Abstract
Water deficit is one of the major limiting factors for apple (Malus domestica) production on the Loess Plateau, a major apple cultivation area in China. The identification of genes related to the regulation of water use efficiency (WUE) is a crucial aspect of crop breeding programs. As a conserved degradation and recycling mechanism in eukaryotes, autophagy has been reported to participate in various stress responses. However, the relationship between autophagy and WUE regulation has not been explored. We have shown that a crucial autophagy protein in apple, MdATG8i, plays a role in improving salt tolerance. Here, we explored its biological function in response to long-term moderate drought stress. The results showed that MdATG8i-overexpressing (MdATG8i-OE) apple plants exhibited higher WUE than wild-type (WT) plants under long-term moderate drought conditions. Plant WUE can be increased by improving photosynthetic efficiency. Osmoregulation plays a critical role in plant stress resistance and adaptation. Under long-term drought conditions, the photosynthetic capacity and accumulation of sugar and amino acids were higher in MdATG8i-OE plants than in WT plants. The increased photosynthetic capacity in the OE plants could be attributed to their ability to maintain optimal stomatal aperture, organized chloroplasts, and strong antioxidant activity. MdATG8i overexpression also promoted autophagic activity, which was likely related to the changes described above. In summary, our results demonstrate that MdATG8i-OE apple lines exhibited higher WUE than WT under long-term moderate drought conditions because they maintained robust photosynthesis, effective osmotic adjustment processes, and strong autophagic activity.
Subject terms: Photosynthesis, Drought, Stomata
Introduction
Drought stress is one of the most widespread environmental constraints and inhibits plant growth and production by affecting every aspect of the plant, especially plant turgor pressure, photosynthetic activity, organelle ultrastructure, metabolism, and hormones1–3. Due to global climate change and the increasingly limited available groundwater for agricultural use, drought stress has become a major threat to crop yields4,5. Hence, it is urgent to develop strategies and technologies to improve the water use efficiency (WUE) of crops. WUE can be defined in different ways; from the perspective of agricultural production, it is described as the amount of plant-produced biomass per unit of water consumed by plants. From a plant physiology viewpoint, WUE is defined as the ratio of the amount of CO2 fixed by photosynthesis to the amount of water vapor lost to the atmosphere6.
Photosynthesis plays a crucial role in plant growth because it produces carbohydrates and oxygen7,8. Greater photosynthetic capacity is one of the key factors involved in improving the WUE of crops without excessive yield penalties under long-term drought conditions. In recent studies, several genes related to photosynthesis have been manipulated to achieve the goal of increased productivity9. Under drought stress, the plant traits that maintain photosynthesis also increase plant growth and WUE10. However, photosynthetic activity is sensitive to water deficits because they result in a rapid decrease in stomatal aperture and damage to the photosynthetic machinery11. Photosystem II (PSII) is vulnerable to damage under various stresses, which results in reductions in electron transport and ATP synthesis12,13. In addition, pigment complexes and chloroplast structures can be destroyed by the excessive reactive oxygen species (ROS) generated under drought stress, resulting in diminished photosynthetic capacity11. Drought stress inhibits all aspects of the photosynthetic system and is ultimately harmful to plant growth.
Plants can alter various metabolic pathways to cope with drought stimuli, such as their carbohydrate and amino acid metabolism14–17. The soluble sugars and polyols accumulated under drought conditions can act as compatible osmolytes, ROS scavengers, energy sources, and signals18–20. Wei et al.21 demonstrated that tetraploid trifoliate orange was more drought tolerant than diploid trifoliate orange due to enhanced sugar accumulation. In addition, drought can induce the accumulation of amino acids, which contribute to maintaining cell turgor and removing excessive ROS22. For instance, proline can serve as an osmoprotectant and ROS scavenger, protecting plants from damage caused by drought and maintaining plant growth under long-term unfavorable environmental conditions23. The capacity to accumulate proline has been shown to be correlated with stress tolerance24. Furthermore, amino acids can also act as alternative energy sources at night, when plant starch resources are limited25.
Autophagy is a conserved eukaryotic mechanism for the degradation of unwanted or damaged proteins and organelles under stress conditions to maintain cell homeostasis26. Researchers have identified >40 autophagy-related genes (ATGs) in yeast27. Among them, 17 core ATGs are reported to participate in the formation of autophagosomes, namely, ATG1-14, ATG16, ATG18, and ATG10128. In particular, ATG8–PE (PE stands for phosphatidylethanolamine) and ATG5–ATG12, which are two ubiquitin-like protein conjugation systems, play a critical role in the expansion of the autophagosome membrane28. The ATG8 protein binds covalently to PE with the mediation of ATG3 and ATG7, and ATG12 binds to ATG5 with the mediation of ATG7 and ATG1029. In addition, the ATG5–ATG12 conjugation system is vital for the conjugation of ATG8 and PE. Over the years, homologs for these core ATGs have been identified in various plant species27.
In plants, autophagy occurs due to multiple environmental stresses, including drought and osmotic conditions30,31. Arabidopsis atg5 and atg7 mutants are hypersensitive to osmotic stress30. Arabidopsis RNAi‐ATG18a plants showed a greater reduction in growth than wild-type (WT) plants under drought and salt stresses32. Accordingly, the overexpression of MdATG18a improved tolerance to severe drought stress in transgenic apple31. The overexpression of MdATG3s isolated from apple in Arabidopsis increased their tolerance to osmotic stress33. In addition, the overexpression of HsfA1a, which regulates the expression of ATG10 and ATG18f in tomatoes, resulted in higher autophagy activity that conferred drought tolerance34. These studies demonstrate that autophagy plays a positive role in improving plant tolerance to drought stress. However, the effect of autophagy on regulating WUE under long-term moderate drought conditions has not been explored.
The semiarid region of the Loess Plateau is one of the largest regions of apple production in China, accounting for nearly 25% of the total national apple production in 201635. However, because of the sufficient light but uneven annual rainfall on the Loess Plateau, water deficit has become the primary limiting factor for apple production. Plants in this region face long-term moderate drought stress. The development of transgenic apple plants with high WUE and production is greatly needed. Previously, we cloned the apple ATG MdATG8i and demonstrated that it functions in apple autophagy in a conserved way36. MdATG8i-overexpressing (MdATG8i-OE) apple lines displayed improved salt tolerance and greater autophagic activity than WT plants37. In this study, we employed MdATG8i-OE apple plants to investigate the function of this gene under long-term moderate drought conditions. We found it particularly interesting that, compared with WT plants, MdATG8i-OE apple plants exhibited higher WUE with minor growth penalties under long-term moderate drought stress, possibly because the OE plants exhibited higher autophagic activity, greater photosynthetic capacity, and better osmotic adjustment. This study provides a promising approach for improving WUE with minimized growth costs in apple under long-term drought stress.
