Abstract
Background: Matrix metalloproteinase-9 (MMP-9), an enzyme with major role in remodeling of extracellular matrix, has been the focus of attention in some previous studies in the field of breast cancer. In the current study the relationship between matrix metalloproteinase-9 and some clinicopathological factors of breast carcinoma has also been evaluated. Methods: Formalin-fixed and paraffin-embedded tissue specimens from three groups including 40 invasive breast carcinoma (tumor group) and their adjacent normal tissue (tumor control), as well as 40 normal mammoplasty specimens (normal control) were studied. The samples were from the pathology archive of Alzahra Hospital, Isfahan, Iran, from 2016 to 2018. The status of intraepithelial MMP-9 were studied and compared in these three groups using immunohistochemistry. Results: The extent of intraepithelial MMP-9 immunostaining in all positive specimens was 100%. The results of intraepithelial MMP-9 staining intensity was as follow: 12.5% strong, 27.5% moderate, 27.5% mild, and 32.5% negative in tumor group; 17.5% strong, 22.5% moderate, 32.5% mild, and 27.5% negative in tumor control group; and 10% strong, 40% moderate, 27.5% mild, and 22.5% negative in normal control group. Intraepithelial MMP-9 immunostaining intensity showed significant difference between tumor and tumor control groups (P<0.001). Intraepithelial MMP-9 immunostaining intensity showed no significant difference between tumor and normal control groups, and between tumor control and normal control groups (P>0.05). No significant relationship was seen between intraepithelial MMP-9 immunostaining intensity and age, tumor size, tumor grade, and lymph node status in tumor group (P>0.05). Conclusion: Intraepithelial MMP-9 expression increases in some breast carcinomas. Normal breast tissue adjacent to carcinoma does not show such increase. However, intraepithelial MMP-9 expression in breast carcinoma does not show any significant relationship with age, tumor size, tumor grade, and lymph node status.
Keywords: Matrix metalloproteinase-9, breast cancer, immunohistochemistry, prognostic factors
Introduction
Breast carcinoma is the most prevalent non-skin carcinoma in women and the second cause of cancer related mortalities after lung cancer. There is a chance of 1:8 for breast cancer in a woman aged around 90 years. In a study performed in 2012 in the United States, there were 40000 deaths among 226000 women with invasive breast carcinoma [1-3].
Matrix metalloproteinases (MMPs) are a huge family of extracellular enzymes dependent to zinc and calcium. These enzymes are categorized based on their function and structures into matrilysin, gelatinases, stromelysin, and collagenases [4].
MMP-9 is one of the most important gelatinases which is highly effective in tumoral invasion and destruction of type IV collagen and other components of extracellular matrix [5,6].
There is low expression of MMPs in normal tissues. Increased activity and expression of these enzymes causes tissue destruction. This situation occurs in inflammatory diseases and tumors. As a result, MMPs have been considered as a marker for tissue destruction [7].
Early diagnosis of breast cancer and its micro-metastases are pivotal factors in cancer treatment. Some studies have found relationship between MMP-9 expression and prevalence of metastasis in breast carcinoma [8,9]. Stromal MMP-9 expression has been shown as a predicting factor for hormonal response and survival in patients with breast cancer [10]. The relationship between MMP-9 expression and lymph node status has also been reported in patients with breast cancer [11].
Matrix metalloproteinases, a family of key molecules in regulating microenvironment of tissues, have been studied as probable prognostic markers of breast cancer. Shen et al evaluated 27-hydroxycholesterol in breast cancer and found this molecule to cause metastasis and more invasive behavior in breast carcinoma by increasing MMP-9 [12].
Milovanovic et al indicated that tamoxifen performs its therapeutic effects on breast carcinoma by influencing MMP-2 [13].
However, the results of previous studies on the issue of MMP-9 expression in breast cancer and its impact on tumor prognosis has not been yet conclusive. The objective of this study has been to assess the frequency of intracellular expression of MMP-9 in invasive breast carcinoma and normal breast tissue, and to investigate the relationship between intracellular expression of this marker in breast carcinoma and some well-known clinicopathological prognostic variables of this cancer.
