Abstract
This systematic review summarizes current evidence on the impact of bariatric surgery (BS) on physical performance, metabolic, and health indices in adults with obesity. This systematic review suggests that BS induced significant reductions in body weight, fat mass, and fat-free mass in individuals with obesity. Additionally, BS may improve many physical fitness and health indicators. Observed improvements manifest during a distinct period of time. To date, studies on BS and performance have been small in number, nonrandomized in design, and not controlled regarding gender distribution and/or post-surgery follow-up. Future studies should further investigate concerns associated with understanding of BS outcomes to improve these outcomes with potential benefits for quality of life, disability, mortality, morbidity, and overall BS success.
Keywords: Obesity, Bariatric surgery, Cardiovascular, Metabolic, Aerobic, Cardiac autonomic function
Introduction
Severe obesity, defined as a body mass index (BMI) of at least 35 kg m2 [1], is strongly associated with several health complications [2–4] along with significant impairments in physical capacity and overall fitness parameters [5–8]. Bariatric surgery (BS) is emerging as an important option for those suffering from severe obesity when nonsurgical weight loss methods have been exhausted. In addition to the direct impact on weight loss, BS improves many health indicators during the post-operative period [9–13]. These changes were correlated with the quality of life and overall health parameters [13].
Changes attributed to BS at post-operative stages have focused mainly on body weight and composition changes, metabolic control, and energy adaptation [9, 10, 14–17] alongside some research that has investigated physical functioning and fitness capacity outcomes. These latter outcomes are known to be relevant in the obesity context especially since they are considered important mediators in developing risk factors for cardiovascular disease in this population [18–20].
In light of what was discussed above, this systematic review aimed to summarize recent findings on the effects of BS alone, without any exercise prescription or lifestyle modification, on the most relevant cardiorespiratory (e.g., oxygen uptake, heart rate), performance (e.g., muscular strength, distance covered), and health (e.g., autonomic nervous system modulation, metabolic parameters) outcomes in adults with obesity undergoing BS.
A good understanding of the effects of BS on cardiorespiratory, performance, and health outcomes is highly recommended for future intervention studies to improve these outcomes with potential benefits for quality of life, disability, mortality, morbidity, and overall BS success.
Methods
Eligibility Criteria
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]. The population, intervention, comparator, outcomes, and study design (PICOS) approach was used to identify the inclusion criteria (Table 1). Only studies with a longitudinal design, of any duration, that have examined effects of BS on anthropometric characteristics and body composition (e.g., body weight, body fat, body mass index), physical performance (e.g., muscular strength, physical capacity), cardiorespiratory fitness and function (e.g., oxygen uptake, heart rate, heart rate variability), and energy expenditure and metabolism parameters (e.g., total energy expenditure, insulin resistance, lipid oxidation), in individuals with obesity undergoing any recognized surgical bariatric procedure, were eligible for inclusion. Studies were included in the current systematic review if they were in accordance with the following criteria: (1) published in peer-reviewed journals; (2) included adults and older of both genders; (3) compared BS outcomes at pre- and at post-surgery. Studies were excluded if they (1) assessed other types of interventions (in addition to the surgery), (2) reported only subjective measures, or (3) were not written in English. Moreover, review articles were not included in the current systematic review.
Table 1.
PICOS criteria for the inclusion of studies
| Parameters | Inclusion criteria |
|---|---|
| Population | Adults with severe obesity |
| Intervention | Bariatric surgery (purely gastric restrictive and gastric bypass with intestinal transposition) |
| Comparator | Pre-surgery versus post-surgery |
| Outcomes | Body composition, weight loss, physical capacity and performance, physical activity level, cardiorespiratory fitness, energy expenditure, metabolic parameters, substrate use, autonomic nervous system modulation |
| Study design | Retrospective, randomized control trial, and prospective studies |
Literature Search Strategy
Literature searches were conducted in four electronic databases, including PubMed, ISI Web of Knowledge, Web of Science, and SPORTDiscus. The following key terms (and synonyms searched for by the MeSH database) were included and combined using the operators “AND,” “OR,” and “NOT”: “anthropometric characteristics” or “body composition” or “physical performance” or “physical capacity” or “fitness” or “physical activity level” or “functional capacity” or “muscular performance” or “muscular strength” or “anaerobic capacity” or “aerobic capacity” or “cardiorespiratory function” or “cardiopulmonary function” or “energy expenditure” or “respiratory quotient” or “energy metabolism” or “cardiac autonomic control” or “heart rate variability” or “metabolic parameters” or and “bariatric surgery” or “obesity surgery” or “weight loss surgery” or “metabolic surgery” or “gastric bypass” or “gastric banding” or “sleeve gastrectomy” or “biliopancreatic diversion” or “duodenal switch.” The search was completed with a manual search of reference lists from key papers. Since the scope of this review is large in terms of outcome measures, a systematic review and not a meta-analysis was performed.
Study Selection
The final screening was performed by the principal investigator (GJ) based on the relevance of the inclusion and exclusion criteria and the identified items for assessing the effects of BS on anthropometric characteristics and body composition (e.g., body weight, body fat, body mass index), physical performance (e.g., muscular strength, physical capacity), cardiorespiratory fitness and function (e.g., oxygen uptake, heart rate, heart rate variability), and energy expenditure and metabolism parameters (e.g., total energy expenditure, insulin resistance, lipid oxidation), in adults with obesity of both gender undergoing BS using PICOS criteria. If the citation showed any potential relevance, the abstract was screened. When abstracts indicated potential inclusion, full-text articles were reviewed.
Results
Study Selection and Description
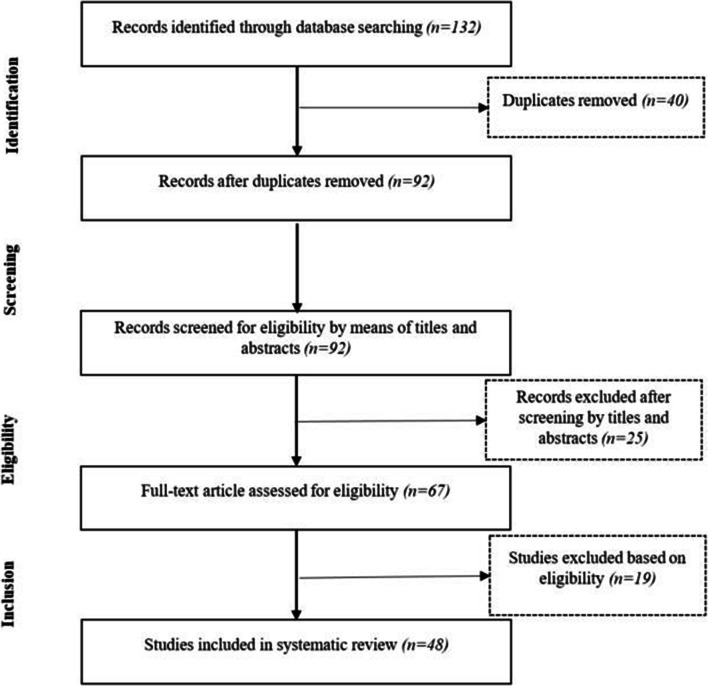
Our search initially identified 132 records (Fig. 1). After screening titles, abstracts, and full texts, 48 studies were included in our final analysis, and the characteristics of these long-term studies are shown in Table 2. The 48 studies reported on a total of 7105 patients; the mean age ranged from 18 to 60 years, and the mean follow-up interval ranged from 1 week to ≥ 24 months (Table 2). All studies had patient samples with a majority of female patients, except Wu et al. [65] who had two similarly sized gender groups (9 F and 9 M). The body mass index reported at baseline ranged from 37 to 55 kg/m2 (Table 2). Thirty-four studies used a gastric bypass (GB) procedure or a version of Roux-en-Y gastric bypass (RYGB) [16, 17, 22, 24–27, 30–32, 34, 36, 38–40, 42–45, 48–50, 52, 53, 55–61, 63, 64, 67], and seven studies reported laparoscopic adjustable gastric banding (LAGB) [22, 25, 33, 41, 43, 58, 67], of which five were combined with another BS method [22, 25, 33, 67]. Thirteen studies reported on laparoscopic sleeve gastrectomy (LSG) [28, 30, 36, 37, 47, 49, 52, 55, 56, 58, 59, 64], of which 8 were combined with another BS method [28, 30, 36, 49, 52, 55, 56, 58, 59, 64]. Three studies enrolled patients undergoing vertical-banded gastroplasty (VBG) [26, 35, 62], and Nault et al. [46] included patients who underwent BDP and biliopancreatic diversion.
Fig. 1.
Flow diagram of included and excluded studies included in this systematic review using the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [21]
Table 2.