Results
Overexpression of MdATG8i improves growth and WUE in apple under long-term moderate drought stress
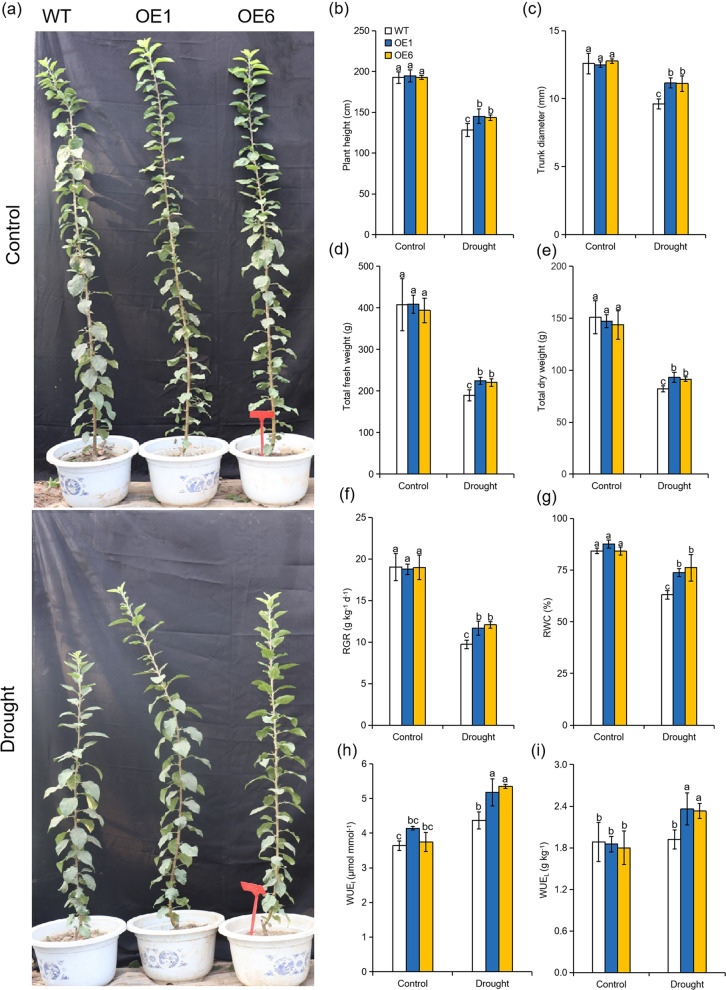
MdATG8i transcription can be induced by sustained moderate drought stress in GL-3 plants (Fig. S1), indicating the potential involvement of MdATG8i in drought tolerance. To analyze the biological function of MdATG8i under long-term moderate drought stress, two previously obtained transgenic lines of MdATG8i-OE apple were used for further treatment37. We investigated the phenotypes of the WT plants and MdATG8i overexpression lines after 80 days of long-term drought treatment (water withheld until 45–50% soil moisture content was reached). The growth phenotypes of the WT and OE plants were not markedly different at the beginning of the long-term experiment or after 80 days normal watering (Fig. S2 and Fig. 1a). After 80 days of the moderate drought treatment, the growth phenotypes were significantly affected in terms of the plant height, trunk diameter, total fresh weight (TFW), total dry weight (TDW), and relative growth rate (RGR). However, the growth of the OE plants was less affected than that of the WT plants under long-term moderate drought conditions. Under sustained moderate drought stress, the transgenic plants had higher plant height and trunk diameter than the WT plants (Fig. 1a–c). Compared with the WT plants, the MdATG8i-OE apple lines accumulated more biomass and maintained a higher RGR after 80 days of the moderate drought treatment (Fig. 1d–f). Additionally, the water deficit treatment for 80 days led to decreased RWC in all genotypes, but the OE lines had significantly higher RWC values than the WT plants (Fig. 1g). High WUE is crucial for plants in adapting to long-term water deficit38. As shown in Fig. 1h, after 80 days of moderate drought, the instantaneous WUE (WUEI) was higher in the MdATG8i-OE plants than in the WT plants. Furthermore, after 80 days of drought treatment, the long-term WUE (WUEL) values of OE1 and OE6 were 22.7 and 21.2%, respectively, higher than that of WT (Fig. 1i). These data indicate that MdATG8i positively regulated the accumulation of biomass and WUE in apple under long-term drought stress.
Fig. 1. Overexpression of MdATG8i promotes growth and water use efficiency (WUE) in apple plants under long-term moderate drought conditions.
Water was withheld to maintain the soil at 45–50% field capacity from June 20, 2018 to September 8, 2018. a Growth phenotypes of WT plants and MdATG8i-overexpressing apple lines after 80 days of growth under normal or moderate drought stress conditions. Comparisons of plant height (b), trunk diameter (c), total fresh weight (d), total dry weight (e), relative growth rate (RGR; f), relative water content (RWC; g), instantaneous water use efficiency (WUEI; h), and long-term water use efficiency (WUEL; i) between WT and transgenic plants under 80 days of normal or moderate drought stress conditions. The data are shown as the means of six replicates with SDs. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Overexpression of MdATG8i enhances photosynthetic capacity in apple under long-term moderate drought stress
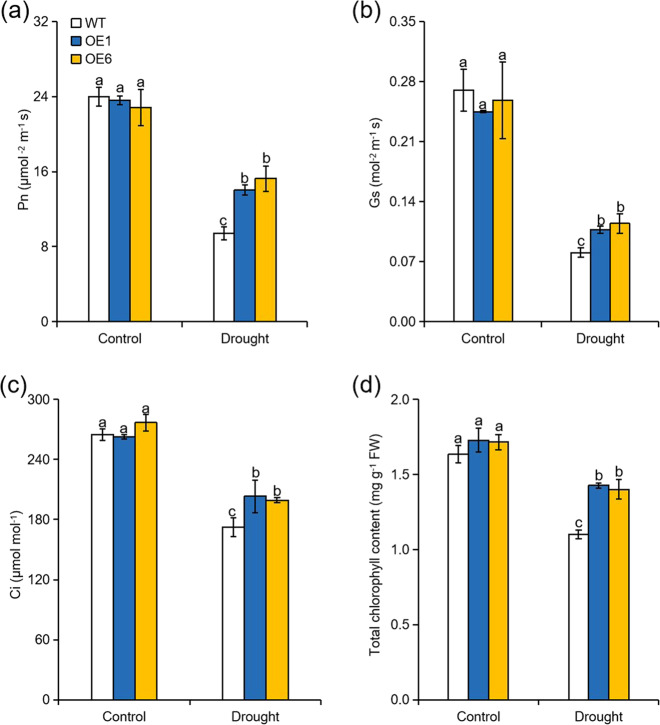
Since there was a difference in growth between the WT and MdATG8i-OE plants after 80 days of drought treatment, we investigated whether there was also a difference in their photosynthetic capacity. We measured the gas exchange parameters and chlorophyll concentrations of all genotypes in the long-term moderate drought treatment. No significant differences in photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), or chlorophyll concentration were observed between the WT and OE plants under normal conditions. These four parameters decreased sharply under sustained moderate drought stress, but the decrease was less severe in the MdATG8i-OE plants than in the WT plants (Fig. 2a–d). After 80 days of the moderate drought treatment, the Pn values of both OE lines were approximately 1.7 times higher than those of the WT plants (Fig. 2a). The Gs, Ci, and chlorophyll concentrations exhibited similar trends (Fig. 2b–d). These results suggest that the MdATG8i overexpression lines exhibited higher photosynthetic capacity than WT under the long-term drought treatment.
Fig. 2. Effect of MdATG8i overexpression in apple on the photosynthetic parameters under normal or moderate drought stress conditions.