Materials and methods
Study design and setting
This cross-sectional study was performed on formalin-fixed and paraffin-embedded tissue blocks from 40 invasive breast carcinomas (tumor group) and 40 normal tissue adjacent to carcinoma (tumor control group), as well as 40 normal breast tissue specimens from mammoplastic surgery (normal control group). The specimens were obtained from pathology archive of Alzahra Hospital, Isfahan, Iran, from 2016 to 2018. The study was approved by ethical committee of Isfahan University of Medical Sciences, Isfahan, Iran (Ethical code: IR.MUI.MED.REC.1398.055). Inclusion criteria for tumor group was breast lumpectomy or mastectomy specimens with documented diagnosis of invasive breast carcinoma having normal tissue adjacent to carcinoma and dissected axillary lymph nodes. Concerning normal control group, only mammoplasty specimens having normal breast tissue without any kind of breast pathology were included in the study. Exclusion criteria for tumor group was those carcinoma specimens without adjacent normal breast tissue and/or lacking dissected axillary lymph nodes. Concerning normal control group, mammoplasty specimens with any kind of breast pathology were excluded from the study.
Lab procedure
After collection of samples, we used immunohistochemistry to stain all 120 specimens with MMP-9 antibody (Rabbit Polyclonal Antibody, Diagnostic BioSystems, Canada). Five micron sections were prepared from tissue blocks and underwent immunohistochemical staining according to the following steps:
48 hours incubation in oven at 37°C, dewaxation by 100% xylol, rehydration by decreasing concentrations of ethanol (100%, 85%, and 75%), rinsing in 10% phosphate-buffered saline (PBS) solution, 30 minutes incubation in 10% H2O2 and methanol to inhibit endogenous peroxidase activity, rinsing in 10% PBS solution, 14 minutes incubation in citrate-buffered solution (PH=6.1) in microwave, rinsing in 10% PBS solution, adding blocking serum for 30 minutes to block endogenous non-specific bindings, drying, adding primary monoclonal antibody, 30 minutes incubation at room temperature, rinsing in 10% PBS solution, adding broad-spectrum secondary antibody for 30 minutes, adding horseradish peroxidase-streptavidin and diaminobenzidine (DAB) for 30 minutes and 10 minutes, respectively, rinsing in 10% PBS solution, dehydration by increasing concentrations of ethanol (75%, 85%, and 100%), and counterstaining with hematoxylin.
The intensity and extent of cytoplasmic MMP-9 immunoreactivity were then examined in epithelial cells of breast carcinoma and normal breast tissue. Epithelial cells, fibroblasts, and extracellular matrix all may show immunoreactivity with MMP-9 antibody [10,11]. However, only MMP-9 expression in epithelial cells has been considered in this study.
Assessments
Scores of staining intensity were defined as follow [10-12,14]: Score 0: Negative; Score 1: Mild; Score 2: Moderate; Score 3: strong.
The percentage of stained epithelial cells irrespective of the intensity of staining was evaluated to determine and classify staining extent as follow [10-12,14]: Score 0: 0 to 10%; Score 1: 11 to 25%; Score 2: 26 to 50%; Score 3: 51 to 75%; Score 4: 76 to 100%.
The intensity and extent of cytoplasmic staining of MMP-9 in epithelial cells was then compared between the three groups. In tumor group, the relationship between intraepithelial MMP-9 expression and some prognostic factors including age, tumor size, tumor grade, and lymph node status was also studied. Data concerning age, tumor size (greatest tumor diameter), tumor grade, and lymph node status were all achieved from pathology archive of the hospital.
Statistical analysis
SPSS software, version 24, was used for data analysis. Data were shown as frequency, mean and standard deviation. To determine the relationship between MMP-9 expression and prognostic variables in tumor group, Chi-Square and one-way ANOVA were used. P-value less than 0.05 was considered as significant.