Baseline characteristics of studies included in the systematic review
| Author (year) | Study design | Operation | Baseline BMI (SD) | Population | Evaluation period | Main outcomes | |
|---|---|---|---|---|---|---|---|
| Mean age, years (SD) | Gender | ||||||
| Alam et al. [22] | Prospective cohort study |
BPD (N = 5) LAGB (N = 6) |
53.0 (7.3) BDP 44.2 (3.2) LGB |
48 (8) | 8 F and 3 M | Pre-surgery and 1, 6, and 12 months post-surgery |
Body weight Autonomic nervous system modulation Metabolic parameters |
| Alba et al. [23] | Prospective cohort study | RYGB | 44 (8) | 45 (12) | 37 F and 10 M | Pre-surgery and 6 and 12 months post-surgery |
Body composition Physical capacity and performance Physical activity level |
| Benedetti et al. [14] | Prospective study | BPD | Not specified | 36.1 (1.62) | 9 F and 5 M | Pre-surgery and 30 months post-surgery |
Body weight Body composition Energy expenditure Metabolic parameters |
| Bobbioni-Harsch et al. [24] | Prospective cohort study | RYGB | 44.6 (1.1) | 39.5 (2) | 12 F | Pre-surgery and 3 and 12 months post-surgery |
Body weight Body composition Metabolic parameters Autonomic nervous system modulation |
| Bond et al. [25] | Prospective cohort study |
LAGB (N = 65%) RYGB (N = 35%) |
50.1 (9.1) | 46.2 (9.8) | 17 F and 3 M | Pre-surgery and 6 months post-surgery | Physical activity level |
| Braga et al. [26] | Prospective cohort study |
VBG (N = 5) RYGB (N = 14) Medical treatment (N = 20) |
41.5 (5.0) | 38.5 (10.6) | 14 F and 6 M | Pre-surgery and 3 months post-surgery |
Body weight Body composition Metabolic parameters Autonomic nervous system modulation |
| Browning et al. [27] | Prospective cohort study | GBS | 42.9 (4.1) | 21–55 | 9 F | Pre-surgery and 3 months post-surgery |
Body weight Body composition Cardiorespiratory fitness |
| Campos et al. [9] | Prospective cohort study | Not specified | 47.42 (5.72) | 40 (7) | 24 F | Pre-surgery and 6 months post-surgery |
Body weight Body composition Physical capacity and performance Cardiorespiratory fitness Physical activity level |
| Carrasco et al. [16] | Prospective cohort study | RYGB | 44.4 (4.8) | 37.3 (11.1) | 27 F and 4 M | Pre-surgery and 6 months post-surgery |
Body weight Body composition Metabolic parameter Energy expenditure Substrate use Physical activity level |
| Colles et al. [17] | Prospective cohort | LAGB | 44.3 (6.8) | 45.2 (11.5) | 103 F and 26 M | Pre-surgery and 4 and 12 months post-surgery |
Body weight Body composition Physical activity level |
| Daniel et al. [28] | Prospective cohort study | LSG | 44.04 (5.84) | 47.0 (9.0) | 14 F and 10 M | Pre-surgery and 5.9 (2.3) months and 15.5 (7.2) months post-surgery |
Body weight Cardiorespiratory fitness |
| Das et al. [29] | Prospective cohort study | GBS | 50.1 (9.3) | 39.0 (9.6) | 24 F and 6 M | Pre-surgery and 14 months post-surgery |
Body weight Body composition Energy expenditure Physical activity level |
| Dereppe et al. [30] | Prospective cohort study |
LSG (N = 18) RYGB (N = 24) |
44 (4) | 42 (13) | 42 F | Pre-surgery and 12 months post-surgery |
Body weight Body composition Metabolic parameters Cardiorespiratory fitness |
| De Souza et al. [31] | Prospective cohort study | RYGB | 49.4 (5.4) | 40.4 (8.4) | 61 F and 4 M | Pre-surgery and 6 and 12 months post-surgery |
Body weight Physical capacity and performance Cardiorespiratory fitness |
| De Souza et al. [32] | Prospective cohort study | RYGB | 51.1 (9.2) | 40.9 (9.2) | 44 F and 7 M | Pre-surgery and 7 and 12 months post-surgery |
Body weight Physical capacity and performance |
| Galtier et al. [33] | Prospective cohort study | LAGB | 44.37 (7.0) | 39.17 (10.4) | 73 F | Pre-surgery and 13.37 (6.0) months post-surgery (group A [6–12 months, n = 39]; group B [12–18 months, n = 21]; group C [418 months, n = 13]) |
Body weight Body composition Metabolic parameters Energy expenditure Substrate use |
| Iannelli et al. [34] | Prospective cohort study | RYGB | 44.6 (5.2) | 39.9 (10) | 115 F | Pre-surgery and 12 months post-surgery |
Body weight Body composition Metabolic parameters Energy expenditure |
| Kanoupakis et al. [35] | Prospective cohort study | VBG | 49 (8) | 22–43 | 10 F and 6 M | Pre-surgery and 6 months post-surgery |
Body weight Cardiorespiratory fitness |
| Kokkinos et al. [36] | Prospective cohort study |
RYGB LAGB |
48.4 (8.2) | 44.2 (10.8) | 450 F and 128 M | Pre-surgery and 12 months post-surgery |
Body weight Body composition Autonomic nervous system modulation Cardiorespiratory fitness |
| Li et al. [37] | Prospective cohort study |
RYGB (N = 14) LSG (N = 23) |
47.9 (6.0) for the RYGB group 51.6 (7.5) for the SG group |
38.0 (7.8) for the RYGB group 40.3 (9.9) for the SG group |
Not specified | Pre-surgery and 3 and 6 months post-surgery |
Body weight Body composition Metabolic parameters Cardiorespiratory fitness Autonomic nervous system modulation |
| Liu et al. [38] | Retrospective cohort study | LSG | 37.2 (6.1) | 35.3 (11.8) | 52 F and 45 M | Pre-surgery and 6 months post-surgery |
Body weight Body composition Energy expenditure Physical activity level |
| Lund et al. [39] | Prospective cohort study | GBS | 44.6 (1.2) | 41.2 (2) | 11 F and 2 M | Pre-surgery and 6 months post-surgery |
Body weight Body composition Metabolic parameters Cardiorespiratory fitness Physical activity level |
| Machado et al. [40] | Prospective cohort study | GBS | 43 (1) | 40 (2) | 22 F and 9 M | Pre-surgery and 4 and 18 months post-surgery |
Body weight Body composition Autonomic nervous system modulation |
| Maniscalco et al. [41] | Prospective cohort study | LAGB | 43 (37 to 56) | 37 (18 to 66) | 42 F and 29 M | Pre-surgery and 6 months post-surgery |
Body weight Body composition Autonomic nervous system modulation Physical capacity and performance |
| Maser et al. [42] | Prospective cohort study | RYGB | 51 (11) | 38 (11) | 29 F and 3 M | Pre-surgery and 6 months post-surgery |
Body weight Metabolic parameters Autonomic nervous system modulation |
| Maser et al. [43] | Prospective cohort study | RYGB | 47.7 (7) | 45 (9) | 22 F and 4 M | Pre-surgery and 6 and 12 months post-surgery |
Body weight Metabolic parameters Autonomic nervous system modulation |
| McCullough et al. [44] | Prospective cohort study | RYGB | 50.4 (6.0) | 46.0 (10.4) | 82 F and 27 M | 30-day period after discharge |
Body weight Cardiorespiratory fitness |
| Mirahmadian et al. [45] | Prospective cohort study |
RYGB (N = 21) LAGB (N = 5) |
47.7 (7) | 45 (9) | 22 F and 4 M | Pre-surgery and 6 and 12 months post-surgery |
Body weight Body composition Energy expenditure |
| Nault et al. [46] | Randomized controlled trials | BPD | 52.3 (7.6) for BPD-DS and 54.3 (10.9) for C | 37.7 (8.5) for BPD-DS and 44.7 (10.8) for C | 6 F and 4 M for BPD-DS and 7 for C | Pre-surgery and 6 and 12 months post-surgery |
Body weight Body composition Metabolic parameters Autonomic nervous system modulation |
| Neunhaeuserer et al. [47] | Prospective cohort study | LSG | 45.2 (5.8) | 48.23 (9.01) | 26 | Pre-surgery and 6 months post-surgery |
Body weight Cardiorespiratory fitness Physical capacity and performance |
| Notarius et al. [48] | Randomized controlled trials | GBS | 46.1 (6.4) | 18–60 | 42 and 21 for C | Pre-surgery and 6 months post-surgery |
Body weight Energy expenditure Cardiorespiratory fitness Physical capacity and performance |
| Otto et al. [49] | Prospective cohort study |
RYGB (N = 16) LSG (N = 9) |
47.40 (6.3) | 36.8 (11.7) for F and 46.7 (9.0) for M | 16 F and 9 M | Pre-surgery and repeated every 6 weeks for 4 months |
Body weight Body composition Physical capacity and performance |
| Perugini et al. [50] | Prospective cohort study | RYGB | 46 (6) | 45 (9) | 21 F and 7 M | Pre-surgery and 6 months post-surgery |
Body weight Autonomic nervous system modulation Metabolic parameters |
| Ravelli et al. [51] | Prospective cohort study | RYGB | 44.9 (2.5) | 20 W | 29.4 (5.1) | Pre-surgery and 6 and 12 months post-surgery |
Body weight Body composition Energy expenditure |
| Remígio et al. [52] | Prospective cohort study |
RYGB LSG |
46.2 (4.9) | 20–45 | 24 | Pre-surgery and 4 months post-surgery |
Body weight Metabolic parameters Cardiorespiratory fitness |
| Sans et al. [53] | Prospective cohort study | RYGB | 43.3 (4.9) | 40.6 (11.2) | 103 F | Pre-surgery and 12 months post-surgery |
Body weight Body composition Metabolic parameters Energy expenditure |
| Seres et al. [54] | Prospective cohort study | Not specified | 51 (4) | 38 (8) | 20 F and 11 M | Pre-surgery and 12 months post-surgery |
Body weight Cardiorespiratory fitness Physical capacity and performance |
| Schneider et al. [55] | Randomized controlled trials |
RYGB LSG |
43.9 (1.3) | 40.3 (10.9) RYGB versus 41.2 (10.4) LSG | 35 F and 7 M | Pre-surgery and 17 ± 5.6 months post-surgery |
Body weight Body composition Energy expenditure Substrate use |
| Silva et al. [56] | Prospective cohort study |
RYGB (N = 15) LSG (N = 2) |
46 (2) | 30 (1) | 13 F and 4 M | Pre-surgery and 3 months post-surgery |
Body weight Body composition Physical capacity and performance |
| Tamboli et al. [57] | Prospective cohort study | RYGB | 46.3 (5.5) | 43.8 (9.6) | 25 F and 4 M | Pre-surgery and 6 and 12 months post-surgery |
Body weight Body composition Energy expenditure Substrate use |
| Tam et al. [58] | Prospective cohort study |
RYGB (N = 5) LSG (N = 9) LAGB (N = 7) |
47.2 (1.5) | 46 (2) | 27 W | Pre-surgery and 8 weeks and 12 months post-surgery |
Body weight Energy expenditure Physical activity level |
| Tettero et al. [59] | Retrospective cohort study |
RYGB (N = 4359) LSG (N = 426) |
44.9 (6.2) | 43.1 (10.7) | 3867 F and 918 M | Pre-surgery and 12 months post-surgery |
Body weight Cardiorespiratory fitness Physical activity level |
| Tompkins et al. [60] | Prospective cohort study | LGB | 45.5 (6.9) | 44 (6.3) | 28 F and 2 M | Pre-surgery and 6 and 12 months post-surgery |
Body weight Body composition Physical capacity and performance |
| Valezi-Machado et al. [61] | Prospective cohort study | RYGB | 41.8 (4.4) | 35.9 (12.2) | 31 F and 12 M | Pre-surgery and 12 months post-surgery |
Body weight Cardiorespiratory fitness |
| Van Gemert et al. [62] | Prospective cohort study | VBG | 48.1 (7.0) | 28 (7) | 7 W and 1 M | Pre-surgery and 3, 6, and 12 months post-surgery |
Body weight Energy expenditure Substrate use |
| Vargas et al. [63] | Prospective cohort study | RYGB | 50.45 (8.5) | 38 (10) | 61 W and 6 M | Pre-surgery and 3 months post-surgery |
Body weight Physical capacity and performance |
| Wilms et al. [64] | Prospective cohort study |
RYGB (N = 16) LSG (N = 2) |
46.3 (6.8) | 42.5 (10.6) | 11 F and 7 M | Pre-surgery and 12 months post-surgery |
Body weight Cardiorespiratory fitness Physical capacity and performance |
| Wu et al. [65] | Prospective cohort study | LSG | 45.4 (6.8) | 34 | 9 F and 9 M | Pre-surgery and 7, 30, 90, and 180 days post-surgery |
Body weight Autonomic nervous system modulation Metabolic parameters |
| Zavorsky et al. [66] | Prospective cohort study | GBS | 47.3 (6.2) | 39 (8) | 11 F and 4 M | Pre-surgery and 2 months post-surgery |
Body weight Body composition Cardiorespiratory fitness |
BDP, biliopancreatic diversion; LAGB, laparoscopic adjustable gastric banding; RYGB, Roux-en-Y gastric bypass; VBG, vertical-banded gastroplasty; GBS, gastric bypass surgery; LSG, laparoscopic sleeve gastrectomy; F, female; M, male; C, control group
Out of 48 studies, 43 were prospective cohorts [9, 14, 16, 17, 22–37, 39–45, 48–54, 56–58, 61–64, 66] and compared pre-operative to post-operative outcomes in adults undergoing BS. Mirahmadian et al. [45], Nault et al. [46], and Schneider et al. [55] were the only randomized control trials. While Mirahmadian et al. [45] and Nault et al. [46] compared patients who were receiving BS with a control group (without BS), Schneider et al. [55] examined whether there were differences between 2 surgical procedures, laparoscopic sleeve gastrectomy (LSG) and Roux-en-Y gastric bypass (RYGB), in terms of their effect on body composition and energy metabolism. The remaining two studies were retrospective cohorts that compared the main outcomes pre- and post-surgery [45, 46].
Post-operative Body Composition Changes and Weight Loss
Due to the research context, all of the studies include post-operative body composition and weight loss as their primary outcome. Body composition changes and weight loss were generally reported as FM (%, kg), FFM (%, kg), BW (kg), BMI (kg/m2), AC (cm), waist circumference (cm), hip circumference (cm), and W/H ratio. All studies reported a significant improvement in post-operative body composition and weight loss (Tables 3 and 4). These improvements were detected at different post-operative follow-up periods [9, 14, 16, 24, 26, 27, 33, 34, 37, 39, 45, 55, 66]. Some studies examined FFM changes over different post-operative periods and reported significant decreases in FFM (kg) values after a short post-operative follow-up (2 months) for up to 2 years.
Table 3.