Effect of MdATG8i overexpression in apple on the photosynthetic capacity under normal or moderate drought stress conditions. Comparisons of photosynthetic rate (Pn; a), stomatal conductance (Gs; b), intercellular CO2 concentration (Ci; c), and chlorophyll concentration (d) between MdATG8i-overexpressing apple lines and WT plants under 80 days of normal or moderate drought conditions. The data are shown as the means of five replicates with SDs. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Overexpression of MdATG8i alters stomatal parameters in apple under long-term moderate drought stress
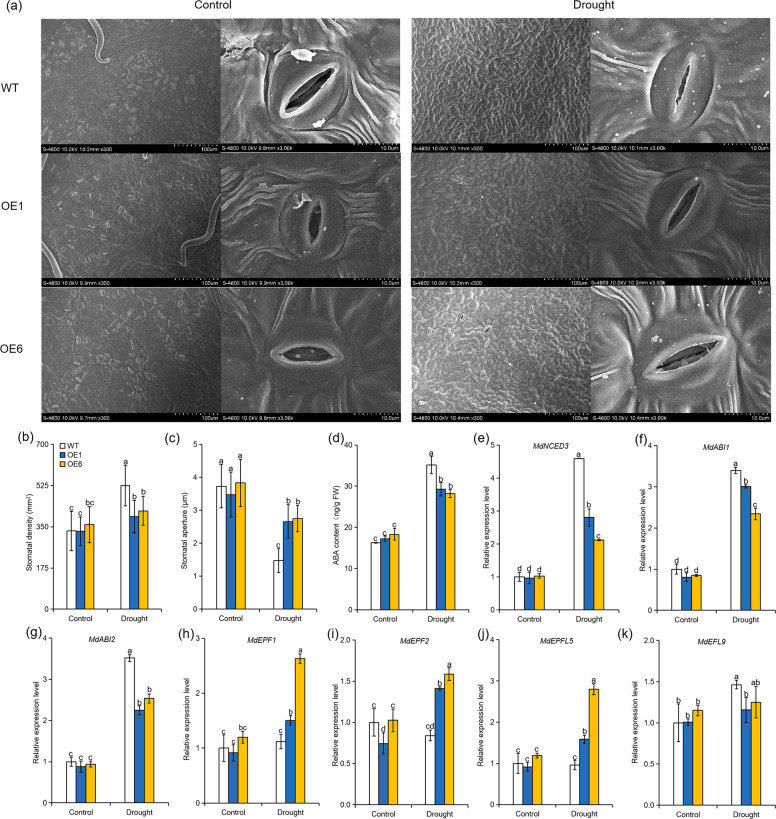
Drought stress can affect stomatal parameters, which are closely related to the photosynthetic activity and water-holding capacity of plants39. Under well-watered growth conditions, there was no difference in stomatal characteristics between the genotypes. The stomatal density was increased in all genotypes after 80 days of drought stress, but the OE plants maintained lower stomatal density than the WT plants (Fig. 3a, b). Meanwhile, the stomatal apertures in the MdATG8i-OE plants were less affected by long-term drought stress than those in the WT plants (Fig. 3c). The ABA level consistently increased more in the WT plants than in the MdATG8i-OE plants in the long-term drought treatment (Fig. 3d). The transcription levels of a key ABA biosynthesis gene and two ABA-responsive genes (MdNCED3, MdABI1, and MdABI2, respectively) were induced more in the WT plants than in the OE plants under drought stress (Fig. 3e–g). In Arabidopsis, epidermal patterning factor (EPF) and EPF-LIKE (EPFL) encode a secreted peptide family (EPF1, EPF2, and EPFL1-9) that plays a vital role in the stomatal development process40. It has been reported that EPF1 and EPF2 negatively regulate stomatal development, whereas EPFL9 regulates stomatal development positively41–43. Jiang et al.42 demonstrated that the ectopic expression of MdEPF2 in tomato led to a reduction in stomatal density in transgenic plants. To investigate the reason for the difference in stomatal development between genotypes under long-term drought conditions, we examined the expression level of several MdEPF family genes, i.e., MdEPF1, MdEPF2, MdEPFL5, and MdEPFL9. As shown in Fig. 3h–j, the transcription levels of MdEPF1, MdEPF2, and MdEPFL5 were more significantly induced by drought stress in the MdATG8i-OE plants than in the WT plants. In contrast, the transcription level of MdEPFL9 was higher in the WT than in the OE plants under drought conditions (Fig. 3k). These data indicate that the overexpression of MdATG8i could influence stomatal behavior and development under sustained moderate drought conditions.
Fig. 3. Stomatal characteristics and ABA content in MdATG8i-overexpressing apple lines and WT plants under normal or moderate drought stress conditions.
a Representative stomatal images observed by scanning electron microscopy (SEM). Comparisons of stomatal density (b), stomatal aperture (c), and ABA content (d) between the WT and transgenic plants. Gene expression analysis of MdNCED3 (e), MdABI (f), MdABI2 (g), MdEPF1 (h), MdEPF2 (i), MdEPFL5 (j), and MdEPFL9 (k) in the WT and OE lines under 80 days of normal or moderate drought stress conditions. The stomatal characteristics data are presented as means ± SDs (n > 30). The ABA content and gene expression data are the means of three replicates with SD. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Overexpression of MdATG8i stimulates ROS scavenging in apple plants under long-term moderate drought stress
Under normal growth conditions, electrolyte leakage and malondialdehyde (MDA) concentrations did not differ substantially among the MdATG8i-OE apple lines and the WT plants. Although the 80-day water deficit led to increased electrolyte leakage and MDA concentrations in apple, the OE plants maintained lower electrolyte leakage values and MDA concentrations than the WT plants (Fig. 4c, d). Drought can induce excessive ROS accumulation, which subsequently damages various cell components. After 80 days of the moderate drought treatment, we stained the leaves with 3,3’-diaminobenzidine (DAB) for hydrogen peroxide (H2O2) detection and nitroblue tetrazolium (NBT) for oxygen free radical (O2−) detection. As shown in Fig. 4a, sustained moderate drought induced ROS accumulation in all genotypes, but more brown areas and blue spots were observed in the leaves of the WT plants than in the leaves of the MdATG8i-OE apple lines. We also measured the ROS levels in guard cells with 2ʹ,7ʹ-dichlorofluorescin diacetate (H2DCFDA), an oxidation-sensitive fluorescence probe. The intracellular ROS levels under drought stress were higher in the WT plants than in the OE lines (Fig. 4b). These observations were consistent with the quantitative measurements of H2O2 and O2−, showing that the OE plants accumulated less ROS than the WT plants under sustained moderate drought conditions (Fig. 4e, f). Furthermore, the activities of superoxide dismutase (SOD) and peroxidase (POD) significantly increased under drought conditions, and a larger increase occurred in the OE lines than in the WT plants (Fig. 4g, h). These results suggest that the MdATG8i-OE lines maintained lower ROS accumulation than WT under drought conditions.