Results
In this study, we evaluated three groups including 40 specimens of invasive breast carcinoma (tumor group) and 40 specimens of normal tissue adjacent to these carcinoma specimens (tumor control group), as well as 40 specimens of normal control specimens from mammoplastic surgery (normal control group). The results of intraepithelial MMP-9 staining intensities were as follow: 12.5% strong, 27.5% moderate, 27.5% mild, and 32.5% negative, in tumor group, 17.5% strong, 22.5% moderate, 32.5% mild, and 27.5% negative in tumor control group; and 10% strong, 40% moderate, 27.5% mild, and 22.5% negative in normal control group (Table 1) (Figure 1).
Table 1.
Intraepithelial MMP-9 staining intensity in tumor, tumor control, and normal control groups
| MMP-9 staining | Tumor | Tumor control | Normal control |
|---|---|---|---|
| Negative | 13 (32.5%) | 11 (27.5%) | 9 (22.5%) |
| Mild | 11 (27.5%) | 13 (32.5%) | 11 (27.5%) |
| Moderate | 11 (27.5%) | 9 (22.5%) | 16 (40%) |
| Strong | 5 (12.5%) | 7 (17.5%) | 4 (10%) |
Figure 1.
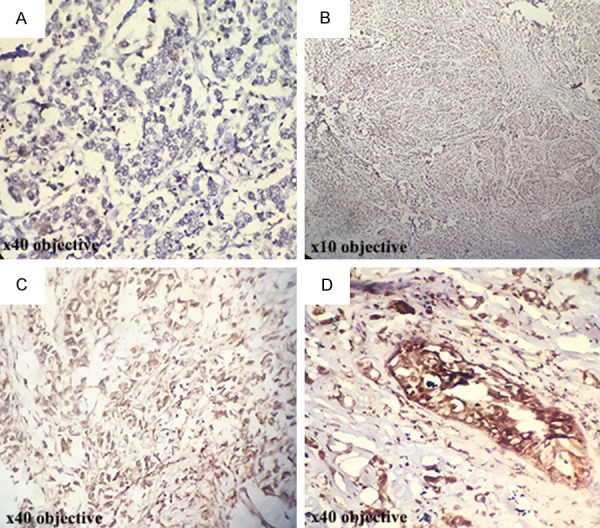
Immunohistochemical staining for evaluation of intraepithelial MMP-9 in breast carcinoma: (A) No staining (×40 objective), (B) Weak staining (×10 objective), (C) Moderate staining (×40 objective), (D) Strong staining (×40 objective).
Two cases in tumor group with negative MMP-9 staining had mild MMP-9 staining in tumor control and intraepithelial MMP-9 immunostaining intensity showed significant difference between tumor and tumor control groups (P<0.001) (Table 2).
Table 2.
Comparison of intraepithelial MMP-9 staining intensity between tumor and tumor control groups
| MMP-9 staining | Tumor group | P-value* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Negative | Mild | Moderate | Strong | |||
| Tumor control group | Negative | 11 (84.6%) | 0 | 0 | 0 | <0.001 |
| Mild | 2 (15.4%) | 7 (63.6%) | 3 (27.3%) | 1 (20%) | ||
| Moderate | 0 | 3 (27.3%) | 5 (45.5%) | 1 (20%) | ||
| Strong | 0 | 1 (9.1%) | 3 (27.3%) | 3 (60%) | ||
Chi Square.
There was no significant difference between normal control with tumor and tumor control based on intraepithelial MMP-9 immunostaining intensity (P>0.05).
In tumor group, the means of age, tumor size, and number of involved lymph nodes were 44.15±9.71 years, 4.37±2.81 cm, and 3.75±4.33, respectively. The frequencies of tumor grading were 10% grade I, 57.5% grade II, and 32.5% grade III. No significant relationship was seen between MMP-9 immunostaining intensity with age, tumor size, tumor grade, and number of involved lymph node in tumor group (P>0.05) (Table 3).
Table 3.