Post-operative body composition, weight loss, physical activity level, performance, cardiorespiratory fitness, energy expenditure, metabolic parameters, substrate use, and autonomic nervous system modulation
| Author (year) | Methods | Results | Post-surgery evaluation period (month) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 12 | 14 | 16 | 17 | 18 | ≥ 24 | |||
| Alam et al. [22] | RR and QT time series | ↓BW (kg) | • | • | • | ||||||||||
| ↓BMI (kg/m2) | |||||||||||||||
| ↓HR (bpm) (at 6th) | |||||||||||||||
| ↑QTVI (1st and 12th) | |||||||||||||||
| ↓SampEn (QT) (at 1st) | |||||||||||||||
|
↓DFAα (NN) (1st) ↓DFAα (QT) (1st) | |||||||||||||||
| ↓RR (6th) | |||||||||||||||
| ↓RMSSD (ms) (at 6th) | |||||||||||||||
| ↓SDNN (ms) (at 6th) | |||||||||||||||
|
↓HbA1c (%) (at 12th) ↓Rest systolic blood pressure (mmHg) (at 6th and 12th) | |||||||||||||||
| ↓ Rest diastolic blood pressure (mmHg) (at 12th) | |||||||||||||||
| Alba et al. [23] |
Gait speed and time to rise from a chair five times + 400-m walk test + Handgrip strength + International Physical Activity Questionnaire (IPAQ) |
↓FFM (kg) ↑Gait speed ↑Time for five chair stands ↓Absolute grip strength (at 6th and 12th) ↑Relative strength (at 6th and 12th) ↔ Self-reported physical activity |
• | • | |||||||||||
| Benedetti et al. [14] | Respiration chamber |
↓FM (kg) ↓FFM (kg) ↓REE ((kcal/24 h)) ↑Fasting npRQ ↓Fasting glucose (mmol/dl) ↓Fasting insulin (mU/ml) ↓Fasting FFA (mM) |
• | ||||||||||||
| Bobbioni-Harsch et al. [24] |
Body impedance analyzer + A 120-min euglycemic, hyperinsulinemic clamp + Plasma levels of glucose and free fatty acids (FFA) were enzymatically determined + Heart rate variability (HRV): electrocardiograph continuously recorded for a 24-h period |
↓BMI (kg/m2) ↓FM (kg) ↓FFM (kg) ↑Glucose uptake (mg/kg LBM/min) ↔ FFA (mEq) ↓Plasma insulin (ng/ml) ↑SDNN (ms) ↑RMS (ms) ↑% pNN 50 |
• | • | |||||||||||
| Bond et al. [25] |
RT3 accelerometers + Paffenbarger Physical Activity Questionnaire (PPAQ) |
55% comply with the recommendation (subjective assessment) versus 5% comply with the recommendation (objective assessment) | • | ||||||||||||
| Braga et al. [26] |
Digital scale and a tape measure + HOMA-IR and glucose were quantified by the glucose oxidase colorimetric method + Endothelial reactivity and HRV analysis were performed by peripheral arterial tonometry (PAT) |
↓FM (kg) ↓FFM (kg) ↓AC (cm) ↔ HOMA-IR (%) ↓Fasting glucose (mg/dl) ↓Fasting insulin (IU/l) ↔ LnRHI ↔ AI ↔ AI@75 ↓HR (bpm) ↑HRV-time domain ↓HRV-frequency domain (↑LF/HF) ↔ Systolic blood pressure (mmHg) ↔ Diastolic blood pressure (mmHg) |
• | ||||||||||||
| Browning et al. [27] |
Treadmill with gas-exchange analysis + Stanford 7-day Physical Activity Recall (PAR) |
↓BMI (kg/m2) ↓BW (kg) ↓FM (kg) ↓FFM (kg) ↓Submaximal HR (bpm) ↔ VO2 (l/min/kg) ↑Time to exhaustion (min) ↔ HRmax (bpm) ↔ Absolute VO2peak (l/min) ↑VO2peak (ml/kg/min) ↔ RERmax ↑VO2peak/pulse (ml/beat/kg) ↔ Post-exercise La (mmol/l) |
• | ||||||||||||
| Campos et al. [9] |
Magnetic bioimpedance device + Lung function: computerized ultrasound spirometer with a flow sensor + Respiratory muscle strength: analog manometer + Functional capacity: incremental shuttle walk test (ISWT) + Baecke questionnaire |
↓BMI (kg/m2) ↓BW (kg) ↓WC (cm) ↔ W/H ↓FM (kg) ↓FFM (kg) ↑SVC (l) ↑FVC (l) ↑FEV1 (l) ↑MIP (cmH2O) ↔ MEP (cmH2O) ↑Distance (m) ↔ PA level |
• | ||||||||||||
| Carrasco et al. [16] |
Digital scale and a scale-mounted stadiometer + isotopic dilution with deuterium oxide (total body water) + Open-circuit indirect calorimetry using a ventilated chamber system + Simple survey to assess PA + HOMA-IR |
↓BMI (kg/m2) ↓BW (kg) ↓WC (cm) ↓FM (kg) ↓FFM (kg) ↓W/HR ↓Fasting glucose (mg/dl) ↓Fasting insulin (μU/ml) ↓HOMA-IR ↓Total cholesterol (mg/dl) ↓LDL cholesterol (mg/dl) ↑HDL cholesterol (mg/dl) ↓Triglycerides (mg/dl) ↓Systolic blood pressure (mmHg) ↓Diastolic blood pressure (mmHg) ↓REE ↑Fasting lipid oxidation (%) ↑PA level |
• | ||||||||||||
| Colles et al. [17] | Medical Outcomes Trust Short Form-36 (SF-36) |
↓BW (kg) ↓BMI (kg/m2) ↑SF-36 PCS score (at 12th) |
• | • | |||||||||||
| Daniel et al. [28] | Treadmill with gas-exchange analysis |
↓BMI (kg/m2) ↑METs max ↑Exercise time (s) ↑VO2peak (ml/min/kg) ↓VO2peak (ml/min) Tau (τ) altered at 6th and improved at 16th ↔ RERmax ↑HR/VO2 slope (at 6th) ↓HR rest (bpm) (at 6th) ↓Rest systolic blood pressure (mmHg) (at 6th) ↓Rest diastolic blood pressure (mmHg) (at 6th) |
• | • | |||||||||||
| Das et al. [29] |
15-day doubly labeled water; indirect Calorimetry + Minnesota Leisure Time Physical Activity (LTPA) questionnaire (structured interview) |
↓BMI (kg/m2) ↓BW (kg) ↓FM (kg) ↓FM (%) ↓TEE (MJ/day) ↓REE (MJ/day) ↔ Physical activity level (TEE/REE) ↔ Reported activity (min/day) |
• | ||||||||||||
| Dereppe et al. [30] | Graded cycle ergometer with gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓ Rest systolic blood pressure (mmHg) ↓ Rest diastolic blood pressure ↓Glucose (mg/dl) ↑HDL cholesterol (mg/dl) ↓LDL cholesterol (mg/dl) ↓Triglycerides (mg/dl) ↓VO2peak (ml/min) ↑VO2peak (ml/min/kg) ↓W (W) ↑RERmax |
• | ||||||||||||
| De Souza et al. [31] | Treadmill with gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↑Distance covered (m) ↑Exercise duration (min) ↑VO2max (ml/kg/min) |
• | • | |||||||||||
| De Souza et al. [32] | 6-min walk test |
↓BW (kg) ↓BMI (kg/m2) ↑Distance covered (m) ↓Perceived exhaustion ↓HR ↓Respiratory frequency |
• | ||||||||||||
| Galtier et al. [33] |
Indirect calorimetry with gas-exchange analysis + HOMA-IR + Bioimpedance analysis |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓ Rest systolic blood pressure (mmHg) ↓ Rest diastolic blood pressure (mmHg) ↓Fat-free mass (kg) ↓Fat mass (%) ↔ Fasting blood glucose (mmol/l) ↓120-min OGTT blood glucose (mmol/l) ↓Fasting plasma insulin (mIU/l) ↓Peak-OGTT plasma insulin (mIU/l) ↓Total cholesterol (mmol/l) ↓Triglycerides (mmol/l) ↑HDL cholesterol (mmol/l) ↔ LDL cholesterol (mmol/l) ↓HOMA-IR ↓REE/FFM ↑REE/BW ↔ Lipid oxidation |
• A |
• A, B |
• B |
||||||||||
| Iannelli et al. [34] |
Bioelectrical impedance analysis + Wall-mounted stadiometer and a digital electronic scale + Indirect calorimetry with gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓FFM (kg) ↓FM (%) ↓REE (kcal/24 h) ↓Glucose levels (mmol/l) ↓HOMA-IR ↓HbA1c (%) ↑HDL cholesterol (mmol/l) ↓LDL cholesterol (mmol/l) ↓Triglycerides (mmol/l) |
• | ||||||||||||
| Kanoupakis et al. [35] |
Treadmill with gas-exchange analysis + M-mode, 2-dimensional, and Doppler echocardiography |
↔ Rest HR (bpm) ↔ Rest systolic blood pressure (mmHg) Anaerobic threshold ↔ HR (bpm) ↔ Systolic blood pressure (mmHg) ↓VO2 (ml/min) ↑VO2 (ml/kg/min) ↔ O2 pulse (ml/beat) Maximal exercise ↔ HR (beats/min) ↔ Systolic blood pressure (mmHg) ↑Time (s) ↓VO2 (ml/min) ↑VO2 (ml/kg/min) ↔ O2 pulse (ml/beat) ↑Ventilation (l/min) ↑VCO2 production (ml/min) ↑METs ↓IVS (mm) ↓PW (mm) ↑E/A ↓IVRT (ms) |
• | ||||||||||||
| Kokkinos et al. [36] |
Heart rate variability (HRV) (frequency domain) + Echocardiography |
↓BMI (kg/m2) (at 3th and 6th) ↓Waist (cm) (at 3th and 6th) ↓Hip (cm) (at 3th and 6th) ↑LF (ms2) (for SG) ↑HF (ms2) (for SG and GB) ↔ LF/HF ratio ↑Total power (ms2) (for SG and GB) ↓Epicardial fat (mm) (at 6th) (for SG and GB) ↓LV Tei index (at 6th month) (for SG and GB) ↓LA diameter (mm) (at 6th) (for SG and GB) ↑EF (%) (at 6th) (for SG and GB) ↑LV mass index (g) (at 6th) (for SG and GB) |
• | • | |||||||||||
| Li et al. [37] |
Glucose oxidase method + High-performance liquid chromatography + Automatic analyzer + Electronic scale and fixed wall stadiometer + Segmental bioelectrical impedance analysis + Gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓FFM (kg) ↓FM (kg) ↓Rest systolic blood pressure (mmHg) ↔ Rest diastolic blood pressure (mmHg) ↓Total cholesterol (mmol/l) ↓Triglycerides (mmol/l) ↑HDL cholesterol (mmol/l) ↓LDL cholesterol (mmol/l) ↓Blood glucose levels ↓HbA1c (%) ↓RQ ↓REE (kcal) ↓REE/BW ↔ REE/FFM |
• | ||||||||||||
| Liu et al. [38] |
Bioelectrical impedance analysis + Dual-energy x-ray absorptiometry + Treadmill with gas-exchange analysis + Accelerometer |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓Fat mass (kg) ↓REE (kcal/day) ↓REE/FFM ↔ Physical activity level |
• | ||||||||||||
| Lund et al. [39] |
Stationary ergometer bike with gas-exchange analysis + Physical function was assessed by the SF-36 questionnaire + CAMB questionnaire |
↓BW (kg) ↓BMI (kg/m2) ↓FFM (kg) ↓FM (%) ↓Fasting insulin (pmol/l) ↓Fasting glucose (mmol/l) ↓HbA1c (mmol/mol) ↓Fasting total cholesterol (mmol/l) ↓Systolic blood pressure (mmHg) ↔ Diastolic blood pressure (mmHg) ↓VO2 (ml/min) ↑VO2 (ml/kg/min) ↔ VO2 (ml/kgFFM/min) ↔ Exercise (h/week) ↔ Physical activity level |
• | • | • | ||||||||||
| Machado et al. [40] |
Electronic anthropometric scale + Heart rate variability (HRV) (time domain) |
↓BMI (kg/m2) ↓WC (cm) ↑NN (ms) ↑SDNN (ms) ↑PNN50 (%) ↑RMSSD (ms) |