Fig. 4. MdATG8i overexpression improves ROS scavenging ability in response to long-term moderate drought conditions.
a Representative images of leaves showing the accumulation of O2− and H2O2 as revealed by staining with NBT and DAB, respectively, in response to drought stress. b Fluorescence detection of ROS accumulation in guard cells of MdATG8i-overexpressing apple lines and WT plants using H2DCFDA under normal or drought stress conditions. Comparisons of electrolyte leakage (c) and MDA concentrations (d) between WT and transgenic plants. Quantitative measurements of the O2− (e) and H2O2 concentrations (f) in apple leaves after 80 days of normal or moderate drought conditions. Activities of superoxide dismutase (SOD; g) and peroxidase (POD; h) after 80 days of normal or moderate drought conditions. The data are the means of three replicates with SDs. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Overexpression of MdATG8i improves energy conversion efficiency in apple under long-term moderate drought stress
The measurement of chlorophyll fluorescence is widely used for obtaining information on the light energy conversion efficiency of PSII. Under well-watered conditions, no significant differences in Fv/Fm, Y(II), ETR(II), and qP were observed among all genotypes (Fig. 5a–d). After 80 days of the moderate drought treatment, the values of Fv/Fm, Y(II), ETR(II), and qP, which reflect energy conversion in PSII, decreased due to the injury caused by sustained moderate drought. However, the values were significantly lower in the WT plants than in the MdATG8i-OE plants (Fig. 5a–d). Here, we also observed changes in the chloroplast structures of the WT and MdATG8i-OE plants under the sustained moderate drought treatment. Under normal conditions, the chloroplasts were well organized, and the grana were stacked closely in the chloroplasts of the leaves of all genotypes (Fig. 5e). However, the WT plants had a much reduced thylakoid membrane network and less grana stacking in the chloroplasts than the OE lines under long-term drought stress. Additionally, there were more starch granules in the chloroplasts of the OE lines under drought stress (Fig. 5e). Accordingly, the starch content in the mature leaves was notably higher in the MdATG8i-OE plants than in the WT plants under the sustained moderate drought treatment (Fig. 5f). Thus, the overexpression of MdATG8i appeared to improve WUE by mitigating damage to the photosynthetic apparatus under long-term drought stress.
Fig. 5. Comparisons of chlorophyll fluorescence parameters and chloroplast structures between MdATG8i-overexpressing and WT plants under 80 days of normal or drought conditions.
a Maximum quantum yield of PSII (Fv/Fm). b Effective quantum yield of PSII [Y(II)]. c Electron transport rate of PSII [ETR (II)]. d Photochemical quenching (qP). e Transmission electron microscopic (TEM) images of chloroplasts in leaves from MdATG8i-overexpressing apple lines and WT plants. T thylakoid, SG starch granules. Scale bars = 1 μm. f Starch concentrations under normal or drought conditions. The data are the means of three replicates with SDs. Values with the different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Overexpression of MdATG8i improves the accumulation of soluble sugars and amino acids in apple under long-term moderate drought stress
Soluble sugars, which can act as compatible solutes, energy sources, and signals, can be induced by various stresses44. In our study, the concentrations of sorbitol, sucrose, and glucose were significantly elevated by drought stress, and their levels were significantly higher in the MdATG8i-OE plants than in the WT plants (Fig. 6a–c). The transcription levels of the genes encoding the glucose sensor hexokinase (MdHXK1), neutral invertase (MdNINV1/2), cell wall invertase (MdCWINV1/2), and sucrose phosphate synthase (MdSPS6) were elevated more in the MdATG8i-OE plants than in the WT plants in response to the sustained drought stress (Fig. S3).
Fig. 6. Accumulations of soluble sugars and amino acids in the leaves of MdATG8i-overexpressing apple lines and WT plants after 80 days of normal or drought stress conditions.
Comparisons of sorbitol (a), sucrose (b), glucose (c), proline (d), histidine (e), tyrosine (f), valine (g), arginine (h), and glutamine (i) contents between WT and MdATG8i-overexpressing apple plants. The data are shown as the means of three replicates with SDs. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
To investigate whether MdATG8i-mediated drought tolerance was also involved in amino acid metabolism, we determined the levels of 18 amino acids in all genotypes from each treatment. The results are listed in Fig. S4 and Fig. 6d–i. In response to sustained moderate drought stress, the transgenic lines accumulated more of almost all the measured amino acids than the WT plants. However, the concentrations of several amino acids were notably different between the WT and MdATG8i-OE plants under long-term drought conditions. For example, the levels of proline in the OE plants were approximately 1.75-fold those in the WT plants after 80 days of drought treatment (Fig. 6d). In addition, the levels of glutamine, histidine, arginine, tyrosine, and valine were approximately 1.4, 1.5, 2.0, 1.88, and 1.97 times higher, respectively, in the MdATG8i-OE lines than in WT under drought conditions (Fig. 6e–i).
Thus, overexpression of MdATG8i appeared to improve adaptation to drought stress in apple by elevating the accumulation of carbohydrates and amino acids.
Overexpression of MdATG8i enhances the autophagic activity under long-term moderate drought stress in apple
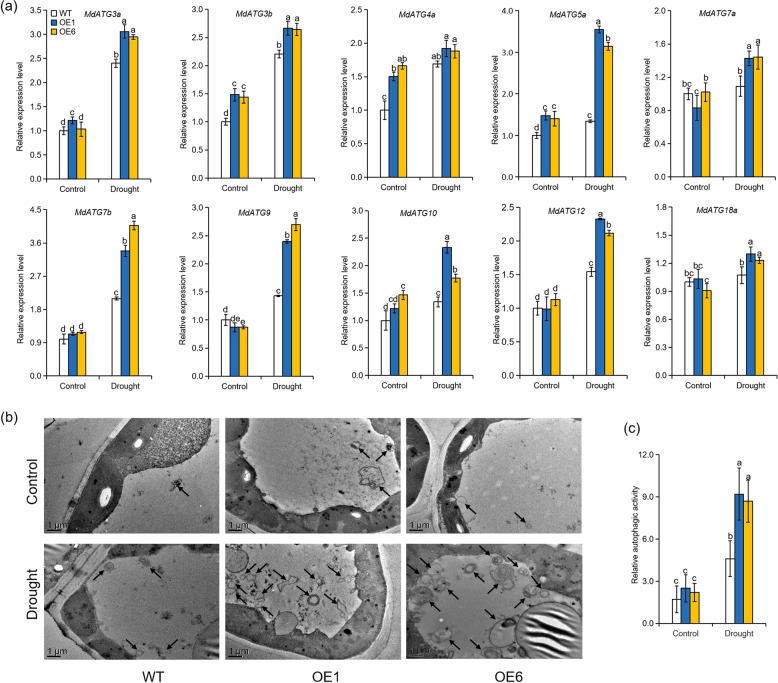
To investigate whether MdATG8i overexpression was related to autophagy under moderate drought conditions, we first determined the transcription levels of several core MdATGs that are ATG homologs in Malus domestica. Under well-watered conditions, the transcription levels of MdATG3a, MdATG7a, MdATG7b, MdATG9, MdATG10, MdATG12, and MdATG18a were similar in all genotypes, whereas the transcription levels of MdATG3b, MdATG4a, and MdATG5a were higher in the MdATG8i-OE plants than in the WT plants (Fig. 7a). As shown in Fig. 7a, the transcription of almost all tested genes could be induced by sustained moderate drought treatment, and this effect was more pronounced in the MdATG8i-OE plants than in the WT plants. To confirm these results, we measured the formation of autophagosomes under long-term drought stress using transmission electron microscopy. Under well-watered conditions, very few autophagic structures were observed in all genotypes. After 80 days of water deficit, the number of autophagic structures in all genotypes increased significantly, and there were nearly twice as many autophagic structures in the MdATG8i-OE plants as in the WT plants (Fig. 7b, c). These results show that the overexpression of MdATG8i contributed to increasing autophagic activity in apple plants exposed to long-term moderate drought stress.
