigated in our study is the enzyme activity of GPx, which was significantly increased in these two patient groups compared to healthy individuals. A distinctive finding in these two groups of patients is that the level of another antioxidant parameter, GSH, was significantly reduced only in the patients with proximal DVT.
Also in another study from my country evaluated association between VTE and oxidative stress. They found serum paraoxonase and arylesterase activities (play a role in the antioxidant and anti-inflammatory properties exerted by high-density lipoprotein (HDL)) were significantly lower in VTE patients. They concluded that oxidative/antioxidative balance shifted towards the oxidative status in venous thromboembolism, which is parallel to our findings [20].
Behçet’s disease is another autoimmune disorder with an increased risk for developing venous thrombosis. A recent study of 98 Behçet’s disease patients has shown that thrombus formation was promoted by oxidatively altered fibrinogen, which was associated with neutrophil activation and enhanced NOX-dependent ROS production [21].
The most important enzymatic antioxydative system in blood platelets are SOD, CAT, GPx, glutathione transferase (GST), glutathione reductase (GSSG-R). GSH is a non-enzymatic substance crucial for free radical removing reactions. Antioxidative enzymes and compounds cooperate in oxidative stress reduction. The blood platelets contain about 1 femtogram of SOD per platelet, which accounts for 20% of this enzyme in leukocytes. This enzyme is involved in a reaction eliminating superoxide radical (O2·) and generating hydrogen peroxide (H2O2), which is subsequently decomposed by CAT and GPx. The synthesis of SOD is stimulated by interleukin 1 and tumour necrosis factor alpha. By increasing endogenous NO bioactivity SOD plays important function in thrombosis prevention [6]. Knockout of the antioxidant enzyme glutathione peroxidase-3 (GPX-3) results in increased platelet-dependent thrombosis in mice [22], whereas mice overexpressing the GPX-1 isoform were protected from platelet hyperactivity and age-dependent increased susceptibility to experimental venous thrombosis after ligation of the inferior vena cava (IVC) [23]. As the same our results there is a significant increase in oxidative damage and at the same time decrease in antioxidant activity, especially low peripheral blood levels of SOD in all patient groups. But we don’t know that if this result is acquired or genetic?
The relationship between DVT and oxidative stress markers has been shown in several studies in the literature. Another study demonstrated significant elevations in the levels of MDA, hydroxynonenal (HNE), and myeloperoxidase (MPO) in DVT patients compared to healthy individuals [24].
Studies in the literature report that blood levels of antioxidant parameters were reduced in patients [25]. Another study determined no changes in the enzyme activities of GPx1 and CAT. The authors have reported that H2O2 is involved in platelet aggregation and that the SOD enzyme converts the superoxide radical to H2O2, being effective in thrombosis [26]. Other tissue and vascular factors of thrombosis and inflammation (plasminogen activator inhibitor-1, P-selectin) may also be actively involved in thrombus formation under aggravated oxidative stress conditions [27]. It has been reported that the genetic deficiency of SOD1, one of the three isoenzymes of SOD, is a significant finding in both arterial and venous thrombosis [28]. SOD1 deficiency was found to be related to the impaired protein C anticoagulant response to thrombin. In the light of these findings, it may be suggested that increased levels of superoxide radical resulting from SOD1 deficiency may impair thrombomodulin-dependent protein C activation and cause thrombosis [28]. These studies strongly indicate that oxidative stress aggravates thrombotic sensitivity and that endogenous SOD is protective against thrombosis; which is induced by multiple oxidation-sensitive pathways, such as superoxide-mediated oxidation of thrombomodulin and disruption of APC production [28].
Our study findings are therefore parallel to those of previous studies in the literature, demonstrating the importance of the oxidative-antioxidative balance in DVT [25]. Also parallel to our results; Blankenberg et al. indicated the importance of the activity of SOD enzyme particularly in the development of DVT [27]. Limitations of our study small numbers of patients groups. For further studies we are planning to perform larger groups for finding the normal reference values of antioxidant enzymes especially SOD for human population. This study performed from a small cardiovascular centre in eastern part Turkey. But all biochemical analyses done manually in a laboratory at the same time in the same day for safer results. For larger groups we need to process commercial laboratory kits. We are of the opinion that these parameters to be tested in the clinical practice will provide results more readily and will be more economical in diagnosing DVT, and that these results obtained in the patient population with DVT will guide the management of high-risk patients or the conduct of prospective cohort studies on patients in the post-treatment period.
Conclusion
In our study, there was a significant increase in MDA levels in all DVT patients compared to the healthy individuals. We believe that this increase in MDA levels caused a significant reduction in the activity of SOD antioxidant enzyme, which is the first line of defence against oxidative injury, and that the increase in GPx antioxidant enzyme activity in both patient groups could not compensate the decrease in the SOD activity and could not prevent the elevation in MDA levels. Oxidative stress in DVT could not be prevented by the antioxidant parameters.
Disclosure of conflict of interest
None.