• | ||||||||||||
| Maniscalco et al. [41] |
Lung volumes and flow rates were determined using automated equipment + 6-min walk test + Oximeter |
↑FVC (% pred) ↑FEV1 (% pred) ↔ FVC/FEV1 ↑TLC (% pred) ↑FRC (% pred) ↑RV (% pred) ↑6-mWT distance (m) ↑HR after 6-mWT (b/min) ↑Baseline SaO2 (%) ↔ SaO2 after 6-mWT (%) ↓Dyspnea score after 6-mWT |
• | ||||||||||||
| Maser et al. [42] |
Measures of HRV (e.g., power spectral analysis, RR variation during deep breathing) + HOMA-IR |
↓BW (kg) ↓HOMA-IR ↓LF ↓HF ↓LF/HF ↑Respiration frequency area |
• | ||||||||||||
| Maser et al. [43] |
RR (interval between R waves of electrocardiographic QRS complexes) + Stadiometer + Finger stick blood glucose readings + Hemoglobin A1c was measured by high-performance ion-exchange liquid chromatography |
↓BMI (kg/m2) ↓Fingerstick glucose (mg/dl) ↓HbA1c (%) ↓Systolic blood pressure (mmHg) ↔ Diastolic blood pressure (mmHg) ↑MCR ↓E/I ratio ↑Valsalva ratio |
• | • | |||||||||||
| McCullough et al. [44] | Bruce treadmill protocols with gas-exchange analysis |
↓BMI (kg/m2) ↔ Systolic blood pressure (mmHg) ↔ Diastolic blood pressure (mmHg) ↑Exercise duration (min) ↑Maximal HR (beats/min) ↔ Perceived exertion (Borg, 6–20) ↓VO2 peak (l/min) ↑VO2 peak (ml/kg/min) ↑V-AT (ml/kg/min) ↔ VE/VCO2 slope |
• | ||||||||||||
| Mirahmadian et al. [45] | Indirect calorimeter with gas-exchange analysis |
↓Body weight (kg) ↓BMI (kg/m2) ↓Fat-free mass (kg and %) ↓Fat mass (kg and %) ↓REE (kcal/day) ↑REE/FM (kcal/kg) |
• | ||||||||||||
| Nault et al. [46] |
Heart rate variability (HRV) (time domain and frequency domain) + Echocardiogram + Biochemical analysis + HOMA-IR |
↓Body weight (kg) ↓BMI (kg/m2) ↓Total cholesterol (mmol/l) ↓Triglycerides (mmol/l) ↑HDL cholesterol (mmol/l) ↓LDL cholesterol (mmol/l) ↓Glucose (mmol/l) ↓Insulin (pmol/l) ↓HOMA-IR ↓HR (beats/min) ↑SDNN (24 h) ↑rMSSD (24 h) ↑pNN50 (24 h) ↑Ln LF (ms2) (24 h) ↑Ln HF (ms2) (24 h) ↓LF/HF (24 h) |
• | • | |||||||||||
| Neunhaeuserer et al. [47] |
Treadmill with gas-exchange analysis + One-repetition maximum (1-RM) |
↑Exercise time (s) ↓VO2peak (l/min) ↑VO2peak (ml/kg/min) ↑VO2/HRmax (ml/bpm) ↓OUES (ml/logl) ↔ RERmax ↑Leg extension (kg) ↔ Handgrip right (kg) ↔ Handgrip left (kg) |
• | ||||||||||||
| Notarius et al. [48] | Treadmill with gas-exchange analysis |
↓TEE ↑Exercise capacity ↓VO2peak (ml/kg/min) |
• | ||||||||||||
| Otto et al. [49] |
Bioelectrical impedance + Handgrip strength |
↓BMI (kg/m2) ↓Fat-free mass (kg) ↓Fat mass (%) ↓Fat mass (kg) ↔ Handgrip strength (kg) dominant hand ↔ Handgrip strength (kg) no dominant hand |
• | • | |||||||||||
| Perugini et al. [50] |
Heart rate variability (HRV) + HOMA-IR |
↓BW (kg) ↓BMI (kg/m2) HRV (improved) ↓HOMA-IR |
• | ||||||||||||
| Ravelli et al. [51] |
Doubly labeled water + Triaxial accelerometer |
↓BW (kg) ↓FM (%) ↓TEE |
• | • | |||||||||||
| Remígio et al. [52] | Treadmill with gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓Systolic blood pressure (mmHg) ↓Diastolic blood pressure (mmHg) ↓Resting HR (bpm) ↓Total cholesterol (mmol/l) ↓LDL cholesterol (mmol/l) ↓Triglycerides (mmol/l) ↔ Glucose (mg/dl) ↔ VO2peak (l/min) ↑VO2peak (ml/min/kg) ↓50%VO2 RP (s) |
• | ||||||||||||
| Sans et al. [53] |
Homeostasis model assessment of insulin resistance (HOMA-IR) + Bioelectrical impedance analysis (BIA) + Gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓HC (cm) ↔ W/H ↓ Brachial circumference (cm) ↓Triceps skinfold thickness (cm) ↓Glucose level (mmol/l) ↓Insulin level (mmol/l) ↓HOMA-IR ↓HbA1c (%) ↑HDL cholesterol (mmol/l) ↓LDL cholesterol (mmol/l) ↓Triglyceride (mmol/l) ↓REE (kcal/day) ↑REE/BW ↓REE/FFM |
• | ||||||||||||
| Seres et al. [54] | Treadmill with gas-exchange analysis |
↑Exercise duration (min) ↑HRmax (bpm) ↑RERmax ↔ VO2peak (l/min) ↑VO2peak (ml/kg/min) ↔ VO2peak/FFM (ml/kg/min) ↔ VO2peak/pulse (ml/beat) ↔ Minute ventilation (l/min) |
• | ||||||||||||
| Schneider et al. [55] |
Dual-energy X-ray absorptiometry + Indirect calorimetry |
↓BW (kg) ↓BMI (kg/m2) ↓FFM (kg) ↓FM (%) ↓REE ↑REE/BW ↓Fat oxidation ↔ CHO oxidation |
• | ||||||||||||
| Silva et al. [56] |
Handgrip dynamometer + Venous occlusion plethysmography + 6-min walk test |
↓BW (kg) ↓BMI (kg/m2) ↓HR (bpm) ↔ Systolic blood pressure (mmHg) ↔ Diastolic blood pressure (mmHg) ↓FVR (units) ↔ 30% handgrip force (Kgf) ↑6-mWT distance (m) ↓Apnea-hypopnea index |
• | ||||||||||||
| Tamboli et al. [57] |
Digital scale + A whole-room indirect calorimeter |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓W/H (at 6th month) ↓TEE (kcal/day) (at 6th month) ↓Total RQ (at 6th month) ↓Sleep RQ (at 12th month) ↓CHO oxidation (g/kg/day) (at 12th month) ↑Fat oxidation (g/kg/day) (at 12th month) |
• | • | |||||||||||
| Tam et al. [58] | Metabolic chamber indirect calorimetry |
↓24hrEE ↓SleepEE ↓REE ↓Spontaneous physical activity |
• | • | |||||||||||
| Tettero et al. [59] |
Baecke questionnaire + Astrand test |
↓BW (kg) ↑VO2max (ml/min/KgFFM) ↑ Leisure physical activity ↑ Sport activity |
• | ||||||||||||
| Tompkins et al. [60] |
Physical ability using SF-36 + 6-mWT + Borg RPE scale |
↓BW (kg) ↓BMI (kg/m2) ↑6-mWT distance (m) ↑Physical functioning ↓Rating of perceived exertion during 6-mWT |
• | • | |||||||||||
| Valezi-Machado et al. [61] |
Treadmill with gas-exchange analysis + Transthoracic echocardiogram |
↑Distance covered (m) ↑METs ↑VO2peak (ml/kg/min) ↑EF ↓Septum |
• | ||||||||||||
| Van Gemert et al. [62] |
Doubly labeled water method + Respiration chamber |
↓TEE ↓Sleep MR ↔ Physical activity index =[TEE/SMR] ↓CHO oxidation |
• | • | |||||||||||
| Vargas et al. [63] |
6-min walking test + Functional Independence Measure (FIM) + Timed Up-and-Go |
↓HR (bpm) ↓Respiratory rate (pm) ↓Systolic arterial pressure (mmHg) ↓Diastolic arterial pressure (mmHg) ↓Borg scale ↑FIM score |
• | ||||||||||||
| Wilms et al. [64] | Bicycle ergospirometry |
↔ Peak workload (W) ↑Peak workload/BW (W kg−1) ↔ Test duration (s) ↔ HRmax (bpm) ↔ RERmax ↔ VO2peak (l/min) ↔ VO2peak (ml/kg/min) ↔ VO2peak/pulse (ml/beat) ↔ Ventilatory equivalent (VE/VO2) |
• | ||||||||||||
| Wu et al. [65] |
Heart rate variability (HRV) + Insulin resistance + HbA1c |
RMSSD improved LF/HF ratio improved ↑Total power ↓HOMA-IR ↓HbA1c |
• | • | • | ||||||||||
| Zavorsky et al. [66] |
Bioelectrical impedance device + Ergocycle with gas-exchange analysis |
↓BW (kg) ↓BMI (kg/m2) ↓WC (cm) ↓HC (cm) ↓W/H ↓FFM (kg) ↓FM (kg) ↓FM (%) Rest ↔ VO2 (ml/kg/min) ↓VO2 (l/min) ↓VE (l/min) BTPS ↔ Breathing frequency (breaths/min) ↓Tidal volume (l/breath) ↓VErest/MVV ↓RER ↓HR (bpm) At peak exercise ↑VO2 (ml/kg/min) ↔ VO2 (l/min) ↔ VE (l/min) BTPS ↔ Breathing frequency (breaths/min) ↑Tidal volume (l/breath) ↔ VEpeak/MVV ↔ RER ↔ HR (beats/min) ↔ Total time of the VO2peak test |
• | ||||||||||||
A, peak late of diastolic filling wave velocity; AC, abdominal circumference; AI, augmentation index; AI@75, AI index standardized for a heart rate of 75 bpm; BMI, body mass index; BTPS, body temperature and pressure saturated; BW, body weight; E, peak early of diastolic filling wave velocity; E/A, velocity ratio; E/I, expiration/inspiration; EF, ejection fraction; ERV, expiratory reserve volume; FEV1, forced expiratory volume in first second; FFA, free fatty acids; FFM, fat-free mass; FM, fat mass; FRC, functional residual capacity; FVC, forced vital capacity; FVR, forearm vascular resistance; HbA1c, glycated hemoglobin; HF, high frequency; HOMA-IR, homeostatic model assessment for insulin resistance; HR, heart rate; IC, inspiratory capacity; IRV, inspiratory reserve volume; IVRT, isovolumic relaxation time; IVS, interventricular septum; La, lactate; LA, left atrium; LF, low frequency; LF/HF, low to high frequency ratio; LnRHI, reactive hyperemia index; LV, Left ventricle; MCR, mean circular resultant; MEP, maximal expiratory pressure; MET, metabolic equivalent of task; MIP, maximal inspiratory pressure; MVV, maximum voluntary ventilation; npRQ, non-protein respiratory quotient; O2-p, oxygen pulse; OGTT, oral glucose tolerance test; OUES, oxygen uptake efficiency slope = (the slope of linear regression of VO2 (L/m) versus log VE (L/m)); pNN 50 (ms), percentage of adjacent NN intervals that differ from each other by more than 50 ms; PW, posterior wall thickness; QTVI, temporal behavior of the QT variability index; REE, resting energy expenditure; RMSSD, root mean square of the successive differences; RQ, respiratory quotient; SampEn, measures of the complexity; SaO2, oxygen saturation; SDNN, standard deviation of NN intervals; SMR, sleeping metabolic ratio; SVC, slow vital capacity; TEE, total energy expenditure; TLC, total lung capacity; V-AT, ventilatory-derived anaerobic threshold; VE/VCO2, the minute ventilation/carbon dioxide production; VO2, oxygen uptake; W, watt; W/H, waist-to-hip ratio; WC, waist circumference, 50%VO2 RP, Post-exercise Oxygen Uptake Recovery Kinetics; ↑ denotes a significant increase; ↓ denotes a significant decrease; ↔, no change
Table 4.