Relationship between intraepithelial MMP-9 staining intensity and age, tumor grade, tumor size, and lymph node status in tumor group
| variable | MMP-9 staining in tumor group | Total (n=40) | P-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Negative (n=13) | Mild (n=11) | Moderate (n=11) | Strong (n=5) | ||||
| Age (mean ± SD) (years) | 42.38±10.71 | 43.55±9.51 | 46.09±7.44 | 45.80±13.64 | 44.15±9.71 | 0.80 | |
| Grade | I | 1 (7.7%) | 1 (9.1%) | 1 (9.1%) | 4 (10%) | 4 (10%) | 0.37 |
| II | 7 (53.8%) | 8 (72.7%) | 4 (36.4%) | 23 (57.5%) | 23 (57.5%) | ||
| III | 5 (38.5%) | 2 (18.2%) | 6 (54.5%) | 13 (32.5%) | 13 (32.5%) | ||
| Size (mean ± SD) [6] | 4.75±3.72 | 3.13±1.26 | 5.31±2.75 | 4.06±2.36 | 4.37±2.81 | 0.31 | |
| Number of Involved Lymph node (mean ± SD) | 3.62±4.40 | 4.55±4.90 | 3.45±4.82 | 3.01±2.01 | 3.75±4.33 | 0.90 | |
Discussion
In this study, intracellular MMP-9 staining intensity showed a significant difference between breast carcinoma and normal tissue adjacent to carcinoma. This finding suggests increased MMP-9 expression as an event in the pathway of transformation of normal breast epithelium to carcinoma cells. Concerning the association between MMP-9 expression and clinicopathological prognostic factors, we could not find any significant relationship between expression of this marker and age, tumor size, tumor grade, and lymph node status. This finding is different from most previous studies on this issue.
Furthermore, some studies have indicated that levels of MMP-9 in both serum and tissue of patients with breast cancer might be a determinant of tumor structure [15-17]. Padala et al showed synergistic effects of MMP-1, MMP-3 and MMP-9 in breast carcinoma [18]. Sun et al showed that higher levels of glucose might influence increased invasiveness of tumors by regulating Glut-1/MMP-2/MMP-9 axis [19]. In another study by Lou et al, arctigenin was found to have therapeutic effects against cancer with the mechanism of reducing MMP-2/-9 expression in MDA-MB-231 cells [15]. These studies emphasize on the hypothesis that MMP-9 might be an important factor in pathogenesis of breast carcinoma. Reggiani et al showed that MMP-9 promotes the protumorigenic effect of white adipose tissue progenitors on local and metastatic breast cancer [16]. Wu et al evaluated 60 tissue samples of breast cancer and investigated MMP-9 and its mRNA in tumor tissue using immunohistochemistry and in situ hybridization. They also evaluated serum and tissue levels of MMP-9 and compared it with clinical and pathological characteristics of patients. Their results showed that serum levels of MMP-9 is highly increased in patients with breast cancer compared to normal population. This increase was also associated with lymph node metastasis, higher tumor grade, shorter relapse-free period, and reduced overall survival [17]. Li et al found MMP-9 and MMP-2 markers to have high prognostic value in lymph node negative breast carcinoma [20]. Mylona et al suggested the possible relationship between cytoplasmic MMP-9 and well-differentiated breast carcinoma, while they found stromal MMP-9 to be associated with an aggressive phenotype of breast cancer [21]. These findings are different from the results of our study. This discrepancy in results may be attributive to different study populations. However, more studies are needed before making any conclusive justification of this discrepancy.
The limitation of this study was its small sample size and lack of evaluation of other clinicopathological prognostic factors of breast carcinoma.
Conclusions
The results of this study suggest increased MMP-9 expression as an event in the pathway of transformation of normal breast epithelium to carcinoma cells. Concerning the association between MMP-9 expression and clinicopathological prognostic factors, we could not find any significant relationship between expression of this marker and age, tumor size, tumor grade, and lymph node status.
Acknowledgements
The authors would like to thank Isfahan University of Medical Sciences vice chancellery for research (Isfahan, Iran) for financial support of this study. This paper is derived from a specialty thesis No. 398017 at Isfahan University of Medical Sciences, Isfahan, Iran. The university vice chancellery for research approved the study in 2018 and financially supplied it.
Disclosure of conflict of interest
None.