Fig. 7. Transcription levels of apple autophagy-related genes and autophagosomes accumulation in the leaves of MdATG8i-overexpressing apple lines and WT plants after 80 days of a moderate drought treatment.
a Expression levels of apple autophagy-related genes in the leaves of WT and MdATG8i-overexpressing apple plants under moderate drought conditions. b Transmission electron microscopic (TEM) images of the autophagic structures in the leaves of WT and MdATG8i-overexpressing apple plants. The autophagic structures are shown by black arrows. Scale bars: 1 μm. c Relative autophagic activity represented as a comparison of the activities of the WT or transgenic plants shown in b. The results are represented as the means ± SDs. Values with different letters differed significantly at P < 0.05 according to Tukey’s multiple range test.
Discussion
Due to global climate change, water deficit has become a serious problem that limits plant growth and crop yield worldwide45. It is important to develop traits in plants that improve WUE without increasing production costs46. Autophagy has been previously reported to play a significant role in improving plant tolerance to various types of stress26,34,47–50. Our group also conducted several studies related to the potential effects of ATGs on abiotic stress tolerance in apple. For example, we identified MdATG3a and MdATG3b and found that their ectopic expression improved osmotic stress tolerance in Arabidopsis33. The overexpression of MdATG18a enhanced drought tolerance in apple by modifying the antioxidant system and increasing autophagic activity31. A recent study showed that MdATG8i regulated photosynthesis and the accumulation of polyamines, thus enhancing salt stress tolerance in apple37. Instead of looking at the effect of autophagy on tolerance to short-term, severe stresses, as our past studies have done, this work examines the role of autophagy in modulating long-term, adaptive response under moderate drought. In both the short-term stress response and long-term adaptation in apple plants, the ATG was found to be related to changes in antioxidant capacity and upregulated autophagy. Notably, this study demonstrated that MdATG8i-OE-mediated autophagy functioned positively in the adaptive response of apple to long-term drought by maintaining plant growth and improving WUE, which may have been achieved by exerting influence on some adaptive strategies (i.e., maintaining the optimal stomatal aperture, developing organized chloroplasts, and making osmotic adjustments). In summary, this research, together with previous studies, leads to the conclusion that autophagy is a key factor regulating both the stress response and the long-term adaptive response in apple.
Photosynthesis is a key physiological process that directly affects plant production and WUE10,51. Enhancing the photosynthetic ability of plants with slight changes in Gs is considered an ideal approach to improving both yield and WUE10. Zhou et al.38 demonstrated that photosynthesis regulation contributed significantly to higher WUE in apple under drought stress. Autophagy may have a positive effect on the photosynthetic capacity of plants. Chloroplast proteins that are involved in photosynthesis are less abundant in autophagy-defective mutants under nutrient-deficient conditions52,53. In this study, drought stress decreased the photosynthetic efficiency of all genotypes. However, we found that the levels of photosynthetic efficiency in the MdATG8i-OE plants were higher than those in the WT plants under moderate drought conditions. Therefore, the overexpression of MdATG8i appeared to modulate WUE by improving the photosynthetic system in apple under long-term moderate drought conditions.
Drought stress affects the photosynthetic efficiency of plants primarily through stomatal closure54. Some studies have suggested that maintaining a certain degree of stomatal opening benefits plant growth under long-term moderate drought stress55,56. Wang et al.56 demonstrated that the apple cultivar “Qinguan,” which has high WUE, exhibited greater stomatal aperture than the cultivar “Honeycrisp,” which has low WUE, under long-term moderate drought conditions. It is more conducive to plant growth and WUE to maintain a certain degree of stomatal opening in a long-term but relatively mild drought. The phytohormone ABA plays a crucial role in regulating stomatal aperture under drought conditions57. It has previously been reported that ABA contents were elevated in the leaves of the autophagy-defective mutant atg12 even under normal conditions52. Accordingly, we found that the Gs and stomatal apertures in the MdATG8i-OE plants were both larger than those in the WT plants under long-term drought stress; this may have been due to the low ABA level in the OE plants. In addition, autophagy has been reported to help keep the stomata open by regulating ROS homeostasis in guard cells58. Here, the low intracellular ROS levels in the guard cells of the OE plants might have been responsible for the stomatal phenotype under drought stress. The optimized stomatal apertures in the OE plants resulted in improved photosynthetic capacity, which eventually contributed to better growth performance and greater WUE in the transgenic lines than in WT.
In addition to stomatal limitations, nonstomatal limiting factors that damage the photosynthetic apparatus under drought stress also affect the photosynthetic capacity of plants11,55. PSII is the part of the photosynthetic apparatus and is the most vulnerable target of multiple stresses13,59. In this study, the MdATG8i-OE plants had higher light energy conversion efficiency than the WT plants under long-term drought conditions, which demonstrated the protective effect of autophagy on PSII. It has been previously reported that autophagy contributes to degrading damaged chloroplasts to control the quality of cell chloroplasts when plants are subjected to ultraviolet B light or heat stress49,60. Consistently, we also found that the transgenic lines showed less damage to their chloroplasts than the WT plants after 80 days of the drought treatment. In this context, MdATG8i-mediated autophagy may play a role in improving photosynthesis by protecting the photosynthetic apparatus in apple under long-term drought conditions. Furthermore, the toxic ROS generated under drought stress can destroy the photosynthetic apparatus11. The improved photosynthesis in the OE plants might in part be the result of better antioxidant system functioning under long-term drought conditions. Moreover, the milder chloroplast damage and more active PSII in the OE plants also led to the generation of fewer toxic ROS during the drought treatment.
Under water-limited conditions, plants accumulate various compatible solutes to make osmotic adjustments, including soluble sugars and amino acids61–64. Previous works have demonstrated that osmotic adjustment is an important drought adaptation strategy that supports plant growth and development65. It has been previously reported that amino acid and sugar metabolism is substantially modified in autophagy mutants66,67. The autophagic process yields sugars, amino acids, and fatty acids that can be utilized by plants as anabolic substrates or for energy production68,69. Sun et al.50 demonstrated that MdATG18a overexpression promoted the accumulation of carbohydrates in apple under N-depletion conditions. In our research, the OE plants accumulated more soluble sugars and amino acids than the WT plants under long-term moderate drought, leading to better osmotic adjustment and stronger protection of the photosynthetic apparatus. The favorable osmotic adjustment in OE plants contributed to mitigating the negative impacts of drought on plant growth. In addition, the accumulated starch, soluble sugars, and amino acids in the OE plants could act as energy sources under drought conditions and might have been partly responsible for the improved growth and WUE25,70. Due to the active metabolism of sugar and amino acids, the MdATG8i-OE plants had higher WUE and were more adaptive to water deficit stress than the WT plants. However, the metabolism of sugar and amino acids can be influenced by multiple factors, and the specific mechanism by which autophagy affects metabolism under drought conditions requires further exploration.