References
- 1.Mirshahi S, Soria C, Kouchakji B, Kierzek G, Borg JY, Varin R, Chidiac J, Drouet L, Mirshahi M, Soria J. New combinational assay using soluble fibrin and d-dimer determinations: a promising strategy for identifying patients with suspected venous thromboembolism. PLoS One. 2014;9:e92379. doi: 10.1371/journal.pone.0092379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogan A, Turker FS. The effect of on-pump and off-pump bypass operations on oxidative damage and antioxidant parameters. Oxid Med Cell Longev. 2017;2017:8271376. doi: 10.1155/2017/8271376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21:1918. doi: 10.3390/ijms21061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herket O, Diebold I, Brandes RP, Hess J, Busse R, Görlach A. NADPH oxidase mediates tissue factor-dependent surface procoagulant activity by thrombin in human vascular smooth muscle cells. Circulation. 2002;105:2030–2036. doi: 10.1161/01.cir.0000014611.28864.1e. [DOI] [PubMed] [Google Scholar]
- 5.Poredoš P, Spirkoska A, Ježovnik K. In patients with superficial vein thrombosis the inflammatory response is increased and related to the recanalization rate. Arch Med Sci. 2019;15:393–401. doi: 10.5114/aoms.2019.83292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stępniewska J, Dołęgowska B, Cecerska-Heryć E, Gołembiewska E, Malinowska-Jędraszczyk A, Marchelek-Myśliwiec M, Ciechanowski K. The activity of antioxidant enzymes in blood platelets in different types of renal replacement therapy: a cross-sectional study. Int Urol Nephrol. 2016;48:593–9. doi: 10.1007/s11255-015-1204-9. [DOI] [PubMed] [Google Scholar]
- 7.Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aviram M. LDL-platelet interaction under oxidative stress induces macrophage foam cell formation. Thromb Haemost. 1995;74:560–4. [PubMed] [Google Scholar]
- 9.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 10.Kelsey JL, Whittemore A, Evans AS, Thompson WD. Methods in observational epidemiology. 2nd edition. Oxford University Press; 1996. [Google Scholar]
- 11.Fleiss JL. Statistical Methods for Rates and Proportions. John Wiley & Son; 1981. [Google Scholar]
- 12.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyldialdehyde) in biochemical systems. Anal Biochem. 1966;16:359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 13.Chavan S, Sava L, Saxena V, Pillai S, Sontakke A, Ingole D. Reduced Glutathione: importance of specimen collection. Indian J Clin Biochem. 2005;20:150–152. doi: 10.1007/BF02893062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Góth LA. Simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta. 1991;196:143–152. doi: 10.1016/0009-8981(91)90067-m. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 16.Beutler E. Red cell metabolism: a manual of biochemical methods. 2nd edition. New York: Grune and Stratton; 1975. [Google Scholar]
- 17.Wessler S, Reimer SM, Sheps MC. Biologic assay of a thrombosis-inducing activity in human serum. J Appl Physiol. 1959;14:943–6. doi: 10.1152/jappl.1959.14.6.943. [DOI] [PubMed] [Google Scholar]
- 18.Mammen EF. Pathogenesis of venous thrombosis. Chest. 1992;102(Suppl):640–644. doi: 10.1378/chest.102.6_supplement.640s. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Zennadi R. Oxidative stress and thrombosis during aging: the roles of oxidative stress in RBCs in venous thrombosis. Int J Mol Sci. 2020;21:4259. doi: 10.3390/ijms21124259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aykal G, Güven R, Yeğin A, Ellidağ HY, Bayindir A, Yilmaz N. The diagnostic value of oxidative/antioxidative balance parameters in venous thromboembolism. Clin Lab. 2015;61:769–75. [PubMed] [Google Scholar]
- 21.Gutmann C, Siow R, Gwozdz AM, Saha P, Smith A. Reactive oxygen species in venous thrombosis. Int J Mol Sci. 2020;21:1918. doi: 10.3390/ijms21061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin RC, Mahoney CE, Coleman Anderson L, Ottaviano F, Croce K, Leopold JA, Zhang YY, Tang SS, Handy DE, Loscalzo J. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation. 2011;123:1963–1973. doi: 10.1161/CIRCULATIONAHA.110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayal S, Wilson KM, Motto DG, Miller FJ, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127:1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Re G, Lanzarini C, Vaona I, Pazzaglia M, Palareti G. Systemically circulating oxidative species in human deep venous thrombosis. Eur J Emerg Med. 1998;5:9–12. [PubMed] [Google Scholar]
- 25.Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–77. doi: 10.4330/wjc.v6.i6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 27.McDonald AP, Meier TR, Hawley AE, Thibert JN, Farris DM, Wrobleski SK, Henle PK, wakefield TW, Myers DD Jr. Aging is associated with impaired thrombus resolution in a Mouse model of stasis induced thrombosis. Thromb Res. 2010;125:72–78. doi: 10.1016/j.thromres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Dayal S, Gu SX, Hutchins RD, Wilson KM, Wang Y, Fu X, Lentz SR. Deficiency of superoxide dismutase impairs protein C activation and enhances susceptibility to experimental thrombosis. Arterioscler Thromb Vasc Biol. 2015;35:1798–1804. doi: 10.1161/ATVBAHA.115.305963. [DOI] [PMC free article] [PubMed] [Google Scholar]