Main analyzed parameters of performance and health indices and type of bariatric surgery
| Main analyzed parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| Body weight | Body mass index | Resting energy expenditure | Total energy expenditure | Heart rate variability | Aerobic capacity | Physical capacity | Plasma insulin | |
| Type of bariatric surgery | ||||||||
| BDP [14, 22, 46] | ↓ [14, 22, 46] | ↓ [14, 22, 46] | ↓ [14] | - | ↑ [14, 46] | - | - | ↓ [14, 46] |
| LAGB [17, 22, 25, 33, 36, 41, 45, 58] | ↓ [17, 22, 33, 45] | ↓ [17, 22, 33, 36, 45] | ↓ [17, 33, 45, 58] | - | ↑ [36] | ↑ [41] | ↑ [41] | ↓ [17, 33] |
| RYGB [16, 23–26, 30–32, 34, 36, 37, 42–45, 49–53, 55–57, 59, 61, 63, 64] | ↓ [16, 23, 24, 26, 30–32, 34, 37, 42, 45, 50–53, 55–57, 59] | ↓ [16, 24, 30–32, 34, 36, 37, 43, 45, 49, 50, 52, 53, 55–57] | ↓ [34, 37, 45, 53, 55] | ↓ [51, 57] | ↑ [24, 26, 36, 42, 50] | ↑ [30, 31, 44, 52, 59, 61] | ↑ [23, 31, 32, 44, 49, 56, 59, 61, 63, 64] | ↓ [16, 24, 53] |
| VBG [26, 35, 62] | ↓ [26] | ↓ [26] | - | ↓ [62] | ↑ [26] | ↑ [35] | ↑ [35, 62] | - |
| GBS [27, 29, 39, 40, 48, 60, 66] | ↓ [27, 29, 39, 60, 66] | ↓ [27, 29, 39, 40, 60, 66] | ↓ [29, 66] | ↓ [29, 48] | ↑ [40] | ↑ [27, 39, 66] | ↑ [48, 60, 66] | ↓ [39] |
| LSG [28, 30, 37, 38, 47, 49, 52, 55, 56, 58, 59, 64, 65] | ↓ [28, 30, 37, 38, 52, 55, 56, 59] | ↓ [30, 37, 38, 49, 52, 55, 56] | ↓ [37, 38, 55, 58] | - | ↑ [65] | ↑ [28, 30, 47, 52, 59] | ↑ [28, 47, 56, 59] | - |
BDP, biliopancreatic diversion; LAGB, laparoscopic adjustable gastric banding; RYGB, Roux-en-Y gastric bypass; VBG, vertical-banded gastroplasty; GBS, gastric bypass surgery; LSG, laparoscopic sleeve gastrectomy; ↑ denotes a significant increase; ↓ denotes a significant decrease; -, not reported
Post-operative Physical Activity Level and Performance
Twenty-four of 48 studies examined the impact of BS on many performance components (Tables 3 and 4) and/or on the post-surgery physical activity levels (Table 3) and used different assessment methods (objective and subjective) to compare outcomes with pre-surgery points. A majority of studies reported the impact of BS on exercise and functional capacity by evaluating various indices, such as gait speed and the time to rise from a chair five times [23]; the distance covered in meters [9, 31, 32, 41, 44]; exercise duration [28, 31, 35, 47, 54, 56, 61, 64, 66]; perceived exhaustion [32, 63]; and the Functional Independence Measure [63]. These studies reported a favorable impact of BS on these outcomes [9, 23, 28, 31, 32, 35, 41, 44, 47, 54, 56, 61, 63]. In contrast, only Wilms et al. [64] did not find any favorable effect of BS on the distance covered ≥ 24 months post-surgery. Muscular performance has been evaluated by reporting absolute and relative grip strength [23, 47, 49, 56], peak power, developed in Watts or relative to body weight [64], or leg extension performance [47]. Some results demonstrated that BS had a beneficial effect on grip strength [23] while other studies found no beneficial effect on grip strength, [47, 49, 56] and that it had a beneficial effect on leg extension performance [47] and on peak power relative to body weight [64].
Nine out of 48 of the selected studies assessed post-surgery physical activity levels and compared them to the pre-surgery period (Tables 3 and 4). Four of the studies used the validated Physical Activity Questionnaire [9, 23, 25, 39] to evaluate subjective physical activity levels and did not report any changes in the post-surgery period compared to baseline. One study that utilized self-developed surveys to assess physical activity [16] showed an increase in the physical activity (PA) level at the 6th month post-surgery evaluation. Bond et al. [25] compared subjective evaluations using the Paffenbarger Physical Activity Questionnaire (PPAQ) and objective measurements using a triaxial accelerometer. They reported that 55% of responders meet the international guideline recommendations when subjectively assessed versus 5% who meet these recommendations when objectively assessed. For Liu et al. [38], the PA level reported via accelerometer did not improve 6 months after BS. Das et al. [29], Tam et al. [58], and Van Germet et al. [62] used a metabolic chamber for indirect calorimetry during the post-surgery period and found no significant changes [29, 62] and even decreases [58] in the PA index among patients.
Post-operative Cardiorespiratory Fitness and Energy Expenditure
Details of the effects of BS on different cardiorespiratory fitness and energy indices expenditure are summarized in Tables 3 and 4. Eleven studies evaluated the effects of BS on cardiorespiratory capacity (oxygen consumption, oxygen uptake efficiency, heart rate max, ventilatory equivalent, lung capacity, and breathing frequency) using a treadmill [27, 28, 31, 35, 44, 47, 48] or an ergometer [30, 39, 64, 66].
Of these 14 studies, 11 reported a significant increase in VO2peak relative to body weight [27, 28, 30, 31, 35, 39, 44, 47, 52, 54, 61], and 5 reported no change [27, 30, 52, 54, 64] or a decrease [28, 30, 35, 39, 44, 47, 66] in absolute VO2peak (7 studies). Only two studies reported a decrease [48] or no change [64] in VO2peak relative to body weight. Other parameters, such as oxygen uptake efficiency, decreased [47], while ventilatory response [66], and ventilatory volume and efficiency [54, 64] improved post-surgery.
The change in total energy expenditure (TEE) between the pre-operative period and follow-up was reported in four studies [29, 48, 51, 60]. Compared with the pre-operative value, the TEE decreased at 6, 12, and 14 months post-operatively. Ten studies [16, 29, 33, 34, 37, 38, 45, 53, 55, 58] reported a reduction in resting energy expenditure (REE) post-surgery. REE/BW was reported in four studies [33, 37, 53, 55], and REE/FFM was reported in five studies [33, 37, 38, 45, 53].
There were significant increases [33, 53, 55] and decreases [37] in REE/BW after BS. REE/FFM decreased [33, 38, 53], increased [45], or did not change [37] after BS.
Five studies [16, 33, 55, 57, 62] reported changes in substrate oxidation during the pre-operative and follow-up period. Compared with the pre-operative value, CHO oxidation decreased at the 3rd [57, 62] and 12th month [57, 62] post-surgery or had not changed at the 14th month post-surgery [55]. In terms of fat oxidation, Carrasco et al. [16] reported a significant increase in fasting lipid oxidation at 6 months post-surgery and a decrease [55] at 17 months post-surgery, and Tamboli et al. reported a decrease at 12 months post-surgery. In contrast, no changes were reported by Galtier et al. [33] at the 6th, 12th, and 18th months post-surgery [33].
Post-operative Metabolic Parameters, Substrate Use, and Autonomic Nervous System Modulation
At ≥ 24 months post-surgery, Benedetti et al. [14] reported significant improvements in metabolic parameters manifested by decreases in fasting glucose, insulin, and FFA levels. For Bobbioni-Harsch et al. [24], plasma glucose and FFA remained unchanged post-surgery. However, plasma insulin decreased at both 3 months and 12 months. Glucose uptake increased at 3 months and 12 months post-surgery. Braga et al. [26] reported no changes in homeostatic model assessment for insulin resistance (HOMA-IR) (%) but a decrease in fasting glucose and insulin at 3 months post-surgery (Table 3). Lipid profiles (total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides) improved significantly at the 4th [52], 6th [16, 33], and 12th [30, 34, 37, 53] months post-surgery as did glucose and HbA1c levels [30, 34, 37, 43, 46, 53] and insulin resistance [33, 34, 46, 53] at the 12th and 6th months [42, 46, 50] post-surgery. Lund et al. [39] reported significant decreases in fasting insulin and glucose levels, as well as in HbA1c and fasting total cholesterol at the 2nd and 4th months post-surgery. Wu et al. [65] reported significant decreases in HOMA-IR and HbA1c at the 1st, 3rd, and 4th months post-surgery.
Alam et al. [22] reported an improvement in the temporal behavior of the QT variability index (QTVI) at the 1st and 12th months following BS. Three other indices (SampEn QT, DFAα (NN), and DFAα (QT)) also improved within 1 month following surgery, and a further four (RR, HR, RMSSD, and SDNN) showed an improvement at 6 months post-surgery. Bobbioni-Harsch et al. [24] reported an improvement in SDNN as well as RMS and % pNN 50 at all follow-up periods. An improvement in both the frequency and time domain has been reported by Braga et al. [26] at the 3rd month and by Nault et al. [46] at the 6th and 12th months post-surgery (Table 3). Kokkinos et al. [36] compared the SG versus GB surgery methods and reported an improvement in frequency domain variables regardless of the groups at 3 and 12 months post-surgery. The HRV-time domain [40] and HRV-frequency [42] domain indices improved at the 6th month [40] post-surgery, and both improved at 6 and 12 months for Nault et al. [46] and at the 1st, 3rd, and 4th months post-surgery for Wu et al. [65]
Other forms of improvement have been reported for heart structure using echocardiography. Two studies reported decreases in IVS, PW, and IVRT and increases in E/A at 6 months post-surgery [35] as well as decreases in epicardial fat, LV Tei index, and LA diameter and increases in EF (%) and LV mass index at 6 months post-surgery for both the SG and GB surgery groups. For endothelial reactivity, no changes were reported for LnRHI, AI, or AI@75 at the 3rd month post-surgery (Table 3) [26].
Discussion
This systematic literature review indicates that undergoing bariatric surgery may procure several health benefits and improve some fitness and performance indicators regardless of the procedure. These improvements may be achieved after short- and/or mid-term post-operative periods. Despite these promising results, more consideration of candidate profiles prior to BS in addition to a longer follow-up with multiple visits is highly recommended to gain a fuller understanding of the influence of BS on the selected outcomes.
Post-operative Body Composition Changes and Weight Loss
Despite heterogeneity in participant age, baseline BMI, the surgical procedure used, and the technique used to assess body composition, studies revealed significant reductions in body weight and fat mass and a decrease in fat-free mass in individuals with obesity who underwent BS.