Usually, plants undergo growth–defense trade-offs when subjected to harsh environmental conditions, and optimal growth traits are achieved at the cost of compromised stress tolerance71. Previous studies have indicated that manipulation of autophagy has an effect on various aspects of plant fitness, including vegetative growth and stress tolerance58,72. Minina et al.73 found that enhanced growth in ATG5- or ATG7-OE Arabidopsis thaliana was accompanied by improved tolerance to oxidative stress. Similarly, in our research, the enhanced autophagic activity in MdATG8i-OE plants resulted in better growth under long-term drought conditions without sacrificing stress tolerance, ultimately leading to improved WUE and drought adaptability. Our results demonstrate that a desirable agronomic trait, high plant WUE with minimal costs to growth, could be developed by modulating autophagy.
Conclusions
In summary, we analyzed the effect of MdATG8i on WUE regulation and plant adaption to drought in apple (Fig. 8). Our results demonstrated that MdATG8i-OE plants exhibited high WUE and drought adaptability with minor biomass penalties under long-term drought conditions. This response could be attributed to the enhanced autophagic activity, greater photosynthetic capacity, and improved osmotic adjustment in the transgenic plants. Under long-term moderate drought conditions, the overexpression of MdATG8i improved photosynthetic efficiency by contributing to maintaining an optimal stomatal aperture, stimulating ROS scavenging, and protecting the photosynthetic apparatus. The higher accumulation of carbohydrates and amino acids in the OE lines than in the WT plants contributed to mitigating osmotic pressure in the OE lines. Furthermore, our results provide new information on the relationship between MdATG8i-mediated autophagy and WUE regulation. The MdATG8i overexpression lines provide an optimal material for the future development of apple varieties that exhibit improved WUE with minor growth penalties under long-term moderate drought conditions.
Fig. 8. A proposed model showing the regulatory function of MdATG8i in response to long-term moderate drought stress in apple.
Under long-term moderate drought conditions, MdATG8i overexpression contributes to maintaining optimal stomatal aperture and organized chloroplasts, resulting in greater photosynthetic capacity and thus higher WUE. Moreover, increased expression of MdATG8i also modulates the accumulation of sugar and amino acids in response to long-term drought stress, resulting in better osmotic adjustment as well as higher WUE and drought adaptability. Most importantly, MdATG8i overexpression promotes autophagic activity, which was likely related to the above changes, eventually resulting in improved WUE and drought adaptability.
Materials and methods
Plant materials and stress treatment
Tissue-cultured GL-3 plantlets of M. domestica (Royal Gala) and MdATG8i-OE lines were cultured as described previously37. The plants were cultivated on MS agar medium containing 0.2 mg L−1 IAA and 0.2 mg L−1 6BA for 30 days. Then, after rooting on MS agar medium containing 0.5 mg L−1 IAA and 0.5 mg L−1 IBA for 40 days, the WT and transgenic seedlings were moved to black plastic pots containing a mixture of soil/roseite/perlite (v:v:v, 3:1:1). After culturing for 30 days in the pots, the seedlings were transferred to larger pots (38 × 23 cm) containing equal mixtures of soil/sand/organic matter (v:v:v, 5:1:1) and were grown in a semiopen greenhouse at Northwest A&F University Yangling (34°20’N, 108°24’E), Shaanxi Province, China under environmental conditions that were similar to field conditions.
Two-year-old plants of similar size were assigned to two treatment groups: (i) normal conditions, daily irrigation to maintain 75–85% field capacity, and (ii) moderate drought conditions, daily irrigation to maintain 45–55% field capacity. On day 80 of this experiment, the 8th to 11th leaves from the base of the stem were collected from 15 plants per treatment. The leaves were rapidly frozen in liquid nitrogen and stored at −80 °C.
RNA extraction and quantitative real-time polymerase chain reaction (PCR) analysis
Total RNA was extracted as previously described by Huo et al.37. Quantitative real-time PCR was carried out following Sun et al.45. The primer sequences used in the expression analysis are listed in Table S1.
Determinations of the growth parameters and WUEL
The measurements of plant height and stem diameter were performed on days 0 and 80 of the long-term drought treatment. Plant heights were measured with a flexible ruler. The stem diameter was determined at 10 cm above the stem base using a digital micrometer (0.001 mm). On days 0 and 80 of this experiment, ten plants in each treatment were harvested. After the TFW was calculated, the samples were dried in an oven at 65 °C for 2 weeks. Subsequently, the TDW was calculated. The RGR was computed as described previously74. The WUEL was calculated using the following formula:
where TDW2 is the TDW of the plant on day 80, TDW1 is the TDW of the plant on day 0, and W is the water consumed during the treatment period75.
Determinations of Pn and chlorophyll fluorescence parameters
Photosynthetic parameters were determined on sunny days (09:00 to 11:00 hours) using a LI-COR 6400 portable photosynthesis system (Li-COR, Huntington Beach, CA, USA). The measurements were performed following Sun et al.31.
Chlorophyll fluorescence was monitored with a Dual-PAM-100 system (Heinz Walz, Effeltrich, Germany), as reported previously76. The chlorophyll fluorescence parameters were measured after the leaves were dark-adapted for 20 min.
Determination of physiological indices
The RWC and relative electrolyte leakage in the leaves were measured as reported previously77,78. The concentration of chlorophyll was obtained based on the method of Lichtenthaler and Wellburn79. The MDA levels of the leaves were measured using the thiobarbituric acid reaction, as previously described by Heath and Packer80.
Determination of ROS accumulation and antioxidant enzyme activity
The accumulation of H2O2 and O2− were measured by staining the leaves in fresh solutions of DAB and NBT, respectively. The activities of SOD and POD and the concentrations of H2O2 and O2− were measured as previously described by Wang et al.56.
Detection of ROS fluorescence in guard cells
The measurements of the ROS level in the guard cells were performed with H2DCFDA. The epidermal strips were transferred to 10 mL of loading buffer (10 mM Tris, 50 mM KCl, pH 6.5) and treated with 50 µM H2DCFDA in the dark for 30–60 min. Afterward, the epidermal strips were washed with loading buffer to remove the excess dye. The fluorescence was determined using confocal microscopy (TCS SP8 SR; Leica) with an excitation wavelength of 460–500 nm.
Determinations of starch, soluble sugars, and amino acids
The starch measurements were performed as previously described by Sun et al.50. The extractions and derivations of the soluble sugars were performed as previously described by Hu et al.81. The extractions of the amino acids were performed as previously described, with minor modifications37. Briefly, 0.1 g of the leaf samples was extracted with 1 mL of 50% ethanol mixed with 0.1 mol/L HCl and centrifuged at 12,000 × g for 10 min. The supernatants were then filtered through 0.22-μm organic filters and diluted 20 times with methanol to measure the concentrations of metabolites using high-performance liquid chromatography–mass spectrometry (QTRAP5500; AB, America).
Observations of the leaf stomata, chloroplasts, and autophagic bodies
After 80 days of the long-term moderate drought treatment, the leaves at the fourth position from the top of the plants were sampled. These samples were immediately cut into small pieces and were fixed in a 4% glutaraldehyde solution in 0.1 M phosphate-buffered saline (pH 6.8) before being maintained at 4 °C for 12 h. Observations of the leaf stomata were performed with a scanning electron microscope (JSM-6360LV; JEOL Ltd., Tokyo, Japan), as previously described by Liang et al.55. Observations of the chloroplast and autophagic bodies were conducted using a transmission electron microscope (JEOL-1230; Hitachi, Tokyo, Japan), as previously reported by Sun et al.31. The stomatal density and aperture were measured with the ImageJ software.