Abbreviations
- MMP
matrix metalloproteinase
References
- 1.Kumar V, Abbas AK, Aster JC. Robbins basic pathology E-book. Elsevier Health Sciences. 2017 [Google Scholar]
- 2.Smith RA, DeSantis CE. Breast cancer epidemiology. Breast Imaging. 2018 [Google Scholar]
- 3.Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59:651–672. doi: 10.1097/GRF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 4.Somiari SB, Somiari RI, Heckman CM, Olsen CH, Jordan RM, Russell SJ, Shriver CD. Circulating MMP2 and MMP9 in breast cancer-potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int J Cancer. 2006;119:1403–1411. doi: 10.1002/ijc.21989. [DOI] [PubMed] [Google Scholar]
- 5.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000;2:252–7. doi: 10.1186/bcr65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liotta L, Tryggvason K, Garbisa S, Hart I, Foltz C, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 7.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 8.Angahar L. An overview of breast cancer epidemiology, risk factors, pathophysiology, and cancer risks reduction. MOJ Biol Med. 2017;1:00019. [Google Scholar]
- 9.Tabatabaei-Malazy O, Qorbani M, Samavat T, Sharifi F, Larijani B, Fakhrzadeh H. Prevalence of dyslipidemia in Iran: a systematic review and meta-analysis study. Int J Prev Med. 2014;5:373. [PMC free article] [PubMed] [Google Scholar]
- 10.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 11.Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen Z, Zhu D, Liu J, Chen J, Liu Y, Hu C, Li Z, Li Y. 27-Hydroxycholesterol induces invasion and migration of breast cancer cells by increasing MMP9 and generating EMT through activation of STAT-3. Environ Toxicol Pharmaco. 2017;51:1–8. doi: 10.1016/j.etap.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Drzewiecka-Jędrzejczyk M, Wlazeł R, Terlecka M, Jabłoński S. Serum metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in lung carcinoma patients. J Thorac Dis. 2017;9:5306. doi: 10.21037/jtd.2017.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutmann B, Mierau A, Hülsdünker T, Hildebrand C, Przyklenk A, Hollmann W, Strüder HK. Effects of physical exercise on individual resting state EEG alpha peak frequency. Neural Plast. 2015;2015:717312. doi: 10.1155/2015/717312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lou C, Zhu Z, Zhao Y, Zhu R, Zhao H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep. 2017;37:179–184. doi: 10.3892/or.2016.5269. [DOI] [PubMed] [Google Scholar]
- 16.Reggiani F, Labanca V, Mancuso P, Rabascio C, Talarico G, Orecchioni S, Manconi A, Bertolini F. Adipose progenitor cell secretion of GM-CSF and MMP9 promotes a stromal and immunological microenvironment that supports breast cancer progression. Cancer Res. 2017;77:5169–5182. doi: 10.1158/0008-5472.CAN-17-0914. [DOI] [PubMed] [Google Scholar]
- 17.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer. 2008;122:2050–2056. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]
- 18.Padala C, Tupurani MA, Puranam K, Gantala S, Shyamala N, Kondapalli MS, kumar Gundapaneni K, Mudigonda S, Galimudi RK, Kupsal K. Synergistic effect of collagenase-1 (MMP1), stromelysin-1 (MMP3) and gelatinase-B (MMP9) gene polymorphisms in breast cancer. PLoS One. 2017;12:e0184448. doi: 10.1371/journal.pone.0184448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun XF, Shao YB, Liu MG, Chen Q, Liu ZJ, Xu B, Luo SX, Liu H. High-concentration glucose enhances invasion in invasive ductal breast carcinoma by promoting Glut1/MMP2/MMP9 axis expression. Oncol Lett. 2017;13:2989–2995. doi: 10.3892/ol.2017.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS, Di GH, Liu G, Li FM, Ou ZL. Prognostic value of matrix metalloproteinases (MMP-2 and MMP-9) in patients with lymph node-negative breast carcinoma. Breast Cancer Res Treat. 2004;88:75–85. doi: 10.1007/s10549-004-1200-8. [DOI] [PubMed] [Google Scholar]
- 21.Mylona E, Nomikos A, Magkou C, Kamberou M, Papassideri I, Keramopoulos A, Nakopoulou L. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology. 2007;50:338–347. doi: 10.1111/j.1365-2559.2007.02615.x. [DOI] [PubMed] [Google Scholar]