The significant body weight loss reported by studies on BS was essentially attributed to reducing energy intake and decreased absorption of nutrients [16, 24]. However, it is important to mention that weight loss is also influenced by variations in the surgical technique, such as the size of the gastric pouch, the alimentary limb length, and the gastrojejunostomy diameter. In fact, the variation in the operative technique affects energy intake among patients leading to the inter-individual variability of weight loss after surgery. On the other hand, many authors suggested that the metabolic adaptation that accompanies weight loss, in addition to variations in plasma levels of mediators derived from adipose tissue, such as leptin, was related mostly to loss of fat mass rather than to a decrease in fat-free mass [16, 68, 69].
Although caloric restriction seems to be the dominant mechanism in body weight reduction and weight loss maintenance, Gemert et al. [62] suggested that a decrease in CHO intake resulted in lower insulin levels, which increased lipolysis and decreased CHO and protein oxidation, procures a beneficial effect on weight loss success.
To conclude, a multitude of factors may be involved in explaining body composition changes and weight loss among BS patients. Considering that an appropriate and permanent reduction in energy intake is essential for long-term weight management in patients who have undergone BS, examining other potential explanations for the variability in weight loss between patients will help potentiate short- and long-term weight loss post-bariatric surgery.
Post-operative Physical Activity Levels and Performance
The effect of BS on post-surgery physical activity levels has been evaluated either subjectively using self-reported questionnaires [9, 23, 25], structured interviews [29], or simple surveys performed by the participants [16], or objectively using an accelerometer [25, 38]. There is a great variation in how exercise is measured and the minimal threshold to define a physically active patient. Of these subjective evaluations, the results reported no changes in physical activity levels during the post-surgery period [9, 23, 29, 39]. For Campos et al. [9], despite the reported improvements in body composition, cardio-respiratory performance, and functional capacity 6 months post-surgery, participants were still sedentary. These results might be related to a lack of consistency in performing physical exercise, as was experienced prior to surgery. Only one study by Carrasco et al. [16] reported an increase in physical activity and a decrease in sedentary behavior. This increase was related to weight loss. When using an accelerometer, Bond et al. [25] reported a near fivefold decrease in MVPA among participants compared to using a self-reported evaluation, and only one participant met the physical activity recommendations. Due to the lack of data on baseline variables regarding a “voluntary” change in physical activity level after bariatric surgery, how to determine post-surgery physical activity practices in bariatric surgery patients is still unknown.
Despite heterogeneity in participant age, baseline profile, surgical procedure used, and test performed, studies reported a positive impact of BS on many performance indicators among the patients. These improvements were related to muscular strength and physical function. For Alba et al. [22], relative muscle strength and physical performance improved between 6 and 12 months post-operatively despite declines in lean mass and absolute muscle strength. Moreover, Alba et al. [22] reported a significant improvement in physical performance, attributed to the person’s ability to perform activities of daily living, as reported recently by Campos et al. [9] among women with morbid obesity 6 months post-operatively after performing the incremental shuttle walk test (ISWT). De Souza et al. [32], Maniscalco et al. [41], Silva et al. [56], Tompkins et al. [60], and Vargas et al. [63] reported an increase in distance when performing a 6-min walking test (6-mWT) with a concomitant decrease in the rating of perceived exertion [32, 60, 63], and body mass and BMI decreases were the strongest predictors of that improvement [41, 56, 63].
A lower muscle strength was associated with the loss of lean body mass, which accompanied the reduction in fat mass, particularly in the first months after surgery [70, 71]. Future studies evaluating both muscle mass and function, as well as fiber-type composition, will help better address this issue.
Post-operative Cardiorespiratory Fitness and Energy Expenditure
Individuals with severe obesity suffer from impaired cardiorespiratory fitness [72–74] that manifests primarily with a decreased VO2peak. In response to BS, studies reported significant increases in VO2peak relative to body weight fitness [27, 28, 30, 31, 35, 39, 44, 47, 52, 54, 61, 66] and in lung function [9, 41, 66], suggesting improved aerobic fitness. However, absolute values were either unchanged [27, 52, 54, 64, 66] or decreased [28, 30, 35, 39, 44, 47]. In the absence of any scheduled physical conditioning or exercise intervention, the improvement in VO2peak relative to weight as well as the decreases in absolute VO2peak post-surgery is mainly attributed to weight loss and body composition changes. However, it is important to note that a significant proportion of weight loss following bariatric surgery comes from muscle mass, especially in the initial post-surgical period [71, 74], and oxidative muscle metabolism [70].
Therefore, it is unclear whether the increased aerobic capacity post-surgery reflects a fundamental improvement at the muscular and cardiorespiratory structure levels or is simply due to a lower energy requirement associated with exercise and reduced strain on the cardiopulmonary system during exercise. More recently, Daniel et al. [28] explored short- and long-term post-surgical effects on aerobic fitness parameters (absolute VO2peak, OUES, and the time constant Tau (τ) in VO2 kinetics) in a homogenous population after LSG. For these authors, the restoration of overall aerobic capacity could be achieved in the long-term post-surgery, allowing an improvement in overall aerobic performance. The latter will also depend on other physiological, environmental, and behavioral characteristics. Consequently, future studies should use multiple time points to give a better understanding as well as have an extended follow-up period, and they should consider other predictors of aerobic performance (e.g., stroke volume, aerobic enzyme, muscle fiber types) that are known to significantly affect these parameters.
Regarding REE, a meta-analysis by Astrup et al. [75] reported that post-operative weight loss is associated with a reduction in REE. For Carrasco et al. [16], the reduction in weight was associated with a significant decrease in the REE/FFM ratio, and greater decreases were shown for those with higher REEs at baseline. In this context, many studies supported that a greater energy expenditure at the pre-surgical stage might be compensatory for energy intake increases when restricted nutritional intake is applied in the post-operative state; this compensation would disappear, leading to a greater reduction in REE in patients with obesity [16, 29]. Another factor that would explain REE decreases in the post-surgical phase is fat mass loss. Carrasco et al. [16] observed a positive correlation between REE changes and the reduction in body fat at the 6-month follow-up. It seems that REE adaptation may be influenced by the reduction in adipose tissue and variations in plasma levels of mediators derived from this tissue in addition to other factors such favorable changes in eating habits, physical activity, and nutritional behavior [76–78], and on the absence of metabolic factors that predispose individuals to regain weight [77, 79].
In terms of REE, TEE decreased significantly post-surgery among patients with obesity [29, 51, 57]. For Tamboli et al. [57], the decrease in TEE did not appear to follow the same pattern as the REE change after BS. A decline in TEE was observed until 6 months post-surgery, while no significant difference was reported at 12 months post-operatively. This decrease was proportional to the weight change within 6 months after surgery, and no further change in TEE occurred with ongoing weight loss. One possible explanation for this is the change in the PA level and diet-induced thermogenesis.
Post-operative Metabolic Parameters, Substrate Use, and Autonomic Nervous System Modulation
Studies have reported immediate improvements in metabolic parameters during the post-surgery period, mainly in the lipid profile (e.g., remission concerning the levels of total cholesterol, LDL cholesterol, and triglycerides) and glycemic control (e.g., improvement in the levels of fasting insulin and the HOMA index, normalization of fasting glycaemia levels, and increases in glucose uptake) [14, 16, 22, 24, 26, 30, 33, 34, 37, 39, 43, 46, 50, 52, 53, 65] and in substrate oxidation (e.g., increases in lipid oxidation and decreases in carbohydrate oxidation) [55, 57, 62]. It has been postulated that these improvements were mainly attributed to body composition changes, mainly to fat mass [16, 80], to visceral fat loss [33, 34], and to changes in intestinal peptides (GLP-1, PYY3–36, etc.) [81, 82], regardless of the surgical procedure.
Obesity alters heart rate variability (HRV) that will manifest as a decrease in HRV due to decreased adrenoreceptor responsiveness, withdrawal of parasympathetic (vagal) tone, and/or increased sympathetic activity [83, 84]. Weight loss improves parasympathetic cardiac modulation, observed as an increase in HRV [85]. Many studies have reported a significant association between weight loss and HRV improvement. For Alam et al. [22], several indices showed a prompt and persistent improvement with progressive weight loss, mainly for the QTVI, which improved as early as 1 month following surgery, and this change was further improved at the 12-month follow-up. Similarly, Maser et al. [42] showed that an average 28% reduction in BMI was accompanied by very significant improvements in all measures of HRV. Other factors, such as hormonal and metabolic factors, have been assessed to elucidate whether one or more of these factors are associated with modifications of cardiac autonomic balance post-surgery. For example, Bobbioni-Harsch et al. [24] showed that in addition to body weight loss, energy intake explained 20% of the variations in the time domain profile 3 months post-surgery. Kokkinos et al. [36] found that PHF and TP were both increased, indicating amelioration of cardiac autonomic function overall and the reversal of vagal impairment. Machado et al. [40] reported an overall HRV increase 6 months post-surgery, and this increase was more evident in men. Moreover, cardiac parasympathetic activity also increased but only in younger patients. Finally, HRV improvement was associated with lipid profile improvement at the 6th and 12th months post-surgery [46] and with insulin resistance decreases [65].
Limitations
It is important to note that the evidence presented in this review comes from different BS’s procedures, e.g., metabolic versus restrictive, which compares different parameters difficult to interpret. While metabolic surgery, mainly gastric bypass and biliopancreatic diversion, is used to treat metabolic diseases, especially type 2 diabetes, the restrictive surgery is considered weight loss surgery. Considering that surgical technique is beyond the scope of this systematic review, however, most of our selected studies have been performed with patients who underwent a gastric bypass (GB) procedure, which may help sort out some interpretation. Moreover, studies were heterogeneous, and full descriptions of inclusion criteria and the adjustment by other covariates such as participant characteristics and duration of follow-up were not always reported. Finally, it is still important to mention that the lack of randomized control trial studies is really significant, and most of the studies recruited are very small in sample size in parallel to a short follow-up time which makes the results less appealing.
Conclusion
This review summarizes the benefits of BS alone for several performance and health indicators in adults with obesity. A key conclusion is that BS has a positive impact on body composition, physical functioning, metabolic parameters, and autonomic nervous system modulation and, to some extent, on energy expenditure, physical activity level, muscular strength, and peak oxygen consumption. As an effective approach to reducing body weight when nonsurgical methods are exhausted, the improvements procured by BS have been achieved both in a shorter period (less than 1 month) and with more extended period (more than 1 year); however, some studies reported that some of these benefits might disappear later on. Therefore, further studies are needed to determine the appropriate recommendations that still imprecise until today, focusing on managing post-surgery outcomes mainly by considering lifestyle modification that is likely to be of significant benefit.
Acknowledgements
Open access funding provided by the Qatar National Library.