Statistical analysis
SPSS 22.0 software was used for statistical data analysis. All experimental data were subjected to one-way analysis of variance, and the differences between means were assessed by Tukey’s multiple range tests (P < 0.05). The values are represented as means ± standard deviations.
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2019YFD1000102), the National Natural Science Foundation of China (31972391), and the earmarked fund for the China Agricultural Research System (CARS-27). The authors are grateful to Dr. Zhihong Zhang from Shenyang Agricultural University for providing the tissue-cultured GL-3 plants.
Author contributions
F.M., X.G., X.J., and K.M. designed the experiments. X.J., K.M., P.W., and Y.W. performed the experiments and analyzed the data, assisted by M.X.J., L.H., X.S., and R.C. X.J. drafted the manuscript. F.M., X.G., and K.M. provided financial support for the study and critically revised the manuscript. All authors provided final approval of this manuscript.
Conflict of interest
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xin Jia, Ke Mao
Contributor Information
Xiaoqing Gong, Email: gongxq0103@nwsuaf.edu.cn.
Fengwang Ma, Email: fwm64@sina.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41438-021-00521-2.
References
- 1.Chaitanya KV, Jutur PP, Sundar D, Reddy AR. Water stress effects on photosynthesis in different mulberry cultivars. Plant Growth Regul. 2003;40:75–80. doi: 10.1023/A:1023064328384. [DOI] [Google Scholar]
- 2.Bhatt RM, Rao N. Influence of pod load on response of okra to water stress. Indian J. Plant Physiol. 2005;10:54–59. [Google Scholar]
- 3.Zhang N, Zhao B, Zhang HJ, Weeda S, Guo YD. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J. Pineal Res. 2012;54:15–23. doi: 10.1111/j.1600-079X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 4.Jury W, Vaux H. The role of science in solving the world’s emerging water problems. Proc. Natl Acad. Sci. USA. 2005;102:15715–15720. doi: 10.1073/pnas.0506467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao HB, Chu LY, Jaleel CA, Zhao CX. Water-deficit stress-induced anatomical changes in higher plants. C R Biol. 2008;331:0–225. doi: 10.1016/j.crvi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Condon AG, Richards R, Rebetzke G, Farquhar G. Breeding for high water-use efficiency. J. Exp. Bot. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- 7.Yamori W, et al. Enhanced leaf photosynthesis as a target to increase grain yield: insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b (6)/f complex. Plant Cell Environ. 2016;39:80–87. doi: 10.1111/pce.12594. [DOI] [PubMed] [Google Scholar]
- 8.Stitt M, Lunn J, Usadel B. Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J. 2010;61:1067–1091. doi: 10.1111/j.1365-313X.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- 9.Simkin A, Lopez-Calcagno P, Raines C. Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019;70:1119–1140. doi: 10.1093/jxb/ery445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condon AG. Drying times: plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 2020;71:2239–2252. doi: 10.1093/jxb/eraa002. [DOI] [PubMed] [Google Scholar]
- 11.Gururani MA, Venkatesh J, Tran LS. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant. 2015;8:1304–1320. doi: 10.1016/j.molp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Joshi R, Karan R, Singla-Pareek SL, Pareek A. Ectopic expression of pokkali phosphoglycerate kinase-2 (ospgk2-p) improves yield in tobacco plants under salinity stress. Plant Cell Rep. 2016;35:27–41. doi: 10.1007/s00299-015-1864-z. [DOI] [PubMed] [Google Scholar]
- 13.Maxwell K, Johnson GN. Chlorophyll fluorescence–a practical guide. J. Exp. Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- 14.Hare PD, Cress WA, Van StadenJ. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 2000;21:535–553. doi: 10.1046/j.1365-3040.1998.00309.x. [DOI] [Google Scholar]
- 15.Rohit J, et al. Enhancing trehalose biosynthesis improves yield potential in marker-free transgenic rice under drought, saline, and sodic conditions. J. Exp. Bot. 2019;71:653–668. doi: 10.1093/jxb/erz462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu L, et al. Genetic, proteomic and metabolic analysis of the regulation of energy storage in rice seedlings in response to drought. Proteomics. 2011;11:4122–4138. doi: 10.1002/pmic.201000485. [DOI] [PubMed] [Google Scholar]
- 17.Zanella M, et al. β-amylase 1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 2016;67:1819–1826. doi: 10.1093/jxb/erv572. [DOI] [PubMed] [Google Scholar]
- 18.Rosa M, et al. Soluble sugars–metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal. Behav. 2009;4:388–393. doi: 10.4161/psb.4.5.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS. Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol. Plant. 2010;3:942–955. doi: 10.1093/mp/ssq044. [DOI] [PubMed] [Google Scholar]
- 20.Sala A, Woodruff DR, Meinzer FC. Carbon dynamics in trees: feast or famine? Tree Physiol. 2012;32:764–775. doi: 10.1093/treephys/tpr143. [DOI] [PubMed] [Google Scholar]
- 21.Wei T, et al. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019;17:1394–1407. doi: 10.1111/pbi.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowne JB, et al. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant. 2012;5:418–429. doi: 10.1093/mp/ssr114. [DOI] [PubMed] [Google Scholar]
- 23.Tan Z, Wen X, Wang Y. Betula platyphylla BpHOX2 transcription factor binds to different cis‐acting elements and confers osmotic tolerance. J. Integr. Plant Biol. 2020;62:1762–1779. doi: 10.1111/jipb.12994. [DOI] [PubMed] [Google Scholar]
- 24.Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:0–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Izumi M, Hidema J, Makino A, Ishida H. Autophagy contributes to nighttime energy availability for growth in. Arabidopsis. Plant Physiol. 2013;161:1682–1693. doi: 10.1104/pp.113.215632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassham DC, et al. Autophagy in development and stress responses of plants. Autophagy. 2006;2:2–11. doi: 10.4161/auto.2092. [DOI] [PubMed] [Google Scholar]
- 27.Marshall RS, Vierstra RD. Autophagy: the master of bulk and selective recycling. Annu. Rev. Plant Biol. 2018;29:173–208. doi: 10.1146/annurev-arplant-042817-040606. [DOI] [PubMed] [Google Scholar]
- 28.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, et al. A protein conjugation system essential for autophagy. Nature. 1998;395:395–398. doi: 10.1038/26506. [DOI] [PubMed] [Google Scholar]
- 30.Luo L, et al. Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 2017;8:1459. doi: 10.3389/fpls.2017.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, et al. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018;16:545–557. doi: 10.1111/pbi.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Xiong Y, Bassham DC. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy. 2009;5:954–963. doi: 10.4161/auto.5.7.9290. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Sun X, Jia X, Ma F. Apple autophagy-related protein MdATG3s afford tolerance to multiple abiotic stresses. Plant Sci. 2017;256:53–64. doi: 10.1016/j.plantsci.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Cai S, Yin L, Shi K, Zhou J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy. 2015;11:2033–2047. doi: 10.1080/15548627.2015.1098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NBSC. National Database (National Bureau of Statistics of China, 2016).