Abbreviations
- A
Peak late of diastolic filling wave velocity
- AC
Abdominal circumference
- AI
Augmentation index
- AI@75
AI index standardized for a heart rate of 75 bpm
- BMI
Body mass index
- BS
Bariatric surgery
- BTPS
Body temperature and pressure saturated
- BW
Body weight
- E
Peak early of diastolic filling wave velocity
- E/A
Velocity ratio
- E/I
Expiration/inspiration
- EF
Ejection fraction
- ERV
Expiratory reserve volume
- FEV1
Forced expiratory volume in first second
- FFA
Free fatty acids
- FFM
Fat-free mass
- FM
Fat mass
- FRC
Functional residual capacity
- FVC
Forced vital capacity
- FVR
Forearm vascular resistance
- HbA1c
Glycated hemoglobin
- HF
High frequency
- HOMA-IR
Homeostatic model assessment for insulin resistance
- HR
Heart rate
- IC
Inspiratory capacity
- IRV
Inspiratory reserve volume
- IVRT
Isovolumic relaxation time
- IVS
Interventricular septum
- La
Lactate
- LA
Left atrium
- LF
Low frequency
- LF/HF
Low to high frequency ratio
- LnRHI
Reactive hyperemia index
- LV
Left ventricle
- MCR
Mean circular resultant
- MEP
Maximal expiratory pressure
- MET
Metabolic equivalent of task
- MIP
Maximal inspiratory pressure
- MVV
Maximum voluntary ventilation
- npRQ
Nonprotein respiratory quotient
- O2-p
Oxygen pulse
- OGTT
Oral glucose tolerance test
- OUES
Oxygen uptake efficiency slope = (the slope of linear regression of VO2 (L/m) versus log VE (L/m))
- pNN 50 (ms)
Percentage of adjacent NN intervals that differ from each other by more than 50 ms
- PW
Posterior wall thickness
- QTVI
Temporal behavior of the QT variability index
- REE
Resting energy expenditure
- RMSSD
Root mean square of the successive differences
- RQ
Respiratory quotient
- SampEn
Measures of the complexity
- SaO2
Oxygen saturation
- SDNN
Standard deviation of NN intervals
- SMR
Sleeping metabolic ratio
- SVC
Slow vital capacity
- TEE
Total energy expenditure
- TLC
Total lung capacity
- V-AT
Ventilatory-derived anaerobic threshold
- VE/VCO2
The minute ventilation/carbon dioxide production
- VO2
Oxygen uptake
- W
Watt
- W/H
Waist-to-hip ratio
- WC
Waist circumference
- 50%VO2 RP
Post-exercise Oxygen Uptake Recovery Kinetics
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization WH. Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity, Geneva, 3–5 June 1997. Geneva: World Health Organization. p. 1998. [PubMed]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 3.Engin A. The definition and prevalence of obesity and metabolic syndrome. In: Engin A, Engin A, editors. Obesity and lipotoxicity. Advances in experimental medicine and biology. Cham: Springer; 2017. [DOI] [PubMed] [Google Scholar]
- 4.Pan F, Laslett L, Blizzard L, Cicuttini F, Winzenberg T, Ding C, Jones G. Associations between fat mass and multisite pain: a five-year longitudinal study. Arthritis Care Res. 2017;69(4):509–516. doi: 10.1002/acr.22963. [DOI] [PubMed] [Google Scholar]
- 5.Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A, Lafortuna CL, Sartorio A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101:51–59. doi: 10.1007/s00421-007-0471-2. [DOI] [PubMed] [Google Scholar]
- 6.Hergenroeder AL, Wert DM, Hile ES, Studenski SA, Brach JS. Association of body mass index with self-report and performance based measures of balance and mobility. Phys Ther. 2011;91:1223–1234. doi: 10.2522/ptj.20100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50:55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 8.Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity. 2007;15:1886–1894. doi: 10.1038/oby.2007.223. [DOI] [PubMed] [Google Scholar]
- 9.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz J-M, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14(1):15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K, Feurer ID, Tamboli RA. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35(1):137–142. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bragge T, Lyytinen T, Hakkarainen M, Vartiainen P, Liikavainio T, Karjalainen PA, Arokoski JP. Lower impulsive loadings following intensive weight loss after bariatric surgery in level and stair walking: a preliminary study. Knee. 2013;21(2):534–540. doi: 10.1016/j.knee.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Li JS, Tsai TY, Clancy MM, Li G, Lewis CL, Felson DT. Weight loss changed gait kinematics in individuals with obesity and knee pain. Gait Posture. 2019;68:461–465. doi: 10.1016/j.gaitpost.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebibo L, Verhaeghe P, Tasseel-Ponche S, Cosse C, Maréchal V, Dhahri A, Doutrellot PL, Regimbeau JM. Does sleeve gastrectomy improve the gait parameters of obese patients? Surg Obes Relat Dis. 2016;12(8):1474–1481. doi: 10.1016/j.soard.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Benedetti G, Mingrone G, Marcoccia S, Benedetti M, Giancaterini A, Greco AV, Castagneto M, Gasbarrini G. Body composition and energy expenditure after weight loss following bariatric surgery. J Am Coll Nutr. 2000;19(2):270–274. doi: 10.1080/07315724.2000.10718926. [DOI] [PubMed] [Google Scholar]
- 15.Butte N, Brandt M, Wong W, Liu Y, Mehta NR, Wilson TA, Adolph AL, Puyau MR, Vohra FA, Shypailo RJ, Zakeri IF. Energetic adaptations persist after bariatric surgery in severely obese adolescents. Obesity (Silver Spring) 2015;23:591–601. doi: 10.1002/oby.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, Díaz E, Rojas J. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 17.Colles SL, Dixon JB, O’Brien PE. Hunger control and regular physical activity facilitate weight loss after laparoscopic adjustable gastric banding. Obes Surg. 2008;18:833–840. doi: 10.1007/s11695-007-9409-3. [DOI] [PubMed] [Google Scholar]
- 18.Sheema UK, Basvaraj MS. A cross-sectional study on effect of body mass index on the spectral analysis of heart rate variability. Natl J Physiol Pharm Pharmacol. 2015;5(3):250–252. [Google Scholar]
- 19.Blimkie CJ, Sale DG, Bar-Or O. Voluntary strength, evoked twitch contractile properties and motor unit activation of knee extensors in obese and non-obese adolescent males. Eur J Appl Physiol Occup Physiol. 1990;61:313–318. doi: 10.1007/BF00357619. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Rohatgi A, Ayers CR, Willis BL, Haskell WL, Khera A, Drazner MH, de Lemos JA, Berry JD. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123:1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Alam IMJ, Lewis MJ, Lewis KE, Stephens JW, Baxter JN. Influence of bariatric surgery on indices of cardiac autonomic control. Auton Neurosci Basic Clin. 2009;151:168–173. doi: 10.1016/j.autneu.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Alba DL, Wu L, Cawthon PM, Mulligan K, Lang T, Patel S, King NJ, Carter JT, Rogers SJ, Posselt AM, Stewart L, Shoback DM, Schafer AL. Changes in lean mass, absolute and relative muscle strength, and physical performance after gastric bypass surgery. J Clin Endocrinol Metab. 2019;104:711–720. doi: 10.1210/jc.2018-00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobbioni-Harsch E, Morel P, Huber O, Chassot G, Lehmann T, Volery M, Golay A. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85(12):4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 25.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, Ryder BA, Sax HC, Wing RR. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity. 2010;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga TG, de Souza MGC, Maranhão PA, Menezes M, Dellatorre-Teixeira L, Bouskela E, Le Roux CW, Kraemer-Aguiar LG. Evaluation of heart rate variability and endothelial function 3 months after bariatric surgery. Obes Surg. 2020;30:2450–2453. doi: 10.1007/s11695-020-04397-4. [DOI] [PubMed] [Google Scholar]
- 27.Browning MG, Franco RL, Herrick JE, Arrowood JA, Evans RK. Assessment of cardiopulmonary responses to treadmill walking following gastric bypass surgery. Obes Surg. 2017;27(1):96–101. doi: 10.1007/s11695-016-2259-0. [DOI] [PubMed] [Google Scholar]
- 28.Daniel N, Francesco C, Andrea G, et al. Cardiorespiratory function and VO2 kinetics after sleeve gastrectomy: a follow-up analysis. Intern Emerg Med. 2020; 10.1007/s11739-020-02279-2. [DOI] [PubMed]
- 29.Das SK, Roberts SB, McCrory MA, Hsu LHG, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition aftermassive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 30.Dereppe H, Forton K, Yaëlle N, Faoro V. Impact of bariatric surgery on women aerobic exercise capacity. Obes Surg. 2019;29:3316–3323. doi: 10.1007/s11695-019-03996-0. [DOI] [PubMed] [Google Scholar]
- 31.de Souza SA, Faintuch J, Sant'anna SF. Effect of weight loss on aerobic capacity in patients with severe obesity before and after bariatric surgery. Obes Surg. 2010;20(7):871–875. doi: 10.1007/s11695-010-0109-z. [DOI] [PubMed] [Google Scholar]
- 32.de Souza SA, Faintuch J, Fabris SM, Nampo FK, Luz C, Fabio TL, Sitta IS, de Batista Fonseca IC. Six-minute walk test: functional capacity of severely obese before and after bariatric surgery. Surg Obes Relat Dis. 2009;5:540–543. doi: 10.1016/j.soard.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Galtier F, Farret A, Verdier R, Barbotte E, Nocca D, Fabre J-M, Bringer J, Renard E. Resting energy expenditure and fuel metabolism following laparoscopic adjustable gastric banding in severely obese women: relationships with excess weight lost. Int J Obes. 2006;30(7):1104–1110. doi: 10.1038/sj.ijo.0803247. [DOI] [PubMed] [Google Scholar]
- 34.Iannelli A, Anty R, Schneck AS, Tran A, Hébuterne X, Gugenheim J. Evolution of low-grade systemic inflammation, insulin resistance, anthropometrics, resting energy expenditure and metabolic syndrome after bariatric surgery: a comparative study between gastric bypass and sleeve gastrectomy. J Visc Surg. 2013;150(4):269–275. doi: 10.1016/j.jviscsurg.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kanoupakis E, Michaloudis D, Fraidakis F, Parthenakis F, Vardas P, Melissas J. Left ventricular function and cardio-pulmonary performance following surgical treatment of morbid obesity. Obes Surg. 2001;11:552–558. doi: 10.1381/09608920160556715. [DOI] [PubMed] [Google Scholar]
- 36.Kokkinos A, Alexiadou K, Liaskos C, Argyrakopoulou BI, Tentolouris N, Moyssakis I, Katsilambros N, Vafiadis I, Alexandrou A, Diamantis T. Improvement in cardiovascular indices after Roux-en-Y gastric bypass or sleeve gastrectomy for morbid obesity. Obes Surg. 2013;23:31–38. doi: 10.1007/s11695-012-0743-8. [DOI] [PubMed] [Google Scholar]
- 37.Li K, Zheng L, Guo J, Shi W, Zhao F, Yang C, Dai Q, Wang B, Li Y. Increased resting energy expenditure/body weight and decreased respiratory quotient correlate with satisfactory weight loss after sleeve gastrectomy: a 6 month follow-up. Obes Surg. 2020;30:1410–1416. doi: 10.1007/s11695-019-04308-2. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Lagoy A, Discenza I, Papineau G, Lewis E, Braden G, Romanelli J, Braun B, Silva JE. Neuroendocrine responses to Roux-en-Y gastric bypass. I: energy balance, metabolic changes, and fat loss. J Clin Endocrinol Metab. 2012;7(8):1440–1450. doi: 10.1210/jc.2012-1016. [DOI] [PubMed] [Google Scholar]
- 39.Lund MT, Hansen M, Wimmelmann CL, Taudorf LR, Helge JW, Mortensen EL, Dela F. Increased postoperative cardiopulmonary fitness in gastric bypass patients is explained by weight loss. Scand J Med Sci Sports. 2016;26:1428–1434. doi: 10.1111/sms.12593. [DOI] [PubMed] [Google Scholar]
- 40.Machado MB, Velasco IT, Augusto ST. Gastric bypass and cardiac autonomic activity: influence of gender and age. Obes Surg. 2009;19:332–338. doi: 10.1007/s11695-008-9665-x. [DOI] [PubMed] [Google Scholar]