- 36.Wang P, et al. Characterization of an autophagy-related gene MdATG8i from apple. Front. Plant Sci. 2016;7:720. doi: 10.3389/fpls.2016.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huo L, et al. MdATG8i functions positively in apple salt tolerance by maintaining photosynthetic ability and increasing the accumulation of arginine and polyamines. Environ. Exp. Bot. 2020;172:103989. doi: 10.1016/j.envexpbot.2020.103989. [DOI] [Google Scholar]
- 38.Zhou S, et al. Physiological and proteome analysis suggest critical roles for the photosynthetic system for high water-use efficiency under drought stress in Malus. Plant Sci. 2015;236:44–60. doi: 10.1016/j.plantsci.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Gómez-Bellot M, et al. Water relations, nutrient content and developmental responses of Euonymus plants irrigated with water of different degrees of salinity and quality. J. Plant Res. 2013;126:567–576. doi: 10.1007/s10265-012-0545-z. [DOI] [PubMed] [Google Scholar]
- 40.Lee JS, et al. Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev. 2012;26:126. doi: 10.1101/gad.179895.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Q, et al. Overexpression of MdEPF2 improves water use efficiency and reduces oxidative stress in tomato. Environ. Exp. Bot. 2019;162:321–332. doi: 10.1016/j.envexpbot.2019.03.009. [DOI] [Google Scholar]
- 43.Kondo T, et al. Stomatal density is controlled by a mesophyll-derived signaling molecule. Plant Cell Physiol. 2010;51:1–8. doi: 10.1093/pcp/pcp180. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, et al. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil. 2016;406:231–249. doi: 10.1007/s11104-016-2877-2. [DOI] [Google Scholar]
- 45.Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018;162:2–12. doi: 10.1111/ppl.12540. [DOI] [PubMed] [Google Scholar]
- 46.Bertolino LT, Caine RS, Gray JE. Impact of stomatal density and morphology on water-use efficiency in a Changing world. Front. Plant Sci. 2019;10:225. doi: 10.3389/fpls.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avin-Wittenberg T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019;42:1045–1053. doi: 10.1111/pce.13404. [DOI] [PubMed] [Google Scholar]
- 48.Huo L, Guo Z, Zhang Z, Jia X, Ma F. The apple autophagy-related gene MdATG9 confers tolerance to low nitrogen in transgenic apple callus. Front. Plant Sci. 2020;11:423. doi: 10.3389/fpls.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huo L, et al. MdATG18a overexpression improves basal thermotolerance in transgenic apple by decreasing damage to chloroplasts. Hortic. Res. 2020;7:21. doi: 10.1038/s41438-020-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X, et al. MdATG18a overexpression improves tolerance to nitrogen deficiency and regulates anthocyanin accumulation through increased autophagy in transgenic apple. Plant Cell Environ. 2017;41:469–480. doi: 10.1111/pce.13110. [DOI] [PubMed] [Google Scholar]
- 51.Long SP, Zhu XG, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006;29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 52.McLoughlin F, et al. Maize multi-omics reveal roles for autophagic recycling in proteome remodelling and lipid turnover. Nat. Plants. 2018;4:1056–1070. doi: 10.1038/s41477-018-0299-2. [DOI] [PubMed] [Google Scholar]
- 53.Marien Havé, et al. Proteomic and lipidomic analyses of the Arabidopsis atg5 autophagy mutant reveal major changes in endoplasmic reticulum and peroxisome metabolisms and in lipid composition. N. Phytol. 2019;223:1461–1477. doi: 10.1111/nph.15913. [DOI] [PubMed] [Google Scholar]
- 54.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2008;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang B, et al. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 2018;9:755. doi: 10.3389/fpls.2018.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q, et al. High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’ apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 2019;39:1880–1895. doi: 10.1093/treephys/tpz093. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, et al. Trithorax-group proteins ARABIDOPSIS TRITHORAX4 (ATX4) and ATX5 function in abscisic acid and dehydration stress responses. N. Phytol. 2017;217:1582–1597. doi: 10.1111/nph.14933. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi S, Mano S, Oikawa K, Hikino K, Takemiya A. Autophagy controls reactive oxygen species homeostasis in guard cells that is essential for stomatal opening. Proc. Natl Acad. Sci. USA. 2019;116:19187–19192. doi: 10.1073/pnas.1910886116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havaux M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993;94:19–33. doi: 10.1016/0168-9452(93)90003-I. [DOI] [Google Scholar]
- 60.Izumi M, Ishida H, Nakamura S, Hidema J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell. 2017;29:377–394. doi: 10.1105/tpc.16.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012;63:1593–1608. doi: 10.1093/jxb/err460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gong X, Liu M, Zhang L, Ruan Y, Wang C. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant. 2014;153:119–136. doi: 10.1111/ppl.12225. [DOI] [PubMed] [Google Scholar]
- 63.Hosseini SA, Hajirezaei MR, Seiler C, Sreenivasulu N, von, Wirén N. A potential role of flag leaf potassium in conferring tolerance to drought-induced leaf senescence in barley. Front. Plant Sci. 2016;7:206. doi: 10.3389/fpls.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rui G, et al. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants. 2018;10:016. doi: 10.1093/aobpla/ply016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017;40:4–10. doi: 10.1111/pce.12800. [DOI] [PubMed] [Google Scholar]
- 66.Guiboileau A, et al. Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. N. Phytol. 2013;199:683–694. doi: 10.1111/nph.12307. [DOI] [PubMed] [Google Scholar]
- 67.Masclaux-Daubresse C, et al. Stitching together the multiple dimensions of autophagy using metabolomics and transcriptomics reveals impacts on metabolism, development, and plant responses to the environment in Arabidopsis. Plant Cell. 2014;26:1857–1877. doi: 10.1105/tpc.114.124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, et al. Autophagy contributes to leaf starch degradation. Plant Cell. 2013;25:1383–1399. doi: 10.1105/tpc.112.108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Du Y, et al. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2019;146:1–12. doi: 10.1016/j.plaphy.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Huot B, Yao J, Montgomery B, He S. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Mol. Plant. 2014;7:1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bozhkov PV. Plant autophagy: mechanisms and functions. J. Exp. Bot. 2018;6:1281–1285. doi: 10.1093/jxb/ery070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minina EA, et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018;6:1415–1432. doi: 10.1093/jxb/ery010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radford JP. Growth analysis formulae - their use and abuse. Crop Sci. 1967;7:171–175. doi: 10.2135/cropsci1967.0011183X000700030001x. [DOI] [Google Scholar]
- 75.Ehdaie B. Variation in water-use efficiency and its components in wheat: II. pot and field experiments. Crop Sci. 1995;35:294–299. doi: 10.2135/cropsci1995.0011183X003500060017x. [DOI] [Google Scholar]
- 76.Deng C, Zhang D, Pan X, Chang F, Wang S. Toxic effects of mercury on PSI and PSII activities, membrane potential and transthylakoid proton gradient in Microsorium pteropus. J. Photochem. Photobiol. B. 2013;127:1–7. doi: 10.1016/j.jphotobiol.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Dionisio-Sese ML, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:0–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- 78.Gaxiola RA, et al. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl Acad. Sci. USA. 2001;98:11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;603:591–592. doi: 10.1042/bst0110591. [DOI] [Google Scholar]
- 80.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 81.Hu L, et al. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensisRehd. Plant Physiol. Biochem. 2018;133:116–126. doi: 10.1016/j.plaphy.2018.10.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.