- 41.Maniscalco M, Zedda A, Giardiello C, Faraone S, Cerbone MR, Cristiano S, Sofia M. Effect of bariatric surgery on the six-minute walk test in severe uncomplicated obesity. Obes Surg. 2006;16:836–841. doi: 10.1381/096089206777822331. [DOI] [PubMed] [Google Scholar]
- 42.Maser RE, Lenhard MJ, Peters MB, Irgau I, Wynn GM. Effects of surgically induced weight loss by Roux-en-Y gastric bypass on cardiovascular autonomic nerve function. Surg Obes Relat Dis. 2013;9(2):221–226. doi: 10.1016/j.soard.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Maser RE, Lenhard MJ. Impact of surgically induced weight loss on cardiovascular autonomic function: one-year follow-up. Obesity. 2007;15(2):364–369. doi: 10.1038/oby.2007.554. [DOI] [PubMed] [Google Scholar]
- 44.McCullough PA, Gallagher MJ, de Jong AT, Sandberg KR, Trivax JE, Alexander D, Kasturi G, Jafri SMA, Krause KR, Chengelis DL, Moy J, Franklin BA. Cardiorespiratory fitness and short term complications after bariatric surgery. Chest. 2006;130:517–525. doi: 10.1378/chest.130.2.517. [DOI] [PubMed] [Google Scholar]
- 45.Mirahmadian M, Hasani M, Taheri E, Qorbani M, Hosseini S. Influence of gastric bypass surgery on resting energy expenditure, body composition, physical activity, and thyroid hormones in morbidly obese patients. Diabetes Metab Syndr Obes Targets Ther. 2018;11:667–672. doi: 10.2147/DMSO.S172028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nault I, Nadreau E, Paquet C, Brassard P, Brassard P, Marceau P, Marceau S, Biron S, Hould F, Lebel S, Richard D, Poirier P. Impact of bariatric surgery–induced weight loss on heart rate variability. Metab Clin Exp. 2007;56:1425–1430. doi: 10.1016/j.metabol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Neunhaeuserer D, Gasperetti A, Savalla F, Gobbo S, Bullo V, Bergamin M, Foletto M, Vettor R, Zaccaria M, Ermolao A. Functional evaluation in obese patients before and after sleeve gastrectomy. Obes Surg. 2017;27(12):3230–3239. doi: 10.1007/s11695-017-2763-x. [DOI] [PubMed] [Google Scholar]
- 48.Notarius CF, Rhode B, MacLean LD, Magder S. Exercise capacity and energy expenditure of morbidly obese and previously obese subjects. Clin Invest Med. 1998;21(2):79–87. [PubMed] [Google Scholar]
- 49.Otto M, Kautt S, Kremer M, Kienle P, Post S, Hasenberg T. Handgrip strength as a predictor for post bariatric body composition. Obes Surg. 2014;24:2082–2088. doi: 10.1007/s11695-014-1299-6. [DOI] [PubMed] [Google Scholar]
- 50.Perugini RA, Li Y, Rosenthal L, Gallagher-Dorval K, Kelly JJ, Czerniach DR. Reduced heart rate variability correlates with insulin resistance but not with measures of obesity in population undergoing laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6(3):237–241. doi: 10.1016/j.soard.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Ravelli MN, Schoeller DA, Crisp AH, Racine NM, Pfrimer K, Junior IR, de Oliveira MRM. Accuracy of total energy expenditure predictive equations after a massive weight loss induced by bariatric surgery. Clin Nutr ESPEN. 2018;26:57–65. doi: 10.1016/j.clnesp.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Remígio MI, Cruz FS, Ferraz Á, Remígio MC, Parente G, Nascimento I, Brandão D, Andrade AFD, Neto FM, Campos J. The impact of bariatric surgery on cardiopulmonary function: analyzing VO2 recovery kinetics. Obes Surg. 2018;28:4039–4044. doi: 10.1007/s11695-018-3469-4. [DOI] [PubMed] [Google Scholar]
- 53.Sans A, Bailly L, Anty R, Sielezenef I, Gugenheim J, Tran A, Gual P, Iannelli A. Baseline anthropometric and metabolic parameters correlate with weight loss in women 1-year after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2017;27:2940–2949. doi: 10.1007/s11695-017-2720-8. [DOI] [PubMed] [Google Scholar]
- 54.Seres L, Lopez-Ayerbe J, Coll R, Rodriguez O, Vila J, Formiguera X, Alastrue A, Rull M, Valle M. Increased exercise capacity after surgically induced weight loss in morbid obesity. Obesity. 2006;14:273–279. doi: 10.1038/oby.2006.35. [DOI] [PubMed] [Google Scholar]
- 55.Schneider J, Peterli R, Gass M, Slawik M, Peters T, Wölnerhanssen BK. Laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass lead to equal changes in body composition and energy metabolism 17 months postoperatively: a prospective randomized trial. Surg Obes Relat Dis. 2016;12(3):563–570. doi: 10.1016/j.soard.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Silva RP, Martinez D, Faria CC, de Carli LA, de Souza WIPB, Meinhardt NG, Souto KEP, Trindade MRM, Ribeiro JP. Improvement of exercise capacity and peripheral metaboreflex after bariatric surgery. Obes Surg. 2013;23:1835–1841. doi: 10.1007/s11695-013-0988-x. [DOI] [PubMed] [Google Scholar]
- 57.Tamboli RA, Hossain HA, Marks PA, Eckhauser AW, Rathmacher JA, Phillips SE, Buchowski MS, Chen KY, Abumrad NN. Body composition and energy metabolism following Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2010;18(9):1718–1724. doi: 10.1038/oby.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tam CS, Redman LM, Greenway F, LeBlanc KA, Haussmann MG, Ravussin E. Energy metabolic adaptation and cardiometabolic improvements one year after gastric bypass, sleeve gastrectomy, and gastric band. J Clin Endocrinol Metab. 2016;101(10):3755–3764. doi: 10.1210/jc.2016-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tettero OM, Aronson T, Wolf RJ, Nuijten MAH, Hopman MTE, Janssen IMV. Increase in physical activity after bariatric surgery demonstrates improvement in weight loss and cardiorespiratory fitness. Obes Surg. 2018;28:3950–3957. doi: 10.1007/s11695-018-3439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tompkins J, Bosch PR, Chenowith R, Tiede JL, Swain JM. Changes in functional walking distance and health-related quality of life after gastric bypass surgery. Phys Ther. 2008;88:928–935. doi: 10.2522/ptj.20070296. [DOI] [PubMed] [Google Scholar]
- 61.Valezi AC, Machado VH. Morphofunctional evaluation of the heart of obese patients before and after bariatric surgery. Obes Surg. 2011;21:1693–1697. doi: 10.1007/s11695-011-0431-0. [DOI] [PubMed] [Google Scholar]
- 62.Van Gemert WG, Westerterp KR, Van Acker BAC, Wagenmakers AJ, Halliday D, Greve JM, Soeters PB. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes. 2000;24(6):711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 63.Vargas CB, Picolli F, Dani C, Padoin AV, Mottin CC. Functioning of obese individuals in pre- and postoperative periods of bariatric surgery. Obes Surg. 2013;23:1590–1595. doi: 10.1007/s11695-013-0924-0. [DOI] [PubMed] [Google Scholar]
- 64.Wilms B, Ernst B, Thurnheer M, Weisser B, Schultes B. Differential changes in exercise performance after massive weight loss induced by bariatric surgery. Obes Surg. 2013;23:365–371. doi: 10.1007/s11695-012-0795-9. [DOI] [PubMed] [Google Scholar]
- 65.Wu JM, Yu HJ, Lai HS, Yang PJ, Lin MT, Lai F. Improvement of heart rate variability after decreased insulin resistance after sleeve gastrectomy for morbidly obesity patients. Surg Obes Relat Dis. 2015;11(3):557–563. doi: 10.1016/j.soard.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Zavorsky GS, Kim DJ, Christou NV. Compensatory exercise hyperventilation is restored in the morbidly obese after bariatric surgery. Obes Surg. 2008;18(5):549–559. doi: 10.1007/s11695-008-9437-7. [DOI] [PubMed] [Google Scholar]
- 67.King WC, Belle SH, Eid GM, Courcoulas AP, Eid GM, Flum DR, Mitchell JE, Pender JR, Smith MD, Steffen KJ, Wolfe BM. Physical activity levels of patients undergoing bariatric surgery in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. Surg Obes Relat Dis. 2008;4(6):721–728. doi: 10.1016/j.soard.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torgerson JS, Carlsson B, Stenlof K, Carlsson LMS, Bringman E, Sjostrom L. A low serum leptin level at baseline and a large early decline in leptin predict a large I-year reduction in energy restricted obese humans. J Clin Endocrinol Metab. 1999;84:4197–4203. doi: 10.1210/jcem.84.11.6089. [DOI] [PubMed] [Google Scholar]
- 69.Verdich C, Toubro S, Buemann B, Holst JJ, Bülow J, Simonsen L, Søndergaard SB, Christensen NJ, Astrup A. Leptin levels are associated with fat oxidation and dietary-induced weight loss in obesity. Obes Res. 2001;9:452–461. doi: 10.1038/oby.2001.59. [DOI] [PubMed] [Google Scholar]
- 70.Frühbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Publ Group. 2015;11:465–477. doi: 10.1038/nrendo.2015.84. [DOI] [PubMed] [Google Scholar]
- 71.Ciangura C, Bouillot J-L, Lloret-Linares C, Poitou C, Veyrie N, Basdevant A, Oppert JM. Dynamics of change in total and regional body composition after gastric bypass in obese patients. Obesity. 2010;18:760–765. doi: 10.1038/oby.2009.348. [DOI] [PubMed] [Google Scholar]
- 72.Vanhecke TE, Franklin BA, Miller WM, de Jong AT, Coleman CJ, McCullough PA. Cardiorespiratory fitness and sedentary lifestyle in the morbidly obese. Clin Cardiol. 2009;32(3):121–124. doi: 10.1002/clc.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rankinen T, Bouchard C. Gene–physical activity interactions: overview of human studies. Obesity. 2008;16(Suppl 3):S47–S50. doi: 10.1038/oby.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santarpia L, Contaldo F, Pasanisi F. Body composition changes after weight-loss interventions for overweight and obesity. Clin Nutr. 2013;32:157–161. doi: 10.1016/j.clnu.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 75.Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69:1117–1122. doi: 10.1093/ajcn/69.6.1117. [DOI] [PubMed] [Google Scholar]
- 76.Silver HJ, Torquati A, Jensen GL, Richards WO. Weight, dietary and physical activity behaviors two years after gastric bypass. Obes Surg. 2006;16:859–864. doi: 10.1381/096089206777822296. [DOI] [PubMed] [Google Scholar]
- 77.Pasman WJ, Saris WHM, Westerterp-Plantenga MS. Predictors of weight maintenance. Obes Res. 1999;7:43–50. doi: 10.1002/j.1550-8528.1999.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 78.Klem ML, Wing RR, Chang CH, Lang W, McGuire MT, Sugerman HJ, Hutchison SL, Makovich AL, Hill JO. A case-control study of successful maintenance of a substantial weight loss: individuals who lost weight through surgery versus those who lost weight through non-surgical means. Int J Obes. 2000;24:573–579. doi: 10.1038/sj.ijo.0801199. [DOI] [PubMed] [Google Scholar]
- 79.Shah M, Miller DS, Geissler CA. Lower metabolic rates of post-obese versus lean woman: thermogenesis, basal metabolic rate and genetics. Eur J Clin Nutr. 1988;42:741–752. [PubMed] [Google Scholar]
- 80.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D. Effect of laparoscopic Roux en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patriti A, Facchiano E, Sanna A, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14:840–848. doi: 10.1381/0960892041590818. [DOI] [PubMed] [Google Scholar]
- 82.Wickremesekera K, Miller G, De Silva T, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 83.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38(3):379–383. doi: 10.1161/01.hyp.38.3.379. [DOI] [PubMed] [Google Scholar]
- 84.Martini G, Rivu P, Tabbia F, Molini V, Ferrero GB, Cerutti F, Carra R, Veglio F. Heart rate variability in childhood obesity. Clin Auton Res. 2001;11:87–91. doi: 10.1007/BF02322051. [DOI] [PubMed] [Google Scholar]
- 85.Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94(11):2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 86.Gallagher MJ, Franklin BA, Ehrman JK, Keteyian SJ, Brawner CA, de Jong AT, McCullough PA. Comparative impact of morbid obesity vs heart failure on cardiorespiratory fitness. Chest. 2006;129(2):493–494. doi: 10.1378/chest.127.6.2197. [DOI] [PubMed] [Google Scholar]